- 1State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Sun Yat-sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 2Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 4Department of Radiation Oncology, Sichuan Cancer Center, School of Medicine, Sichuan Cancer Hospital & Institute, University of Electronic Science and Technology of China, Chengdu, China
- 5Department of Radiotherapy, Sun Yat-sen University Cancer Center, Guangzhou, China
- 6Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China
Background: Nasopharyngeal carcinoma (NPC) patients receiving concurrent chemoradiotherapy (CCRT) frequently develop low skeletal muscle mass (SMM), but, little is known about the impacts of low SMM on health-related quality of life (QOL).
Methods: We retrospectively assessed 56 patients with locoregionally advanced NPC enrolled in a prospective trial. Low SMM was determined on routine computed tomography simulation (CT-sim) scans taken before radiotherapy, at the third cervical (C3) vertebral level with validated sex-specific cutoffs. QOL was assessed using the World Health Organization Quality of Life Questionnaire-100 at baseline and after 3 weeks. Pain was scored every 24 h using a numerical rating scale (NRS). Characteristics related to low SMM were identified by logistic regression. The chi-square test was used to examine the association of low SMM with QOL and pain.
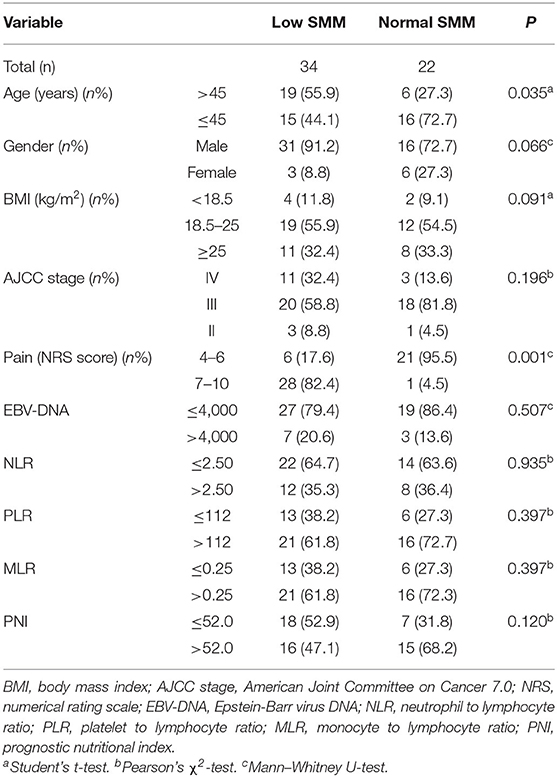
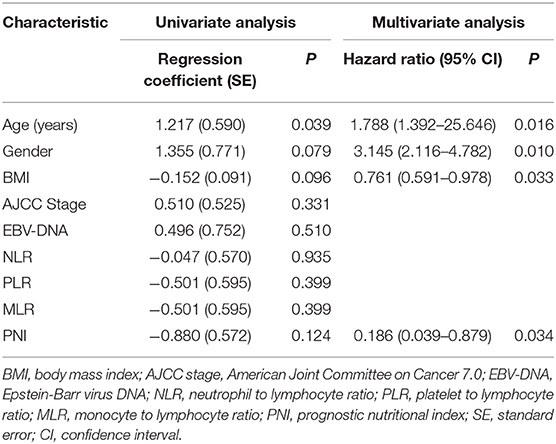
Results: Of the 56 participants (mean age 44.20 ± 10.93 years), over half (60.71%) developed low SMM. Patients with low SMM were more likely to be older (P = 0.035), male (P = 0.066), have a lower body-mass index (BMI; P = 0.091), and have a higher pain score (P = 0.001). Older age (hazard ratio [HR] = 1.788, P = 0.016), being male (HR = 3.145, P = 0.010), lower BMI (HR = 0.761, P = 0.033), and lower prognostic nutritional index (HR = 0.186, P = 0.034) were associated with higher risk of low SMM. Low SMM was associated with poorer baseline QOL scores (P = 0.072), especially in the physical domain (P = 0.002) and its three facets: pain (P = 0.003), energy (P = 0.021), and sleep (P = 0.007). Low SMM was also associated with significantly worse QOL scores (P = 0.006) at 3 weeks, especially in the physical (P = 0.002), psychological (P = 0.046), independence (P = 0.003), social domains (P = 0.023), and in general health condition (P = 0.043). For pain score, low SMM group had worse overall changes from baseline to week 3 (P = 0.011).
