Neural Processes Underlying Mirror-Induced Visual Illusion: An Activation Likelihood Estimation Meta-Analysis
- 1Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hong Kong, China
- 2Department of Physiotherapy, Yobe State University Teaching Hospital, Damaturu, Nigeria
- 3Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria
- 4Applied Cognitive Neuroscience Laboratory, The Hong Kong Polytechnic University, Hong Kong, China
- 5University Research Facility in Behavioral and Systems Neuroscience, The Hong Kong Polytechnic University, Hong Kong, China
Introduction: Neuroimaging studies on neural processes associated with mirror-induced visual illusion (MVI) are growing in number. Previous systematic reviews on these studies used qualitative approaches.
Objective: The present study conducted activation likelihood estimation (ALE) meta-analysis to locate the brain areas for unfolding the neural processes associated with the MVI.
Method: We searched the CINAHL, MEDLINE, Scopus, and PubMed databases and identified eight studies (with 14 experiments) that met the inclusion criteria.
Results: Contrasting with a rest condition, strong convergence in the bilateral primary and premotor areas and the inferior parietal lobule suggested top-down motor planning and execution. In addition, convergence was identified in the ipsilateral precuneus, cerebellum, superior frontal gyrus, and superior parietal lobule, clusters corresponding to the static hidden hand indicating self-processing operations, somatosensory processing, and motor control. When contrasting with an active movement condition, additional substantial convergence was revealed in visual-related areas, such as the ipsilateral cuneus, fusiform gyrus, middle occipital gyrus (visual area V2) and lingual gyrus, which mediate basic visual processing.
Conclusions: To the best of our knowledge, the current meta-analysis is the first to reveal the visualization, mental rehearsal and motor-related processes underpinning the MVI and offers theoretical support on using MVI as a clinical intervention for post-stroke patients.
Introduction
A plane mirror provides an instant visual feedback of body appearance and posture, thereby influencing self-awareness and aiding in complex visually guided tasks (Jenkinson and Preston, 2017). A plane mirror inverts the reflected image; e.g., the left limb appears as the right when positioned at the midsagittal plane (Bähr et al., 2018). The visual feedback created by the illusion of the left-to-right limb has been adopted as the basis of mirror therapy for patients with neurological disorders, e.g., stroke survivors (Matthys et al., 2009).
Mirror-induced visual illusion (MVI) was first introduced in late 1990s by Ramachandran to alleviate phantom limb pain in patients (Ramachandran and Rogers-Ramachandran, 1996). MVI was later adopted as clinical intervention for treating hemiparesis due to stroke (Altschuler et al., 1999). During mirror therapy, a patient places the paretic hand behind the mirror, whereas the unaffected hand stays in front of the reflecting surface of the mirror (Guerraz, 2015). The mirror is placed in an erect position corresponding to the body midline of the patient such that the paretic upper limb is hidden from the view (Guerraz, 2015). Common MVI protocols involve the unaffected hand engaging in different movements whilst the patient looks into the mirror and observes the movements as if they are performed by the paretic hand hidden behind the mirror. MVI was found to facilitate the motor recovery of paretic limbs amongst stroke survivors (Rosén and Lundborg, 2005). Evidence gathered from clinical reviews support the effectiveness of MVI on improving the functional regains of the upper and lower limbs (Broderick et al., 2018; Thieme et al., 2018; Zeng et al., 2018).
Deconinck et al. (2015) and Arya (2016) qualitatively collated the results of neuroimaging studies on neural substrates that mediate MVI. Deconinck et al. (2015) observed that MVI appeared to facilitate activities in the motor network despite the findings being largely inconsistent. The MVI effects were related to the increase in attention control via “increased cognitive penetration” (Deconinck et al., 2015). Deconinck et al. (2015) explained that the inconsistent results may be due to the small sample sizes and methodological variations across the studies reviewed. Supplementing Deconinck et al.'s study, Arya (2016) concluded that MVI facilitated the ipsilesional primary motor cortex (M1) via a top-down influence on the ipsilesional premotor cortex, resulting in the augmentation of neuroplasticity in the affected hemisphere amongst stroke survivors. Both papers reported widespread mirror-induced activations of the fronto-temporo-parietal, occipital and cerebellar brain regions. Findings from qualitative analyses would have the advantage of collating the results of studies and might align with the interests of researchers. However, researchers would face the challenge of whether the observations are robust and occur more than by chance. The present study attempted to use a meta-analytic method to test MVI hypotheses by pooling the activations of neural substrates found in functional brain imaging studies on MVI. The results offer evidence on the possible effects and underlying neural processes of MVI and hence increase our understanding on its potential role in the motor recovery of post-stroke patients.
Activation likelihood estimation (ALE) is a coordinate-based statistical method for consolidating the foci of the neural substrate(s) being activated when subjects engage in a task; such method is used across individual studies that report experiment(s) (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012). Previous ALE meta-analyses were conducted to collate and locate the neural substrates associated with motor imagery (Hétu et al., 2013) and action observation (Caspers et al., 2010). Furthermore, a recent ALE meta-analysis approach was used to identify the neural substrates associated with movement execution, motor imagery and action observation (Hardwick et al., 2018).
The current study aimed to use the ALE method to collate and identify the neural substrates that sub-serve the MVI processes in healthy adults and to examine how the sides of the hand (i.e., left vs. right) would modulate brain activations during task manipulation. Studies that recruited post-stroke patients were not included because of the inadequate number of studies and the limitations against the standards set by the ALE method. Understanding the effects of MVI on healthy adults contributes to knowledge on neural mechanisms and is essential to understanding the effect of treatment on post-stroke patients under the influence of brain lesions and functional abnormalities. In this study, we conceptualized MVI is to visualize moving hand images over-imposing on the “static” hand, thereby producing visual illusions. These motor-related visualizations result in top-down sensorimotor planning, execution and control processes. We hypothesized that in contrast to a condition involving movement without mirror visual feedback, the visualization in MVI would yield a significant convergence of activations in visual and motor-associated areas. Moreover, in contrast to a resting or control condition, the MVI effect would yield activations in the motor-associated network, particularly the premotor cortex, M1 and cerebellum, in line with increased top-down motor facilitation and activations in the precuneus and inferior parietal lobule ipsilateral to the moved hand.
Methods
Study Selection
The search for eligible studies for inclusion was conducted in accordance with the preferred reporting items for systematic review and meta-analysis protocol (PRISMA-P) (Moher et al., 2015). The search covered functional brain neuroimaging studies on MVI in the CINAHL, MEDLINE, Scopus, and PubMed databases from inception until November 2019. No restriction was set on the year of publication. The search terms were constructed under two themes: mirror therapy and neuroimaging modalities. Related and similar terms for each theme were developed, and the search used Boolean “OR” for individual terms and Boolean “AND” to combine the terms of the two themes. The search terms under mirror therapy included “mirror therapy,” “mirror visual illusion,” and “mirror illusion,” whereas those under neuroimaging modalities included “functional magnetic resonance imaging,” “positron emission tomography,” “fMRI,” and “PET.” Additional articles were manually searched from the reference lists of the included articles and existing systematic reviews.
The title, abstract and full text of each study were obtained in accordance with the method described above. The inclusion criteria were as follows: (1) test protocol on the instant effect of MVI involving the active movement of the right or left hand whilst observing mirror images superimposed on the opposite static hidden hand, (2) healthy adults (18 years or above) as subjects, (3) whole-brain group analysis results of functional magnetic resonance imaging or positron emission topography, (4) inclusion of a minimum of five participants, and (5) mapping of brain coordinates using Montreal Neurological Institute (MNI) or Talairach and Tournoux space.
Data Extraction
The activation coordinates, number of experiments and sample size of each experiment were extracted and organized in accordance with the guideline provided by GingerALE 3.0.2 (available at: http://brainmap.org). Brain coordinates reported in the Talairach and Tournoux reference space were converted to the MNI reference space using the brain coordinate conversion options in GingerALE software (Lancaster et al., 2007).
