Association between red blood cell distribution width to albumin ratio and prognosis of patients with sepsis: A retrospective cohort study
- 1Department of Emergency, First People's Hospital of Foshan, Foshan, China
- 2The Poison Treatment Centre of Foshan, First People's Hospital of Foshan, Foshan, China
Background: Red blood cell distribution width (RDW) to albumin ratio (RAR) is associated with poor prognosis in diabetic comorbidities and cancer. However, the association between RAR and prognosis in patients with sepsis remains unclear, which was investigated in this study.
Methods: We conducted a retrospective cohort study based on the Medical Information Mart for Intensive Care (MIMIC) IV version 2.0 database. The primary outcome of this study was 28-day mortality. Secondary outcomes included 90-day mortality, in-hospital mortality, length of hospital stay, and length of intensive care unit (ICU) stay. Multivariate regression analysis and subgroup analysis were performed to investigate the association between RAR and prognosis in patients with sepsis.
Results: A total of 14,639 participants were included in this study. The mean age of the participants was 65.2 ± 16.3 years and the mean RAR was 5.5 ± 1.9 % /g/dl. For 28-day mortality, after adjusting for covariates, HRs [95% confidence intervals (CIs)] for tertiles 2 (4.4–5.8) and 3 (RAR > 5.8) were 1.33 (1.20, 1.46) and 1.98 (1.79, 2.19), respectively. Similar results were observed for 90-day mortality and in-hospital mortality. According to Kaplan-Meier curve analysis, the higher RAR group had higher 28-day mortality and 90-day mortality.
Conclusion: Our study shows that RAR is significantly associated with poor clinical prognosis in sepsis. The higher the RAR, the higher the 28-day, 90-day, and in-hospital mortality.
Introduction
Sepsis is a clinical syndrome caused by infection that manifests as systemic inflammation, and the host can suffer from organ dysfunction and even life-threatening effects caused by a dysregulated immune response to infection (1). Although some progress has been made in the management of sepsis, with morbidity and mortality rates trending downward year by year, reports indicate that the incidence of sepsis still ranges from 30 to 80% annually (2) and remains the leading cause of hospital deaths (3). Accurate prediction of the prognosis of sepsis is vital, which will facilitate early aggressive intervention (4). Unfortunately, although some scoring systems have been shown to correlate with outcomes in patients with sepsis (5–8), these scoring systems are inconvenient to use due to the numerous indicators involved, and cannot be used as a satisfactory predictive tool in clinical practice. Therefore, there is an urgent need for convenient biomarkers with good predictive power to help physicians identify high-risk patients and make treatment decisions.
Red blood cell distribution width (RDW) is a common clinical hematology indicator that reflects the heterogeneity of red blood cell size. As a simple and inexpensive parameter, RDW has been successfully used to predict the prognosis of many diseases, including cardiovascular disease, kidney disease, diabetes mellitus, and liver disease (9). Recent studies have also shown that RDW is significantly associated with the mortality of sepsis (10–15). The predictive power of RDW for mortality in sepsis is comparable to that of the Sepsis Associated Organ Failure Assessment (SOFA) and the Acute Physiology and Chronic Health Evaluation-II (APACHE-II) (16). Serum albumin is a negative phase protein that not only reflects systemic nutritional status but also has anti-inflammatory effects by reducing oxidative stress and inhibiting apoptosis of endothelial cells (17, 18). Serum albumin has also been reported to be a biomarker of prognosis in patients with sepsis (19). RDW to albumin ratio (RAR) is a novel and simple biomarker of inflammation. Previous studies have demonstrated that RAR is associated with the prognosis of diabetic ketoacidosis (20), diabetic retinopathy (21), and cancer (22). Previous studies have demonstrated the effectiveness of RAR in predicting the prognosis of diseases such as diabetic ketoacidosis, diabetic retinopathy, and cancer. However, it is unclear whether RAR is associated with the prognosis of patients with sepsis.
In this study, we evaluated the association between RAR and the prognosis of patients with sepsis. We presented the following articles according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) report checklist (23).
Methods
Data sources
Data for this retrospective study was extracted from the Medical Information Mart for Intensive Care (MIMIC)-IV version 2.0 database, which contains comprehensive data on 315,460 inpatients from 2008 to 2019. To access the MIMIC-IV version 2.0 database, we completed a training course on the National Institutes of Health (NIH) website and passed the “Protecting Human Research Participants” exam (author certification number: 46450588). The database was approved by the Institutional Review Board of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. To protect patient privacy, all private information in the database depository has been removed. Thus, informed consent and the ethical approval statement were waived for this study. The study was consistent with the Declaration of Helsinki-compliant principles.
Study population
To screen participants for this study, participants who met the following criteria were included in the study: (1) participants with a diagnosis of sepsis; (2) age ≥18 years; and (3) length of intensive care unit (ICU) stay ≥24 h. Exclusion criteria were as follows: (1) Patients with Sequential Organ Failure Assessment (SOFA) score < 2 were excluded; (2) Patients without RDW or albumin recording were excluded. The diagnosis of sepsis was based on the sepsis 3.0 criteria, which defined sepsis as a suspected or confirmed infection with a SOFA score of 2 or more (1).
Data extraction
We extracted the following variables from the MIMIC-IV version 2.0 database: sex, age, ethnicity, weight, comorbidities [myocardial infarction, congestive heart failure (CHF), cerebrovascular disease, chronic lung disease, liver disease, diabetes, renal disease, and malignancy], mean blood pressure (MBP), respiratory rate, heart rate, temperature, pulse oxygen saturation (SpO2), SOFA score, Simplified Acute Physiology Score (SAPS) II, ventilator use, vasopressors use and renal replacement therapy (RRT) use. Laboratory variables included white blood cells (WBC), hemoglobin (HGB), platelets (PLT), hematocrit (HCT), RDW, albumin, anion gap, sodium, chloride, glucose, blood urea nitrogen (BUN), serum creatinine (Scr), partial thromboplastin time (PPT), alanine transaminase (ALT). Exposure factor RAR is equal to RDW divided by albumin. Data were extracted through Navicat Premium version 15.0. For missing data in continuous variables, we impute with the median of non-missing values. Covariates will be excluded if they have ≥10% missing values.
