- 1International Institute for Integrative Sleep Medicine (WPI-IIIS), University of Tsukuba, Tsukuba, Japan
- 2Research and Development, VA North Texas Health Care System, Dallas, TX, United States
- 3Department of Psychiatry, The University of Texas Southwestern Medical Center, Dallas, TX, United States
- 4Department of Neuroscience, The University of Texas Southwestern Medical Center, Dallas, TX, United States
Roughly one-third of the human lifetime is spent in sleep, yet the reason for sleep remains unclear. Understanding the physiologic function of sleep is crucial toward establishing optimal health. Several proposed concepts address different aspects of sleep physiology, including humoral and circuit-based theories of sleep-wake regulation, the homeostatic two-process model of sleep regulation, the theory of sleep as a state of adaptive inactivity, and observations that arousal state and sleep homeostasis can be dissociated in pathologic disorders. Currently, there is no model that places the regulation of arousal and sleep homeostasis in a unified conceptual framework. Adenosine is well known as a somnogenic substance that affects normal sleep-wake patterns through several mechanisms in various brain locations via A1 or A2A receptors (A1Rs or A2ARs). Many cells and processes appear to play a role in modulating the extracellular concentration of adenosine at neuronal A1R or A2AR sites. Emerging evidence suggests that A1Rs and A2ARs have different roles in the regulation of sleep. In this review, we propose a model in which A2ARs allow the brain to sleep, i.e., these receptors provide sleep gating, whereas A1Rs modulate the function of sleep, i.e., these receptors are essential for the expression and resolution of sleep need. In this model, sleep is considered a brain state established in the absence of arousing inputs.
Introduction
Sleep is a highly conserved behavior that is vital to survival among all living organisms with a nervous system, from worms to humans. Chronic sleep loss is linked to a wide range of deleterious physiologic changes, such as altered food intake, weight loss or gain, skin lesions, compromised thermoregulation, and even death (Rechtschaffen et al., 1989; Siegel, 2008). Humans spend roughly one-third of their lives asleep. While we know why we eat, drink, and mate, we do not yet know why we sleep. The neuroscience community has therefore increased efforts to gain knowledge of the physiologic function of sleep.
During sleep, cortical neurons alternate between periods of firing and periods of silence. The switching between the two states, also known as ON and OFF states, is widely synchronized across neurons and represented by slow wave activity (SWA) in encephalography. SWA is observed as slow, oscillatory neocortical activity (0.5–4.5 Hz) that intensifies in correlation with wake duration and declines during sleep, but is expressed only during slow wave sleep (SWS). Because SWS-SWA increases as sleep loss is prolonged and decreases as sleep progresses, it is widely used as a marker of mammalian sleep homeostasis. The rates of SWA build-up and decay can be altered by extreme sleep loss or by pharmacologic or genetic manipulations in mammals, especially those affecting adenosine systems of the central nervous system (CNS). The adenosine system can also affect the gating of SWS-SWA expression by modulating the arousal level, thereby altering the duration of time during which sleep homeostasis and function can occur.
Adenosine is the key building block of every cell’s energy source, i.e., adenosine triphosphate (ATP), and the related adenosine mono- and di-phosphates (AMP and ADP, respectively). Adenosine fulfills a wide range of physiologic and pathophysiologic functions (Fredholm, 2014). In the nervous system, adenosine acts as a neuromodulator through metabotropic receptors. Although adenosine acts on four evolutionarily well-conserved receptors present on most cells, it is believed to modulate sleep need and arousal by acting through A1 and A2A receptors (A1Rs and A2ARs), respectively.
In light of the emerging roles of adenosine and its receptors in regulating different aspects of sleep, we propose a model for the gating and function of sleep. In our model, A2ARs allow the brain to sleep, i.e., these receptors provide sleep gating, whereas A1Rs modulate the function of sleep, i.e., these receptors are essential for the expression and resolution of the sleep need.
Aspects of Sleep/Wake Regulation
Humoral Theory of Sleep-Wake Regulation
The humoral theory of sleep-wake regulation posits that during wakefulness, one or more endogenous somnogenic factors is produced and accumulated. Brain activity decreases when the concentration of somnogenic substances increases to a certain threshold. These substances are gradually metabolized during sleep, which leads to a return to the waking state. Rosenbaum (1892) hypothesized that sleep is regulated by humoral factors; i.e., excess water accumulation due to oxidative processes in nerve cells during wakefulness depresses neuronal excitability and removal of the excess water during sleep restores full brain activity, resulting in wakefulness. A few years later, Ishimori (1909) and Legendre and Pieron (1913) independently demonstrated the existence of sleep-promoting hypnogenic substances, also known as “hypnotoxins,” in the cerebrospinal fluid of sleep-deprived dogs (Kubota, 1989; Inoué et al., 1995).
The hypnotic effect of adenosine in the mammalian brain was discovered in 1954 (Feldberg and Sherwood, 1954). Adenosine as a neuromodulator with somnogenic properties should thus be classified as a sleep substance. Extensive evidence also suggests that components of the immune system, such as pro-inflammatory cytokines (Krueger et al., 1984, 2001; Mullington et al., 2000, 2001; Krueger and Majde, 2003) [for review, see (Krueger et al., 2011)] and prostaglandins (Ushikubi et al., 1998; Lazarus et al., 2007; Urade and Lazarus, 2013; Oishi et al., 2015) [for review, see (Urade and Lazarus, 2013)], are interrelated with the regulation of sleep. The involvement of other putative hypnogenic substances, including anandamide (Garcia-Garcia et al., 2009), urotensin-II peptide (Huitron-Resendiz et al., 2005), and the Drosophila peptide NEMURI (Toda et al., 2019), is also implicated in the sleep process.
Circuit-Based Theories of Sleep-Wake Regulation
A slow humoral process, however, cannot sufficiently explain the reversibility of sleep, especially rapid transitions from sleep to wake in response to external stimuli. Experimental work by Constantin von Economo in the early 20th century produced findings that inspired circuit-based theories of sleep/wake regulation. In 1916, von Economo began to see patients with a new type of encephalitis eventually referred to as encephalitis lethargica or von Economo’s sleeping sickness. The disorder was characterized by lesions in the anterior hypothalamus leading to prolonged insomnia or lesions at the junction of the brainstem and forebrain leading to prolonged sleepiness (von Economo, 1917; Economo, 1930). Von Economo concluded that these brain areas must play a role in sleep/wake regulation. The “passive theory,” which dominated in the 1940/1950s, suggested that sleep occurs passively due to decreased activity of the brainstem reticular formation (Bremer, 1938; Moruzzi and Magoun, 1949). Importantly, this “passive theory” implicates a necessary active neuronal modulation to maintain a behavioral state of wake via the ascending reticular activating system. Although overly restrictive to the reticular activating system with regard to the wake-modulatory components, the principle of a necessary activation for wake cannot be ruled out; nor can an active sleep-promoting modulation be ruled out, as these are not mutually exclusive types of modulation.
Many decades later, neurons that are active when animals sleep were identified in the ventrolateral preoptic area (VLPO) near the third ventricle in the anterior part of the hypothalamus (Sherin et al., 1996; Chung et al., 2017). Studies demonstrated that sleep is promoted by projections from the GABAergic preoptic area (POA), including the VLPO, to the tuberomammillary nucleus (TMN) in the posterior hypothalamus (Sherin et al., 1998; Chung et al., 2017), which contains neurons that produce histamine, a neurotransmitter having an important role in arousal (Huang et al., 2006; Haas et al., 2008; Oishi et al., 2008). These findings provided strong evidence of sleep control by the POA-TMN neural pathway.
Neural circuits in the brainstem and basal ganglia also regulate sleep/wake behavior. The parafacial zone (PZ) in the medulla contains sleep-promoting GABAergic neurons (Anaclet et al., 2012, 2014) that project to the parabrachial nucleus (PB), a critical nucleus for cortical activation as lesions of the PB result in a comatose state (Fuller et al., 2011).
More recently, the involvement of dopaminergic neurons in the ventral tegmental area (VTA) was strongly implicated in the arousal effect (Eban-Rothschild et al., 2016; Oishi et al., 2017a). A role for dopamine in arousal is also supported by evidence that amphetamine, which induces the release of monoamines (including dopamine), increases alertness and psychomotor performance in sleep-deprived individuals (Bonnet et al., 2005). Dopamine transporters are necessary for the wake-promoting effects of amphetamine (Wisor et al., 2001). Ablating or suppressing GABAergic neurons in the ventral medial midbrain/pons (VMP), including the VTA, produces wakefulness and prevents sleep mainly through dopaminergic systems (Takata et al., 2018; Yu et al., 2019). Furthermore, the ability of dopamine neurons in the VTA to promote wakefulness is at least in part mediated by projections to the nucleus accumbens (NAc; Eban-Rothschild et al., 2016). Medium spiny GABAergic neurons in the NAc can be divided into two groups that respond differentially to stimulation by dopamine or adenosine. Direct pathway neurons express excitatory dopamine D1 receptors and inhibitory adenosine A1Rs, whereas neurons of the indirect pathway express inhibitory dopamine D2 receptors and excitatory A2ARs. In fact, recent studies showed that NAc direct pathway neurons induce wakefulness (Luo et al., 2018) and A2AR-expressing indirect pathway neurons strongly induce SWS (Oishi et al., 2017b). The indirect pathway neurons in the NAc produce sleep by inhibiting the ventral pallidum (VP) in the basal forebrain (BF), although these neurons also have sparse to moderate projections to other well-known arousal-promoting areas, such as the lateral hypothalamus, which produces orexin, the TMN, and the VTA. Interestingly, chemogenetic activation of the BF, including the VP, largely reduces sleep (Anaclet et al., 2015).
