Adenosine A2A Receptors in the Rat Prelimbic Medial Prefrontal Cortex Control Delay-Based Cost-Benefit Decision Making
- 1CNC-Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal
- 2Department of Biochemistry, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
- 3Department of Neurobiology, Fluminense Federal University, Niterói, Brazil
- 4Post-Graduate Program in Medical Sciences, Faculty of Medicine, Federal University of Ceará, Fortaleza, Brazil
- 5Faculty of Medicine, University of Coimbra, Coimbra, Portugal
Adenosine A2A receptors (A2ARs) were recently described to control synaptic plasticity and network activity in the prefrontal cortex (PFC). We now probed the role of these PFC A2AR by evaluating the behavioral performance (locomotor activity, anxiety-related behavior, cost-benefit decision making and working memory) of rats upon downregulation of A2AR selectively in the prelimbic medial PFC (PLmPFC) via viral small hairpin RNA targeting the A2AR (shA2AR). The most evident alteration observed in shA2AR-treated rats, when compared to sh-control (shCTRL)-treated rats, was a decrease in the choice of the large reward upon an imposed delay of 15 s assessed in a T-maze-based cost-benefit decision-making paradigm, suggestive of impulsive decision making. Spontaneous locomotion in the open field was not altered, suggesting no changes in exploratory behavior. Furthermore, rats treated with shA2AR in the PLmPFC also displayed a tendency for higher anxiety levels in the elevated plus maze (less entries in the open arms), but not in the open field test (time spent in the center was not affected). Finally, working memory performance was not significantly altered, as revealed by the spontaneous alternation in the Y-maze test and the latency to reach the platform in the repeated trial Morris water maze. These findings constitute the first direct demonstration of a role of PFC A2AR in the control of behavior in physiological conditions, showing their major contribution for the control of delay-based cost-benefit decisions.
Introduction
Adenosine A2A receptors (A2ARs) are mostly known to control long-term synaptic plasticity throughout the brain (reviewed in Cunha, 2016), namely in the prefrontal cortex (PFC) where they facilitate long-term potentiation (LTP) in excitatory synapses onto fast spiking interneurons and control network activity (Kerkhofs et al., 2018). The PFC mediates cognitive and executive functions including working memory, attention and inhibitory control (Goldman-Rakic, 1999; Fuster, 2001), which are disrupted in major neuropsychiatric disorders such as attention deficit and hyperactivity disorder (ADHD), addiction and schizophrenia (Arnsten et al., 2015). Notably, the antagonism of A2AR has been tied to the improvement of mood and memory deficits in several neuropsychiatric disorders (Chen, 2014; Kaster et al., 2015; Viana da Silva et al., 2016). The contribution of A2AR in the PFC is suggested by the observation that the antagonism of adenosine receptors with their general antagonist caffeine improves attention and short-term memory in animal models of ADHD (Caballero et al., 2011; Pandolfo et al., 2013). Furthermore, A2AR antagonism increases impulsivity (Oliveros et al., 2017), attenuate the effects of dopamine D2 receptor antagonism on effort-based decision making (Pardo et al., 2012) and attenuate working memory deficits in rats with PFC dopamine depletion (Horita et al., 2013).
While optogenetic activation of A2AR signaling pathways in the medial PFC improves maintenance of spatial working memory (Li et al., 2018), there are still no direct evidence supporting a role for the endogenous activation of A2AR in the PFC to modulate behavior. This is of particular relevance since A2AR are present in different areas of the forebrain (i.e., cerebral cortex, hippocampus and striatum) with different impacts on different behavioral outputs, as heralded by the striking opposite phenotypes resulting from the selective deletion of A2AR from only the striatum or forebrain neurons (Shen et al., 2008, 2013; Wei et al., 2014). Thus, to better understand the role of the endogenous activation of A2AR in the PFC, we now selectively downregulated A2AR in the rat prelimbic medial PFC (PLmPFC) and evaluated the consequences on PFC-related behaviors such as working memory, anxiety-related behavior and delay-based cost-benefit decision-making. Our findings reveal that the downregulation of A2AR in the PLmPFC decreased the choice of the large reward in a T-maze-based cost-benefit paradigm in which the cost was delay, suggesting an increase in impulsive decision making, a finding relevant for disorders with impaired decision making, such as Parkinson’s disease, schizophrenia, ADHD and addiction (Lee, 2013).
Materials and Methods
Animals
Male Wistar rats (7-week-old) were purchased from Charles River (Barcelona, Spain) and housed in a temperature and humidity-controlled environment with 12 h light on/off cycles and ad libitum access to food and water. All studies were conducted in accordance with the principles and procedures outlined as “3Rs” in the EU guidelines (210/63), FELASA, and the National Centre for the 3Rs (the ARRIVE; Kilkenny et al., 2010), and were approved by the Animal Care Committee of the Center for Neuroscience and Cell Biology (ORBEA 78/2013).
Generation and Bilateral Administration of Lentiviral Vectors Into the PLmPFC
A small hairpin RNA targeting A2AR (shA2AR, nt 419–437) was inserted into a lentivector together with an enhanced green fluorescent protein (EGFP) reporter gene, as previously detailed (Simões et al., 2016; Viana da Silva et al., 2016). This shA2AR has been shown to cause a 68% decrease of A2AR mRNA expression and a 55% decrease of A2AR protein density in the striatum, where the high density of A2AR allows a faithful quantification (Viana da Silva et al., 2016). A hairpin targeting the coding region of red fluorescent protein (nt 22–41) was used as an internal control (shCTRL). These lentivectors (1 μL per hemisphere at 750,000 ng of p24 antigen/mL) were stereotaxically delivered into the PLmPFC of the two hemispheres at an infusion rate of 0.2 μL/min in the following coordinates: antero-posterior: +3.20 mm; lateral: ±0.60 mm; dorso-ventral: −3.80 mm (Paxinos and Watson, 2009).
Radioligand Binding Assay in Total Membranes From the PLmPFC
The amount of tissue allowed a single point radioligand binding which was carried out with slight modification to our previous studies (Cunha et al., 1999; Ferreira et al., 2015). Three male Wistar rats of 6–8 weeks of age were bilaterally injected shA2AR in their PLmPFC, while four Wistar rats were bilaterally injected with the shCTRL. At 5 weeks post-injection, rats were decapitated under halothane anesthesia, and their brains transferred to ice-cold artificial cerebrospinal fluid (composition in mM: NaCl 125, KCl 3, MgSO4 1, CaCl2 2, Na2HPO4 1.25, NaHCO3 25–26 and glucose 11, pH 7.4 (osmolality of 300 mOsmol/kg), oxygenated with carbogen (95% O2 + 5% CO2). We obtained coronal brain slices from which we dissected the PLmPFC, which was homogenized in 1.8 mL of ice-cold membrane preparation solution of the following composition: sucrose (320 mM), EDTA (2 mM), MgCl2 (3 mM), HEPES (15 mM), pH 7.4, supplied with a protease inhibitor cocktail (Sigma-Aldrich, 1 μL/mL). The homogenates were then centrifuged at 1,000 g for 30 min, at 4°C to decant intracellular debris. The membrane-rich supernatant was then re-centrifuged at 20,000 g for 30 min, and the pellets were vigorously resuspended in 450 μL binding assay buffer of the following composition: NaCl (100 mM), Tris-HCl (50 mM), EDTA (1 mM), MgCl2 (3 mM), protease inhibitor (1 μL/mL), pH 7.4. Next, 100 μL of the protein suspension was mixed with 200 μL of assay buffer containing adenosine deaminase (Sigma-Aldrich; final concentration, 3 U/mL), guanosine 5′-diphosphate (Abcam; 100 μM), and either the A2AR-selective antagonist, SCH58261 (Tocris; 1 μM) to measure non-specific binding or its vehicle, DMSO (0.1% v/v) to yield the total binding. This mixture also contained the A2AR-selective radioligand 3H-ZM241385 (American Radiolabeled Chemicals, St. Louis, MO, USA; specific activity, 30 mCi/mmol) at a final concentration of 2.63 nM. The binding assay was carried out in duplicate. The remaining 50 μL of protein aliquots were used to determine protein concentration with the BCA method. The mixtures (containing 20.6 ± 1.4 μg of protein) were left to incubate for 2 h at room temperature in Eppendorf-tubes, then were rapidly transferred into 15 mL of ice-cold washing solution (Tris-HCl, 50 mM, BSA 0.1% v/w), and instantly vacuum-filtered with the help of a Millipore filtration unit, containing Whatman GF/B glass microfiber filters, which had been soaked overnight in Tris-HCl (10 mM), pH 9.1, containing 0.25% v/v of the cationic polymer polyethylenimine (Sigma; Bruns et al., 1983). The glass tubes were rinsed with an additional 15 mL of washing solution onto the filters. The filters then were harvested into 3 mL of Aquasafe scintillation liquid and after 24 h, were counted for tritium with the help of a Tricarb β-counter (PerkinElmer). Binding values are expressed as fmol binding sites per mg protein.
