Association between obesity and age-related cataract: an updated systematic review and dose–response meta-analysis of prospective cohort studies
- 1Translational Ophthalmology Research Center, Farabi Eye Hospital, Tehran, Iran
- 2Ophthalmic Research Center, Research Institute for Ophthalmology and Vision Science, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3John A. Moran Eye Center, University of Utah, Salt Lake City, UT, United States
- 4Department of Ophthalmology and Visual Science, Rutgers New Jersey Medical School, Newark, NJ, United States
- 5Clinical Research Development Center, Shahid Modarres Educational Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 6Negah Specialty Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 7Instituto de Olhos Renato Ambrósio, Rio de Janeiro, Brazil
- 8Rio de Janeiro Corneal Tomography and Biomechanics Study Group, Rio de Janeiro, Brazil
- 9BrAIN: Brazilian Artificial Intelligence Networking in Medicine, Rio de Janeiro, Brazil
- 10Department of Ophthalmology, Federal University of the State of Rio de Janeiro (UNIRIO), Rio de Janeiro, Brazil
- 11Department of Ophthalmology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
Objective: There are inconsistent findings on the association between obesity and age-related cataract (ARC). This systematic review was done to summarize available findings on the association between obesity [defined by body mass index (BMI)] and ARC by performing a dose–response meta-analysis on eligible prospective cohort studies.
Methods: We performed a systematic search in PubMed, Scopus, ISI Web of Knowledge, and Google Scholar until June 2022 to identify eligible publications.
Results: In total, 16 studies with a total sample size of 1,607,125 participants were included. Among all of these studies, there were 103,897 cases of ARC. In the follow-up periods ranging between 4 and 28 years, 4,870 cases of nuclear cataract, 1,611 cases of cortical cataract, and 1,603 cases of posterior subcapsular cataracts (PSC) were detected. By comparing the highest and lowest categories of BMI, we found that higher BMI was associated with an increased risk of ARC (RR: 1.18, 95% CI: 1.09–1.28) and PSC (RR: 1.44, 95% CI: 1.08–1.90). In the dose–response analysis, each 5 kg/m2 increase in BMI was associated with a 6 and 27% increased risk of ARC (RR: 1.06, 95% CI: 1.01–1.12) and PSC (RR: 1.27, 95% CI: 1.14–1.41), respectively. In addition, we found a positive association for cortical cataract among high-quality studies, in which higher BMI was associated with a 20% increased risk of cortical cataract (RR: 1.20, 95% CI: 1.02–1.42). In terms of nuclear cataract, we found no significant association either in the comparison between the highest and lowest categories of BMI or in the dose–response meta-analysis.
Conclusion: Obesity (defined by BMI) was associated with an increased risk of ARC, PSC, and cortical cataract in adults. However, such a positive association was not seen for nuclear cataract.
PROSPERO registration: CRD42022357132.
1 Introduction
Cataract is one of the top causes of visual impairment and blindness among the elderly (1, 2). The subtypes of cataracts include cortical, nuclear, and posterior subcapsular cataracts (PSC), with an age-standard pooled prevalence of 8.05, 8.22, and 2.24%, respectively, in the general population (3). In addition to morbidities, the presence of cataracts is associated with increased mortality (4).
Research has focused on age-related cataracts (ARC) as an inevitable aging issue, which has an increased risk in the presence of genetic and environmental factors (5). Among environmental and lifestyle factors, it has been shown that illiteracy, smoking, wine drinking, as well as underlying diseases such as hypertension and diabetes mellitus (DM) are the risk factors for ARC (6). Since obesity contributes to the etiology of DM and hypertension, it may affect the risk of ARC (7–9). However, the results from epidemiological studies investigating the association between obesity and ARC are inconsistent (10–25). In a cohort study of 1,312,051 adults, Floud et al. reported a significant positive association between obesity and ARC risk. However, some studies reported no significant association in this regard (10, 13, 14). Also, we found a cohort study in which obesity was associated with a reduced risk of ARC (22).
Two meta-analyses of Ye et al. (26) and Pan et al. (2), published in 2014, assessed the association between obesity and ARC and reported a significant positive association for ARC. However, findings from the two meta-analyses for cataract subtypes were different: Pan et al. reported a significant positive association in terms of nuclear and cortical cataracts, while Ye et al. did not find any significant association in this regard. It should be noted that these meta-analyses missed some eligible studies (14–18, 20, 25). In addition, these meta-analyses included some studies that were not eligible for this topic. For instance, in the association between body mass index (BMI) and ARC, they included the study of Chang et al. in which the link between weight change and ARC was evaluated (27). Also, Ye et al. (26) included the study of Lindblad et al. in which the relation between waist circumference and ARC was examined (28). These limitations may affect the results of these meta-analyses. Furthermore, none of these meta-analyses determined linear and non-linear dose–response associations between BMI and ARC. Therefore, the current systematic review and dose–response meta-analysis was conducted to determine the relation between BMI and ARC by summarizing available findings from prospective cohort studies.
2 Methods
2.1 Search strategy and study selection
This study was conducted using the preferred reporting items for systematic review and meta-analysis (PRISMA) standards (29). To find pertinent papers up to June 2022, we conducted a thorough search of the online databases of PubMed, Scopus, and ISI Web of Science. In the systematic search, we utilized both MeSH (medical subject heading terms) and non-MeSH terms (Supplementary Table 1). The publishing schedule and language of the pieces were both unrestricted. Following the thorough search, all of the results were imported into Endnote software before the screening process began. In Endnote, duplicate citations were eliminated. We also performed a web-based search in Google Scholar using the phrases “body mass index” and “cataract” in addition to the databases already listed. In order to ensure that we did not overlook any publications, we lastly checked the reference list of the chosen articles.
The following criteria were considered to select eligible studies in the screening step: (1) studies with prospective cohort designs, such as prospective cohort, nested case–control, and case-cohort studies; (2) studies on healthy adults (18 years); (3) studies measuring BMI to assess general obesity; (4) studies taking into account ARC or its subtypes, such as nuclear, cortical, or PSC as an outcome variable; and (5) studies reporting hazard ratio (HR), risk ratio (RR), and odds ratio (OR), with 95% confidence intervals for the association between obesity and ARC. If we found two papers that were published on a population, only the paper with higher quality or the most number of cases was included in our systematic review and meta-analysis. We disregarded retrospective, case–control, and cross-sectional studies as well as cohort studies that enrolled critically ill patients or people with chronic illnesses including diabetes mellitus and chronic kidney disease. Additionally, studies that did not report relative risks for the link between obesity and cataracts or lacked the necessary information to calculate these effect sizes were disregarded. Two independent reviewers chose the studies by taking the inclusion and exclusion criteria into account.
2.2 Data extraction and quality assessment
Two independent reviewers extracted data from each selected article and entered the data in an Excel-based form that was previously designed. Any discrepancies were discussed with a third reviewer in order to be rectified. Based on the form, the following information was extracted from each article: first author’s name, year of publication, cohort name, geographical region, characteristics of participants (age and gender), sample size, number of cases with ARC, follow-up period, methods used for the assessment of obesity and cataract, relative risk estimates, including ORs, RRs, and HRs for the link between obesity and ARC risk, and confounding variables adjusted in statistical analysis. If research did not offer the necessary estimations, we calculated them using conventional techniques.
We used the Newcastle Ottawa Scale (NOS), designed for prospective cohort studies, to assess the quality of included studies (30). Based on the NOS, each cohort study can get a maximum of nine points: four for the selection of participants, two for comparability, and three for the assessment of outcomes. In the current study, we categorized studies based on the median score of NOS in which studies with a score more than the median were considered high-quality ones.
