Power Comparisons and Clinical Meaning of Outcome Measures in Assessing Treatment Effect in Cancer Cachexia: Secondary Analysis From a Randomized Pilot Multimodal Intervention Trial
- 1Department of Clinical and Molecular Medicine, Faculty of Medicine and Health Sciences, NTNU–Norwegian University of Science and Technology, Trondheim, Norway
- 2Cancer Clinic, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
- 3Palliative Care, Pain Therapy and Rehabilitation Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 4Edinburgh Cancer Research Centre (IGMM), University of Edinburgh, Edinburgh, United Kingdom
- 5Department of Oncology, European Palliative Care Research Centre (PRC), Oslo University Hospital and Institute of Clinical Medicine, University of Oslo, Oslo, Norway
- 6Department of Nursing and Health Promotion, Faculty of Health Sciences, OsloMet–Oslo Metropolitan University, Oslo, Norway
- 7St. Columba's Hospice, Edinburgh, United Kingdom
- 8Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, United Kingdom
Background: New clinical trials in cancer cachexia are essential, and outcome measures with high responsiveness to detect meaningful changes are crucial. This secondary analysis from a multimodal intervention trial estimates sensitivity to change and between treatment effect sizes (ESs) of outcome measures associated with body composition, physical function, metabolism, and trial intervention.
Methods: The study was a multicenter, open-label, randomized pilot study investigating the feasibility of a 6-week multimodal intervention [exercise, non-steroidal anti-inflammatory drugs, and oral nutritional supplements containing polyunsaturated fatty acids (n−3 PUFAs)] vs. standard cancer care in non-operable non-small-cell lung cancer and advanced pancreatic cancer. Body composition measures from computerized tomography scans and circulating biomarkers were analyzed.
Results: Forty-six patients were randomized, and the analysis included 22 and 18 patients in the treatment and control groups, respectively. The between-group ESs were high for body weight (ES = 1.2, p < 0.001), small for body composition and physical function [handgrip strength (HGS)] measures (ES < 0.25), moderate to high for n-3 PUFAs and 25-hydroxyvitamin D (25-OH vitamin D) (ES range 0.64–1.37, p < 0.05 for all), and moderate for serum C-reactive protein (ES = 0.53, p = 0.12). Analysis within the multimodal treatment group showed high sensitivity to change for adiponectin (ES = 0.86, p = 0.001) and n-3 PUFAs (ES > 0.8, p < 0.05 for all) and moderate for 25-OH vitamin D (ES = 0.49, p = 0.03). In the control group, a moderate sensitivity to change for body weight (ES = −0.84, p = 0.002) and muscle mass (ES = −0.67, p = 0.016) and a high sensitivity to change for plasma levels of 25-OH vitamin D (ES = −0.88, p = 0.002) were found.
Conclusion: Demonstrating high sensitivity to change and between treatment ES and body composition measures, body weight still stands out as a clinical and relevant outcome measure in cancer cachexia. Body composition and physical function measures clearly are important to address but demand large sample sizes to detect treatment group differences.
Trial registration: ClinicalTrials.gov identifier: NCT01419145.
Introduction
Cancer cachexia is a complex multifactorial syndrome resulting in progressive weight loss due to loss of skeletal muscle mass with or without depletion of adipose tissue, leading to progressive loss of physical function (1). Discussion of how to evaluate the effect of any anti-cachexia therapy is continuously ongoing, and there is no consensus as to the optimal outcome measures in clinical trials (2, 3). Weight loss is the defining factor of cachexia according to the international cachexia definition but may not always be a valid indicator (2). Weight gain might be due to edema and/or ascites and may conceal muscle loss due to adiposity. Change in lean body mass is regularly used as an outcome measure in clinical trials, but the magnitude of clinically relevant changes has not yet been established. The loss of lipid reserves may also contribute to the cachexia phenotype. Depletion of fat depots is more prominent and often precedes loss of muscle mass in cancer patients (4, 5), but the significance of fat mass as an outcome measure in cachexia trials is not well-studied. Candidate outcome measures should be responsive to change, which implies that they need to be specific to the cachexia pathophysiology. Ideally, such outcome measures should not be significantly influenced by other factors contributing to wasting, such as antineoplastic therapy or immobilization. Nevertheless, this is practically impossible as the cachexia pathophysiology is complex, and any cachexia treatment may be influenced by effects of antineoplastic treatment, as treating cancer is also a treatment for cachexia.
The clinical need for early diagnosis and treatment of cachexia supports the need to identify specific biomarkers that precociously detect the wasting process (6). If cachexia intervention trials can demonstrate beneficial effects on body composition measures, an important question is whether circulating biomarkers representing key metabolic alterations can be used complementary to such clinical outcomes and add information about the underlying pathophysiology. So far, a limited number of clinical outcome measures have been explored in cachexia trials, most likely a consequence of ongoing definitional ambiguities together with the complexity of the condition. There is a need to establish reliable clinical outcomes, including circulating biomarkers, and evaluate their sensitivity to change in patients with cancer cachexia.
