Motoneuronal Spinal Circuits in Degenerative Motoneuron Disease
- Section on Developmental Neurobiology, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States
The most evident phenotype of degenerative motoneuron disease is the loss of motor function which accompanies motoneuron death. In both amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA), it is now clear that dysfunction is not restricted to motoneurons but is manifest in the spinal circuits in which motoneurons are embedded. As mounting evidence shows that motoneurons possess more elaborate and extensive connections within the spinal cord than previously realized, it is necessary to consider the role of this circuitry and its dysfunction in the disease process. In this review article, we ask if the selective vulnerability of the different motoneuron types and the relative disease resistance of distinct motoneuron groups can be understood in terms of their intraspinal connections.
Introduction
Degenerative motoneuron diseases are devastating conditions whose underlying causes are poorly understood. Two of these diseases, amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA), result in loss of motoneurons, leading to reduced motor function and ultimately death (Cleveland and Rothstein, 2001; Wee et al., 2010). Although both disorders have been considered autonomous motoneuron diseases, it is now clear that their pathology is not restricted to motoneurons and that dysfunction is more widespread, particularly within the brainstem and spinal circuits in which the motoneurons are embedded (Schütz, 2005; Ling et al., 2010; Mentis et al., 2011). Because of this, the primary, cell-autonomous pathologies caused by the conditions are compounded by secondary effects that result from disruptions in the spinal circuitry. The resulting motor deficits are, therefore, due to the interactions between the primary and secondary processes.
In this review article, we will first discuss the spinal circuit abnormalities in both SMA and ALS. We will then describe the sensitivity of the different motoneuron types to the diseases, and finally, we will consider these differences in susceptibility in light of recent discoveries showing that the intraspinal connections of motoneurons are more extensive than previously appreciated (Bhumbra and Beato, 2018; Chopek et al., 2018). These new connections create the likelihood of additional secondary effects that will further complicate interpretation of the disease process. We will conclude the review by considering these novel findings in relation to the motor dysfunction, and we will ask if the known susceptibility of different motoneuron types and different motoneuron pools to disease can be understood by considering differences in their intraspinal connectivity.
Motoneuronal Circuits in Degenerative Motoneuron Disease
The SMNΔ7 mouse model of SMA lacks the SMN gene but expresses two copies of the human SMN2 gene (Le et al., 2005). These mice exhibit several motor defects, including weakness and an inability to right themselves, and they eventually die at 2 weeks of age. The proximal muscles are more affected than the distal muscles, with the epaxial and hypaxial muscles being the most severely weakened (Montes et al., 2009; Mentis et al., 2011). One of the first pathological changes in the disease is a decline in the strength of muscle spindle afferent synaptic input to motoneurons (Ling et al., 2010; Mentis et al., 2011; Fletcher and Mentis, 2017; Fletcher et al., 2017). This loss of muscle afferent input to motoneurons is due to a decrease in the amount of glutamate released from the afferents onto motoneurons (Fletcher et al., 2017). In addition to a loss of proprioceptive input to motoneurons, there is a reduction in the number of vesicular glutamate transporter (VGLUT)2+ terminals on motoneurons in SMNΔ7 mice (Ling et al., 2010) that can be derived from local (Ling et al., 2010) or descending (Du Beau et al., 2012) glutamatergic interneurons. Loss of somatic vesicular gamma aminobutyric acid (GABA) transporter (VGAT) terminals was not observed, suggesting that the inhibitory inputs to motoneurons are less affected in the disease than excitatory inputs (Ling et al., 2010).
The decreased glutamate output from primary muscle spindle afferents triggers several changes in the properties of motoneurons, including an increase in input impedance and a downregulation of the Kv2.1 potassium channel (Fletcher et al., 2017). These responses are probably compensatory because they also occur in wild-type mice in which transmitter release from muscle afferents is abrogated by tetanus toxin (Fletcher et al., 2017). Proof of the secondary nature of the altered motoneuron electrical properties in SMA comes from experiments in which the SMN protein was selectively restored in afferents or motoneurons (Fletcher et al., 2017). Restoration of the protein in afferents, but not in motoneurons, normalized Kv2.1 expression and partially restored the firing of motoneurons to current injection (Fletcher et al., 2017). These findings illustrate that the pathology exhibited in SMA is a combination of cell-autonomous abnormalities, secondary changes due to the interaction of motoneurons with abnormally functioning afferents, and the compensatory responses of both motoneurons and afferents to their primary and secondary defects (Brownstone and Lancelin, 2018). Although secondary, the motoneuronal changes contribute significantly to the motor deficits in SMA.
If the reduced synaptic input from primary afferents to motoneurons reflected generalized afferent dysfunction and was independent of motoneuron pathology, then we would predict that afferent loss should also be observed on other intraspinal targets of primary afferents. This idea was tested by examining the number of VGLUT1 primary afferent terminals on Renshaw cells (RCs) in SMNΔ7 neonatal mice (Thirumalai et al., 2013). However, in contrast to the findings in motoneurons, the number of VGLUT1 terminals on RCs was increased rather than decreased. While this suggests that not all branches of proprioceptive afferents exhibit the same fate, it is not known if the proprioceptive synapses on RCs are functional. The cause of this increased afferent innervation is unknown, but one possibility is that primary afferents sprout in response to the loss of inputs to motoneurons. Interestingly, the number of cholinergic vesicular acetylcholine transporter (VAChT)+ terminals was also increased onto RCs in the rostral lumbar segments at P13 even though there was a substantial loss of motoneurons in these segments. Again, the mechanisms responsible for this are unclear, but it may also represent sprouting because the remaining motoneurons will have lost a significant portion of their motoneuronal targets (Nishimaru et al., 2005; Bhumbra and Beato, 2018). The consequences of these changes in connectivity within motor circuits are not known. An increased innervation of RCs, if functional, could serve to inhibit motoneuron firing, thereby exacerbating the weakness exhibited by these animals.
There are several mouse models of ALS, but here we will concentrate on the superoxide dismutase (SOD)1 G93A model because most work has been done using this line (Rosen et al., 1993). Unlike the SMNΔ7 model of SMA, the natural history of the disease in the SOD1 G93A mouse is much more prolonged with animals living to 150 days. Furthermore, in contrast to the findings in SMA, inhibitory spinal circuits exhibit abnormalities early in the disease. Some of these changes can be detected even before birth. For example, in SOD-93 mice, the GABA equilibrium potential recorded in motoneurons is more depolarized than in wild-type animals, indicating an alteration in chloride homeostasis at E17.5 (Branchereau et al., 2019). At this early stage, there is also a deficiency of inhibitory synaptic terminals on motoneurons which persists into postnatal life (Martin and Chang, 2012; Branchereau et al., 2019). Studies of cultured motoneurons and interneurons showed that glycine currents are smaller in motoneurons from the mutant mice compared to their wild-type counterparts. The loss of glycinergic function appears to be specific for large motoneurons because it is not observed in presumed gamma and small, fatigue-resistant (S-type) motoneurons that innervate type I muscle fibers (Chang and Martin, 2011). The reduced inhibitory input could be due to loss of inhibitory interneurons or to weaker inputs from inhibitory neurons (Chang and Martin, 2009; Wootz et al., 2013). Consistent with the latter idea, Wootz et al. (2013) showed that the innervation of RCs by motoneurons was lost at early stages of the disease and was associated with a downregulation of VAChT in motoneurons. Eventually, a majority of the motoneuronal synapses on RCs are lost. It seems likely that the synaptic projections of motoneurons to other motoneurons and to V3 interneurons will also be lost at some stage in the disease.
Muscle spindle afferent inputs to motoneurons are also affected in the SOD1 mouse model of ALS. VGLUT1 immunoreactivity, presumed to originate from proprioceptive afferents, is reduced in the motor nucleus at day 110 and is almost absent at day 130, indicating loss of muscle spindle afferent input to motoneurons (Schütz, 2005). This was confirmed by Vaughan et al. (2015) who showed that proprioceptive nerve endings initially degenerate in the periphery, and this is followed by loss of their central projections onto motoneurons. Electrophysiological studies of monosynaptic afferent connections in sacral motoneurons have shown that the evoked response recorded from the ventral roots declines with age, and although this was attributed to a loss of motoneurons (Jiang et al., 2009), it seems likely that it also reflects loss of proprioceptive input to motoneurons. Proprioceptive afferents in the mesencephalic nucleus of the SOD1 mouse exhibit reduced excitability at P11 due to reduced expression of Nav1.6-type Na+ currents, which could lead to compensatory increases in the excitability of their target motoneurons (Seki et al., 2019).