Behavioral Experiments
Behavioral analyses started 21 days post-surgery and were conducted between 8:00 AM and 6:00 PM under a low intensity red light (12 lx), after habituation of the animals to the room for at least 1 h and with care to clean all apparatus with ethanol after testing each animal to eliminate olfactory cues. We carried out two groups of experiments, all video-taped and analyzed using the ANY-maze video tracking system (Stoelting, Wood Dale, IL, USA).
Experimental Set I
The first group of rats were sequentially exposed to the following behavioral tests with a 24 h interval in between them: the elevated plus maze, in order to assess anxiety-like behavior; the open field test to assess locomotor activity as well as anxiety-like behavior; and the splash test in order to evaluate mood alterations.
The elevated plus maze was carried out in an elevated plus-shaped maze with two open arms arranged perpendicularly to two closed arms, as previously described (Kaster et al., 2015). Rats were allowed to explore the maze for 10 min. The general principle of this test is that more “anxious” animals will likely explore less the risky open arms as opposed to the closed arms, which are perceived as safer. Thus, anxiety-like behavior was measured as a lower percentage of open arm entries (Pellow et al., 1985). Entries were counted whenever all the four paws of the animal crossed into one of the arms. The open field test was carried out in a square-shaped arena (1 × 1 m) with defined peripheral and central (36% of total area) zones. Rats were allowed to explore the arena for 10 min and only the first 5 min were analyzed (Gonçalves et al., 2015). Locomotor activity was measured as the total distance traveled and anxiety-like behavior was measured by the time spent in the center zone of the arena, which is perceived as a more threatening area (Choleris et al., 2001; Prut and Belzung, 2003).
The splash test was used as a measure of anxiety- and depressive-like behavior as previously described (Kaster et al., 2015). We measured grooming bouts (head washing and nose/face and body grooming) over 5 min after a 10% sucrose solution was splashed on the dorsal coat of the animal (Yalcin et al., 2005).
Experimental Set II
This second set of experiments sequentially tested rats in a delay-based cost-benefit decision making paradigm in a T-maze, followed by spatial working memory tests using a Y-maze and a repeated trial Morris water maze (MWM).
Delay-Based Cost-Benefit Decision-Making Paradigm in a T-maze
This test is based on delay aversion, which is used as a measure of impulsive decision making or impulsive choice (Pattij and Vanderschuren, 2008). Animals had to choose between a large-but-delayed and small-but-immediate reward (adapted from Bizot et al., 2007). The testing apparatus was a gray-colored T-maze, built out of PVC, with 50-cm-high walls, consisting of a starting runway, ending in two perpendicular 50-cm-long, 15-cm-wide arms. Four removable guillotine wood doors were vertically inserted at the entry and 10-cm from the end of each arm. The space between doors in each arm was enough to accommodate a rat. One week before starting the behavioral tests, rats were food-restricted to achieve 90% of their original weight. During that time, palatable dog chow pellets (Royal Canin Junior®) were given to the rats to habituate them to the new food. The task was divided into three different phases: habituation, training and testing phase.
Habituation
Rats were individually placed on the starting runway and allowed to freely explore the apparatus. Each arm had three pellets, including the starting runway. After 5 min, the number of pellets ingested was verified. If an animal had not eaten all the pellets, it was subjected to a new habituation trial. Up to five trials were conducted each day. After eating all the pellets, the rats progressed to the training phase. The number of habituation trials to reach training criterion was recorded.
Training Phase
Rats were run in the maze where one arm of the apparatus had a small reward (0.5 pellet) and the other had a large (two pellets) reward. The arm where the large reward was placed was randomly selected for each rat, but it was always on the same side throughout the experiment for a given rat. Rats were individually placed on the starting runway and had equal access to both arms. Both doors in the chosen arm were opened when the animal turned to its direction. As soon as the first door was crossed, it was closed to prevent the rat from escaping. The second door in that arm remained open to allow the animal to eat the reward. Then, another trial was carried out until a session of five trials was complete. Up to two sessions of five trials were conducted in the same day. The criterion to progress to the testing phase was choosing the large reward at least four times in five trials in two consecutive sessions. Otherwise, further trials were carried out in the next day. The number of training sessions to reach testing criterion was recorded.
Testing Phase
The test was conducted in five consecutive days, each day consisting of five trials. A delay of 15 s was imposed before the rat had access to the large reward, i.e., after choosing the arm with the large reward, both doors were closed right after the animal crossed the first one, keeping the animal between doors during this period. No delay was imposed after entering the small reward arm. The number of choices of the large reward was recorded for each day.
Working Memory Tests
The Y-maze spontaneous alternation test was carried out as previously described (Augusto et al., 2013). The rats explored the maze for 8 min. The spontaneous alternation test takes advantage of the natural tendency of animals to choose a different arm than the one previously chosen (Dudchenko, 2004). In a correct sequence, a rat chooses a different arm in each of the successive three entries. The percentage of alternation in correct sequences was used to evaluate spatial working memory.
The repeated trial MWM was carried out in a circular pool (100 cm in diameter, 55 cm high), filled with water at 26°C. A platform (10 cm in diameter) was placed just under the surface of the water. The extra-maze cues in the testing room were kept constant. The test was adapted from a four-trial repeated acquisition protocol described in previous studies (Whishaw, 1985; Zhou et al., 2009) with four consecutive daily trials repeated during four consecutive days. The interval between trials was less than 1 min. The platform was moved to a new quadrant every day, but kept in the same position for all trials on the same day. The rats were allowed to swim until they reach the platform. Working memory was evaluated through the latency of escape from the starting point to the platform.
Statistical Analysis
Statistical analyses were performed using Prism 6 GraphPad Software. Data are expressed as mean ± standard error of the mean (SEM). Data were analyzed using unpaired Student’s t-test and two-way ANOVA for repeated measures, followed by Bonferroni post hoc test as appropriate. p < 0.05 was taken as statistically significant.
Results
We generated lentivectors, with neuronal tropism (Lundberg et al., 2008), encoding shRNAs to selectively neutralize A2AR (shA2AR) together with EGFP. These lentivectors were injected into the PLmPFC (Figure 1A) of rats. Upon dissection of the PLmPFC 5 weeks post-injection, we performed radioligand biding assay with 3H-ZM241385, an A2AR ligand, to assess A2AR density. The density of A2AR in PLmPFC total membranes was 42.28 ± 13.46 fmol/mg protein for shCTRL-treated rats (n = 4) and 17.13 ± 16.03 fmol/mg protein in PLmPFC total membranes from shA2AR-treated rats Figure 1B), representing a 59 ± 18% decrease in A2AR protein density as compared to shCTRL-treated rats (n = 3–4), a down-regulation similar to that achieved in the striatum (Viana da Silva et al., 2016).