2.3 Statistical analysis
We included the RRs, HRs, or ORs and 95% CIs reported for the association between obesity and ARC risk into the meta-analysis. These RRs were calculated based on the comparison between the highest versus lowest categories of BMI. However, it should be noted that some studies reported RRs of ARC per one-SD increment in BMI. To include in the meta-analysis, we converted the per SD increment risk estimates to the RRs for the comparison of the top versus bottom tertiles of BMI using the method suggested by Danesh et al. in which the log risk estimates reported for the comparison between the top and bottom tertiles of exposure variable are equivalent to 2.18 times of the log risk estimates for a 1-SD increase in that variable (31). This method assumes that the exposure is a normally distributed variable and that the association with disease risk is log-linear. To combine the RRs of ARC, a random-effects model was used. Random-effects models take into consideration different sources of uncertainties including within-study (sampling or estimation) error and between-studies variance (32, 33). To assess heterogeneity among studies, we used Cochran’s Q test and the I2 statistic. For the I2 statistic, we considered the I2 values of >50% as high between-study heterogeneity (34). To find possible sources of heterogeneity, subgroup analyses were conducted. Publication bias was examined using Egger’s linear regression test for the associations with more than 10 effect sizes (35). In the case of substantial publication bias, the trim-and-fill method was used to detect the effect of possibly missing studies on the overall RR (36). To assess the dependency of overall ES on one study, sensitivity analysis was done using a random-effects model in which each study was excluded to examine the influence of that study on the overall estimate.
Since the highest and lowest categories of BMI were different across the included studies, we performed a dose–response meta-analysis to determine the RR of ARC at different levels of BMI. We applied the method described by Crippa et al. to do a dose–response meta-analysis (37). In this method, the number of participants and cases of cataract and also the RR of ARC in each category of exposure (BMI) were required. In each category of BMI, we considered the median or mean amount of BMI as the corresponding RR of ARC. For studies that reported BMI as ranges, we estimated the midpoint in each category by calculating the mean of the lower and upper bound. When the highest or lowest category was open-ended, the length of the open-ended interval was assumed to be the same as that of the adjacent interval. We conducted the one-stage dose–response meta-analysis using restricted maximum likelihood estimation to assess linear and non-linear associations (37). This method estimates the study-specific slopes and combines them to obtain an overall average slope in a single stage, and is a more precise, flexible, and efficient method than the traditional two-stage method. Statistical analyses were conducted using STATA version 14.0. p < 0.05 was considered statistically significant for all tests, including Cochran’s Q test.
3 Results
3.1 Findings from the systematic search
In our initial search, we found 3,777 articles among the online databases, of them, 750 papers were duplicated. After excluding duplicate papers, we screened the remaining articles (n = 3,027) and disqualified any research that failed to fulfill the inclusion requirements (n = 2,997) (Supplementary Figure 1). After full-text reviews, nine articles were excluded because of being conducted on patients who underwent kidney transplantation or those with chronic diseases (n = 2) (38, 39), having a case–control or cross-sectional design (40–42), assessing waist circumference, weight or weight changes rather than BMI (28, 43, 44), and reporting incomplete data (45). Also, we excluded the study of Yuan et al. because they genetically predicted BMI (46). In addition, we found three different articles from the Physicians’ Health Study (21, 47, 48), two different papers from the UK Biobank (12, 49), and two different publications from the Blue Mountains Eye Study (22, 50). Since these publications evaluated similar associations, only the study with the highest quality or the greatest number of cases was considered for each dataset (12, 21, 22) and the duplicate papers were excluded (47–50). Moreover, two articles were published on the Beaver Dam Eye Study; however, both assessed different exposure and outcome variables in terms of BMI and cataract, and therefore, both were included (14, 16). After these exclusions, 16 articles containing 16 prospective cohort studies were included in the current systematic review and meta-analysis (10–25): 10 articles assessed BMI and risk of ARC (10–14, 21–25), 10 articles evaluated BMI and risk of nuclear cataract (13–15, 17–23), 6 papers assessed BMI and risk of cortical cataract (13, 16, 17, 20–22), and 7 publications assessed BMI and risk of PSC (13, 16, 17, 20–23). The flowchart of study selection is shown in Supplementary Figure 1.
3.2 Characteristics of studies
Characteristics of prospective studies included in the current systematic review and meta-analysis are shown in Table 1. The sample size of these studies ranged from 372 to 1,312,051 participants. In total, these studies recruited 1,607,125 participants with an age range of ≥40 years. In addition, during follow-up periods ranging between 4 and 28 years, 103,897 cases of ARC, 4870 cases of nuclear cataract, 1,611 cases of cortical cataract, and 1,603 cases of PSC were detected. The included articles were published between 1998 and 2016. Among the 16 articles, one article included only males (21), two articles performed analysis on only females (12, 19), and the remaining articles included both genders in statistical analysis (10, 11, 13, 15–18, 20, 22) or presented gender-stratified risk estimates (14, 23–25). In terms of geographical region, included studies were conducted in the US (11, 13, 14, 16, 18–21, 23, 24), Europe (10, 12, 15), Asia (17, 25), and Australia (22). In 7 articles, researchers measured weight and height using a standard protocol for calculating BMI (13, 15, 17–20, 22), while in 9 studies, self-reported weight and height were used (10–12, 14, 16, 21, 23–25). Regarding outcome assessment, researchers performed a direct examination for cataract diagnosis in four articles (13, 17–19) and used data from medical records/registries in 3 articles (10, 12, 15). Among the remaining articles, self-reported data were used for cataract assessment. Of the 16 included articles, 13 papers presented adjusted risk estimates for the association between BMI and cataract risk (10–16, 19, 21–25). Some important confounding variables including age (n = 12), smoking (n = 9), and having diabetes mellitus (n = 8) were adjusted in these studies. By considering the median NOS score of 7, 13 articles, of the 16 papers, had high quality or low risk of bias in most components of NOS (10–16, 19–24) (Supplementary Table 2).

Table 1. Characteristics of prospective cohort studies investigating the association between BMI and risk of ARC in adults.
3.3 Findings from the systematic review
Of the 10 articles that assessed the association between BMI and risk of ARC, 6 papers showed a significant positive association (11, 12, 21, 23–25) and others did not find any significant association. In addition, two articles, among the 10 publications on the link between BMI and risk of nuclear cataract, indicated a significant positive association (19, 21), while the remaining articles reported a non-significant association. None of the studies that examined the association between BMI and risk of cortical cataract revealed a significant association. In terms of BMI and risk of PSC, a significant positive association was reported in 3 articles (16, 21, 23) of the 7 publications.
3.4 Findings from the meta-analysis
In this section, we included all studies that were evaluated in the systematic review. Below, findings from the meta-analysis were reported for ARC and its subtypes.
3.4.1 Meta-analysis on BMI and risk of ARC
Ten articles that included 11 studies assessed the association between BMI and risk of ARC (10–14, 21–25). The overall RR by comparing the highest and lowest categories of BMI was 1.18 (95% CI: 1.09–1.28, I2 = 67.7, Pheterogeneity < 0.001), indicating a significant positive association between BMI and ARC (Figure 1). However, between-study heterogeneity was significant in this regard. Subgroup analyses based on geographical region, gender, follow-up duration, and study quality reduced the heterogeneity, otherwise, these variables can be considered as possible sources of the observed heterogeneity. In the subgroup analyses, we found a significant positive association between BMI and risk of ARC in all subgroups except for studies that had a sample size of <10,000 participants.