This report presents secondary analyses of data from a randomized phase II multimodal intervention trial for the treatment of cachexia evaluating implementation and effect of oral nutritional supplements (ONSs) containing polyunsaturated fatty acids (n−3 PUFAs), exercise, and non-steroidal anti-inflammatory drugs (NSAIDs) compared to standard cancer care (7). The multimodal intervention resulted in a stabilization of body weight, while patients in the control arm lost weight (7). The overall aim of the present study was to estimate sensitivity to change and between treatment effect sizes (ESs) of outcome measures associated with body composition, physical function, metabolism, as well as markers of the trial intervention. Considering these outcome measures, implications for trial design with regard to sample size will be discussed.
Materials and Methods
Trial Design and Patients
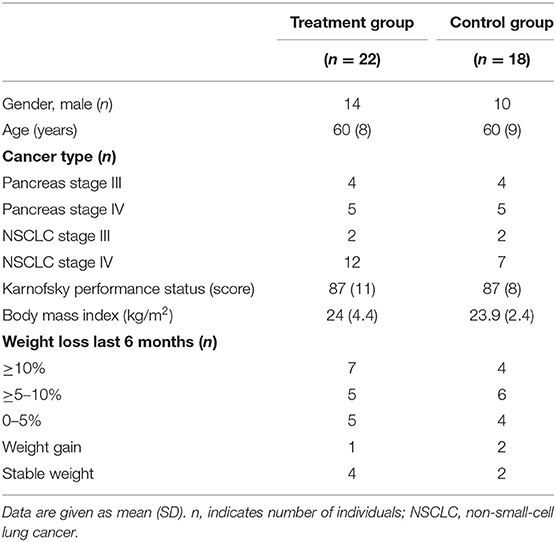
The study was a multicenter, open-label, pilot randomized phase II study investigating the feasibility of a 6-week multimodal intervention for cachexia vs. standard cancer care. This study recruited those with non-operable non-small-cell lung cancer (NSCLC) (stage III–IV) or advanced pancreatic cancer starting antineoplastic therapy (7). The primary aim of the feasibility study was to assess recruitment, compliance, and contamination in the control arm (7), and a phase III efficacy study is now ongoing (MENAC Trial, ClinicalTrials.gov: NCT02330926) (8). Forty-six patients were included in the study; three patients in each group were excluded due to missing blood samples at week 6. The present analysis includes 22 and 18 patients in the treatment and control groups, respectively (7). Characteristics of the study participants indicate that the two groups were comparable at baseline in terms of gender, age, cancer type, Karnofsky performance score, body mass index (BMI), and pre-inclusion weight loss (Table 1). The protocol received ethics and medical agency approval from all centers, and written informed consent was obtained from all patients. The study is registered at ClinicalTrials.gov (NCT01419145).
Body Composition Measures
Anthropometric measurements for body weight (kg) and height (cm) were obtained from all participating patients, and BMI was calculated (kg/m2). Total muscle mass and adipose tissue area were quantified using computerized tomography (CT) imaging covering the abdomen area at the third lumbar vertebra (L3) taken at baseline and after 6 weeks (9, 10). Axial images were selected out and analyzed using the Automatic Body composition Analyzer using Computed tomography image Segmentation (ABACS) software (11). Adipose tissue cross-sectional areas were calculated using standard Hounsfield unit (HU) thresholds of −150 to −50 HU for visceral adipose tissue, −190 to −30 HU for subcutaneous adipose tissue, and −29 to +150 HU for muscle tissue (12, 13). Tissue cross-sectional areas (cm2) were calculated by adding up the given tissue pixels and multiplying by the pixel surface area. Visceral and subcutaneous adipose tissue cross-sectional areas were summarized to estimate total adipose tissue areas. The total muscle and adipose area were normalized for patient height to calculate total muscle and adipose index (cm2/m2).
Physical Function
Handgrip strength (HGS) (kg) was collected at baseline and after 6 weeks and measured with a hydraulic handheld dynamometer (JAMAR). The test was performed using the dominant hand, and three test trials were performed (7, 14).
Collection, Storing, and Processing of Biological Samples
Baseline samples were collected before the start of chemotherapy and at endpoint (week 6 ± 1 week allowed according to the protocol). C-reactive protein (CRP) was collected using standard analytical methods applied by local hospitals. Blood samples from ethylenediaminetetraacetic acid (EDTA) containers for isolation of plasma and container without additive for isolation of serum were centrifuged at 2,200 g for 10 min, aliquoted to cryotubes, and stored at −80°C. During blood sample analysis, researchers were blinded to both the sample randomization results and clinical data. All samples were analyzed in duplicate, and a fresh aliquot was used for each analysis with no prior freeze–thaw cycles.
Analysis of Adiponectin, Zink-α2 Glycoprotein, Insulin-Like Growth Factor 1, Glycerol, and Lipolysis
Plasma levels of adiponectin, zink-α2 glycoprotein (ZAG), and insulin-like growth factor 1 (IGF-1) were measured using ELISA (R&D Systems, Abingdon, UK). A standard concentration curve was made for each ELISA plate with the manufacturer's control solution and used to calculate plasma concentrations in the samples assayed. A coefficient of variability among sample replicates calculated by dividing the standard deviation (SD) by the mean of the set of measurements expressed as a percentage of variation to the mean below 0.10 was determined to be acceptable. Glycerol was measured calorimetrically from serum in μmol/L concentrations (Lipolysis kit LIP-3-NC, Zen-Bio, Durham, NC, USA). Lipolysis is presented as glycerol umol/L/total adipose index (cm2/m2) (15).
