Acceptability of Iron- and Zinc-Biofortified Pearl Millet (ICTP-8203)-Based Complementary Foods among Children in an Urban Slum of Mumbai, India
- 1Division of Nutritional Sciences, Cornell University, Ithaca, NY, United States
- 2Department of Food Science and Nutrition, SNDT Women’s University, Mumbai, India
- 3St. John’ Research Institute, Division of Nutrition, Bangalore, India
- 4Institute for Nutritional Sciences, Global Health, and Technology (INSiGHT), Cornell University, Ithaca, NY, United States
Biofortification, a method for increasing micronutrient content of staple crops, is a promising strategy for combating major global health problems, such as iron and zinc deficiency. We examined the acceptability of recipes prepared using iron- and zinc-biofortified pearl millet (FeZnPM) (~80 ppm Fe, ~34 ppm Zn, varietal ICTP-8203), compared to conventional pearl millet (CPM) (~20 ppm Fe, ~19 ppm Zn) in preparation for an efficacy trial. Our objective was to examine the acceptability of FeZnPM compared to CPM among young children and mothers living in the urban slums of Mumbai. Standardized traditional feeding program recipes (n = 18) were prepared with either FeZnPM or CPM flour. The weight (g) of each food product was measured before and after consumption by children (n = 125) and the average grams consumed over a 3-day period were recorded. Mothers (n = 60) rated recipes using a 9-point hedonic scale. Mean intakes and hedonic scores of each food product were compared using t-tests across the two types of pearl millet. There were no statistically significant differences in consumption by children (FeZnPM: 25.27 ± 13.0 g; CPM: 21.72 ± 6.90 g) across the food products (P = 0.28). Overall mean hedonic scores for all recipes were between 7 to 9 points. CPM products were rated higher overall (8.22 ± 0.28) compared to FeZnPM products (7.95 ± 0.35) (P = 0.01). FeZnPM and CPM were similarly consumed and had high hedonic scores, demonstrating high acceptability in this population. These results support using these varieties of pearl millet in a proposed trial [http://Clinicaltrials.gov ID: NCT02233764; Clinical Trials Registry of India (CTRI), reference number REF/2014/10/007731, CTRI number CTRI/2015/11/006376] testing the efficacy of FeZnPM for improving iron status and growth.
Introduction
Iron deficiency is the most common micronutrient deficiency in the world and particularly impacts child health in resource-limited settings such as urban slums in India (1). With or without anemia, low iron status impairs growth (2), immune (3), and cognitive functions (4) in infants and young children. Preliminary data from children in our study population in Mumbai showed that iron intake was 35% that of the Indian RDA (5, 6) and that nearly 60% of children were iron deficient (serum ferritin < 12 ng/mL) (7). Zinc deficiency affects nearly 2 billion people worldwide and is associated with short stature, delayed development, and increased morbidity particularly from diarrhea (8). Collectively, these deficits, particularly during the first 1,000 days of life, result in long-term health consequences such as poor school performance and decreased work capacity (9).
Biofortification of staple crops with iron is a sustainable agricultural approach that can help prevent and treat iron deficiency in vulnerable populations (10). In the process of biofortification, the concentration and bioavailability of essential micronutrients in staple food crops is increased by traditional plant breeding techniques (11, 12). Pearl millet (Pennisetum glaucum, and locally known as bajra in Hindi) is a nutritious, hardy, drought-tolerant grain that is a staple of the traditional diet in many areas of India including Maharashtra, Gujarat, and Rajasthan, where per-capita consumption is 80–100 g (10, 13).