Study endpoints
In this study, the primary outcome was 28-day mortality. Secondary outcomes included 90-day mortality, in-hospital mortality, length of hospital stay, and length of ICU stay.
Statistical analysis
All participants were divided into three groups according to the tertiles of RAR for descriptive analysis. Data for continuous variables were expressed as mean ± standard deviation (SD) or median (IQR), and between-group differences were compared by t-test or one-way ANOVA. For categorical variables, data were expressed as frequencies or percentages and analyzed using Chi-square or Fisher test.
Multivariate Cox proportional hazards models for the association between RAR and 28 and 90-day mortality were constructed, and the hazard ratio (HR) of mortality was calculated. We also used logistic regression and linear regression to evaluate the association between RAR and in-hospital mortality, length of ICU stay, and length of hospital stay, respectively. In the multiple regression analysis models, adjusted covariates were selected based on the association of the covariates with clinical outcomes or a change in the effective estimate of more than 10%. In model I, no covariates was adjusted. Model II adjusted for sex and age. In model III, the covariates included sex, age, ethnicity, weight, SAPS II score, Charlson Comorbidity Index, SOFA score, septic shock, myocardial infarction, CHF, cerebrovascular disease, chronic lung disease, liver disease, diabetes, renal disease, malignancy. In model IV, respiratory rate, heart rate, temperature, SpO2, MBP, WBC, HGB, PLT, anion gap, sodium, chloride, Scr, BUN, glucose, PTT, ALT, RRT use, ventilator use, and vasopressor use were further adjusted.
We used a smoothed curve fit to assess the association between RAR and sepsis 28-day mortality. Kaplan-Meier curve method was used to compare the probability of survival for different levels of RAR groups. ROC curve analysis was applied to evaluate whether RAR combined with SAPS II score and SOFA score could improve the predictive value of 28-day mortality in sepsis. Statistical analyses and plotting were performed using the R software (version 4.0.0) and free statistics software (version 1.5). A two-sided p < 0.05 was considered statistically significant.
Subgroup analysis and sensitivity analysis
To assess the robustness of the findings, we explored whether the associations differed between different subgroups, including sex, age, septic shock, myocardial infarction, CHF, chronic lung disease, liver disease, diabetes mellitus, renal disease, malignancy, SOFA score, and SAPS II score. We performed two sensitivity analyses for the results. First, sensitivity analysis was performed after removing patients with missing values. Second, considering the infusion of red blood cells or human serum albumin before ICU admission, there may be effects on the exposure factor RAR. Therefore, another sensitivity analysis was performed after removing patients who were transfused with red blood cells and human serum albumin2 days before ICU admission.
Results
Population and baseline characteristics
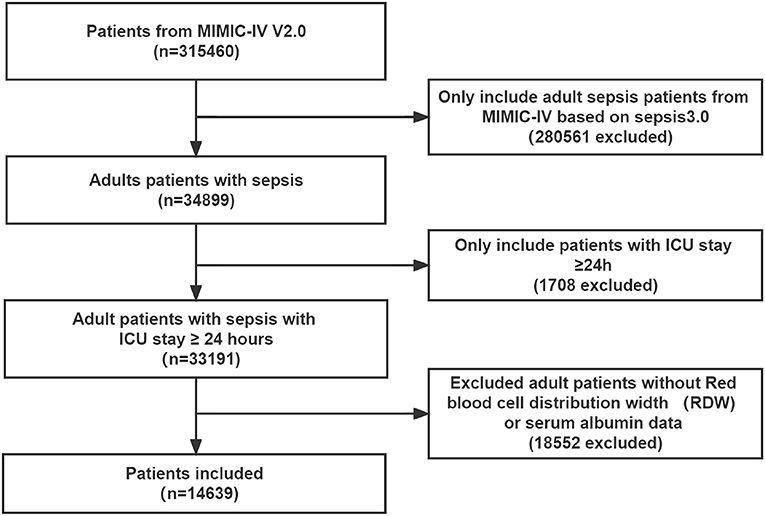
Of the 315,460 patients in the MIMIC-IV version 2.0 database, a total of 34,899 met the sepsis-3.0 definition. All patients were ≥18 years old, of which 1708 records were excluded due to length of ICU stay < 24 h, and 18,552 records were excluded due to lack of RDW or albumin information. Therefore, 14,639 patients with sepsis were finally included in this study (Figure 1).