Figure 1. Forest plot for the association between BMI and ARC risk by comparing the highest and lowest categories of BMI. The overall RR was obtained from a random-effects model. RR, relative risk; CI, confidence interval; BMI, body mass index; ARC, age-related cataract; NHS, nurse health study; HPFS, health professional follow-up study.
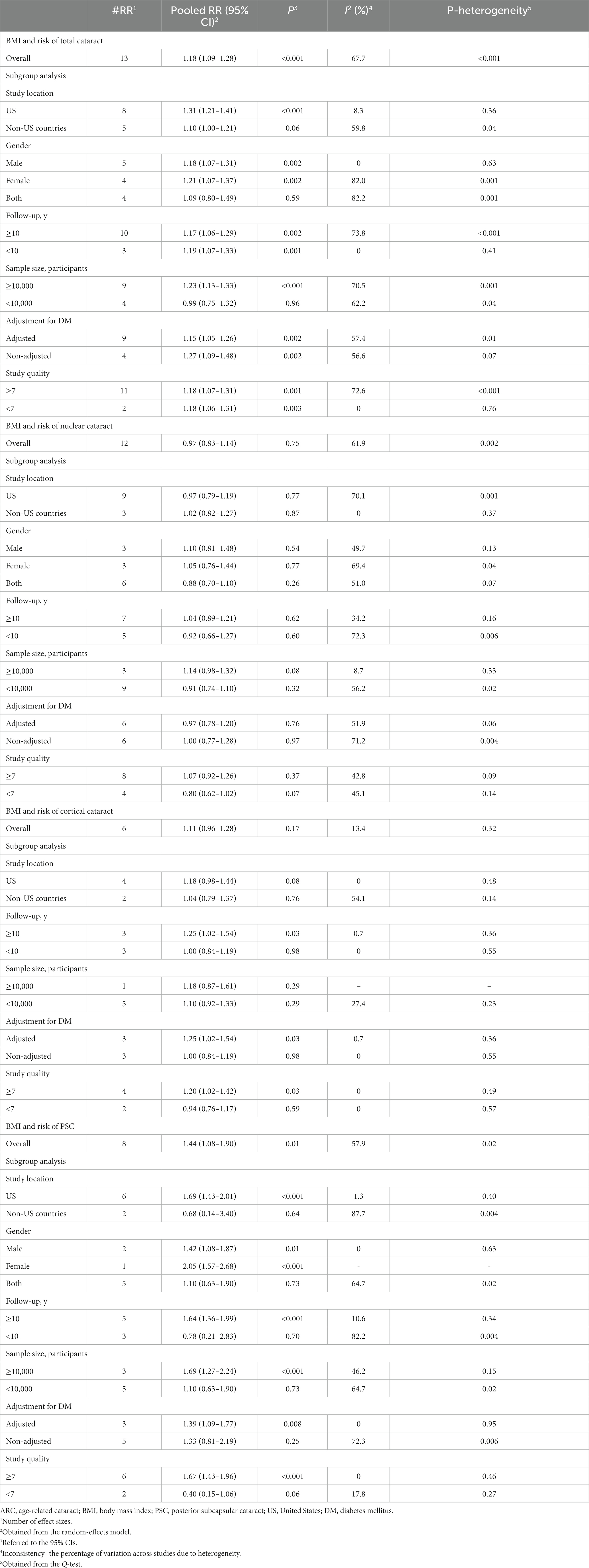
All articles in this section were included in the dose–response meta-analysis. We found a significant linear association between BMI and risk of ARC (Figure 2A); such that, the overall RRs of ARC per 1, 5, and 10 kg/m2 increase in BMI were 1.01 (95% CI: 1.00–1.02), 1.06 (95% CI: 1.01–1.12), 1.13 (95% CI: 1.02–1.26). In the non-linear dose–response meta-analysis, we found no evidence of a non-linear association between BMI and risk of ARC (P non-linearity = 0.39) (Figure 3A).
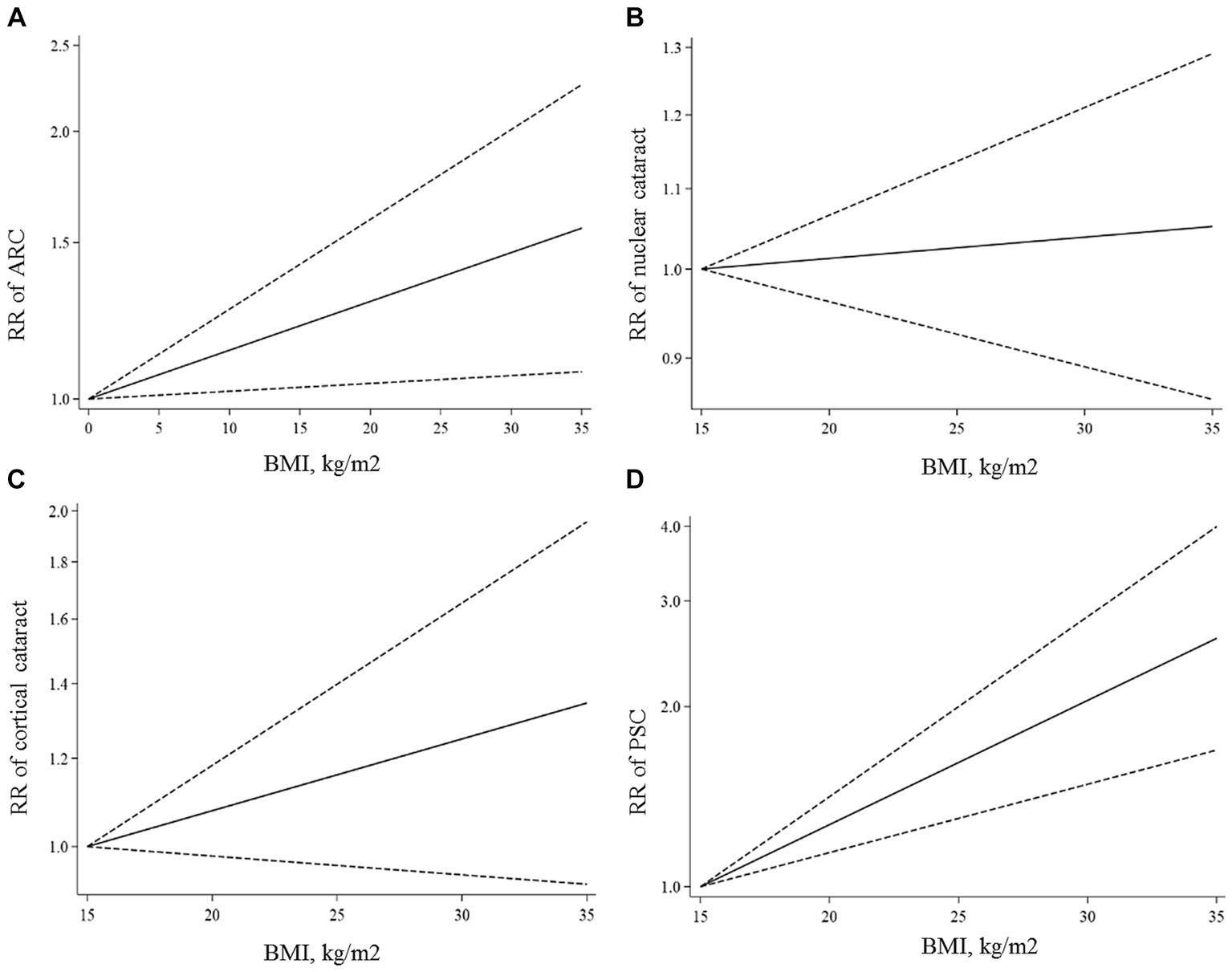
Figure 2. Linear dose–response associations of BMI with ARC (A), nuclear (B), cortical (C), and PSC (D) cataracts. The solid lines indicate the overall RRs. The dashed lines present the 95% CIs. RR, relative risk; CI, confidence interval; BMI, body mass index; ARC, age-related cataract; PSC, posterior subcapsular cataract.