A locally grown variety of iron- and zinc-biofortified pearl millet (ICTP-8203 or FeZnPM) with four times higher iron concentration than conventional pearl millet (CPM) has the potential to improve iron status in these populations (10, 14). Traditionally, pearl millet is ground into flour, roasted, and consumed in the form of non-leavened breads called bhakri or roti as part of the daily diet. ICTP-8203, shown to have comparable if not higher yield than conventional varieties (15), has demonstrated efficacy in improving iron status in older children who consumed bhakri twice daily for 6 months (10, 16). However, flatbreads like bhakri are not ideal weaning or complementary foods for young children and infants to consume due to their tougher and chewier texture. The main objective of this study was to formulate and test the acceptability (in terms of volume consumed and sensory characteristics) of new pearl millet-based palatable complementary food products for weaning infants. The food products with highest acceptability would be ideal candidates for a randomized controlled trial testing the efficacy of biofortified pearl millet for improving iron status in infants and young children.
Materials and Methods
Intervention
Pearl millets CPM and ICTP-8203 were transported from Maharashtra and Gujarat, respectively, in 50-kg gunny bags and stored in a climate-controlled space (humidity level < 50%, 25°C). Pearl millet was transferred from the gunny bags into steel canisters and transported to SNDT Women’s University, Mumbai, India, for storage and preparation. The concentrations of iron, zinc, and aluminum in whole grain were quantified after nitric acid digestion using inductively coupled plasma optical emission spectrometry (ICP-OES) at the International Crops Research Institute for the Semi-Arid Tropics research laboratory in Telangana, India. Confirmatory testing in the USDA-ARS lab at Cornell University (courtesy, Dr. Ray Glahn), was used to test for iron and zinc concentrations, along with assessment of potential contamination via aluminum concentrations using ICP-OES.
Recipe Formulation and Production
Ingredients and formulations were based on traditional weaning and complementary foods in this region. The teaching food laboratory at SNDT Women’s University was utilized for recipe development. The recipes were prepared by Master of Science in Food, Science, and Nutrition students and staff from the department of Food Science and Nutrition under the guidance of the study investigators and community coordinator. Standard hygiene and sanitation procedures were maintained throughout.
Study Setting
The study site was a feeding center within a large slum known locally as Nehru Nagar, in Vile Parle, a suburb in Mumbai. The feeding center was a room generally used for Integrated Child Development Services activities (17). This room was equipped with electricity and fans.
Study Design and Protocol
This study was conducted from January to December 2015. Mothers were given an information sheet describing the pearl millet crop and its potential health benefits. Community health workers explained to mothers that they would be given foods prepared using both high-iron/zinc and conventional pearl millets to consume.
Based on preliminary data indicating preferred times of day for feeding, mothers were requested to come to the feeding center between 11:00 a.m. and 5:00 p.m. each day to maximize participation. If a child refused food or if mothers needed to leave before the child finished, mothers were requested to return to the center to continue feeding. Mothers were also encouraged to come between breast-feeding periods to encourage consumption of the complementary food. Participants consumed each food product over a 3-day period to allow the child to become familiar with any potential new tastes or flavors. First, all CPM products were tested for acceptability (days 1–3), followed by all FeZnPM products (days 4–6).
Research assistants weighed each food product before and after feeding to the nearest 0.01 g to determine the net number of grams consumed of each food product. Both research assistants and community health workers assisted mothers with feeding, if needed. The proportion of pearl millet consumed in each food product was then back-calculated via the proportion of pearl millet initially used to prepare the given recipe.
Concurrently with their infants, mothers were also given a sample of each food on the day of its introduction and were asked to rate its acceptability using a 9-point hedonic scale while a research assistant assisted with feeding their child. The hedonic score was based on a 9-point scale (1 = lowest rating, “dislike extremely”; 9 = highest rating, “like extremely”) similar to scales used in previous studies of consumer acceptance and assessed four parameters: taste, smell, color, and overall acceptability (18–20).