Conclusions: The incidence of low SMM, as evaluated using routine CT-sim scans, is high in patients receiving CCRT for locoregionally advanced NPC. Low SMM results in poorer QOL and higher pain scores, which underscores the requirement for nutritional and functional interventions to address low SMM early in the treatment course.
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma with distinct pathophysiological characteristics in contrast with other head and neck cancer (1, 2). The mainstay of treatment of locoregionally advanced NPC is concurrent chemoradiotherapy (CCRT) (3). The most common CCRT-related toxicities include mucositis, dermatitis, nausea, poor appetite, and dysphagia (4). Intensity-modulated radiotherapy (IMRT), which is currently the most common photon-based radiotherapy technique used in clinical practice, does to some degree reduce treatment-related toxicity and improve patient quality of life (QOL) (5). However, adding chemotherapy to IMRT inevitably increases the treatment-related toxicities and deteriorates patient QOL, thereby impairing treatment compliance and even having life-threatening consequences. Almost all patients who undergo CCRT develop some toxicities, which increases the risk of decreased physical activity, reduced appetite, and metabolic disorders (6).
Sarcopenia was defined as generalized and progressive loss of skeletal muscle strength (SMS) and skeletal muscle mass (SMM) in various clinical settings (7). For many cancer patients with sarcopenia, especially for those treated by sequential chemotherapy and radiotherapy, may experience more serious chemotherapy dose-limiting toxicity, poorer treatment tolerance and worse survival (8, 9) which also occurs in locoregionally advanced head and neck cancers (10, 11). While many researchers have investigated the effects of sarcopenia on prognosis in head and neck cancers, the association with patient-reported QOL remains unclear (8, 10–12). Our previous study found that QOL can affect patient response to treatment, as well as long-term survival (13, 14). Importantly, the deterioration in QOL caused by sarcopenia often appears gradually, as the cumulative dose of radio- and chemotherapy increases. Hence, clinicians may only notice the profound deterioration in QOL during the later stages of treatment, when it is too late to reverse by intervention. We should determine the associations between sarcopenia and patient QOL, to facilitate earlier intervention.
Here, we retrospectively analyzed data from a prospective clinical trial which evaluated the efficacy of oxycodone in reducing treatment-related mucositis pain of CCRT for NPC. We used loss of SMM as an indicator of sarcopenia to determine the association between sarcopenia before treatment and the QOL at different timepoints. And relationship between low SMM and patient characteristics and therapeutic response were also assessed in detail.
Methods
Study Population
This study enrolled 56 NPC patients who received CCRT between 19th May 2015 and 23rd January 2018 at Sun Yat-sen University Cancer Center, Guangzhou, China, as part of a prospective clinical trial. The details of this trial have been described by Hua et al. (13). In brief, all patients received triweekly cisplatin (100 mg/m2)-based CCRT concurrently with IMRT following the guidelines of our institute (15), and reported moderate or severe pain, as indicated by numerical rating scale [NRS] pain scores of 4–10. This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center. Written informed consent was obtained from all patients.
Routine Assessments
We collected pretreatment laboratory data from within 1 week of CCRT as well as clinicopathological data from patient medical records. Plasma Epstein-Barr virus DNA levels (copies/mL) were measured using a real-time quantitative polymerase chain reaction assay, and its cutoff value classified as previously described (16, 17). Representative hematological biomarkers, such as the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), monocyte to lymphocyte ratio (MLR), and prognostic nutritional index (PNI) were assessed, and their cutoffs classified as previously described (18–20). All patients were observed from fraction 15th to 30th of radiotherapy for 3 weeks. And pain was assessed daily using the NRS. Nasopharyngoscopic examinations were conducted weekly and magnetic resonance imaging (MRI) was conducted at 30th radiotherapy fraction to compare the therapeutic effects of CCRT. Complete response (CR) was defined as complete regression of observable nasopharyngeal primary tumors by imaging methods above.