Plasma n−3 Polyunsaturated Fatty Acids and 25-Hydroxyvitamin D Analysis
Phospholipids (PLs) from blood plasma were extracted and fatty acids from the PL were transmethylated with boron-tri-fluoride in methanol. Quantification of n−3 PUFAs [eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), docosapentaenoic acid (DPA)] from PL was performed using gas chromatography. The quantification is based on the use of an internal standard with known concentration and the instrument Agilent 6890N gas chromatograph with GC ChemStation software was used. PL concentration of n−3 PUFAs was calculated as % of total fatty acids in plasma PL. Plasma levels of 25-hydroxyvitamin D (25-OH vitamin D) were measured based on an ultra-performance liquid chromatography technique and detection by tandem mass spectrometry [Acquity UPLC® I Class med Xevo TQS MSMS (Waters)]. This assay measures both 25-OH calcidiol (vitamin D3) and 25-OH calciferol (vitamin D2), and the sum of these two are presented. Both n−3 PUFAs and 25-OH vitamin D analyses were done at the Department of Medical Biochemistry, St. Olavs University Hospital, Trondheim, Norway.
Statistics
Descriptive statistics are presented as means and SDs. All analyses were carried out on the modified intention-to-treat population (defined as all randomized patients with both baseline and week 6 assessments). Comparisons between groups were conducted using t-tests for independent samples, while paired sample t-tests were used to evaluate changes within each study group. For each outcome, ESs within and between groups (ESWG and ESBG) were calculated using appropriate formulas. ESWG was calculated using Cohen's d for one-sample pre–post design to estimate sensitivity to change over time in each treatment group separately (16). Positive and negative values of ESWG indicate, respectively, an increase and a decrease in the outcome over time. ESBG was calculated using Hedges' g for two-independent sample design on the pre–post variations to estimate between treatment effects (16). A positive ESBG value indicated an advantage for the treatment arm with respect to the control. Reference values for small (<0.2), medium (<0.5), and large (>0.8) ESs were used for result interpretation (17). Sample size per treatment arm by ESBG of the various outcome measures was calculated by t-test for independent samples (alpha error = 0.05, power 0.9) and plotted in order to compare the relative power of the different outcome measures. Analyses were performed using IBM SPSS Statistic Software 25 for Windows and Stata 15.1 for Windows (StataCorp, College Station, Texas, USA).
