Ultra-processed food consumption and chronic kidney disease risk: a systematic review and dose–response meta-analysis
- 1Department of Digestion, Zhejiang Hospital, Hangzhou, Zhejiang, China
- 2Department of Nutrition, Zhejiang Hospital, Hangzhou, Zhejiang, China
- 3School of Public Health, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Background: High intake of ultra-processed food (UPF) has been associated with increased risk of chronic kidney disease(CKD), but the results remain inconsistent. We therefore performed this systematic review and dose–response meta-analysis of observational studies that shed light on the association between UPF consumption and the risk of CKD.
Methods: A systematic literature search of PubMed, Embase, Web of Science, Scopus and China National Knowledge Infrastructure (CNKI) databases was carried out to find the eligible articles published up to October 31, 2023. Random-effects or fixed-effects models were used to pool the relative risks(RRs) and their 95% confidence intervals (CIs).The potential sources of heterogeneity across studies were examined using the Cochran’s Q test and I-square(I2). Publication bias was examined using the visual inspection of asymmetry in funnel plots and quantified by Begg’s and Egger’s tests.
Results: Eight studies (six cohort and two cross-sectional studies) exploring the association between UPF consumption and risk of CKD, were included in the final analysis. The pooled analyses revealed that high consumption of UPF was associated with an increased risk of CKD (RR = 1.25; 95%CI: 1.09–1.42, p < 0.0001). Moreover, a 10% increase of UPF consumption was associated with a 7% higher risk of CKD (RR = 1.07; 95%CI: 1.04–1.10, p < 0.001). Dose–response analysis of all included studies showed a linear association between UPF consumption and the risk of CKD (RR = 1.02; 95%CI:0.99–1.05, Pdose–response = 0.178, Pnonlinearity = 0.843).
Conclusion: Our findings indicate that high consumption of UPF is significantly associated with an increased risk of CKD. Future research with prospective design is required to confirm this positive association.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023478483, PROSPERO identifier CRD42023478483.
Introduction
Chronic kidney disease(CKD) is an emerging global public health problem, affecting approximately 8% ~ 16% of the world’s population (1). According to the United States (U.S) Renal Data System Annual Data Report, the prevalence of CKD among U.S. adults has been relatively stable at just under 15% (2). In the period 2018–2019, an analysis of nationally representative survey with 176, 874 participants conducted in China, reported a CKD prevalence of 8.2% in adults aged ≥18 years (3). Given the highly prevalent and socioeconomic burden of CKD, urgent public health preventive measures is paramount importance. As is known to all, CKD is considered as a multifactorial chronic disease, which may be associated with multiple risk factors, including genetic factors, smoking, use of nephrotoxic medications, diabetes, obesity and cardiovascular disease (4, 5). Apart from the aforementioned risk factors, diet, one of modifiable environmental factors, has continuously been recognized as etiological and prognostic factor of CKD (6).
In the past few decades, numerous epidemiological studies have focused on the associations between the intakes of individual nutrients, food groups or whole dietary patterns and CKD risk (7–9). Nonetheless, little is known concerning the impact of the degree of food processing on CKD incidence. Since 2010, Brazilian researchers proposed the concept of the NOVA food classification, and classified foods and beverages into four different groups, including ultra-processed food (UPF) (10). Notably, UPF is usually ready-to-eat, cheap, highly palatable, and high in energy, salt, fats, added sugars, and low in fiber, protein, vitamins and minerals (11). Meanwhile, there has been a global nutrition transition, with food consumption shifting from minimally processed foods to UPFs, a hallmark of Western diet (11). Currently, UPF consumption has drastically increased in some middle- and high-income countries around the world, accounting for 25% ~ 60% of total daily energy intake (12, 13). Thus far, many epidemiological studies have shown the significant positive associations between high UPF consumption and increased risks of various diet-related chronic non- communicable diseases, such as overweight/obesity, type 2 diabetes, cardiovascular disease (CVD), and certain cancers (14–17). Moreover, multiple previous systematic reviews and meta-analyses have also confirmed these positive associations (13, 18, 19). Notwithstanding, only a few epidemiological studies have specially explored the potential relationship between UPF consumption and the risk of CKD (20–27). However, findings from these published studies are still inconsistent. While the majority of published studies have consistently shown that high intake of UPF was associated with an increased risk of CKD (20–22, 24–26), other studies also found the null association (23). For instance, in the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) and UK Biobank cohort studies, Gu et al. observed that higher UPF consumption was associated with a higher risk of CKD (26). Similarly, in a large prospective cohort of 15,792 black and white men and women aged 45–64 years, Du et al. also found that higher ultraprocessed food consumption was associated with a higher risk of incident CKD [hazard ratio(HR) = 1.24, 95%CI: 1.15–1.35] (22). Contrary to the above findings, Sullivan et al. followed 3,939 from Chronic Renal Insufficiency Cohort (CRIC) Study participants and found a null association between high UPF consumption and risk of CKD (HR = 1.07, 95%CI: 0.91–1.25) (23). Recently, Xiao et al. published a meta-analysis of four cohort studies assessing the relationship between UPF consumption and the risk of CKD (28). However, the aforementioned meta-analysis has several methodological limitations. For instance, due to the limited number of publications, Xiao et al. did not perform dose–response and subgroup analyses. Therefore, we aimed to carry out a comprehensive systematic review and dose–response meta-analysis of observational studies on the association between UPF consumption and risk of CKD.
Methods
Protocol and registration
This study complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29). This systematic review and meta-analysis has been registered in the International Prospective Register of Systematic reviews (PROSPERO) with registration number CRD42023478483.
Search strategy
An electronic literature search in PubMed, Embase, Web of Science, Scopus, and CNKI database was carried out to retrieve relevant articles written in English or Chinese languages published from inception up to October 2023, with the following keywords or combinations: (“fast foods” OR “processed food” OR “ultra-processed food” OR “processed meat” OR “hamburger” OR “salami” OR “bacon” OR “sausage” OR “luncheon meats”) AND (“chronic kidney disease” OR “kidney disease” OR “End-Stage Renal Disease” OR “ESKD” OR “CKD”). In addition, we also conducted the manual searches of reference lists from the selected articles and systematic reviews to identify additional relevant articles. Meanwhile, the search for gray literature was not conducted in this paper. The complete search strategy could be found in the Supplementary Table S1.The initial literature search was performed by two independent authors (XH and XZ). Discrepancies were resolved through discussion or consultation with the corresponding author (LS).