Activation Likelihood Estimation (ALE) Method
The analyses were performed in accordance with a recent coordinate-based meta-analysis guideline (Müller et al., 2018). ALE software version 3.0.2 (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012) was used to conduct all the data analyses, which began with generated modeled activation maps by pooling the task-related activations at the voxel level in the foci identified across the experiments (Turkeltaub et al., 2012). The ALE scores were yielded by pooling all the activation maps (Hardwick et al., 2018). Cluster-level FWE thresholding was used to guide the meta-analysis because it has greater sensitivity and specificity and is less prone to type-1 error in terms of convergence in comparison with voxel-wise thresholding (Eickhoff et al., 2016). The brain coordinates from experiments involving the same group of subjects were pooled into one experiment to control for sample overlap (Turkeltaub et al., 2012; Müller et al., 2018). Statistical significance was set at a corrected threshold of p < 0.05 (threshold permutation at 1000 cluster-forming threshold at a voxel level of p < 0.05) (Zheng et al., 2019). The foci labeling which showed significant pooled convergence used the probabilistic cytoarchitectonic maps of human brain in the SPM Anatomy Toolbox v2.1 (Eickhoff et al., 2005). MRIcro software with an MNI template (www.mricro.com) was used to visualize the results of the meta-analyses.
Data Analyses
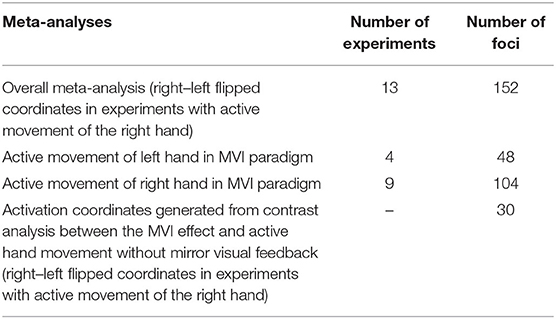
The overall meta-analysis entails pooling the activation foci from all the experiments involving the active movement of either the right or left hand in the MVI paradigm. To isolate the MVI effect on one side of the brain, flipping of activation coordinates was performed as previously reported (Witteman et al., 2012; Favre et al., 2014). We flipped the activation coordinates generated by experiments in which the right hand was actively moved to isolate the MVI effect on the left hemisphere. This process involved multiplying the x-coordinates of the foci yielded on the basis of right hand movements by “−1” (Witteman et al., 2012). The coordinates generated by the active movement of the right hand were flipped in association with the generation of reliable findings in motor-associated areas and on the basis of interhemispheric discrepancies in the brain mask size according to the ALE method (Eickhoff et al., 2005).
To examine the effect of the actively moving hand on brain activations, we conducted separate analyses by pooling the activation coordinates generated during the active movement of the right or left hand (without flipping the coordinates).
Activation foci were also pooled separately for studies that conducted second-level analysis by contrasting the MVI effect against active hand movement without mirror visual feedback as a control condition.
Results
Number and Description of Included Articles
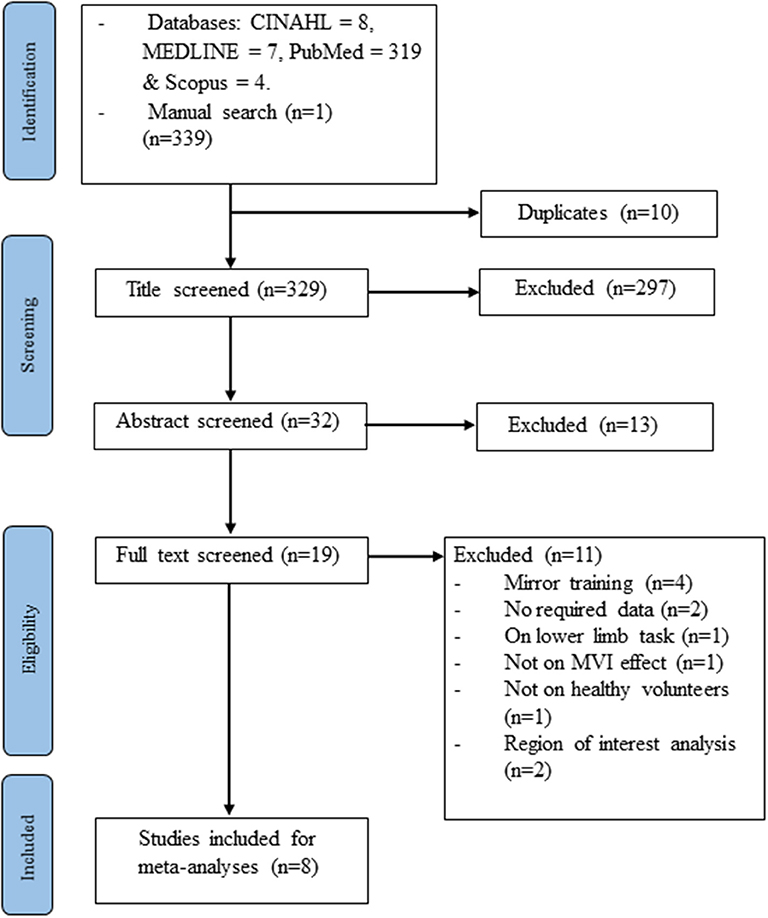
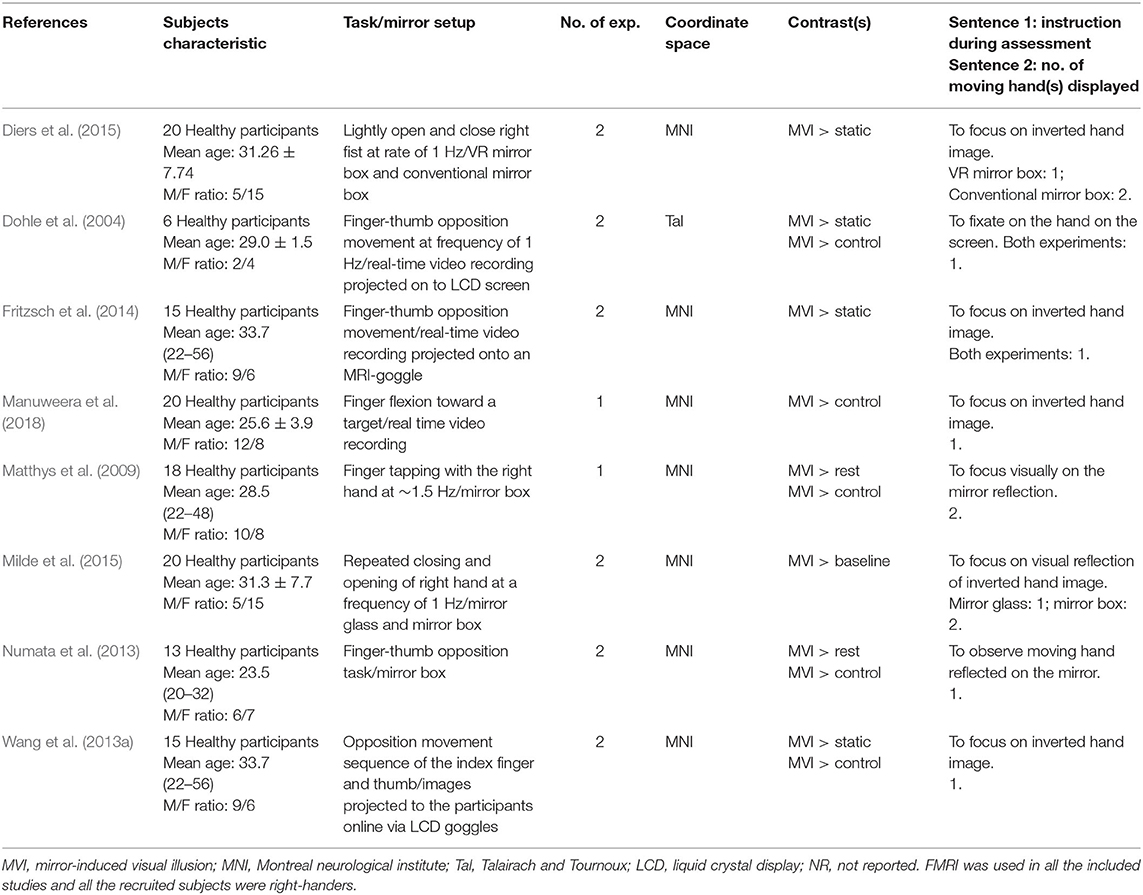
The electronic and manual search yielded 339 studies. After the removal of 10 duplicate records and the retrieval of the full information, another 321 studies were excluded. Eight studies (Dohle et al., 2004; Matthys et al., 2009; Numata et al., 2013; Wang et al., 2013a; Fritzsch et al., 2014; Diers et al., 2015; Milde et al., 2015; Manuweera et al., 2018) that reported experiments met the inclusion criteria (see Figure 1 and Table 1). All the studies tested the effect of MVI experiments involving upper limb movements. A total of 14 experiments reported 182 foci amongst 127 healthy subjects. Four studies (Dohle et al., 2004; Matthys et al., 2009; Numata et al., 2013; Wang et al., 2013a) reported results of contrast analysis between the MVI effect against a baseline/resting condition and a control condition (involving hand movement without mirror visual feedback). Three studies (Fritzsch et al., 2014; Diers et al., 2015; Milde et al., 2015) reported results of contrast analysis between the MVI effect and a baseline/resting condition, whereas a single study reported the result of contrast analysis between the MVI effect and a control condition (Manuweera et al., 2018). Nine experiments in seven studies (Dohle et al., 2004; Matthys et al., 2009; Numata et al., 2013; Wang et al., 2013a; Fritzsch et al., 2014; Diers et al., 2015; Milde et al., 2015) required the participants to engage in active movements of the right hand in the MVI paradigm, whereas four experiments in four studies involved the active movement of the left hand (Dohle et al., 2004; Numata et al., 2013; Wang et al., 2013a; Fritzsch et al., 2014) (Table 2).