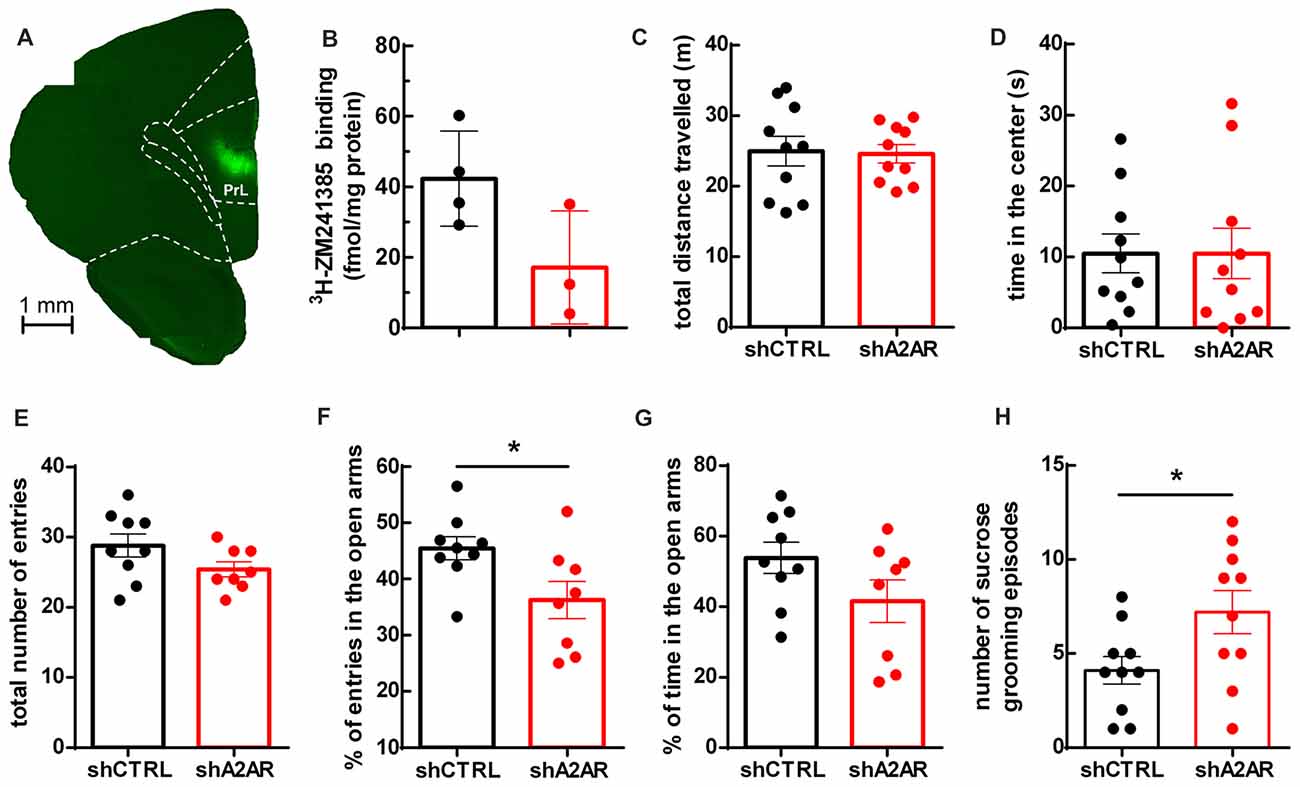
Figure 1. Effect of downregulatingadenosine A2A receptors (A2AR) in the prelimbic medial prefrontal cortex (PLmPFC) on locomotion and anxiety- and depressive-like behavior. (A) Lentivector constructs containing small hairpin RNA targeting A2AR (shA2AR) together with enhanced green fluorescent protein (EGFP) reporter gene was effectively transduced in the PLmPFC, as shown by EGFP labeling in the region. (B) Specific binding of the selective A2AR antagonist 3H-ZM241385 shows a decrease in A2AR protein density in shA2AR- as compared to shCTRL-treated rats (n = 3–4, p = 0.07). (C) The analysis of the performance in the open field test suggests no alteration of exploratory activity, since the total distance traveled was similar between shA2AR- and shCTRL-treated rats. (D) There was also no change in the time spent in the center of the open field between shA2AR- and shCTRL-treated rats. (E) In the elevated plus maze there was no change in the total number of arm entries. (F) The performance in the elevated plus maze test suggests a mild anxiogenic effect resulting from the downregulation of PLmPFC A2AR, since shA2AR-treated rats entered less in the open arms when compared with shCTRL-treated rats. (G) Time spent in the open arms was not significantly affected (p = 0.1173). (H) Likewise, the performance in the splash test (with 10% sucrose solution) revealed an increased number of sucrose grooming episodes in shA2AR- compared with shCTRL-treated rats. Behavioral data are mean ± SEM of 9–10 rats per group; *p < 0.05, unpaired Student’s t-test.
Downregulation of A2AR in the PLmPFC Induces Slight Mood Alterations With No Changes of Locomotor Activity
We then evaluated if the downregulation of A2AR in the PLmPFC impacted on locomotor activity. As shown in Figure 1C, there was no difference in the total distance traveled between shA2AR- and shCTRL-treated rats (24.59 ± 1.28 m for shA2AR-treated rats vs. 24.96 ± 2.10 m for shCTRL-treated rats, n = 10, p = 0.8830), suggesting that the exploratory behavior was not affected. Regarding anxiety-like behavior, the results were less clear: there was no difference in the time spent in the center of the open field between shA2AR- and shCTRL-treated rats (10.48 ± 3.58 s for shA2AR-treated rats vs. 10.49 ± 2.72 s for shCTRL-treated rats, n = 10, p = 0.9983; Figure 1D). However, in the elevated plus maze test, while total number of arm entries remained unchanged (25.38 ± 1.07 n = 8, for shA2AR-treated rats vs. 28.78 ± 1.64, n = 9, for shCTRL-treated, p = 0.1116; Figure 1E), shA2AR-treated rats entered less in the open arms as compared with shCTRL-treated rats (36.24 ± 3.32%, n = 8, for shA2AR-treated rats vs. 53.86 ± 4.46%, n = 9, for shCTRL-treated, p = 0.0289; Figure 1F). In contrast, the time spent in the open arms was not significantly altered (41.56 ± 6.04% n = 8, for shA2AR-treated rats vs. 53.86 ± 4.46%, n = 9, for shCTRL-treated, p = 0.1173; Figure 1G). In the splash test, there was an increase in sucrose grooming frequency (7.20 ± 1.14 events for shA2AR-treated rats vs. 4.10 ± 0.737 events for shCTRL-treated rats, n = 10, p = 0.0351; Figure 1H). Altogether, these data suggest that downregulating PLmPFC A2AR might result in a discrete anxiogenic profile.
Downregulation of A2AR in the PLmPFC Renders Rats More Averse to Delay
We used a delay-based cost-benefit decision making paradigm in a T-maze (Figures 2A,B) to evaluate preference for a small immediate reward over a larger, but delayed reward. In the habituation phase, shA2AR- and shCTRL-treated rats needed a similar number of habituation trials to reach training criterion, i.e., they learned similarly that there was a reward at the end of two arms (shA2AR-treated rats learned over 7.25 ± 0.88 trials, whereas shCTRL-treated rats learned over 8.38 ± 1.64 trials, n = 8, p = 0.5575; Figure 2C). In the training phase, shA2AR-treated rats needed a lower number of training sessions to reach testing criterion, i.e., they learned faster to choose the larger reward as compared to shCTRL-treated rats (shA2AR-treated rats learned over 3.25 ± 0.16 sessions, whereas shCTRL-treated rats learned over 6.00 ± 0.82 sessions, n = 8, p = 0.0122; Figure 2D). However, in the testing phase, shA2AR-treated rats were more intolerant to a 15-s-imposed delay as compared to shCTRL-treated rats, suggesting an increase in impulsive choice upon down-regulation of A2AR in the PLmPFC. A repeated measures ANOVA analysis indicated a decrease in the choices of the large reward with increased number of sessions (F(5,70) = 28.08, p < 0.0001), with shA2AR treatment (F(1,14) = 13.64, p = 0.0024), and a session × shA2AR treatment interaction (F(5,70) = 3.10, p = 0.0138; Figure 2E). The total number of choices of the large reward was 8.63 ± 0.82 in shA2AR-treated rats and 16.00 ± 1.67 in shCTRL-treated rats (n = 8, p = 0.0026; Figure 2F).
