A Nexus Model of Restricted Interests in Autism Spectrum Disorder
- 1Institute of Cognitive Science, University of Colorado Boulder, Boulder, CO, United States
- 2Department of Psychology and Neuroscience, University of Colorado Boulder, Boulder, CO, United States
- 3JFK Partners, Department of Psychiatry and Pediatrics, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
- 4School of Medicine, Carolina Institute for Developmental Disabilities, The University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 5Department of Psychiatry, The University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 6Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Restricted interests (RIs) in autism spectrum disorder (ASD) are clinically impairing interests of unusual focus or intensity. They are a subtype of restricted and repetitive behaviors which are one of two diagnostic criteria for the disorder. Despite the near ubiquity of RIs in ASD, the neural basis for their development is not well understood. However, recent cognitive neuroscience findings from nonclinical samples and from individuals with ASD shed light on neural mechanisms that may explain the emergence of RIs. We propose the nexus model of RIs in ASD, a novel conceptualization of this symptom domain that suggests that RIs may reflect a co-opting of brain systems that typically serve to integrate complex attention, memory, semantic, and social communication functions during development. The nexus model of RIs hypothesizes that when social communicative development is compromised, brain functions typically located within the lateral surface of cortex may expand into social processing brain systems and alter cortical representations of various cognitive functions during development. These changes, in turn, promote the development of RIs as an alternative process mediated by these brain networks. The nexus model of RIs makes testable predictions about reciprocal relations between the impaired development of social communication and the emergence of RIs in ASD and suggests novel avenues for treatment development.
Background
Restricted interests (RIs) in autism spectrum disorder (ASD) are clinically impairing interests of unusual focus or intensity that are a subtype of the restrictive and repetitive behaviors symptom domain of ASD (American Psychiatric Association, 2013). RIs are strongly associated with ASD (Gal, 2011), are typically challenging to treat (Dawson et al., 2010), and are described in some of the earliest accounts of ASD (Kanner, 1943; Asperger, 1944). Additionally, they are less often studied than social communication symptoms of ASD (Richler et al., 2007), making them a promising avenue for improving our understanding of ASD. The manifestations of RIs are highly variable in expression and intensity (Baron-Cohen and Wheelwright, 1999), and early descriptions of RIs in ASD characterized their content or topic area (Kanner, 1943; Asperger, 1944; Baron-Cohen and Wheelwright, 1999; South et al., 2005), though the focus of RIs often change over time and are not easily semantically categorized (Klin et al., 2007). Although the neural bases of RIs are not well understood, recent research has highlighted that RIs engage mesolimbic motivational brain systems (Clements et al., 2018), compete with social stimuli for attentional resources (Sasson and Touchstone, 2014), and prompt effort expenditure to seek out RIs (Traynor et al., 2019) in individuals with ASD.
The goal of this paper is to provide a framework to explain the neural mechanisms underlying the development of RIs. We propose a nexus model of RIs as a brain-based developmental bridge between social communication impairments and RIs in ASD. The nexus model emphasizes that RIs may be conceptualized as a preferred mode of engaging with the world (Baron-Cohen et al., 2003; Klin et al., 2007) that first emerges early in life and suggests that RIs may reflect cognitive abilities and supporting neural systems that are strengthened during development relative to those of individuals without ASD. RIs involve altered development of both the social cognition and reward systems; they are linked and develop in concert. Without social motivation, the perceptual and modeling areas do not develop because social stimuli do not elicit behavior to attend or approach. Without social cognition, social motivation cannot develop optimally because actors and predictions must be neurally represented to be motivating. We recognize the extensive literature documenting associations between the reward system and RIs [e.g., (Dichter et al., 2012; Cascio et al., 2014; Kohls et al., 2018; Harrop et al., 2019) and the role motivation plays in the formation of RIs (Chevallier et al., 2012; Dichter, 2018)]. The mechanism we propose here reflects a complementary framework addressing the influence of motivation on RI development (Dawson et al., 2005; Clements et al., 2018; Dichter, 2018): namely that RIs arise as motivation and cognitive systems are mutually constraining during development in ASD. We hold the description of the interaction between reward systems and social cognitive development for later work. Here, we focus on how the altered development of social cognitive systems could produce RIs.
The Nexus Model of Social Cognition Highlights the Role of the Temporal-Parietal Junction in Social Processing
The nexus model of social cognition (Carter and Huettel, 2013) describes the convergence of cognitive processing streams that are related to preferred modes of engagement. As an example, the temporal-parietal junction (TPJ) is a multimodal brain region involved in attention (Mitchell, 2008; Corbetta and Shulman, 2011; Geng and Vossel, 2013), memory (Berryhill et al., 2007; Cabeza et al., 2012), language (Binder et al., 2009; Donaldson et al., 2015; Redcay et al., 2016), and social processing (Gallagher and Frith, 2003; Saxe and Kanwisher, 2003; Young et al., 2010) functions (Figure 1). This model asserts that novel, complex, predictive social cognitive functions emerge from the functional and anatomical intersection of attention, memory, language, and social processing streams in the brain (Carter and Huettel, 2013), and the combination of output from these processes provides a means to predict future social action by others (Hill et al., 2017). Each constituent process has its own developmental progression that must be successfully completed before it can be combined with other necessary functions to support complex social cognition and social communication (Carter and Huettel, 2013). Given this, a change in the developmental progression of these processing streams may give rise to alternative preferred modes of engagement.
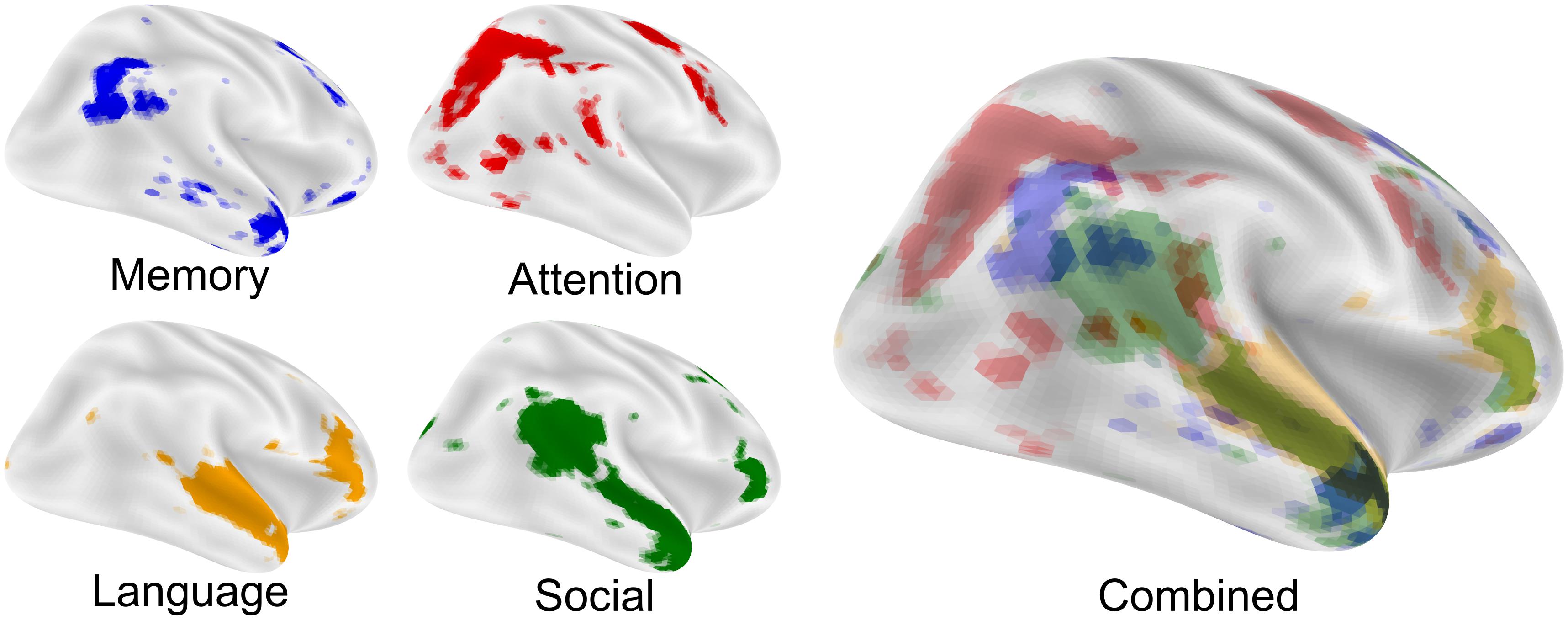
Figure 1. The nexus model of social function. The nexus model hypothesizes that complex social functions arise where memory, attention, language, and social processing come into close proximity and can be combined. Each image is an FDR corrected reverse-inference (likelihood of term given activation) statistical image from neurosynth.org overlaid on an inflated cortical surface using nilearn. Maps were taken from the 200-topic cognitive maps: Memory is topic 28; Attention is topic 64; Language is topic 93 and Social is topic 145. All maps were downloaded in the summer of 2019 from the July 2018 LDA 200-topic model from neurosynth.org based on 14,371 studies.