Anthropometry
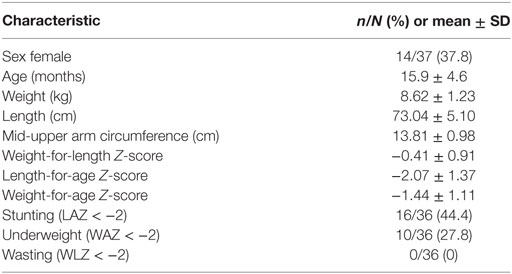
Mothers of N = 38 children consented to anthropometric measurements. Recumbent length (centimeters) (Meditrin Infantometer, Mumbai, India), weight (kilograms) (Meditrin scale model 385, Mumbai, India), and mid-upper-arm circumference (centimeters) (Hardik Meditech model #HM009, New Delhi, India) were measured by trained study personnel.
Ethical Approval
Intersystems Biomedica Ethics Committee, Mumbai, India, approved the study protocol (ISBEC/NR-17/KM-JVJ/2014). The nature of the study was explained to the mothers and written informed consent was obtained from the mothers or the caregivers. All participants gave written informed consent.
Data Analysis
Two outcome variables in this study were used: the average net number of grams of pearl millet consumed in each food product among children and the mean hedonic score for each food product among mothers. To compare the mean intake of each pearl millet variety as a proportion of the total food product, as well as mean hedonic scores, data were first tested for normality. Because data were not normally distributed, results from the non-parametric Hodges–Lehmann–Sen test were compared with results from two-sample t-tests. Results were similar, thus we report means and t-tests here for comparability with other publications. Anthropometric z-scores [weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length (WLZ)] were calculated using World Health Organization guidelines; underweight, stunting, and wasting were defined as WAZ < −2, LAZ < −2, and WLZ < −2 SDs from the WHO reference standard, respectively (21). SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all analysis.
Results
Development of Pearl Millet Products
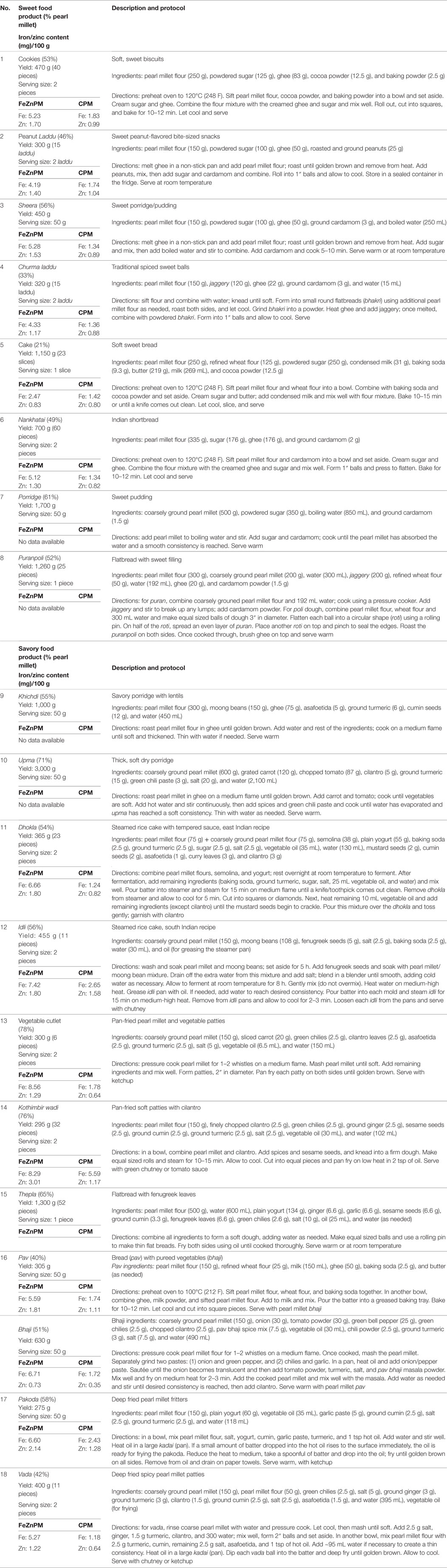
A total of 18 food products were tested in an open cohort of 125 children aged 6–24 months living in Nehru Nagar, Vile Parle, Mumbai. These food products included freshly prepared and shelf stable foods, both sweet (#1–8) and savory (#9–18) recipes (Table 1). We formulated recipes appropriate to various stages of development, i.e., a softer porridge-like consistency food product (khichdi) for very young children and more solid foods such as pakoda and thepla for older children. Iron and zinc concentration in the biofortified pearl millet was 82.74 and 34.17 ppm, respectively; in the conventional pearl millet, iron and zinc concentration were 21.24 and 19.34 ppm, respectively, and aluminum concentrations indicated low levels of contamination (<10 ppm per varietal).