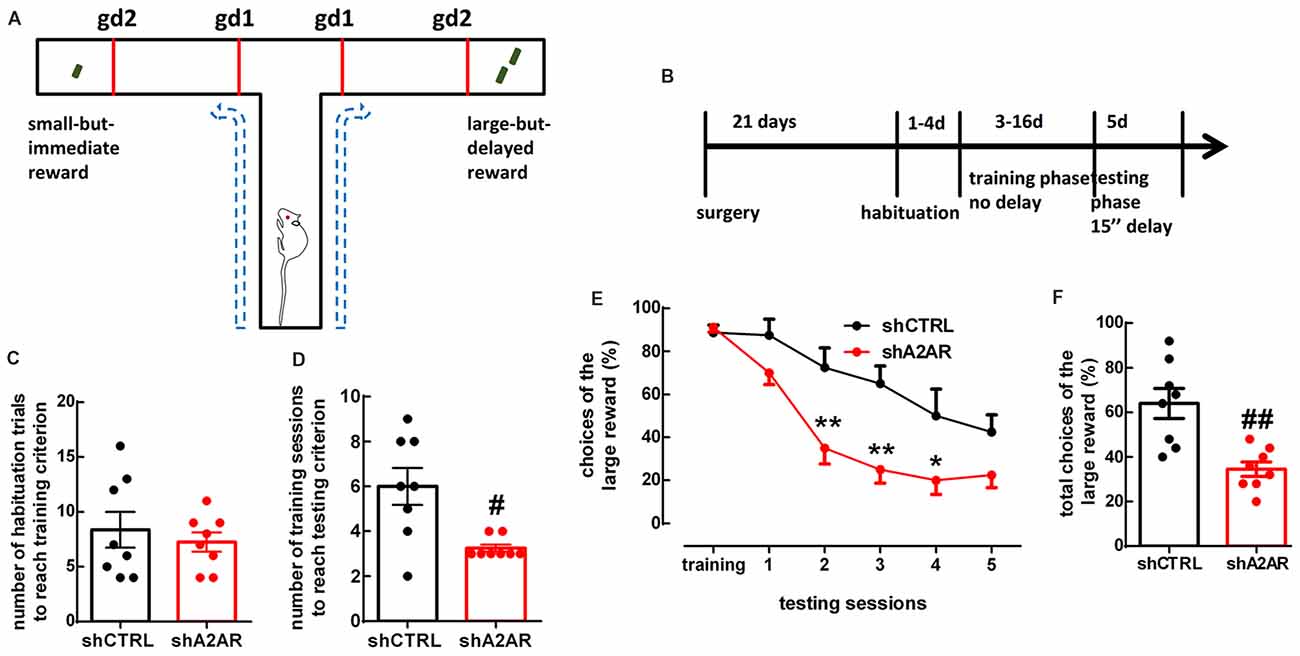
Figure 2. Downregulation of A2AR in the PLmPFC increases impulsive choice. (A) Scheme of the testing apparatus (gd, guillotine door). (B) Scheme of the experimental design. (C) shA2AR- and shCTRL-treated rats required a similar number of habituation trials to reach training criterion. (D) shA2AR-treated rats required less training sessions than shCTRL-treated rats to reach testing criterion (when they learn to choose the large reward); (E,F) with a 15-s-imposed delay, shA2AR-treated rats were more intolerant to delay as compared to shCTRL-treated rats, as shown by their decreased choice of the large reward. Data are mean ± SEM. #p < 0.05 and ##p < 0.01, unpaired Student’s t-test; *p < 0.05 and **p < 0.01, two-way analysis of variance (ANOVA) for repeated measures, followed by Bonferroni post hoc test.
Downregulation of A2AR in the PLmPFC Does Not Affect Spatial Working Memory
There were no differences in spontaneous alternation in the Y-maze test between shA2AR- and shCTRL-treated rats (65.87 ± 5.61%, n = 9, for shA2AR-treated rats vs. 66.44 ± 3.99%, n = 5, for shCTRL-treated rats, p = 0.9350; Figure 3A). In the MWM test, repeated measures ANOVA revealed an effect for trial (F(15,180) = 4.63, p < 0.0001), but not for shA2AR treatment (F(1,12) = 0.31, p = 0.5853; Figure 3B). Overall, these data suggest that A2AR in the PLmPFC have no impact on spatial working memory.
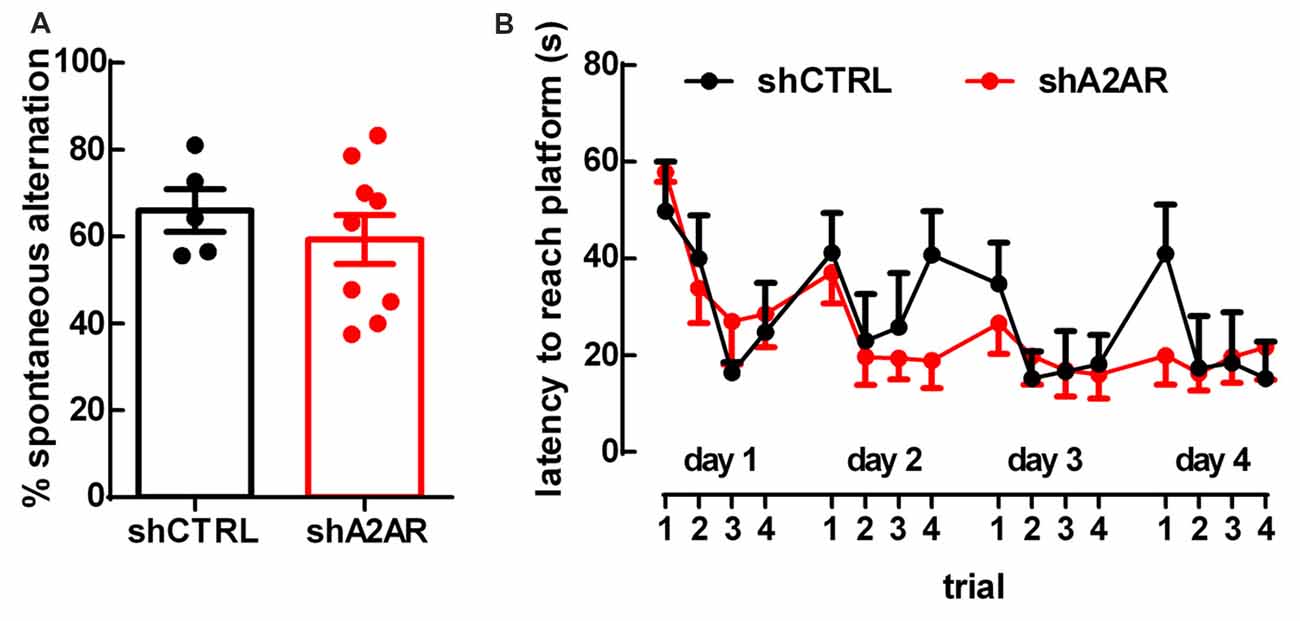
Figure 3. Downregulation of A2AR in the PLmPFC does not affect spatial working memory. (A) The performance in the Y-maze test revealed no change of the percentage of spontaneous alternation in shA2AR-compared to shCTRL-treated rats. (B) The repeated trial Morris water maze (MWM) paradigm revealed that shA2AR-treated rats had no changes in the latency to reach the platform as compared to shCTRL-treated rats. Data are mean ± SEM of n = 5 for shCTRL- and n = 9 for shA2AR-treated rats.
Discussion
We have recently reported that A2AR in the PLmPFC control long-term plasticity of excitatory synaptic transmission onto fast spiking interneurons (Kerkhofs et al., 2018) and that they are necessary for dopamine-induced decrease in population activity (Real et al., 2018). Thus, we anticipated a role for PFC A2AR in the control of PFC-dependent behaviors, which include anxiety-like behavior (Calhoon and Tye, 2015; Tovote et al., 2015), cost-benefit decision-making (Bailey et al., 2016) and working memory (Goldman-Rakic, 1999; Fuster, 2001). The most evident effect resulting from the down-regulation of A2AR selectively in the PLmPFC was increased aversion to delay, suggestive of increased impulsive decision making, while only a discrete increase in anxiety-like behavior was observed, and spatial working memory was not significantly affected.
A2AR, in particular those in the nucleus accumbens, have been consistently implicated in cost-benefit decision-making in which the cost is physical effort (e.g., Font et al., 2008; Mingote et al., 2008; Pardo et al., 2012; Nunes et al., 2013). Specifically, activation of A2AR decreased lever pressing for the preferred food as opposed to eating the readily available less preferred chow (Font et al., 2008) and disrupted performance in an instrumental task with high work demands (Mingote et al., 2008), while A2AR blockade or genetic deletion attenuated haloperidol (dopamine D2 receptor antagonist)-induced decrease in the choice of the high reward arm of a T-maze that was accessible after climbing a barrier (Pardo et al., 2012). Now, we show that PLmPFC A2AR have the opposite effect on a cost-benefit decision-making task in which the cost was delay. Decisions about different, yet interrelated, types of costs have dissociable neural circuits and neurochemical mechanisms (Rudebeck et al., 2006; Floresco et al., 2008; Bailey et al., 2016), which may have contributed to this difference. Furthermore, it is known that A2AR are able to modulate the same behavior in opposite direction, depending on the brain region that is being manipulated. That is, for instance, the case for fear memory (Wei et al., 2014; Simões et al., 2016) and psychomotor activity (Shen et al., 2008). Thus, a more comprehensive study involving region-selective manipulations of A2AR will be necessary to dissect their role across different types of decision costs.