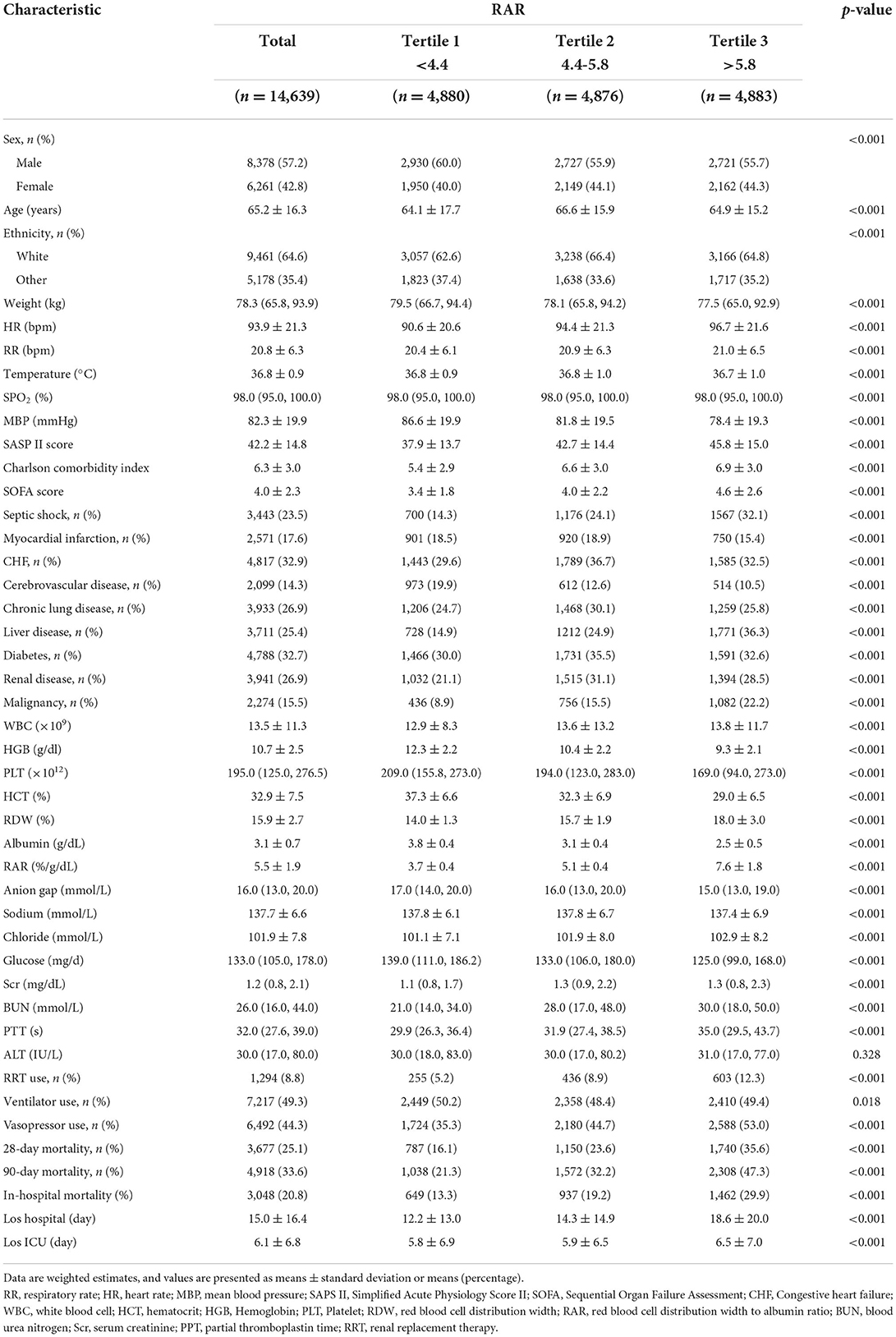
The demographic sample characteristics of all participants are shown in Table 1. The mean age of the patients was 65.2 years, of which 6,261 (42.8%) were female and 8,379 (57.2%) were male. The mean baseline value of RAR was 5.5 ± 1.9 %/g/dl. The majority of patients were white (64.6%). The main comorbidities were CHF (32.9%) and diabetes mellitus (32.7%). Based on RAR values, participants in this study were divided into tertiles (< 4.4, 4.4–5.8, and >5.8). Patients with higher RAR had a higher SOFA score, SAPS II score, and RDW, but lower HGB, HCT, and albumin. Among patients with high RAR, the prevalence of liver disease and malignancy is significantly higher, and the probability of progression to septic shock is also greater. There was a trend toward increased use of vasopressor and RRT in patients with higher RAR. For the study endpoints, the 28, 90-day, and in-hospital mortality were 25.1, 35.6, and 20.8%, respectively. The mean length of hospital stay and ICU stay were 15.0 ± 16.4 days and 6.1 ± 6.8 days, respectively. Patients with elevated RAR had significantly higher 28, 90-day, and in-hospital mortality, and longer length of hospital stay and ICU stay (all P < 0.001).
Association between RAR and clinical outcomes
To evaluate the linear association between RAR and mortality in patients with sepsis, we performed a smooth curve fitting. After adjusting for confounding variables, a linear association between RAR and 28-day mortality was observed (Figure 2). In addition, a similar linear association was observed in the analysis of 90-day mortality or in-hospital mortality (Supplementary Figures S1, S2).
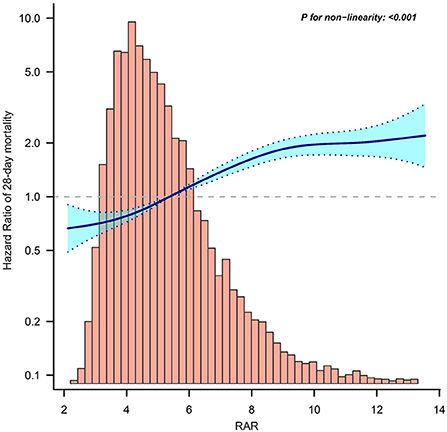
Figure 2. Curve fitting of RAR and 28-day mortality in patients with sepsis. RAR, Red blood cell distribution width to albumin ratio.
To assess cumulative survival at different levels of RAR, we generated 28-day survival curves for patients with sepsis by stratifying according to the RAR tertiles. Kaplan-Meier analysis showed that patients in the low RAR group had a significantly higher 28-day survival (p < 0.001). In addition, similar results were observed in the 90-day survival curves (Figure 3).