Acceptability: Infants and Mothers
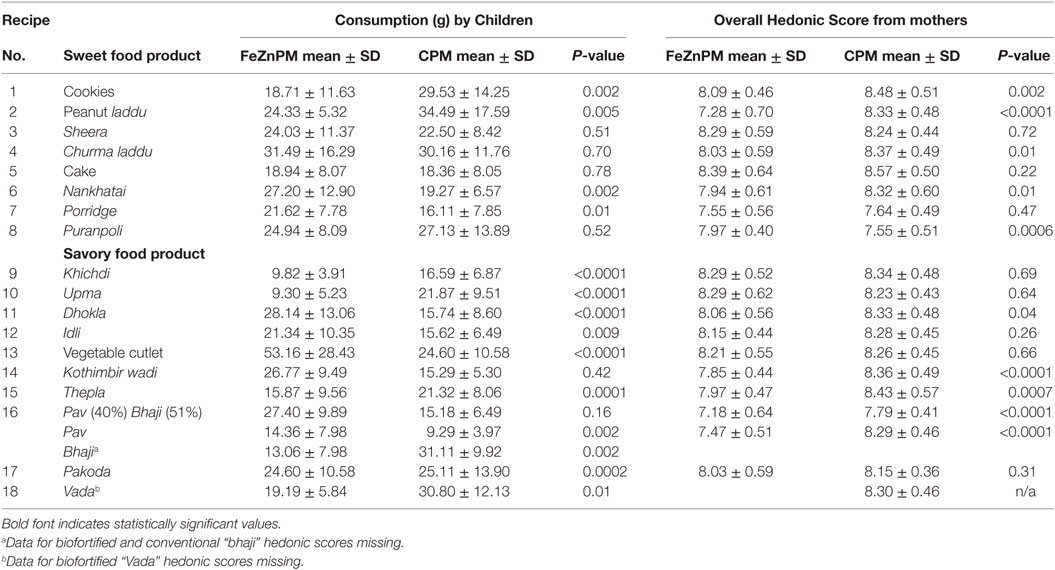
Population characteristics of an N = 38 subset of children may be found in Table 2. No statistically significant differences in infants’ overall mean consumption of foods developed from ICTP-8203-Fe PM (mean ± SD; 25.3 ± 13.0 g), were found, compared to the conventional pearl millet (21.7 ± 6.9 g) (P = 0.28) (Table 3). There was no difference in mean grams consumed from either sweet or savory food products in the CPM (P = 0.1) and FeZnPM (P = 0.6) groups. FeZnPM products with the highest acceptability among children included churma laddu (31.5 g), vegetable cutlet (53.2 g), and dhokla (28.1 g), while the least-consumed FeZnPM food products included upma (9.3 g), khichdi (9.8 g), and pav (14.4 g).