The impact of PFC A2AR on delay-based decision making could either be due to a control of: (i) aversion to the holding chamber before the reward; (ii) an altered goal-directed to habit-based strategy; (iii) an altered subjective value of the reward; (iv) an altered spatial memory encoding; and (v) impulsivity. This is compatible with the main involvement of the accumbens-PFC-amygdala circuitry (Floresco and Ghods-Sharifi, 2007; Hauber and Sommer, 2009) as well as of the dorsal hippocampus (Liu et al., 2016) in T-maze based analysis of effort-based decision making, and with the ability of the PFC to control aversive memories (Courtin et al., 2013), goal-directed behavior (Gourley and Taylor, 2016), the subjective value of rewards (Kable and Glimcher, 2007), processing of spatial memory (Jin and Maren, 2015) and impulsivity (Kim and Lee, 2011). However, the previous analysis of the role of A2AR in these different behaviors leads us to propose that PFC-A2AR mostly control delay-based decision making by controlling impulsivity. This contention stems from observations that: (i) A2AR control aversive memories, but this is fully accounted by the impact of amygdalar A2AR (Simões et al., 2016); (ii) A2AR control the shift from goal-directed to habit-based strategies, but this is fully accounted by the activity of A2AR in medial spiny neurons of different striatal regions (Yu et al., 2009; Li et al., 2016); (iii) A2AR control reward, but this is dependent on A2AR in the nucleus accumbens rather than PFC A2AR (Harper et al., 2006; Wydra et al., 2018); and (iv) A2AR control spatial memory, but this is fully accounted by A2AR in the dorsal hippocampus (Li P. et al., 2015; Pagnussat et al., 2015). Furthermore, we now report that PFC A2AR have a discrete impact on anxiety-like behaviors. Therefore, it is likely that the control by PFC A2AR of delay-based decision making might be a consequence of an ability of PFC A2AR to control impulsivity, which is often inferred from the analysis of delay-based decision tasks (Dalley et al., 2011; Kim and Lee, 2011). This contention that PFC A2AR might control delay-based decision making by controlling impulsivity is in agreement with the key role of the PFC in gating impulsivity (Sripada et al., 2011; Mason et al., 2014). However, it should be made clear that this is an indirect inference rather than a direct demonstration and future work should address if PFC A2AR also control others forms of impulsivity apart from impulsive intertemporal choice, such as impulsivity based on speed instead of accuracy (see Kim and Lee, 2011).
Our finding of increased delay aversion upon decreased function of PLmPFC A2AR seems to fully account for the exacerbation of waiting impulsivity observed upon systemic antagonism of A2AR (Oliveros et al., 2017). Likewise, there also seems to be a positive correlation between the intake of caffeine (Grant and Chamberlain, 2018) or caffeinated alcoholic beverages (Amlung et al., 2013; Heinz et al., 2013) and higher impulsivity. Although caffeine is a mixed antagonist of A1/A2AR antagonist (Fredholm et al., 2005), and its intake is already biased by the predisposition to impulsivity (Waldeck and Miller, 1997; Jones and Lejuez, 2005), animal studies indicate that caffeine can actually reduce impulsive choice behavior only in the sub-population of rodents with medium-to-high basal level of impulsivity (Barbelivien et al., 2008). Thus, blockade of adenosine receptors seems to work as a normalizer of function, bolstering impulsivity in low-impulsivity individuals and dampening impulsivity when it is already elevated. This putative shift of A2AR function may be associated with stressful conditions in the brain (reviewed in Cunha, 2016) or with a disbalance among the PFC, dorsal hippocampus and nucleus accumbens in the control of impulsivity (Kim and Lee, 2011; Monterosso et al., 2001), since synaptic plasticity is differently regulated by A2AR in each of these brain areas (D’Alcantara et al., 2001; Costenla et al., 2011; Kerkhofs et al., 2018). This possibility allows reconciling the presently observed increased impulsivity upon selective downregulation of A2AR in the PLmPFC with the beneficial effect of caffeine and A2AR antagonists in processes such as memory deterioration (Cunha and Agostinho, 2010; Chen, 2014), ADHD (Pandolfo et al., 2013), schizophrenia (Rial et al., 2014), effort-based decision-making (e.g., Pardo et al., 2012; Nunes et al., 2013), ethanol consumption (Nam et al., 2013) or psychomotor responses triggered by drugs of abuse (e.g., Shen et al., 2008; Matos et al., 2015), all of which are worsened with increased impulsivity.
The role of A2AR in the control of anxiety is not straightforward (reviewed in Cunha et al., 2008; Yamada et al., 2014). Accordingly, there was a discrete effect upon downregulating A2AR selectively in the PLmPFC on anxiety-like behavior in the elevated plus maze test and in the splash test, whereas no effect was observed in the open field test. Although previous human genetic association studies implicate polymorphisms of the A2AR gene in caffeine-induced anxiety (Alsene et al., 2003; Tsai et al., 2006), there is some discrepancy on the impact on anxiety-like behaviors of A2AR genetic deletions and A2AR pharmacological antagonism (e.g., Ledent et al., 1997; El Yacoubi et al., 2000; Kaster et al., 2015) as well as A2AR overexpression (Giménez-Llort et al., 2007; Coelho et al., 2014). The inconsistency in these global manipulations of A2AR might result from a differential contribution of A2AR in different brain regions. This is exemplified by the observations that the deletion of A2AR in striatal neurons does not affect anxiety-like behavior, while deletion of A2AR in the entire forebrain or focal deletion of hippocampal A2AR both produce an anxiolytic phenotype (Wei et al., 2014). Our results add further complexity to the A2AR-mediated modulation of anxious behavior and warrants future region-selective studies to unravel the impact of A2AR in different circuits in the control this behavior.
Given that the downregulation of A2AR in the PLmPFC resulted in a discrete anxious phenotype, caution must be taken when inferring impulsive choice behavior from a T-maze delay-based cost-benefit decision making analysis. The enclosure of animals with an anxious phenotype in a small compartment between two arms of the T-maze during the delay period could have induced a context aversion, leading the rats to choose the small reward solely as a result of a cost-benefit re-evaluation rather than impulsivity. Furthermore, the subjective value of the large food reward was greater in shA2AR- as compared to shCTRL-treated rats as they needed lower number of training sessions to reach testing criterion. Because of this, the extinction of this subjective value could also be faster, adding a confound to our observations. It is known that PLmPFC and A2AR regulate fear responses. However, the involvement of A2AR in fear responses is complex, as the nature of regulation depends on the manipulated brain region (Wei et al., 2014; Simões et al., 2016). If A2AR in the PLmPFC also regulates fear responses is not known. Interestingly, impulsive choice behavior has been shown to predict greater anxiety-like behavior in rats (Stein et al., 2015), and in humans, anxious individuals were shown to be impulsive decision-makers in the delay discounting task (Xia et al., 2017), both in agreement with our findings. Thus, future studies to clarify the role of A2AR in anxiety and fear responses and their relationship to impulsive behavior will be useful to dissociate between the impact of PLmPFC A2AR on impulsive decision making vs. on reward value- and context-dependent re-evaluation of cost-benefit during decision making.
The final PFC-related behavioral output that was investigated was working memory, which is bolstered upon pharmacological and genetic ablation of A2AR, both in physiological and pathological situations (reviewed in Chen, 2014), whereas transgenic overexpression of A2AR in the cortex of rats impairs working memory (Giménez-Llort et al., 2007). Working memory is a short-lasting on-line memory buffer system that holds behaviorally relevant information to ongoing tasks and relies on a network of brain regions connected to and orchestrated by the PFC (Goldman-Rakic, 1999; Fuster, 2001). However, we now show that the genetic downregulation of A2AR selectively in the PLmPFC of rats does not affect spatial working memory when assessed as the spontaneous alternation in the Y-maze and it has an inconsistent effect on working memory assessed in the repeated trial MWM test. Our findings are in line with a recent report that optogenetic activation of A2AR signaling pathways in the mPFC did not affect spatial working in the Y-maze test (Li et al., 2018); in contrast, the selective manipulation of A2AR in the striatum is sufficient to control working memory (Zhou et al., 2009; Wei et al., 2011) in a manner equivalent to the improvement of spatial working memory upon systemic antagonism of A2AR (Augusto et al., 2013; Li et al., 2018). Thus, it seems that striatal A2AR override mPFC A2AR in controlling working memory performance in physiological conditions. However, it remains to be determined to which extent PFC A2AR might contribute to the recovery of the deterioration of working memory performance afforded by A2AR blockade in different pathological conditions (Horita et al., 2013; Li W. et al., 2015).