Results
Body Mass and Body Composition
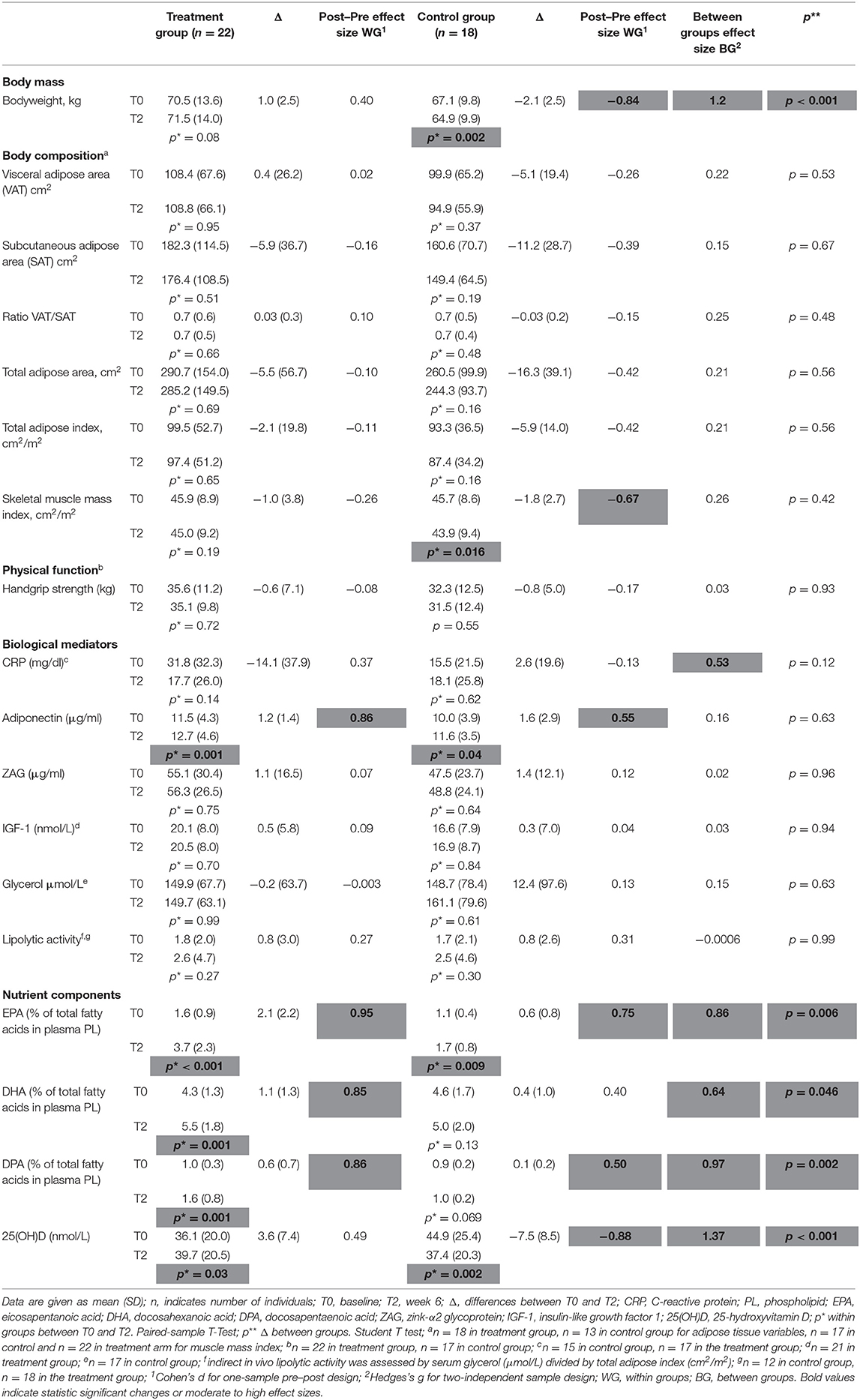
At baseline, the degree of weight loss was equally distributed between the two arms (Table 1). Mean (SD) change in body weight from baseline to week 6 within the two groups showed a small increase within the treatment arm [1.0 (2.5), p = 0.08, ESWG = 0.40] and a moderate, significant decrease within the control group [−2.1 (2.5), p = 0.002, ESWG = −0.84] (Table 2). A significant difference between the two arms was found (p < 0.001) with a high ESBG = 1.2 (Table 2).
When analyzing body composition measures (Table 2), significant time change was found for skeletal muscle mass index, which decreased within the control group (−1.8 cm2/m2, p = 0.016, ESWG = −0.67; Table 2). Most ESWG in both groups were negative, indicating a decline from baseline to week 6, but these were very small in absolute magnitude within the treatment group (range −0.26 to +0.10) and higher in the control group (range −0.67 to −0.15). All ESBG indicate small effects in favor of the treatment group (all below 0.26 and none of them statistically significant; penultimate column, Table 2). The sample size needed to detect ESBG as those observed for body weight would be 15 participants with completed outcome measures per arm (orange color line in Figure 1), and in comparison, ~300–900 participants per arm for body composition measures (blue lines in Figure 1; sample sizes not shown for ESBG < 0.2).
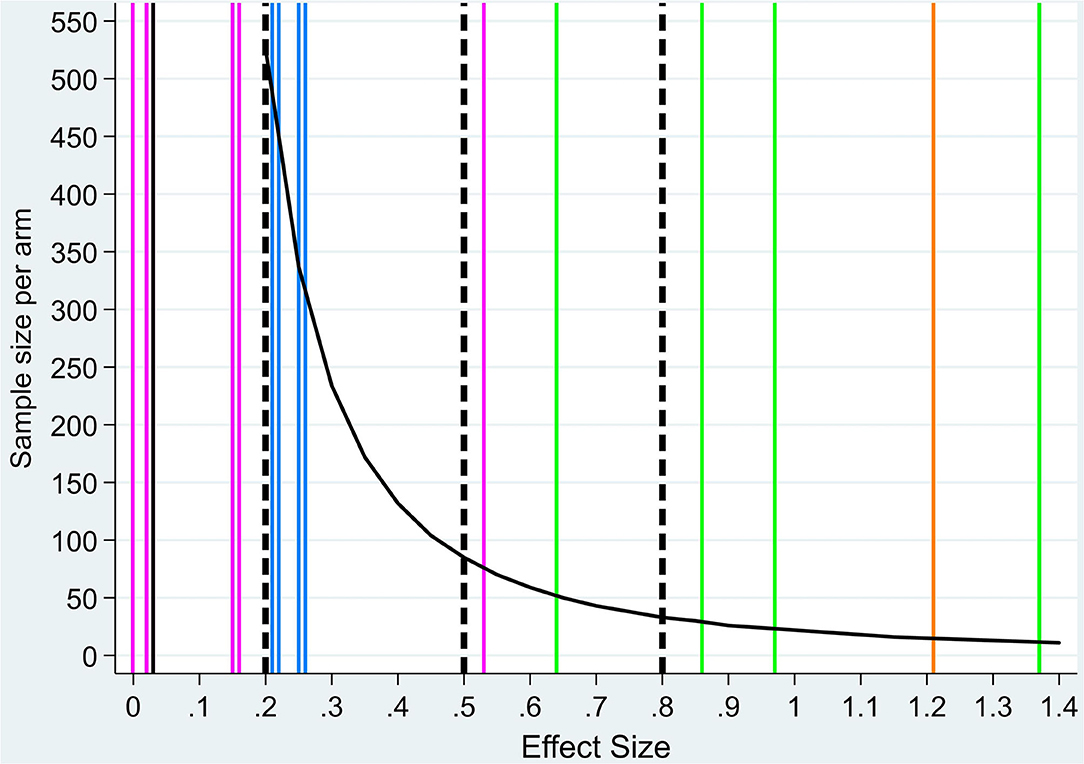
Figure 1. Sample size per treatment arm by effect size values. Sample size by treatment arm by effect size (ES) values (black curve). Dashed vertical lines indicate reference value for small (<0.2), medium (<0.5), and large (>0.8) ESs (17). Colored vertical lines indicate ESBG for each outcome measure: body weight (orange, n = 1), body composition (blue, n = 6, two overlap, one overlaps with metabolism outcome), physical function (black, n = 1), metabolic mediators (pink, n = 6, two overlap), and nutrient components (green, n = 4) (exact values are reported in Table 2). Sample size values for ES < 0.2 are higher than 1,000 and not shown in the figure.