Motoneuron Classes and Their Susceptibility to the Disease Process
Degenerative diseases do not affect all motoneuron classes uniformly. For instance, in both SMA and ALS, the motoneurons innervating the extraocular muscles and the anal and bladder sphincters are spared (Comley et al., 2016; Nijssen et al., 2017). In this section, we consider the different motoneuron types and ask if their susceptibility to the disease differs and whether this is correlated with any features of their intraspinal circuitry.
In mammals, motoneurons innervating skeletal muscles comprise three classes (for a review, see Manuel and Zytnicki, 2011): α-motoneurons that innervate the extrafusal fibers, γ-motoneurons that innervate intrafusal muscle fibers (Kuffler et al., 1951), and β-motoneurons that innervate both (Bessou et al., 1963). α-Motoneurons can be further subdivided into fast-twitch fatigable (FF) motoneurons that control type IIb muscle fibers, fast-twitch fatigue-resistant (FR) motoneurons that control type IIa muscle fibers, and slow (S) motoneurons that control type I muscle fibers (Burke et al., 1971). There are two types of γ-motoneurons: static type that innervates the bag2 and chain fibers of the spindle, and dynamic type that innervates the bag1 fiber of the spindle (Matthews, 1963; Brown et al., 1965). Static β-motoneurons innervate type II extrafusal muscle fibers and the bag 2 and the chain fibers of the muscle spindle, whereas the dynamic β-motoneurons preferentially innervate type I skeletal muscle fibers and the muscle spindle bag1 fiber. β-Motoneurons innervate from 30% to 70% of the muscle spindles (McWilliam, 1975) and constitute from 11% to 30% of the axons supplying the extrafusal muscle fibers (Emonet-Dénand and Laporte, 1975; McWilliam, 1975). In both ALS and SMA, the largest motoneurons (FF) are the most vulnerable, followed by the FR, with the S motoneurons being the last to degenerate (for a review, see Kanning et al., 2010). While γ-motoneurons are resistant to ALS and SMA, β-motoneurons appear to be as vulnerable to the disease as α-motoneurons (Lalancette-Hebert et al., 2016; Powis and Gillingwater, 2016).
In addition, the external anal sphincter of the cat (innervated by motoneurons in Onuf’s nucleus) is a slow twitch muscle (Bowen and Bradley, 1973) that is presumably innervated by type S motoneurons. This might explain some of the resistance of these motoneurons to disease. In contrast, the extraocular muscles comprise six different types of muscle fiber including slow- and fast-twitch fibers and multiply-innervated non-twitch fibers (Evinger et al., 1979; Yu et al., 2005; Nijssen et al., 2017), suggesting that the resistance of the motoneurons to disease is not explained by the types of muscle fiber they innervate, consistent with transplant studies between SOD1-G93A and wild-type mice (Carrasco et al., 2010).
Many explanations have been proposed to account for the differences in the susceptibility of motoneurons to disease pathology. Here, we focus on the synaptic inputs and outputs of motoneurons innervating the hind limb and ask if any aspect of this connectivity is correlated with the susceptibility of the different motoneuron types to disease.
Synaptic Inputs to Motoneurons
One difficulty in drawing general conclusions is that the work on motoneuron connectivity has been done in different species at different ages and on a relatively limited set of motoneuron pools. Electron microscopy studies in cat have shown that α-motoneurons have four main types of boutons (McLaughlin, 1972b; Conradi et al., 1979a; Brännström, 1993): S-type boutons (small diameter with spherical synaptic vesicles), F-type boutons (small diameter with flattened synaptic vesicles), C-type boutons (large diameter with subsynaptic cisterns), and M-type boutons (large diameter that disappear after dorsal root section). The S-type boutons have been associated with excitatory synapses, the F-type with inhibitory synapses (Uchizono, 1965; Brännström, 1993), M-type with afferent inputs (McLaughlin, 1972a), and C-type with cholinergic inputs arising from V0c neurons expressing the pituitary homeobox (PITX)-2 transcription factor (Hellström et al., 2003; Zagoraiou et al., 2009). Ia afferent synapses are either S-type (Fyffe and Light, 1984) or the larger M-type that are apposed to P-type presynaptic boutons (Ornung et al., 1995).
The different motoneuron classes do not receive the same number or type of inputs. In particular, C-boutons are much more frequent on F-type motoneurons than on S-type motoneurons (Conradi et al., 1979b; Kellerth et al., 1979; Brännström, 1993; Hellström et al., 2003), and the number of Ia synaptic contacts is higher on FF motoneurons than on the S-type (Burke and Glenn, 1996). γ-Motoneurons appear to lack C-boutons and Ia contacts in cats and rodents and have less diversity in their synaptic inputs than α-motoneurons with only S- and F-type boutons on their proximal dendrites and their cell bodies lacking the M-, a C-type synapse found on α-motoneurons (Arvidsson et al., 1987; Simon et al., 1996; Ichiyama et al., 2006).
A review of literature reveals that the synaptic efficacy of the inputs to motoneurons, measured as the size of the excitatory postsynaptic potential (EPSP) or inhibitory postsynaptic potential (IPSP), is generally highest in the type S motoneurons followed by the type FR and then the type FF (Figure 1). This is true for the monosynaptic inputs from muscle spindle afferents (Burke and Rymer, 1976) even though the number of afferent synaptic contacts on motoneurons exhibits the reverse distribution (Burke and Glenn, 1996). The discrepancy between the distribution of synaptic efficacy and the number of afferent terminals reflects differences in the input impedance of the different motoneurons, with the highest in type S and the lowest in type F. The synaptic efficacy of inhibitory inputs, including disynaptic 1a inhibition (Burke and Rymer, 1976) and recurrent inhibition (Hultborn et al., 1988), is also highest in type S motoneurons and weakest in type FF motoneurons. In contrast, γ-motoneurons lack monosynaptic primary afferent inputs but do receive polysynaptic excitatory and inhibitory inputs from other afferents (Eccles et al., 1960; Appelberg et al., 1983a,b,c) as well as inhibition from RCs (Ellaway and Murphy, 1981; Appelberg et al., 1983d).
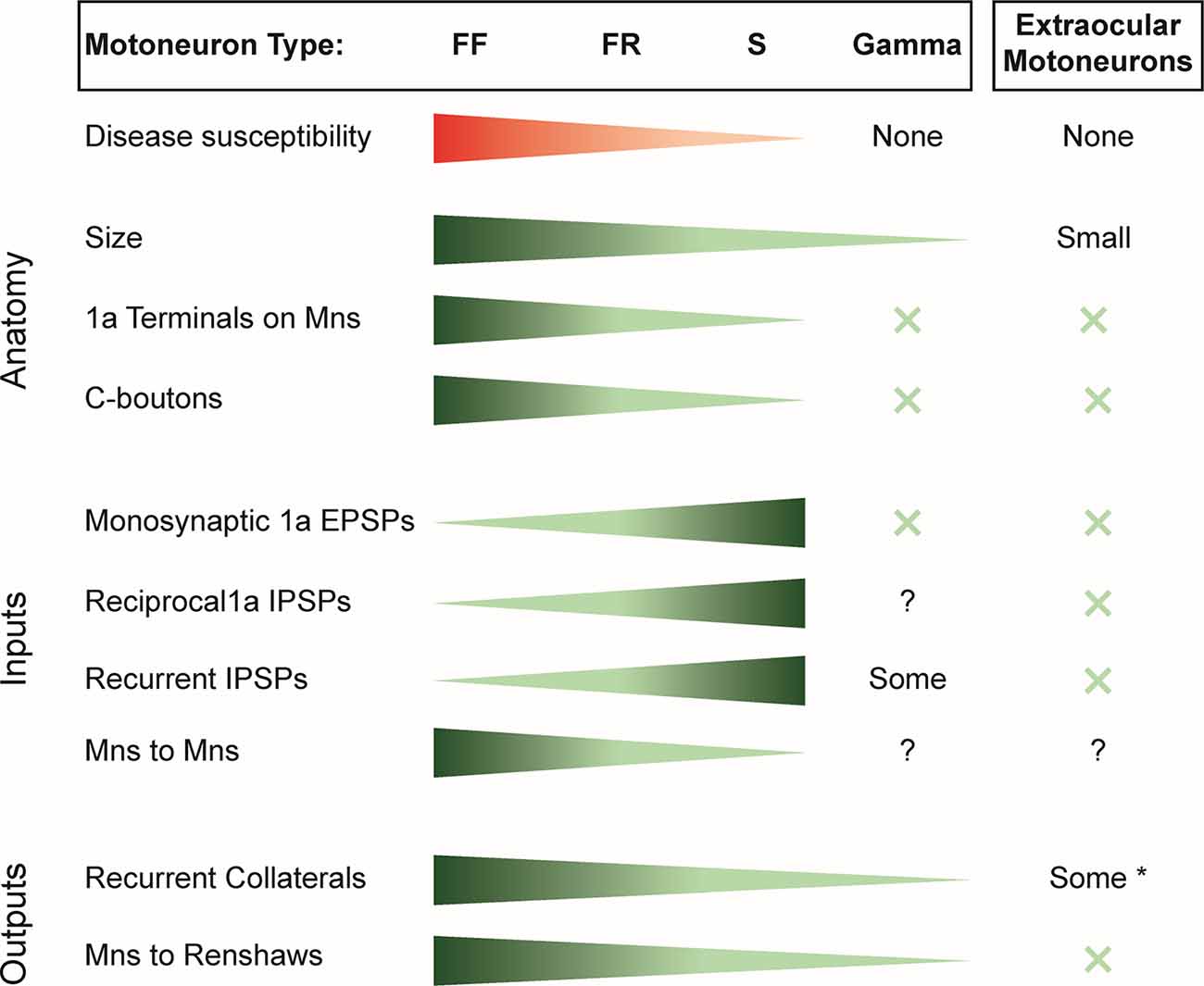
Figure 1. Summary of the susceptibility of motor unit types to spinal muscle atrophy (SMA) and amyotrophic lateral sclerosis (ALS) and the distribution of inputs and outputs of the different motoneuron classes. X indicates the absence of the variable considered. *Note that only some extraocular motoneurons have recurrent collaterals; others have none.EPSP, excitatory postsynaptic potential; IPSP, inhibitory postsynaptic potential; FF, fast-twitch fatigable motoneuron; FR, fast-twitch fatigue-resistant motoneuron; S, slow motoneuron.