Eight studies on MVI involving post-stroke patients were identified (Merians et al., 2009; Michielsen et al., 2011a,b; Bhasin et al., 2012; Wang et al., 2013b, 2017; Saleh et al., 2014, 2017; Novaes et al., 2018). Amongst them, majority (Merians et al., 2009; Bhasin et al., 2012; Wang et al., 2013b, 2017; Novaes et al., 2018) reported findings based on the region of interest method and do not satisfy the inclusion criteria (Müller et al., 2018).
Overall Meta-Analysis
The overall results involved the contrast of pooled brain activations elicited in the MVI moving hand condition, i.e., observing the mirror on which images of the movements superimposed on the hand (static) hidden behind the mirror in contrast to those elicited under the baseline/resting condition (without movement of both hands). Consistent convergence was revealed in the bilateral M1 (Figure 2, Z-score: contralateral to moving hand = 7.63; ipsilateral to moving hand = 5.15), the premotor cortex (6.12; 4.34) and the inferior parietal lobule (3.32; 3.45) clusters. In terms of the Z-score, the strength of convergence in the primary and premotor cortices were stronger in the hemisphere contralateral to the moving hand in comparison with that in the ipsilateral hemisphere. Regions that were consistently found in the hemisphere ipsilateral to the moving hand were in the primary somatosensory cortex (Z-score = 4.70), superior frontal gyrus (3.61), superior parietal lobule (4.10), precuneus (2.98), cerebellum-anterior lobe (5.60) and the cerebellum-posterior lobe (3.26). The brain coordinates, Z-scores and their ALE values can be found in Appendix A.
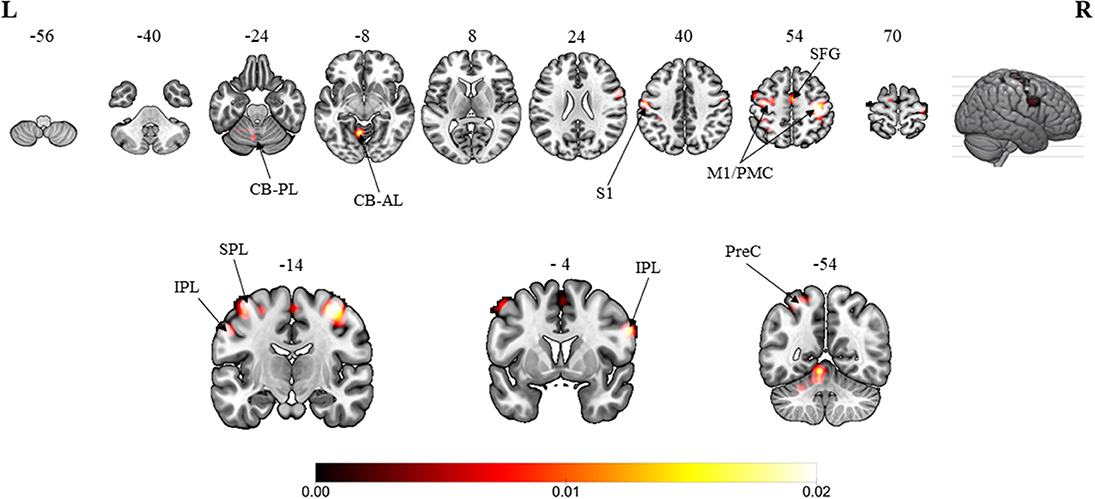
Figure 2. Overall meta-analytic results showing convergence of brain areas found to associate with the MVI condition when contrasted with the baseline/resting condition (13 experiments with 152 foci). Note: The MVI tasks involved moving left hand (right-left flipped coordinates for moving right hand). Labels: CB-PL, cerebellum-posterior lobe; CB-AL, cerebellum-anterior lobe; S1, primary somatosensory cortex; M1/PMC, primary motor cortex/premotor cortex; SFG, superior frontal gyrus; IPL, inferior parietal lobulem; SPL, superior parietal lobule; PreC, precuneus.
MVI Moving Left Hand (vs. Baseline/Resting Condition)
The moving left hand MVI protocols involved active movements of the left hand. Significantly strong convergence was found in the bilateral M1, premotor cortex and inferior parietal lobule. Regions that were consistently found specific to the ipsilateral (i.e., left side) hemisphere included the superior frontal gyrus, medial frontal gyrus, superior parietal lobule and precuneus. By contrast, regions specific to the contralateral (i.e., right side) hemisphere included the primary somatosensory cortex and insula cortex (Table 3).
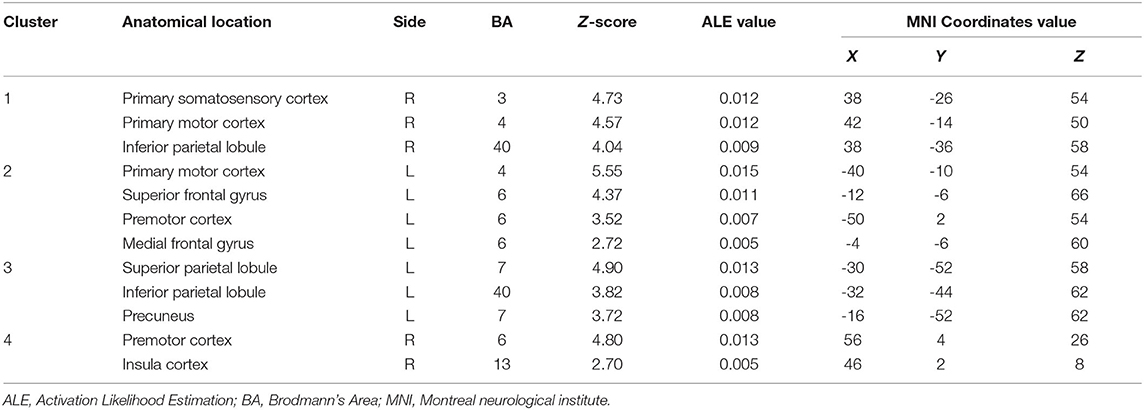
Table 3. Convergent brain clusters found associated with moving left hand in the MVI paradigm (4 experiments with 48 foci).