Altogether, it is impossible to abolish sleep completely by lesioning the afore-mentioned inhibitory circuits, including the POA-TMN, PZ-PB, and NAc-VP pathways, making it unlikely that the regulation of sleep time depends on a single center (i.e., a master switch for sleep in the brain may not exist). On the contrary, the existence of various neural circuits controlling sleep suggests that sleep is gated by different processes. All of these sleep/wake circuits clearly modulate an animal’s level of arousal (i.e., vigilance) to determine the behavioral state and GABA is the key neurotransmitter for promoting the transition from wake to sleep and the duration of sleep. For example, the observation that the level of wakefulness is regulated by VMP GABAergic neurons (Takata et al., 2018) indicates the ability of the brain to adapt an animal’s sleep/wake time to its behavior.
The transition from waking to sleep may be essential, at least under physiologic conditions, for the facilitation of sleep function to occur. A sufficiently increased level of arousal, as may be experimentally or environmentally induced, prevents sleep occurrence and, accordingly, sleep function. The resulting increase in sleep need is normally reflected by an increase in SWA in the ensuing sleep episode. During this ensuing episode, SWS-SWA resolves toward a non-sleep deprived baseline and the threshold for arousal to waking decreases. As a matter of fact, local sleep, i.e., a phenomenon in which discrete regions of cortical neurons go “offline” similar to during sleep, but other regions do not, is insufficient for sleep function to occur (Vyazovskiy et al., 2011), most likely as a result of the brain’s massive interconnectivity. Thus the integration of local sleep events into a global sleep state is necessary for effective sleep function, even at a local level.
Homeostatic Regulation of Sleep (Two-Process Model)
In 1982, Alexander Borbély at the University of Zürich in Switzerland proposed a two-process model of sleep regulation (Borbély, 1982) that currently prevails as a major conceptual framework in sleep research. In a simplified version of the two-process model of sleep regulation, sleep propensities in homeostatic and circadian processes are commonly plotted against the time of day and interactions of the two processes determine the cardinal aspects of sleep regulation. The “homeostatic” process is controlled by the sleep pressure or need that builds up during the waking period and dissipates during sleep. In contrast, the “circadian” process, i.e., the sleep/wake cycle during the day and night, is controlled by a circadian pacemaker or biologic clock. Although it was originally hypothesized that the circadian process is independent of prior sleep and waking, experiments in mice lacking clock genes revealed that clock gene knockout (KO) disrupts not only circadian processes, but also sleep homeostatic processes (Franken, 2013). This suggests overlapping functions for the circadian genes in sleep homeostatic control.
Consistent with the two-process model, a homeostatic response to sleep loss, namely sleep rebound in an animal after sleep deprivation, is considered an essential criterion of sleep. Rebound can reflect an increase in SWS-SWA power and/or an increase in SWS duration along with an increase in consolidation. Of these two rebound parameters, SWS-SWA is better correlated with prior waking time. Although a rebound increase in SWA during SWS is often associated with an increase in SWS time or consolidation, its occurrence may be dissociated from an effect on sleep time (Douglas et al., 2007; Bjorness et al., 2009; Suzuki et al., 2013). Importantly, SWA during SWS is considered to be an indicator of sleep intensity (Borbély and Neuhaus, 1979), providing a dimension beyond time in the recovery from prolonged waking. Interestingly, although sleep rebound is widely observed after sleep loss, some species skip sleep in favor of migration, mating, or other social interactions and do not catch up on lost sleep (Berger and Phillips, 1994; Rattenborg et al., 2004; Lyamin et al., 2005; Fuchs et al., 2009; Thimgan et al., 2010; Lesku et al., 2012). Recently, scientists at the Imperial College London demonstrated that male flies in the presence of another male fly undergo sleep loss that results in a sleep rebound once the male intruder is removed, whereas a resident fly also loses sleep in the presence of a female fly, but shows no sleep rebound when the female fly is removed (Beckwith et al., 2017), suggesting that sexual arousal in flies prevents a homeostatic response to sleep loss. Altogether, there is ample evidence in nature challenging the view that sleep rebound is an inescapable outcome of sleep loss. Nevertheless, the “rebound” in these cases refers only to sleep duration and not to SWS-SWA intensity. This potential dissociation of SWS duration from SWS-SWA expression (i.e., SWS duration may not reflect the SWA changes shaping sleep homeostasis) suggests that it may not be possible to fully interpret rebound sleep or the lack thereof in the absence of SWS-SWA assessment.
Another limitation of the two-process model is that it defines circadian input as the only allostatic component that drives the balance between waking and sleep. Sleep/wake behavior is also influenced by cognitive and emotional factors (Saper et al., 2005; Fernandez-Mendoza et al., 2014; Mullins et al., 2014) or other basic drives, such as a lack of food, predator confrontation, mating pressure, and seasonal migration (Yamanaka et al., 2003; Cano et al., 2008). The mechanisms by which motivational stimuli or stressors interact with sleep/wake behavior are not easily accounted for by the two-process model. On the other hand, if the exceptional conditions mentioned above primarily affect arousal level and thus gating of sleep, then sleep homeostasis, conceptualized as “process S” in the two-process model, may still occur. Sleep homeostasis, although related to arousal (sleep need can dissipate to the largest extent only during sleep) appears to follow an exponential rate of decay (Franken et al., 2001; Bjorness et al., 2016). With greater sleep need, there is greater rebound SWS-SWA, but the rate of decay is slowed, further enhancing the amount of SWS-SWA expressed (Bjorness et al., 2016).
The increased sleep duration and consolidation associated with increased sleep need may reflect a decreased level of arousal needed for waking although both external sensory input as well as the internal state (likely to include circadian drive, need for food, predator threat, sex drive, etc.) remain as effective determinants. Accordingly, level of arousal and sleep duration are dynamic, relying on the integration of multiple factors in addition to previous waking time.
Sleep as a State of Adaptive Inactivity
An alternative view proposes that sleep enforces adaptive inactivity to conserve energy when activity is not beneficial (Siegel, 2009). The wide variability in sleep duration across the animal kingdom (Preston et al., 2009) suggests the sleep amount of an animal may be adapted to the species’ behavior that is critical for survival. Consequently, animals may have the ability to dispense with sleep when varying ecologic demands favor wakefulness; e.g., the ability of male pectoral sandpipers to maintain high neurobehavioral performance despite greatly reduced sleeping time when competing for mating opportunities in a short annual window of female fertility (Lesku et al., 2012) may contradict the notion that decreased performance is an inescapable outcome of sleep loss. A model of sleep as a state of adaptive inactivity challenges the hypothesis that the sleep state persists because it has a vital physiologic function and proposes that sleep has not evolved for what happens when we are asleep, but rather for the energy-saving absence of activity during sleep. The magnitude of energy savings gained through sleep is still unknown, although a new framework for determining relative energy savings during sleep was recently described (Schmidt et al., 2017).
The teleological problem of sleep function may arise from the presumption of sleep’s evolution from a default state of waking. Humans are likely biased toward this presumption by the egocentricity of waking consciousness. The adaptive inactivity model could be modified by reorientation of the question “why do we sleep” to “why do we wake?” In this model, sleep is considered the state that facilitates vegetative functions like anabolism and replacement of proteins, complex carbohydrates, and complex lipids and organelles. The vegetative functions are clearly not inactivity or passive in terms of energy conservation. On the contrary, there is evidence for increased energy utilization in sleep, such as ATP mobilization and AMP dephosphorylation (Dworak et al., 2010). Moreover, the cellular metabolism of brain tissue does not coincide with the systemic eating and digestion of food. From this perspective, an organism is driven to waking primarily by non-vegetative, life-essential pursuits, such as foraging for food, avoiding predators, and, occasionally, sex, along with an integrated circadian timer (also controlling arousal). This model is thus consistent with an active drive or activating system needed to maintain wake.
Dissociation of the Arousal State and Sleep Homeostasis
An increase in the response threshold to external stimuli is a core feature of sleep (Zepelin, 1994) and is critical for defining sleep in animals that lack a cortex (for review, see Ho and Sehgal, 2005). Prolonged waking by sleep deprivation increases the arousal threshold during subsequent sleep (Williams et al., 1964; Bonnet, 1985), and stronger stimuli are necessary to prevent sleeping/prolong waking (Blumberg et al., 2004). As with spontaneous waking, arousal during sleep deprivation is modulated by internal and external stimuli, but within the context of sleep deprivation the arousal threshold is typically increased. Arousal-related brain regions show greater activity, as measured by c-fos, under sleep deprivation mediated by exposure to novel environments or social interaction compared with gentle handling alone (Deurveilher et al., 2013). Furthermore, the nature of the homeostatic response to prolonged waking varies across development with increases in sleep time preceding the appearance of increased SWA power (Frank et al., 1998). Finally, SWA power, commonly used as an indicator of homeostatic sleep need, is increased within waking during prolonged sleep deprivation (Franken et al., 2001; Vyazovskiy et al., 2011), but the relationship between SWA and sleep homeostasis can be dissociated in pathologic disorders. SWA power is increased during waking and rapid eye movement (REM) sleep in Alzheimer’s disease (Hassainia et al., 1997; Jeong, 2004); whereas SWA is increased during waking but decreased during sleep in schizophrenia (Keshavan et al., 1998; Hoffmann et al., 2000; Fehr et al., 2003). Conversely, faster EEG activity, such as beta and gamma rhythms commonly used as an indicator of arousal, is high during sleep in a subset of insomniacs (Perlis et al., 2001; Dolsen et al., 2017).
Adenosine and Sleep
Adenosine Metabolism and Levels During Sleep and Wakefulness
Hydrolysis of AMP and S-adenosylhomocysteine (SAH) produces adenosine (Schrader, 1983; Fredholm, 2007). Adenosine is generated from SAH by SAH hydrolase, which also acts to trap adenosine in the presence of excess L-homocysteine. This takes place intracellularly and the bidirectional actions of the enzyme ensure the constant presence of a particular concentration of adenosine in the cell. Whether SAH hydrolase is involved in generating adenosine in the brain, however, is controversial (Latini and Pedata, 2001). Adenosine is formed intracellularly and extracellularly from 5′-AMP by different 5′-nucleotidase (5′-NT) (Zimmermann, 2000). A cascade of actions by an ecto-5′-NT, together with ecto-ATPases, terminates the action of ATP as extracellular signaling molecules (Zimmermann, 2000, 2006; Yegutkin, 2008; Kovacs et al., 2013).