The development of complex social processes necessarily depends on the prior development of simpler social processes. These social building blocks are bootstrapped through interactive specialization (Johnson, 2011), by which early experiences produce responses that drive the development of more complex functions. For example, analogous to social development, regions of the brain anatomically close to sensory areas drive the functional specialization of regions further from sensory areas as development progresses. Representations grow increasingly abstract as visual information travels from V1 along the ventral visual stream, supporting object recognition (Goodale and Milner, 1992; DiCarlo et al., 2012). A similar increase in complexity is evident for social cognition. Meta-analyses of neuroimaging data show a pattern of activation in response to complex visual social stimuli that predict future actions (Carter and Huettel, 2013). Moving superior and anterior from the lateral-occipital face processing area, cortical regions are sensitive to more complex and abstracted social-cognitive representations (Figure 2). This pattern of increasing complexity in representations of social stimuli is not thought to exist at birth but occurs sequentially in a developmental cascade (Johnson, 2011). In this way, development proceeds sequentially, focusing at first on simpler processes which form the basis for more complex behaviors (Nelson, 1999). This progression is constructivist in that the development of each cognitive function depends upon previous and less complex and abstract functions (Quartz and Sejnowski, 1997), but also relies on nativist principles. In particular, patches of cortex involved in social processing are reliably associated with specific computations (Srihasam et al., 2014; Livingstone et al., 2017) and are consistently anatomically located across individuals. Functions within these same areas are also subject to change by experience (Dehaene et al., 2010) in a manner that relies upon their particular functional network (Mahon and Caramazza, 2011), commonly referred to as experience-dependent plasticity. Social development thus relies on the emergence of specific brain functions at birth and on the reinforcing interactions that occur as these functions are successfully used. The neural development of complex social cognition recapitulates the order of social-developmental milestones in infancy and childhood with implications for ASD (Shultz et al., 2018): should the development of each social area of cortex depend on earlier, simpler areas, as is predicted by interactive specialization, decreased orienting to social stimuli, or any deficiency in the ability to represent those stimuli, may leave areas of cortex critical for social cognition underdeveloped.
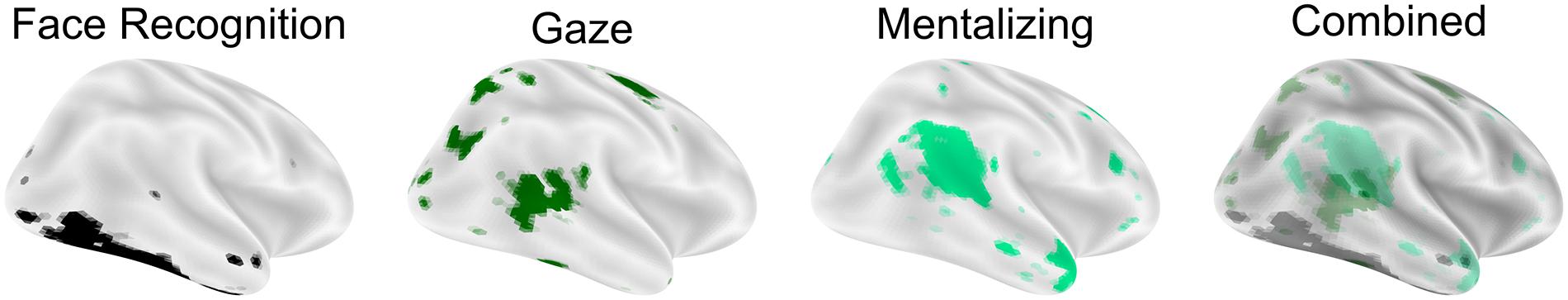
Figure 2. Hierarchical construction of flexible social cognition. Social cognitive processes begin with face recognition on the lateral occipital surface and more superior/anterior regions representing more complex aspects of social cognition. Images are surface overlay of a reverse-inference maps for the terms “face recognition,” “gaze,” and “mentalizing” downloaded from neurosynth.org in the summer of 2019 (based on 14,371 studies).
Altered Developmental Trajectories Lead to Preferred Modes of Engagement in ASD
In the absence of valued (Chevallier et al., 2012; Dichter et al., 2012; Abrams et al., 2013; Chen et al., 2015) or easily interpretable (Sasson et al., 2013; Hohwy and Palmer, 2014) social input, neural systems supporting complex social cognitive hierarchies fail to develop, leaving cortical areas that are critical for social cognitive functions potentially capable of assuming, at least in part, alternative functions. When typical input to a brain region is disrupted, the region often takes on meaningful functions using alternative or indirect input. For example, neurons in visual cortex in the congenitally or early blind are selectively active during braille reading (Sadato et al., 1996, 2004; Cheung et al., 2009), speech processing (Kujala et al., 2005; Hertrich et al., 2009; Dietrich et al., 2013), or even patterned electrical stimulation to the tongue (Ptito et al., 2005; Matteau et al., 2010). For reasons that are not clearly understood, social stimuli activate reward systems, including the dorsal and ventral striatum and medial prefrontal cortex, to a lesser degree in ASD (Clements et al., 2018; Kohls et al., 2018; Supekar et al., 2018). A critical implication of the nexus model of RIs in ASD is that when rewarding responses to social stimuli are dampened early in development, areas of the brain that govern more complex social cognitive processes will develop in a delayed fashion. Critically, experience-dependent and experience-expectant social-cognitive brain areas would show altered developmental trajectories and thus would be relatively more responsive to other, non-social sources of input (Butz et al., 2009; Holtmaat and Svoboda, 2009). In the same way that visual cortex utilizes new inputs for spatial processing when visual information is unavailable, areas of cortex that typically predict future states of complex social stimuli may instead make predictions about RI-related stimuli. Extending the nexus model, any of the cognitive processes that typically neighbor social processing in the cortex (Figure 3) may expand into underdeveloped social areas of the brain. This biased flexibility in processing is inherent in the theory of interactive specialization and may provide a mechanism for better characterizing regional brain functions. The interactive development of cortex is also hypothesized to underlie the development of language-specific cortical areas. Through a mechanism described as cortical recycling, Dehaene and colleagues hypothesize that the visual word form area develops from cortical tissues previously devoted to face and object processing (Dehaene and Cohen, 2011). This link between face and language processing is akin to the repurposing of social communication brain regions for RI processing.
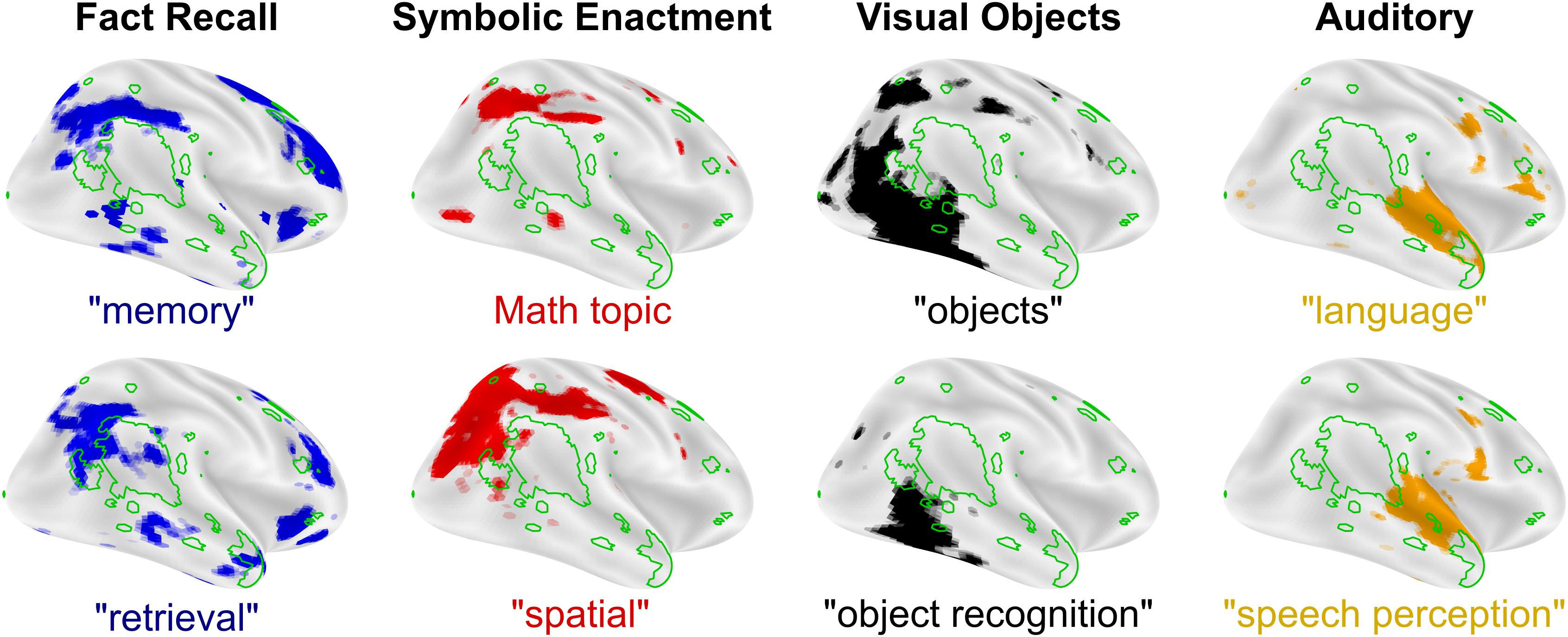
Figure 3. Cognitive processes and preferred modes of engagement. Undeveloped complex social functions like mentalizing (Neurosynth reverse inference “mentalizing,” green outline) may leave areas of cortex open to expansion from neighboring cognitive functions that can be mapped to preferred modes-of-engagement for RIs in autism. FDR corrected, reverse inference (likelihood of term given activation) statistical images from neurosynth.org are overlaid on inflated cortical maps using Nilearn for each term in quotes. “Math topic” is Topic number 2 from version 5 (July 2018) of the Neurosynth set of LDA derived topic maps with prominent terms like: problem(s), arithmetic, solving, and calculation. All maps were downloaded in the summer of 2019 (based on 14,371 studies).