SMM Assessment
On routine computed tomography simulation (CT-sim) images before radiotherapy, we measured the cross-sectional area (CSA) of the sternocleidomastoid and paravertebral muscles on an axial slice at the level of the third cervical (C3) vertebrae, the CT Hounsfield unit thresholds was −29 to +150 for skeletal muscle (Figure 1), as previously described (8, 21). Monaco TPS software (version 5.1, Elekta CMS, Maryland Heights, MO, USA) was used for image evaluation, registration, and mapping at our center for radiotherapy planning. The contours of the muscles were hand-drawn by a senior radiotherapy specialist (LG) and reviewed by another senior specialist (HXL). The CSA of skeletal muscles at the third lumbar (L3) vertebral level was calculated using the formula described by Jung et al. (21): CSA at L3 (cm2) = 81.059 + 0.874 × CSA at C3 (cm2) + 0.956 × Weight (kg) – 28.127 × Sex (value = 1 for female and 2 for male) – 0.257 × Age (y). The skeletal muscle index (SMI) which represented generalized SMM was calculated as follows: SMI (cm2/m2) = CSA at L3 (cm2)/height2 (m2). Low SMM was defined (8) as an SMI of <41 cm2/m2, regardless of body-mass index (BMI) in the case of female patients, and as an SMI of <43 cm2/m2 in male patients with BMI ≤ 25 or an SMI of <53 cm2/m2 in male patients with BMI >25.
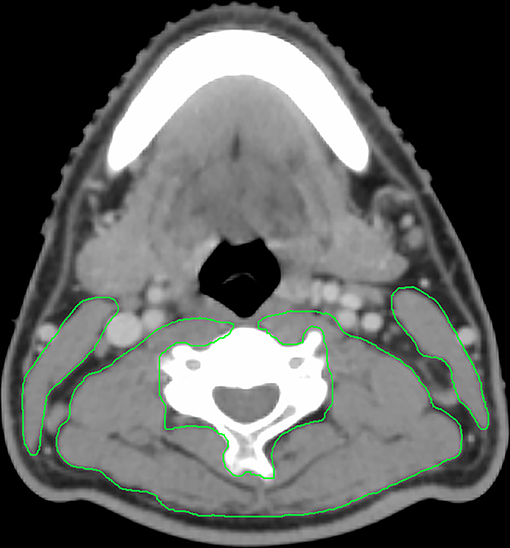
Figure 1. Example contour of the sternocleidomastoid and paravertebral muscles on an axial slice at the C3 vertebral body.
QOL Assessment
QOL was assessed at baseline and after 3 weeks using the World Health Organization QOL Questionnaire-100 (WHO QoL-100) (22). The WHO QOL-100 contains 100 items with subscales assessing wellbeing across six domains: physical health, level of independence, social relations, psychological, environment, and spirituality/religion/personal beliefs. Higher scores on the WHO QOL-100 indicate better QOL. An additional question concerning appetite was assessed and analyzed separately.
Statistical Analyses
Data were analyzed using SPSS software (v. 19.0; IBM Corp., Armonk, NY, USA). Missing data in QOL analysis were handled using mean/mode imputation. After test of normality of distribution, student's t-test or Mann–Whitney U-test were used to compare continuous variables, and the chi-squared test and Fisher's exact test to compare categorical variables. Logistic regression, adjusted for variables with P < 0.25 in univariate analysis, was used to identify patient characteristics independently associated with the presence of low SMM. All statistical tests were two-sided. P ≤ 0.05 was considered statistically significant.
Results
Patient Characteristics
The present observational, retrospective study evaluated data for patients (n = 56) who were enrolled in our previous prospective trial. Their baseline characteristics are shown in Table 1. The participants (mean age, 44.20 ± 10.93 years) were primarily male, and over half (60.71%) had low SMM. Patients in low SMM group were more likely to be above 45 years old (P = 0.035), male (P = 0.066), have a lower BMI (P = 0.091), and have a higher NRS pain score (P = 0.001) (Table 1).
Associations Between Low SMM and Patient Characteristics
Using univariate and multivariate logistic regressions, we found that older age (hazard ratio [HR] = 1.788, P = 0.016) and being male (HR = 3.145, P = 0.010) were associated with higher risk of low SMM, while higher BMI (HR = 0.761, P = 0.033) and PNI (hazard ratio = 0.186, P = 0.034) were associated with lower risk (Table 2).
Associations Between Low SMM and QOL
Figures 2, 3 and Table 3 show the mean QOL scores at baseline and after 3 weeks in both groups. At baseline, low SMM was associated with worse QOL scores (P = 0.072), especially in the physical health domain (P = 0.002), which includes the following three facets: pain and discomfort (P = 0.003), energy and fatigue (P = 0.021), and sleep and rest (P = 0.007). At the end of week 3, low SMM was associated with significantly worse QOL scores (P = 0.006) in the domains physical health (P = 0.002), psychological health (P = 0.046), level of independence (P = 0.003), and social relations (P = 0.023), and in general health condition (P = 0.043). There was a significant decrease in independence (low SMM group: P = 0.004, normal SMM group: P = 0.028) and pain (low SMM group: P = 0.001, normal SMM group: P = 0.001) in both groups between baseline and the end of week 3. During the same time interval, the normal SMM group alone showed an increase in the general health condition score (P = 0.032). The overall changes in the QOL score from baseline to the end of week 3 were comparable between the groups.