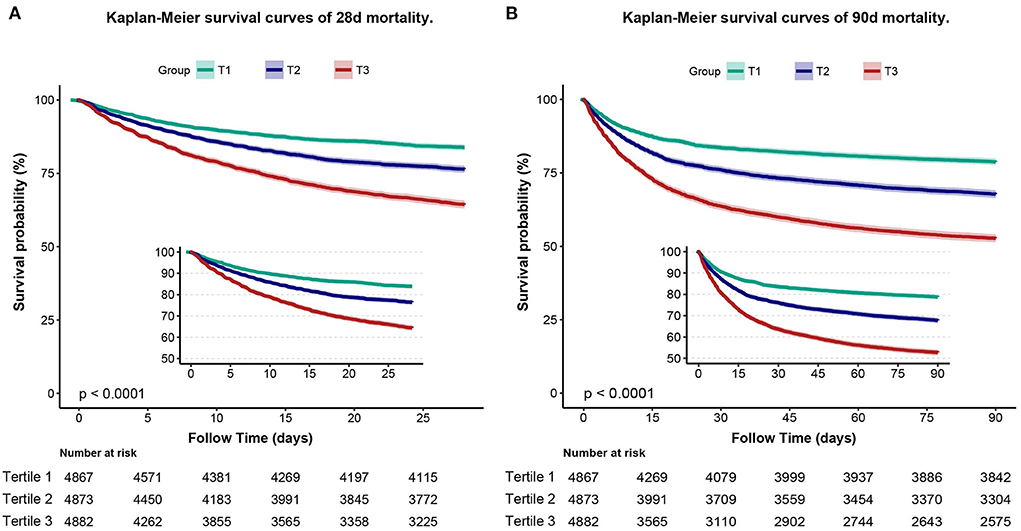
Figure 3. Kaplan–Meier curve of 28-day mortality for patients with sepsis (A). Kaplan–Meier curve of 90-day mortality for patients with sepsis (B).
After univariate Cox regression analysis (Supplementary Table S1), extended multivariate models revealed significant associations between RAR and different clinical outcomes (Table 2). For 28-day mortality, in model I unadjusted for variables, the HRs (95% CI) for tertile 2 and tertile 3 were 1.52 (1.39, 1.67) and 2.47 (2.27, 2.69), respectively, compared with the reference group tertile 1 (p < 0.001). This association remained statistically significant even after adjusting for sex, age, ethnicity, weight, myocardial infarction, CHF, cerebrovascular disease, chronic lung disease, liver disease, diabetes, kidney disease, malignancy, HR, RR, temperature, SpO2, MBP, WBC, HGB, PLT, HCT, anon gap, sodium, chloride, Scr, BUN, glucose, PTT, ALT, RRT use, ventilator use, and vasopressor use. In model IV, the adjusted HR (95% CI) was 1.26 (1.14, 1.39) and 1.74 (1.57, 1.93) for tertile 2 and tertile 3, respectively, compared to the reference group tertile 1 (p < 0.001). When analyzed as a continuous variable, RAR was associated with 28-day mortality. HRs (95% CI) in the four models were 1.14 (1.13, 1.15), 1.14 (1.13, 1.15), 1.09 (1.08, 1.11), and 1.09 (1.08, 1.11), respectively (all p < 0.001). In the analysis of in-hospital mortality and 90-day mortality, similar results were observed. In addition, we analyzed the association between RAR and length of hospital stay or ICU stay. Linear regression results showed that the beta values (95% CIs) for the length of ICU stay in the four models were 0.15 (0.10, 0.21), 0.16 (0.10, 0.21), 0.08 (0.02, 0.14), and 0.09 (0.03, 0.16), respectively. The beta values (95% CIs) for length of hospital stay in the four models were 1.39 (1.26, 1.53), 1.41 (1.27, 1.54), 1.17 (1.02, 1.31) and 0.78 (0.62, 0.94), respectively (all p < 0.05).
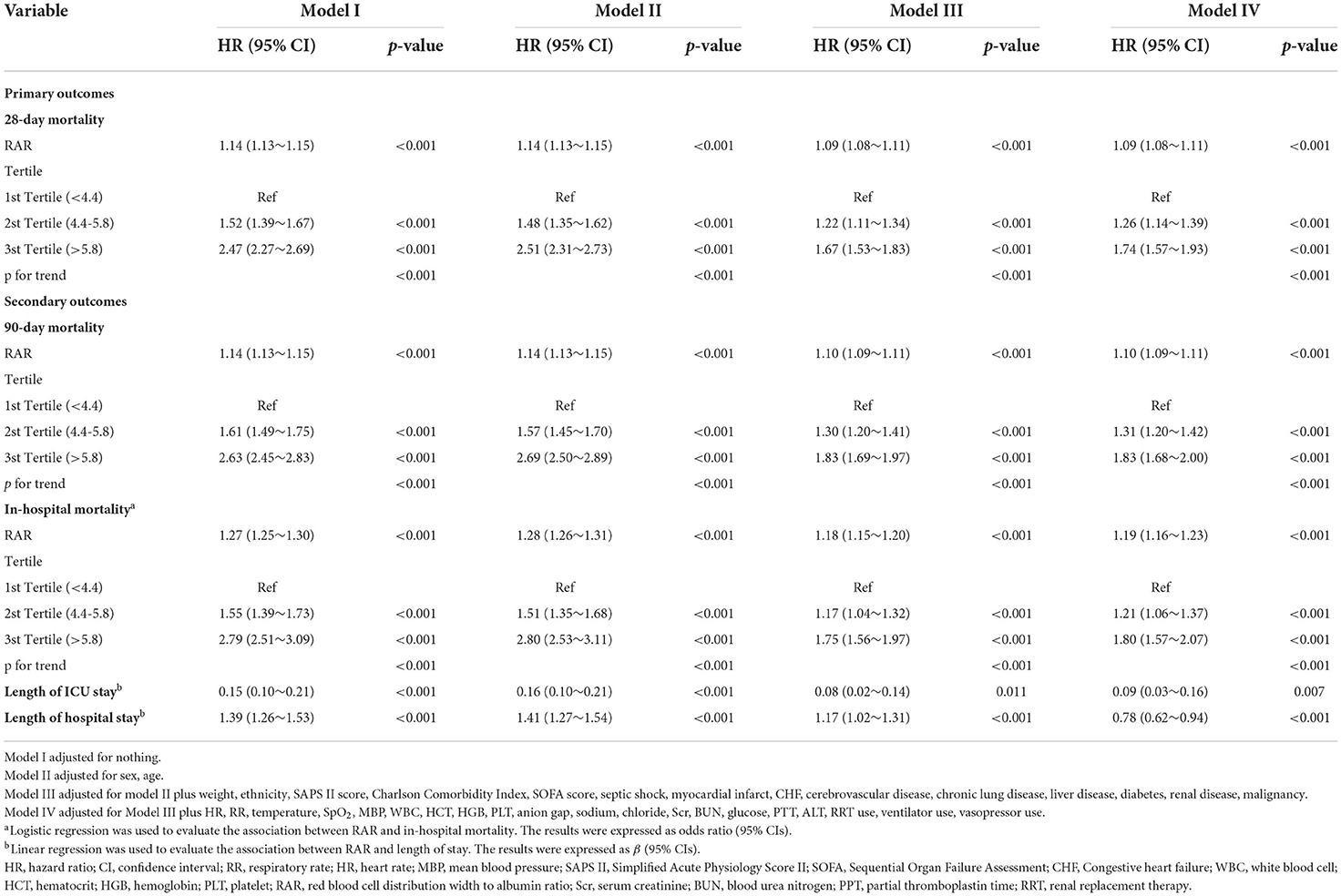
Table 2. Hazard ratio (HR) [95% confidence intervals (CIs)] for mortality across groups of ratio of red blood cell distribution width (RDW) to albumin (RAR) level.