Thus, within the α-motoneuron population, there is an inverse relation between the strength of synaptic inputs and the susceptibility of the different motoneuron types to disease. Accordingly α-motoneurons have the largest inputs from muscle spindle afferents, 1a inhibitory interneurons, and recurrent inhibition. However, this correlation fails when the disease-resistant, γ-motoneurons and extraocular motoneurons are considered because they receive only weak or no input from these synaptic sources (Figure 1).
Synaptic Outputs of Motoneurons and Their Effects on Spinal Circuits
The best described output connection of motoneurons is to the inhibitory Renshaw cell population (Renshaw, 1946; Eccles et al., 1954, 1961). In the adult cat, it has been estimated that the largest inputs to RCs are from FF motoneurons with progressively fewer from FR and type S motoneurons (Hultborn et al., 1988). The distribution of inputs from motoneurons to RCs appears to reflect the number of collateral swellings, presumed to be presynaptic terminals, which is greatest on the type FF motoneurons followed by FR and S (Cullheim and Kellerth, 1978). In addition, γ-motoneurons have very few recurrent collaterals (Cullheim and Ulfhake, 1979; Westbury, 1982). It was also known from work in adult cats that α-motoneuron recurrent collaterals project to other α-motoneurons irrespective of their motor unit type (Cullheim et al., 1977, 1984). However, at least in the adult cat, it is not clear that these are functional because no reports have described excitatory synaptic connections between feline motoneurons.
Recently, new evidence has emerged, showing that motoneurons have more extensive intraspinal synaptic targets that were previously realized. In 2005, it was demonstrated that motoneurons release an excitatory amino acid—probably glutamate—in addition to acetylcholine at their central connections with RCs in the neonatal mouse spinal cord (Mentis et al., 2005; Nishimaru et al., 2005) and confirmed a few years later in both the neonate (Lamotte d’Incamps and Ascher, 2008) and the adult mouse (Lamotte d’Incamps et al., 2017). Subsequent work in neonatal and juvenile mice showed that motoneurons make powerful glutamatergic connections with each other, with the largest inputs to type F motoneurons (Figure 2A; Bhumbra and Beato, 2018). This surprising finding indicates that motoneurons release different transmitters at different axonal branches apparently contravening Dale’s principle (Dale, 1935; Eccles et al., 1954). However, it is possible that both transmitters are present at the motoneuronal terminals on motoneurons because the failure to detect cholinergic responses could be due to the absence of postsynaptic acetylcholine receptors at the synapse. In addition, motoneurons in the neonatal mouse spinal cord also project exclusively glutamatergic synapses to a class of glutamatergic, commissural spinal interneurons called V3 interneurons (Chopek et al., 2018).
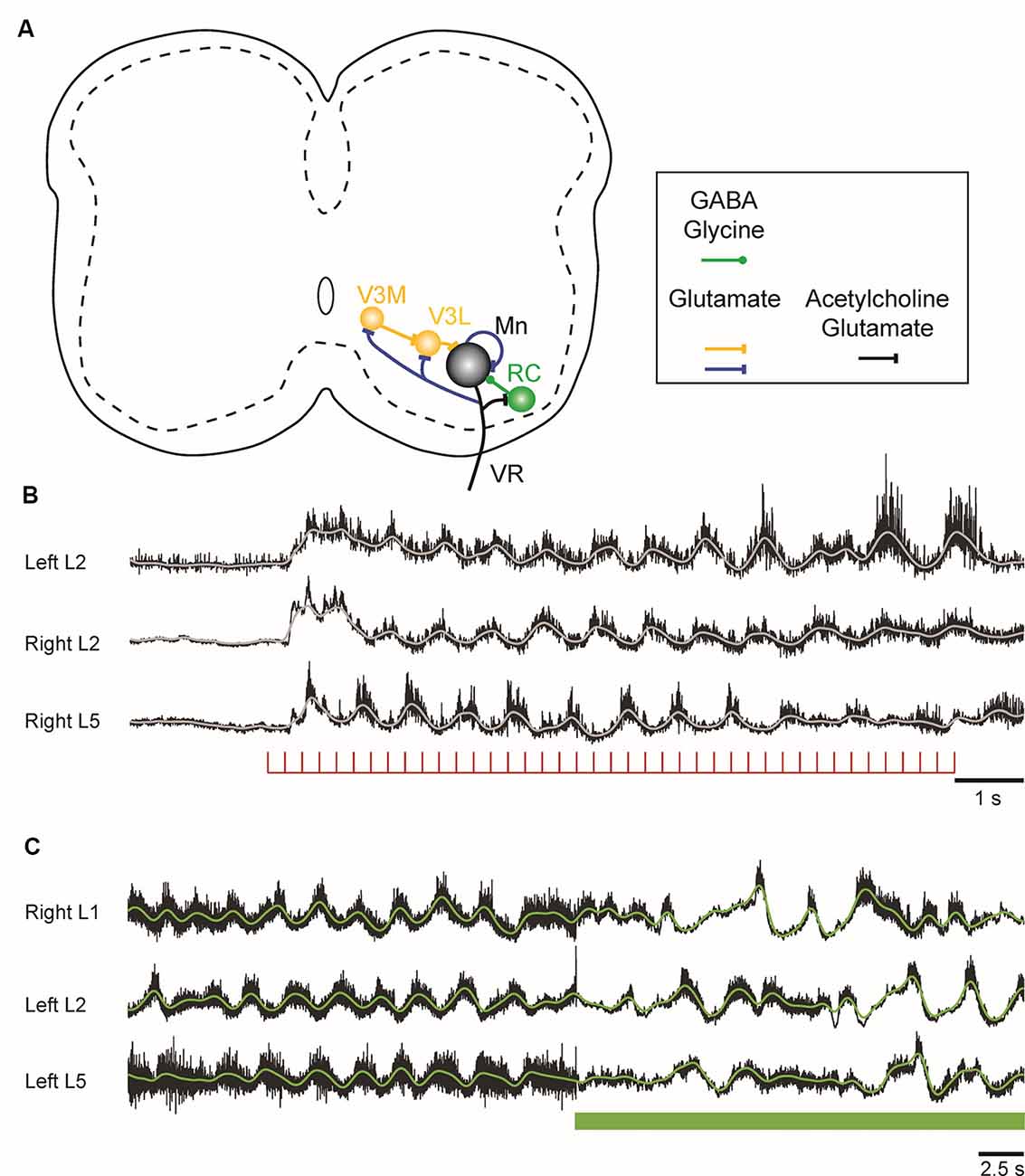
Figure 2. Figure 2. (A) Schematic showing the connections of motoneurons within the lumbar spinal cord of the neonatal mouse. Motoneurons project to each other, to inhibitory Renshaw cells (RCs), and to a medial (V3M) and a lateral (V3L) population of V3 interneurons. The lateral V3 population and RCs project back to motoneurons. The neurotransmitters released at the different sites are indicated in the box under the schematic. (B) Locomotor-like activity can be evoked in the neonatal mouse cord by a train of stimuli applied to the ventral roots. The records are the neurograms recorded from the indicated ventral roots in response to a train of stimuli applied to the right L6 ventral root. The continuous traces are the integrated records of ventral root discharge. (C) Optogenetic reduction of motoneuron firing slows and disrupts the locomotor-like rhythm induced by drugs. Ventral root recordings of locomotor-like activity induced by bath application of serotonin and N-methyl-D-aspartate (NMDA) on a spinal cord expressing the inhibitory opsin archaerhodopsin in cholinergic neurons. On exposure to green light (green bar), the neurons expressing the opsin are hyperpolarized, leading to a slowing and disruption of the locomotor rhythm.