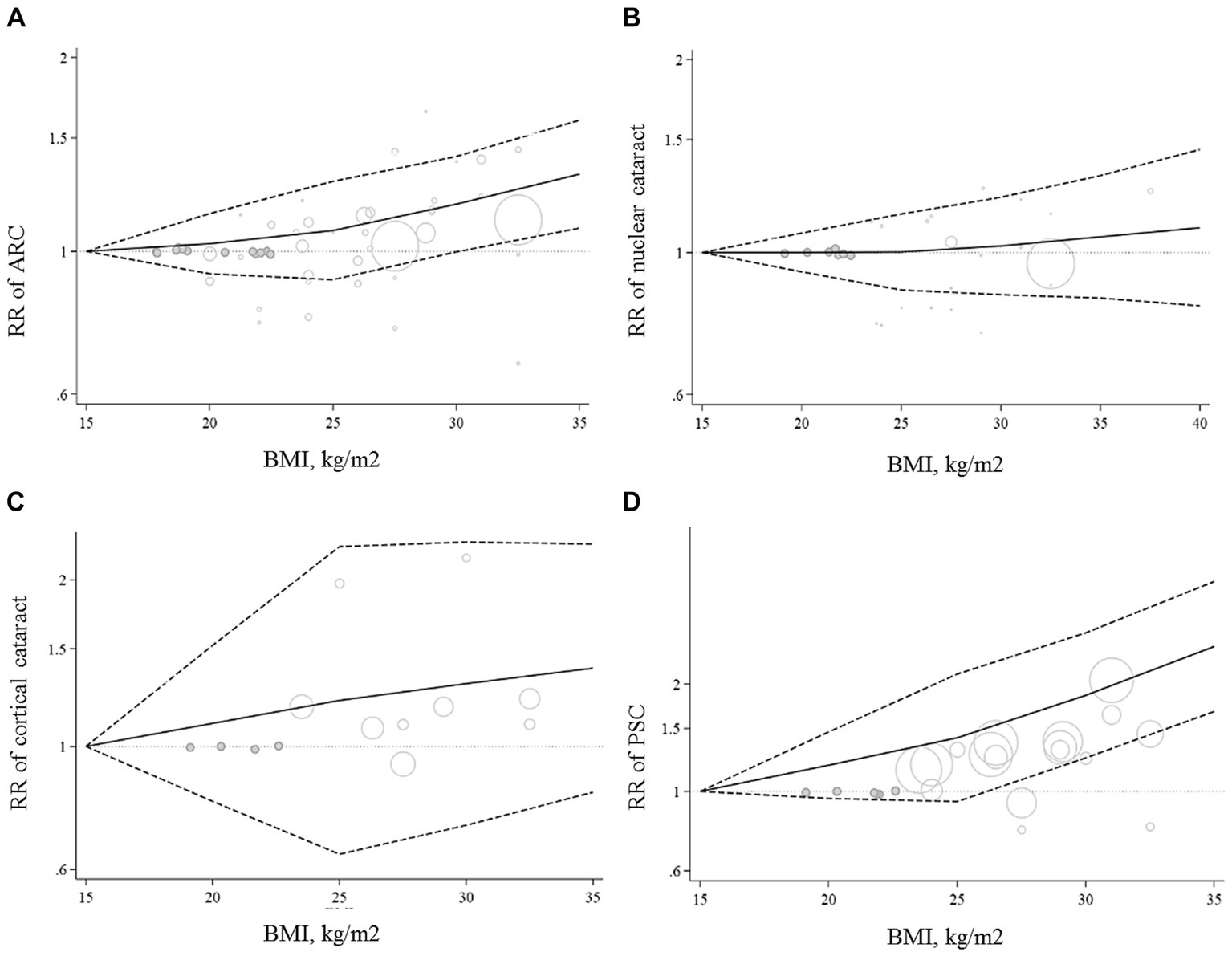
Figure 3. Non-linear dose–response associations of BMI with ARC (A), nuclear (B), cortical (C), and PSC (D) cataracts. The solid lines indicate the overall RRs. The dashed lines present the 95% CIs. RR, relative risk; CI, confidence interval; BMI, body mass index; ARC, age-related cataract; PSC, posterior subcapsular cataract.
3.4.2 Meta-analysis on BMI and risk of nuclear cataract
Ten papers containing 11 studies were included in the meta-analysis of BMI and nuclear cataract (13–15, 17–23). Combining the RRs of nuclear cataract reported for the highest versus lowest categories of BMI revealed a non-significant association between BMI and nuclear cancer (Pooled RR: 0.97, 95% CI: 0.83–1.14, I2 = 61.9, Pheterogeneity = 0.002) (Figure 4). Such a non-significant association was also seen in the subgroup analyses (Table 2). In these analyses, we found that different characteristics of studies including geographical location, follow-up duration, sample size, and quality of included studies contributed to the significant heterogeneity observed in the overall analysis. In addition to the highest versus comparison, we found no significant association in the dose–response analysis (P linearity = 0.62, P non-linearity = 0.52) (Figures 2B, 3B).
Figure 4. Forest plot for the association between BMI and the risk of nuclear cataract by comparing the highest and lowest categories of BMI. The overall RR was obtained from a random-effects model. RR, relative risk; CI, confidence interval; BMI, body mass index; ARC, age-related cataract.
3.4.3 Meta-analysis on BMI and risk of cortical cataract
In total, we included 6 studies (from 6 papers) in this meta-analysis (13, 16, 17, 20–22). There was no significant association between BMI and risk of cortical cataract when we compared risk between the highest and lowest categories of BMI (Pooled RR: 1.11, 95% CI: 0.96–1.28, I2 = 13.4, Pheterogeneity = 0.32) (Figure 5). Between-study heterogeneity was not significant in this association. Regarding subgroup analyses, we found a significant positive association between BMI and risk of cortical cataract among cohort studies with a follow-up duration of ≥10 years (Pooled RR: 1.25, 95% CI: 1.02–1.54, I2 = 0.7, Pheterogeneity = 0.36), those that adjusted for diabetes in their analysis, and those with high quality (Pooled RR: 1.20, 95% CI: 1.02–1.42, I2 = 0, Pheterogeneity = 0.49) (Table 2). In the dose–response analysis of four articles containing required data, we found no evidence of linear (P linearity = 0.12) or non-linear (P non-linearity = 0.90) association between BMI and risk of cortical cataract (Figures 2C, 3C).