The nexus model of RIs suggests that brain regions that typically mediate social communication, including most centrally the TPJ, demonstrate a functional shift during development towards processing information related to RIs. This co-opting highlights a perspective that RIs reflect a preferred mode of engaging with the world in individuals with ASD. Whereas early descriptions of RIs in ASD characterized the specific content or topic area of RIs (Kanner, 1943; Asperger, 1944; Baron-Cohen and Wheelwright, 1999; South et al., 2005) (e.g., folk physics, objects, or machines), a broader perspective on RIs (as a preferred mode of engagement) accounts for changes in RIs over time. It also accounts for the fact that RIs are not always easily categorized semantically, and emphasizes types of interactions that may be part of an individual’s RI (e.g., fact memorizing, counting, spatial manipulation, and categorizing), (Attwood, 2003; Baron-Cohen et al., 2003; South et al., 2005; Klin et al., 2007). The perspective that RIs reflect a preferred mode of engagement suggests that RIs reflect cognitive abilities that are relatively enhanced and consequently pursued (Spiker et al., 2012) and represents a more parsimonious account of RIs as reflecting altered functioning of brain systems that mediate higher-order cognitive processes in individuals with ASD, as will be described in the next section.
A Nexus Model of RIs in ASD
In this section, we focus on four cognitive functions represented in cortical and neighboring social processing brain regions that may be linked to preferred modes of engagement and RIs in ASD: (1) memory (e.g., fact recall RIs), (2) spatial attention (e.g., calculating, building, and mapping RIs), (3) object processing (e.g., object expertise and reading RIs), and (4) auditory processing (e.g., tone and speech RIs).
Memory recall is associated with activation in the posterior parietal cortex, an area of the brain that anatomically neighbors mentalizing functions in the TPJ (Figure 3, left). There are some documented changes in the hippocampus in ASD (Barnea-Goraly et al., 2014; Trontel et al., 2015; Cooper et al., 2017). However, if the hippocampus were the primary driver of memory deficits in ASD, these deficits found in individuals with ASD would be expected to be general in nature. Instead, the majority of studies that find changes in memory in ASD report specific impairments, and not a general deficiency (Boucher, 1981; Renner et al., 2000; Toichi and Kamio, 2002, 2003). Higher functioning individuals with ASD perform well on standard cued recall and paired-association learning (Boucher and Warrington, 1976; Minshew and Goldstein, 2001; Williams et al., 2006) and recall tasks involving non-social items, such as buildings and leaves, but have been found to perform poorly on face memory tasks (Blair et al., 2002). Neurobiologically, specific memory deficits like these are unlikely to be due to changes in the hippocampus, as patients with hippocampal lesions show broad memory deficiencies (Winocur and Weiskrantz, 1976; Shimamura and Squire, 1988; Holdstock et al., 2002). Instead, these specific deficits are better attributed to memory recall functions in the posterior parietal cortex (Hadjikhani et al., 2004), which, when lesioned, produce subtle and selective memory deficits resembling episodic memory impairments (Boucher and Mayes, 2012). Thus, parietal cortical mechanisms are implicated in memory-based RIs and improved memory recall observed in ASD. Developmentally, early changes in motivation or exposure to social stimuli may alter development of the lateral surface of the cortex in ASD. Variations in the precise timing of these changes would result in memory alterations in ASD, ranging from memory deficiencies to memory enhancement (Rimland, 1978), which may result in memory-related RIs in some individuals with ASD.
Second, spatial attention, numeracy, and timing are related to automatic and volitional attentional control (Hartje, 1987; Walsh, 2003), and these functions each produce activity adjacent to social processing brain areas in the intraparietal sulcus (Hubbard et al., 2005; Ansari et al., 2007; DeWind et al., 2015) (Figure 3, left-middle) that are themselves implicated in ASD (Allman et al., 2011b; Dichter, 2012). Some individuals with ASD exhibit evidence of compromised functioning of attentional streams reflected in less frequent (Baranek, 1999) and slower (Wainwright and Bryson, 1996; Keehn et al., 2010) orienting. Others studies, however, have documented evidence of enhanced spatial attention in ASD (Mottron et al., 2006), reflected in faster responses on a conjunctive visual search task (Jarrold et al., 2005) and greater accuracy in the embedded figures (Jolliffe and Baron-Cohen, 1997) and block design (Tymchuk et al., 1977; Siegel et al., 1996) tasks. There are also examples of compromised numeracy (Meaux et al., 2014) and timing (Allman et al., 2011a) in ASD. In functional neuroimaging studies of selective attention, there is evidence that activation in the dorsal attention stream (including the intraparietal sulcus) is greater or more variable in ASD (Belmonte and Yurgelun-Todd, 2003). A popular theory for explaining better visual performance in some cases is that individuals with ASD have enhanced perceptual function (Mottron et al., 2006), focusing on simpler, rather than more abstract, dimensions (Bertone et al., 2005) or on local, rather than global, configurations (Rinehart et al., 2000) of visual stimuli. The nexus model of RIs would hypothesize that these differences may reflect improved spatial attention, numeracy, and timing due to expanded representations into cortical brain regions, including specifically the TPJ.
Third, object sensitive areas of the brain are arranged along the lateral occipital surface of the brain (Figure 3), adjacent to areas of the brain that respond to faces, emotions, and eye-gaze (Figure 2). Enhanced visual perception has been reported in a number of contexts in ASD, including fine pattern discrimination (Plaisted et al., 1998a), conjunction search (Plaisted et al., 1998b), orientation (Bertone et al., 2005), and other tasks (Dakin and Frith, 2005). Performance improvements in visual perception in ASD tend to be constrained to lower-order abilities, whereas deficits in integrative and holistic tasks are commonly reported in ASD (Happé and Frith, 2006). Brain areas that mediate many of these enhanced processes border the lateral occipital face area of the brain, raising the possibility that small differences in performance on these tasks in early childhood may developmentally expand these cortical representations, leading to expanded lower-order visual processing and by extension reduced focus on social stimuli. In fact, a common occurrence in ASD is hyperlexia, a syndrome that relies on character and word recognition areas of the brain in the ventral visual pathway that borders face processing brain areas (Ostrolenk et al., 2017), offering a potential mechanistic explanation for the co-occurrence of diminished social function and hyperlexia in ASD.
Relatedly, an early hypothesis for social communication deficits in ASD is a diminished ability to process faces (Dawson et al., 2002). Although this deficit is not universally true, individuals with ASD tend to have a diminished ability to recognize unfamiliar faces (Weigelt et al., 2012; Tang et al., 2015), which in turn may also impact social functions including social memory (Ewbank et al., 2017), social eyescan paths (Pelphrey et al., 2002), social emotion recognition (Adolphs et al., 2001; Bal et al., 2010), and face inversion effects (Scherf et al., 2008), though there may be compensation for familiar or enhanced stimuli (Pierce et al., 2004; Pierce and Redcay, 2008). Proposed explanations for diminished face recognition in ASD are that it reflects lower-level perceptual impairments (Behrmann et al., 2006), reduced generalization (Plaisted, 2001), and enhanced perceptual function (Mottron et al., 2006) [for review see Mottron et al. (2009); Thye et al. (2018)]. Although low-level perceptual changes explain enhanced functions in ASD, understanding higher-order impairments has traditionally relied on an additional proposed mechanism, such as diminished veridical mapping and weak central coherence. While not typically described in terms of a specific brain mechanism, these compromised higher-order processes are mediated by the TPJ (Dakin and Frith, 2005). As in memory and attentional RIs, this heterogeneity of visual phenotypes in ASD hints at variations in developmental trajectories that depend on preferred modes of engagement and the development of lower-level processes that support a specific RI class. Because object and text sensitive brain areas (Figure 3, right-middle) abutt brain regions specialized for face and emotional processing, they are positioned to expand in cases where face processing is compromised during development. This link between objects/text and social brain areas predicts that face processing areas of the brain may respond preferentially to RIs that focus on text and objects. For example, a case study of a boy with ASD found greater brain activation to his RI (a DigimonTM cartoon character) in the fusiform face area (FFA) compared to human face stimuli (Grelotti et al., 2005). This study demonstrated the possibility of plasticity in FFA responses in ASD and served as evidence that lower-level face processing brain regions may develop to respond to non-face stimuli in ASD. Foss-Feig et al. (2016) compared responses to object RIs of individuals with ASD to responses to intense interests of typically developing individuals. Both groups exhibited greater activation in the FFA in response to their own RIs. In addition, activation was more robust for object RIs in ASD compared to responses of typically developing individuals. Numerous additional studies have shown that the FFA of individuals with ASD responded more to non-social stimuli (Pierce and Redcay, 2008; Perlman et al., 2011; Foss-Feig et al., 2016; Whyte et al., 2016). We include cartoon and video-game characters as object RIs since their treatment is mechanistic in nature. In typically developing individuals, the study of expertise has shown increased activation in the FFA when viewing the focus of their expertise (Gauthier et al., 2000), supporting the argument for repurposing based on interest more broadly. We hypothesize that this modified development of functional specialization can drive changes in the ascending social cognitive hierarchy, reshaping dynamic social cognitive areas of the brain to respond to dynamic aspects of RIs.