In the neonatal mouse cord, stimulation of motor axons can initiate locomotor-like activity (Figure 2B; Mentis et al., 2005; Pujala et al., 2016), and optogenetic manipulations of motoneuron firing regulate the frequency of the locomotor rhythm during drug-induced locomotor-like activity (Figure 2C; Falgairolle et al., 2017). The mechanisms by which motoneurons influence the central pattern generator (CPG) are not fully understood (Falgairolle and O’Donovan, 2019a). Surprisingly, it occurs in the absence of cholinergic transmission and depends instead on glutamatergic transmission (Mentis et al., 2005; Falgairolle et al., 2017). It has been proposed that these excitatory effects of motoneuron stimulation on spinal circuitry are most simply explained by the presence of an excitatory interneuron interposed between the locomotor central pattern generator and motoneurons (Machacek and Hochman, 2006; Bonnot et al., 2009; Falgairolle et al., 2017). The glutamatergic V3 interneuronal population is clearly a candidate for such a neuron because it receives direct monosynaptic input from motoneurons. The V3 interneurons comprise a medial and a lateral group. The medial V3 neurons project to the lateral group which in turn projects back to motoneurons, thus forming a recurrent excitatory connection with motoneurons (Figure 2A; Chopek et al., 2018). The existence of this pathway probably explains earlier observations that revealed recurrent, disynaptic excitation of motoneurons in the neonatal rat (Schneider and Fyffe, 1992; Ichinose and Miyata, 1998). However, the effects of genetic silencing or elimination of V3 interneurons on the locomotor rhythm are not the same as optogenetic hyperpolarization of motoneurons. The silencing experiments show that V3 neurons are required to balance the excitatory locomotor drive to both sides of the cord (Zhang et al., 2008), whereas hyperpolarization of motoneurons slows the locomotor frequency (Falgairolle et al., 2017). Furthermore, optogenetic excitation of V3 interneurons expressing channelrhodopsin slows the locomotor-like rhythm (Danner et al., 2019), in contrast to the acceleration that occurs when motoneurons are optogenetically excited. This suggests that the V3 neurons do not mediate the excitatory effects of motoneurons on the locomotor CPG, raising the possibility that other—currently unidentified—classes of interneurons are targeted by motoneurons.
Furthermore, it is not clear if glutamate release from the VGLUT2 from motoneurons mediates the excitatory effects of motoneurons because selective elimination of VGLUT2 from cholinergic neurons—including motoneurons—has no effect on locomotor-like activity (Caldeira et al., 2017). However, because this was a chronic study, some type of compensation may have occurred to offset the absence of motoneuronal glutamatergic inputs. Alternatively, glutamate release from motoneurons may not be mediated exclusively by VGLUT2, although it is difficult to support this idea given that the glutamatergic component of the motoneuron–Renshaw synapse is abolished in the VGLUT2 knockout (Talpalar et al., 2011).
In the adult zebrafish, motoneurons have also been shown to modulate locomotion (Song et al., 2016). In this animal, motoneurons have reciprocal hybrid chemical/electrical synapses with a class of excitatory interneurons (V2a) that are believed to be important in generating the swimming rhythm (Eklöf-Ljunggren et al., 2012). Motoneuron membrane polarization can modulate transmitter release from the V2a interneurons by polarizing the V2a terminals on motoneurons. In addition, motoneuron membrane potential can directly modulate the firing of V2a interneurons and the swimming frequency. In the neonatal mouse spinal cord, this mechanism does not appear to be responsible for the effects of motoneuron activity on the rhythm because blockade of gap junctions with carbenoxolone does not attenuate the effect of motoneuron activity on the frequency of the rhythm (Falgairolle et al., 2017). Consistent with this idea, application of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist NBQX, blocked the effects of motoneuron activity on the rhythm, suggesting that motoneuronal connections to the CPG are mediated by an excitatory interneuron contacted by motoneurons (Machacek and Hochman, 2006; Bonnot et al., 2009; Falgairolle et al., 2017). Furthermore, it has also been shown that V2a interneurons do not receive synaptic or electrical inputs from motoneurons in the neonatal mouse (Bhumbra and Beato, 2018).
Synaptic Connections of Motoneurons and Their Susceptibility to Disease
In the final section of this review, we ask if any of the input or output connections of the different motoneuron types, the extraocular motoneurons, and Onuf’s nucleus (Comley et al., 2016; Nijssen et al., 2017) are correlated with their susceptibility to disease.
Several correlations are apparent in the data of Figure 1. For example, the most resistant motoneurons are the smallest, and they receive the fewest and the least diverse synaptic inputs. Thus, within the α-motoneuron population, the number of 1a terminals progressively decreases from type FF to S and are absent on γ-motoneurons, extraocular motoneurons (Keller and Robinson, 1971), and Onuf’s nucleus (Lalancette-Hebert et al., 2016). Similarly, C-boutons are absent on extraocular motoneurons (Hellström et al., 2003; Rozani et al., 2019) and γ-motoneurons (Arvidsson et al., 1987), with a gradient of inputs from type S to type F α-motoneurons (Hellström et al., 2003). However, at least for primary afferents, their presence or absence does not seem to be associated with motoneuron cell death. For instance, although the number of VGLUT1+ primary afferents on motoneurons decreases before motoneuron death, their restoration does not prevent motoneuron death (Fletcher et al., 2017). In ALS, by contrast, ablation of primary afferents exerts a protective effect on α-motoneurons (Lalancette-Hebert et al., 2016). Similarly, although the least susceptible motoneurons lack C-boutons in both diseases, during the progression of ALS, C-boutons become more numerous on vulnerable α-motoneurons, and the number of cholinergic interneurons in Lamina X (presumably the source of C-boutons) increases. Although both decrease toward the end of the disease, the initial changes may reflect compensatory adaptations to maintain motoneuron excitability (Milan et al., 2015). In SMA, the number of cholinergic interneurons does not change (Powis and Gillingwater, 2016), and furthermore, when the C-boutons are restored in the ALS mouse model, they extend survival time (Lasiene et al., 2016), suggesting that the presence of C-boutons and their normal function may facilitate motoneuron survival. Collectively, these observations suggest that the distribution of primary muscle spindle afferents and C-boutons on motoneurons is probably not the factor that contributes to their vulnerability.
A more consistent association emerges when we consider the number of recurrent collaterals produced by the different motoneurons. Those with the greatest number of intraspinal collaterals (type F) are the most susceptible, and those with the fewest γ-motoneurons and extraocular motoneurons are the least (Evinger et al., 1979). About half the motoneurons in Onuf’s nucleus have no recurrent collaterals (Sasaki, 1994). This relationship may also extend to the well-known difference in the sensitivity of proximal and distal muscles to the disease process in both SMA and ALS. In the adult cat, the most distal limb muscles, including many of the foot and forepaw muscles, lack recurrent collaterals (Hörner et al., 1991; McCurdy and Hamm, 1992; Illert and Kümmel, 1999), and recurrent inhibition is much more pronounced in motoneurons innervating the muscles of the elbow than of the wrist (Hahne et al., 1988). Furthermore, motoneurons innervating the intercostal muscles have been shown to have axon collaterals and receive recurrent inhibition (Kirkwood et al., 1981; Lipski and Martin-Body, 1987), and axial motoneurons receive recurrent inhibition (Jankowska and Odutola, 1980), suggesting that they have axon collaterals projecting to RCs as do α-motoneurons. Why would the number of motoneuron collaterals be associated with disease susceptibility? Before motoneurons die, their intraspinal connections to RCs are lost (Wootz et al., 2013) and any functions associated with these connections will also be lost. For example, loss of motoneuron input to the locomotor CPG could compromise locomotion, although this could be compensated by interneurons that also influence locomotor function (Gosgnach et al., 2006; Dougherty et al., 2013; Talpalar et al., 2013; Falgairolle and O’Donovan, 2019b).