Physical Function
Physical function measured using HGPs showed no significant change between the two groups (p = 0.93) with a very low ESBW = 0.03. Within group analysis, a small mean (SD) reduction in HPS of −0.6 (7.1) (ESWG = −0.08) for the treatment group and −0.8 (5.0) (ESWG = −0.17) for the control group was found. Sample size by ES for HGS would be >1,000 per treatment arm (black horizontal line in Figure 1; sample sizes not shown for ES < 0.2).
Biological Mediators
As for serum CRP levels, a nonsignificant decrease was found within the treatment group with a mean (SD) of −14.1 (37.9), medium ESWG = 0.37, p = 0.14 (Table 2). Within the control group, a low nonsignificant mean (SD) increase of 2.6 (19.6), ESWG = −0.13, p = 0.53, was observed with a medium ESBG (0.53) in favor of the treatment group when comparing the two groups (p = 0.12). For CRP, sample size by ES would be 75 participants per treatment arm (blue color line in Figure 1). Plasma levels of adiponectin increased significantly within both groups from baseline to week 6 with a mean (SD) change of 1.2 (1.4) μg/ml, p = 0.001, with a high ESWG = 0.86 for the treatment group and 1.6 (2.9) μg/ml, p = 0.04, and moderate ESWG = 0.55 for the control group (Table 2). No significant differences in change of adiponectin levels between the groups were observed (p = 0.63), low ESBG = 0.16. No significant change within groups or between groups were found for plasma levels of ZAG, IGF-1, glycerol, or lipolysis (Table 2) (p > 0.05 for all). ESWG for ZAG, IGF-1, glycerol, and lipolysis in both arms were very small (<0.20), indicating no change from baseline to week 6. For adiponectin, a large ESWG in the treatment arm (>0.80) and a medium ESWG in the control arm (>0.50) was observed. The ESBG for all variables were very small [all <0.20 in favor of the treatment arm except for lipolysis (−0.001)]. Sample sizes by ESs as those observed for adiponectin, ZAG, glycerol, or lipolysis would consequently range from around 1,000 or more participants per treatment arm (pink lines in Figure 1; sample sizes not shown for ES < 0.2).
Nutrient Components
The recommended intake of n−3 PUFA containing ONS in the treatment group was two containers/day; however, the actual mean (SD) intake among the 22 patients was 1.1 (0.73) containers (range 0–2 containers/day) (7). Changes in plasma level (% of total fatty acids in plasma PL) from baseline to week 6 for EPA, DHA, and DPA are shown in Table 2. In the treatment group, significant mean (SD) increase for EPA [2.1% (2.2%), p < 0.001], DHA [1.1% (1.3%), p = 0.001], and DPA [0.6% (0.7%), p = 0.001] was demonstrated. In the control group, a significant increase was observed for EPA [0.6% (0.8%), p = 0.009]. Mean (SD) changes in EPA, DHA, and DPA from baseline to week 6 were statistically significantly increased in the treatment group compared to the control group (Table 2; p < 0.05 for all).
A significant mean (SD) increase of 25-OH vitamin D was observed in the treatment group [3.6 (7.4) nmol/L, p = 0.03] compared to a significant mean (SD) decrease in the control group [−7.5 (8.5) nmol/L, p = 0.03]. The change in 25-OH vitamin D levels was significant between the two groups (Table 2; p < 0.001). ESWG for EPA, DHA, and DPA was large (>0.80 for all) and medium (0.49) for 25-OH vitamin D in the treatment arm and medium for EPA (0.75), DHA (0.40), and DPA (0.5) and large for 25-OH vitamin D (−0.88) in the control arm. The ESBG were medium for DHA (0.64) and large (>0.8) for EPA, DPA, and 25-OH vitamin D in favor of the treatment arm. Accordingly, green lines in Figure 1 show that small sample sizes are needed per treatment arm for this set of variables if chosen as outcome measures (52 participants for DHA, 29 for EPA, 23 for DPA, and 12 for 25-OH vitamin D).
Discussion
The selection of valid and useful outcome measures is a critical step when designing cancer cachexia trials. In the present study, we investigated cachexia outcome measures for their sensitivity to change and ESs between treatment groups. Outcomes investigated were related to body mass and body composition, physical function, as well as circulating biomarkers representing metabolism and the nutritional intervention. The outcome measures examined changed predominantly in favor of the treatment arm, although high ESBG were demonstrated for body weight and the nutrient component biomarkers only. Furthermore, our sample size estimations show a large difference between sample sizes for body weight (n = 15), body composition measures (~300–900 participants) and HGS (n > 1,000) if used as primary outcome.