Fourth, auditory areas of the brain are organized along the superior temporal sulcus just anterior to mentalizing areas in the TPJ (Figure 3, right). Auditory processing in ASD is atypical in a number of ways, including hyper- and hypo-sensitivity and unusual abilities like absolute pitch [for review see Samson et al. (2006)]. As with visual perception, individuals with ASD commonly show enhanced lower-order perception (Bonnel et al., 2003) and diminished abilities when working with complex voice stimuli (Gervais et al., 2004). It is, however, important to note that, as with visual stimuli, familiar voice recognition is not impaired in ASD (Boucher et al., 2000). Impairments in processing of complex auditory stimuli in ASD include both spectral and temporal aspects with compensation occuring when lower-order auditory processing may be leveraged to complete the task (Samson et al., 2006). Accordingly, auditory RIs in ASD are often related to music (Sacks, 2008), for which preferences are similar to typically developing individuals (Boso et al., 2009). In fact, impaired functional brain connectivity during speech processing may be improved by a transition to singing in ASD (Sharda et al., 2015), consistent with greater emotional comprehension for music that has been observed in ASD (Molnar-Szakacs and Heaton, 2012). In line with these findings, neuroimaging studies show a familiar pattern: areas of the brain that typically respond to speech respond more strongly to music in ASD (Lai et al., 2012). This cortical shift from processing social stimuli to processing non-social stimuli parallels the findings in the ventral visual stream discussed above, and may reflect a co-opting of typically social processing pathways in favor of responding to RIs. This repurposing of neural regions again indicates that areas of the brain that typically respond to voices would respond preferentially to auditory RIs in ASD.
Although we have described each of these cognitive functions as separate, they likely compete for processing resources during development, suggesting that both in ASD and typically developing individuals these processes may be more or less dominant. It also suggests that combinations of preferred modes of engagement would be common, as long as preferred functions related to RIs are mediated by brain areas typically used by functions that have been diminished. For example, as noted in this section, responses to music may be mediated by brain regions that typically respond to voices and responses to text or objects may be mediated by brain regions that typically respond to social stimuli such as faces. The increased processing of non-social stimuli in lower-order social processing brain areas would drive the development of non-social functional specialization in integrative brain areas, including the TPJ. We next explore preliminary evidence for such an outcome.
Restricted Interests as Both Strengths and Challenges
Restricted interests (RIs) are a prominent characteristic of ASD that, by definition, cause impairment. RIs interfere with social development (Attwood, 2003; Turner-Brown et al., 2011), restrict the experiences of young children with ASD (Pierce and Courchesne, 2001) and interfere with learning adaptive behaviors (Koegel and Covert, 1972; Koegel et al., 1974; Varni et al., 1979). Direct tradeoffs between engaging with social stimuli and RIs have been observed in eye-tracking experiments (Sasson et al., 2008, 2011; Sasson and Touchstone, 2014; Unruh et al., 2016) and in reports of peer engagement (Boyd et al., 2007; Jordan and Caldwell-Harris, 2012) in individuals with ASD. RIs and social stimuli may directly compete for neural and attentional resources (Richey et al., 2014; Unruh et al., 2016). However, RIs may also represent areas of cognitive strengths (Grigorenko and Sternberg, 1997; Céline et al., 2000; Armstrong, 2011, 2014; Caldwell-Harris and Jordan, 2014). If social processing areas of the brain develop to preferentially respond to a preferred mode of engagement, then performance within that mode may exceed performance in other modes and may exceed that of typically developing individuals. Savant syndrome is a condition in which individuals who show substantial performance deficits in many areas have very specific areas in which they excel. This condition occurs in only ∼10% of individuals with ASD (Rimland, 1978) but approximately half of individuals with savant syndrome also have ASD (Treffert, 2009). Rimland’s 1978 survey found prodigious memory in nearly all individuals with savant syndrome with 53% focused on music, 40% on memorization, 25% on mathematical or calculating skills and 19% on art, categories that fit well with the nexus model of RIs. Even in individuals with ASD without savant syndrome, there are performance increases in narrow skill areas (Tymchuk et al., 1977). Approximately half of individuals with ASD show substantially better performance on the block design portion of intelligence tests compared to 2% of typically developing individuals (Caron et al., 2006). Enhanced performance on the block design task for individuals with ASD is generally attributed to enhanced perceptual function (Caron et al., 2006), which is also consistent with enhanced processing of auditory stimuli such as pitch and height discrimination of pure tones (O’Riordan and Passetti, 2006), complex tones (Heaton et al., 2008) and musical material (Mottron et al., 2000). Enhanced block-design performance may be due to additional processing resources in the spatial attention areas of the brain. Accordingly, individuals with non-spatial/symbolic preferred modes of engagement may show performance peaks on other tasks matched to their preferred mode of engagement.
Temple Grandin describes the importance of helping a child with ASD to find their strengths (Grandin, 2011), and indeed RIs can be leveraged to improve social functioning in ASD intervention contexts (Kasari et al., 2006; Boyd et al., 2007; Schertz and Odom, 2007). RIs have also been shown to be positive targets for therapy (Charlop-Christy and Haymes, 1996; Carnett et al., 2014; Patten Koenig and Hough Williams, 2017) and can positively affect social abilities when they are incorporated into treatment (Boyd et al., 2007; Koegel et al., 2012, 2013; Harrop et al., 2019). The Early Start Denver model, for example, is an approach that aims to develop skills by rewarding pro-social behaviors during early developmental periods when repurposing of neural pathways is most likely (Dawson et al., 2010). In the film Life Animated, a child with ASD has an RI that is focused on sidekicks in Disney movies. His parents were able to engage with their son by using the voices of these characters and through this interaction, gradually encouraged him to increase his social interactions more broadly. Modern virtual reality approaches raise a new possibility that dynamic and interactive worlds can be produced based on individual RIs (Thies et al., 2016), potentially enabling the automated development of teachers based on preferred mode of engagements that could dramatically improve the lives of some individuals with ASD.
Challenges to the Nexus Model of RIs, Future Directions, and Conclusions
There are several clear challenges to the nexus model of RIs. First, there is mixed support for a relationship between RI intensity and social impairment. While some studies of RIs have found such a relationship (Turner-Brown et al., 2011; Jordan and Caldwell-Harris, 2012), others have failed to do so (Lam et al., 2008). Whereas the relationship between social and non-social behaviors may be complex and non-linear, neuroimaging studies testing the nexus model of RIs would need to be sufficiently powered to address this potential inconsistency. Second, clearly brain regions outside of social processing systems are implicated in ASD. For example, recent work in a large, multi-study sample did not find reduced activation in the TPJ during a false belief task in individuals with ASD (Dufour et al., 2013). Third, a preference for systematizing has been shown to be greater in individuals with ASD (Turner-Brown et al., 2011), but it is not clear how this preference relates to the nexus model. Finally, rather than a co-opting of social communication brain networks, RIs have also been hypothesized to reflect impaired executive function that results in perseverative behaviors (Russell, 1997; Turner, 1999), an account that differs in testable ways from the implications of the nexus model of RI development. A related hypothesis suggests that insistence on sameness and habitual behavior could be due to changes in the basal ganglia (Calderoni et al., 2014; Sinha et al., 2014; Kohls et al., 2018), which is related to habitual behaviors in typically developing individuals.
A hypothesized framework whereby RIs reflect canalization of social processing brain systems during development in ASD makes a number of testable predictions. First, RIs should reflect preferred modes of engagement rather than specific topics. While there is evidence for this conceptualization of RIs (Baron-Cohen et al., 2003; Klin et al., 2007), future RI studies should assess modes of engagement to allow for a formal comparison of this and more topic-driven models. Second, individuals with a particular RI should have thicker or expanded cortical gray matter in brain areas associated with processing that specific RI. For example, an individual with a symbolic enactment RI (e.g., counting) should show greater gray-matter thickening or larger cortical areas near the intraparietal sulcus. Additionally, areas of the brain typically responsive to social stimuli (especially those that are spatially proximal) should respond to RIs to a greater degree in individuals with ASD. For example, the TPJ would be hypothesized to show more activation to spatial attention and numeric tasks than in typically developing individuals given that their preferred mode of engagement is attention-oriented. Lastly, using brain network analysis, the nexus model of RIs would predict that the processing stream associated with the preferred mode of engagement should be more locally integrated than processing streams associated with less preferred modes of engagement. Similar functional processing streams (Margulies et al., 2016) as well as their alteration in ASD (Hong et al., 2019) lend early support for this possibility.
Conclusion
We have described a possible developmental neural mechanism that may lead to RIs in which the altered development of social communication processing brain areas result in preferred modes of engagement and ultimately RIs. The nexus model of RIs suggests testable hypotheses about the reciprocal relation of RIs and impaired social communication and highlights novel avenues for treatment development. Most critically, although ASD research has often addressed social communication impairments and restricted and repetitive behaviors as distinct symptom domains, the nexus model of RIs suggests that social communication skills and RIs may be functionally linked during development, and that any comprehensive intensive early ASD intervention must address both social communication and RIs to be maximally effective.
Data Availability Statement
Meta analyses figures were generated from publicly available utilities (neurosynth.org).
Author Contributions
All authors participated in the preparation and review of the manuscript. RC generated figures.
Funding
This work was supported by a Young Investigator Award from the Brain Behavior Research Foundation, a Beverly Sears Graduate Student Grant, and the Institute of Cognitive Science at the University of Colorado Boulder. GD was also supported by MH110933.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Tim Curran and Isabel Gauthier for valuable comments on the manuscript. We would also like to thank the staff and students of the Temple Grandin School for their role in the development of these ideas and for their commitment to the community.
Abbreviations
ASD, autism spectrum disorder; FFA, fusiform face area; RI, Restricted Interest; TPJ, Temporal-Parietal Junction.