Study selection
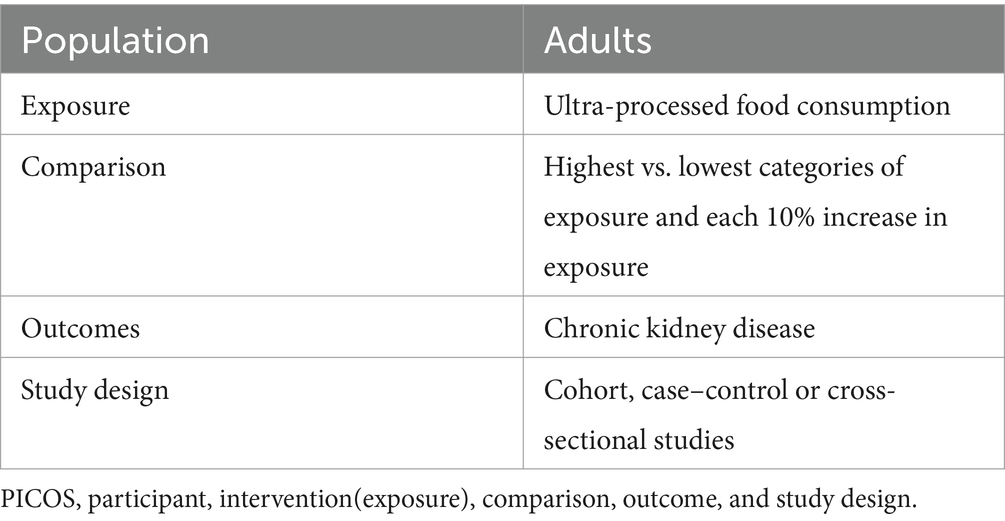
In the initial search, two authors (XH and XZ) independently screened the titles and abstracts of the retrieved articles and excluded duplicates and irrelevant articles. Subsequently, the full-text versions of the articles were reviewed basing on the inclusion and exclusion criteria of this systematic review and meta-analysis. To be included in our analyses, studies must meet all the following eligibility criteria: (1) observational studies (e.g., cohort, case–control or cross-sectional studies) performed in participants aged ≥18 years; (2) UPF consumption as defined by the NOVA food classification system; (3) studies exploring the association between UPF consumption and risk of CKD; (4) studies providing the adjusted relative risk(RRs), odds ratios (ORs), HRs with their corresponding 95% confidence intervals(CIs) of CKD (or sufficient data to calculate them); (5) If the original data in the retrieved studies lacked sufficient detail, the corresponding author of this study would be contacted by email. Similarly, studies were excluded if they met one of the following criteria: (1) animal, cell culture, and in vitro studies; (2) non-observational studies, including conference abstracts, editorials, reviews, case-reports, book chapters, and letters; (3) the NOVA food classification system was not used to define UPF (only specific food or food groups, e.g., sugar-sweetened drinks, processed meat were assessed); (4) did not provide the HRs, RRs or ORs with corresponding 95%CIs; (5) irrelevant articles. The PICOS criteria for inclusion and exclusion of studies is summarized in Table 1.
Data extraction
Two authors (QZ and CS) independently extracted the following data from all eligible articles: first author’s last name, year of publication, study region, study design, sample size, number of CKD cases, mean age/age range, duration of follow-up in cohort studies, method used for the assessment of UPF, adjustment for confounders and risk estimates(ORs, HRs or RRs) with their corresponding 95%CIs for the association between UPF consumption and risk of CKD. Any discrepancies arising during the data extraction were resolved via discussion with the corresponding author (LS).
Definitions of ultra-processed food and CKD
According to the NOVA classification system, all foods and beverages were classified into four groups, including unprocessed/minimally processed food, processed culinary ingredients, processed food and UPFs (11). The UPFs are usually ready-to-eat, hyper-palatable, cheap and characterized by high in energy density, salt, fats, added sugar and low in fiber, vitamins and minerals (13). Examples of UPF included sugar-sweetened drinks, sweet, crisps, cookies and cakes, and many ready-to-heat products such as pizza, burgers, noodles and desserts. Moreover, based on the CKD Epidemiology Collaboration (CKD-EPI) equation, incident CKD was defined by an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73m2, albumin-to-creatinine ratio ≥ 30 mg/g, or as having a clinical diagnosis of CKD (26).
Quality assessment
The Newcastle-Ottawa Scale (NOS) was utilized by two authors (Y.-L.F. and Q.Z.) to assess the quality of the included studies in this study (30). This scale is composed of eight items that evaluate quality in three domains: study selection, comparability of participants, and assessment of outcome/exposure of interest, with a maximum score of nine. Only those studies with NOS scores ≥7 points were considered to be of high quality (9). Moreover, these two authors also used the NutriGrade scoring system to assess the credibility of evidence. NutriGrade tool includes eight items: (1) risk of bias, study quality, and study limitations (0–2 points); (2) precision (0–1 point); (3) heterogeneity (0–1 point); (4) directness (0–1 point); (5) publication bias (0–1 point); (6) funding bias (0–1 point); (7) effect size (0–2 points); and (8) dose–response (0–1 point). Based on the overall NutriGrade score, ≥ 8 points, 6–7.99 points, 4–5.99 points and 0–3.99 points were classified as high, moderate, low and very low, respectively (31). Any discrepancies between two authors were resolved by corresponding author (LS) to reach a consensus.
Statistical analyses
For the current study, we utilized RRs and 95%CIs as the risk estimate for the primary analysis. Meanwhile, we assumed that the HR was approximately equal to the RR (32). For the ORs, they were converted into RRs using the following formula: RR = OR/[(1-P0) + (P0*OR)], in which P0 shows the incidence of CKD in the non-exposed group (33). We performed this meta-analysis by summarizing the RRs and 95% CIs for the highest comprising the lowest categories of UPF consumption in relation to the risk of CKD. The Cochran’s Q test and I2 statistical were used to measure the heterogeneity across studies. In our analyses, if p-values of Cochran’s Q test >0.10 or I2 > 50% demonstrated the high heterogeneity in the included studies, and a DerSimonnian and Laird random-effects model was used to pool the RRs. Conversely, a p value of Q-test >0.10 or I2 < 50% indicated an absence of heterogeneity among studies, and a fixed-effects model was used to calculate the pooled RRs (34). If there was significant heterogeneity between studies, sensitivity and subgroup analyses were further used to identify potential sources of heterogeneity. Subgroup analyses were conducted based on study design (cohort or cross-sectional studies), study region (Western or Asian countries), mean age(≥50 or < 50), sample size(<5,000 or ≥ 5,000), study quality (≥7 or < 7), and methods for assessing UPF consumption (food frequency questionnaire(FFQ) or other). Sensitivity analysis was carried out to confirm whether the pooled RRs were robust or sensitive to the impact of a certain study. Publication bias was assessed via the visual inspection of the funnel plots and quantified by both Begg’s and Egger’s tests (35). If publication bias was found, the trim and fill method was used to re-calculate our results (36). Finally, we also performed a dose–response meta-analysis to estimate the RRs for each 10% increment in energy (grams) from UPF consumption. A two-stage GLST model based on generalized least squares was applied to examine the linear or non-linear dose–response relationship between UPF consumption and CKD risk. Statistical analyses were performed using Stata/SE, version 12.0 (StataCorp, College Station, TX, United States). All p value were reported as two-sided, and statistical significance was set at p ≤ 0.05 unless otherwise specified.