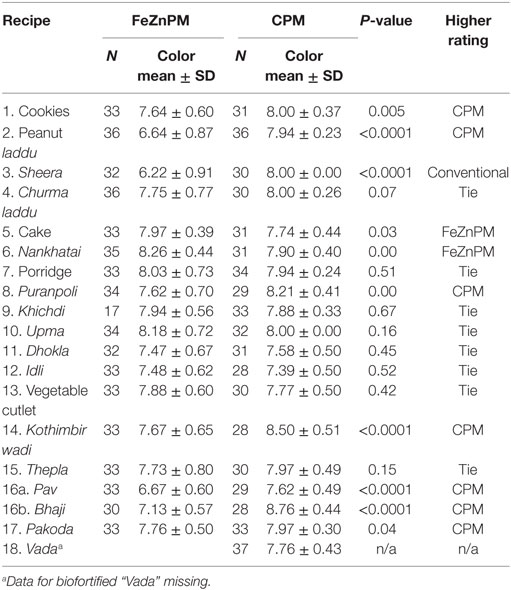
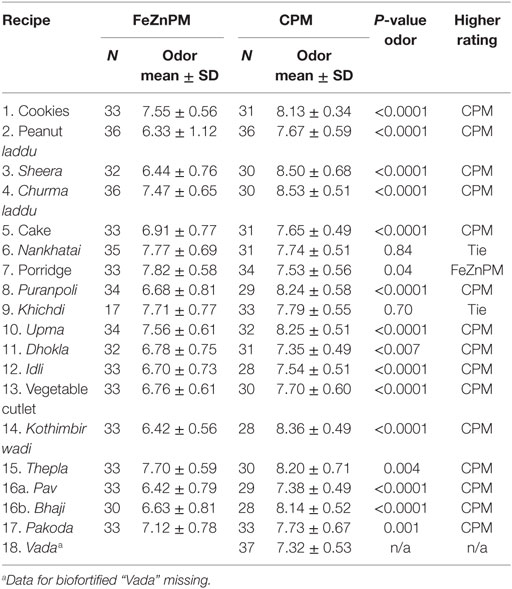
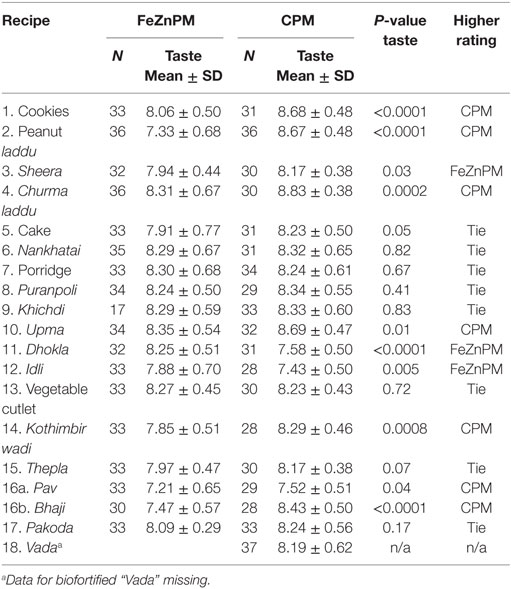
Mothers gave higher hedonic scores to foods made with CPM (“Overall” mean hedonic score: 8.22) compared to FeZnPM (“Overall” mean hedonic score: 7.95; P = 0.01) but had no preference or preferred FeZnPM in terms of color, taste, and overall score in at least 50% of recipes (Tables 3–6). In contrast, mothers tended to give higher ratings to CPM in terms of Odor (Table 5). For specific food items, Mothers gave the highest hedonic ratings to churma laddu, upma, and nankhatai (Table 3).
Anthropometry
Data for sex and age were missing from two participants; therefore, anthropometric z-scores were calculated for the remaining sample of n = 36. On average, mean anthropometric z-scores were below the reference standard for weight-for-length, length-for-age, or weight-for-age (Table 2). Stunting (LAZ < −2) affected nearly half of the participants, while 27% of participants were underweight (WAZ < −2).
Discussion
The present study is the first to develop several pearl millet-based complementary foods and to assess the acceptability of these food products among infants living in the urban slums of Mumbai, India. The acceptability of complementary food products was primarily determined by each child’s 3-day mean food intake along with mothers’ hedonic scores. Complementary food products developed from both varieties of pearl millet were highly accepted by children and their mothers.