High adenosine levels are reduced by the actions of adenosine deaminase (ADA), or are taken up by cells where adenosine is rapidly phosphorylated to AMP by adenosine kinase (AdK), an enzyme that effectively controls the intracellular adenosine concentration (Figure 1; Fredholm et al., 2005; Oishi et al., 2008; Parkinson et al., 2011). Importantly, AdK binds a molecule of ATP and adenosine, catalyzes the transfer of a phosphate group from ATP to adenosine and produces ADP and AMP. As a result, the rate of adenosine metabolism is reflected by the [ATP]/[ADP][AMP] ratio, linking the rate of adenosine metabolism to the metabolic state of the cell. In the adult CNS, AdK expression occurs predominately in the glia (Studer et al., 2006), and thus the concentration of adenosine is controlled by the metabolic state of the glia.
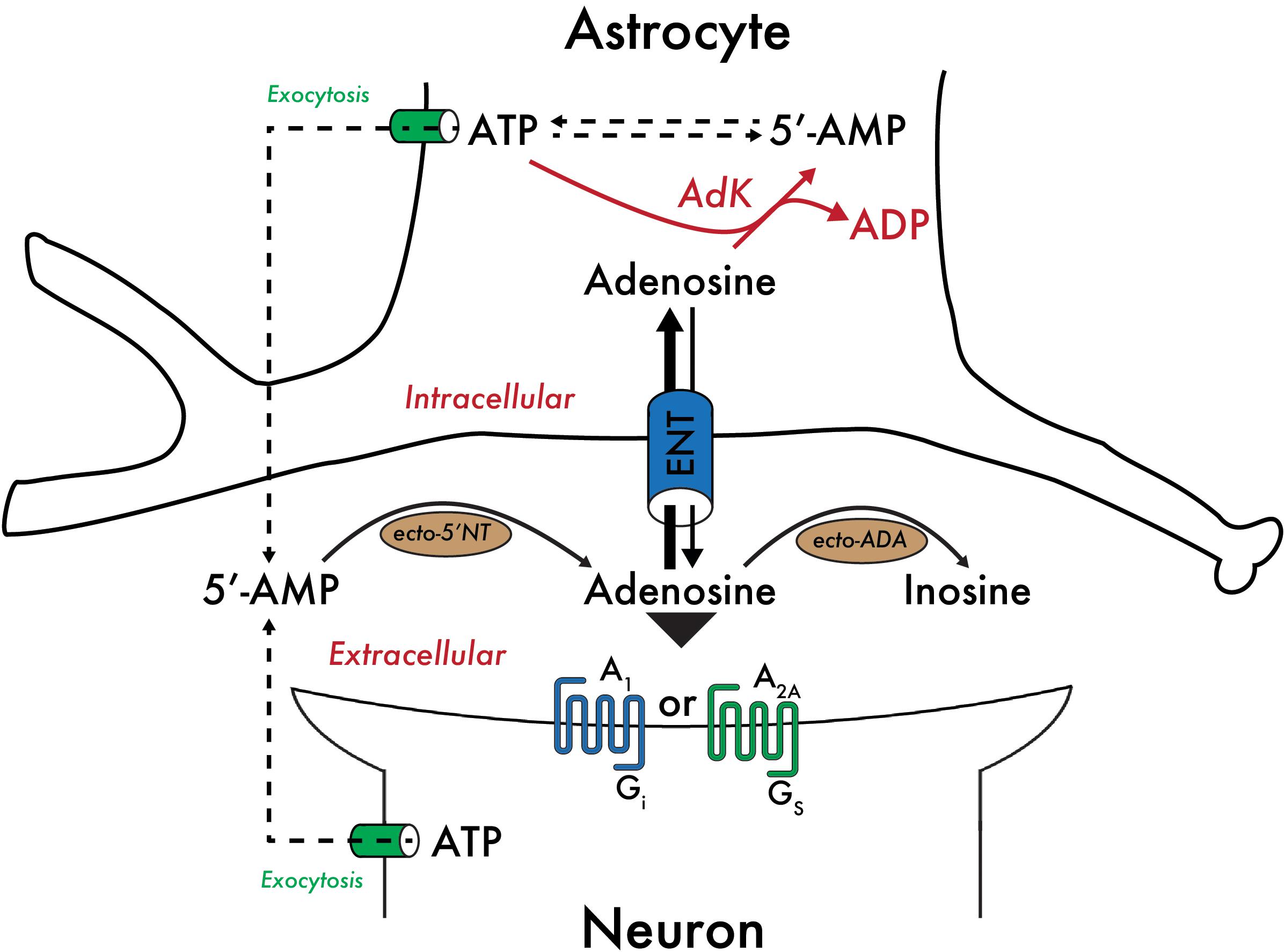
Figure 1. Control of the adenosine concentration by the metabolic state of astrocytes. Adenosine taken up by astrocytes is rapidly phosphorylated to AMP by adenosine kinase (AdK), an enzyme expressed predominantly in glia in the adult CNS. AdK effectively controls the intracellular adenosine concentration by catalyzing the transfer of a phosphate group from ATP to adenosine to produce ADP and AMP. As a result, the rate of adenosine metabolism is reflected by the [ATP]/[ADP][AMP] ratio, linking the rate of adenosine metabolism to the metabolic state of the cell. Equilibrative nucleoside transporters (ENT) bi-directionally regulate the concentration of adenosine available to pre- and post-synaptic A1Rs and A2ARs. Other abbreviations used: 5′-NT, 5′-nucleotidase; ADA, adenosine deaminase.
Bi-directional equilibrative nucleoside transporters regulate the concentration of adenosine available to cell surface adenosine receptors (Parkinson et al., 2011; Dos Santos-Rodrigues et al., 2014). Therefore, adenosine levels are dependent on the formation and removal of extracellular adenosine. Extracellular adenosine levels are low under basal conditions – approximately 30 to 300 nM (Ballarin et al., 1991), but may exceed 1 μM under more extreme conditions, such as mild hypoxia or strenuous exercise, and can reach up to several tens of micromolar concentration in severely traumatic situations, including local ischemia (Fredholm, 2007).
Adenosine triphosphate depletion and an increase of extracellular adenosine levels are positively correlated (Kalinchuk et al., 2003) and positively associated with sleep (Rainnie et al., 1994; Porkka-Heiskanen et al., 1997). Thus, adenosine may represent a state of relative energy deficiency. During spontaneous sleep/wake behavior in cats, adenosine levels in several brain regions are higher during SWS than wakefulness (Porkka-Heiskanen et al., 1997, 2000). Moreover, in vivo microdialysis studies in cats revealed that adenosine concentrations increase 2-fold in the BF during a prolonged 6-h period of wakefulness compared with that at the beginning of sleep deprivation. Under more chronic sleep deprivation protocols, however, increases in adenosine concentrations during prolonged waking are no longer observed (Clasadonte et al., 2014), suggesting that loss of the adenosine response may mark a shift from a homeostatic response to an allostatic response following reduced sleep.
Six decades after the discovery of adenosine’s role in sleep, the mammalian brain cell types involved in the sleep-promoting effects of adenosine remain unclear (Feldberg and Sherwood, 1954). ATP, which is rapidly degraded to adenosine, and adenosine are released from glial cells and neurons. In genetically engineered mice in which the release of ATP is non-specifically blocked in astrocytes by selective expression of a dominant negative SNARE domain, decreased concentrations of extracellular adenosine are observed (Pascual et al., 2005). While the amounts of wakefulness, SWS, and REM sleep in these mice are indistinguishable from those in wild-type mice, these mice exhibit reduced SWA and recovery sleep after sleep deprivation (Halassa et al., 2009). Furthermore, reducing AdK in astrocytes, thereby increasing the adenosine tone, is sufficient to increase SWS-SWA and sleep consolidation, reduce the decrease in SWA across the light phase, and slow the decay of SWS-SWA within an average SWS episode, whereas selectively reducing AdK in neurons has no effect (Bjorness et al., 2016). These observations suggest that adenosine mediates the sleep deprivation-induced homeostatic sleep response. The source of the released adenosine, however, remains controversial. Some of the adenosine may originate from astrocytes and the majority may originate from neurons, but direct proof is lacking and thus the exact source of adenosine remains unknown. On the other hand, control of extracellular adenosine modulating sleep need clearly involves glial metabolism mediated by AdK (Bjorness et al., 2016).
Radulovacki et al. (1983) extensively investigated the effects of adenosine on wakefulness. They found that increasing the levels of adenosine in the central nervous system of rats by systemic administration of the ADA inhibitor deoxycoformycin led to increases in REM and SWS. In addition, Oishi et al. (2008) reported that focal administration of the ADA inhibitor coformycin into the rat TMN, where ADA is dominantly expressed, increases SWS, further supporting a hypnotic role for adenosine.