Results
Eligible studies
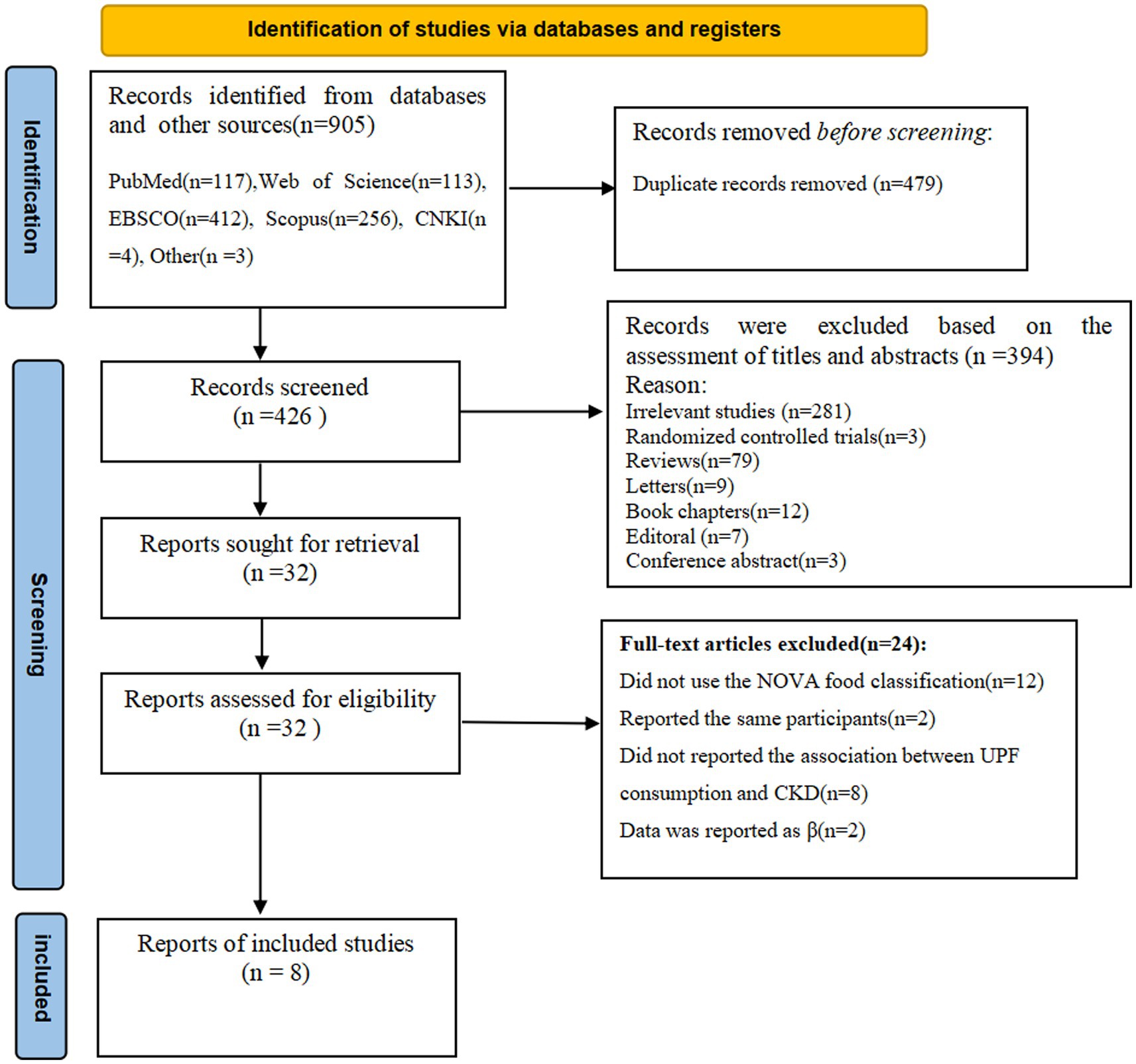
The flow chart of literature search process is shown in Figure 1. In the initial literature search, a total of 905 potentially relevant articles were retrieved (117 from PubMed, 113 from Web of Science, 412 from EBSCO, 256 from Scopus, 4 from CNKI and 3 from others). After excluding 479 duplicates, 426 articles were identified. Whereafter, 394 articles were excluded based on an evaluation of the titles and/or abstracts of the retrieved articles. Thirty-two full-text articles were reviewed by two independent authors. After reviewing the full-text versions of the remaining 32 articles, 24 articles were excluded for the following reasons: 2 studies reported the same participants, 12 studies did not use the NOVA food classification, 8 studies did not report the association between UPF consumption and CKD, and 2 studies reported data as β coefficient. Finally, eight articles were included in this meta-analysis.
Study characteristics
The characteristics of eligible studies are presented in Table 2. Eight articles with 513,440 participants and 20,637 CKD cases were included in the final analysis. Of the eight eligible studies, six were cohort designs (20, 22–26), and the remaining two were cross-sectional designs (21, 27). Two of the eight eligible studies were conducted in the United States (22, 23), two in Spain (25, 27), one in the United Kingdom (20), one in Korea (26), one in China and the United Kingdom (23), and one in Netherlands (24). All included studies were published after 2021. Sample size of included studies ranged from 1,312 to 153,985. The follow-up duration for cohort studies ranged from 3.6 to 24 years. The age of study participants across studies ranged from ages 18 to 90. All the included studies classified UPF consumption basing on the NOVA food classification systems (20–26). For dietary assessment, four of included studies used FFQs (21–24, 26, 27), one study used interviews (25), and the remaining one study used the 24 h dietary recall (20) to collect dietary information. Taken in total, seven out of eight studies were recognized as of high quality studies (20, 22–27), and the remaining one was recognized as of medium quality study (21), based on the NOS scores.