It might seem paradoxical that an absence of recurrent collaterals, and presumably Renshaw inhibition, would be associated with a protection against the disease process. Enhanced motoneuron excitability, particularly at early stages of the disease, is often proposed as one of the mechanisms contributing to pathophysiology of motoneurons (Kuo et al., 2004, 2005; Jiang et al., 2017). The Renshaw pathway exerts a powerful inhibitory effect on motoneurons (Moore et al., 2015) and would therefore be expected to temper any increases in motoneuron excitability. However, motoneurons also receive monosynaptic glutamatergic input from other motoneurons and recurrent excitation from V3 glutamatergic interneurons. If motoneuronal inputs to inhibitory RCs are lost before those to motoneurons, this would result in powerful, recurrent glutamatergic excitation of motoneurons unbalanced by recurrent inhibition particularly in the type-F population which receives the strongest excitatory input from other motoneurons (Bhumbra and Beato, 2018). This could lead to glutamate toxicity and a compensatory reduction of motoneuron excitability. Consistent with this suggestion, a reduction of motoneuron excitability in type-F motoneurons is observed to precede denervation in the SOD1-G93A and FUS-P525L mouse models of ALS (Martinez-Silva et al., 2018).
As with their central connections, type FF motoneurons have the most intramuscular synaptic connections (Burke, 1978). Because synapses are energetically demanding (Harris et al., 2012), the FF motoneurons have the highest metabolic demands (Le Masson et al., 2014) which may increase their susceptibility to the disease given that mutant SOD1 can compromise mitochondrial function (Pasinelli et al., 2004).
An alternative and complementary interpretation for the relation between the number of recurrent collaterals and disease susceptibility derives from the idea that a neuron is dependent on all its synaptic targets for trophic support. It is well known that during development, motoneuron survival depends on its target muscle for survival, but as the animal matures, this dependence is reduced (de la Cruz et al., 1996). What is less clear is the extent to which the functions and properties of motoneurons also depend on trophic support from their synaptic targets within the central nervous system. The recent discoveries that spinal motoneurons have novel synaptic targets within the cord mean that trophic support from these neuronal populations has necessarily been underappreciated. According to this idea, as motoneurons disconnect from their synaptic targets within the spinal cord (Wootz et al., 2013), they lose the trophic support normally provided by these targets. The motoneurons lacking recurrent collaterals would thus be resistant to this process because they presumably derive their trophic needs from other sources including the motoneurons themselves and the muscles they innervate. Consistent with this hypothesis, the disease-resistant extraocular muscles express higher levels of neurotrophins than other brainstem neurons that are sensitive to disease (Hernández et al., 2017; Silva-Hucha et al., 2017). Extraocular muscles also contain high levels of insulin-like growth factor (IGF) compared to other cranial or spinal motoneurons (Allodi et al., 2016). Remarkably, IGF-2 delivered by viruses to spinal motoneurons preserves the motoneurons and induces nerve regeneration in ALS (Allodi et al., 2016). It is not known if the different types of α-motoneuron or γ-motoneurons differ in their expression of trophic factors. γ-Motoneurons uniquely express the glial cell-derived neurotrophic factor (GDNF) receptor and require GDNF derived from the muscle spindle for their survival (Shneider et al., 2009). Unfortunately, trials of neurotrophins in humans have not been successful, but this is complicated by difficulties in delivering the molecules to neurons and because the appropriate neurotrophins may not have been discovered (for a review, see Kanning et al., 2010).
One observation that appears to contradict this hypothesis is the finding that motoneuron cell death is associated with an increase in the number of VAChT+ terminals on RCs in the SMNΔ7 model of SMA (Thirumalai et al., 2013). It is not known if these additional synapses originate exclusively from motoneurons. However, if they do, then this behavior differs from the loss of motoneuron terminals on RCs that precedes motoneuron cell death in the SOD1-G93A mouse model of ALS (Wootz et al., 2013). This difference in behavior may reflect the different ages at which motoneurons die in the two diseases. In the SMNΔ7 mouse model, motoneuron cell death begins in the neonatal period when motoneurons are not fully mature and may have an enhanced sprouting ability. It is possible therefore that the sprouting of motoneuron axons, which presumably occurs in the remaining motoneurons, is a characteristic of their immaturity rather than a fundamental difference between the two diseases.
Author Contributions
MO’D and MF wrote and approved the article.
Funding
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Allodi, I., Comley, L., Nichterwitz, S., Nizzardo, M., Simone, C., Benitez, J. A., et al. (2016). Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS. Sci. Rep. 6:25960. doi: 10.1038/srep25960
Appelberg, B., Hulliger, M., Johansson, H., and Sojka, P. (1983a). Actions on gamma-motoneurones elicited by electrical stimulation of group I muscle afferent fibres in the hind limb of the cat. J. Physiol. 335, 237–253. doi: 10.1113/jphysiol.1983.sp014531
Appelberg, B., Hulliger, M., Johansson, H., and Sojka, P. (1983b). Actions on gamma-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. J. Physiol. 335, 255–273. doi: 10.1113/jphysiol.1983.sp014532
Appelberg, B., Hulliger, M., Johansson, H., and Sojka, P. (1983c). Actions on gamma-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. J. Physiol. 335, 275–292. doi: 10.1113/jphysiol.1983.sp014533
Appelberg, B., Hulliger, M., Johansson, H., and Sojka, P. (1983d). Recurrent actions on gamma-motoneurones mediated via large and small ventral root fibres in the cat. J. Physiol. 335, 293–305. doi: 10.1113/jphysiol.1983.sp014534
Arvidsson, U., Svedlund, J., Lagerbäck, P. A., and Cullheim, S. (1987). An ultrastructural study of the synaptology of gamma-motoneurones during the postnatal development in the cat. Brain Res. 465, 303–312. doi: 10.1016/0165-3806(87)90251-3
Bessou, P., Laporte, Y., and Emonetdenand, F. (1963). Occurrence of intrafusal muscle fibres innervation by branches of slow alpha motor fibres in cat. Nature 198, 594–595. doi: 10.1038/198594a0
Bhumbra, G. S., and Beato, M. (2018). Recurrent excitation between motoneurones propagates across segments and is purely glutamatergic. PLoS Biol. 16:e2003586. doi: 10.1371/journal.pbio.2003586
Bonnot, A., Chub, N., Pujala, A., and O’Donovan, M. J. (2009). Excitatory actions of ventral root stimulation during network activity generated by the disinhibited neonatal mouse spinal cord. J. Neurophysiol. 101, 2995–3011. doi: 10.1152/jn.90740.2008
Bowen, J. M., and Bradley, W. E. (1973). Some contractile and electrophysiological properties of external anal-sphincter muscle of cat. Gastroenterology 65, 919–928. doi: 10.1016/s0016-5085(19)32985-3
Branchereau, P., Martin, E., Allain, A. E., Cazenave, W., Supiot, L., Hodeib, F., et al. (2019). Relaxation of synaptic inhibitory events as a compensatory mechanism in fetal SOD spinal motor networks. Elife 8:e51402. doi: 10.7554/eLife.51402