Effects of A1 Receptors and Sleep Homeostasis
Adenosine acting through A1Rs facilitates sleep as non-selective and selective A1R agonists increase sleep and SWA (Radulovacki et al., 1984; Benington et al., 1995), whereas A1R antagonists decrease sleep and SWA (Virus et al., 1990; Methippara et al., 2005; Thakkar et al., 2008). Furthermore, A1R antagonism within the BF reduces the homeostatic sleep and SWA response following acute sleep deprivation (Gass et al., 2009). Conditional KO of A1Rs predominantly affecting forebrain glutamatergic neurons prevents sleep deprivation-induced increases in SWA, indicating that A1Rs are necessary for normal sleep homeostasis (Bjorness et al., 2009). In mixed background mice with constitutive KO of A1Rs, the normal sleep homeostatic response is maintained as measured by slow wave energy [SWE; SWA (0.5–4.5 Hz) × time] in SWS (Stenberg et al., 2003). Further, acute application of a selective A1R antagonist blocks the homeostatic response of increased SWS-SWA in sleep-deprived wild-type mice, but is ineffective in the constitutive KO mice under the same conditions (Stenberg et al., 2003). This finding suggests the presence of compensatory mechanisms in mice with constitutive KO that were not present in mice with conditional KO. Sleep facilitation via A1Rs occurs through inhibition of wake-active neurons in several brain areas, including both the brainstem and forebrain regions of the cholinergic arousal system [mesopontine tegmentum (Rainnie et al., 1994) and BF (Alam et al., 1999; Thakkar et al., 2003)], and the lateral hypothalamus containing hypocretin/orexin neurons (Liu and Gao, 2007). Additionally, administration of a selective A1R agonist into the TMN decreases histamine in the frontal cortex while increasing sleep and SWA (Oishi et al., 2008), suggesting that adenosine also inhibits activity of this wake-promoting neurotransmitter system. An additional mechanism by which adenosine facilitates sleep through A1Rs is by disinhibiting sleep-active neurons in the VLPO and anterior hypothalamic area (Chamberlin et al., 2003; Morairty et al., 2004). Finally, A1Rs mediate homeostatic sleep pressure based on astrocytic gliotransmission (Halassa et al., 2009) and as part of a glial-neuronal circuit (Bjorness et al., 2016).
Prolonged waking through sleep deprivation increases the expression of A1Rs in both humans and rodents (Basheer et al., 2007; Elmenhorst et al., 2007), with expression levels normalizing after recovery sleep in humans (Elmenhorst et al., 2017).
As mentioned above, SWA power is the primary indicator of homeostatic sleep need. SWA power reflects both the number of cells firing at SWA frequencies, which is an intrinsic feature of thalamocortical neurons (McCormick and Pape, 1990; Dossi et al., 1992), and the synchronicity of firing across neurons, which is a circuit effect involving cortical neurons, thalamocortical neurons, and neurons of the reticular nucleus of the thalamus (Steriade et al., 1993). Activation of A1Rs influences SWA by both direct and indirect mechanisms; the direct mechanism is based on presynaptic inhibition of cortical and thalamic neurons, which results in relative functional deafferentation along with an A1R-induced increase in whole cell, GIRK channel conductance and decreased hyperpolarization activated currents (Ih), such that adenosine enhances slow oscillations in thalamocortical neurons (Pape, 1992). The indirect mechanism is a reduction of cholinergic tone by A1R-mediated inhibition of cholinergic arousal neurons (Rainnie et al., 1994; Porkka-Heiskanen et al., 1997). Acetylcholine inhibits slow oscillation in thalamocortical neurons (Curro Dossi et al., 1991; Steriade et al., 1991; McCormick, 1993); thus reduction of cholinergic tone is permissive for the expression of SWA.
Effects of A2A Receptors and Control of Arousal
Infusion of the selective A2AR agonist CGS21680 into the subarachnoid space below the ventral surface region of the rostral BF in rats or into the lateral ventricle of mice produces robust increases in SWS and REM sleep (Satoh et al., 1996; Urade et al., 2003). In vivo microdialysis experiments, infusing CGS21680 into the BF dose-dependently decreases histamine release in the frontal cortex and medial preoptic area, and increases the release of GABA in the TMN, but not in the frontal cortex (Hong et al., 2005). Infusion of the GABA antagonist picrotoxin into the TMN attenuates the CGS21680-induced inhibition of histamine release, suggesting that the A2AR agonist induces sleep by inhibiting the histaminergic system through increasing the release of GABA in the TMN. Intracellular recordings of VLPO neurons in rat brain slices demonstrated that two distinct types of VLPO neurons exist in terms of their responses to serotonin and adenosine. VLPO neurons are inhibited by noradrenaline, acetylcholine, and an A1R agonist, whereas serotonin inhibits type-1 neurons, but excites type-2 neurons. An A2AR agonist post-synaptically excites type-2, but not type-1, neurons. These findings suggest that type-2 neurons are involved in initiating sleep, whereas type-1 neurons may contribute to sleep consolidation, because they are only activated in the absence of inhibitory effects from wake-inducing systems (Gallopin et al., 2005).
Administration of CGS21680 into the rostral BF, however, produces c-fos expression not only in the VLPO, but also within the NAc shell and the medial portion of the olfactory tubercle (Satoh et al., 1999; Scammell et al., 2001). Direct infusion of the A2AR agonist into the NAc induces SWS that corresponds to approximately 75% of the sleep amount measured when the A2AR agonist is infused into the subarachnoid space (Satoh et al., 1999). This observation may indicate that activating A2ARs within or close to the NAc induces sleep. Acting opposite to adenosine, caffeine, which is the most widely consumed psychostimulant in the world, enhances wakefulness because it acts to antagonize both A1R and A2AR subtypes. At doses commonly consumed by humans, caffeine partially (estimated as 25–50%) and non-selectively (similar affinity for both A1Rs and A2ARs) blocks adenosine receptors (Fredholm et al., 1999). Experiments using mice with global genetic A1R and A2AR KO revealed that A2ARs, but not A1Rs, mediate the wakefulness-inducing effect of caffeine (Huang et al., 2005), while single nucleotide mutations of the A2AR gene confer sensitivity to caffeine and sleep deprivation (Bodenmann et al., 2012). The specific role of A2ARs in the striatum was investigated in conditional A2AR KO mice based on the Cre/lox technology and local infection with AAV carrying short-hairpin RNA of the A2AR to silence the expression of the receptor. Selective deletion of the A2ARs in the NAc shell blocked caffeine-induced wakefulness (Lazarus et al., 2011).
For caffeine to be effective as an A2AR antagonist, adenosine must tonically activate excitatory A2ARs within the NAc shell. This activation likely occurs in the NAc shell because A2ARs are abundantly expressed throughout the striatum, including the NAc shell and sufficient levels of adenosine are available under basal conditions (Rosin et al., 1998; Svenningsson et al., 1999). A recent study showed that chemogenetic or optogenetic activation of NAc A2AR core neurons projecting to the VP in the BF strongly induces SWS, whereas chemogenetic inhibition of these neurons prevents sleep induction, but does not affect homoeostatic sleep rebound (Oishi et al., 2017b). Interestingly, motivational stimuli suppress sleep and inhibit the activity of VP-projecting NAc A2AR neurons. In addition, another recent study revealed that adenosine is a plausible candidate molecule for activating NAc core A2AR neurons to induce SWS because elevated adenosine levels in the NAc core promote SWS via A2ARs (Zhou et al., 2019).
The sleep-gating ability of the NAc indirect pathway may explain the tendency toward falling asleep in boring situations. Interestingly, excessive daytime sleepiness is common in children with attention-deficit/hyperactivity disorder, who frequently start napping or daydream when they are bored (Weinberg and Brumback, 1990). Dopamine produced by VTA neurons has a key role in processing reward, aversive, or cognitive signals (Wise, 2004; Bromberg-Martin et al., 2010; Schultz, 2015), and projections from VTA dopaminergic neurons to the NAc, commonly known as the mesolimbic pathway, constitute a well-characterized reward circuit in the brain (Russo and Nestler, 2013; Volkow and Morales, 2015). Two independent studies recently examined the contribution of VTA dopaminergic neurons to wakefulness under baseline conditions by chemogenetic inhibition. One study found that chemogenetic inhibition of VTA dopamine neurons decreases the amount of wakefulness, thus suggesting that these neurons are necessary for baseline wakefulness in mice (Eban-Rothschild et al., 2016). The other study showed that chemogenetic inhibition of VTA dopamine neurons does not significantly affect wakefulness at baseline in mice (Oishi et al., 2017a). A plausible explanation for the differences in the observations in these studies is different ectopic Cre expression (Lammel et al., 2015) in the midbrain of the tyrosine hydroxylase-Cre mice used by Eban-Rothschild et al. (2016) or the dopamine transporter-Cre mice used by Oishi et al. (2017a).
Unified Model of Sleep-Wake Regulation: Gating of Sleep Homeostasis by Arousal
As knowledge of the molecular and circuit bases of sleep/wake regulation expands, new roles of adenosine receptors in modulating different aspects of sleep emerge. For example, A2ARs appear to promote sleep by suppressing arousal, whereas sleep need and the response to sleep deprivation are mediated by A1Rs, and these receptors may thus play a crucial role in the function of sleep. In light of the dissociable effects of adenosine for gating sleep and mediating sleep need at the receptor level, we propose a model of sleep-wake regulation in which the sleep state is regulated by arousal when an organism must consolidate wakefulness in response to environmental changes (Figure 2). A typical example is motivated behavior that efficiently suppresses sleep of all stages and produces arousal by utilizing mesolimbic dopaminergic systems, whereas the wake state is suppressed in the absence of motivating stimuli by activation of A2ARs in the NAc (Oishi and Lazarus, 2017; Oishi et al., 2017b). The circadian and hypothalamic feeding systems have indirect influences by driving internally generated arousal, e.g., increasing motivation to forage according to the circadian phase. Thus in the absence of motivating/external arousing stimuli, the loss of the arousing influence of the circadian system (the sleep phase) may be sufficient to allow transition to sleep. On the other hand, sleep is necessary for SWS-SWA to facilitate the expression of sleep need and for the resolution of sleep need, a process in which A1Rs play a crucial role.