Receiver operating characteristic analysis
To further evaluate the predictive value of RAR, RAW, albumin, SOFA score, SAPS II score, and RAR combined with different scoring systems in patients with sepsis for 28-day mortality, we constructed receiver operating characteristic (ROC) curves (Supplementary Figure S3; Supplementary Table S2). The results showed that the area under the ROC curve (AUC) (95% CI) for RAR, RDW, albumin, SOFA score, and SAPS II score were 0.633 (0.623, 0.644), 0.614 (0.604, 0.625), 0.602 (0.591, 0.613), 0.603 (0.593, 0.614), and 0.726 (0.717, 0.735), respectively. In addition, the AUC (95% CI) for RAR combined with SOFA score or SAPS II score was 0.656 (0.646, 0.667), 0.743 (0.733, 0.752).
Subgroup analysis and sensitivity analysis
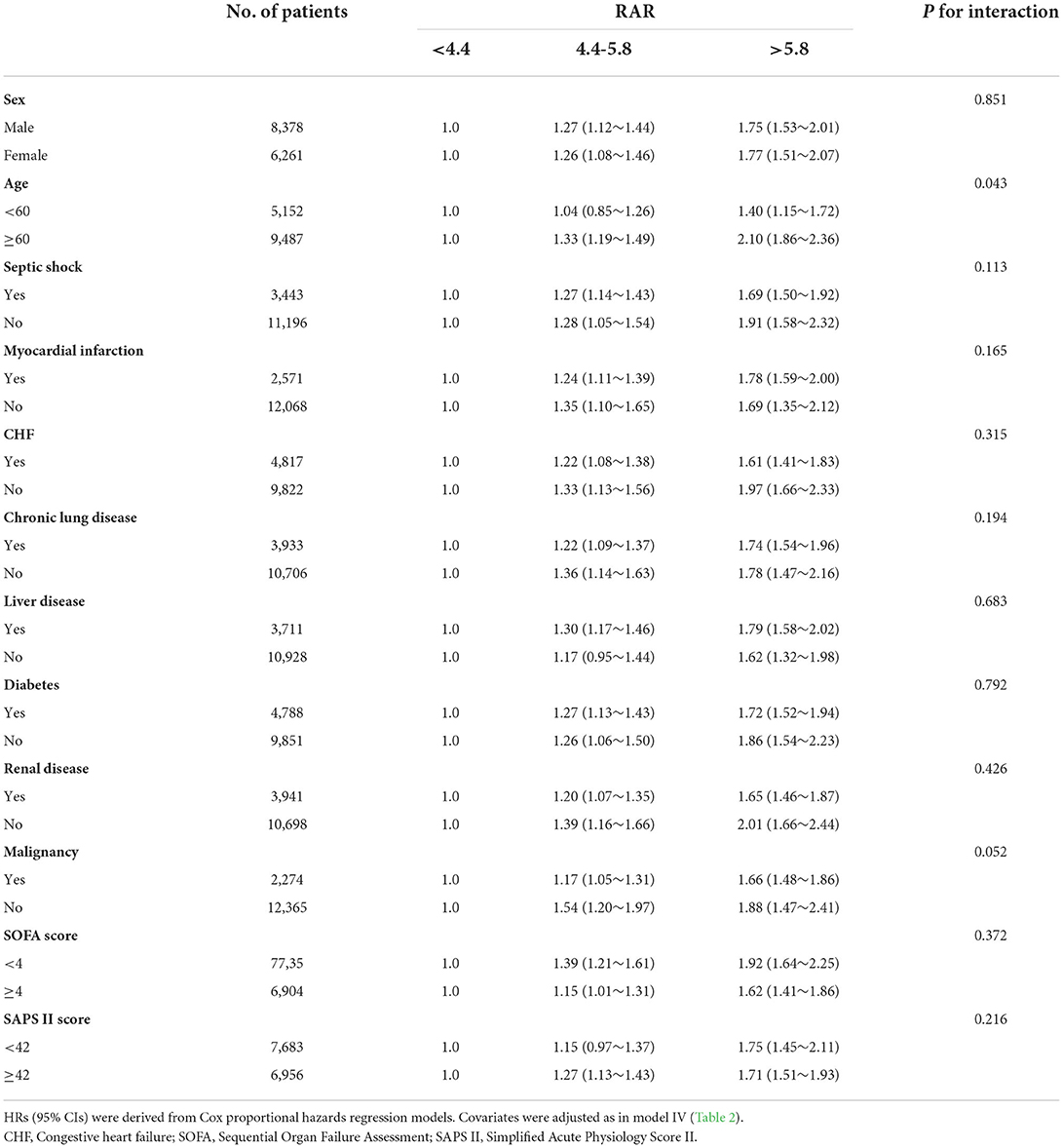
Table 3 shows the results of the subgroup analysis. There was an interaction between age and RAR on 28-day mortality (p for interaction =0.043). For elderly patients (≥60 years), 28-day mortality was higher with increasing RAR. The HRs (95% CI) for tertile 2 and tertile 3 were 1.33 (1.19, 1.49) and 2.10 (1.86, 2.36), respectively, compared with tertile 1 (p < 0.001). No significant interactions were observed in other subgroups (p for interaction > 0.05).