Brännström, T. (1993). Quantitative synaptology of functionally different types of cat medial gastrocnemius alpha-motoneurons. J. Comp. Neurol. 330, 439–454. doi: 10.1002/cne.903300311
Brown, M. C., Crowe, A., and Matthews, P. B. (1965). Observations on the fusimotor fibres of the tibialis posterior muscle of the cat. J. Physiol. 177, 140–159. doi: 10.1113/jphysiol.1965.sp007582
Brownstone, R. M., and Lancelin, C. (2018). Escape from homeostasis: spinal microcircuits and progression of amyotrophic lateral sclerosis. J. Neurophysiol. 119, 1782–1794. doi: 10.1152/jn.00331.2017
Burke, R. E. (1978). Motor units—physiological-histochemical profiles, neural connectivity and functional specializations. Am. Zool. 18, 127–134. doi: 10.1093/icb/18.1.127
Burke, R. E., and Glenn, L. L. (1996). Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J. Comp. Neurol. 372, 465–485. doi: 10.1002/(sici)1096-9861(19960826)372:3<465::aid-cne9>3.0.co;2-0
Burke, R. E., Levine, D. N., and Zajac, F. E. III. (1971). Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science 174, 709–712. doi: 10.1126/science.174.4010.709
Burke, R. E., and Rymer, W. Z. (1976). Relative strength of synaptic input from short-latency pathways to motor units of defined type in cat medial gastrocnemius. J. Neurophysiol. 39, 447–458. doi: 10.1152/jn.1976.39.3.447
Caldeira, V., Dougherty, K. J., Borgius, L., and Kiehn, O. (2017). Spinal Hb9::Cre-derived excitatory interneurons contribute to rhythm generation in the mouse. Sci. Rep. 7:41369. doi: 10.1038/srep41369
Carrasco, D. I., Bichler, E. K., Seburn, K. L., and Pinter, M. J. (2010). Nerve terminal degeneration is independent of muscle fiber genotype in SOD1(G93A) mice. PLoS One 5:e9802. doi: 10.1371/journal.pone.0009802
Chang, Q., and Martin, L. J. (2009). Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice a quantitative confocal analysis. Am. J. Pathol. 174, 574–585. doi: 10.2353/ajpath.2009.080557
Chang, Q., and Martin, L. J. (2011). Motoneuron subtypes show specificity in glycine receptor channel abnormalities in a transgenic mouse model of amyotrophic lateral sclerosis. Channels 5, 299–303. doi: 10.4161/chan.5.4.16206
Chopek, J. W., Nascimento, F., Beato, M., Brownstone, R. M., and Zhang, Y. (2018). Sub-populations of spinal V3 interneurons form focal modules of layered pre-motor microcircuits. Cell Rep. 25, 146.e3–156.e3. doi: 10.1016/j.celrep.2018.08.095
Cleveland, D. W., and Rothstein, J. D. (2001). From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2, 806–819. doi: 10.1038/35097565
Comley, L. H., Nijssen, J., Frost-Nylen, J., and Hedlund, E. (2016). Cross-disease comparison of amyotrophic lateral sclerosis and spinal muscular atrophy reveals conservation of selective vulnerability but differential neuromuscular junction pathology. J. Comp. Neurol. 524, 1424–1442. doi: 10.1002/cne.23917
Conradi, S., Kellerth, J. O., and Berthold, C. H. (1979a). Electron microscopic studies of serially sectioned cat spinal alpha-motoneurons: II. A method for the description of architecture and synaptology of the cell body and proximal dendritic segments. J. Comp. Neurol. 184, 741–754. doi: 10.1002/cne.901840407
Conradi, S., Kellerth, J. O., Berthold, C. H., and Hammarberg, C. (1979b). Electron microscopic studies of serially sectioned cat spinal alpha-motoneurons. IV. Motoneurons innervating slow-twitch (type S) units of the soleus muscle. J. Comp. Neurol. 184, 769–782. doi: 10.1002/cne.901840409
Cullheim, S., and Kellerth, J. O. (1978). A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J. Physiol. 281, 301–313. doi: 10.1113/jphysiol.1978.sp012423
Cullheim, S., Kellerth, J. O., and Conradi, S. (1977). Evidence for direct synaptic interconnections between cat spinal alpha-motoneurons via recurrent axon collaterals—morphological-study using intracellular injection of horseradish-peroxidase. Brain Res. 132, 1–10. doi: 10.1016/0006-8993(77)90702-8
Cullheim, S., Lipsenthal, L., and Burke, R. E. (1984). Direct monosynaptic contacts between type-identified alpha-motoneurons in the cat. Brain Res. 308, 196–199. doi: 10.1016/0006-8993(84)90937-5
Cullheim, S., and Ulfhake, B. (1979). Observations on the morphology of intracellularly stained gamma-motoneurons in relation to their axon conduction-velocity. Neurosci. Lett. 13, 47–50. doi: 10.1016/0304-3940(79)90073-9
Dale, H. (1935). Pharmacology and nerve-endings (walter ernest dixon memorial lecture): (section of therapeutics and pharmacology). Proc. R. Soc. Med. 28, 319–332.
Danner, S. M., Zhang, H., Shevtsova, N. A., Borowska-Fielding, J., Deska-Gauthier, D., Rybak, I. A., et al. (2019). Spinal V3 interneurons and left-right coordination in mammalian locomotion. Front. Cell. Neurosci. 13:516. doi: 10.3389/fncel.2019.00516
de la Cruz, R. R., Pastor, A. M., and Delgado-García, J. M. (1996). Influence of the postsynaptic target on the functional properties of neurons in the adult mammalian central nervous system. Rev. Neurosci. 7, 115–149. doi: 10.1515/revneuro.1996.7.2.115
Dougherty, K. J., Zagoraiou, L., Satoh, D., Rozani, I., Doobar, S., Arber, S., et al. (2013). Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 80, 920–933. doi: 10.1016/j.neuron.2013.08.015
Du Beau, A., Shakya Shrestha, S., Bannatyne, B. A., Jalicy, S. M., Linnen, S., and Maxwell, D. J. (2012). Neurotransmitter phenotypes of descending systems in the rat lumbar spinal cord. Neuroscience 227, 67–79. doi: 10.1016/j.neuroscience.2012.09.037
Eccles, J. C., Eccles, R. M., Iggo, A., and Lundberg, A. (1960). Electrophysiological studies on gamma motoneurones. Acta Physiol. Scand. 50, 32–40. doi: 10.1111/j.1748-1716.1960.tb02070.x
Eccles, J. C., Eccles, R. M., Iggo, A., and Lundberg, A. (1961). Electrophysiological investigations on Renshaw cells. J. Physiol. 159, 461–478. doi: 10.1113/jphysiol.1961.sp006821
Eccles, J. C., Fatt, P., and Koketsu, K. (1954). Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J. Physiol. 126, 524–562. doi: 10.1113/jphysiol.1954.sp005226
Eklöf-Ljunggren, E., Haupt, S., Ausborn, J., Dehnisch, I., Uhlén, P., Higashijima, S., et al. (2012). Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc. Natl. Acad. Sci. U S A 109, 5511–5516. doi: 10.1073/pnas.1115377109
Ellaway, P. H., and Murphy, P. R. (1981). A comparison of the recurrent inhibition of alpha- and gamma-motoneurones in the cat. J. Physiol. 315, 43–58. doi: 10.1113/jphysiol.1981.sp013731
Emonet-Dénand, F., and Laporte, Y. (1975). Proportion of muscles spindles supplied by skeletofusimotor axons (beta-axons) in peroneus brevis muscle of the cat. J. Neurophysiol. 38, 1390–1394. doi: 10.1152/jn.1975.38.6.1390
Evinger, C., Baker, R., and McCrea, R. A. (1979). Axon collaterals of cat medial rectus motoneurons. Brain Res. 174, 153–160. doi: 10.1016/0006-8993(79)90810-2
Falgairolle, M., and O’Donovan, M. J. (2019a). Feedback regulation of locomotion by motoneurons in the vertebrate spinal cord. Curr. Opin. Physiol. 8, 50–55. doi: 10.1016/j.cophys.2018.12.009
Falgairolle, M., and O’Donovan, M. J. (2019b). V1 interneurons regulate the pattern and frequency of locomotor-like activity in the neonatal mouse spinal cord. PLoS Biol. 17:e3000447. doi: 10.1371/journal.pbio.3000447
Falgairolle, M., Puhl, J. G., Pujala, A., Liu, W., and O’Donovan, M. J. (2017). Motoneurons regulate the central pattern generator during drug-induced locomotor-like activity in the neonatal mouse. Elife 6:e26622. doi: 10.7554/eLife.26622
Fletcher, E. V., and Mentis, G. Z. (2017). “Motor circuit dysfunction in spinal muscular atrophy,” in Spinal Muscular Atrophy: Disease Mechanisms and Therapy, eds C. J. Sumner, S. Paushkin and C. P. Ko (London: Elsevier), 153–165.