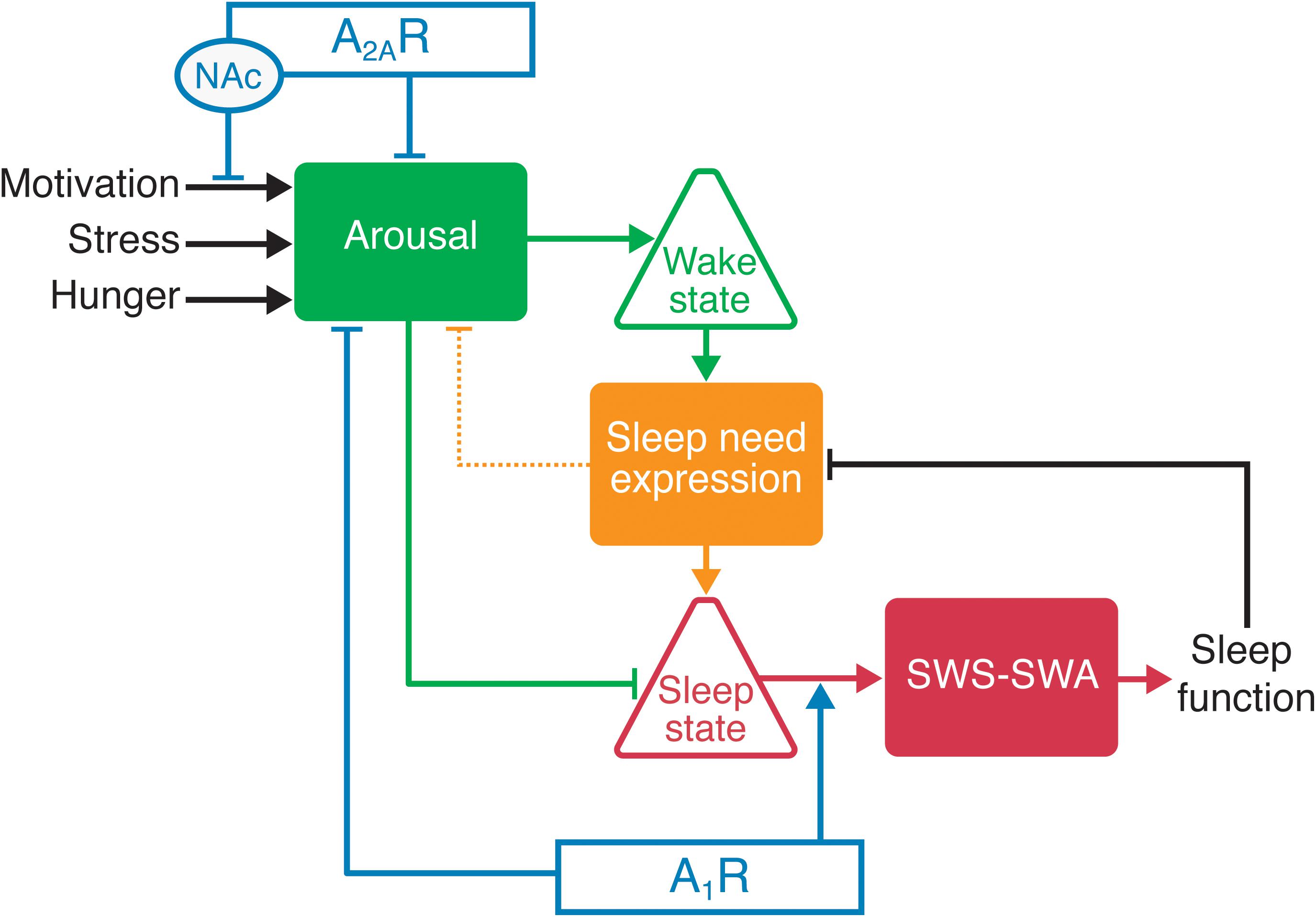
Figure 2. Adenosine receptors influence sleep/wake behavior by modulating the arousal level through A2ARs or A1Rs and the sleep need through A1Rs. Increased activity of the arousal centers promotes wakefulness. For example, activation of A2ARs in the nucleus accumbens (NAc) and hypothalamus facilitates sleep through the inhibition of arousal-promoting neurons. The duration of wake time positively correlates with sleep need and the buildup of extracellular adenosine. The buildup of adenosine in the cortex and thalamus increases SWS-SWA through the activation of A1Rs. Sleep need also increases the probability of a state change from wake to sleep, primarily by decreasing arousal center activity (in part by activating A1Rs in arousal centers and A2ARs in the NAc). The sleep state is permissive for sleep function that resolves the sleep need (as sleep function is accomplished), as reflected by the resolution of rebound SWS-SWA.
Conclusion
Adenosine is a well-known somnogenic substance that affects normal sleep-wake patterns. While the source of the adenosine involved in sleep remains poorly understood, the metabolism of adenosine in the CNS is significantly mediated by adenosine kinase, which modulates the concentration of adenosine at neuronal A1R sites. Similarly, adenosine promotes sleep by several mechanisms in various locations via A1Rs or A2ARs.
Adenosine receptor stimulation should be considered as a potential treatment for insomnia. Insomnia is a sleep disorder affecting millions of people around the world and frequently co-occurs with a wide range of psychiatric disorders (Roth, 2007; de Zambotti et al., 2018; Seow et al., 2018). Although A2AR agonists strongly induce sleep, classical A2AR agonists have adverse cardiovascular effects and cannot be used clinically to treat sleep disorders. Moreover, the development of adenosine analogs for treating central nervous system disorders, including insomnia, is hampered by the poor transport of these drugs across the blood-brain barrier. A small blood brain barrier-permeable monocarboxylate was recently demonstrated to induce sleep by enhancing A2AR signaling in the brain, and surprisingly did not exhibit the typical cardiovascular effects of A2AR agonists (Korkutata et al., 2017). Therefore, molecules that allosterically enhance A2AR signaling could help people with insomnia fall asleep and may also be a potential treatment for psychiatric illness. Similarly, molecules that enhance A1R signaling might enhance sleep efficiency.
Author Contributions
All authors wrote the review, and approved it for publication.
Funding
Our work was generously supported by the Japan Society for the Promotion of Science [Grants-in-Aid for Scientific Research B (grant number 17H02215) to ML]; the Japan Science and Technology Agency [CREST (grant number JPMJCR1655) to ML]; the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan [Grants-in-Aid for Scientific Research on Innovative Areas “Living in Space” (grant numbers 15H05935, 15K21745, and 18H04966) and “WillDynamics” (grant number 19H05004) to ML]; the World Premier International Research Center Initiative (WPI) from MEXT (to ML, YO, and RG.); a research grant from the Astellas Foundation for Research on Metabolic Disorders (to ML); the National Institutes of Health (grant numbers MH 06777 and NS075545 to RG); and the Department of Veterans Affairs through Dallas VA Medical Center (grant numbers MH79710 and MH083711 to RG). The contents of this review do not represent the views of the United States Department of Veterans Affairs or the United States Government.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate the research spirit and diligence of all the colleagues who contributed to this fascinating and lively research area.
References
Alam, M. N., Szymusiak, R., Gong, H., King, J., and Mcginty, D. (1999). Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J. Physiol. 521(Pt 3), 679–690. doi: 10.1111/j.1469-7793.1999.00679.x
Anaclet, C., Ferrari, L., Arrigoni, E., Bass, C. E., Saper, C. B., Lu, J., et al. (2014). The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat. Neurosci. 17, 1217–1224. doi: 10.1038/nn.3789
Anaclet, C., Lin, J.-S., Vetrivelan, R., Krenzer, M., Vong, L., Fuller, P. M., et al. (2012). Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J. Neurosci. 32, 17970–17976. doi: 10.1523/JNEUROSCI.0620-12.2012
Anaclet, C., Pedersen, N. P., Ferrari, L. L., Venner, A., Bass, C. E., Arrigoni, E., et al. (2015). Basal forebrain control of wakefulness and cortical rhythms. Nat. Commun. 6:8744. doi: 10.1038/ncomms9744
Ballarin, M., Fredholm, B. B., Ambrosio, S., and Mahy, N. (1991). Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol. Scand. 142, 97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x
Basheer, R., Bauer, A., Elmenhorst, D., Ramesh, V., and Mccarley, R. W. (2007). Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport 18, 1895–1899. doi: 10.1097/wnr.0b013e3282f262f6
Beckwith, E. J., Geissmann, Q., French, A. S., and Gilestro, G. F. (2017). Regulation of sleep homeostasis by sexual arousal. eLife 6:e27445. doi: 10.7554/eLife.27445
Benington, J. H., Kodali, S. K., and Heller, H. C. (1995). Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 692, 79–85. doi: 10.1016/0006-8993(95)00590-m
Berger, R. J., and Phillips, N. H. (1994). Constant light suppresses sleep and circadian rhythms in pigeons without consequent sleep rebound in darkness. Am. J. Physiol. 267, R945–R952.
Bjorness, T. E., Dale, N., Mettlach, G., Sonneborn, A., Sahin, B., Fienberg, A. A., et al. (2016). An adenosine-mediated glial-neuronal circuit for homeostatic sleep. J. Neurosci. 36, 3709–3721. doi: 10.1523/JNEUROSCI.3906-15.2016
Bjorness, T. E., Kelly, C. L., Gao, T., Poffenberger, V., and Greene, R. W. (2009). Control and function of the homeostatic sleep response by adenosine A1 receptors. J. Neurosci. 29, 1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009
Blumberg, M. S., Middlemis-Brown, J. E., and Johnson, E. D. (2004). Sleep homeostasis in infant rats. Behav. Neurosci. 118, 1253–1261. doi: 10.1037/0735-7044.118.6.1253
Bodenmann, S., Hohoff, C., Freitag, C., Deckert, J., Retey, J. V., Bachmann, V., et al. (2012). Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br. J. Pharmacol. 165, 1904–1913. doi: 10.1111/j.1476-5381.2011.01689.x
Bonnet, M. H. (1985). Effect of sleep disruption on sleep, performance, and mood. Sleep 8, 11–19. doi: 10.1093/sleep/8.1.11
Bonnet, M. H., Balkin, T. J., Dinges, D. F., Roehrs, T., Rogers, N. L., and Wesensten, N. J. (2005). The use of stimulants to modify performance during sleep loss: a review by the sleep deprivation and stimulant task force of the American Academy of Sleep Medicine. Sleep 28, 1163–1187. doi: 10.1093/sleep/28.9.1163
Borbély, A. A., and Neuhaus, H. U. (1979). Sleep-deprivation: effects on sleep and EEG in the rat. J. Comp. Physiol. 133, 71–87. doi: 10.1007/BF00663111
Bremer, F. (1938). L’activité électrique de l’écorce cerebral etle probléme physiologique du sommeil. Boll. Soc. Ital. Biol. Sper. 13, 271–290.