Two sensitivity analyses were performed to assess the robustness of the study results. After removal of patients with missing values, sensitivity analyses showed that the association between RAR and clinical outcomes in patients with sepsis remained strong (Supplementary Table S3). In addition, after removing patients who had received red blood cells and human serum albumin infusion 2 days before ICU admission, sensitivity analyses were performed again, and the results were consistent with our main finding that RAR remained significantly associated with clinical outcomes in patients with sepsis (Supplementary Table S4).
Discussion
In this study, we investigate for the first time the association between RAR and poor clinical outcomes in patients with sepsis. We found that in patients with sepsis, elevated RAR was significantly associated with 28 days, 90 days, in-hospital mortality, length of ICU stay, and length of hospital stay. ROC curves showed that RAR had good predictive power for 28-day mortality in patients with sepsis.
Hematopoietic dysfunction occurs frequently during sepsis. Systemic infections and inflammation can inhibit erythropoietin production and affect erythrocyte maturation, leading to an increased proportion of immature erythrocytes in the circulation (24). Inflammatory factors can reduce iron utilization and promote erythrocyte apoptosis, leading to the development of sepsis-associated anemia (25, 26). In addition, inflammatory factors can also affect the cell membrane glycoproteins and ion channels of erythrocytes, resulting in altered erythrocyte morphology (27, 28). All of these pathological changes increase the heterogeneity of red blood cell volume and lead to elevated RDW. A recent study reported that all-cause mortality in patients with sepsis increased with increasing RDW values and RDW was an effective predictor of sepsis prognosis (15). Serum albumin is an important protein with inflammatory, nutritional, and blood rheological properties, inhibiting platelet activation and aggregation (29, 30). Reduced serum albumin levels are usually associated with increased blood viscosity and impaired endothelial function (31). Albumin has been proposed as a reliable predictor of prognosis in critically ill patients (32). Recent studies have shown that hypoalbuminemia is associated with poor prognosis in patients with sepsis (33, 34).
RAR is a new indicator combining RDW and albumin, which is widely studied in various inflammation-related diseases. Long et al. revealed that RAR is a risk factor for prognosis in patients with aortic aneurysms (35). Zhou et al. reported that high RAR was significantly associated with increased all-cause mortality in diabetic ketoacidosis and an increased incidence of DKA-related infections (20). In addition, RAR showed good predictive power for the prognosis of cancer (22) and acute respiratory distress syndrome (36), both of which are associated with sepsis (37). However, no studies have reported an association between RAR and sepsis. Therefore, we hypothesized that RAR is also associated with prognosis in sepsis. This study enrolled 14,639 patients with sepsis in the intensive care unit. The results showed that RAR was associated with increased patient mortality at 28, 90 days and in-hospital mortality, longer length of stay and ICU stay. It is suggested that the higher the RAR the poorer the clinical prognosis of patients with sepsis.
It is worth mentioning that SOFA score, SAPS II score are clinically used disease severity scores which are associated with prognosis of patients with sepsis. In the present study, we observed that patients with a higher RAR had a higher SOFA score, SAPS II score, a greater probability of progression to septic shock, and a higher proportion of vasopressors and RRT use. Superficially, RAR organically combines RDW and albumin, and reflects the levels of the two factors. In terms of clinical significance, RAR can comprehensively reflect the two pathological states of hematopoietic dysfunction and hypoalbuminemia. The results of the study suggest that there may be an association between RAR and the severity of the disease in patients with sepsis in ICU. In our further subgroup analysis, age (≥60 years) showed an interaction with RARin 28-day mortality, suggesting that RAR may be an important biomarker for predicting the prognosis of elderly patients with sepsis. We are cautious about the findings of subgroup analyses since these results may be influenced by heterogeneity among different populations. These results require further research to be confirmed.
In the present study, we observed that the area under the ROC curve of RAR was superior to that of RDW or albumin alone, suggesting that RAR has a higher predictive value for 28-d morbidity and mortality in sepsis patients than RDW or albumin alone. This can be explained as follows. Although both RDW and albumin are associated with sepsis prognosis and reflect a systemic inflammatory response, they show opposite responses in terms of inflammation. A strong inflammatory response can lead to a significant increase in RAR. In comparison to RDW and albumin alone, The RAR reflects not only the hematopoietic and nutritional status of the patient but also the severity of inflammation. Weng et al. reported that RAR was superior to RDW or albumin alone in predicting prognosis in patients after percutaneous coronary intervention (38), which is similar to the result of this study. In addition, we observed that the area under the ROC curve increased after RAR was combined with SAPS II score or SOFA score, suggesting that RAR combined with SAPS II or SOFA score further improves the predictive power of septic mortality.