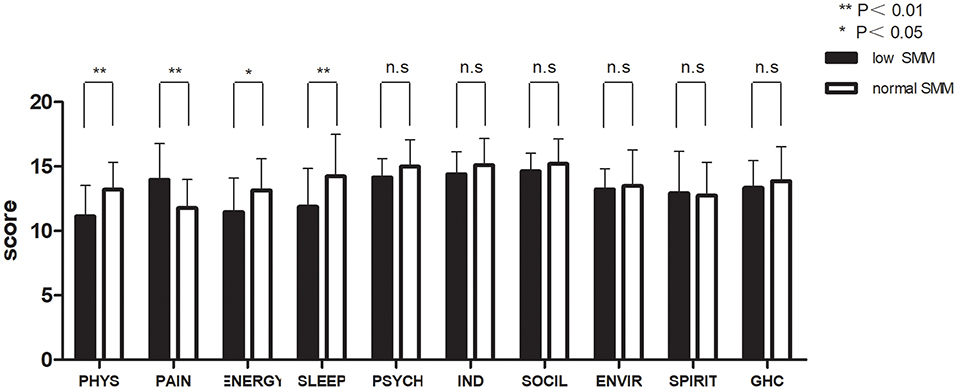
Figure 2. Patient-reported World Health Organization QOL Questionnaire-100 (WHO QOL-100 scores) at baseline in patients with low and normal SMM. Data are expressed as mean ± SD. PHYS, physical; PSYCH, psychological; IND, independence; SOCIL, social; ENVIR, environment; GHC, general health condition.
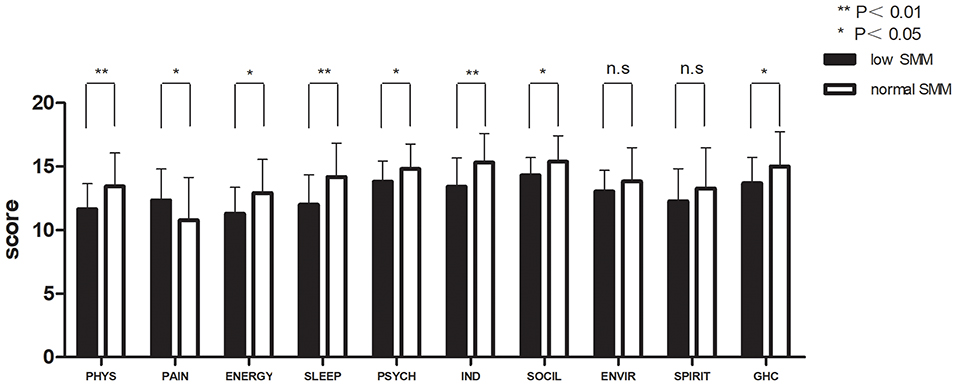
Figure 3. Patient-reported WHO QOL-100 scores at the end of week 3 in patients with low and normal SMM. Data are expressed as mean ± SD. PHYS, physical; PSYCH, psychological; IND, independence; SOCIL, social; ENVIR, environment; GHC, general health condition.