Bromberg-Martin, E. S., Matsumoto, M., and Hikosaka, O. (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834. doi: 10.1016/j.neuron.2010.11.022
Cano, G., Mochizuki, T., and Saper, C. B. (2008). Neural Circuitry of Stress-induced Insomnia in rats. J. Neurosci. 28, 10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008
Chamberlin, N. L., Arrigoni, E., Chou, T. C., Scammell, T. E., Greene, R. W., and Saper, C. B. (2003). Effects of adenosine on gabaergic synaptic inputs to identified ventrolateral preoptic neurons. Neuroscience 119, 913–918. doi: 10.1016/s0306-4522(03)00246-x
Chung, S., Weber, F., Zhong, P., Tan, C. L., Nguyen, T. N., Beier, K. T., et al. (2017). Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545, 477–481. doi: 10.1038/nature22350
Clasadonte, J., Mciver, S. R., Schmitt, L. I., Halassa, M. M., and Haydon, P. G. (2014). Chronic sleep restriction disrupts sleep homeostasis and behavioral sensitivity to alcohol by reducing the extracellular accumulation of adenosine. J. Neurosci. 34, 1879–1891. doi: 10.1523/JNEUROSCI.2870-12.2014
Curro Dossi, R., Pare, D., and Steriade, M. (1991). Short-lasting nicotinic and long-lasting muscarinic depolarizing responses of thalamocortical neurons to stimulation of mesopontine cholinergic nuclei. J. Neurophysiol. 65, 393–406. doi: 10.1152/jn.1991.65.3.393
de Zambotti, M., Goldstone, A., Colrain, I. M., and Baker, F. C. (2018). Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med. Rev. 39, 12–24. doi: 10.1016/j.smrv.2017.06.009
Deurveilher, S., Ryan, N., Burns, J., and Semba, K. (2013). Social and environmental contexts modulate sleep deprivation-induced c-Fos activation in rats. Behav. Brain Res. 256, 238–249. doi: 10.1016/j.bbr.2013.08.029
Dolsen, M. R., Cheng, P., Arnedt, J. T., Swanson, L., Casement, M. D., Kim, H. S., et al. (2017). Neurophysiological correlates of suicidal ideation in major depressive disorder: hyperarousal during sleep. J. Affect. Disord. 212, 160–166. doi: 10.1016/j.jad.2017.01.025
Dos Santos-Rodrigues, A., Grane-Boladeras, N., Bicket, A., and Coe, I. R. (2014). Nucleoside transporters in the purinome. Neurochem. Int. 73, 229–237. doi: 10.1016/j.neuint.2014.03.014
Dossi, R. C., Nunez, A., and Steriade, M. (1992). Electrophysiology of a slow (0.5-4 Hz) intrinsic oscillation of cat thalamocortical neurones in vivo. J. Physiol. 447, 215–234. doi: 10.1113/jphysiol.1992.sp018999
Douglas, C. L., Vyazovskiy, V., Southard, T., Chiu, S. Y., Messing, A., and Tononi, G., et al. (2007). Sleep in Kcna2 knockout mice. BMC Biol. 5:42. doi: 10.1186/1741-7007-5-42
Dworak, M., McCarley, R. W., Kim, T., Kalinchuk, A. V., and Basheer, R. (2010). Sleep and brain energy levels: ATP changes during sleep. J. Neurosci. 30, 9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010
Eban-Rothschild, A., Rothschild, G., Giardino, W. J., Jones, J. R., and De Lecea, L. (2016). VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 19, 1356–1366. doi: 10.1038/nn.4377
Economo, C. V. (1930). Sleep as a problem of localization. J. Nerv. Mental Dis. 71, 249–259. doi: 10.1097/00005053-193003000-00001
Elmenhorst, D., Elmenhorst, E. M., Hennecke, E., Kroll, T., Matusch, A., Aeschbach, D., et al. (2017). Recovery sleep after extended wakefulness restores elevated A1 adenosine receptor availability in the human brain. Proc. Natl. Acad. Sci. U.S.A. 114, 4243–4248. doi: 10.1073/pnas.1614677114
Elmenhorst, D., Meyer, P. T., Winz, O. H., Matusch, A., Ermert, J., Coenen, H. H., et al. (2007). Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J. Neurosci. 27, 2410–2415. doi: 10.1523/jneurosci.5066-06.2007
Fehr, T., Kissler, J., Wienbruch, C., Moratti, S., Elbert, T., Watzl, H., et al. (2003). Source distribution of neuromagnetic slow-wave activity in schizophrenic patients–effects of activation. Schizophr. Res. 63, 63–71. doi: 10.1016/s0920-9964(02)00213-x
Feldberg, W., and Sherwood, S. L. (1954). Injections of drugs into the lateral ventricle of the cat. J. Physiol. 123, 148–167. doi: 10.1113/jphysiol.1954.sp005040
Fernandez-Mendoza, J., Shaffer, M. L., Olavarrieta-Bernardino, S., Vgontzas, A. N., Calhoun, S. L., Bixler, E. O., et al. (2014). Cognitive–emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family study. J. Sleep Res. 23, 489–498. doi: 10.1111/jsr.12168
Frank, M. G., Morrissette, R., and Heller, H. C. (1998). Effects of sleep deprivation in neonatal rats. Am. J. Physiol. 275, R148–R157. doi: 10.1152/ajpregu.1998.275.1.R148
Franken, P. (2013). A role for clock genes in sleep homeostasis. Curr. Opin. Neurobiol. 23, 864–872. doi: 10.1016/j.conb.2013.05.002
Franken, P., Chollet, D., and Tafti, M. (2001). The homeostatic regulation of sleep need is under genetic control. J. Neurosci. 21, 2610–2621. doi: 10.1523/jneurosci.21-08-02610.2001
Fredholm, B. B. (2007). Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 14, 1315–1323. doi: 10.1038/sj.cdd.4402132
Fredholm, B. B. (2014). Adenosine–a physiological or pathophysiological agent? J. Mol. Med. 92, 201–206. doi: 10.1007/s00109-013-1101-6
Fredholm, B. B., Bättig, K., Holmén, J., Nehlig, A., and Zvartau, E. E. (1999). Actions of Caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 51, 83–133.
Fredholm, B. B., Chen, J.-F., Cunha, R. A., Svenningsson, P., and Vaugeois, J.-M. (2005). Adenosine and brain function. Int. Rev. Neurobiol. 63, 191–270.
Fuchs, T., Maury, D., Moore, F. R., and Bingman, V. P. (2009). Daytime micro-naps in a nocturnal migrant: an EEG analysis. Biol. Lett. 5, 77–80. doi: 10.1098/rsbl.2008.0405
Fuller, P., Sherman, D., Pedersen, N. P., Saper, C. B., and Lu, J. (2011). Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 519, 3817–3817. doi: 10.1002/cne.22559
Gallopin, T., Luppi, P. H., Cauli, B., Urade, Y., Rossier, J., Hayaishi, O., et al. (2005). The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience 134, 1377–1390. doi: 10.1016/j.neuroscience.2005.05.045
Garcia-Garcia, F., Acosta-Pena, E., Venebra-Munoz, A., and Murillo-Rodriguez, E. (2009). Sleep-inducing factors. CNS Neurol Disord. Drug Targets 8, 235–244. doi: 10.2174/187152709788921672
Gass, N., Porkka-Heiskanen, T., and Kalinchuk, A. V. (2009). The role of the basal forebrain adenosine receptors in sleep homeostasis. Neuroreport 20, 1013–1018. doi: 10.1097/WNR.0b013e32832d5859
Haas, H. L., Sergeeva, O. A., and Selbach, O. (2008). Histamine in the nervous system. Physiol. Rev. 88, 1183–1241.
Halassa, M. M., Florian, C., Fellin, T., Munoz, J. R., Lee, S. Y., Abel, T., et al. (2009). Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219. doi: 10.1016/j.neuron.2008.11.024
Hassainia, F., Petit, D., Nielsen, T., Gauthier, S., and Montplaisir, J. (1997). Quantitative EEG and statistical mapping of wakefulness and REM sleep in the evaluation of mild to moderate Alzheimer’s disease. Eur. Neurol. 37, 219–224. doi: 10.1159/000117446
Ho, K. S., and Sehgal, A. (2005). “Drosophila melanogaster: An Insect Model for Fundamental Studies of Sleep,” in Methods in Enzymology, ed. M. W. Young (Cambridge: Academic Press), 772–793. doi: 10.1016/s0076-6879(05)93041-3
Hoffmann, R., Hendrickse, W., Rush, A. J., and Armitage, R. (2000). Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Res. 95, 215–225. doi: 10.1016/s0165-1781(00)00181-5
Hong, Z.-Y., Huang, Z.-L., Qu, W.-M., Eguchi, N., Urade, Y., and Hayaishi, O. (2005). An adenosine A2A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J. Neurochem. 92, 1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x
Huang, Z. L., Mochizuki, T., Qu, W. M., Hong, Z. Y., Watanabe, T., Urade, Y., et al. (2006). Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 103, 4687–4692. doi: 10.1073/pnas.0600451103
Huang, Z.-L., Qu, W.-M., Eguchi, N., Chen, J.-F., Schwarzschild, M. A., Fredholm, B. B., et al. (2005). Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 8, 858–859. doi: 10.1038/nn1491
Huitron-Resendiz, S., Kristensen, M. P., Sánchez-Alavez, M., Clark, S. D., Grupke, S. L., Tyler, C., et al. (2005). Urotensin II modulates rapid eye movement sleep through activation of brainstem cholinergic neurons. J. Neurosci. 25, 5465–5474. doi: 10.1523/jneurosci.4501-04.2005
Inoué, S., Honda, K., and Komoda, Y. (1995). Sleep as neuronal detoxification and restitution. Behav. Brain Res. 69, 91–96. doi: 10.1016/0166-4328(95)00014-k
Ishimori, K. (1909). True cause of sleep: a hypnogenic substance as evidenced in the brain of sleep-deprived animals. Tokyo Igakkai Zasshi 23, 429–457.