Fletcher, E. V., Simon, C. M., Pagiazitis, J. G., Chalif, J. I., Vukojicic, A., Drobac, E., et al. (2017). Reduced sensory synaptic excitation impairs motor neuron function via Kv2.1 in spinal muscular atrophy. Nat. Neurosci. 20, 905–916. doi: 10.1038/nn.4561
Fyffe, R. E., and Light, A. R. (1984). The ultrastructure of group Ia afferent fiber synapses in the lumbosacral spinal cord of the cat. Brain Res. 300, 201–209. doi: 10.1016/0006-8993(84)90831-x
Gosgnach, S., Lanuza, G. M., Butt, S. J., Saueressig, H., Zhang, Y., Velasquez, T., et al. (2006). V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440, 215–219. doi: 10.1038/nature04545
Hahne, M., Illert, M., and Wietelmann, D. (1988). Recurrent inhibition in the cat distal forelimb. Brain Res. 456, 188–192. doi: 10.1016/0006-8993(88)90362-9
Harris, J. J., Jolivet, R., and Attwell, D. (2012). Synaptic energy use and supply. Neuron 75, 762–777. doi: 10.1016/j.neuron.2012.08.019
Hellström, J., Oliveira, A. L. R., Meister, B., and Cullheim, S. (2003). Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J. Comp. Neurol. 460, 476–486. doi: 10.1002/cne.10648
Hernández, R. G., Silva-Hucha, S., Morcuende, S., de la Cruz, R. R., Pastor, A. M., and Benitez-Temiño, B. (2017). Extraocular motor system exhibits a higher expression of neurotrophins when compared with other brainstem motor systems. Front. Neurosci. 11:399. doi: 10.3389/fnins.2017.00399
Hörner, M., Illert, M., and Kümmél, H. (1991). Absence of recurrent axon collaterals in motoneurones to the extrinsic digit extensor muscles of the cat forelimb. Neurosci. Lett. 122, 183–186. doi: 10.1016/0304-3940(91)90853-l
Hultborn, H., Katz, R., and Mackel, R. (1988). Distribution of recurrent inhibition within a motor nucleus. II. Amount of recurrent inhibition in motoneurones to fast and slow units. Acta Physiol. Scand. 134, 363–374. doi: 10.1111/j.1748-1716.1988.tb08503.x
Ichinose, T., and Miyata, Y. (1998). Recurrent excitation of motoneurons in the isolated spinal cord of newborn rats detected by whole-cell recording. Neurosci. Res. 31, 179–187. doi: 10.1016/s0168-0102(98)00043-1
Ichiyama, R. M., Brown, J., Edgerton, V. R., and Havton, L. A. (2006). Ultrastructural synaptic features differ between alpha- and gamma-motoneurons innervating the tibialis anterior muscle in the rat. J. Comp. Neurol. 499, 306–315. doi: 10.1002/cne.21110
Illert, M., and Kümmel, H. (1999). Reflex pathways from large muscle spindle afferents and recurrent axon collaterals to motoneurones of wrist and digit muscles: a comparison in cats, monkeys and humans. Exp. Brain Res. 128, 13–19. doi: 10.1007/s002210050812
Jankowska, E., and Odutola, A. (1980). Crossed and uncrossed synaptic actions on moto-neurons of back muscles in the cat. Brain Res. 194, 65–78. doi: 10.1016/0006-8993(80)91319-0
Jiang, M. C., Adimula, A., Birch, D., and Heckman, C. J. (2017). Hyperexcitability in synaptic and firing activities of spinal motoneurons in an adult mouse model of amyotrophic lateral sclerosis. Neuroscience 362, 33–46. doi: 10.1016/j.neuroscience.2017.08.041
Jiang, M., Schuster, J. E., Fu, R., Siddique, T., and Heckman, C. J. (2009). Progressive changes in synaptic inputs to motoneurons in adult sacral spinal cord of a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 29, 15031–15038. doi: 10.1523/jneurosci.0574-09.2009
Kanning, K. C., Kaplan, A., and Henderson, C. E. (2010). Motor neuron diversity in development and disease. Annu. Rev. Neurosci. 33, 409–440. doi: 10.1146/annurev.neuro.051508.135722
Keller, E. L., and Robinson, D. A. (1971). Absence of a stretch reflex in extraocular muscles of the monkey. J. Neurophysiol. 34, 908–919. doi: 10.1152/jn.1971.34.5.908
Kellerth, J. O., Berthold, C. H., and Conradi, S. (1979). Electron microscopic studies of serially sectioned cat spinal alpha-motoneurons: III. Motoneurons innervating fast-twitch (type FR) units of the gastrocnemius muscle. J. Comp. Neurol. 184, 755–767. doi: 10.1002/cne.901840408
Kirkwood, P. A., Sears, T. A., and Westgaard, R. H. (1981). Recurrent inhibition of intercostal motoneurones in the cat. J. Physiol. 319, 111–130. doi: 10.1113/jphysiol.1981.sp013895
Kuffler, S. W., Hunt, C. C., and Quilliam, J. P. (1951). Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J. Neurophysiol. 14, 29–54. doi: 10.1152/jn.1951.14.1.29
Kuo, J. J., Schonewille, M., Siddique, T., Schults, A. N., Fu, R., Bär, P. R., et al. (2004). Hyperexcitability of cultured spinal motoneurons from presymptomatic ALS mice. J. Neurophysiol. 91, 571–575. doi: 10.1152/jn.00665.2003
Kuo, J. J., Siddique, T., Fu, R., and Heckman, C. J. (2005). Increased persistent Na+ current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J. Physiol. 563, 843–854. doi: 10.1113/jphysiol.2004.074138
Lalancette-Hebert, M., Sharma, A., Lyashchenko, A. K., and Shneider, N. A. (2016). Gamma motor neurons survive and exacerbate alpha motor neuron degeneration in ALS. Proc. Natl. Acad. Sci. U S A 113, E8316–E8325. doi: 10.1073/pnas.1605210113
Lamotte d’Incamps, B., and Ascher, P. (2008). Four excitatory postsynaptic ionotropic receptors coactivated at the motoneuron-Renshaw cell synapse. J. Neurosci. 28, 14121–14131. doi: 10.1523/jneurosci.3311-08.2008
Lamotte d’Incamps, B., Bhumbra, G. S., Foster, J. D., Beato, M., and Ascher, P. (2017). Segregation of glutamatergic and cholinergic transmission at the mixed motoneuron Renshaw cell synapse. Sci. Rep. 7:4037. doi: 10.1038/s41598-017-04266-8
Lasiene, J., Komine, O., Fujimori-Tonou, N., Powers, B., Endo, F., Watanabe, S., et al. (2016). Neuregulin 1 confers neuroprotection in SOD1-linked amyotrophic lateral sclerosis mice via restoration of C-boutons of spinal motor neurons. Acta Neuropathol. Commun. 4:15. doi: 10.1186/s40478-016-0286-7
Le Masson, G., Przedborski, S., and Abbott, L. F. (2014). A computational model of motor neuron degeneration. Neuron 83, 975–988. doi: 10.1016/j.neuron.2014.07.001
Le, T. T., Pham, L. T., Butchbach, M. E. R., Zhang, H. L., Monani, U. R., Coovert, D. D., et al. (2005). SMN Delta 7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 14, 845–857. doi: 10.1093/hmg/ddi078
Ling, K. K., Lin, M. Y., Zingg, B., Feng, Z., and Ko, C. P. (2010). Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PLoS One 5:e15457. doi: 10.1371/journal.pone.0015457
Lipski, J., and Martin-Body, R. L. (1987). Morphological properties of respiratory intercostal motoneurons in cats as revealed by intracellular injection of horseradish-peroxidase. J. Comp. Neurol. 260, 423–434. doi: 10.1002/cne.902600308
Machacek, D. W., and Hochman, S. (2006). Noradrenaline unmasks novel self-reinforcing motor circuits within the mammalian spinal cord. J. Neurosci. 26, 5920–5928. doi: 10.1523/jneurosci.4623-05.2006
Manuel, M., and Zytnicki, D. (2011). Alpha, beta and gamma motoneurons: functional diversity in the motor system’s final pathway. J. Integr. Neurosci. 10, 243–276. doi: 10.1142/s0219635211002786
Martin, L. J., and Chang, Q. (2012). Inhibitory synaptic regulation of motoneurons: a new target of disease mechanisms in amyotrophic lateral sclerosis. Mol. Neurobiol. 45, 30–42. doi: 10.1007/s12035-011-8217-x
Martinez-Silva, M. D., Imhoff-Manuel, R. D., Sharma, A., Heckman, C. J., Shneider, N. A., Roselli, F., et al. (2018). Hypoexcitability precedes denervation in the large fast-contracting motor units in two unrelated mouse models of ALS. Elife 7:e30955. doi: 10.7554/elife.30955
Matthews, P. B. (1963). The response of de-efferented muscle spindle receptors to stretching at different velocities. J. Physiol. 168, 660–678. doi: 10.1113/jphysiol.1963.sp007214
McCurdy, M. L., and Hamm, T. M. (1992). Recurrent collaterals of motoneurons projecting to distal muscles in the cat hindlimb. J. Neurophysiol. 67, 1359–1366. doi: 10.1152/jn.1992.67.5.1359
McLaughlin, B. J. (1972a). Dorsal root projections to the motor nuclei in the cat spinal cord. J. Comp. Neurol. 144, 461–473. doi: 10.1002/cne.901440405