Although frequently used, body composition is a challenging primary outcome measure in cancer cachexia trials. Body composition, either measured as total lean mass (entire body weight minus fat), skeletal muscle mass, or fat mass, is in general extremely variable across the general population and in patients with cancer (18). This introduces the necessity of large sample sizes in clinical trials, which again can emphasize statistical differences that are not necessarily clinically relevant (19).
Furthermore, as a prognostic indicator, CT is considered the “gold standard” measurement providing high precision (<2% error) (20) and, demonstrating high correlation with assessment by dual-energy X-ray absorptiometry (DXA) (21). However, as an outcome measure, there are uncertainties to whether the same cross-sectional area, such as L3 level used in the present trial, captures treatment effects, especially if strength exercise intervention mainly involves large muscle groups in the upper and lower extremities (7, 8). Considering fat mass, previous studies have also reported that a single CT image slice does not accurately predict adipose tissue changes during weight loss (22). Nevertheless, compared to lean body mass measurements from DXA, muscle mass quantification from CT images yields information on a tissue-organ level reflecting striated muscle only- and skeletal muscle mass-specific changes.
Comparable trials testing the effect of novel anti-cachexia drugs [e.g., anamorelin or selective androgen receptor modulators (SARMs)] have used body composition measurement such as lean body mass (total or appendicular) as outcome measure (23–25). Different methodologies make comparison of ESBG for body composition across trials challenging, and furthermore, there is an abundance of well-validated outcome measures for this purpose. Recent trials have added measures that capture changes in physical function in conjunction with skeletal muscle mass to test the efficacy of anti-cachexia treatments. Albeit endorsed by regulatory authorities, the use of such co-primary endpoints has so far had limited success, as corresponding effects are not demonstrated (26). The magnitude of muscle mass loss in the control arm in this study does not evoke a corresponding reduction in HGS. Low muscle mass is associated with reduced physical function; however, the relationship is nonlinear and, likely, there is a variable impact on physical function outcomes depending on the magnitude of changes in muscle mass (14). The potential of physical function outcomes such as HGS (and other performance testing) to detect change relative to muscle/weight changes in cancer cachexia remains unclear.
Cachexia is considered a multiorgan syndrome (27), and emerging evidence suggests there is a crosstalk between adipose tissue and skeletal muscle (28). For instance, muscle wasting seems to be preceded by signals generated from inflamed and dysregulated adipose tissue, which may be present prior to detectable loss of fat mass. The use of circulating biomarkers as outcome measures in clinical trials could potentially overcome several of these challenges by representing specific metabolic pathways. In the present study, there were neither within- nor between-group changes in any fat mass compartments or for biomarkers representing loss of fat mass such as plasma levels of ZAG, glycerol, and lipolysis. This may indicate that adipose tissue biomarkers and fat mass correspond over time. It remains to be investigated whether any of these circulating biomarkers, or others not investigated in this study, demonstrates corresponding changes with body composition. Further, the prognostic and predictive value for loss of muscle mass independent of loss of adipose tissue needs further investigation.
To understand the anti-cachexic mechanisms of any intervention, it is of importance to explore how interventions act on regulators of metabolism and inflammation. The loss of muscle mass within the control group was not followed by a corresponding change in IGF-1, a strong modulator of muscle mass synthesis. The effect of the multimodal intervention might prevent loss of muscle mass by targeting systemic inflammation and thus acting anti-catabolic rather than being anabolic. This seems supported by the change in CRP in favor of the multimodal treatment with a medium ESBG of 0.53.
Adiponectin is involved in the regulation of glucose and lipid metabolism and has insulin-sensitizing and anti-inflammatory properties (29). To our knowledge, this is the first study to evaluate how adiponectin corresponds to change in body weight and body composition over time as well as response to anti-cachexic treatment. The increased levels of adiponectin within the control arm might be due to weight and muscle loss, which is also shown in cross-sectional studies comparing cachexic cancer patients to non-cachexic and healthy controls (30–32). In the intervention group, the increased adiponectin levels might be a response to the intake of n−3 PUFAs (33, 34). Further studies investigating the role of adipokines in cancer cachexia are necessary, as the direction and clinical meaning of change are not fully outlined.
Biomarkers may in some cases be related to parts of the intervention targeting cachexia, e.g., they may provide information about contamination and compliance and might represent a relevant outcome. The nutritional intervention biomarkers (n−3 PUFAs and 25-OH vitamin D) yielded the largest within- and between-group ESs corresponding to intake of the ONS. The moderate increase in EPA also within the control group may be explained by contamination if patients start taking supplements or mimic parts of the intervention (7). In unblinded randomized controlled trial (RCT) designs with nutrition and exercise interventions, outcome measures of compliance, and contamination are important to be able to assess risk of bias.