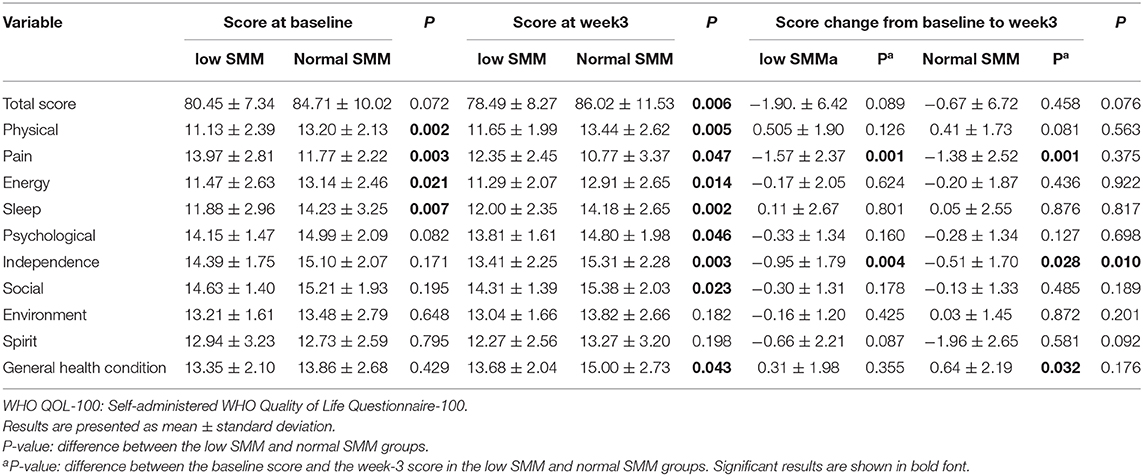
Table 3. Patient-reported WHO QOL-100 scores at the baseline and at the end of week 3 in patients with low and normal SMM.
NRS Pain Score
In all group patients, pain was significantly reduced over the first 3 days, then effectively and steadily controlled from day 4 (Figure 4). The low SMM group reported slightly higher pain scores than the normal SMM group and the overall changes in pain score were significant (3.27 ± 0.98 vs. 2.57 ± 0.69, P = 0.011).
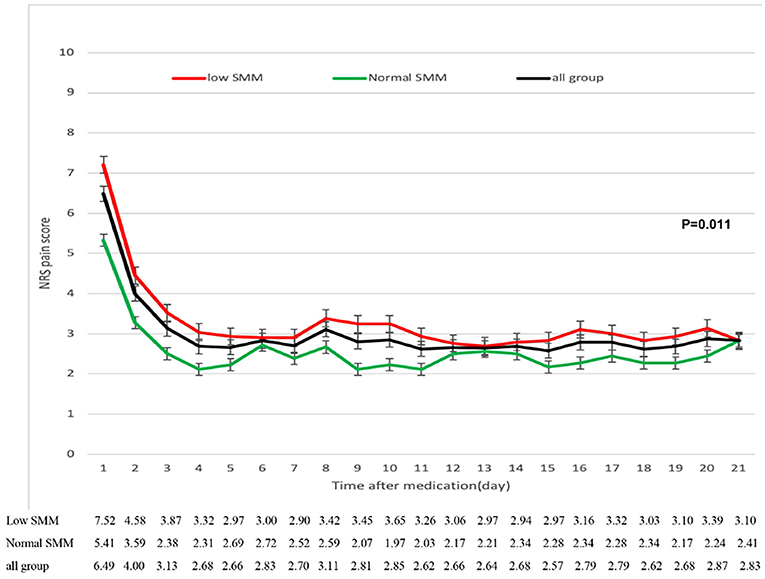
Figure 4. Reduction in numerical rating scale (NRS) pain scores from baseline to week 3 in patients with low and normal SMM. The error bars represent standard deviations.
Appetite
Patients in the normal SMM group had a significantly better appetite at both baseline (3.32 ± 0.99 vs. 2.68 ± 0.64; P = 0.005) and at the end of week 3 (3.22 ± 1.11 vs. 2.38 ± 0.89; P = 0.003) compared with those in the low SMM group.
Response to CCRT
The mean rate of CR was 66.1% (37/56). The rate of CR was higher (in the normal SMM group [68.2% (22/34)] than in the low SMM group [64.7% (15/22)], but the p-value is not significant (P = 0.788).
Discussion
Here, in NPC patients who received CCRT, low SMM was associated with worse patient-reported QOL both at the baseline and at the end of CCRT. We also demonstrated a significant correlation between low SMM and certain patient characteristics, including age, sex, and BMI. Additionally, we found that although patients with low SMM had a lower appetite and higher pain scores than patients without low SMM, the response to CCRT and the overall change in pain scores were comparable in the two groups.
In this study, as an indicator of sarcopenia, low SMM was indirectly measured using a low SMI at the C3 vertebral level. In most studies (12), low SMM has been determined using CT or positron-emission tomography (PET)-CT scans at the L3 vertebral level, which had been routinely performed for diagnostic purposes. These types of scan are not routinely performed for NPC patients. However, comparisons of skeletal muscle CSA at the C3 and L3 levels have demonstrated that CT scans taken at the C3 level can be used to reliably and effectively determine low SMM in patients with head and neck cancer (23). Notably, studies with large sample sizes have assessed and confirmed the efficacy of this method in European and Asian cohorts (8, 21). Every NPC patient routinely completes comprehensive pretreatment CT examinations that includes scans taken from the level of frontal sinus to the level of clavicle, which includes the C3 vertebrae. Therefore, CSA assessment at the C3 level is a reasonable and efficient method of detecting low SMM in these patients, as this method does not involve additional expense or radiation exposure to the patients.