Our study had some limitations. First, owing to the retrospective design, the population was a heterogeneous mixture of infection etiologies, although we tried our best to adjust for potential confounding confounders and perform subgroup analyses, it was still difficult to avoid selection bias and confounding bias, which is a limitation of all retrospective studies. Second, some variables in the MIMIC-IV database had a high number of missing values, including C-reactive protein, N-terminal pro-B-type natriuretic peptide (NT-proBNP), troponin t, lactate, and blood gas analysis. Considering that too many missing values may have an impact on the study results, we ultimately did not include these covariates in the regression analysis. Third, we only measured RAR at ICU admission, which may have influenced the results if the patient had been transfused with human serum albumin before ICU admission. Therefore, we performed a sensitivity analysis after excluding this cohort. Fourth, some comorbidities, such as chronic liver disease and chronic kidney disease, may have an impact on RDW and albumin levels. We performed subgroup analyses on these populations to verify the robustness of the results. Finally, our results suggested that RAR is associated with poor clinical prognosis of sepsis. These findings are hypothesis-generating and should be considered exploratory. We believe that a carefully designed, multicenter prospective study is needed to validate our results.
Conclusions
RAR is a potential prognostic indicator for patients with sepsis and is associated with a poor clinical prognosis. The higher the RAR, the higher the 28, 90-day, and in-hospital mortality.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://physionet.org/content/mimiciv.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Written informed consent for participation was not required for this study in accordance with the National Legislation and the Institutional requirements.
Author contributions
WX and JJ designed the study. JX, GC, and JH conducted data collection and data analysis. WX wrote the manuscript. KY, ZH, and LP analyzed and interpreted the result. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center for the MIMIC-IV database. We thank Dr. Jie Liu of the Department of Vascular and Internal Medicine, People's Liberation Army General Hospital, China, for his assistance. Dr. Jie Liu, Department of Vascular and Internal Medicine, People's Liberation Army General Hospital, China, for his contributions to statistical support, study design consultation, and comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1019502/full#supplementary-material
Abbreviations
RR, respiratory rate; HR, heart rate; MBP, mean blood pressure; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; CHF, Congestive heart failure; WBC, white blood cell; HCT, hematocrit; HGB, Hemoglobin; PLT, Platelet; RDW, red blood cell distribution width; RAR, red blood cell distribution width to albumin ratio; BUN, blood urea nitrogen; Scr, serum creatinine; PPT, partial thromboplastin time; RRT, renal replacement therapy.
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health. (2012) 2:010404. doi: 10.7189/jogh.01.010404
3. Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. (2014) 312:90–2. doi: 10.1001/jama.2014.5804
4. Chen KF, Liu SH Li CH, Wu CC, Chaou CH, Tzeng IS, et al. Development and validation of a parsimonious and pragmatic CHARM score to predict mortality in patients with suspected sepsis. Am J Emerg Med. (2017) 35:640–6. doi: 10.1016/j.ajem.2016.10.075
5. Wu Q, Xiao Z, Pu Y, Zhou J, Wang D, Huang Z, et al. TnI and IL-18 levels are associated with prognosis of sepsis. Postgrad Med J. (2019) 95:240–4. doi: 10.1136/postgradmedj-2018-136371
6. Chen Q, Zhang L, Ge S, He W, Zeng M. Prognosis predictive value of the Oxford acute severity of illness score for sepsis: a retrospective cohort study. PeerJ. (2019) 7:e7083. doi: 10.7717/peerj.7083
7. Letendre JA, Goggs R. Determining prognosis in canine sepsis by bedside measurement of cell-free DNA and nucleosomes. J Vet Emerg Crit Care (San Antonio). (2018) 28:503–11. doi: 10.1111/vec.12773
8. Liu J, Shi K, Chen M, Xu L, Hong J, Hu B, et al. Elevated miR-155 expression induces immunosuppression via CD39(+) regulatory T cells in sepsis patient. Int J Infect Dis. (2015) 40:135–41. doi: 10.1016/j.ijid.2015.09.016
9. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52:86–105. doi: 10.3109/10408363.2014.992064
10. Seppa K, Sillanaukee P. High red cell distribution width in alcoholics: not due to liver disease. JAMA. (1992) 268:1413. doi: 10.1001/jama.268.11.1413c
11. Baqar MS, Khurshid M, Molla A. Does red blood cell distribution width (RDW) improve evaluation of microcytic anaemias? J Pak Med Assoc. (1993) 43:149–51.