In this study, we estimated sensitivity to change and between treatment ESs from a pilot study. Albeit underpowered and not designed to compare the efficacy of an intervention, pilot studies are considered legitimate to estimate sample sizes. Still, caution is advised as estimates might be biased or unrealistic due to chance factors related to the small sample size (35). Our results revealed that >300 participants were needed per arm to detect an ES of 0.2 for skeletal muscle mass index, which are numbers comparable to the numbers of participants included in other cachexia trials with lean body mass and HGS as co-primary outcomes (24). The ongoing phase III MENAC trial is powered on body weight with a moderate ESBG (0.5) as main outcome including 90 completed patients per arm (8). In parallel arm RCTs, the between-group analysis is the correct analysis approach (36). In this secondary analysis, we also analyzed within-group ESs to estimate sensitivity to change of the various outcomes explored as it can be informative when choosing the most appropriate outcomes. Evaluation of the control group receiving standard care, which to a certain extent also is anti-cachexia treatment, is consequently of importance.
In conclusion, body weight remains a clinical and relevant outcome measure in cancer cachexia, as body composition measures, HGS, and some circulation biomarkers demand large sample sizes to detect differences. So far, research has not been able to demonstrate superiority for any measure of body composition or specific biomarkers, although clearly, these are important to address in order to understand the underlying pathophysiology of weight loss in cancer cachexia. Research in cancer cachexia still needs to address both testing of treatments and evaluation of relevant outcomes until an evidence-based consensus on what to measure is reached.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Regional Committee for Medical and Health Research Ethics (Reference 2010/2620) and Norwegian Medicines Agency (Reference 11/01673-8). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made substantial, direct and intellectual contribution to the work and approved it for publication.
Funding
preMENAC was funded by Liaison Committee for education, research and innovation in Central Norway; Nordic Cancer Union; Norwegian Cancer Society; St Olav Cancer Fund; Cancer Clinic, St. Olavs hospital, Trondheim University Hospital; Clinical Trials Unit (CTU), Cancer Research UK. The ONS (ProSure) was received free of charge from Abbott Nutrition.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the following: Liaison Committee for Education, Research and Innovation in Central Norway; Nordic Cancer Union; Norwegian Cancer Society; St. Olav Cancer Fund; Cancer Clinic, St. Olavs Hospital, Trondheim University Hospital; Clinical Trials Unit (CTU), The Central Norway Regional Health Authority (HMN); West of Scotland Hepatobiliary and Pancreatic Surgery Department; Respiratory Oncology Team based in the New Victoria Hospital/Southern General Hospital, Glasgow; Cancer Research UK; Clinical Trials Unit, based in the Beatson West of Scotland Cancer Centre. The ONS (ProSure) was received free of charge from Abbott Nutrition, Columbus, OH.
References
1. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. (2011) 12:489–95. doi: 10.1016/S1470-2045(10)70218-7
2. Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle. (2015) 6:272–4. doi: 10.1002/jcsm.12083
3. Laird BJA, Balstad TR, Solheim TS. Endpoints in clinical trials in cancer cachexia: where to start? Curr Opin Support Palliat Care. (2018) 12:445–52. doi: 10.1097/SPC.0000000000000387
4. Bing C. Lipid mobilization in cachexia: mechanisms and mediators. Curr Opin Support Palliat Care. (2011) 5:356–60. doi: 10.1097/SPC.0b013e32834bde0e
5. Tsoli M, Swarbrick MM, Robertson GR. Lipolytic and thermogenic depletion of adipose tissue in cancer cachexia. Semin Cell Dev Biol. (2016) 54:68–81. doi: 10.1016/j.semcdb.2015.10.039
6. Loumaye A, Thissen JP. Biomarkers of cancer cachexia. Clin Biochem. (2017) 50:1281–8. doi: 10.1016/j.clinbiochem.2017.07.011
7. Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. (2017) 8:778–88. doi: 10.1002/jcsm.12201
8. Solheim TS, Laird BJA, Balstad TR, Bye A, Stene G, Baracos V, et al. Cancer cachexia: rationale for the MENAC (multimodal-exercise, nutrition and anti-inflammatory medication for cachexia) trial. BMJ Support Palliat Care. (2018) 8:258–65. doi: 10.1136/bmjspcare-2017-001440
9. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. (2008) 33:997–1006. doi: 10.1139/H08-075
10. Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. (2004) 97:2333–8. doi: 10.1152/japplphysiol.00744.2004
11. Popuri K, Cobzas D, Esfandiari N, Baracos V, Jagersand M. Body composition assessment in axial CT images using FEM-based automatic segmentation of skeletal muscle. IEEE Trans Med Imaging. (2016) 35:512–20. doi: 10.1109/TMI.2015.2479252
12. Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. (1998) 351:871–5. doi: 10.1016/S0140-6736(97)11518-5
13. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. (1998) 85:115–22. doi: 10.1152/jappl.1998.85.1.115
14. Stene GB, Balstad TR, Leer ASM, Bye A, Kaasa S, Fallon M, et al. Deterioration in muscle mass and physical function differs according to weight loss history in cancer cachexia. Cancers (Basel). (2019) 11:1925. doi: 10.3390/cancers11121925
15. Agustsson T, Ryden M, Hoffstedt J, van Harmelen V, Dicker A, Laurencikiene J, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. (2007) 67:5531–7. doi: 10.1158/0008-5472.CAN-06-4585
16. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. (2013) 4:863. doi: 10.3389/fpsyg.2013.00863
17. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd edn. New York, NY: Taylor & Francis Inc (1988).