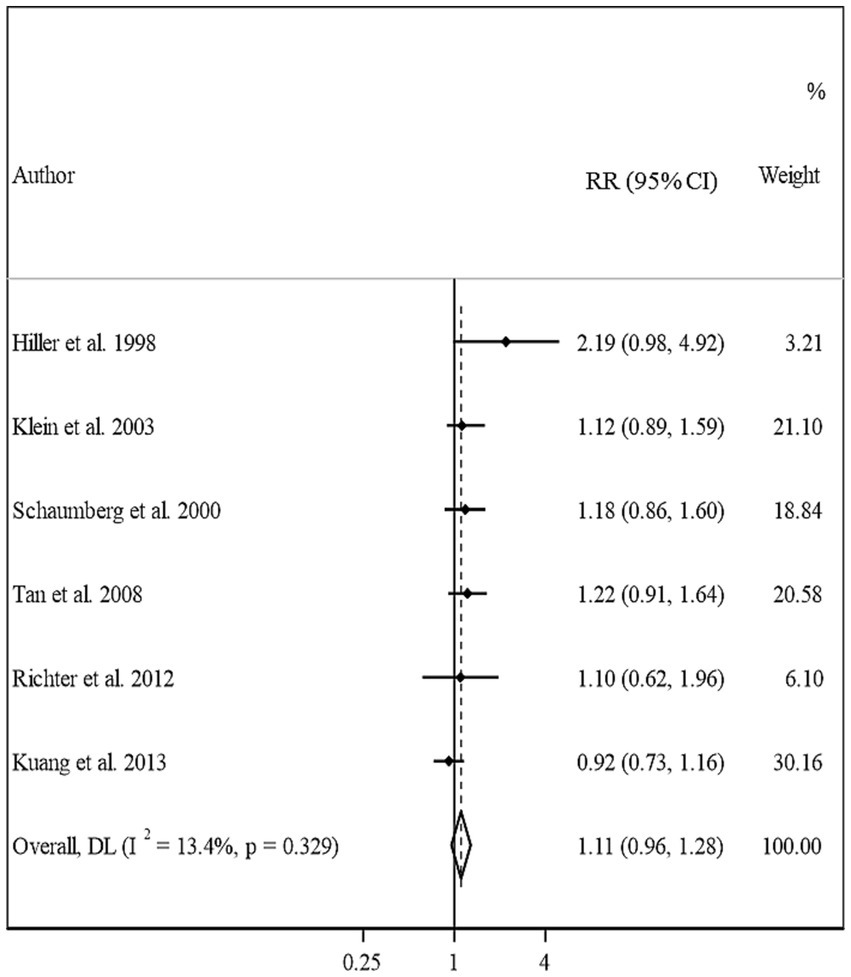
Figure 5. Forest plot for the association between BMI and the risk of cortical cataract by comparing the highest and lowest categories of BMI. The overall RR was obtained from a random-effects model. RR, relative risk; CI, confidence interval; BMI, body mass index; ARC, age-related cataract.
3.4.4 Meta-analysis on BMI and risk of PSC
Overall, eight studies from seven articles were assessed in the meta-analysis on BMI and risk of PSC (13, 16, 17, 20–23). We found a significant positive association in this regard; such that, people in the highest categories of BMI had a 44% higher risk of PSC compared with those in the lowest category (Pooled RR: 1.44, 95% CI: 1.08–1.90, I2 = 57.9, Pheterogeneity = 0.02) (Figure 5). However, we found evidence of moderate heterogeneity in this association. Subgroup analyses showed that participants’ gender, follow-up duration, study location, and study quality were possible reasons for the observed heterogeneity (Table 2). From these analyses, we also found a significant positive association between BMI and PSC risk among cohort studies conducted in the US and those with high quality such as studies with high follow-up duration, those with larger sample sizes, and studies that controlled their analysis for diabetes.
In the dose–response meta-analysis, five papers (6 studies) on the link between BMI and PSC had required data, and therefore, were included in the dose–response meta-analysis (13, 20–23). There was evidence of a linear association between BMI and risk of PSC (Figure 2D) so that each 1, 5, and 10 kg/m2 increase in BMI was associated with a 5% (Pooled RR: 1.05, 95% CI: 1.03–1.07), 27% (Pooled RR: 1.27, 95% CI: 1.14–1.41), and 61% (Pooled RR: 1.61, 95% CI: 1.30–2.00) higher risk of PSC in adults. We found no evidence of a non-linear association in this regard (Figure 3D).
3.4.5 Publication bias and sensitivity analysis
In the sensitivity analysis, when we excluded the study of Leske et al., the non-significant positive association between BMI and risk of nuclear cataract became significant (Pooled RR: 1.11, 95% CI: 1.01–1.22). Sensitivity analyses for other associations showed that the overall RRs obtained in the current meta-analysis were robust and did not depend on one study. We assessed publication bias using Egger’s linear regression test for associations with ≥10 risk estimates and found no substantial publication bias (Figure 6).
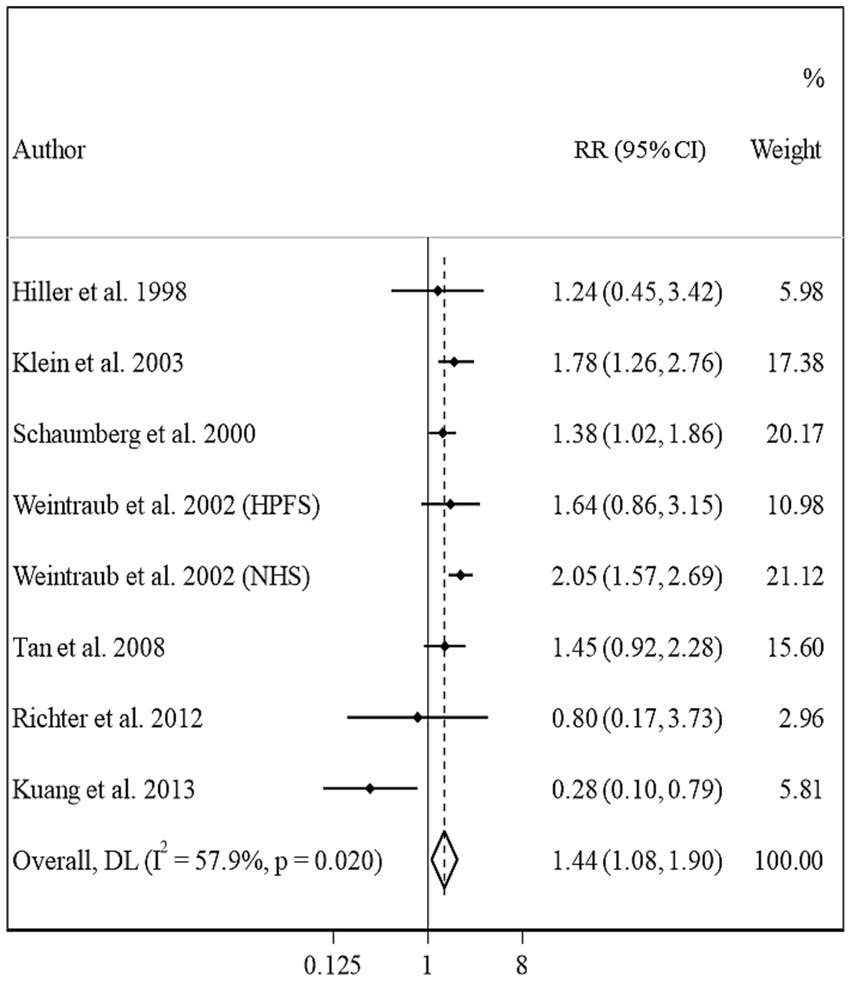
Figure 6. Forest plot for the association between BMI and the risk of PSC by comparing the highest and lowest categories of BMI. The overall RR was obtained from a random-effects model. RR, relative risk; CI, confidence interval; BMI, body mass index; ARC, age-related cataract; PSC, posterior subcapsular cataract.
4 Discussion
In the current meta-analysis, we found a significant positive association between BMI and risk of ARC and PSC in adults so that each 5 kg/m2 increase in BMI was associated with a 6 and 27% increased risk of ARC and PSC, respectively. In terms of nuclear and cortical cataracts, we found no significant association in the overall analysis; however, in the subgroup analyses, a significant positive association was seen between BMI and cortical cataract among studies with high quality.