MVI Moving Right Hand (vs. Baseline/Resting Condition)
In comparison with the moving-left-hand paradigm, the moving of the right hand resulted in convergence (i.e., left side) in the contralateral M1 and premotor cortex clusters. Specific to the ipsilateral hemisphere (i.e., left side) brain areas were the transverse temporal gyrus, supramarginal gyrus, superior temporal gyrus and insula cortex (Table 4).
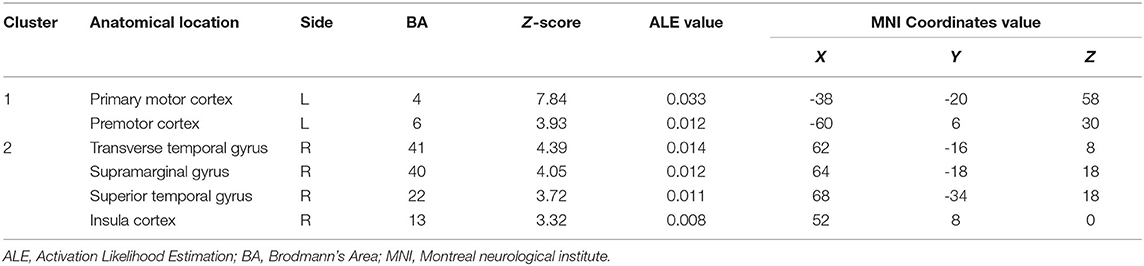
Table 4. Convergent brain clusters found associated with moving right hand in the MVI paradigm (9 experiments with 104 foci).
Other MVI Analysis (vs. Active Hand Movement Without Mirror Visual Feedback)
When compared with active hand movement without mirror visual feedback, the convergent clusters associated with the MVI processes were in the ipsilateral cuneus (Z-score = 4.69), lingual gyrus (4.50), middle occipital gyrus (4.16), superior temporal (fusiform) gyrus (2.87), precuneus (2.87), and posterior lobe of the cerebellum (2.87) (Figure 3 and Appendix B).
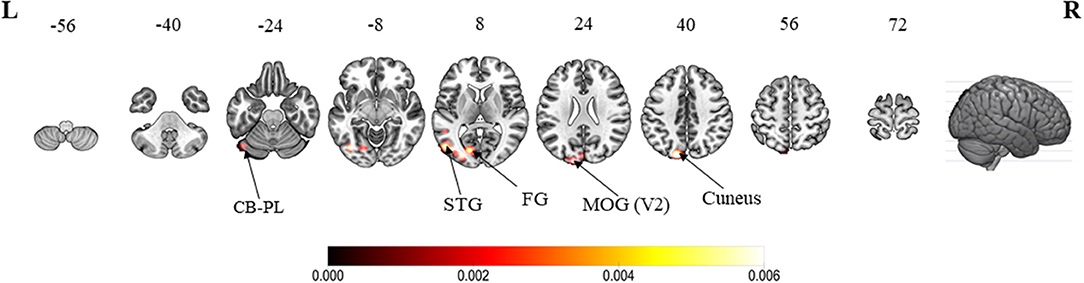
Figure 3. ALE meta-analytic results showing convergent brain clusters as a result of contrast between MVI condition with active hand movement without mirror visual feedback (30 foci). Note: The MVI tasks involved movements of the left hand (right–left flipped coordinates for right hand movements). Labels: CB-PL, cerebellum-posterior lobe; STG, superior temporal gyrus; FG, fusiform gyrus; MOG, middle occipital gyrus.
Discussion
Overall Meta-Analysis
This study attempts to explore the cluster convergence induced by the MVI to reveal its possible involvement in visualization and motor-related processes. The first major finding is that the MVI showed strong convergence in the bilateral M1, premotor cortex and inferior parietal lobule. The bilateral hemispheric convergence, particularly those in the ipsilateral hemisphere, may have been contaminated by the activations associated with the movements of the “moved” hand. In the ipsilateral hemisphere, our findings show convergence in the cuneus, lingual gyrus, middle occipital gyrus, superior temporal (fusiform) gyrus, precuneus, and the posterior lobe of the cerebellum.
The premotor cortex plays a major role in decoding visuo-motor movements (Schluter et al., 2001) and sensory feedback (Rushworth et al., 1998; Hoshi and Tanji, 2007). Together with the M1, it mediates movement execution (Hoshi and Tanji, 2007). The premotor cortex also mediates fine motor coordination (Hardwick et al., 2018), and its activation is considered the precursor for MVI-related ipsilateral M1 activation (Hamzei et al., 2012). In contrast to a baseline/resting condition, the convergent results of the clusters in bilateral M1 and premotor cortex are consistent with those reported in existing reviews on the MVI effect (Deconinck et al., 2015; Arya, 2016). The contrasts with the contralateral “moved” hand condition in this study demonstrate that the ipsilateral M1 and premotor cortex are likely not involved in the MVI processes. This finding offers further evidence to support the notion that the results reported in previous studies may be due to interhemispheric transcallosal transfer (Tinazzi and Zanette, 1998) and top-down sensorimotor facilitation via attention control (Deconinck et al., 2015). Future studies should collect further evidence on confirming these confounds.
Using transcranial magnetic stimulation, other studies have revealed that MVI results in increases in the amplitude of motor-evoked potentials in the hemisphere ipsilateral to the “moved” hand; a marker of M1 activity amongst healthy volunteers (Garry et al., 2005; Fukumura et al., 2007) and stroke survivors (Kang et al., 2011, 2012). MVI also results in increases in the amplitudes of the lateralized readiness potential and event-related desynchronization in the ipsilateral M1 regions (Lee et al., 2015; Debnath and Franz, 2016). Some MVI studies do not report significant ipsilateral M1 involvement (Funase et al., 2007; Mehnert et al., 2013). These inconsistent results may be due to the variation in the content of mirror images (i.e., the speed, complexity, and clarity of movement images); mirror therapy setups and task designs could further serve as sources of heterogeneity in mirror therapy research.
The second major finding in this study is that MVI shows specific convergence in the hemisphere ipsilateral to the “moved” hand in the precuneus, cerebellum (anterior and posterior lobe), primary somatosensory cortex, superior frontal gyrus, and superior parietal lobule. Amongst these clusters, the precuneus and superior parietal lobule seem to play a key role in linking the visualization process of the mirrored images of the “moved” hand (see below) to motor-related planning, execution, and control processes (primary somatosensory cortex, superior frontal gyrus, and cerebellum). The precuneus has been identified as a hub for coordinating cognitive and motor-related processes (Zigmond et al., 2014). These processes include recollection and memory, self-processing operations, retrieval of spatial information during motor imagery, body image representations and visuospatial perception (Ogiso et al., 2000; Cavanna and Trimble, 2006; Zigmond et al., 2014; Deconinck et al., 2015). In this case, a crucial step in MVI is to include the processing of the mirrored images of the “moved” hand in the form of visuospatial hand information, such as finger and wrist movements (Wolbers et al., 2003). It is also plausible that the gestures of the “moved” hand could be perceived as motor programming of the “static” hand (Eng et al., 2007). Such propositions are further supported by the convergences revealed in the supramarginal gyrus and the insular cortex from the contrast between MVI (moving right hand) and the baseline/resting condition. Our finding on the involvement of the precuneus in the MVI is consistent with studies on stroke survivors (Wang et al., 2013b; Saleh et al., 2014, 2017). Different studies have reported the association of activations in the precuneus with viewing mirror-inverted images of one's own “moved” limb (Dohle et al., 2011; Mehnert et al., 2013).