18. Prado CM, Purcell SA, Alish C, Pereira SL, Deutz NE, Heyland DK, et al. Implications of low muscle mass across the continuum of care: a narrative review. Ann Med. (2018) 50:675–93. doi: 10.1080/07853890.2018.1511918
19. Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press J Orthod. (2014) 19:27–9. doi: 10.1590/2176-9451.19.4.027-029.ebo
20. MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross-sectional body composition analysis. Curr Opin Support Palliat Care. (2011) 5:342–9. doi: 10.1097/SPC.0b013e32834c49eb
21. Baracos V, Kazemi-Bajestani SM. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int J Biochem Cell Biol. (2013) 45:2302–8. doi: 10.1016/j.biocel.2013.06.016
22. Shen W, Chen J, Gantz M, Velasquez G, Punyanitya M, Heymsfield SB. A single MRI slice does not accurately predict visceral and subcutaneous adipose tissue changes during weight loss. Obesity (Silver Spring, Md). (2012) 20:2458–63. doi: 10.1038/oby.2012.168
23. Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. (2013) 14:335–45. doi: 10.1016/S1470-2045(13)70055-X
24. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. (2016) 17:519–31. doi: 10.1016/S1470-2045(15)00558-6
25. Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. (2015) 16:108–16. doi: 10.1016/S1470-2045(14)71154-4
26. Ramage MI, Skipworth RJE. The relationship between muscle mass and function in cancer cachexia: smoke and mirrors? Curr Opin Support Palliat Care. (2018) 12:439–44. doi: 10.1097/SPC.0000000000000381
27. Tsoli M, Robertson G. Cancer cachexia: malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol Metab. (2013) 24:174–83. doi: 10.1016/j.tem.2012.10.006
28. Daas SI, Rizeq BR, Nasrallah GK. Adipose tissue dysfunction in cancer cachexia. J Cell Physiol. (2018) 234:13–22. doi: 10.1002/jcp.26811
29. Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. (2020) 292:1–9. doi: 10.1016/j.atherosclerosis.2019.10.021
30. Kerem M, Ferahkose Z, Yilmaz UT, Pasaoglu H, Ofluoglu E, Bedirli A, et al. Adipokines and ghrelin in gastric cancer cachexia. World J Gastroenterol. (2008) 14:3633–41. doi: 10.3748/wjg.14.3633
31. Diakowska D, Markocka-Maczka K, Szelachowski P, Grabowski K. Serum levels of resistin, adiponectin, and apelin in gastroesophageal cancer patients. Dis Markers. (2014) 2014:619649. doi: 10.1155/2014/619649
32. Smiechowska J, Utech A, Taffet G, Hayes T, Marcelli M, Garcia JM. Adipokines in patients with cancer anorexia and cachexia. J Investig Med. (2010) 58:554–9. doi: 10.2310/JIM.0b013e3181cf91ca
33. von Frankenberg AD, Silva FM, de Almeida JC, Piccoli V, do Nascimento FV, Sost MM, et al. Effect of dietary lipids on circulating adiponectin: a systematic review with meta-analysis of randomised controlled trials. Br J Nutr. (2014) 112:1235–50. doi: 10.1017/S0007114514002013
34. Wu JH, Cahill LE, Mozaffarian D. Effect of fish oil on circulating adiponectin: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. (2013) 98:2451–9. doi: 10.1210/jc.2012-3899
35. Abbott JH. The distinction between randomized clinical trials (RCTs) and preliminary feasibility and pilot studies: what they are and are not. J Orthop Sports Phys Ther. (2014) 44:555–8. doi: 10.2519/jospt.2014.0110
Keywords: cachexia, multimodal management, outcome measures, biomarkers, body composition, effect size, sample size (n)
Citation: Balstad TR, Brunelli C, Pettersen CH, Schønberg SA, Skorpen F, Fallon M, Kaasa S, Bye A, Laird BJA, Stene GB and Solheim TS (2021) Power Comparisons and Clinical Meaning of Outcome Measures in Assessing Treatment Effect in Cancer Cachexia: Secondary Analysis From a Randomized Pilot Multimodal Intervention Trial. Front. Nutr. 7:602775. doi: 10.3389/fnut.2020.602775
Received: 04 September 2020; Accepted: 01 December 2020;
Published: 14 January 2021.
Edited by:
Zeno Stanga, Bern University Hospital, SwitzerlandReviewed by:
Vickie Baracos, University of Alberta, CanadaVera C. Mazurak, University of Alberta, Canada
Copyright © 2021 Balstad, Brunelli, Pettersen, Schønberg, Skorpen, Fallon, Kaasa, Bye, Laird, Stene and Solheim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trude R. Balstad, trude.r.balstad@ntnu.no
 Trude R. Balstad
Trude R. Balstad Cinzia Brunelli3
Cinzia Brunelli3  Barry J. A. Laird
Barry J. A. Laird Guro B. Stene
Guro B. Stene