ARC is a common disorder among older adults (51). Previous studies have shown that lifestyle-related factors such as smoking, alcohol consumption, and exposure to radiation or environmental pollution contribute to the etiology of ARC (52–54). However, the genetic potential of individuals plays an important role (55). Recently, some cohort studies have shown that obesity may affect the risk of cataract among older adults (10–25). However, findings from these studies were not conclusive. In the current meta-analysis, we found that higher BMI was associated with an increased risk of ARC. Also, in the dose–response meta-analysis, a 5 kg/m2 increase in BMI was associated with a 6% higher risk of ARC. In a 2014 meta-analysis, Ye et al. reported that obesity is a potential risk factor for ARC (26). In another review article, Line et al. concluded that obesity has a direct association with age-related eye diseases (56). Despite the positive association, some cohort studies included in the current meta-analysis indicated a non-significant association between BMI and risk of ARC (10, 13, 14, 22). This might be explained by the different sample sizes of the studies that were mostly low. Also, in the subgroup analyses, we found a significant positive association between BMI and ARC among studies with higher sample sizes, while this was not significant among small cohorts. In addition, different adjustments in the statistical analysis might be another reason for inconsistent results among the included studies. For instance, three studies that did not include any confounders in models showed no significant association between BMI and ARC and surprisingly indicated a significant inverse association for nuclear and PSC cataracts (17, 18, 20). In contrast, most studies that controlled their analysis for potential confounders revealed a significant positive association between BMI and ARC risk. Further studies are needed to confirm the positive association.
In the current study, we found a significant positive association between BMI and the risk of PSC. Also, each 5 kg/m2 increase in BMI was associated with a 27% higher risk of PSC. This was in line with a previous meta-analysis in which elevated BMI increased the risk of PSC. However, the dose–response association between BMI and PSC was not assessed in that meta-analysis (26). Also, in a prospective cohort study, Lindblad et al. reported that metabolic syndrome with the combination of abdominal adiposity, diabetes, and hypertension was associated with an increased risk for cataract extraction (28). Such a positive association was also reported in another cohort study (49). Among 7 papers included in the meta-analysis of BMI and PSC (13, 16, 17, 20–23), 3 articles reported a significant positive association (16, 21, 23), 3 indicated no significant association (13, 20, 22), and one showed an inverse association between BMI and PSC risk (17). The lack of significant positive association among the four studies might be due to the short duration of follow-up, low sample size, and totally low quality of these studies. The involvement of these variables was confirmed in the subgroup analyses in which we found a significant positive association between BMI and PSC risk in studies with high quality, long duration of follow-up (≥10 years), and high sample size (≥10,000 participants).
Elevated BMI or obesity is associated with several complications such as diabetes mellitus, hypertension, and hyperlipidemia (57, 58). These complications are known risk factors of ARC (59). In the subgroup analyses, we found a significant positive association between BMI and ARC among studies that adjusted for diabetes mellitus in their analysis. It means that there are other plausible pathophysiological pathways in addition to obesity complications through which elevated BMI increases the risk of ARC. It has been shown that obese individuals have increased levels of leptin which has a role in the elevation of oxidative stress (60). The role of oxidative stress in the progression of ARC has been well-established (61). In addition, obesity is linked with increased levels of inflammatory biomarkers which are involved in the development of ARC (62, 63).
In the current study, BMI had no significant association with nuclear and cortical cataracts in the overall analysis, however, in the subgroup analyses, a significant positive association was seen between BMI and cortical cataract among studies with high quality. In contrast with our findings, a 2014 meta-analysis showed a significant positive association between obesity and risk of nuclear and cortical cataracts (64). This inconsistency is explained by entering eligible articles, published after 2014, into the current meta-analysis. Unlike the cortical cataract, we found no significant association between BMI and nuclear cataract in any subgroups of the included studies. The lack of significant association for nuclear cataract might be due to the different patterns of formation and progression of this subtype compared with other subtypes of cataracts (65). For instance, PSC is highly overrepresented among extracted cataracts, while other subtypes are less common (61). Therefore, this might be a reason for the stronger association between BMI and PSC compared with other subtypes of cataracts.
Strengths of our meta-analysis included the linear and non-linear dose–response analyses on prospective cohort studies, which help us to draw the shape of the association between BMI and ARC. Since we included prospective cohort studies in the current meta-analysis, our findings are less susceptible to recall and selection bias which is common among retrospective case–control studies. In addition, to combine RRs, we used a random-effects model, that takes between-study variation into account. Despite the strengths, our meta-analysis had some limitations that should be considered when interpreting our results. The methods used for the definition of cataract were different among the included studies and some defined cataract based on self-reported data that may induce underestimates of the number of cataract cases. This problem was also the case for BMI which was calculated based on self-reported weight and height in some studies. Furthermore, in the comparison between the highest and lowest categories of BMI, we observed different cut-off points for the definition of these categories among included studies. However, we handled this problem by performing the dose–response meta-analysis.
In the current meta-analysis, we concluded that increased BMI is associated with a higher risk of ARC, particularly PSC, in adults. Moreover, we found that a 5 kg/m2 increase in BMI was associated with a 6 and 27% increased risk of ARC and PSC, respectively. We also found a significant positive association between BMI and risk of cortical cataract in high-quality studies. No significant association was seen for nuclear cataract. Future studies should assess the link between abdominal obesity and the risk of ARC.
Author contributions
SN and MM contributed to the literature search and data extraction. MD and SN contributed to data analysis. FD and FN drafted the manuscript which was critically revised for important intellectual content by all authors. RA contributed to the manuscript editing. FD supervised the study. All authors have read and approved the final manuscript.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study has been exclusively funded by the team working on it.
Acknowledgments
Thanks to Dr. Omid Sadeghi for his expertise and assistance throughtout editing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1215212/full#supplementary-material
Abbreviations
ARC, Age-related cataract; BMI, Body mass index; CI, Confidence interval; DM, Diabetes mellitus; HR, Hazard Ratio; MeSH, Medical subject heading terms; NOS, Newcastle Ottawa Scale; OR, Odds Ratio; PSC, Posterior subcapsular cataracts; PRISMA, Preferred reporting items for systematic review and meta-analysis; RR, Relative risk.