Other MVI Analysis
Four visual-related convergent clusters worth mentioning are the ipsilateral cuneus, lingual gyrus, fusiform gyrus and middle occipital gyrus (MOG) which were revealed in the contrast between the MVI and “moved” hand without mirror visual feedback conditions. Both the cuneus and MOG are involved in the early visual processing of object orientation, motion, form and color (Gegenfurtner et al., 1996; Vanni et al., 2001). The lingual and fusiform gyri mediate the object color attribute judgement task (Wang X. et al., 2013). Meanwhile, the fusiform gyrus is associated with shape and color information processing (Simmons et al., 2007; Yang et al., 2015). Taken together, these results suggest that visualizing the mirrored images of the “moved” hand would have begun rather early (MOG) and been perceived as external to the body (i.e., cuneus) intensively, whereas the images would have been rich in context (i.e., lingual and fusiform gyri Table 5).
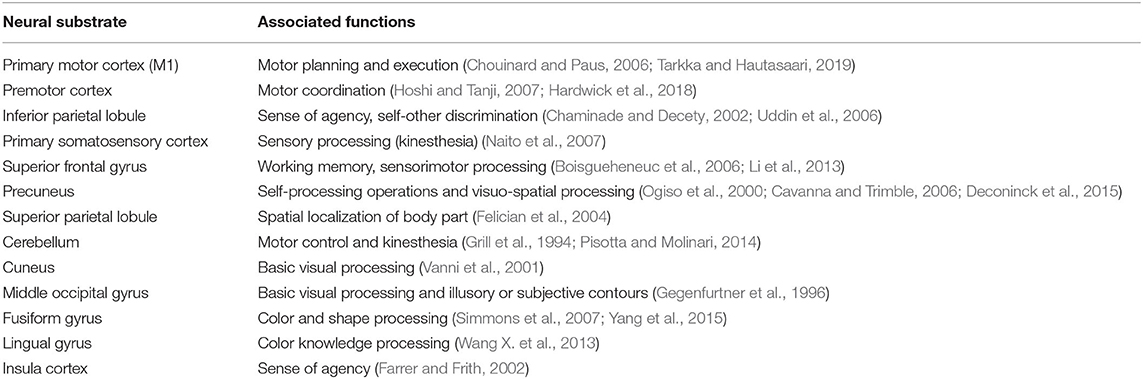
Table 5. Summary of neural substrates showing convergent clustering and their associated mental processes.
Motor Imagery and MVI Processes
Two major theories that have been adopted by researchers for describing the MVI phenomenon are mirror neuron system theory (Matthys et al., 2009; Hamzei et al., 2012) and motor imagery (Stevens and Stoykov, 2003; Fukumura et al., 2007). The results of this study, particularly those in the ipsilateral (to the “moved” hand) convergent clusters, support the latter theory more than the former. Results of an earlier meta-analytic study on the mirror properties of mirror neuron system suggested substantially larger involvements of brain areas including posterior inferior frontal gyrus, inferior parietal lobule, superior parietal lobule, middle temporal gyrus, dorsal and ventral premotor cortices, and the cerebellum (Molenberghs et al., 2012). Earlier studies associated mirror-liked properties with neural activities in the premotor cortex, superior temporal gyrus, middle temporal gyrus, and inferior frontal gyrus (Rizzolatti et al., 1996; Strafella and Paus, 2000). The limited overlaps in the premotor cortex between these studies and the present study suggests that the mental processes sub-serving the MVI are likely beyond the mirror neuron system. This proposition concurs with the observation made by Deconinck et al. (2015). Mental imagery theory stipulates that the rehearsal of the motor images throughout the imagery processes involves activations in motor-related cortices, as well as in the precuneus, superior and inferior parietal lobules, insula cortex, dorsolateral prefrontal cortex, putamen, and cerebellum (Hanakawa et al., 2003; Kuhtz-Buschbeck et al., 2003; Fourkas et al., 2006; Higuchi et al., 2007; Guillot et al., 2009; Glover and Baran, 2017). These neural substrates largely overlap with the ipsilateral clusters, i.e., primary somatosensory cortex, premotor cortex, precuneus, cerebellum, superior, and inferior parietal lobules and insula cortex, found in this study. The overlaps in these neural substrates suggest other major mental processes when engaging in MVI. They include access to sensorimotor representation, maintenance, and transformation of visuo-motor images, and motor preparation (Hétu et al., 2013; Simos et al., 2017). With similar widespread neural networks (Hétu et al., 2013; Hardwick et al., 2018), motor imagery could serve as the theoretical underpinning for MVI processes. In addition, the involvement of the primary somatosensory cortex in MVI is an indication of the increased ipsilateral sensory processing of the observed hand movement. This finding could explain the integration of kinesthetic sensation associated with the hidden static hand when subjects engage with the mirror therapy setup (Diers et al., 2010; Hadoush et al., 2013; Chancel et al., 2016).
Comparing the Active Movement of the Right or Left Hand in the MVI Paradigm
The convergence of activations in the bilateral M1 and premotor cortices was only found in the left- but not in the right-hand movement condition. The left hand was the non-dominant hand. By contrast, the right-hand-movement condition yielded convergence in the contralateral hemisphere. Two studies using magnetoencephalography revealed higher involvement of the ipsilateral M1 in the left-hand-movement condition (Tominaga et al., 2009; Hadoush et al., 2013). These results seem to suggest that left hand movements may elicit stronger activations in the ipsilateral M1 in comparison with those of the right hand. Hadoush et al. (2013) speculated that the stronger ipsilateral activations may be attributed to the mirror-inverted right-hand image (more dominant) generated during left hand (less dominant) movements. Future studies should address the effect of hand dominance in MVI.
Implications of the Findings
The revealed convergence clusters explain the effect of MVI. Observing hand movements results in increasing activations in the clusters in the motor-related system, i.e., M1 and premotor cortex. The motor-related effects appear to be mediated by a series of additional cognitive processes, resulting in activations in clusters in the parietal and visual regions. These clusters suggest the heavy involvement of visuo-motor imagery processes in MVI, including the maintenance, visualization and transformation of somatosensory and motor images. However, different opinions on the theoretical underpinning which accounts for the MVI effect exist. Our findings provide quantitative neural substrates and the mental processes which may sub-serve the MVI effect. Understanding these processes is important for the design of MVI protocols when conducting future experiments and providing clinical intervention for post-stroke patients. Subjects or patients should receive clear instructions to ensure that proper visuo-motor imagery process is engaged throughout the protocol. In addition, conducting the protocol in a quiet and appropriately illuminated environment can also enhance the quality of the images for processing.
Strength and Limitations of Our Study
The present ALE meta-analysis provides quantitative neural substrates that modulate the MVI processes. Through subsequent sub-analyses, we reported the effects of hand dominance on the neural activity in MVI. To control for the sample overlap, we combined brain coordinates generated from different experiments that involve the same participants, as recommended (Turkeltaub et al., 2012). We restricted studies to those reporting on the instant effects of performing a motor task in the mirror therapy paradigm to ensure homogeneity. As a result only 14 experiments (with 152 foci) met the inclusion for the meta-analysis, which is less than the number of experiments (17–20) recommended to perform an ALE meta-analysis (Eickhoff et al., 2016). This would have lowered the power of part of the analyses and readers should interpret the results with caution. Among the experiments included in the analyses, the instructions given to the participants appeared to be rather brief which were to observe movements of the inverted virtual or mirrored hand. As a consequence, there could have been variability among the participants in how observations were made such as on the entire or part of the hand. Another variability identified was in the number of hands displayed for observation by the participants such as unilateral or bilateral hands. Previous studies revealed activations in the visual and motor cortices were modulated by participants' observing one versus two limbs (Hadoush et al., 2013; Deconinck et al., 2015). These variabilities could have confounded the results and is regarded as a limitation of the study.
Conclusion
Neural substrates that subserve the mental processes of MVI could only be traced from individual neuroimaging studies and reviews with qualitative approaches. The present study provided the first quantitative account on neural substrates that are found collectively associated with the neural processes underlying MVI. The findings from this study showed the effect of MVI in facilitating neural activities in the diverse regions of the human brain, including the fronto-temporo-parietal, occipital and cerebellar regions. The lateralization of basic visual processing toward the ipsilateral hemisphere is indicative that MVI can potentially influence the neural system.