In our study, the incidence rate of low SMM was 60.71%, which is higher than the incidence rates reported in other publications on low SMM in head and neck cancers, ranging from 6.6 to 64.6% (8, 10, 24, 25). A possible explanation for this may be that the present study enrolled patients with locoregionally advanced cancers who were receiving CCRT, whereas more patients in early stages were included in prior studies. Additionally, the treatments in previous studies consisted of radiotherapy or chemotherapy administered separately, rather than the highly intensive CCRT regimen used in our study. The high incidence of low SMM in our study cohort, combined with its negative effect on health-related outcomes, underscores the urgency to appreciate and address low SMM at an early stage of CCRT for NPC.
Multiple studies have found that QOL is an important prognostic factor in patients with head and neck cancers, including NPC, and our previous large-scale prospective trial confirmed this finding (26, 27). In the present study, we demonstrated that low SMM impairs health-related QOL in NPC patients receiving CCRT. It is valuable to have a comprehensive understanding of the frequency and effects of low SMM in this population to: (1) identify characteristics of patients at risk of developing low SMM; (2) determine how low SMM can impact health-related outcomes; and (3) provide additional nutritional and functional assistance to support this population. By showing the negative effect of low SMM on patient QOL, our results provide strong evidence for supportive interventions. Rehabilitation and nutrition experts should participate in the multidisciplinary management of cancer patients in order to identify and treat patients with low SMM earlier in the course of cancer treatment. Such interventions may allow clinicians to better manage their patients, improve QOL, and even improve prognosis.
In our study, older patients were more likely to develop low SMM, consistent with previous publications (28, 29). Studies suggest that the decreased SMM and changes in muscle density observed in older patients may be attributable to neurological, nutritional, hormonal, metabolic, and physical activity-related factors (30, 31). Patient mobility and functional dependence can be affected by these changes in muscle, leading to disability and falls (32). These results indicate that for older cancer patients, screening and assessing SMM and nutritional and functional status may need to be early performed during the course of treatment. We also demonstrated that patients with lower PNI were more likely to have low SMM. PNI is a reflect factor for nutritional and immune status of cancer patients, which is a meaningful prognostic factor in NPC patients (20, 33). A possible reason for the above finding may be that stronger anticancer T-cell immunity may improve muscle quality and function through interaction with immune cells (34), it is more likely that a good muscle mass allows a better immune response, being a reserve for the production of acute-phase proteins. These results indicate that oncologists should screen and assess older cancer patients for sarcopenia and nutritional and functional status early in the course of treatment. Consistent with previous studies, we also found that male patients were more likely to develop low SMM (28, 35), which may be attributable to differences in the patterns of muscle loss between men and women. Moreover, the gender-specific cutoffs for low SMM, though adopted from consensus guidelines, may differ in specific populations. Overall, our results may help identify individuals who are at a high risk for developing low SMM, so that they may receive tailored interventions that meet their needs.
We are the first to report that low SMM, as evaluated using routine CT-sim images, impairs the health-related QOL in NPC patients receiving CCRT. Some researchers believe that low SMM-related physical decline has a negative impact on the QOL of cancer patients by reducing their functional ability (14). The association of pain symptoms with dysphagia and reduced physical activity may be a new explanation for the link between low SMM and pain in cancer patients. Importantly, our results will provide evidence for future efforts to evaluate and treat low SMM in the early stages of cancer treatment, which may improve the prognosis of patients with CCRT-related adverse events.
There are several limitations to this study. First, this study enrolled a small number of patients limited racial diversity from a single cancer center in an area where NPC is endemic. Thus, our results may not be generalizable, multiregional studies with larger samples are needed to confirm our results. Second, we enrolled patients from a prospective clinical trial; these patients may have different characteristics from those excluded from the prospective trial. Third, due to the lack of longitudinal CT scans, we could not determine changes in muscle mass throughout the course of cancer (patients may develop low SMM during or after cancer treatment) and their further effects on patient QOL. Finally, because our study was cross-sectional, we assessed the relationship between low SMM and patient QOL only over a short time; further research is needed to clarify the precise nature of this relationship.