References
Abrams, D. A., Lynch, C. J., Cheng, K. M., Phillips, J., Supekar, K., Ryali, S., et al. (2013). Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc. Natl. Acad. Sci. U.S.A. 110, 12060–12065. doi: 10.1073/pnas.1302982110
Adolphs, R., Sears, L., and Piven, J. (2001). Abnormal processing of social information from faces in autism. J. Cogn. Neurosci. 13, 232–240. doi: 10.1162/089892901564289
Allman, M. J., DeLeon, I. G., and Wearden, J. H. (2011a). Psychophysical assessment of timing in individuals with autism. Am. J. Intellect. Dev. Disabil. 116, 165–178. doi: 10.1352/1944-7558-116.2.165
Allman, M. J., Pelphrey, K. A., and Meck, W. H. (2011b). Developmental neuroscience of time and number: implications for autism and other neurodevelopmental disabilities. Front. Integr. Neurosci. 6:7. doi: 10.3389/fnint.2012.00007
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Washington, DC: American Psychiatric Pub.
Ansari, D., Lyons, I. M., van Eimeren, L., and Xu, F. (2007). Linking visual attention and number processing in the brain: the role of the temporo-parietal junction in small and large symbolic and nonsymbolic number comparison. J. Cogn. Neurosci. 19, 1845–1853. doi: 10.1162/jocn.2007.19.11.1845
Armstrong, K. A. (2014). Interests in Adults with Autism Spectrum Disorder. Ph.D. thesis, Simon Fraser University, Burnaby.
Armstrong, T. (2011). The Power of Neurodiversity: Unleashing the Advantages of Your Differently Wired Brain (published in Hardcover as Neurodiversity). Boston, MA: Da Capo Press.
Asperger, H. (1944). Die autistischen Psychopathen im Kindesalter. Arch. Psychiatr. Nervenkr. 117, 76–136. doi: 10.1007/bf01837709
Attwood, T. (2003). “Understanding and managing circumscribed interests,” in Learning and Behavior Problems in Asperger Syndrome, ed. M. Prior (New York, NY: Guilford Press), 126–147.
Bal, E., Harden, E., Lamb, D., Van Hecke, A. V., Denver, J. W., and Porges, S. W. (2010). Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. J. Autism Dev. Disord. 40, 358–370. doi: 10.1007/s10803-009-0884-3
Baranek, G. T. (1999). autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J. Autism Dev. Disord. 29, 213–224. doi: 10.1023/A:1023080005650
Barnea-Goraly, N., Frazier, T. W., Piacenza, L., Minshew, N. J., Keshavan, M. S., Reiss, A. L., et al. (2014). A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 124–128. doi: 10.1016/j.pnpbp.2013.09.010
Baron-Cohen, S., Richler, J., Bisarya, D., Gurunathan, N., and Wheelwright, S. (2003). The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 361–374. doi: 10.1098/rstb.2002.1206
Baron-Cohen, S., and Wheelwright, S. (1999). “Obsessions” in children with autism or Asperger syndrome. Content analysis in terms of core domains of cognition. Br. J. Psychiatry 175, 484–490. doi: 10.1192/bjp.175.5.484
Behrmann, M., Thomas, C., and Humphreys, K. (2006). Seeing it differently: visual processing in autism. Trends Cogn. Sci. 10, 258–264. doi: 10.1016/j.tics.2006.05.001
Belmonte, M. K., and Yurgelun-Todd, D. A. (2003). Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res. Cogn. Brain Res. 17, 651–664. doi: 10.1016/s0926-6410(03)00189-7
Berryhill, M. E., Phuong, L., Picasso, L., Cabeza, R., and Olson, I. R. (2007). Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J. Neurosci. 27, 14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007
Bertone, A., Mottron, L., Jelenic, P., and Faubert, J. (2005). Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain 128, 2430–2441. doi: 10.1093/brain/awh561
Binder, J. R., Desai, R. H., Graves, W. W., and Conant, L. L. (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 19, 2767–2796. doi: 10.1093/cercor/bhp055
Blair, R. J. R., Frith, U., Smith, N., Abell, F., and Cipolotti, L. (2002). Fractionation of visual memory: agency detection and its impairment in autism. Neuropsychologia 40, 108–118. doi: 10.1016/s0028-3932(01)00069-0
Bonnel, A., Mottron, L., Peretz, I., Trudel, M., Gallun, E., and Bonnel, A. M. (2003). Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. J. Cogn. Neurosci. 15, 226–235. doi: 10.1162/089892903321208169
Boso, M., Comelli, M., Vecchi, T., Barale, F., and Politi, P. (2009). Exploring musical taste in severely autistic subjects: preliminary data. Ann. N. Y. Acad. Sci. 1169, 332–335. doi: 10.1111/j.1749-6632.2009.04853.x
Boucher, J. (1981). Memory for recent events in autistic children. J. Autism Dev. Disord. 11, 293–301. doi: 10.1007/bf01531512
Boucher, J., Lewis, V., and Collis, G. M. (2000). Voice processing abilities in children with autism, children with specific language impairments, and young typically developing children. J. Child Psychol. Psychiatry 41, 847–857. doi: 10.1111/1469-7610.00672
Boucher, J., and Mayes, A. (2012). Memory in ASD: have we been barking up the wrong tree? Autism 16, 603–611. doi: 10.1177/1362361311417738
Boucher, J., and Warrington, E. K. (1976). Memory deficits in early infantile autism: some similarities to the amnesic syndrome. Br. J. Psychol. 67, 73–87. doi: 10.1111/j.2044-8295.1976.tb01499.x
Boyd, B. A., Conroy, M. A., Mancil, G. R., Nakao, T., and Alter, P. J. (2007). Effects of circumscribed interests on the social behaviors of children with autism spectrum disorders. J. Autism Dev. Disord. 37, 1550–1561. doi: 10.1007/s10803-006-0286-8
Butz, M., Wörgötter, F., and van Ooyen, A. (2009). Activity-dependent structural plasticity. Brain Res. Rev. 60, 287–305. doi: 10.1016/j.brainresrev.2008.12.023
Cabeza, R., Ciaramelli, E., and Moscovitch, M. (2012). Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn. Sci. 16, 338–352. doi: 10.1016/j.tics.2012.04.008
Calderoni, S., Bellani, M., Hardan, A. Y., Muratori, F., and Brambilla, P. (2014). Basal ganglia and restricted and repetitive behaviours in autism spectrum disorders: current status and future perspectives. Epidemiol. Psychiatr. Sci. 23, 235–238. doi: 10.1017/S2045796014000171
Caldwell-Harris, C. L., and Jordan, C. J. (2014). Systemizing and special interests: characterizing the continuum from neurotypical to autism spectrum disorder. Learn. Individ. Differ. 29, 98–105. doi: 10.1016/j.lindif.2013.10.005
Carnett, A., Raulston, T., Lang, R., Tostanoski, A., Lee, A., Sigafoos, J., et al. (2014). Effects of a perseverative interest-based token economy on challenging and on-task behavior in a child with autism. J. Behav. Educ. 23, 368–377. doi: 10.1007/s10864-014-9195-7
Caron, M.-J., Mottron, L., Berthiaume, C., and Dawson, M. (2006). Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain 129, 1789–1802. doi: 10.1093/brain/awl072
Carter, R. M., and Huettel, S. A. (2013). A nexus model of the temporal-parietal junction. Trends Cogn. Sci. 17, 328–336. doi: 10.1016/j.tics.2013.05.007
Cascio, C. J., Foss-Feig, J. H., Heacock, J., Schauder, K. B., Loring, W. A., Rogers, B. P., et al. (2014). Affective neural response to restricted interests in autism spectrum disorders. J. Child Psychol. Psychiatry 55, 162–171. doi: 10.1111/jcpp.12147
Céline, M., Laurent, M., and Sylvie, B. (2000). A psychosocial study on restricted interests in high functioning persons with pervasive developmental disorders. Autism 4, 406–425. doi: 10.1177/1362361300004004006
Charlop-Christy, M. H., and Haymes, L. K. (1996). Using obsessions as reinforcers with and without mild reductive procedures to decrease inappropriate behaviors of children with autism. J. Autism Dev. Disord. 26, 527–546. doi: 10.1007/BF02172274
Chen, Y.-W., Bundy, A. C., Cordier, R., Chien, Y. L., and Einfeld, S. L. (2015). Motivation for everyday social participation in cognitively able individuals with autism spectrum disorder. Neuropsychiatr. Dis. Treat. 11, 2699–2709. doi: 10.2147/NDT.S87844
Cheung, S.-H., Fang, F., He, S., and Legge, G. E. (2009). Retinotopically specific reorganization of visual cortex for tactile pattern recognition. Curr. Biol. 19, 596–601. doi: 10.1016/j.cub.2009.02.063
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S., and Schultz, R. T. (2012). The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. doi: 10.1016/j.tics.2012.02.007
Clements, C. C., Zoltowski, A. R., Yankowitz, L. D., Yerys, B. E., Schultz, R. T., and Herrington, J. D. (2018). Evaluation of the social motivation hypothesis of autism: a systematic review and meta-analysis. JAMA Psychiatry 75, 797–808. doi: 10.1001/jamapsychiatry.2018.1100
Cooper, R. A., Richter, F. R., Bays, P. M., Plaisted-Grant, K. C., Baron-Cohen, S., and Simons, J. S. (2017). Reduced hippocampal functional connectivity during episodic memory retrieval in autism. Cereb. Cortex 27, 888–902. doi: 10.1093/cercor/bhw417
Corbetta, M., and Shulman, G. L. (2011). Spatial neglect and attention networks. Annu. Rev. Neurosci. 34, 569–599. doi: 10.1146/annurev-neuro-061010-113731
Dakin, S., and Frith, U. (2005). Vagaries of visual perception in autism. Neuron 48, 497–507. doi: 10.1016/j.neuron.2005.10.018
Dawson, G., Carver, L., Meltzoff, A. N., Panagiotides, H., McPartland, J., and Webb, S. J. (2002). Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 73, 700–717. doi: 10.1111/1467-8624.00433
Dawson, G., Rogers, S., Munson, J., Smith, M., Winter, J., Greenson, J., et al. (2010). Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics 125, e17–e23. doi: 10.1542/peds.2009-0958
Dawson, G., Webb, S. J., and McPartland, J. (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 27, 403–424. doi: 10.1207/s15326942dn2703_6
Dehaene, S., and Cohen, L. (2011). The unique role of the visual word form area in reading. Trends Cogn. Sci. 15, 254–262. doi: 10.1016/j.tics.2011.04.003
Dehaene, S., Pegado, F., Braga, L. W., Ventura, P., Nunes Filho, G., Jobert, A., et al. (2010). How learning to read changes the cortical networks for vision and language. Science 330, 1359–1364. doi: 10.1126/science.1194140
DeWind, N. K., Adams, G. K., Platt, M. L., and Brannon, E. M. (2015). Modeling the approximate number system to quantify the contribution of visual stimulus features. Cognition 142, 247–265. doi: 10.1016/j.cognition.2015.05.016
DiCarlo, J. J., Zoccolan, D., and Rust, N. C. (2012). How does the brain solve visual object recognition? Neuron 73, 415–434. doi: 10.1016/j.neuron.2012.01.010
Dichter, G. S. (2012). Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin. Neurosci. 14, 319–351.