Author Contributions
UB: conceptualization, methodology, formal analysis, data curation, writing—original draft, and visualization. GK: methodology, validation, and writing—review and editing. SW: conceptualization, methodology, resources, writing—review and editing, and supervision. CC: conceptualization, methodology, writing—review and editing, and supervision. All authors: contributed to the article and approved the submitted version.
Funding
Work of UB was supported by Ph.D. studentship of The Hong Kong Polytechnic University. Publication of open access was supported by the Hong Kong Polytechnic University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the University Research Facility in Behavioral and Systems Neuroscience (UBSN) for providing technical support on data management and analyses. We would like to thank Mr. Jack Jiaqi Zhang for assisting in the article search and screening processes.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2020.00276/full#supplementary-material
References
Altschuler, E. L., Wisdom, S. B., Stone, L., Foster, C., Galasko, D., Llewellyn, D. M. E., et al. (1999). Rehabilitation of hemiparesis after stroke with a mirror. Lancet 353, 2035–2036. doi: 10.1016/S0140-6736(99)00920-4
Arya, K. N. (2016). Underlying neural mechanisms of mirror therapy: implications for motor rehabilitation in stroke. Neurol. India 64:38. doi: 10.4103/0028-3886.173622
Bähr, F., Ritter, A., Seidel, G., Puta, C., Gabriel, H. H., and Hamzei, F. (2018). Boosting the motor outcome of the untrained hand by action observation: mirror visual feedback, video therapy, or both combined-what is more effective? Neural Plast. 2018:10. doi: 10.1155/2018/8369262
Bhasin, A., Srivastava, M. P., Kumaran, S. S., Bhatia, R., and Mohanty, S. (2012). Neural interface of mirror therapy in chronic stroke patients: a functional magnetic resonance imaging study. Neurol. India 60:570. doi: 10.4103/0028-3886.105188
Boisgueheneuc, Fd., Levy, R., and Volle, E. (2006). Functions of the left superior frontal gyrus in humans: a lesion study. Brain 129, 3315–3328. doi: 10.1093/brain/awl244
Broderick, P., Horgan, F., Blake, C., Ehrensberger, M., Simpson, D., and Monaghan, K. (2018). Mirror therapy for improving lower limb motor function and mobility after stroke: a systematic review and meta-analysis. Gait Posture 63, 208–220. doi: 10.1016/j.gaitpost.2018.05.017
Caspers, S., Zilles, K., Laird, A. R., and Eickhoff, S. B. (2010). ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 50, 1148–1167. doi: 10.1016/j.neuroimage.2009.12.112
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. doi: 10.1093/brain/awl004
Chaminade, T., and Decety, J. (2002). Leader or follower? Involvement of the inferior parietal lobule in agency. Neuroreport 13, 1975–1978. doi: 10.1097/00001756-200210280-00029
Chancel, M., Brun, C., Kavounoudias, A., and Guerraz, M. (2016). The kinaesthetic mirror illusion: how much does the mirror matter? Exp. Brain Res. 234, 1459–1468. doi: 10.1007/s00221-015-4549-5
Chouinard, P. A., and Paus, T. (2006). The primary motor and premotor areas of the human cerebral cortex. The neurosci. 12, 143–152. doi: 10.1177/1073858405284255
Debnath, R., and Franz, E. A. (2016). Perception of hand movement by mirror reflection evokes brain activation in the motor cortex contralateral to a non-moving hand. Cortex 81, 118–125. doi: 10.1016/j.cortex.2016.04.015
Deconinck, F. J., Smorenburg, A. R., Benham, A., Ledebt, A., Feltham, M. G., and Savelsbergh, G. J. (2015). Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehab. Neural Repair. 29, 349–361. doi: 10.1177/1545968314546134
Diers, M., Christmann, C., Koeppe, C., Ruf, M., and Flor, H. (2010). Mirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb pain. PAIN® 149, 296–304. doi: 10.1016/j.pain.2010.02.020
Diers, M., Kamping, S., Kirsch, P., Rance, M., Bekrater-Bodmann, R., Foell, J., et al. (2015). Illusion-related brain activations: a new virtual reality mirror box system for use during functional magnetic resonance imaging. Brain Res.1594, 173–182. doi: 10.1016/j.brainres.2014.11.001
Dohle, C., Kleiser, R., Seitz, R. J., and Freund, H.-J. (2004). Body scheme gates visual processing. J. Neurophysiol. 91, 2376–2379. doi: 10.1152/jn.00929.2003
Dohle, C., Stephan, K., Valvoda, J., Hosseiny, O., Tellmann, L., Kuhlen, T., et al. (2011). Representation of virtual arm movements in precuneus. Exp. Brain Res. 208, 543–555. doi: 10.1007/s00221-010-2503-0
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., and Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., and Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. doi: 10.1002/hbm.20718
Eickhoff, S. B., Nichols, T. E., Laird, A. R., Hoffstaedter, F., Amunts, K., Fox, P. T., et al. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70–85. doi: 10.1016/j.neuroimage.2016.04.072
Eickhoff, S. B., Stephan, K. E., Mohlberg, H., Grefkes, C., Fink, G. R., Amunts, K., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25, 1325–1335. doi: 10.1016/j.neuroimage.2004.12.034
Eng, K., Siekierka, E., Pyk, P., Chevrier, E., Hauser, Y., Cameirao, M., et al. (2007). Interactive visuo-motor therapy system for stroke rehabilitation. Med. Biol. Eng. Comput. 45, 901–907. doi: 10.1007/s11517-007-0239-1
Farrer, C., and Frith, C. D. (2002). Experiencing oneself vs. another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage 15, 596–603. doi: 10.1006/nimg.2001.1009
Favre, I., Zeffiro, T. A., Detante, O., Krainik, A., Hommel, M., and Jaillard, A. (2014). Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta-analysis. Stroke 45, 1077–1083. doi: 10.1161/STROKEAHA.113.003168
Felician, O., Romaiguére, P., Anton, J. L., Nazarian, B., Roth, M., and Poncet, M. (2004). The role of human left superior parietal lobule in body part localization. Ann. Neurol. 55, 749–751. doi: 10.1002/ana.20109
Fourkas, A. D., Avenanti, A., Urgesi, C., and Aglioti, S. M. (2006). Corticospinal facilitation during first and third person imagery. Exp. Brain Res. 168, 143–151. doi: 10.1007/s00221-005-0076-0
Fritzsch, C., Wang, J., dos Santos, L. F., Mauritz, K.-H., Brunetti, M., and Dohle, C. (2014). Different effects of the mirror illusion on motor and somatosensory processing. Restor. Neurol. Neurosci. 32, 269–280. doi: 10.3233/RNN-130343
Fukumura, K., Sugawara, K., Tanabe, S., Ushiba, J., and Tomita, Y. (2007). Influence of mirror therapy on human motor cortex. Int. J. Neurosci. 117, 1039–1048. doi: 10.1080/00207450600936841
Funase, K., Tabira, T., Higashi, T., Liang, N., and Kasai, T. (2007). Increased corticospinal excitability during direct observation of self-movement and indirect observation with a mirror box. Neurosci. Lett. 419, 108–112. doi: 10.1016/j.neulet.2007.04.025
Garry, M., Loftus, A., and Summers, J. (2005). Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp. Brain Res. 163, 118–122. doi: 10.1007/s00221-005-2226-9