McLaughlin, B. J. (1972b). The fine structure of neurons and synapses in the motor nuclei of the cat spinal cord. J. Comp. Neurol. 144, 429–460. doi: 10.1002/cne.901440404
McWilliam, P. N. (1975). The incidence and properties of beta axons to muscle spindles in the cat hind limb. Q. J. Exp. Physiol. Cogn. Med. Sci. 60, 25–36. doi: 10.1113/expphysiol.1975.sp002287
Mentis, G. Z., Alvarez, F. J., Bonnot, A., Richards, D. S., Gonzalez-Forero, D., Zerda, R., et al. (2005). Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc. Natl. Acad. Sci. U S A 102, 7344–7349. doi: 10.1073/pnas.0502788102
Mentis, G. Z., Blivis, D., Liu, W. F., Drobac, E., Crowder, M. E., Kong, L., et al. (2011). Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron 69, 453–467. doi: 10.1016/j.neuron.2010.12.032
Milan, L., Courtand, G., Cardoit, L., Masmejean, F., Barriere, G., Cazalets, J. R., et al. (2015). Age-related changes in pre- and postsynaptic partners of the cholinergic c-boutons in wild-type and SOD1G93A lumbar motoneurons. PLoS One 10:e0135525. doi: 10.1371/journal.pone.0135525
Montes, J., Gordon, A. M., Pandya, S., De Vivo, D. C., and Kaufmann, P. (2009). Clinical outcome measures in spinal muscular atrophy. J. Child Neurol. 24, 968–978. doi: 10.1177/0883073809332702
Moore, N. J., Bhumbra, G. S., Foster, J. D., and Beato, M. (2015). Synaptic connectivity between renshaw cells and motoneurons in the recurrent inhibitory circuit of the spinal cord. J. Neurosci. 35, 13673–13686. doi: 10.1523/jneurosci.2541-15.2015
Nijssen, J., Comley, L. H., and Hedlund, E. (2017). Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathol. 133, 863–885. doi: 10.1007/s00401-017-1708-8
Nishimaru, H., Restrepo, C. E., Ryge, J., Yanagawa, Y., and Kiehn, O. (2005). Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc. Natl. Acad. Sci. U S A 102, 5245–5249. doi: 10.1073/pnas.0501331102
Ornung, G., Ragnarson, B., Grant, G., Ottersen, O. P., Storm-Mathisen, J., and Ulfhake, B. (1995). Ia boutons to CCN neurones and motoneurones are enriched with glutamate-like immunoreactivity. Neuroreport 6, 1975–1980. doi: 10.1097/00001756-199510010-00006
Pasinelli, P., Belford, M. E., Lennon, N., Bacskai, B. J., Hyman, B. T., Trotti, D., et al. (2004). Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron 43, 19–30. doi: 10.1016/j.neuron.2004.06.021
Powis, R. A., and Gillingwater, T. H. (2016). Selective loss of alpha motor neurons with sparing of gamma motor neurons and spinal cord cholinergic neurons in a mouse model of spinal muscular atrophy. J. Anat. 228, 443–451. doi: 10.1111/joa.12419
Pujala, A., Blivis, D., and O’Donovan, M. J. (2016). Interactions between dorsal and ventral root stimulation on the generation of locomotor-like activity in the neonatal mouse spinal cord. eNeuro 3:ENEURO.0101-16.2016. doi: 10.1523/eneuro.0101-16.2016
Renshaw, B. (1946). Central effects of centripetal impulses in axons of spinal ventral roots. J. Neurophysiol. 9, 191–204. doi: 10.1152/jn.1946.9.3.191
Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., et al. (1993). Mutations in Cu/Zn superoxide-dismutase gene are associated with familial amyotrophic-lateral-sclerosis. Nature 362, 59–62. doi: 10.1038/362059a0
Rozani, I., Tsapara, G., Witts, E. C., Deaville, S. J., Miles, G. B., and Zagoraiou, L. (2019). Pitx2 cholinergic interneurons are the source of C bouton synapses on brainstem motor neurons. Sci. Rep. 9:4936. doi: 10.1038/s41598-019-39996-4
Sasaki, M. (1994). Morphological analysis of external urethral and external anal sphincter motoneurones of cat. J. Comp. Neurol. 349, 269–287. doi: 10.1002/cne.903490209
Schneider, S. P., and Fyffe, R. E. (1992). Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J. Neurophysiol. 68, 397–406. doi: 10.1152/jn.1992.68.2.397
Schütz, B. (2005). Imbalanced excitatory to inhibitory synaptic input precedes motor neuron degeneration in an animal model of amyotrophic lateral sclerosis. Neurobiol. Dis. 20, 131–140. doi: 10.1016/j.nbd.2005.02.006
Seki, S., Yamamoto, T., Quinn, K., Spigelman, I., Pantazis, A., Olcese, R., et al. (2019). Circuit-specific early impairment of proprioceptive sensory neurons in the SOD1(G93A) mouse model for ALS. J. Neurosci. 39, 8798–8815. doi: 10.1523/jneurosci.1214-19.2019
Shneider, N. A., Brown, M. N., Smith, C. A., Pickel, J., and Alvarez, F. J. (2009). Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural Dev. 4:42. doi: 10.1186/1749-8104-4-42
Silva-Hucha, S., Hernandez, R. G., Benitez-Temino, B., Pastor, A. M., De La Cruz, R. R., and Morcuende, S. (2017). Extraocular motoneurons of the adult rat show higher levels of vascular endothelial growth factor and its receptor Flk-1 than other cranial motoneurons. PLoS One 12:e0178616. doi: 10.1371/journal.pone.0178616
Simon, M., Destombes, J., Horchollebossavit, G., and Thiesson, D. (1996). Postnatal development of alpha- and gamma-peroneal motoneurons in kittens: An ultrastructural study. Neurosci. Res. 25, 77–89. doi: 10.1016/0168-0102(96)01030-9
Song, J., Ampatzis, K., Bjornfors, E. R., and El Manira, A. (2016). Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature 529, 399–402. doi: 10.1038/nature16497
Talpalar, A. E., Bouvier, J., Borgius, L., Fortin, G., Pierani, A., and Kiehn, O. (2013). Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500, 85–88. doi: 10.1038/nature12286
Talpalar, A. E., Endo, T., Low, P., Borgius, L., Hagglund, M., Dougherty, K. J., et al. (2011). Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron 71, 1071–1084. doi: 10.1016/j.neuron.2011.07.011
Thirumalai, V., Behrend, R. M., Birineni, S., Liu, W., Blivis, D., and O’Donovan, M. J. (2013). Preservation of VGLUT1 synapses on ventral calbindin-immunoreactive interneurons and normal locomotor function in a mouse model of spinal muscular atrophy. J. Neurophysiol. 109, 702–710. doi: 10.1152/jn.00601.2012
Uchizono, K. (1965). Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature 207, 642–643. doi: 10.1038/207642a0
Vaughan, S. K., Kemp, Z., Hatzipetros, T., Vieira, F., and Valdez, G. (2015). Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J. Comp. Neurol. 523, 2477–2494. doi: 10.1002/cne.23848
Wee, C. D., Kong, L. L., and Sumner, C. J. (2010). The genetics of spinal muscular atrophies. Curr. Opin. Neurol. 23, 450–458. doi: 10.1097/WCO.0b013e32833e1765
Westbury, D. R. (1982). A comparison of the structures of alpha and gamma-spinal motoneurones of the cat. J. Physiol. 325, 79–91. doi: 10.1113/jphysiol.1982.sp014137
Wootz, H., Fitzsimons-Kantamneni, E., Larhammar, M., Rotterman, T. M., Enjin, A., Patra, K., et al. (2013). Alterations in the motor neuron-renshaw cell circuit in the Sod1(G93A) mouse model. J. Comp. Neurol. 521, 1449–1469. doi: 10.1002/cne.23266
Yu, C. Y., Man, W., Chinnery, P. F., and Griffiths, P. G. (2005). Extraocular muscles have fundamentally distinct properties that make them selectively vulnerable to certain disorders. Neuromuscul. Disord. 15, 17–23. doi: 10.1016/j.nmd.2004.10.002
Zagoraiou, L., Akay, T., Martin, J. F., Brownstone, R. M., Jessell, T. M., and Miles, G. B. (2009). A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64, 645–662. doi: 10.1016/j.neuron.2009.10.017
Keywords: locomotion, spinal muscular atrophy, amyotrophic lateral sclerosis, central pattern generator, recurrent collaterals
Citation: Falgairolle M and O’Donovan MJ (2020) Motoneuronal Spinal Circuits in Degenerative Motoneuron Disease. Front. Mol. Neurosci. 13:74. doi: 10.3389/fnmol.2020.00074
Received: 21 February 2020; Accepted: 15 April 2020;
Published: 25 May 2020.
Edited by:
George Mentis, Columbia University, United StatesReviewed by:
Francisco Javier Alvarez, Emory University, United StatesAngel M. Pastor, University of Seville, Spain
Marco Beato, University College London, United Kingdom
Copyright © 2020 Falgairolle and O’Donovan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. O’Donovan, odonovm@ninds.nih.gov
 Mélanie Falgairolle
Mélanie Falgairolle Michael J. O’Donovan
Michael J. O’Donovan