Dichter, G. S. (2018). Motivational impairments in autism may be broader than previously thought. JAMA Psychiatry 75, 773–774. doi: 10.1001/jamapsychiatry.2018.1078
Dichter, G. S., Felder, J. N., Green, S. R., Rittenberg, A. M., Sasson, N. J., and Bodfish, J. W. (2012). Reward circuitry function in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 7, 160–172. doi: 10.1093/scan/nsq095
Dietrich, S., Hertrich, I., and Ackermann, H. (2013). Ultra-fast speech comprehension in blind subjects engages primary visual cortex, fusiform gyrus, and pulvinar - a functional magnetic resonance imaging (fMRI) study. BMC Neurosci. 14:74. doi: 10.1186/1471-2202-14-74
Donaldson, P. H., Rinehart, N. J., and Enticott, P. G. (2015). Noninvasive stimulation of the temporoparietal junction: a systematic review. Neurosci. Biobehav. Rev. 55, 547–572. doi: 10.1016/j.neubiorev.2015.05.017
Dufour, N., Redcay, E., Young, L., Mavros, P. L., Moran, J. M., Triantafyllou, C., et al. (2013). Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One 8:e75468. doi: 10.1371/journal.pone.0075468
Ewbank, M. P., Pell, P. J., Powell, T. E., von dem Hagen, E. A. H., Baron-Cohen, S., and Calder, A. J. (2017). Repetition suppression and memory for faces is reduced in adults with autism spectrum conditions. Cereb. Cortex 27, 92–103. doi: 10.1093/cercor/bhw373
Foss-Feig, J. H., McGugin, R. W., Gauthier, I., Mash, L. E., Ventola, P., and Cascio, C. J. (2016). A functional neuroimaging study of fusiform response to restricted interests in children and adolescents with autism spectrum disorder. J. Neurodev. Disord. 8:15. doi: 10.1186/s11689-016-9149-6
Gal, E. (2011). “Nosology and theories of repetitive and restricted behaviours and interests,” in International Handbook of Autism and Pervasive Developmental Disorders Autism and Child Psychopathology Series, eds J. Matson, and P. Sturmey (New York, NY: Springer), 115–125. doi: 10.1007/978-1-4419-8065-6_8
Gallagher, H. L., and Frith, C. D. (2003). Functional imaging of “theory of mind.”. Trends Cogn. Sci. 7, 77–83. doi: 10.1016/s1364-6613(02)00025-6
Gauthier, I., Skudlarski, P., Gore, J. C., and A-Feb, A. W. (2000). Expertise for cars and birds recruits brain areas involved in face recognition. Nat. Neurosci. 3, 191–197. doi: 10.1038/72140
Geng, J. J., and Vossel, S. (2013). Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci. Biobehav. Rev. 37, 2608–2620. doi: 10.1016/j.neubiorev.2013.08.010
Gervais, H., Belin, P., Boddaert, N., Leboyer, M., Coez, A., Sfaello, I., et al. (2004). Abnormal cortical voice processing in autism. Nat. Neurosci. 7, 801–802. doi: 10.1038/nn1291
Goodale, M. A., and Milner, A. D. (1992). Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25. doi: 10.1016/0166-2236(92)90344-8
Grandin, T. (2011). The Way I See it: A Personal Look at Autism & Asperger’s. Future Horizons. Available at: https://market.android.com/details?id=book-05lyDb7hqX8C (accessed January 8, 2018).
Grelotti, D. J., Klin, A. J., Gauthier, I., Skudlarski, P., Cohen, D. J., Gore, J. C., et al. (2005). fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia 43, 373–385. doi: 10.1016/j.neuropsychologia.2004.06.015
Grigorenko, E. L., and Sternberg, R. J. (1997). Styles of thinking, abilities, and academic performance. Except. Child. 63, 295–312. doi: 10.1177/001440299706300301
Hadjikhani, N., Joseph, R. M., Snyder, J., Chabris, C. F., Clark, J., Steele, S., et al. (2004). Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage 22, 1141–1150. doi: 10.1016/j.neuroimage.2004.03.025
Happé, F., and Frith, U. (2006). The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 36, 5–25. doi: 10.1007/s10803-005-0039-0
Harrop, C., Amsbary, J., Towner-Wright, S., Reichow, B., and Boyd, B. A. (2019). That’s what I like: the use of circumscribed interests within interventions for individuals with autism spectrum disorder. A systematic review. Res. Autism Spectr. Disord. 57, 63–86. doi: 10.1016/j.rasd.2018.09.008
Hartje, W. (1987). “The effect of spatial disorders on arithmetical skills” in Mathematical Disabilities: A Cognitive Neuropsychological Perspective, eds G. Deloche, and X. Seron (Hillsdale, NJ: Lawrence Erlbaum Associates, Inc), 121–135.
Heaton, P., Williams, K., Cummins, O., and Happé, F. (2008). autism and pitch processing splinter skills: a group and subgroup analysis. Autism 12, 203–219. doi: 10.1177/1362361307085270
Hertrich, I., Dietrich, S., Moos, A., Trouvain, J., and Ackermann, H. (2009). Enhanced speech perception capabilities in a blind listener are associated with activation of fusiform gyrus and primary visual cortex. Neurocase 15, 163–170. doi: 10.1080/13554790802709054
Hill, C. A., Suzuki, S., Polania, R., Moisa, M., O’Doherty, J. P., and Ruff, C. C. (2017). A causal account of the brain network computations underlying strategic social behavior. Nat. Neurosci. 20, 1142–1149. doi: 10.1038/nn.4602
Hohwy, J., and Palmer, C. (2014). “Social cognition as causal inference: implications for common knowledge and autism,” in Perspectives on Social Ontology and Social Cognition, eds M. Gallotti, and J. Michael (Dordrecht: Springer), 167–189. doi: 10.1007/978-94-017-9147-2_12
Holdstock, J. S., Mayes, A. R., Roberts, N., Cezayirli, E., Isaac, C. L., O’Reilly, R. C., et al. (2002). Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus 12, 341–351. doi: 10.1002/hipo.10011
Holtmaat, A., and Svoboda, K. (2009). Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10, 647–658. doi: 10.1038/nrn2699
Hong, S.-J., Vos de Wael, R., Bethlehem, R. A. I., Lariviere, S., Paquola, C., Valk, S. L., et al. (2019). Atypical functional connectome hierarchy in autism. Nat. Commun. 10:1022. doi: 10.1038/s41467-019-08944-1
Hubbard, E. M., Piazza, M., Pinel, P., and Dehaene, S. (2005). Interactions between number and space in parietal cortex. Nat. Rev. Neurosci. 6, 435–448. doi: 10.1038/nrn1684
Jarrold, C., Gilchrist, I. D., and Bender, A. (2005). Embedded figures detection in autism and typical development: preliminary evidence of a double dissociation in relationships with visual search. Dev. Sci. 8, 344–351. doi: 10.1111/j.1467-7687.2005.00422.x
Johnson, M. H. (2011). Interactive specialization: a domain-general framework for human functional brain development? Dev. Cogn. Neurosci. 1, 7–21. doi: 10.1016/j.dcn.2010.07.003
Jolliffe, T., and Baron-Cohen, S. (1997). Are people with autism and Asperger syndrome faster than normal on the embedded figures test? J. Child Psychol. Psychiatry 38, 527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x
Jordan, C. J., and Caldwell-Harris, C. L. (2012). Understanding differences in neurotypical and autism spectrum special interests through internet forums. Intellect. Dev. Disabil. 50, 391–402. doi: 10.1352/1934-9556-50.5.391
Kasari, C., Freeman, S., and Paparella, T. (2006). Joint attention and symbolic play in young children with autism: a randomized controlled intervention study. J. Child Psychol. Psychiatry 47, 611–620. doi: 10.1111/j.1469-7610.2005.01567.x
Keehn, B., Lincoln, A. J., Müller, R.-A., and Townsend, J. (2010). Attentional networks in children and adolescents with autism spectrum disorder. J. Child Psychol. Psychiatry 51, 1251–1259. doi: 10.1111/j.1469-7610.2010.02257.x
Klin, A., Danovitch, J. H., Merz, A. B., and Volkmar, F. R. (2007). Circumscribed interests in higher functioning individuals with autism spectrum disorders: an exploratory study. Res. Pract. Persons Severe Disabil. 32, 89–100. doi: 10.2511/rpsd.32.2.89