Gegenfurtner, K. R., Kiper, D. C., and Fenstemaker, S. B. (1996). Processing of color, form, and motion in macaque area V2. Vis. Neurosci. 13, 161–172. doi: 10.1017/S0952523800007203
Glover, S., and Baran, M. (2017). The motor-cognitive model of motor imagery: evidence from timing errors in simulated reaching and grasping. J. Exp. Psychol. Hum. Percept. Perform. 43, 1359–1375. doi: 10.1037/xhp0000389
Grill, S. E., Hallett, M., Marcus, C., and McShane, L. (1994). Disturbances of kinaesthesia in patients with cerebellar disorders. Brain 117, 1433–1447. doi: 10.1093/brain/117.6.1433
Guerraz, M. (2015). The mirror paradigm and mirror therapy: does the “virtual hand” have a beneficial impact on motor behavior? Ther. Targ. Neurol. Dis. 2, 1–4. doi: 10.14800/ttnd.518
Guillot, A., Collet, C., Nguyen, V. A., Malouin, F., Richards, C., and Doyon, J. (2009). Brain activity during visual versus kinesthetic imagery: an fMRI study. Hum. Brain Mapp. 30, 2157–2172. doi: 10.1002/hbm.20658
Hadoush, H., Mano, H., Sunagawa, T., Nakanishi, K., and Ochi, M. (2013). Optimization of mirror therapy to excite ipsilateral primary motor cortex. NeuroRehabilitation 32, 617–624. doi: 10.3233/NRE-130884
Hamzei, F., Läppchen, C. H., Glauche, V., Mader, I., Rijntjes, M., and Weiller, C. (2012). Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehab. Neural Repair. 26, 484–496. doi: 10.1177/1545968311427917
Hanakawa, T., Immisch, I., Toma, K., Dimyan, M. A., Van Gelderen, P., and Hallett, M. (2003). Functional properties of brain areas associated with motor execution and imagery. J. Neurophysiol. 89, 989–1002. doi: 10.1152/jn.00132.2002
Hardwick, R. M., Caspers, S., Eickhoff, S. B., and Swinnen, S. P. (2018). Neural correlates of action: comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 94, 31–44. doi: 10.1016/j.neubiorev.2018.08.003
Hétu, S., Grégoire, M., Saimpont, A., Coll, M.-P., Eugène, F., Michon, P.-E., et al. (2013). The neural network of motor imagery: an ALE meta-analysis. Neurosci. Biobehav. Rev. 37, 930–949. doi: 10.1016/j.neubiorev.2013.03.01
Higuchi, S., Imamizu, H., and Kawato, M. (2007). Cerebellar activity evoked by common tool-use execution and imagery tasks: an fMRI study. Cortex 43, 350–358. doi: 10.1016/S0010-9452(08)70460-X
Hoshi, E., and Tanji, J. (2007). Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr. Opin. Neurobiol. 17, 234–242. doi: 10.1016/j.conb.2007.02.003
Jenkinson, P. M., and Preston, C. (2017). The “not-so-strange” body in the mirror: a principal components analysis of direct and mirror self-observation. Conscious. Cognit. 48, 262–272. doi: 10.1016/j.concog.2016.12.007
Kang, Y., Park, H., Kim, H., Lim, T., Ku, J., Cho, S., et al. (2012). Upper extremity rehabilitation of stroke: facilitation of corticospinal excitability using virtual mirror paradigm. J. NeuroEng. Rehab. 9:71. doi: 10.1186/1743-0003-9-71
Kang, Y. J., Ku, J., Kim, H. J., and Park, H. K. (2011). Facilitation of corticospinal excitability according to motor imagery and mirror therapy in healthy subjects and stroke patients. Ann. Rehab. Med. 35:747. doi: 10.5535/arm.2011.35.6.747
Kuhtz-Buschbeck, J., Mahnkopf, C., Holzknecht, C., Siebner, H., Ulmer, S., and Jansen, O. (2003). Effector-independent representations of simple and complex imagined finger movements: a combined fMRI and TMS study. Eur. J. Neurosci. 18, 3375–3387. doi: 10.1111/j.1460-9568.2003.03066.x
Lancaster, J. L., Tordesillas-Gutiérrez, D., Martinez, M., Salinas, F., Evans, A., Zilles, K., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 28, 1194–1205. doi: 10.1002/hbm.20345
Lee, H.-M., Li, P.-C., and Fan, S.-C. (2015). Delayed mirror visual feedback presented using a novel mirror therapy system enhances cortical activation in healthy adults. J. NeuroEng. Rehab. 12, 1–11. doi: 10.1186/s12984-015-0053-1
Li, W., Qin, W., Liu, H., Fan, L., Wang, J., and Jiang, T. (2013). Subregions of the human superior frontal gyrus and their connections. Neuroimage 78, 46–58. doi: 10.1016/j.neuroimage.2013.04.011
Manuweera, T., Yarossi, M., Adamovich, S., and Tunik, E. (2018). Parietal activation associated with target-directed right hand movement is lateralized by mirror feedback to the ipsilateral hemisphere. Front. Hum. Neurosci. 12. doi: 10.3389/fnhum.2018.00531
Matthys, K., Smits, M., Van der Geest, J. N., Van der Lugt, A., Seurinck, R., Stam, H. J., et al. (2009). Mirror-induced visual illusion of hand movements: a functional magnetic resonance imaging study. Arch. Phys. Med. Rehab. 90, 675–681. doi: 10.1016/j.apmr.2008.09.571
Mehnert, J., Brunetti, M., Steinbrink, J. M., Niedeggen, M., and Dohle, C. (2013). Effect of a mirror-like illusion on activation in the precuneus assessed with functional near-infrared spectroscopy. J. Biomed. Opt. 18:066001. doi: 10.1117/1.JBO.18.6.066001
Merians, A., Tunik, E., Fluet, G., Qiu, Q., and Adamovich, S. (2009). Innovative approaches to the rehabilitation of upper extremity hemiparesis using virtual environments. Eur. J. Phys. Rehab. Med. 45:123.
Michielsen, M. E., Selles, R. W., van der Geest, J. N., Eckhardt, M., Yavuzer, G., Stam, H. J., et al. (2011a). Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehab. Neural Repair. 25, 223–233. doi: 10.1177/1545968310385127
Michielsen, M. E., Smits, M., Ribbers, G. M., Stam, H. J., Van Der Geest, J. N., Bussmann, J. B., et al. (2011b). The neuronal correlates of mirror therapy: an fMRI study on mirror induced visual illusions in patients with stroke. J. Neurol. Neurosurg. Psychiatr. 82, 393–398. doi: 10.1136/jnnp.2009.194134
Milde, C., Rance, M., Kirsch, P., Trojan, J., Fuchs, X., Foell, J., et al. (2015). Do mirror glasses have the same effect on brain activity as a mirror box? Evidence from a functional magnetic resonance imaging study with healthy subjects. PLoS ONE 10:e0127694. doi: 10.1371/journal.pone.0127694
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4:1. doi: 10.1186/2046-4053-4-1
Molenberghs, P., Cunnington, R., and Mattingley, J. B. (2012). Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 36, 341–349. doi: 10.1016/j.neubiorev.2011.07.004
Müller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012
Naito, E., Nakashima, T., Kito, T., Aramaki, Y., Okada, T., and Sadato, N. (2007). Human limb-specific and non-limb-specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur. J. Neurosci. 25, 3476–3487. doi: 10.1111/j.1460-9568.2007.05587.x
Novaes, M. M., Palhano-Fontes, F., Peres, A., Mazzetto-Betti, K., Pelicioni, M., Andrade, K. C., et al. (2018). Neurofunctional changes after a single mirror therapy intervention in chronic ischemic stroke. Int. J. Neurosci. 128, 1–9. doi: 10.1080/00207454.2018.1447571
Numata, K., Murayama, T., Takasugi, J., Monma, M., and Oga, M. (2013). Mirror observation of finger action enhances activity in anterior intraparietal sulcus: a functional magnetic resonance imaging study. J. Jpn. Phys. Ther. Assoc. 16, 1–6. doi: 10.1298/jjpta.Vol16_001