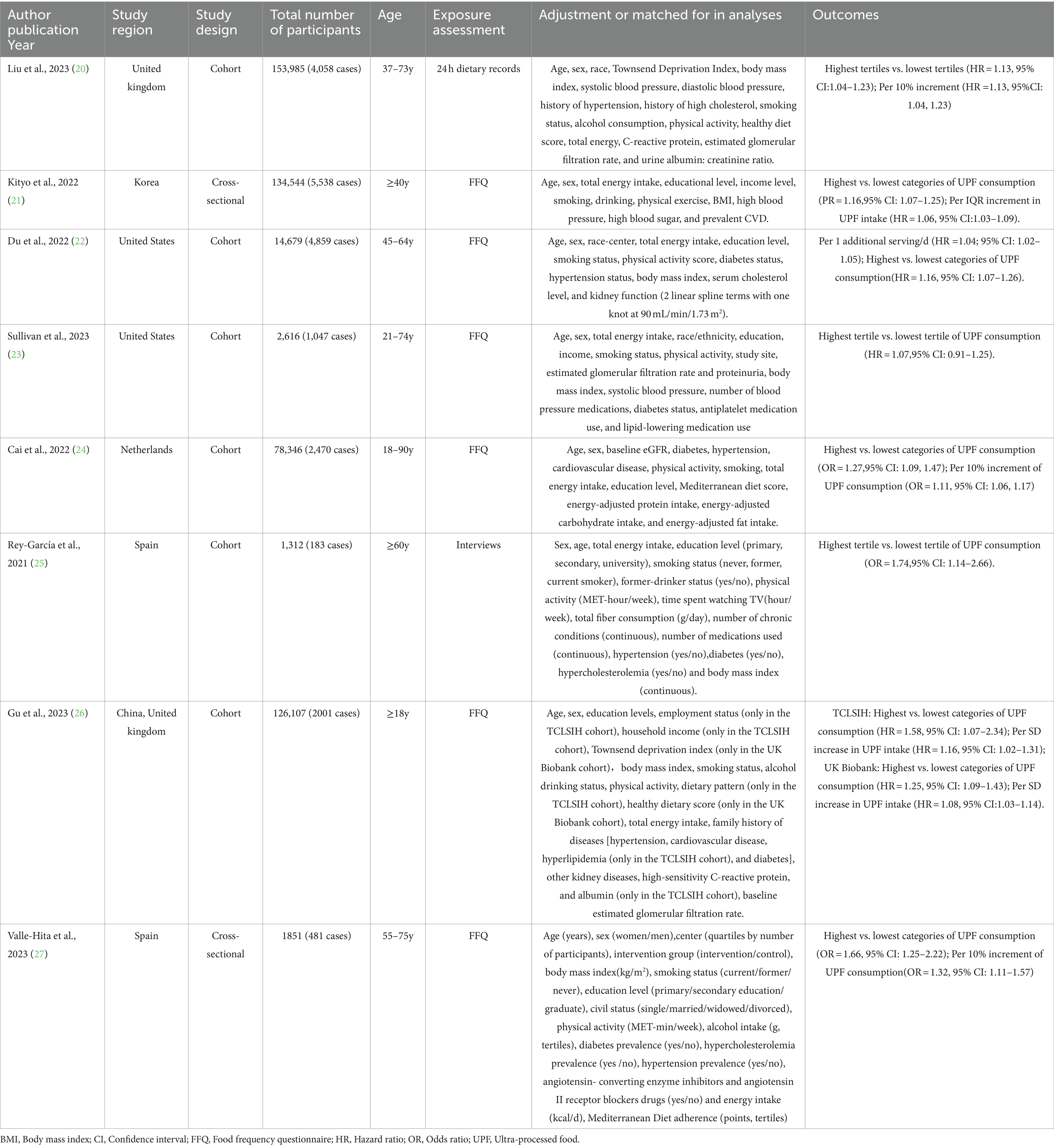
Table 2. Characteristics of included studies on the association between UPF consumption and CKD risk.
Ultra-processed food and CKD risk
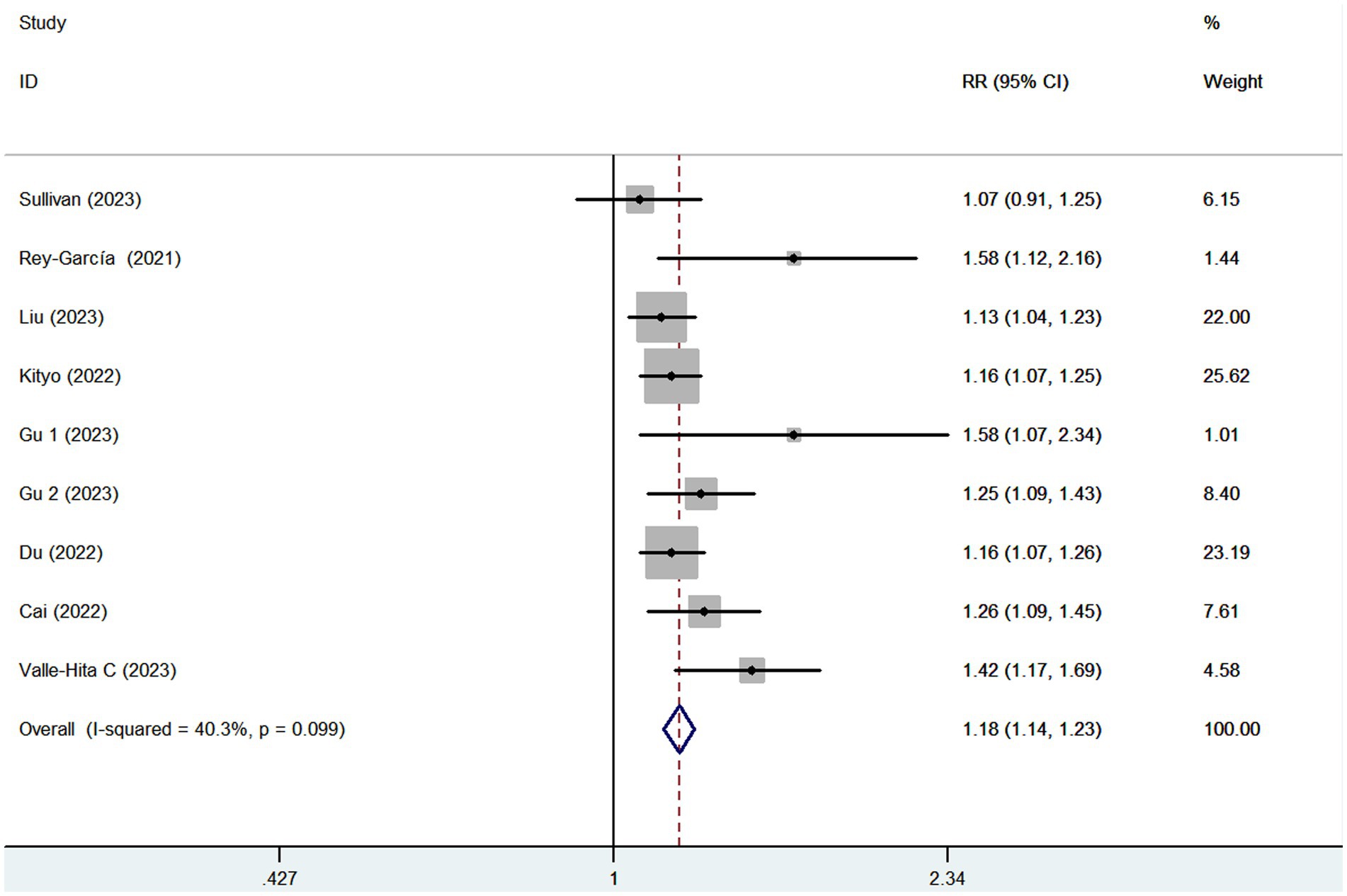
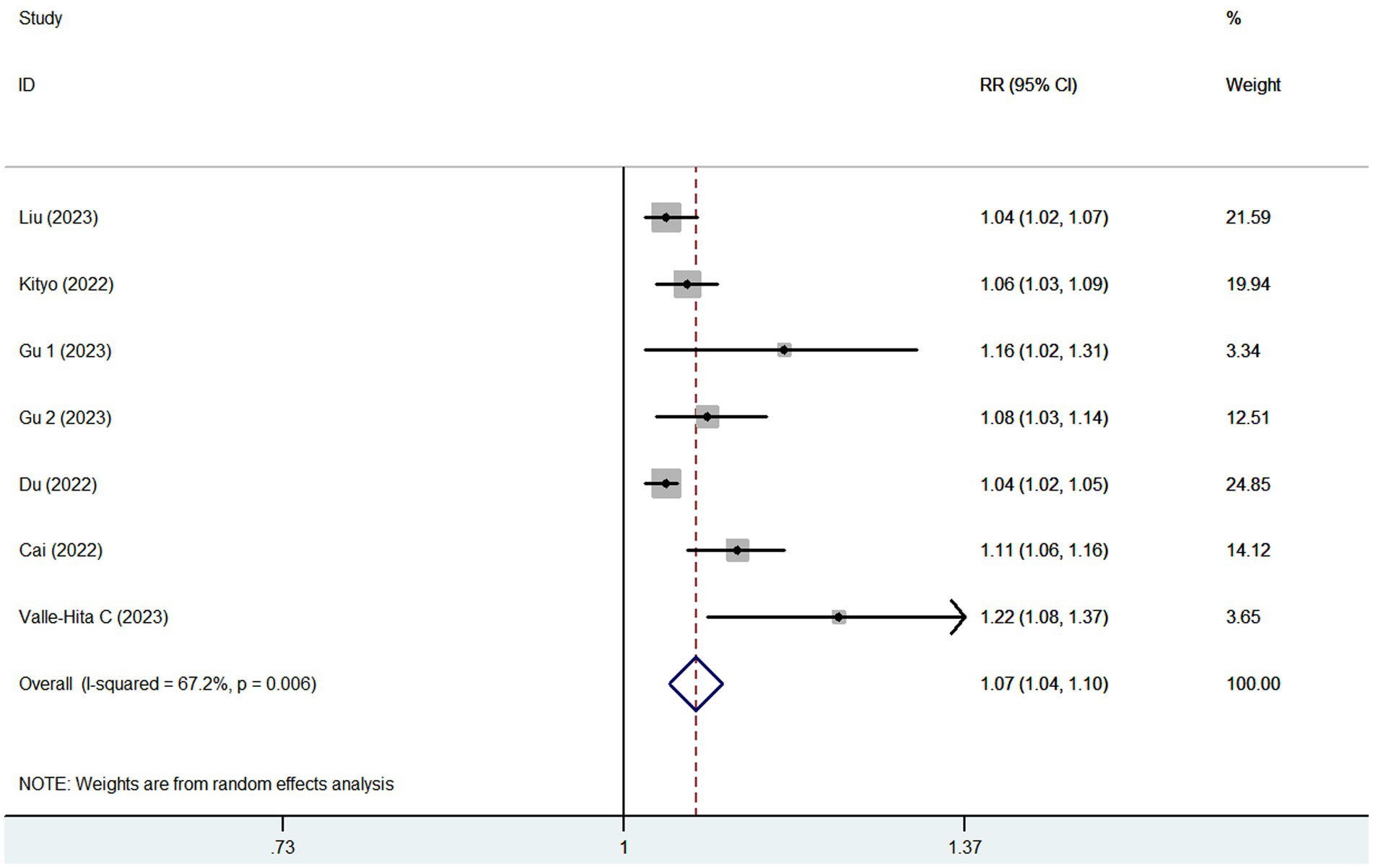
Eight articles reporting 9 studies (513,440 participants and 20,637 CKD cases), were included in this meta-analysis. Figure 2 showed that the highest category of UPF consumption had a 18% higher risk of CKD than the lowest category(RR = 1.18; 95%CI: 1.14–1.23, p < 0.001). Moderate heterogeneity was found in this meta-analysis (I2 = 40.3%; p = 0.099), thus the fixed-effects model was used to calculate the combined RRs. Meanwhile, Figure 3 showed that each 10% increase in UPF consumption was associated with a 7% higher risk of CKD (RR = 1.07; 95% CI:1.04–1.10, I2 = 67.2%; p = 0.006).
Dose–response analysis
Seven studies (21–27) containing 5 cohort and 2 cross-sectional studies, were included in the dose–response analysis for the link between UPF consumption and CKD risk (Figure 4). The dose–response meta-analysis indicated a linear association between UPF consumption and the risk of CKD in the analysis of all included studies (RR = 1.02; 95%CI:0.99–1.05, Pdose–response = 0.178, Pnonlinearity = 0.843).