There are limited studies on the acceptability of biofortified pearl millet-based complementary foods among young children (22). A recently published randomized controlled trial from our research group studied the effect of consuming biofortified pearl millet on iron status indicators compared to conventional pearl millet in school children from Maharashtra, India (10). These school children regularly consumed pearl millet-based unleavened bread (bhakri) for 6 months. Similar to our findings, no significant differences were observed between the consumption of biofortified and conventional pearl millet, and consumption of bhakri was high, indicating the high acceptance by school children (10). In a hospital-based feeding trial from southern India, the investigators studied the absorption of iron and zinc from biofortified pearl millet-based complementary foods (sheera, upma, and roti) compared to the same food products prepared using conventional pearl millet in young children (22–35 months of age) (16, 23). Children consumed an average of ~60 g pearl millet flour per day, again indicating the acceptance of pearl millet-based food products in 22- to 35-month-old children. Acceptability of other biofortified crops such as cassava has also been tested in young children and their caregivers (22). An acceptability study from Kenya compared the sensory acceptability of the consumption of foods prepared using biofortified cassava vs. white cassava in school children (7–12 years of age) and their caregivers (24). This study demonstrated that the biofortified cassava was well-accepted by the children and their caregivers compared to white cassava (24).
Consistent with our results, high acceptability of cereal and lipid-based complementary foods by toddlers was observed in other feeding studies from Peru (25) and Ghana (26). In our study, though the overall hedonic score responses from the mothers showed that CPM was preferred to FeZnPM, the quantitative data demonstrate that the consumption and acceptability of complementary foods prepared with both FeZnPM and CPM were equally high in this population. Similar hedonic score responses were observed in other studies from Peru (25) and Ghana (26) indicating that mothers’ responses can be biased, and direct measure of food consumption by the toddlers are more conclusive evidence of food acceptability, as reported in Bangladesh (27).
We observed variability in consumption volume among children, which may be attributed to the age range included (12–18 months) wherein some children are accustomed to consuming solid food (thereby eating more) while others are just beginning complementary feeding (who may still be breastfeeding, limiting their capacity for the pearl millet). This variability may be minimized by increasing the sample size in future acceptability studies, or excluding children who have already started complementary feeding.
In the preliminary phase of acceptability study, we observed some limitations including mothers’ refusal to feed their children in the presence of other caregivers or in a group due to presence of existing socio-cultural myths and taboos. Another important limitation we observed was that some mothers fed their child at home before bringing them to the feeding center as the child were reported to be very hungry. If the child refused to eat the study food product, mothers tended to breastfeed them which may have influenced their child’s hunger levels and consumption of the study food products.
In this study, we determined which biofortified pearl millet food products are the most accepted and would, therefore, have a higher likelihood of being consumed as part of the daily diet among young children. For example, churma laddu (described in Table 1) made with FeZnPM variety ICTP-8203 was most accepted by both mothers and infants. This indicates that similar recipes would be ideal candidates for inclusion in the proposed randomized controlled feeding trial (ClinicalTrials.gov ID: NCT02233764; Clinical Trials Registry of India (CTRI), reference number REF/2014/10/007731, CTRI number CTRI/2015/11/006376) to test the efficacy of consuming iron- and zinc-biofortified pearl millet like ICTP-8203 in improving iron status, growth, immune function, and cognition among young children.
Ethics Statement
This study was carried out in accordance with the recommendations of Intersystem Biomedica Ethics Committee (ISBEC) (Ethic Committee Registration No. ECR/108/Indt/MH/2013) with written informed consent from all participants. All participants gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Intersystem Biomedica Ethics Committee (ISBEC).