References
1. Prokofyeva, E, Wegener, A, and Zrenner, E. Cataract prevalence and prevention in Europe: a literature review. Acta Ophthalmol. (2013) 91:395–405. doi: 10.1111/j.1755-3768.2012.02444.x
2. Pan, CW, and Lin, Y. Overweight, obesity, and age-related cataract: a meta-analysis. Optom Vis Sci. (2014) 91:478–83. doi: 10.1097/OPX.0000000000000243
3. Hashemi, H, Pakzad, R, Yekta, A, Aghamirsalim, M, Pakbin, M, Ramin, S, et al. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye (Lond). (2020) 34:1357–70. doi: 10.1038/s41433-020-0806-3
4. Song, E, Sun, H, Xu, Y, Ma, Y, Zhu, H, and Pan, CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. (2014) 9:e112054. doi: 10.1371/journal.pone.0112054
5. Tran Ba Huy, P. Age-related decline of vision, hearing, and balance: pathophysiology and midlife prevention. Prevention of chronic diseases and age-related disability. Springer, Cham; (2019) 129–136
6. Chen, X, Zhou, DY, Shen, J, Wu, YB, Sun, QZ, Dong, JM, et al. Prevalence and risk factors on age-related cataract and surgery in adults over 50 years old in Binhu District, Wuxi, China. Int J Ophthalmol. (2020) 13:445–51. doi: 10.18240/ijo.2020.03.12
7. Akhavanfar, R, Hojati, A, Kahrizi, MS, Farhangi, MA, and Ardekani, AM. Adherence to lifelines diet score and risk factors of metabolic syndrome among overweight and obese adults: a cross-sectional study. Front Nutr. (2022) 9:961468. doi: 10.3389/fnut.2022.961468
8. Ma, YL, Jin, CH, Zhao, CC, Ke, JF, Wang, JW, Wang, YJ, et al. Waist-to-height ratio is a simple and practical alternative to waist circumference to diagnose metabolic syndrome in type 2 diabetes. Front Nutr. (2022) 9:986090. doi: 10.3389/fnut.2022.986090
9. Hu, J, Zhong, Y, Ge, W, Lv, H, Ding, Z, Han, D, et al. Comparisons of tri-ponderal mass index and body mass index in discriminating hypertension at three separate visits in adolescents: a retrospective cohort study. Front Nutr. (2022) 9:1028861. doi: 10.3389/fnut.2022.1028861
10. Appleby, PN, Allen, NE, and Key, TJ. Diet, vegetarianism, and cataract risk. Am J Clin Nutr. (2011) 93:1128–35. doi: 10.3945/ajcn.110.004028
11. Chodick, G, Bekiroglu, N, Hauptmann, M, Alexander, BH, Freedman, DM, Doody, MM, et al. Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol. (2008) 168:620–31. doi: 10.1093/aje/kwn171
12. Floud, S, Kuper, H, Reeves, GK, Beral, V, and Green, J. Risk factors for cataracts treated surgically in postmenopausal women. Ophthalmology. (2016) 123:1704–10. doi: 10.1016/j.ophtha.2016.04.037
13. Hiller, R, Podgor, MJ, Sperduto, RD, Nowroozi, L, Wilson, PWF, D’Agostino, RB, et al. A longitudinal study of body mass index and lens opacities. The Framingham studies. Ophthalmology. (1998) 105:1244–50. doi: 10.1016/s0161-6420(98)97029-4
14. Howard, KP, Klein, BE, Lee, KE, and Klein, R. Measures of body shape and adiposity as related to incidence of age-related eye diseases: observations from the beaver dam eye study. Invest Ophthalmol Vis Sci. (2014) 55:2592–8. doi: 10.1167/iovs.13-13763
15. Karppi, J, Laukkanen, JA, and Kurl, S. Plasma lutein and zeaxanthin and the risk of age-related nuclear cataract among the elderly Finnish population. Br J Nutr. (2012) 108:148–54. doi: 10.1017/S0007114511005332
16. Klein, BE, Klein, R, Lee, KE, and Moore, EL. Do multiple families alter estimates of risk for age-related cataract in a population-based study? The beaver dam eye study. Ophthalmic Epidemiol. (2003) 10:97–106. doi: 10.1076/opep.10.2.97.13896
17. Kuang, TM, Tsai, SY, Liu, CJ, Ko, YC, Lee, SM, and Chou, P. Seven-year incidence of age-related cataracts among an elderly Chinese population in Shihpai, Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. (2013) 54:6409–15. doi: 10.1167/iovs.13-12582
18. Leske, MC, Wu, SY, Nemesure, B, and Hennis, A, Barbados Eye Studies G. Risk factors for incident nuclear opacities. Ophthalmology. (2002) 109:1303–8. doi: 10.1016/s0161-6420(02)01094-1
19. Mares, JA, Voland, R, Adler, R, Tinker, L, Millen, AE, Moeller, SM, et al. Healthy diets and the subsequent prevalence of nuclear cataract in women. Arch Ophthalmol. (2010) 128:738–49. doi: 10.1001/archophthalmol.2010.84
20. Richter, GM, Choudhury, F, Torres, M, Azen, SP, and Varma, R, Los Angeles Latino Eye Study G. Risk factors for incident cortical, nuclear, posterior subcapsular, and mixed lens opacities: the Los Angeles Latino eye study. Ophthalmology. (2012) 119:2040–7. doi: 10.1016/j.ophtha.2012.05.001
21. Schaumberg, DA, Glynn, RJ, Christen, WG, Hankinson, SE, and Hennekens, CH. Relations of body fat distribution and height with cataract in men. Am J Clin Nutr. (2000) 72:1495–502. doi: 10.1093/ajcn/72.6.1495
22. Tan, JS, Wang, JJ, and Mitchell, P. Influence of diabetes and cardiovascular disease on the long-term incidence of cataract: the Blue Mountains eye study. Ophthalmic Epidemiol. (2008) 15:317–27. doi: 10.1080/09286580802105806
23. Weintraub, JM, Willett, WC, Rosner, B, Colditz, GA, Seddon, JM, and Hankinson, SE. A prospective study of the relationship between body mass index and cataract extraction among US women and men. Int J Obes Relat Metab Disord. (2002) 26:1588–95. doi: 10.1038/sj.ijo.0802158
24. Williams, PT. Prospective epidemiological cohort study of reduced risk for incident cataract with vigorous physical activity and cardiorespiratory fitness during a 7-year follow-up. Invest Ophthalmol Vis Sci. (2009) 50:95–100. doi: 10.1167/iovs.08-1797
25. Yoshida, M, Inoue, M, Iwasaki, M, and Tsugane, S, Group JS. Association of body mass index with risk of age-related cataracts in a middle-aged Japanese population: the JPHC study. Environ Health Prev Med. (2010) 15:367–73. doi: 10.1007/s12199-010-0153-2
26. Ye, J, Lou, LX, He, JJ, and Xu, YF. Body mass index and risk of age-related cataract: a meta-analysis of prospective cohort studies. PLoS One. (2014) 9:e89923. doi: 10.1371/journal.pone.0089923
27. Chang, JR, Koo, E, Agron, E, Hallak, J, Clemons, T, Azar, D, et al. Risk factors associated with incident cataracts and cataract surgery in the age-related eye disease study (AREDS): AREDS report number 32. Ophthalmology. (2011) 118:2113–9. doi: 10.1016/j.ophtha.2011.03.032
28. Lindblad, BE, Hakansson, N, Philipson, B, and Wolk, A. Metabolic syndrome components in relation to risk of cataract extraction: a prospective cohort study of women. Ophthalmology. (2008) 115:1687–92. doi: 10.1016/j.ophtha.2008.04.004
29. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
30. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
31. Danesh, J, Collins, R, Appleby, P, and Peto, R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. (1998) 279:1477–82. doi: 10.1001/jama.279.18.1477
32. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
33. Salari-Moghaddam, A, Sadeghi, O, Keshteli, AH, Larijani, B, and Esmaillzadeh, A. Metformin use and risk of fracture: a systematic review and meta-analysis of observational studies. Osteoporos Int. (2019) 30:1167–73. doi: 10.1007/s00198-019-04948-1
34. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
35. van Enst, WA, Ochodo, E, Scholten, RJ, Hooft, L, and Leeflang, MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol. (2014) 14:70. doi: 10.1186/1471-2288-14-70