Conclusions
We have demonstrated that locoregionally advanced NPC patients receiving CCRT have a high incidence rate of low SMM as evaluated using routine head and neck CT simulation scans. Patients with low SMM experience worse QOL and higher pain scores, which highlights the urgency of nutritional and functional interventions to address improve SMM early during the course of treatment. Further researches should focus on when and how to evaluate and improve SMM to enhance the health-related QOL and pain control in NPC patients receiving CCRT.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author. The data and materials of this study will be included at RDD (http://www.researchdata.org.cn/).
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Sun Yat-sen University Cancer Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LG and H-XL: conceptualization, writing (review and editing), supervision, and project administration. XH, J-FL, and SL: methodology, formal analysis, and writing (original draft preparation). JZ, Z-QL, and XH: software. XH, H-YH, and W-WZ: validation. H-YH, XH, WW, SL, and J-FL: investigation. LG, H-XL, and W-WZ: resources and funding acquisition. XH and H-XL: data curation. W-WZ and H-YH: visualization.
Funding
This work was partly supported by the National Natural Science Foundation of China (Nos. 81772877, 81773103, 81572848), the Science and Technology Department of Guangdong Province, China (No. 2017A030310422).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
3. Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. (2015) 33:3356–64. doi: 10.1200/JCO.2015.60.9347
4. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
5. Tang LL, Chen L, Mao YP, Li WF, Sun Y, Liu LZ, et al. Comparison of the treatment outcomes of intensity-modulated radiotherapy and two-dimensional conventional radiotherapy in nasopharyngeal carcinoma patients with parapharyngeal space extension. Radiother Oncol. (2015) 116:167–73. doi: 10.1016/j.radonc.2015.07.038
6. Zheng B, Zhu X, Liu M, Yang Z, Yang L, Lang J, et al. Randomized, double-blind, placebo-controlled trial of shuanghua baihe tablets to prevent oral mucositis in patients with nasopharyngeal cancer undergoing chemoradiation therapy. Int J Radiat Oncol Biol Phys. (2018) 100:418–26. doi: 10.1016/j.ijrobp.2017.10.013
7. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afz046
8. Ganju RG, Morse R, Hoover A, TenNapel M, Lominska CE. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol. (2019) 137:117–24. doi: 10.1016/j.radonc.2019.04.023
9. Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. (2017) 71:26–33. doi: 10.1016/j.oraloncology.2017.05.012
10. Cho Y, Kim JW, Keum KC, Lee CG, Jeung HC, Lee IJ. Prognostic significance of sarcopenia with inflammation in patients with head and neck cancer who underwent definitive chemoradiotherapy. Front Oncol. (2018) 8:457. doi: 10.3389/fonc.2018.00457
11. Grossberg AJ, Chamchod S, Fuller CD, Mohamed AS, Heukelom J, Eichelberger H, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol. (2016) 2:782–9. doi: 10.1001/jamaoncol.2015.6339
12. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
13. Hua X, Chen LM, Zhu Q, Hu W, Lin C, Long ZQ, et al. Efficacy of controlled-release oxycodone for reducing pain due to oral mucositis in nasopharyngeal carcinoma patients treated with concurrent chemoradiotherapy: a prospective clinical trial. Support Care Cancer. (2019) 29:mdy438.006. doi: 10.1093/annonc/mdy438.006
14. Keogh JW, MacLeod RD. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manage. (2012) 43:96–110. doi: 10.1016/j.jpainsymman.2011.03.006
15. Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. (2014) 110:398–403. doi: 10.1016/j.radonc.2013.10.020
16. Tang LQ, Li CF, Li J, Chen WH, Chen QY, Yuan LX, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. (2016) 108:djv291. doi: 10.1093/jnci/djv291
17. Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, et al. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. (2004) 100:1162–70. doi: 10.1002/cncr.20099
18. Jiang W, Chen Y, Huang J, Xi D, Chen J, Shao Y, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Oncotarget. (2017) 8:66075–86. doi: 10.18632/oncotarget.19796