Altogether, our findings that PLmPFC A2AR mediate impulsive choice constitutes the first direct demonstration of a role of A2AR in the control of behavior in physiological conditions. We have recently shown that PFC A2AR LTP in excitatory synapses onto fast spiking interneurons and control PLmPFC network activity (Kerkhofs et al., 2018). Thus, future research targeting selectively A2AR in PLmPFC fast spiking interneurons will be needed to clarify whether specifically A2AR located on glutamatergic synapses impinging on fast spiking interneurons control decision making and impulsive choice, or these behaviors are rather dependent on cooperation among A2AR located in different cell types. Furthermore, the differences observed between the selective manipulation of A2AR in the PLmPFC and more global alterations of A2AR function clearly warrant the need of future studies to dissect the hierarchy of the different roles of A2AR in different brain regions in the control of mood and cognition. Additionally, since decision making and impulsive choice is also modulated by dopamine receptors, it will also be interesting to probe whether the effect of A2AR on impulsive choice involves interaction with dopamine D2R, especially because antagonism and genetic deletion of A2AR dampen dopamine-mediated decrease in PFC network activity (Real et al., 2018). Finally, the up-regulation of A2AR in synapses upon brain disease condition (reviewed in Cunha, 2016), namely in the PFC (Pandolfo et al., 2013), heralds the potential of A2AR as relevant players controlling the pathophysiology of several neuropsychiatric disorders (Cunha et al., 2008), which still remains to be explored.
Author Contributions
DL, PP, NG, RC and SF designed the research. DL, PP, NG, NM, CS, JR, HS, AK and SF performed the experiments and analyzed the data. SF wrote the first draft of the manuscript. All authors commented on the manuscript text.
Funding
This work was supported by Maratona da Saúde, Fundacion LaCaixa, Pepita-FMUC and Banco Santander-Totta, NARSAD, Centro 2020 (project CENTRO-01-0145-FEDER-000008:BrainHealth 2020 and CENTRO-01-0246-FEDER-000010), and FCT (projects POCI-01-0145-FEDER-007440 and PTDC/NEU-NMC/4154/2016) to RC.
Conflict of Interest Statement
RC is a scientific consultant for the Institute for Scientific Information on Coffee.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alsene, K., Deckert, J., Sand, P., and de Wit, H. (2003). Association between A2A receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 28, 1694–1702. doi: 10.1038/sj.npp.1300232
Amlung, M., Few, L. R., Howland, J., Rohsenow, D. J., Metrik, J., and MacKillop, J. (2013). Impulsivity and alcohol demand in relation to combined alcohol and caffeine use. Exp. Clin. Psychopharmacol. 21, 467–474. doi: 10.1037/a0034214
Arnsten, A. F. T., Wang, M., and Paspalas, C. D. (2015). Dopamine’s actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol. Rev. 67, 681–696. doi: 10.1124/pr.115.010512
Augusto, E., Matos, M., Sévigny, J., El-Tayeb, A., Bynoe, M. S., Müller, C. E., et al. (2013). Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 33, 11390–11399. doi: 10.1523/jneurosci.5817-12.2013
Bailey, M. R., Simpson, E. H., and Balsam, P. D. (2016). Neural substrates underlying effort, time and risk-based decision making in motivated behavior. Neurobiol. Learn. Mem. 133, 233–256. doi: 10.1016/j.nlm.2016.07.015
Barbelivien, A., Billy, E., Lazarus, C., Kelche, C., and Majchrzak, M. (2008). Rats with different profiles of impulsive choice behavior exhibit differences in responses to caffeine and d-amphetamine and in medial prefrontal cortex 5-HT utilization. Behav. Brain Res. 187, 273–283. doi: 10.1016/j.bbr.2007.09.020
Bizot, J. C., Chenault, N., Houzé, B., Herpin, A., David, S., Pothion, S., et al. (2007). Methylphenidate reduces impulsive behaviour in juvenile Wistar rats, but not in adult Wistar, SHR and WKY rats. Psychopharmacology 193, 215–223. doi: 10.1007/s00213-007-0781-4
Bruns, R. F., Lawson-Wendling, K., and Pugsley, T. A. (1983). A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal. Biochem. 132, 74–81. doi: 10.1016/0003-2697(83)90427-x
Caballero, M., Núñez, F., Ahern, S., Cuffí, M. L., Carbonell, L., Sánchez, S., et al. (2011). Caffeine improves attention deficit in neonatal 6-OHDA lesioned rats, an animal model of attention deficit hyperactivity disorder (ADHD). Neurosci. Lett. 494, 44–48. doi: 10.1016/j.neulet.2011.02.050
Calhoon, G. G., and Tye, K. M. (2015). Resolving the neural circuits of anxiety. Nat. Neurosci. 18, 1394–1404. doi: 10.1038/nn.4101
Chen, J. F. (2014). Adenosine receptor control of cognition in normal and disease. Int. Rev. Neurobiol. 119, 257–307. doi: 10.1016/b978-0-12-801022-8.00012-x
Choleris, E., Thomas, A. W., Kavaliers, M., and Prato, F. S. (2001). A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 25, 235–260. doi: 10.1016/s0149-7634(01)00011-2
Coelho, J. E., Alves, P., Canas, P. M., Valadas, J. S., Shmidt, T., Batalha, V. L., et al. (2014). Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion and anxiety. Front. Psychiatry 5:67. doi: 10.3389/fpsyt.2014.00067
Costenla, A. R., Diógenes, M. J., Canas, P. M., Rodrigues, R. J., Nogueira, C., Maroco, J., et al. (2011). Enhanced role of adenosine A2A receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur. J. Neurosci. 34, 12–21. doi: 10.1111/j.1460-9568.2011.07719.x
Courtin, J., Bienvenu, T. C., Einarsson, E. Ö., and Herry, C. (2013). Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience 240, 219–242. doi: 10.1016/j.neuroscience.2013.03.001
Cunha, R. A. (2016). How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 139, 1019–1055. doi: 10.1111/jnc.13724
Cunha, R. A., and Agostinho, P. M. (2010). Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J. Alzheimers Dis. 20, S95–S116. doi: 10.3233/jad-2010-1408
Cunha, R. A., Constantino, M. D., and Ribeiro, J. A. (1999). G protein coupling of CGS 21680 binding sites in the rat hippocampus and cortex is different from that of adenosine A1 and striatal A2A receptors. Naunyn Schmiedebergs Arch. Pharmacol. 359, 295–302. doi: 10.1007/pl00005355
Cunha, R. A., Ferré, S., Vaugeois, J. M., and Chen, J. F. (2008). Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr. Pharm. Des. 14, 1512–1524. doi: 10.2174/138161208784480090
D’Alcantara, P., Ledent, C., Swillens, S., and Schiffmann, S. N. (2001). Inactivation of adenosine A2A receptor impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience 107, 455–464. doi: 10.1016/S0306-4522(01)00372-4
Dalley, J. W., Everitt, B. J., and Robbins, T. W. (2011). Impulsivity, compulsivity and top-down cognitive control. Neuron 69, 680–694. doi: 10.1016/j.neuron.2011.01.020
Dudchenko, P. A. (2004). An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 28, 699–709. doi: 10.1016/j.neubiorev.2004.09.002
El Yacoubi, M., Ledent, C., Parmentier, M., Costentin, J., and Vaugeois, J. M. (2000). The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A2A adenosine receptor antagonists. Psychopharmacology 148, 153–163. doi: 10.1007/s002130050037
Ferreira, S. G., Gonçalves, F. Q., Marques, J. M., Tomé, Â. R., Rodrigues, R. J., Nunes-Correia, I., et al. (2015). Presynaptic adenosine A2A receptors dampen cannabinoid CB1 receptor-mediated inhibition of corticostriatal glutamatergic transmission. Br. J. Pharmacol. 172, 1074–1086. doi: 10.1111/bph.12970
Floresco, S. B., and Ghods-Sharifi, S. (2007). Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb. Cortex 17, 251–260. doi: 10.1093/cercor/bhj143
Floresco, S. B., Tse, M. T. L., and Ghods-Sharifi, S. (2008). Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33, 1966–1979. doi: 10.1038/sj.npp.1301565
Font, L., Mingote, S., Farrar, A. M., Pereira, M., Worden, L., Stopper, C., et al. (2008). Intra-accumbens injections of the adenosine A2A agonist CGS21680 affect effort-related choice behavior in rats. Psychopharmacology 199, 515–526. doi: 10.1007/s00213-008-1174-z