Jeong, J. (2004). EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 115, 1490–1505.
Kalinchuk, A. V., Urrila, A.-S., Alanko, L., Heiskanen, S., Wigren, H.-K., Suomela, M., et al. (2003). Local energy depletion in the basal forebrain increases sleep. Eur. J. Neurosci. 17, 863–869. doi: 10.1046/j.1460-9568.2003.02532.x
Keshavan, M. S., Reynolds, C. F. III, Miewald, M. J., Montrose, D. M., Sweeney, J. A., Vasko, R. C., et al. (1998). Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch. Gen. Psychiatry 55, 443–448.
Korkutata, M., Saitoh, T., Feng, D., Murakoshi, N., Sugiyama, F., Cherasse, Y., et al. (2017). Allosteric modulation of adenosine A sub 2A sub receptors in mice induces slow-wave sleep without cardiovascular effects. Sleep Med. 40:e181. doi: 10.1016/j.sleep.2017.11.530
Kovacs, Z., Dobolyi, A., Kekesi, K. A., and Juhasz, G. (2013). 5’-nucleotidases, nucleosides and their distribution in the brain: pathological and therapeutic implications. Curr. Med. Chem. 20, 4217–4240. doi: 10.2174/0929867311320340003
Krueger, J. M., Clinton, J. M., Winters, B. D., Zielinski, M. R., Taishi, P., Jewett, K. A., et al. (2011). Involvement of cytokines in slow wave sleep. Prog. Brain Res. 193, 39–47. doi: 10.1016/B978-0-444-53839-0.00003-X
Krueger, J. M., and Majde, J. A. (2003). Humoral links between sleep and the immune system: research issues. Ann. N. Y. Acad. Sci. 992, 9–20. doi: 10.1111/j.1749-6632.2003.tb03133.x
Krueger, J. M., Obal, F. Jr., Fang, J., Kubota, T., and Taishi, P. (2001). The role of cytokines in physiological sleep regulation. Ann. N. Y. Acad. Sci. 933, 211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x
Krueger, J. M., Walter, J., Dinarello, C. A., Wolff, S. M., and Chedid, L. (1984). Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am. J. Physiol. 246, R994–R999.
Kubota, K. (1989). Kuniomi Ishimori and the first discovery of sleep-inducing substances in the brain. Neurosci. Res. 6, 497–518. doi: 10.1016/0168-0102(89)90041-2
Lammel, S., Steinberg, E. E., Földy, C., Wall, N. R., Beier, K., Luo, L., et al. (2015). Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 85, 429–438. doi: 10.1016/j.neuron.2014.12.036
Latini, S., and Pedata, F. (2001). Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 79, 463–484. doi: 10.1046/j.1471-4159.2001.00607.x
Lazarus, M., Shen, H.-Y., Cherasse, Y., Qu, W.-M., Huang, Z.-L., Bass, C. E., et al. (2011). Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J. Neurosci. 31, 10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011
Lazarus, M., Yoshida, K., Coppari, R., Bass, C. E., Mochizuki, T., Lowell, B. B., et al. (2007). EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 10, 1131–1133. doi: 10.1038/nn1949
Legendre, R., and Pieron, H. (1913). Recherches sur le besoin de sommeil consécutif à une veille prolongée. Z. Allegem. Physiol. 14, 235–262.
Lesku, J. A., Rattenborg, N. C., Valcu, M., Vyssotski, A. L., Kuhn, S., Kuemmeth, F., et al. (2012). Adaptive sleep loss in polygynous pectoral sandpipers. Science 337, 1654–1658. doi: 10.1126/science.1220939
Liu, Z. W., and Gao, X. B. (2007). Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J. Neurophysiol. 97, 837–848. doi: 10.1152/jn.00873.2006
Luo, Y.-J., Li, Y.-D., Wang, L., Yang, S.-R., Yuan, X.-S., and Wang, J., et al. (2018). Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat. Commun. 9:1576. doi: 10.1038/s41467-018-03889-3
Lyamin, O., Pryaslova, J., Lance, V., and Siegel, J. (2005). Animal behaviour: Continuous activity in cetaceans after birth. Nature 435, 1177–1177. doi: 10.1038/4351177a
McCormick, D. A. (1993). Actions of acetylcholine in the cerebral cortex and thalamus and implications for function. Prog. Brain Res. 98, 303–308. doi: 10.1016/s0079-6123(08)62412-7
McCormick, D. A., and Pape, H. C. (1990). Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. 431, 291–318. doi: 10.1113/jphysiol.1990.sp018331
Methippara, M. M., Kumar, S., Alam, M. N., Szymusiak, R., and Mcginty, D. (2005). Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1715–R1723.
Morairty, S., Rainnie, D., Mccarley, R., and Greene, R. (2004). Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience 123, 451–457. doi: 10.1016/j.neuroscience.2003.08.066
Moruzzi, G., and Magoun, H. W. (1949). Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1, 455–473. doi: 10.1016/0013-4694(49)90066-8
Mullington, J., Korth, C., Hermann, D. M., Orth, A., Galanos, C., Holsboer, F., et al. (2000). Dose-dependent effects of endotoxin on human sleep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R947–R955.
Mullington, J. M., Hinze-Selch, D., and Pollmacher, T. (2001). Mediators of inflammation and their interaction with sleep: relevance for chronic fatigue syndrome and related conditions. Ann. N. Y. Acad. Sci. 933, 201–210. doi: 10.1111/j.1749-6632.2001.tb05825.x
Mullins, H. M., Cortina, J. M., Drake, C. L., and Dalal, R. S. (2014). Sleepiness at work: a review and framework of how the physiology of sleepiness impacts the workplace. J. Appl. Psychol. 99, 1096–1112. doi: 10.1037/a0037885
Oishi, Y., Huang, Z. L., Fredholm, B. B., Urade, Y., and Hayaishi, O. (2008). Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. U.S.A. 105, 19992–19997. doi: 10.1073/pnas.0810926105
Oishi, Y., and Lazarus, M. (2017). The control of sleep and wakefulness by mesolimbic dopamine systems. Neurosci. Res. 118, 66–73. doi: 10.1016/j.neures.2017.04.008
Oishi, Y., Suzuki, Y., Takahashi, K., Yonezawa, T., Kanda, T., Takata, Y., et al. (2017a). Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D2-like receptors in mice. Brain Struct. Funct. 222, 2907–2915. doi: 10.1007/s00429-017-1365-7
Oishi, Y., Xu, Q., Wang, L., Zhang, B.-J., Takahashi, K., Takata, Y., et al. (2017b). Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat. Commun. 8:734. doi: 10.1038/s41467-017-00781-4
Oishi, Y., Yoshida, K., Scammell, T. E., Urade, Y., Lazarus, M., and Saper, C. B. (2015). The roles of prostaglandin E2 and D2 in lipopolysaccharide-mediated changes in sleep. Brain Behav. Immun. 47, 172–177. doi: 10.1016/j.bbi.2014.11.019
Pape, H. C. (1992). Adenosine promotes burst activity in guinea-pig geniculocortical neurones through two different ionic mechanisms. J. Physiol. 447, 729–753. doi: 10.1113/jphysiol.1992.sp019026
Parkinson, F. E., Damaraju, V. L., Graham, K., Yao, S. Y., Baldwin, S. A., Cass, C. E., et al. (2011). Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr. Top. Med. Chem. 11, 948–972. doi: 10.2174/156802611795347582
Pascual, O., Casper, K. B., Kubera, C., Zhang, J., Revilla-Sanchez, R., Sul, J.-Y., et al. (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. doi: 10.1126/science.1116916
Perlis, M. L., Smith, M. T., Andrews, P. J., Orff, H., and Giles, D. E. (2001). Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep 24, 110–117. doi: 10.1093/sleep/24.1.110
Porkka-Heiskanen, T., Strecker, R. E., and Mccarley, R. W. (2000). Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99, 507–517. doi: 10.1016/s0306-4522(00)00220-7
Porkka-Heiskanen, T., Strecker, R. E., Thakkar, M., Bjorkum, A. A., Greene, R. W., and Mccarley, R. W. (1997). Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276, 1265–1268. doi: 10.1126/science.276.5316.1265
Preston, B., Capellini, I., Mcnamara, P., Barton, R., and Nunn, C. (2009). Parasite resistance and the adaptive significance of sleep. BMC Evol. Biol. 9:7. doi: 10.1186/1471-2148-9-7
Radulovacki, M., Virus, R. M., Djuricic-Nedelson, M., and Green, R. D. (1983). Hypnotic effects of deoxycorformycin in rats. Brain Res. 271, 392–395. doi: 10.1016/0006-8993(83)90309-8
Radulovacki, M., Virus, R. M., Djuricic-Nedelson, M., and Green, R. D. (1984). Adenosine analogs and sleep in rats. J. Pharmacol. Exp. Ther. 228, 268–274.
Rainnie, D. G., Grunze, H. C., Mccarley, R. W., and Greene, R. W. (1994). Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science 263, 689–692. doi: 10.1126/science.8303279
Rattenborg, N. C., Mandt, B. H., Obermeyer, W. H., Winsauer, P. J., Huber, R., Wikelski, M., et al. (2004). Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii). PLoS Biol. 2:e212. doi: 10.1371/journal.pbio.0020212
Rechtschaffen, A., Bergmann, B. M., Everson, C. A., Kushida, C. A., and Gilliland, M. A. (1989). Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep 12, 68–87. doi: 10.1093/sleep/25.1.68
Rosenbaum, E. (1892). Warum Müssen wir Schlafen? Eine Neue Theorie des SCHLAFES. Berlin: August Hirschwald.
Rosin, D. L., Robeva, A., Woodard, R. L., Guyenet, P. G., and Linden, J. (1998). Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J. Comp. Neurol. 401, 163–186. doi: 10.1002/(sici)1096-9861(19981116)401:2<163::aid-cne2>3.3.co;2-4
Roth, T. (2007). Insomnia: definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 3, S7–S10.