36. Abudurexiti, M, Zhu, W, Wang, Y, Wang, J, Xu, W, Huang, Y, et al. Targeting CPT1B as a potential therapeutic strategy in castration-resistant and enzalutamide-resistant prostate cancer. Article Prostate. (2020) 80:950–61. doi: 10.1002/pros.24027
37. Crippa, A, Discacciati, A, Bottai, M, Spiegelman, D, and Orsini, N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. (2019) 28:1579–96. doi: 10.1177/0962280218773122
38. Albert, K, Sennesael, J, and Haentjens, P. Incidence and risk factors for posttransplant subcapsular cataract: a long-term retrospective cohort study. Transplant Proc. (2011) 43:3465–9. doi: 10.1016/j.transproceed.2011.10.007
39. Janghorbani, MB, Jones, RB, and Allison, SP. Incidence of and risk factors for cataract among diabetes clinic attenders. Ophthalmic Epidemiol. (2000) 7:13–25. doi: 10.1076/0928-6586(200003)711-2FT013
40. Jee, D, and Park, S. Hyperglycemia and hypo-HDL-cholesterolemia are primary risk factors for age-related cataract, and a Korean-style balanced diet has a negative association, based on the Korean genome and epidemiology study. J Korean Med Sci. (2021) 36:e155. doi: 10.3346/jkms.2021.36.e155
41. Klein, BE, Klein, R, Lee, KE, and Jensen, SC. Measures of obesity and age-related eye diseases. Ophthalmic Epidemiol. (2001) 8:251–62. doi: 10.1076/opep.8.4.251.1612
42. Zhu, Z, Wang, L, Scheetz, J, and He, M. Age-related cataract and 10-year mortality: the Liwan Eye Study. Acta Ophthalmol. (2020) 98:e328–32. doi: 10.1111/aos.14258
43. Age-Related Eye Disease Study Research G. Risk factors associated with age-related nuclear and cortical cataract: a case-control study in the Age-Related Eye Disease Study, AREDS Report No. 5. Ophthalmology. (2001) 108:1400–8. doi: 10.1016/s0161-6420(01)00626-1
44. Lindblad, BE, Hakansson, N, and Wolk, A. Metabolic syndrome and some of its components in relation to risk of cataract extraction. A prospective cohort study of men. Acta Ophthalmol. (2019) 97:409–14. doi: 10.1111/aos.13929
45. Zhang, JS, Xu, L, Wang, YX, You, QS, Wang, JD, and Jonas, JB. Five-year incidence of age-related cataract and cataract surgery in the adult population of greater Beijing: the Beijing eye study. Ophthalmology. (2011) 118:711–8. doi: 10.1016/j.ophtha.2010.08.021
46. Yuan, S, Wolk, A, and Larsson, SC. Metabolic and lifestyle factors in relation to senile cataract: a Mendelian randomization study. Sci Rep. (2022) 12:409. doi: 10.1038/s41598-021-04515-x
47. Glynn, RJ, Christen, WG, Manson, JE, Bernheimer, J, and Hennekens, CH. Body mass index. An independent predictor of cataract. Arch ophthalmol. (1995) 113:1131–7. doi: 10.1001/archopht.1995.01100090057023
48. Glynn, RJ, Rosner, B, and Christen, WG. Evaluation of risk factors for cataract types in a competing risks framework. Ophthalmic Epidemiol. (2009) 16:98–106. doi: 10.1080/09286580902737532
49. Shang, X, Zhu, Z, Zhang, X, Huang, Y, Tan, Z, Wang, W, et al. Adiposity by differing measures and the risk of cataract in the UK biobank: the importance of diabetes. Invest Ophthalmol Vis Sci. (2021) 62:19. doi: 10.1167/iovs.62.14.19
50. Ghaem Maralani, H, Tai, BC, Wong, TY, Tai, ES, Li, J, Wang, JJ, et al. Metabolic syndrome and risk of age-related cataract over time: an analysis of interval-censored data using a random-effects model. Invest Ophthalmol Vis Sci. (2013) 54:641–6. doi: 10.1167/iovs.12-10980
51. Liu, YC, Wilkins, M, Kim, T, Malyugin, B, and Mehta, JS. Cataracts. Lancet. (2017) 390:600–12. doi: 10.1016/S0140-6736(17)30544-5
52. Ye, J, He, J, Wang, C, Wu, H, Shi, X, Zhang, H, et al. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci. (2012) 53:3885–95. doi: 10.1167/iovs.12-9820
53. Wang, W, and Zhang, X. Alcohol intake and the risk of age-related cataracts: a meta-analysis of prospective cohort studies. PLoS One. (2014) 9:e107820. doi: 10.1371/journal.pone.0107820
54. Shin, J, Lee, H, and Kim, H. Association between exposure to ambient air pollution and age-related cataract: a nationwide population-based retrospective cohort study. Int J Environ Res Public Health. (2020) 17:9231. doi: 10.3390/ijerph17249231
55. Shiels, A, and Hejtmancik, JF. Mutations and mechanisms in congenital and age-related cataracts. Exp Eye Res. (2017) 156:95–102. doi: 10.1016/j.exer.2016.06.011
56. Ng Yin Ling, C, Lim, SC, Jonas, JB, and Sabanayagam, C. Obesity and risk of age-related eye diseases: a systematic review of prospective population-based studies. Int J Obes. (2021) 45:1863–85. doi: 10.1038/s41366-021-00829-y
57. Askarpour, M, Hadi, A, Symonds, ME, Miraghajani, M, Omid Sadeghi,, Sheikhi, A, et al. Efficacy of l-carnitine supplementation for management of blood lipids: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metabol Cardiovas Dis. (2019) 29:1151–67. doi: 10.1016/j.numecd.2019.07.012
58. Parohan, M, Sadeghi, A, Nasiri, M, Maleki, V, Khodadost, M, Pirouzi, A, et al. Dietary acid load and risk of hypertension: a systematic review and dose-response meta-analysis of observational studies. Nutr Metabol Cardiovas. Dis. (2019) 29:665–75. doi: 10.1016/j.numecd.2019.03.009
59. Drinkwater, JJ, Davis, WA, and Davis, TME. A systematic review of risk factors for cataract in type 2 diabetes. Diabetes Metab Res Rev. (2019) 35:e3073. doi: 10.1002/dmrr.3073
60. Yamagishi, S, Amano, S, Inagaki, Y, Okamoto, T, Takeuchi, M, and Inoue, H. Pigment epithelium-derived factor inhibits leptin-induced angiogenesis by suppressing vascular endothelial growth factor gene expression through anti-oxidative properties. Microvasc Res. (2003) 65:186–90. doi: 10.1016/s0026-2862(03)00005-0
61. Beebe, DC, Holekamp, NM, and Shui, YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. (2010) 44:155–65. doi: 10.1159/000316481
62. Ahmad, A, and Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J Immunoassay Immunochem. (2020) 41:257–71. doi: 10.1080/15321819.2020.1726774
63. Asbaghi, O, Sadeghian, M, Nazarian, B, Sarreshtedari, M, Mozaffari-Khosravi, H, Maleki, V, et al. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomized clinical trials. Sci Rep. (2020) 10:17234. doi: 10.1038/s41598-020-73741-6
64. Wu, JX, Wang, Y, Chen, N, Chen, LC, Bai, PG, and Pan, JJ. In the era of total mesorectal excision: adjuvant radiotherapy may be unnecessary for pT3N0 rectal cancer. Radiat Oncol. (2014) 9:159. doi: 10.1186/1748-717X-9-159
Keywords: age-related cataract, body mass index, meta-analysis, obesity, cataract
Citation: Niazi S, Moshirfar M, Dastjerdi MH, Niazi F, Doroodgar F and Ambrósio R Jr (2024) Association between obesity and age-related cataract: an updated systematic review and dose–response meta-analysis of prospective cohort studies. Front. Nutr. 10:1215212. doi: 10.3389/fnut.2023.1215212
Edited by:
Yukiko Wagatsuma, University of Tsukuba, JapanReviewed by:
Smitha Jasper, Christian Medical College and Hospital, IndiaHun Lee, University of Ulsan, Republic of Korea
Copyright © 2024 Niazi, Moshirfar, Dastjerdi, Niazi, Doroodgar and Ambrósio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farideh Doroodgar, f-doroodgar@farabi.tums.ac.ir
 Sana Niazi1,2
Sana Niazi1,2  Farideh Doroodgar
Farideh Doroodgar