Author Contributions
SM, JH, JF, SU, PG, and AK contributed to the concept and design of the work; VT, AS, and AT contributed to acquisition of data for the work; SH analyzed the data; SH, SV, SM, JH, JF, SU, PG, and AK interpreted the data; SH and SV wrote the initial draft of the manuscript; all authors contributed to critical revisions of the work; all authors gave final approval of the version to be published, SM had primary responsibility for the final content; all authors are in agreement that they will be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
SM is an unpaid board member for a diagnostic start up focused on developing point-of-care assays for nutritional status informed by his research as a faculty member at Cornell University. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the mothers and children participating in this study and the Community Health Workers.
Funding
This work was supported by HarvestPlus, through grant number 2014H8302 awarded to Cornell University.
References
1. Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet (2016) 387:907–16. doi:10.1016/S0140-6736(15)60865-0
2. Vucic V, Berti C, Vollhardt C, Fekete K, Cetin I, Koletzko B, et al. Effect of iron intervention on growth during gestation, infancy, childhood, and adolescence: a systematic review with meta-analysis. Nutr Rev (2013) 71:386–401. doi:10.1111/nure.12037
3. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr (2001) 131:568S–79S; discussion 580S.
4. Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr (2011) 141:740S–6S. doi:10.3945/jn.110.131169
5. I.C.M.R. Nutrient Requirements and Recommended Dietary Allowances for Indians. Expert Group of the Indian Council of Medical Research, editor. Hyderabad: National Institute of Nutrition, Indian Council of Medical Research (2009).
6. Venkatramanan S, Haas JD, Finkelstein JL, Salvi A, Thorat A, Huey SL, et al. Dietary intake and nutritional status in infants from the urban slums of Mumbai, India. FASEB J (2016) 30(1 Suppl):891.14
7. Huey SL, Powis LE, Venkatramanan S, Udipi SA, Finkelstein JL, Ghugre P, et al. Pre-intervention characterization of nutritional status to estimate burden and potential to benefit among mothers and their children living in urban slums of Mumbai, India. International Congress of Nutrition (Forthcoming). Buenos Aires: International Union of Nutritional Sciences (2017).
8. Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr (2003) 133:1485S–9S.
9. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet (2013) 382:427–51. doi:10.1016/S0140-6736(13)60937-X
10. Finkelstein JL, Mehta S, Udipi SA, Ghugre PS, Luna SV, Wenger MJ, et al. A randomized trial of iron-biofortified pearl millet in school children in India. J Nutr (2015) 145:1576–81. doi:10.3945/jn.114.208009
11. Bouis HE. Plant breeding: a new tool for fighting micronutrient malnutrition. J Nutr (2002) 132:491S–4S.
12. Nestel P, Bouis HE, Meenakshi JV, Pfeiffer W. Biofortification of staple food crops. J Nutr (2006) 136:1064–7.
13. Basavaraj G, Rao PP, Bhagavatula S, Ahmed W. Availability and utilization of pearl millet in India. J SAT Agric Res (2010) 8:1–13.
14. Finkelstein JL, Haas JD, Mehta S. Iron-biofortified staple food crops for improving iron status: a review of the current evidence. Curr Opin Biotechnol (2017) 44:138–45. doi:10.1016/j.copbio.2017.01.003
15. I.C.R.I.S.A.T. Pearl millet open-pollinated variety ICTP 8203. Plant Material Description No. 18. International Crops Research Institute for the Semi-Arid Tropics (1989). p. 1–4.
16. Kodkany BS, Bellad RM, Mahantshetti NS, Westcott JE, Krebs NF, Kemp JF, et al. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J Nutr (2013) 143:1489–93. doi:10.3945/jn.113.176677
17. Prinja S, Verma R, Lal S. Role of ICDS program in delivery of nutritional services and functional integration between anganwadi and health worker in north India. J Nutr Wellness (2008) 5:8–15.