12. Hammarsten O, Jacobsson S, Fu M. Red cell distribution width in chronic heart failure: a new independent marker for prognosis? Eur J Heart Fail. (2010) 12:213–4. doi: 10.1093/eurjhf/hfp208
13. Saigo K, Jiang M, Tanaka C, Fujimoto K, Kobayashi A, Nozu K, et al. Usefulness of automatic detection of fragmented red cells using a hematology analyzer for diagnosis of thrombotic microangiopathy. Clin Lab Haematol. (2002) 24:347–51. doi: 10.1046/j.1365-2257.2002.00473.x
14. Cakal B, Akoz AG, Ustundag Y, Yalinkilic M, Ulker A, Ankarali H. Red cell distribution width for assessment of activity of inflammatory bowel disease. Dig Dis Sci. (2009) 54:842–7. doi: 10.1007/s10620-008-0436-2
15. Zhang L, Yu CH, Guo KP, Huang CZ, Mo LY. Prognostic role of red blood cell distribution width in patients with sepsis: a systematic review and meta-analysis. BMC Immunol. (2020) 21:40. doi: 10.1186/s12865-020-00369-6
16. Lorente L, Martin MM, Argueso M, Sole-Violan J, Perez A, Marcos YRJA, et al. Association between red blood cell distribution width and mortality of COVID-19 patients. Anaesth Crit Care Pain Med. (2021) 40:100777. doi: 10.1016/j.accpm.2020.10.013
17. Wang X, Wang J, Wu S, Ni Q, Chen P. Association between the neutrophil percentage-to-albumin ratio and outcomes in cardiac intensive care unit patients. Int J Gen Med. (2021) 14:4933–43. doi: 10.2147/IJGM.S328882
18. Zhao N, Hu W, Wu Z, Wu X, Li W, Wang Y, et al. The red blood cell distribution width-albumin ratio: a promising predictor of mortality in stroke patients. Int J Gen Med. (2021) 14:3737–47. doi: 10.2147/IJGM.S322441
19. Yin M, Si L, Qin W, Li C, Zhang J, Yang H, et al. Predictive value of serum albumin level for the prognosis of severe sepsis without exogenous human albumin administration: a prospective cohort study. J Intensive Care Med. (2018) 33:687–94. doi: 10.1177/0885066616685300
20. Zhou D, Wang J, Li X. The red blood cell distribution width-albumin ratio was a potential prognostic biomarker for diabetic ketoacidosis. Int J Gen Med. (2021) 14:5375–80. doi: 10.2147/IJGM.S327733
21. Zhao F, Liu M, Kong L. Association between red blood cell distribution width-to-albumin ratio and diabetic retinopathy. J Clin Lab Anal. (2022) 36:e24351. doi: 10.1002/jcla.24351
22. Lu C, Long J, Liu H, Xie X, Xu D, Fang X, et al. Red blood cell distribution width-to-albumin ratio is associated with all-cause mortality in cancer patients. J Clin Lab Anal. (2022) 36:e24423. doi: 10.1002/jcla.24423
23. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
24. Macdougall IC, Cooper AC. Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant. (2002) 17 (Suppl 11):39–43. doi: 10.1093/ndt/17.suppl_11.39
25. Bateman RM, Sharpe MD, Singer M, Ellis CG. The effect of sepsis on the erythrocyte. Int J Mol Sci. (2017) 18:1932. doi: 10.3390/ijms18091932
26. Ekregbesi P, Shankar-Hari M, Bottomley C, Riley EM, Mooney JP. Relationship between anaemia, haemolysis, inflammation and haem oxygenase-1 at admission with sepsis: a pilot study. Sci Rep. (2018) 8:11198. doi: 10.1038/s41598-018-29558-5
27. Piagnerelli M, Boudjeltia KZ, Brohee D, Piro P, Carlier E, Vincent JL, et al. Alterations of red blood cell shape and sialic acid membrane content in septic patients. Crit Care Med. (2003) 31:2156–62. doi: 10.1097/01.CCM.0000079608.00875.14
28. Todd JC. 3rd, Mollitt DL. Sepsis-induced alterations in the erythrocyte membrane. Am Surg. (1994) 60:954–7.
29. Ozcan Cetin EH, Konte HC, Temizhan A. Blood viscosity should not be overlooked when evaluating the fibrinogen to albumin ratio. Angiology. (2019) 70:465–6. doi: 10.1177/0003319718822244
30. Gresele P, Deckmyn H, Huybrechts E, Vermylen J. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol. (1984) 33:2083–8. doi: 10.1016/0006-2952(84)90577-X
31. Joles JA, Willekes-Koolschijn N, Koomans HA. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. (1997) 52:761–70. doi: 10.1038/ki.1997.393
32. Lee JH, Kim J, Kim K, Jo YH, Rhee J, Kim TY, et al. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care. (2011) 26:287–94. doi: 10.1016/j.jcrc.2010.10.007
33. Artero A, Zaragoza R, Camarena JJ, Sancho S, Gonzalez R, Nogueira JM. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J Crit Care. (2010) 25:276–81. doi: 10.1016/j.jcrc.2009.12.004
34. Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. (1997) 50:693–703. doi: 10.1016/S0895-4356(97)00015-2
35. Long J, Xie X, Xu D, Huang C, Liu Y, Meng X, et al. Association between red blood cell distribution width-to-albumin ratio and prognosis of patients with aortic aneurysms. Int J Gen Med. (2021) 14:6287–94. doi: 10.2147/IJGM.S328035
36. Yoo JW, Ju S, Lee SJ, Cho YJ, Lee JD, Kim HC. Red cell distribution width/albumin ratio is associated with 60-day mortality in patients with acute respiratory distress syndrome. Infect Dis. (2020) 52:266–70. doi: 10.1080/23744235.2020.1717599
37. Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. (2017) 318:1241–9. doi: 10.1001/jama.2017.13836
38. Weng Y, Peng Y, Xu Y, Wang L, Wu B, Xiang H, et al. the ratio of red blood cell distribution width to albumin is correlated with all-cause mortality of patients after percutaneous coronary intervention - a retrospective cohort study. Front Cardiovasc Med. (2022) 9:869816. doi: 10.3389/fcvm.2022.869816
Keywords: sepsis, red blood cell distribution width, albumin, RAR, prognosis, MIMIC-IV
Citation: Xu W, Huo J, Chen G, Yang K, Huang Z, Peng L, Xu J and Jiang J (2022) Association between red blood cell distribution width to albumin ratio and prognosis of patients with sepsis: A retrospective cohort study. Front. Nutr. 9:1019502. doi: 10.3389/fnut.2022.1019502
Received: 15 August 2022; Accepted: 05 September 2022;
Published: 23 September 2022.
Edited by:
Francesco Alessandri, Sapienza University of Rome, ItalyReviewed by:
Iokfai Cheang, Nanjing Medical University, ChinaMenghuan Li, Nanjing Medical University, China
Copyright © 2022 Xu, Huo, Chen, Yang, Huang, Peng, Xu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Jiang, jiangjungd2@163.com
†ORCID: Weigan Xu orcid.org/0000-0002-4440-7246
 Weigan Xu
Weigan Xu Jianyang Huo1,2
Jianyang Huo1,2