Russo, S. J., and Nestler, E. J. (2013). The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625. doi: 10.1038/nrn3381
Saper, C. B., Cano, G., and Scammell, T. E. (2005). Homeostatic, circadian, and emotional regulation of sleep. J. Comp. Neurol. 493, 92–98. doi: 10.1002/cne.20770
Satoh, S., Matsumura, H., Koike, N., Tokunaga, Y., Maeda, T., and Hayaishi, O. (1999). Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur. J. Neurosci. 11, 1587–1597. doi: 10.1046/j.1460-9568.1999.00569.x
Satoh, S., Matsumura, H., Suzuki, F., and Hayaishi, O. (1996). Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc. Natl. Acad. Sci.U.S.A. 93, 5980–5984. doi: 10.1073/pnas.93.12.5980
Scammell, T. E., Gerashchenko, D. Y., Mochizuki, T., Mccarthy, M. T., Estabrooke, I. V., Sears, C. A., et al. (2001). An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 107, 653–663. doi: 10.1016/s0306-4522(01)00383-9
Schmidt, M. H., Swang, T. W., Hamilton, I. M., and Best, J. A. (2017). State-dependent metabolic partitioning and energy conservation: A theoretical framework for understanding the function of sleep. PLoS One 12:e0185746. doi: 10.1371/journal.pone.0185746
Schrader, J. (1983). “Metabolism of Adenosine and Sites of Production in the Heart,” in Regulatory Function of Adenosine, eds R. Berne, T. Rall, and R. Rubio (New York, NY: Springer), 133–156. doi: 10.1007/978-1-4613-3909-0_9
Schultz, W. (2015). Neuronal reward and decision signals: from theories to data. Physiol. Rev. 95, 853–951. doi: 10.1152/physrev.00023.2014
Seow, L. S. E., Abdin, E., Chang, S., Chong, S. A., and Subramaniam, M. (2018). Identifying the best sleep measure to screen clinical insomnia in a psychiatric population. Sleep Med. 41, 86–93. doi: 10.1016/j.sleep.2017.09.015
Sherin, J. E., Elmquist, J. K., Torrealba, F., and Saper, C. B. (1998). Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 18, 4705–4721. doi: 10.1523/jneurosci.18-12-04705.1998
Sherin, J. E., Shiromani, P. J., Mccarley, R. W., and Saper, C. B. (1996). Activation of ventrolateral preoptic neurons during sleep. Science 271, 216–219. doi: 10.1126/science.271.5246.216
Siegel, J. M. (2008). Do all animals sleep? Trends Neurosci. 31, 208–213. doi: 10.1016/j.tins.2008.02.001
Siegel, J. M. (2009). Sleep viewed as a state of adaptive inactivity. Nat. Rev. Neurosci. 10, 747–753. doi: 10.1038/nrn2697
Stenberg, D., Litonius, E., Halldner, L., Johansson, B., Fredholm, B. B., and Porkka-Heiskanen, T. (2003). Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J. Sleep Res. 12, 283–290. doi: 10.1046/j.0962-1105.2003.00367.x
Steriade, M., Contreras, D., Curro Dossi, R., and Nunez, A. (1993). The slow (1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J. Neurosci. 13, 3284–3299. doi: 10.1523/jneurosci.13-08-03284.1993
Steriade, M., Dossi, R. C., and Nunez, A. (1991). Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J. Neurosci. 11, 3200–3217. doi: 10.1523/jneurosci.11-10-03200.1991
Studer, F. E., Fedele, D. E., Marowsky, A., Schwerdel, C., Wernli, K., Vogt, K., et al. (2006). Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience 142, 125–137. doi: 10.1016/j.neuroscience.2006.06.016
Suzuki, A., Sinton, C. M., Greene, R. W., and Yanagisawa, M. (2013). Behavioral and biochemical dissociation of arousal and homeostatic sleep need influenced by prior wakeful experience in mice. Proc. Natl. Acad. Sci. U.S.A. 110, 10288–10293. doi: 10.1073/pnas.1308295110
Svenningsson, P., Le Moine, C., Fisone, G., and Fredholm, B. B. (1999). Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog. Neurobiol. 59, 355–396. doi: 10.1016/s0301-0082(99)00011-8
Takata, Y., Oishi, Y., Zhou, X.-Z., Hasegawa, E., Takahashi, K., Cherasse, Y., et al. (2018). Sleep and wakefulness are controlled by ventral medial midbrain/pons GABAergic neurons in mice. J. Neurosci. 38, 10080–10092. doi: 10.1523/JNEUROSCI.0598-18.2018
Thakkar, M. M., Engemann, S. C., Walsh, K. M., and Sahota, P. K. (2008). Adenosine and the homeostatic control of sleep: effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience 153, 875–880. doi: 10.1016/j.neuroscience.2008.01.017
Thakkar, M. M., Winston, S., and Mccarley, R. W. (2003). A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J. Neurosci. 23, 4278–4287. doi: 10.1523/jneurosci.23-10-04278.2003
Thimgan, M. S., Suzuki, Y., Seugnet, L., Gottschalk, L., and Shaw, P. J. (2010). The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 8:e1000466. doi: 10.1371/journal.pbio.1000466
Toda, H., Williams, J. A., Gulledge, M., and Sehgal, A. (2019). A sleep-inducing gene, nemuri, links sleep and immune function in Drosophila. Science 363, 509–515. doi: 10.1126/science.aat1650
Urade, Y., Eguchi, N., Qu, W. M., Sakata, M., Huang, Z. L., Chen, J. F., et al. (2003). Sleep regulation in adenosine A2A receptor-deficient mice. Neurology 61, S94–S96.
Urade, Y., and Lazarus, M. (2013). “Prostaglandin D2 in the regulation of sleep,” in The Genetic Basis of Sleep and Sleep Disorders, eds P. J. Shaw, M. Tafti, and M. J. Thorpy (Cambridge: Cambridge University), 73–83. doi: 10.1017/cbo9781139649469.010
Ushikubi, F., Segi, E., Sugimoto, Y., Murata, T., Matsuoka, T., Kobayashi, T., et al. (1998). Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395, 281–284. doi: 10.1038/26233
Virus, R. M., Ticho, S., Pilditch, M., and Radulovacki, M. (1990). A comparison of the effects of caffeine, 8-cyclopentyltheophylline, and alloxazine on sleep in rats. Possible roles of central nervous system adenosine receptors. Neuropsychopharmacology 3, 243–249.
Volkow, N. D., and Morales, M. (2015). The brain on drugs: from reward to addiction. Cell 162, 712–725. doi: 10.1016/j.cell.2015.07.046
Vyazovskiy, V. V., Olcese, U., Hanlon, E. C., Nir, Y., Cirelli, C., and Tononi, G. (2011). Local sleep in awake rats. Nature 472:443. doi: 10.1038/nature10009
Weinberg, W. A., and Brumback, R. A. (1990). Primary disorder of vigilance: a novel explanation of inattentiveness, daydreaming, boredom, restlessness, and sleepiness. J. Pediatr. 116, 720–725. doi: 10.1016/s0022-3476(05)82654-x
Williams, H. L., Hammack, J. T., Daly, R. L., Dement, W. C., and Lubin, A. (1964). Responses to auditory stimulation, sleep loss and the eeg stages of sleep. Electroencephalogr. Clin. Neurophysiol. 16, 269–279. doi: 10.1016/0013-4694(64)90109-9
Wise, R. A. (2004). Dopamine, learning and motivation. Nat. Rev. Neurosci. 5:483. doi: 10.1038/nrn1406
Wisor, J. P., Nishino, S., Sora, I., Uhl, G. H., Mignot, E., and Edgar, D. M. (2001). Dopaminergic role in stimulant-induced wakefulness. J. Neurosci. 21, 1787–1794. doi: 10.1523/jneurosci.21-05-01787.2001
Yamanaka, A., Beuckmann, C. T., Willie, J. T., Hara, J., Tsujino, N., Mieda, M., et al. (2003). Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38, 701–713. doi: 10.1016/s0896-6273(03)00331-3
Yegutkin, G. G. (2008). Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783, 673–694. doi: 10.1016/j.bbamcr.2008.01.024
Yu, X., Li, W., Ma, Y., Tossell, K., Harris, J. J., Harding, E. C., et al. (2019). GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat. Neurosci. 22, 106–119. doi: 10.1038/s41593-018-0288-9
Zepelin, H. (1994). “Mammalian Sleep,” in Principles and Practice of Sleep Medicine, eds M. H. Kryger, T. Roth, and W. C. Dement (Philadelphia: W.B. Saunders Company).
Zhou, X., Oishi, Y., Cherasse, Y., Korkutata, M., Fujii, S., Lee, C.-Y., et al. (2019). Extracellular adenosine and slow-wave sleep are increased after ablation of nucleus accumbens core astrocytes and neurons in mice. Neurochem. Int. 124, 256–263. doi: 10.1016/j.neuint.2019.01.020
Zimmermann, H. (2000). Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 362, 299–309. doi: 10.1007/s002100000309
Keywords: adenosine, slow-wave sleep, A2A receptor, A1 receptor, slow-wave activity, sleep homeostasis, dopamine, motivation
Citation: Lazarus M, Oishi Y, Bjorness TE and Greene RW (2019) Gating and the Need for Sleep: Dissociable Effects of Adenosine A1 and A2A Receptors. Front. Neurosci. 13:740. doi: 10.3389/fnins.2019.00740
Received: 31 March 2019; Accepted: 02 July 2019;
Published: 17 July 2019.
Edited by:
Ritchie Edward Brown, VA Boston Healthcare System, United StatesReviewed by:
Marcos G. Frank, Washington State University Health Sciences Spokane, United StatesDavid Elmenhorst, Jülich Research Centre, Germany
Copyright © 2019 Lazarus, Oishi, Bjorness and Greene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Lazarus, lazarus.michael.ka@u.tsukuba.ac.jp; Robert W. Greene, RobertW.Greene@UTSouthwestern.edu
 Michael Lazarus
Michael Lazarus Yo Oishi
Yo Oishi Theresa E. Bjorness
Theresa E. Bjorness Robert W. Greene
Robert W. Greene