Fredholm, B. B., Chen, J. F., Cunha, R. A., Svenningsson, P., and Vaugeois, J. M. (2005). Adenosine and brain function. Int. Rev. Neurobiol. 63, 191–270. doi: 10.1016/S0074-7742(05)63007-3
Fuster, J. M. (2001). The prefrontal cortex—an update: time is of the essence. Neuron 30, 319–333. doi: 10.1016/s0896-6273(01)00285-9
Giménez-Llort, L., Schiffmann, S. N., Shmidt, T., Canela, L., Camón, L., Wassholm, M., et al. (2007). Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol. Learn. Mem. 87, 42–56. doi: 10.1016/j.nlm.2006.05.004
Goldman-Rakic, P. S. (1999). The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol. Psychiatry 46, 650–661. doi: 10.1016/s0006-3223(99)00130-4
Gonçalves, F. Q., Pires, J., Pliassova, A., Beleza, R., Lemos, C., Marques, J. M., et al. (2015). Adenosine A2B receptors control A1 receptor-mediated inhibition of synaptic transmission in the mouse hippocampus. Eur. J. Neurosci. 41, 878–888. doi: 10.1111/ejn.12851
Gourley, S. L., and Taylor, J. R. (2016). Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat. Neurosci. 19, 656–664. doi: 10.1038/nn.4275
Grant, J. E., and Chamberlain, S. R. (2018). Caffeine’s influence on gambling behavior and other types of impulsivity. Addict. Behav. 76, 156–160. doi: 10.1016/j.addbeh.2017.08.007
Harper, L. K., Beckett, S. R., Marsden, C. A., McCreary, A. C., and Alexander, S. P. (2006). Effects of the A2A adenosine receptor antagonist KW6002 in the nucleus accumbens in vitro and in vivo. Pharmacol. Biochem. Behav. 83, 114–121. doi: 10.1016/j.pbb.2005.12.014
Hauber, W., and Sommer, S. (2009). Prefrontostriatal circuitry regulates effort-related decision making. Cereb. Cortex 19, 2240–2247. doi: 10.1093/cercor/bhn241
Heinz, A. J., de Wit, H., Lilje, T. C., and Kassel, J. D. (2013). The combined effects of alcohol, caffeine and expectancies on subjective experience, impulsivity and risk-taking. Exp. Clin. Psychopharmacol. 21, 222–234. doi: 10.1037/a0032337
Horita, T. K., Kobayashi, M., Mori, A., Jenner, P., and Kanda, T. (2013). Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology 23, 345–352. doi: 10.1007/s00213-013-3158-x
Jin, J., and Maren, S. (2015). Prefrontal-hippocampal interactions in memory and emotion. Front. Syst. Neurosci. 9:170. doi: 10.3389/fnsys.2015.00170
Jones, H. A., and Lejuez, C. W. (2005). Personality correlates of caffeine dependence: the role of sensation seeking, impulsivity and risk taking. Exp. Clin. Psychopharmacol. 13, 259–266. doi: 10.1037/1064-1297.13.3.259
Kable, J. W., and Glimcher, P. W. (2007). The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 10, 1625–1633. doi: 10.1038/nn2007
Kaster, M. P., Machado, N. J., Silva, H. B., Nunes, A., Ardais, A. P., Santana, M., et al. (2015). Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc. Natl. Acad. Sci. U S A 112, 7833–7838. doi: 10.1073/pnas.1423088112
Kerkhofs, A., Canas, P. M., Timmerman, A. J., Real, J. I., Xavier, C., Cunha, R. A., et al. (2018). Adenosine A2A receptors control glutamatergic synaptic plasticity in fast spiking interneurons of the prefrontal cortex. Front. Pharmacol. 9:133. doi: 10.3389/fphar.2018.00133
Kilkenny, C., Browne, W., Cuthill, I. C., Emerson, M., Altman, D. G., and NC3Rs Reporting Guidelines Working Group. (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x
Kim, S., and Lee, D. (2011). Prefrontal cortex and impulsive decision making. Biol. Psychiatry 69, 1140–1146. doi: 10.1016/j.biopsych.2010.07.005
Ledent, C., Vaugeois, J. M., Schiffmann, S. N., Pedrazzini, T., El Yacoubi, M., Vanderhaeghen, J. J., et al. (1997). Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2A receptor. Nature 388, 674–678. doi: 10.1038/41771
Lee, D. (2013). Decision making: from neuroscience to psychiatry. Neuron 78, 233–248. doi: 10.1016/j.neuron.2013.04.008
Li, Z., Chen, X., Wang, T., Gao, Y., Li, F., Chen, L., et al. (2018). The corticostriatal adenosine A2A receptor controls maintenance and retrieval of spatial working memory. Biol. Psychiatry 83, 530–541. doi: 10.1016/j.biopsych.2017.07.017
Li, Y., He, Y., Chen, M., Pu, Z., Chen, L., Li, P., et al. (2016). Optogenetic activation of adenosine A2A receptor signaling in the dorsomedial striatopallidal neurons suppresses goal-directed behavior. Neuropsychopharmacology 41, 1003–1013. doi: 10.1038/npp.2015.227
Li, P., Rial, D., Canas, P. M., Yoo, J. H., Li, W., Zhou, X., et al. (2015). Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol. Psychiatry 20, 1339–1349. doi: 10.1038/mp.2015.43
Li, W., Silva, H. B., Real, J., Wang, Y.-M., Rial, D., Li, P., et al. (2015). Inactivation of adenosine A2A receptors reverses working memory deficits at early stages of Huntington’s disease models. Neurobiol. Dis. 79, 70–80. doi: 10.1016/j.nbd.2015.03.030
Liu, K. C., Li, J. Y., Xie, W., Li, L. B., Zhang, J., Du, C. X., et al. (2016). Activation and blockade of serotonin6 receptors in the dorsal hippocampus enhance T maze and hole-board performance in a unilateral 6-hydroxydopamine rat model of Parkinson’s disease. Brain Res. 1650, 184–195. doi: 10.1016/j.brainres.2016.09.009
Lundberg, C., Björklund, T., Carlsson, T., Jakobsson, J., Hantraye, P., Déglon, N., et al. (2008). Applications of lentiviral vectors for biology and gene therapy of neurological disorders. Curr. Gene Ther. 8, 461–473. doi: 10.2174/156652308786847996
Mason, L., O’Sullivan, N., Montaldi, D., Bentall, R. P., and El-Deredy, W. (2014). Decision-making and trait impulsivity in bipolar disorder are associated with reduced prefrontal regulation of striatal reward valuation. Brain 137, 2346–2355. doi: 10.1093/brain/awu152
Matos, M., Shen, H. Y., Augusto, E., Wang, Y., Wei, C. J., Wang, Y. T., et al. (2015). Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol. Psychiatry 78, 763–774. doi: 10.1016/j.biopsych.2015.02.026
Mingote, S., Font, L., Farrar, A. M., Vontell, R., Worden, L. T., Stopper, C. M., et al. (2008). Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J. Neurosci. 28, 9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008
Monterosso, J., Ehrman, R., Napier, K. L., O’Brien, C. P., and Childress, A. R. (2001). Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction 96, 1825–1837. doi: 10.1080/09652140120089571
Nam, H. W., Hinton, D. J., Kang, N. Y., Kim, T., Lee, M. R., Oliveros, A., et al. (2013). Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J. Neurosci. 33, 4329–4338. doi: 10.1523/JNEUROSCI.3094-12.2013
Nunes, E. J., Randall, P. A., Podurgiel, S., Correa, M., and Salamone, J. D. (2013). Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine and muscarinic acetylcholine receptors. Neurosci. Biobehav. Rev. 37, 2015–2025. doi: 10.1016/j.neubiorev.2013.04.002
Oliveros, A., Cho, C. H., Cui, A., Choi, S., Lindberg, D., Hinton, D., et al. (2017). Adenosine A2A receptor and ERK-driven impulsivity potentiates hippocampal neuroblastproliferation. Transl. Psychiatry 7:e1095. doi: 10.1038/tp.2017.64
Pagnussat, N., Almeida, A. S., Marques, D. M., Nunes, F., Chenet, G. C., Botton, P. H., et al. (2015). Adenosine A2A receptors are necessary and sufficient to trigger memory impairment in adult mice. Br. J. Pharmacol. 172, 3831–3845. doi: 10.1111/bph.13180
Pandolfo, P., Machado, N. J., Köfalvi, A., Takahashi, R. N., and Cunha, R. A. (2013). Caffeine regulates frontocorticostriatal dopamine transporter density and improves attention and cognitive deficits in an animal model of attention deficit hyperactivity disorder. Eur. Neuropsychopharmacol. 23, 317–328. doi: 10.1016/j.euroneuro.2012.04.011
Pardo, M., Lopez-Cruz, L., Valverde, O., Ledent, C., Baqi, Y., Müller, C. E., et al. (2012). Adenosine A2A receptor antagonism and genetic deletion attenuate the effects of dopamine D2 antagonism on effort-based decision making in mice. Neuropharmacology 62, 2068–2077. doi: 10.1016/j.neuropharm.2011.12.033
Pattij, T., and Vanderschuren, L. J. (2008). The neuropharmacology of impulsive behaviour. Trends Pharmacol. Sci. 29, 192–199. doi: 10.1016/j.tips.2008.01.002
Paxinos, G., and Watson, C. (2009). The Rat Brain In Stereotaxic Coordinates. 6th Edn. London: Elsevier Academic Press.