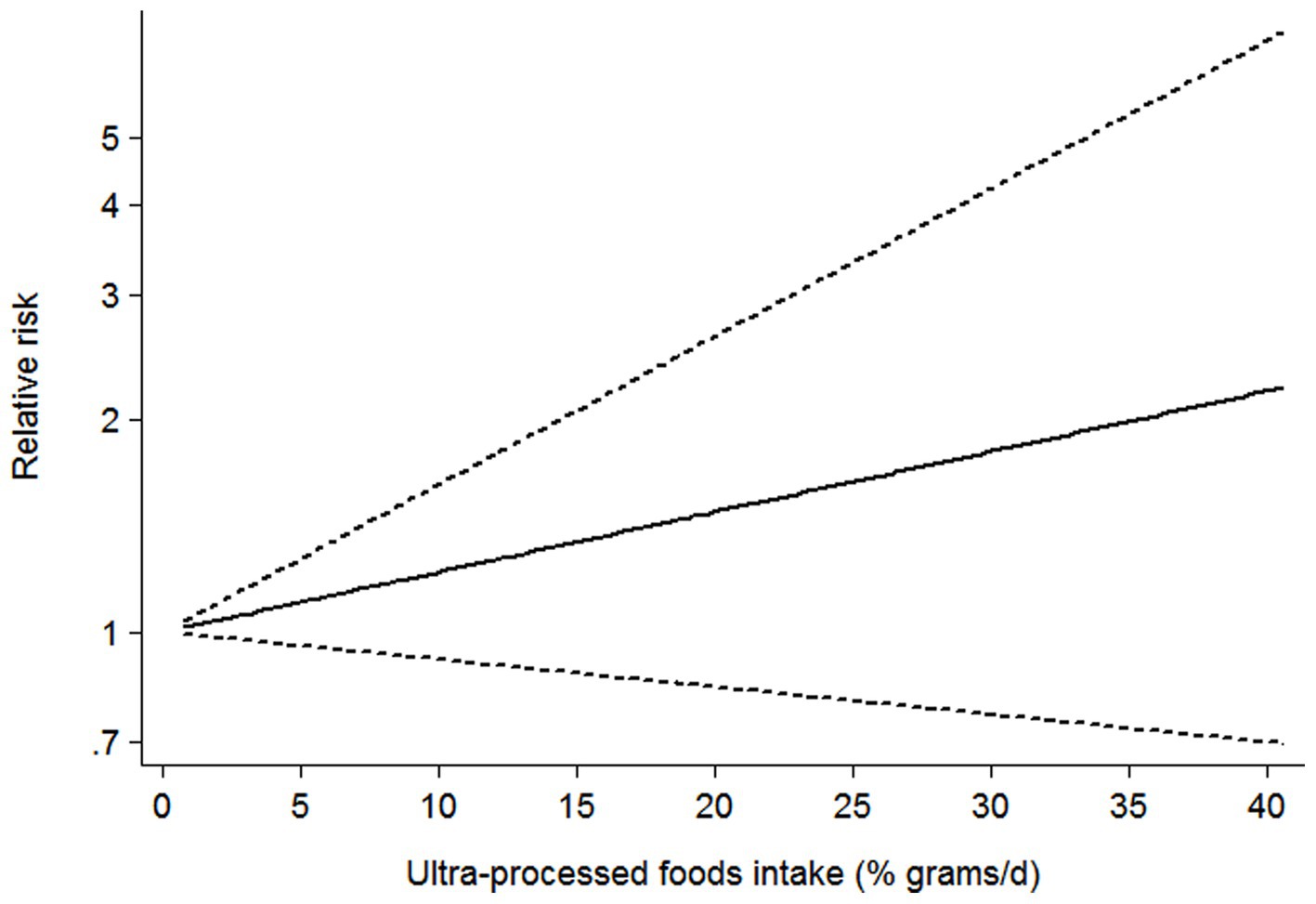
Figure 4. Dose–response association between UPF consumption and CKD in the analysis of all included studies.
Subgroup analyses
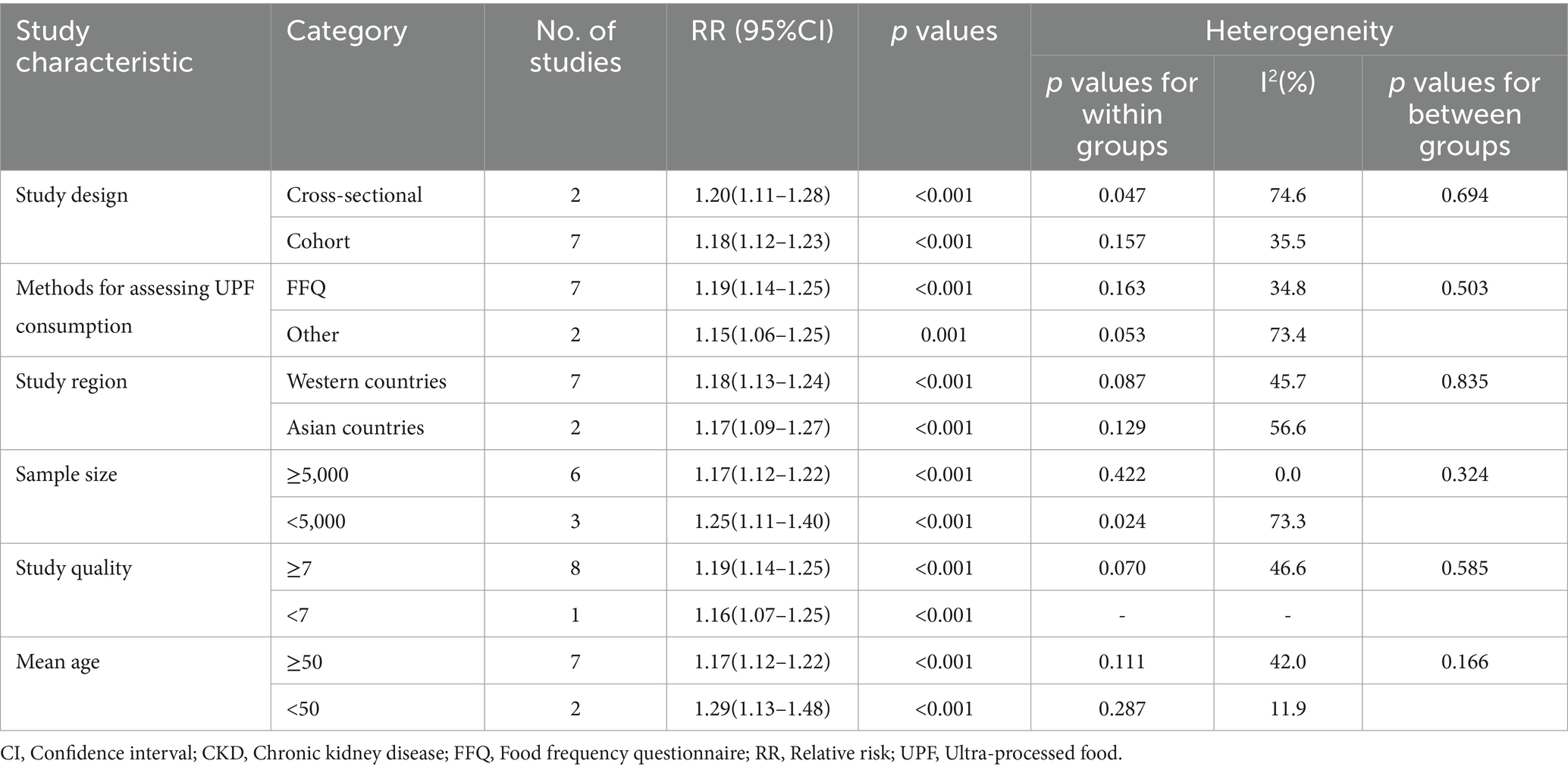
Subgroup analyses were performed to further evaluate the cause of heterogeneity across studies. We performed subgroup analyses basing on study design, study region, mean age, sample size, study quality, and methods for assessing UPF consumption (Table 3). The results showed that there were significant associations between UPF consumption and CKD risk in the all subgroups. Specifically, for study design, we found a positive association in cohort studies (RR = 1.18; 95% CI: 1.12–1.23, p < 0.001), and there was less evidence of heterogeneity (p = 0.157; I2 = 35.5%). For sample size, the results of subgroup analysis showed a positive relationship between UPF consumption and risk of CKD in sample size≥5,000 (RR = 1.17; 95% CI:1.12–1.22, p < 0.001) and there was no evidence of heterogeneity (p = 0.422; I2 = 0.0%).For mean age, we found the significant positive association in mean age < 50 (RR = 1.29; 95% CI:1.13–1.48, p < 0.001), and there was less heterogeneity (p = 0.287; I2 = 11.9%). In the subgroup analysis of methods for assessing UPF consumption, the results showed a positive association in FFQs (RR = 1.19; 95% CI: 1.14–1.25, p < 0.001), and less evidence of heterogeneity (p = 0.163; I2 = 34.8%).