18. Tomlins K, Manful J, Gayin J, Kudjawu B, Tamakloe I. Study of sensory evaluation, consumer acceptability, affordability and market price of rice. J Sci Food Agric (2007) 87:1564–75. doi:10.1002/jsfa.2889
19. Shamim AA, Hanif AA, Merrill RD, Campbell RK, Kumkum MA, Shaikh S, et al. Preferred delivery method and acceptability of Wheat-Soy Blend (WSB++) as a daily complementary food supplement in northwest Bangladesh. Ecol Food Nutr (2015) 54:74–92. doi:10.1080/03670244.2014.930030
20. Banerji A, Birol E, Karandikar B, Rampal J. Information, branding, certification, and consumer willingness to pay for high-iron pearl millet: evidence from experimental auctions in Maharashtra, India. Food Policy (2016) 62:133–41. doi:10.1016/j.foodpol.2016.06.003
21. WHO. WHO child growth standards and the identification of severe acute malnutrition in infants and children. A Joint Statement by the WHO and UNICEF. Geneva: WHO, UNICEF (2009).
22. Garcia-Casal MN, Pena-Rosas JP, Giyose B; Consultation Working Group. Staple crops biofortified with increased vitamins and minerals: considerations for a public health strategy. Ann N Y Acad Sci (2016) 1:1–11. doi:10.1111/nyas.13293
23. Kodkany B, Bellad R, Mahantshetti N, Westcott J, Krebs N, Kemp J, et al. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorbtion of these minerals above physiologic requirements in young children – erratum. J Nutr (2013) 143:2055. doi:10.3945/jn.113.176677
24. Talsma EF, Melse-Boonstra A, De Kok BP, Mbera GN, Mwangi AM, Brouwer ID. Biofortified cassava with pro-vitamin A is sensory and culturally acceptable for consumption by primary school children in Kenya. PLoS One (2013) 8:e73433. doi:10.1371/journal.pone.0073433
25. Pachon H, Dominguez MR, Creed-Kanashiro H, Stoltzfus RJ. Acceptability and safety of novel infant porridges containing lyophilized meat powder and iron-fortified wheat flour. Food Nutr Bull (2007) 28:35–46. doi:10.1177/156482650702800104
26. Adu-Afarwuah S, Lartey A, Zeilani M, Dewey KG. Acceptability of lipid-based nutrient supplements (LNS) among Ghanaian infants and pregnant or lactating women. Matern Child Nutr (2011) 7:344–56. doi:10.1111/j.1740-8709.2010.00286.x
Keywords: pearl millet, ICTP-8203, iron, iron deficiency, biofortification, acceptability, children
Citation: Huey SL, Venkatramanan S, Udipi SA, Finkelstein JL, Ghugre P, Haas JD, Thakker V, Thorat A, Salvi A, Kurpad AV and Mehta S (2017) Acceptability of Iron- and Zinc-Biofortified Pearl Millet (ICTP-8203)-Based Complementary Foods among Children in an Urban Slum of Mumbai, India. Front. Nutr. 4:39. doi: 10.3389/fnut.2017.00039
Received: 10 May 2017; Accepted: 31 July 2017;
Published: 25 August 2017
Edited by:
Michael A. Grusak, USDA-ARS Children’s Nutrition Research Center, United StatesReviewed by:
Alexander Arthur Theodore Johnson, University of Melbourne, AustraliaJames Stangoulis, Flinders University, Australia
Copyright: © 2017 Huey, Venkatramanan, Udipi, Finkelstein, Ghugre, Haas, Thakker, Thorat, Salvi, Kurpad and Mehta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saurabh Mehta, smehta@cornell.edu
†Joint first authorship.
 Samantha Lee Huey
Samantha Lee Huey Sudha Venkatramanan
Sudha Venkatramanan Shobha A. Udipi
Shobha A. Udipi Julia Leigh Finkelstein
Julia Leigh Finkelstein Padmini Ghugre2
Padmini Ghugre2
 Varsha Thakker
Varsha Thakker Saurabh Mehta
Saurabh Mehta