Ogiso, T., Kobayashi, K., and Sugishita, M. (2000). The precuneus in motor imagery: a magnetoencephalographic study. Neuroreport 11, 1345–1349. doi: 10.1097/00001756-200004270-00039
Pisotta, I., and Molinari, M. (2014). Cerebellar contribution to feedforward control of locomotion. Front. Hum. Neurosci. 8, 1–5. doi: 10.3389/fnhum.2014.00475
Ramachandran, V. S., and Rogers-Ramachandran, D. (1996). Synaesthesia in phantom limbs induced with mirrors. Proc. R. Soc. Lond. Ser. B Biol. Sci. 263, 377–386. doi: 10.1098/rspb.1996.0058
Rizzolatti, G., Fadiga, L., Matelli, M., Bettinardi, V., Paulesu, E., Perani, D., et al. (1996). Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp. Brain Res. 111, 246–252. doi: 10.1007/BF00227301
Rosén, B., and Lundborg, G. (2005). Training with a mirror in rehabilitation of the hand. Scand. J. Plast. Reconstr. Surg. Hand Surg. 39, 104–108. doi: 10.1080/02844310510006187
Rushworth, M. F., Nixon, P. D., Wade, D. T., Renowden, S., and Passingham, R. E. (1998). The left hemisphere and the selection of learned actions. Neuropsychologia 36, 11–24. doi: 10.1016/S0028-3932(97)00101-2
Saleh, S., Adamovich, S. V., and Tunik, E. (2014). Mirrored feedback in chronic stroke: recruitment and effective connectivity of ipsilesional sensorimotor networks. Neurorehab. Neural Repair. 28, 344–354. doi: 10.1177/1545968313513074
Saleh, S., Yarossi, M., Manuweera, T., Adamovich, S., and Tunik, E. (2017). Network interactions underlying mirror feedback in stroke: a dynamic causal modeling study. NeuroImage Clin. 13:46. doi: 10.1016/j.nicl.2016.11.012
Schluter, N., Krams, M., Rushworth, M., and Passingham, R. (2001). Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia 39, 105–113. doi: 10.1016/S0028-3932(00)00105-6
Simmons, W. K., Ramjee, V., Beauchamp, M. S., McRae, K., Martin, A., and Barsalou, L. W. (2007). A common neural substrate for perceiving and knowing about color. Neuropsychologia 45, 2802–2810. doi: 10.1016/j.neuropsychologia.2007.05.002
Simos, P. G., Kavroulakis, E., Maris, T., Papadaki, E., Boursianis, T., Kalaitzakis, G., et al. (2017). Neural foundations of overt and covert actions. Neuroimage 152, 482–496. doi: 10.1016/j.neuroimage.2017.03.036
Stevens, J. A., and Stoykov, M. E. P. (2003). Using motor imagery in the rehabilitation of hemiparesis 1. Arch. Phys. Med. Rehab. 84, 1090–1092. doi: 10.1016/S0003-9993(03)00042-X
Strafella, A. P., and Paus, T. (2000). Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport 11, 2289–2292. doi: 10.1097/00001756-200007140-00044
Tarkka, I. M., and Hautasaari, P. (2019). Motor action execution in reaction-time movements: magnetoencephalographic study. Am. J. Phys. Med. Rehab. 98, 771–776. doi: 10.1097/PHM.0000000000001187
Thieme, H., Morkisch, N., Mehrholz, J., Pohl, M., Behrens, J., Borgetto, B., et al. (2018). Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 7, 1–155. doi: 10.1002/14651858.CD008449.pub3
Tinazzi, M., and Zanette, G. (1998). Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neurosci. Lett. 244, 121–124. doi: 10.1016/S0304-3940(98)00150-5
Tominaga, W., Matsubayashi, J., Deguchi, Y., Minami, C., Kinai, T., Nakamura, M., et al. (2009). A mirror reflection of a hand modulates stimulus-induced 20-Hz activity. Neuroimage 46, 500–504. doi: 10.1016/j.neuroimage.2009.02.021
Turkeltaub, P. E., Eickhoff, S. B., Laird, A. R., Fox, M., Wiener, M., and Fox, P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 33, 1–13. doi: 10.1002/hbm.21186
Uddin, L. Q., Molnar-Szakacs, I., Zaidel, E., and Iacoboni, M. (2006). rTMS to the right inferior parietal lobule disrupts self-other discrimination. Soc. Cognit. Affect. Neurosci. 1, 65–71. doi: 10.1093/scan/nsl003
Vanni, S., Tanskanen, T., Seppä, M., Uutela, K., and Hari, R. (2001). Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc. Natl. Acad. Sci. U.S.A. 98, 2776–2780. doi: 10.1073/pnas.041600898
Wang, H., Zhao, Z., Jiang, P., Li, X., Lin, Q., and Wu, Q. (2017). Effect and mechanism of mirror therapy on rehabilitation of lower limb motor function in patients with stroke hemiplegia. Biomed. Res. 28, 10165–10170.
Wang, J., Fritzsch, C., Bernarding, J., Holtze, S., Mauritz, K.-H., Brunetti, M., et al. (2013a). A comparison of neural mechanisms in mirror therapy and movement observation therapy. J. Rehab. Med. 45, 410–413. doi: 10.2340/16501977-1127
Wang, J., Fritzsch, C., Bernarding, J., Krause, T., Mauritz, K.-H., Brunetti, M., et al. (2013b). Cerebral activation evoked by the mirror illusion of the hand in stroke patients compared to normal subjects. NeuroRehabilitation 33, 593–603. doi: 10.3233/NRE-130999
Wang, X., Han, Z., He, Y., Caramazza, A., Song, L., and Bi, Y. (2013). Where color rests: spontaneous brain activity of bilateral fusiform and lingual regions predicts object color knowledge performance. Neuroimage 76, 252–263. doi: 10.1016/j.neuroimage.2013.03.010
Witteman, J., Van Heuven, V. J., and Schiller, N. O. (2012). Hearing feelings: a quantitative meta-analysis on the neuroimaging literature of emotional prosody perception. Neuropsychologia 50, 2752–2763. doi: 10.1016/j.neuropsychologia.2012.07.026
Wolbers, T., Weiller, C., and Büchel, C. (2003). Contralateral coding of imagined body parts in the superior parietal lobe. Cereb. Cortex 13, 392–399. doi: 10.1093/cercor/13.4.392
Yang, Y.-L., Deng, H.-X., Xing, G.-Y., Xia, X.-L., and Li, H.-F. (2015). Brain functional network connectivity based on a visual task: visual information processing-related brain regions are significantly activated in the task state. Neural Regen. Res. 10:298. doi: 10.4103/1673-5374.152386
Zeng, W., Guo, Y., Wu, G., Liu, X., and Fang, Q. (2018). Mirror therapy for motor function of the upper extremity in patients with stroke: a meta-analysis. J. Rehab. Med. 50, 8–15. doi: 10.2340/16501977-2287
Zheng, G., Ye, B., Zheng, Y., Xiong, Z., Xia, R., Qiu, P., et al. (2019). The effects of exercise on the structure of cognitive related brain regions: a meta-analysis of functional neuroimaging data. Int. J. Neurosci. 129, 406–415. doi: 10.1080/00207454.2018.1508135
Keywords: mirror-induced visual illusion, activation likelihood estimation, meta-analysis, cuneus, premotor
Citation: Bello UM, Kranz GS, Winser SJ and Chan CCH (2020) Neural Processes Underlying Mirror-Induced Visual Illusion: An Activation Likelihood Estimation Meta-Analysis. Front. Hum. Neurosci. 14:276. doi: 10.3389/fnhum.2020.00276
Received: 31 March 2020; Accepted: 18 June 2020;
Published: 31 July 2020.
Edited by:
Ferdinand Binkofski, RWTH Aachen University, GermanyReviewed by:
Hasan Ayaz, Drexel University, United StatesSoha Saleh, Kessler Foundation, United States
Copyright © 2020 Bello, Kranz, Winser and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stanley John Winser, stanley.j.winser@polyu.edu.hk; Chetwyn C. H. Chan, chetwyn.Chan@polyu.edu.hk
 Umar Muhammad Bello
Umar Muhammad Bello Georg S. Kranz
Georg S. Kranz Stanley John Winser
Stanley John Winser Chetwyn C. H. Chan
Chetwyn C. H. Chan