Publication bias
As shown in Supplementary Figure 1, an examination of the funnel plots found little evidence of asymmetry. Begg’s publication bias test was not statistically significant (highest compared with lowest categories of UPF consumption: p = 0.118). Conversely, Egger’s publication bias test had statistical significance (p = 0.016). Thus, we used the trim and fill analysis to re-estimate the pooled risk estimates(Supplementary Figure 2). After running trim and fill analysis, two studies were added to the funnel plot, which showed a low degree of asymmetry and no drastic change in the overall risk estimates (RR = 1.19;95% CI:1.12–1.26, p < 0.01).
Sensitivity analysis and quality assessment
In sensitivity analysis(Supplementary Figure 3), our results showed that the link between UPF consumption and CKD risk was robust. The quality assessment of included studies is shown in Table 4. Seven out of eight included studies received NOS scores ≥7 points, and were classified as of high-quality (20, 22–27). In addition, the remaining one article was classified as of medium-quality (21). Based on the NutriGrade score, the credibility of evidence was moderate considering studies that assessed the exposure with the NOVA food classification system (Table 5).
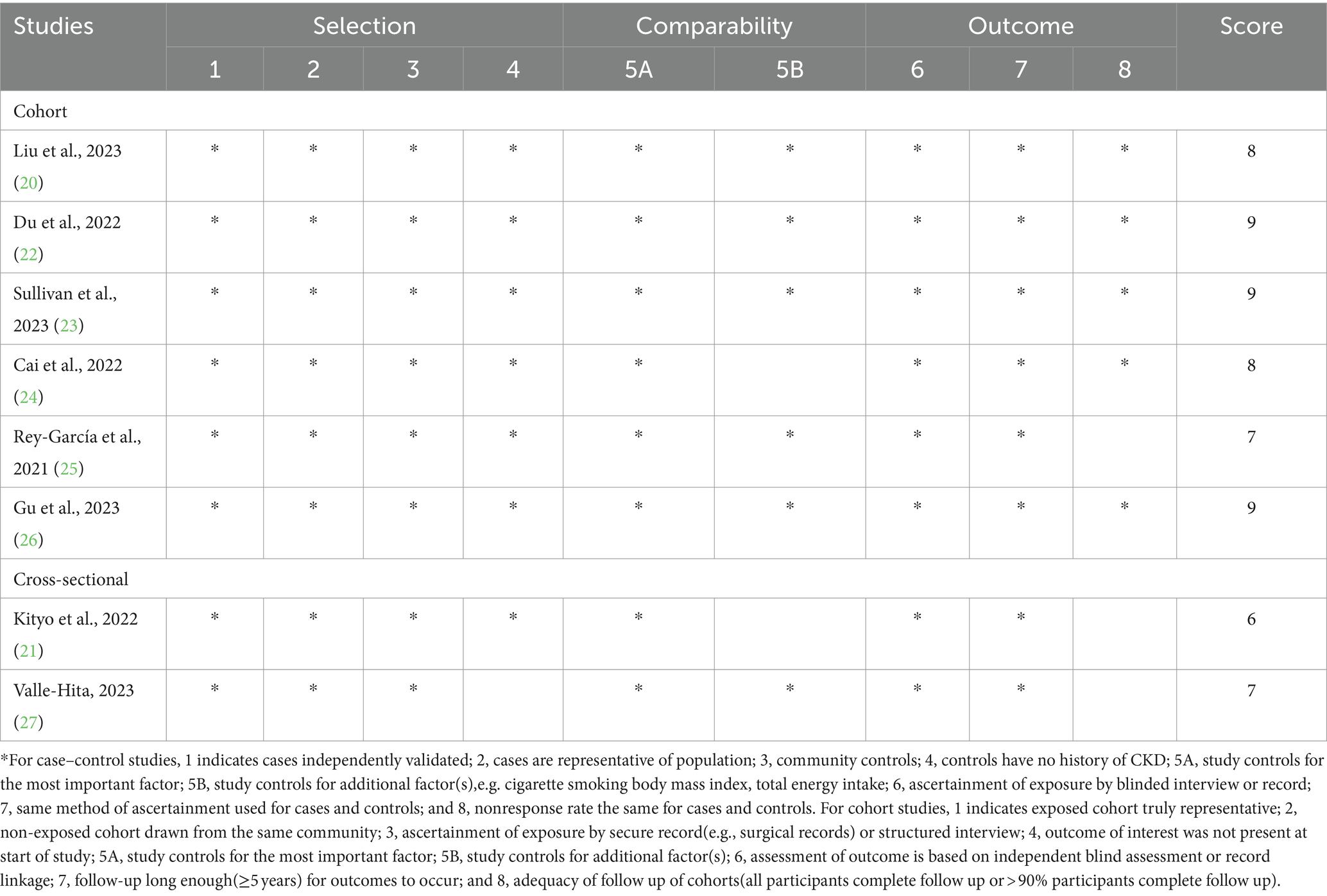
Table 4. Ultra-processed food consumption and risk of chronic kidney disease: assessment of study quality.