Koegel, L. K., Vernon, T., Koegel, R. L., Koegel, B. L., and Paullin, A. W. (2012). Improving social engagement and initiations between children with autism spectrum disorder and their peers in inclusive settings. J. Posit. Behav. Interv. 14, 220–227. doi: 10.1177/1098300712437042
Koegel, R., Kim, S., Koegel, L., and Schwartzman, B. (2013). Improving socialization for high school students with ASD by using their preferred interests. J. Autism Dev. Disord. 43, 2121–2134. doi: 10.1007/s10803-013-1765-3
Koegel, R. L., and Covert, A. (1972). The relationship of self-stimulation to learning in autistic children. J. Appl. Behav. Anal. 5, 381–387. doi: 10.1901/jaba.1972.5-381
Koegel, R. L., Firestone, P. B., Kramme, K. W., and Dunlap, G. (1974). Increasing spontaneous play by suppressing self-stimulation in autistic children. J. Appl. Behav. Anal. 7, 521–528. doi: 10.1901/jaba.1974.7-521
Kohls, G., Antezana, L., Mosner, M. G., Schultz, R. T., and Yerys, B. E. (2018). Altered reward system reactivity for personalized circumscribed interests in autism. Mol. Autism 9:9. doi: 10.1186/s13229-018-0195-7
Kujala, T., Palva, M. J., Salonen, O., Alku, P., Huotilainen, M., Järvinen, A., et al. (2005). The role of blind humans’ visual cortex in auditory change detection. Neurosci. Lett. 379, 127–131. doi: 10.1016/j.neulet.2004.12.070
Lai, G., Pantazatos, S. P., Schneider, H., and Hirsch, J. (2012). Neural systems for speech and song in autism. Brain 135, 961–975. doi: 10.1093/brain/awr335
Lam, K. S. L., Bodfish, J. W., and Piven, J. (2008). Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. J. Child Psychol. Psychiatry 49, 1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x
Livingstone, M. S., Vincent, J. L., Arcaro, M. J., Srihasam, K., Schade, P. F., and Savage, T. (2017). Development of the macaque face-patch system. Nat. Commun. 8:14897. doi: 10.1038/ncomms14897
Mahon, B. Z., and Caramazza, A. (2011). What drives the organization of object knowledge in the brain? Trends Cogn. Sci. 15, 97–103. doi: 10.1016/j.tics.2011.01.004
Margulies, D. S., Ghosh, S. S., Goulas, A., Falkiewicz, M., Huntenburg, J. M., Langs, G., et al. (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. U.S.A. 113, 12574–12579. doi: 10.1073/pnas.1608282113
Matteau, I., Kupers, R., Ricciardi, E., Pietrini, P., and Ptito, M. (2010). Beyond visual, aural and haptic movement perception: hMT+ is activated by electrotactile motion stimulation of the tongue in sighted and in congenitally blind individuals. Brain Res. Bull. 82, 264–270. doi: 10.1016/j.brainresbull.2010.05.001
Meaux, E., Taylor, M. J., Pang, E. W., Vara, A. S., and Batty, M. (2014). Neural substrates of numerosity estimation in autism. Hum. Brain Mapp. 35, 4362–4385. doi: 10.1002/hbm.22480
Minshew, N. J., and Goldstein, G. (2001). The pattern of intact and impaired memory functions in autism. J. Child Psychol. Psychiatry 42, 1095–1101. doi: 10.1111/1469-7610.00808
Mitchell, J. P. (2008). Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb. Cortex 18, 262–271. doi: 10.1093/cercor/bhm051
Molnar-Szakacs, I., and Heaton, P. (2012). Music: a unique window into the world of autism. Ann. N. Y. Acad. Sci. 1252, 318–324. doi: 10.1111/j.1749-6632.2012.06465.x
Mottron, L., Dawson, M., and Soulières, I. (2009). Enhanced perception in savant syndrome: patterns, structure and creativity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1385–1391. doi: 10.1098/rstb.2008.0333
Mottron, L., Dawson, M., Soulières, I., Hubert, B., and Burack, J. (2006). Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 36, 27–43. doi: 10.1007/s10803-005-0040-7
Mottron, L., Peretz, I., and Menard, E. (2000). Local and global processing of music in high-functioning persons with autism: beyond central coherence? J. Child Psychol. Psychiatry 41, 1057–1065. doi: 10.1111/1469-7610.00693
Nelson, C. A. (1999). Neural plasticity and human development. Curr. Dir. Psychol. Sci. 8, 42–45. doi: 10.1111/1467-8721.00010
O’Riordan, M., and Passetti, F. (2006). Discrimination in autism within different sensory modalities. J. Autism Dev. Disord. 36, 665–675. doi: 10.1007/s10803-006-0106-1
Ostrolenk, A., Forgeot d’Arc, B., Jelenic, P., Samson, F., and Mottron, L. (2017). Hyperlexia: systematic review, neurocognitive modelling, and outcome. Neurosci. Biobehav. Rev. 79, 134–149. doi: 10.1016/j.neubiorev.2017.04.029
Patten Koenig, K., and Hough Williams, L. (2017). Characterization and utilization of preferred interests: a survey of adults on the autism spectrum. Occup. Ther. Ment. Health 33, 129–140. doi: 10.1080/0164212X.2016.1248877
Pelphrey, K. A., Sasson, N. J., Reznick, J. S., Paul, G., Goldman, B. D., and Piven, J. (2002). Visual scanning of faces in autism. J. Autism Dev. Disord. 32, 249–261.
Perlman, S. B., Hudac, C. M., Pegors, T., Minshew, N. J., and Pelphrey, K. A. (2011). Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Soc. Neurosci. 6, 22–30. doi: 10.1080/17470911003683185
Pierce, K., and Courchesne, E. (2001). Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry 49, 655–664. doi: 10.1016/s0006-3223(00)01008-8
Pierce, K., Haist, F., Sedaghat, F., and Courchesne, E. (2004). The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain 127, 2703–2716. doi: 10.1093/brain/awh289
Pierce, K., and Redcay, E. (2008). Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biol. Psychiatry 64, 552–560. doi: 10.1016/j.biopsych.2008.05.013
Plaisted, K., O’Riordan, M., and Baron-Cohen, S. (1998a). Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. J. Child Psychol. Psychiatry 39, 765–775. doi: 10.1111/1469-7610.00375
Plaisted, K., O’Riordan, M., and Baron-Cohen, S. (1998b). Enhanced visual search for a conjunctive target in autism: a research note. J. Child Psychol. Psychiatry 39, 777–783. doi: 10.1017/s0021963098002613
Plaisted, K. C. (2001). “Reduced generalization in autism: an alternative to weak central coherence,” in The Development of Autism: Perspectives from Theory and Research, eds J. A. Burack, T. Charman, N. Yirmiya, and P. R. Zelazo (Hillsdale, NJ: Lawrence Erlbaum Associates, Inc), 149–169.
Ptito, M., Moesgaard, S. M., Gjedde, A., and Kupers, R. (2005). Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain 128, 606–614. doi: 10.1093/brain/awh380
Quartz, S. R., and Sejnowski, T. J. (1997). The neural basis of cognitive development: a constructivist manifesto. Behav. Brain Sci. 20, 537–556. doi: 10.1017/s0140525x97001581
Redcay, E., Velnoskey, K. R., and Rowe, M. L. (2016). Perceived communicative intent in gesture and language modulates the superior temporal sulcus. Hum. Brain Mapp. 37, 3444–3461. doi: 10.1002/hbm.23251
Renner, P., Klinger, L. G., and Klinger, M. R. (2000). Implicit and explicit memory in autism: is autism an amnesic disorder? J. Autism Dev. Disord. 30, 3–14.
Richey, J. A., Rittenberg, A., Hughes, L., Damiano, C. R., Sabatino, A., Miller, S., et al. (2014). Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Soc. Cogn. Affect. Neurosci. 9, 367–377. doi: 10.1093/scan/nss146
Richler, J., Bishop, S. L., Kleinke, J. R., and Lord, C. (2007). Restricted and repetitive behaviors in young children with autism spectrum disorders. J. Autism Dev. Disord. 37, 73–85.
Rimland, B. (1978). “Savant capabilities of autistic children and their cognitive implications,” in Cognitive Defects in the Development of Mental Illness, ed. G. Serban (New York, NY: Brunner/Mazel), 43–65.