19. Yao JJ, Zhu FT, Dong J, Liang ZB, Yang LW, Chen SY, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced nasopharyngeal carcinoma: a large institution-based cohort study from an endemic area. BMC Cancer. (2019) 19:37. doi: 10.1186/s12885-018-5236-2
20. Miao J, Xiao W, Wang L, Han F, Wu H, Deng X, et al. The value of the prognostic nutritional index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol. (2017) 143:1263–73. doi: 10.1007/s00432-017-2360-3
21. Jung AR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY. Efficacy of head and neck computed tomography for skeletal muscle mass estimation in patients with head and neck cancer. Oral Oncol. (2019) 95:95–9. doi: 10.1016/j.oraloncology.2019.06.009
22. The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. (1998) 46:1569–85. doi: 10.1016/S0277-9536(98)00009-4
23. Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KM, de Bree R, et al. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. (2016) 62:28–33. doi: 10.1016/j.oraloncology.2016.09.006
24. Jung AR, Roh JL, Kim JS, Kim SB, Choi SH, Nam SY, et al. Prognostic value of body composition on recurrence and survival of advanced-stage head and neck cancer. Euro J Cancer. (2019) 116:98–106. doi: 10.1016/j.ejca.2019.05.006
25. Fattouh M, Chang GY, Ow TJ, Shifteh K, Rosenblatt G, Patel VM, et al. Association between pretreatment obesity, sarcopenia, and survival in patients with head and neck cancer. Head Neck. (2018) 41:707–14. doi: 10.1002/hed.25420
26. Guo SS, Hu W, Chen QY, Li JM, Zhu SH, He Y, et al. Pretreatment quality of life as a predictor of survival for patients with nasopharyngeal carcinoma treated with IMRT. BMC Cancer. (2018) 18:114. doi: 10.1186/s12885-018-4003-8
27. Fang FM, Tsai WL, Chien CY, Chen HC, Hsu HC, Huang TL, et al. Pretreatment quality of life as a predictor of distant metastasis and survival for patients with nasopharyngeal carcinoma. J Clin Oncol. (2010) 28:4384–9. doi: 10.1200/JCO.2010.28.8324
28. Nipp RD, Fuchs G, El-Jawahri A, Mario J, Troschel FM, Greer JA, et al. Sarcopenia is associated with quality of life and depression in patients with advanced cancer. Oncologist. (2018) 23:97–104. doi: 10.1634/theoncologist.2017-0255
29. Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. (2010) 13:1–7. doi: 10.1097/MCO.0b013e328333c1c1
30. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol. (2015) 205:W255–66. doi: 10.2214/AJR.15.14635
31. Welle S. Cellular and molecular basis of age-related sarcopenia. Can J Appl Physiol. (2002) 27:19–41. doi: 10.1139/h02-002
32. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. (2012) 31:652–8. doi: 10.1016/j.clnu.2012.02.007
33. Du XJ, Tang LL, Mao YP, Guo R, Sun Y, Lin AH, et al. Value of the prognostic nutritional index and weight loss in predicting metastasis and long-term mortality in nasopharyngeal carcinoma. J Transl Med. (2015) 13:364. doi: 10.1186/s12967-015-0729-0
34. Narsale A, Moya R, Ma J, Anderson LJ, Wu D, Garcia JM, et al. Cancer-driven changes link T cell frequency to muscle strength in people with cancer: a pilot study. J Cachexia Sarcopenia Muscle. (2019) 10:827–43. doi: 10.1002/jcsm.12424
Keywords: nasopharyngeal carcinoma, low skeletal muscle mass, pain, quality of life, concurrent chemoradiotherapy
Citation: Hua X, Liao J-F, Liu S, Zhang J, Huang H-Y, Wen W, Long Z-Q, Zhang W-W, Guo L and Lin H-X (2020) Low Skeletal Muscle Mass Impairs Quality of Life in Nasopharyngeal Carcinoma Patients Treated With Concurrent Chemoradiotherapy. Front. Nutr. 6:195. doi: 10.3389/fnut.2019.00195
Received: 14 September 2019; Accepted: 20 December 2019;
Published: 15 January 2020.
Edited by:
Daniel W. D. West, University of Toronto, CanadaReviewed by:
Emanuele Rinninella, Agostino Gemelli University Polyclinic, ItalyKirsten Bell, University of Waterloo, Canada
Copyright © 2020 Hua, Liao, Liu, Zhang, Huang, Wen, Long, Zhang, Guo and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Guo, Z3VvbGluZ0BzeXN1Y2Mub3JnLmNu; Huan-Xin Lin, bGluaHhAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
 Xin Hua
Xin Hua Jun-Fang Liao3†
Jun-Fang Liao3† Wen-Wen Zhang
Wen-Wen Zhang