Pellow, S., Chopin, P., File, S. E., and Briley, M. (1985). Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167. doi: 10.1016/0165-0270(85)90031-7
Prut, L., and Belzung, C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33. doi: 10.1016/s0014-2999(03)01272-x
Real, J. I., Simões, A. P., Cunha, R. A., Ferreira, S. G., and Rial, D. (2018). Adenosine A2A receptors modulate the dopamine D2 receptor-mediated inhibition of synaptic transmission in the mouse prefrontal cortex. Eur. J. Neurosci. 47, 1127–1134. doi: 10.1111/ejn.13912
Rial, D., Lara, D. R., and Cunha, R. A. (2014). The adenosine neuromodulation system in schizophrenia. Int. Rev. Neurobiol. 119, 395–449. doi: 10.1016/b978-0-12-801022-8.00016-7
Rudebeck, P. H., Walton, M. E., Smyth, A. N., Bannerman, D. M., and Rushworth, M. F. S. (2006). Separate neural pathways process different decision costs. Nat. Neurosci. 9, 1161–1168. doi: 10.1038/nn1756
Shen, H. Y., Canas, P. M., Garcia-Sanz, P., Lan, J. Q., Boison, D., Moratalla, R., et al. (2013). Adenosine A2A receptors in striatal glutamatergic terminals and GABAergic neurons oppositely modulate psychostimulant action and DARPP-32 phosphorylation. PLoS One 8:e80902. doi: 10.1371/journal.pone.0080902
Shen, H. Y., Coelho, J. E., Ohtsuka, N., Canas, P. M., Day, Y. J., Huang, Q. Y., et al. (2008). A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knockouts. J. Neurosci. 28, 2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008
Simões, A. P., Machado, N. J., Gonçalves, N., Kaster, M. P., Simões, A. T., Nunes, A., et al. (2016). Adenosine A2A receptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology 41, 2862–2871. doi: 10.1038/npp.2016.98
Sripada, C. S., Gonzalez, R., Phan, K. L., and Liberzon, I. (2011). The neural correlates of intertemporal decision-making: contributions of subjective value, stimulus type and trait impulsivity. Hum. Brain Mapp. 32, 1637–1648. doi: 10.1002/hbm.21136
Stein, J. S., Renda, C. R., Barker, S. M., Liston, K. J., Shahan, T. A., and Madden, G. J. (2015). Impulsive choice predicts anxiety-like behavior, but not alcohol or sucrose consumption, in male long-evans rats. Alcohol. Clin. Exp. Res. 39, 932–940. doi: 10.1111/acer.12713
Tovote, P., Fadok, J. P., and Lüthi, A. (2015). Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331. doi: 10.1038/nrn3945
Tsai, S. J., Hong, C. J., Hou, S. J., and Yen, F. C. (2006). Association study of adenosine A2A receptor (1976C>T) genetic polymorphism and mood disorders and age of onset. Psychiatr. Genet. 16:185. doi: 10.1097/01.ypg.0000218627.26622.eb
Viana da Silva, S., Haberl, M. G., Zhang, P., Bethge, P., Lemos, C., Gonçalves, N., et al. (2016). Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat. Commun. 7:11915. doi: 10.1038/ncomms11915
Waldeck, T. L., and Miller, L. S. (1997). Gender and impulsivity differences in licit substance use. J. Subst. Abuse 9, 269–275. doi: 10.1016/s0899-3289(97)90021-3
Wei, C. J., Augusto, E., Gomes, C. A., Singer, P., Wang, Y., Boison, D., et al. (2014). Regulation of fear responses by striatal and extrastriatal adenosine A2A receptors in forebrain. Biol. Psychiatry 75, 855–863. doi: 10.1016/j.biopsych.2013.05.003
Wei, C. J., Singer, P., Coelho, J., Boison, D., Feldon, J., Yee, B. K., et al. (2011). Selective inactivation of adenosine A2A receptors in striatal neurons enhances working memory and reversal learning. Learn. Mem. 18, 459–474. doi: 10.1101/lm.2136011
Whishaw, I. Q. (1985). Formation of a place learning-set by the rat: a new paradigm for neurobehavioral studies. Physiol. Behav. 35, 139–143. doi: 10.1016/0031-9384(85)90186-6
Wydra, K., Suder, A., Frankowska, M., Borroto Escuela, D. O., Fuxe, K., and Filip, M. (2018). Effects of intra-accumbal or intra-prefrontal cortex microinjections of adenosine 2A receptor ligands on responses to cocaine reward and seeking in rats. Psychopharmacology 235, 3509–3523. doi: 10.1007/s00213-018-5072-8
Xia, L., Gu, R., Zhang, D., and Luo, Y. (2017). Anxious individuals are impulsive decision-makers in the delay discounting task: an ERP study. Front. Behav. Neurosci. 11:5. doi: 10.3389/fnbeh.2017.00005
Yalcin, I., Aksu, F., and Belzung, C. (2005). Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur. J. Pharmacol. 514, 165–174. doi: 10.1016/j.ejphar.2005.03.029
Yamada, K., Kobayashi, M., and Kanda, T. (2014). Involvement of adenosine A2A receptors in depression and anxiety. Int. Rev. Neurobiol. 119, 373–393. doi: 10.1016/b978-0-12-801022-8.00015-5
Yu, C., Gupta, J., Chen, J. F., and Yin, H. H. (2009). Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J. Neurosci. 29, 15100–15103. doi: 10.1523/JNEUROSCI.4215-09.2009
Keywords: adenosine A2A receptors, impulsive choice, prefrontal cortex (PFC), anxiety, working memory, cost-benefit decision making
Citation: Leffa DT, Pandolfo P, Gonçalves N, Machado NJ, de Souza CM, Real JI, Silva AC, Silva HB, Köfalvi A, Cunha RA and Ferreira SG (2018) Adenosine A2A Receptors in the Rat Prelimbic Medial Prefrontal Cortex Control Delay-Based Cost-Benefit Decision Making. Front. Mol. Neurosci. 11:475. doi: 10.3389/fnmol.2018.00475
Received: 22 May 2018; Accepted: 05 December 2018;
Published: 20 December 2018.
Edited by:
David Blum, INSERM U1172 Centre de Recherche Jean Pierre Aubert, FranceReviewed by:
Joana Esteves Coelho, Instituto de Medicina Molecular (IMM), PortugalRyan K. Bachtell, University of Colorado Boulder, United States
Copyright © 2018 Leffa, Pandolfo, Gonçalves, Machado, de Souza, Real, Silva, Silva, Köfalvi, Cunha and Ferreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samira G. Ferreira, carsamira@gmail.com
† These authors have contributed equally to this work
 Douglas T. Leffa
Douglas T. Leffa Pablo Pandolfo
Pablo Pandolfo Nélio Gonçalves
Nélio Gonçalves Nuno J. Machado
Nuno J. Machado Carolina M. de Souza
Carolina M. de Souza Joana I. Real
Joana I. Real António C. Silva
António C. Silva Henrique B. Silva
Henrique B. Silva Attila Köfalvi
Attila Köfalvi Rodrigo A. Cunha
Rodrigo A. Cunha Samira G. Ferreira
Samira G. Ferreira