Rinehart, N. J., Bradshaw, J. L., Moss, S. A., Brereton, A. V., and Tonge, B. J. (2000). Atypical interference of local detail on global processing in high-functioning autism and Asperger’s disorder. J. Child Psychol. Psychiatry 41, 769–778. doi: 10.1111/1469-7610.00664
Sadato, N., Okada, T., Kubota, K., and Yonekura, Y. (2004). Tactile discrimination activates the visual cortex of the recently blind naive to Braille: a functional magnetic resonance imaging study in humans. Neurosci. Lett. 359, 49–52. doi: 10.1016/j.neulet.2004.02.005
Sadato, N., Pascual-Leone, A., Grafman, J., Ibañez, V., Deiber, M. P., Dold, G., et al. (1996). Activation of the primary visual cortex by Braille reading in blind subjects. Nature 380, 526–528. doi: 10.1038/380526a0
Samson, F., Mottron, L., Jemel, B., Belin, P., and Ciocca, V. (2006). Can spectro-temporal complexity explain the autistic pattern of performance on auditory tasks? J. Autism Dev. Disord. 36, 65–76. doi: 10.1007/s10803-005-0043-4
Sasson, N. J., Elison, J. T., Turner-Brown, L. M., Dichter, G. S., and Bodfish, J. W. (2011). Brief report: circumscribed attention in young children with autism. J. Autism Dev. Disord. 41, 242–247. doi: 10.1007/s10803-010-1038-3
Sasson, N. J., Nowlin, R. B., and Pinkham, A. E. (2013). Social cognition, social skill, and the broad autism phenotype. Autism 17, 655–667. doi: 10.1177/1362361312455704
Sasson, N. J., and Touchstone, E. W. (2014). Visual attention to competing social and object images by preschool children with autism spectrum disorder. J. Autism Dev. Disord. 44, 584–592. doi: 10.1007/s10803-013-1910-z
Sasson, N. J., Turner-Brown, L. M., Holtzclaw, T. N., Lam, K. S. L., and Bodfish, J. W. (2008). Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Res. 1, 31–42. doi: 10.1002/aur.4
Saxe, R., and Kanwisher, N. (2003). People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. Neuroimage 19, 1835–1842. doi: 10.1016/s1053-8119(03)00230-1
Scherf, K. S., Behrmann, M., Minshew, N., and Luna, B. (2008). Atypical development of face and greeble recognition in autism. J. Child Psychol. Psychiatry 49, 838–847. doi: 10.1111/j.1469-7610.2008.01903.x
Schertz, H. H., and Odom, S. L. (2007). Promoting joint attention in toddlers with autism: a parent-mediated developmental model. J. Autism Dev. Disord. 37, 1562–1575. doi: 10.1007/s10803-006-0290-z
Sharda, M., Midha, R., Malik, S., Mukerji, S., and Singh, N. C. (2015). Fronto-temporal connectivity is preserved during sung but not spoken word listening, across the autism spectrum. Autism Res. 8, 174–186. doi: 10.1002/aur.1437
Shimamura, A. P., and Squire, L. R. (1988). Long-term memory in amnesia: cued recall, recognition memory, and confidence ratings. J. Exp. Psychol. Learn. Mem. Cogn. 14, 763–770. doi: 10.1037/0278-7393.14.4.763
Shultz, S., Klin, A., and Jones, W. (2018). Neonatal Transitions in Social Behavior and Their Implications for autism. Trends Cogn. Sci. 22, 452–469. doi: 10.1016/j.tics.2018.02.012
Siegel, D. J., Minshew, N. J., and Goldstein, G. (1996). Wechsler IQ profiles in diagnosis of high-functioning autism. J. Autism Dev. Disord. 26, 389–406. doi: 10.1007/bf02172825
Sinha, P., Kjelgaard, M. M., Gandhi, T. K., Tsourides, K., Cardinaux, A. L., Pantazis, D., et al. (2014). autism as a disorder of prediction. Proc. Natl. Acad. Sci. U.S.A. 111, 15220–15225. doi: 10.1073/pnas.1416797111
South, M., Ozonoff, S., and McMahon, W. M. (2005). Repetitive behavior profiles in Asperger syndrome and high-functioning autism. J. Autism Dev. Disord. 35, 145–158. doi: 10.1007/s10803-004-1992-8
Spiker, M. A., Lin, C. E., Van Dyke, M., and Wood, J. J. (2012). Restricted interests and anxiety in children with autism. Autism 16, 306–320. doi: 10.1177/1362361311401763
Srihasam, K., Vincent, J. L., and Livingstone, M. S. (2014). Novel domain formation reveals proto-architecture in inferotemporal cortex. Nat. Neurosci. 17, 1776–1783. doi: 10.1038/nn.3855
Supekar, K., Kochalka, J., Schaer, M., Wakeman, H., Qin, S., Padmanabhan, A., et al. (2018). Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism. Brain 141, 2795–2805. doi: 10.1093/brain/awy191
Tang, J., Falkmer, M., Horlin, C., Tan, T., Vaz, S., and Falkmer, T. (2015). Face recognition and visual search strategies in autism spectrum disorders: amending and extending a recent review by Weigelt et al. PLoS One 10:e0134439. doi: 10.1371/journal.pone.0134439
Thies, J., Zollhöfer, M., Stamminger, M., Theobalt, C., and Nießner, M. (2016). “Face2Face: real-time face capture and reenactment of RGB videos,” in Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, 2387–2395. doi: 10.1109/CVPR.2016.262
Thye, M. D., Bednarz, H. M., Herringshaw, A. J., Sartin, E. B., and Kana, R. K. (2018). The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 29, 151–167. doi: 10.1016/j.dcn.2017.04.010
Toichi, M., and Kamio, Y. (2002). Long-term memory and levels-of-processing in autism. Neuropsychologia 40, 964–969. doi: 10.1016/s0028-3932(01)00163-4
Toichi, M., and Kamio, Y. (2003). Long-term memory in high-functioning autism: controversy on episodic memory in autism reconsidered. J. Autism Dev. Disord. 33, 151–161.
Traynor, J. M., Gough, A., Duku, E., Shore, D. I., and Hall, G. B. C. (2019). Eye tracking effort expenditure and autonomic arousal to social and circumscribed interest stimuli in autism spectrum disorder. J. Autism Dev. Disord. 49, 1988–2002. doi: 10.1007/s10803-018-03877-y
Treffert, D. A. (2009). The savant syndrome: an extraordinary condition. A synopsis: past, present, future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1351–1357. doi: 10.1098/rstb.2008.0326
Trontel, H. G., Duffield, T. C., Bigler, E. D., Abildskov, T. J., Froehlich, A., Prigge, M. B. D., et al. (2015). Mesial temporal lobe and memory function in autism spectrum disorder: an exploration of volumetric findings. J. Clin. Exp. Neuropsychol. 37, 178–192. doi: 10.1080/13803395.2014.997677
Turner, M. (1999). Annotation: repetitive behaviour in autism: a review of psychological research. J. Child Psychol. Psychiatry 40, 839–849. doi: 10.1111/1469-7610.00502
Turner-Brown, L. M., Lam, K. S. L., Holtzclaw, T. N., Dichter, G. S., and Bodfish, J. W. (2011). Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism 15, 437–456. doi: 10.1177/1362361310386507
Tymchuk, A. J., Simmons, J. Q., and Neafsey, S. (1977). Intellectual characteristics of adolescent childhood psychotics with high verbal ability. J. Ment. Defic. Res. 21, 133–138. doi: 10.1111/j.1365-2788.1977.tb00033.x
Unruh, K. E., Sasson, N. J., Shafer, R. L., Whitten, A., Miller, S. J., Turner-Brown, L., et al. (2016). Social orienting and attention is influenced by the presence of competing nonsocial information in adolescents with autism. Front. Neurosci. 10:586. doi: 10.3389/fnins.2016.00586
Varni, J. W., Lovaas, O. I., Koegel, R. L., and Everett, N. L. (1979). An analysis of observational learning in autistic and normal children. J. Abnorm. Child Psychol. 7, 31–43. doi: 10.1007/bf00924508
Wainwright, J. A., and Bryson, S. E. (1996). Visual-spatial orienting in autism. J. Autism Dev. Disord. 26, 423–438. doi: 10.1007/BF02172827
Walsh, V. (2003). A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn. Sci. 7, 483–488. doi: 10.1016/j.tics.2003.09.002
Weigelt, S., Koldewyn, K., and Kanwisher, N. (2012). Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci. Biobehav. Rev. 36, 1060–1084. doi: 10.1016/j.neubiorev.2011.12.008
Whyte, E. M., Behrmann, M., Minshew, N. J., Garcia, N. V., and Scherf, K. S. (2016). Animal, but not human, faces engage the distributed face network in adolescents with autism. Dev. Sci. 19, 306–317. doi: 10.1111/desc.12305
Williams, D. L., Goldstein, G., and Minshew, N. J. (2006). The profile of memory function in children with autism. Neuropsychology 20, 21–29. doi: 10.1037/0894-4105.20.1.21
Winocur, G., and Weiskrantz, L. (1976). An investigation of paired-associate learning in amnesic patients. Neuropsychologia 14, 97–110. doi: 10.1016/0028-3932(76)90011-7
Keywords: autism, restricted interests, cognitive neural development, fMRI, social perception
Citation: Carter RM, Jung H, Reaven J, Blakeley-Smith A and Dichter GS (2020) A Nexus Model of Restricted Interests in Autism Spectrum Disorder. Front. Hum. Neurosci. 14:212. doi: 10.3389/fnhum.2020.00212
Received: 18 January 2020; Accepted: 11 May 2020;
Published: 03 June 2020.
Edited by:
Carol Seger, Colorado State University, United StatesReviewed by:
Pengmin Qin, University of Ottawa, CanadaPeter G. Enticott, Deakin University, Australia
Filippo Muratori, Fondazione Stella Maris (IRCCS), Italy
Copyright © 2020 Carter, Jung, Reaven, Blakeley-Smith and Dichter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. McKell Carter, mckell.carter@colorado.edu; mckellcarter@gmail.com
 R. McKell Carter
R. McKell Carter Heejung Jung
Heejung Jung Judy Reaven3
Judy Reaven3  Gabriel S. Dichter
Gabriel S. Dichter