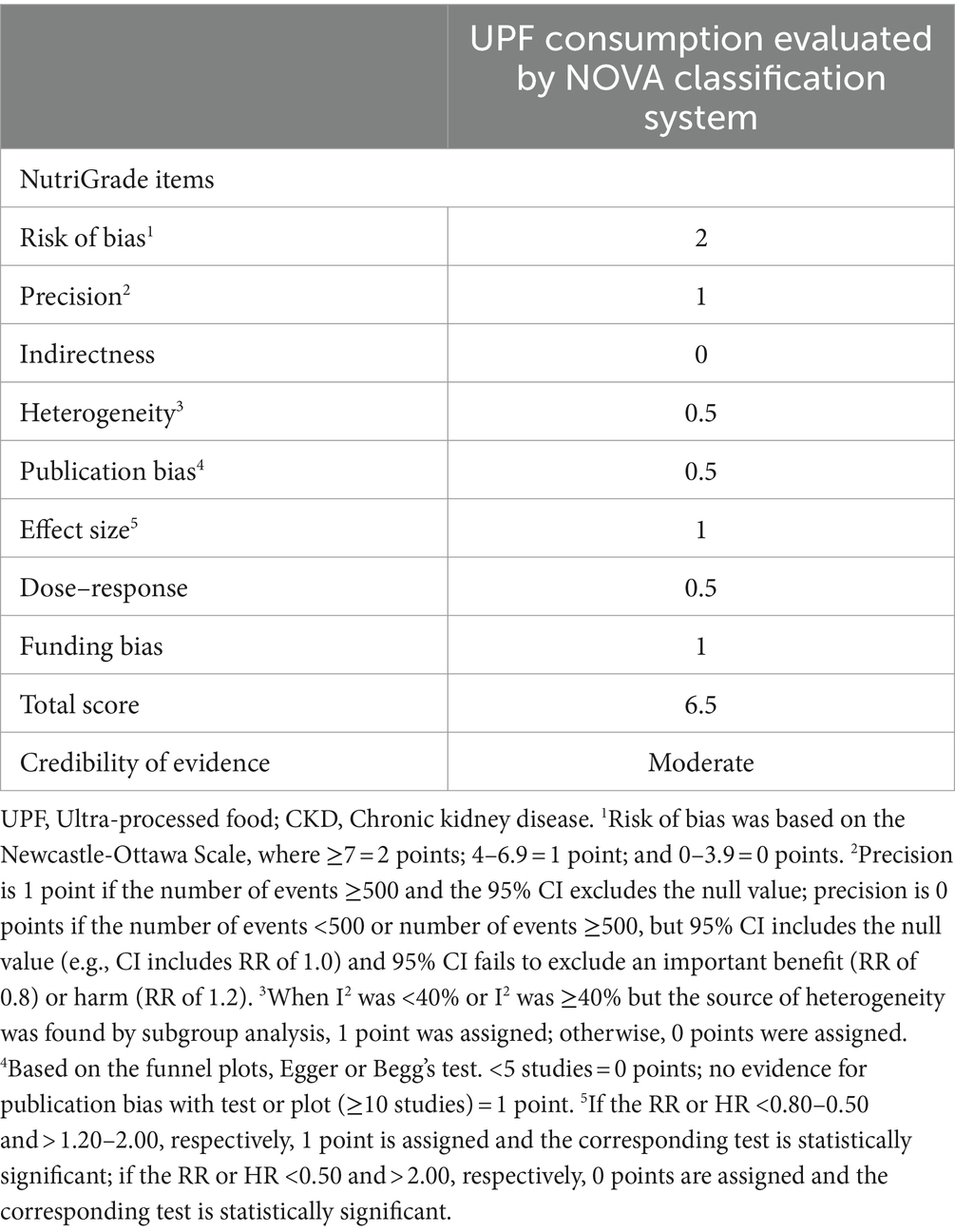
Table 5. Credibility of evidence using NutriGrade tool for relationship between UPF consumption and CKD.
Discussion
To the best of our knowledge, this study is the first systematic review and dose–response meta-analysis to examine the association between high UPF consumption and risk of CKD. In this study, our results showed that high UPF consumption was significantly related to a higher risk of CKD. Moreover, a 10% increase in the consumption of UPF was associated with a 7% higher risk of CKD. Dose–response analysis of all included studies indicated a linear association between UPF consumption and the risk of CKD. Our findings reinforce the existing evidence about the negative effect of high UPF consumption on CKD, and support the need to highlight the importance of decreasing UPF consumption in the prevention of CKD.
Although our results revealed a positive association between high UPF consumption and the risk of CKD, moderate heterogeneity of this meta-analysis was also found (I2 = 40.3%; p = 0.099). We therefore conducted subgroup analyses to examine the possible sources of heterogeneity. In the current study, subgroup analyses were performed based on some factors, including study design, study region, mean age, sample size, study quality and dietary assessment methods. Our results suggested that moderate heterogeneity might be explained by the differences in study design, sample size, mean age, and assessment of UPF consumption. When subgroup analysis was performed in sample size≥5,000, the heterogeneity in this meta-analysis decreased from 40.3 to 0.0%. In fact, some possible explanations for this heterogeneity have been proposed. First, two out of eight included studies were cross-sectional studies. Thus, we could not determine the causality of observed associations due to the observational nature of cross-sectional study. Also, in the observational studies, these results were susceptible to recall bias, resulting from assessment method of UPF consumption, such as FFQs and 24 h dietary recall. Second, three of included studies were sample size<5,000. Thus, small sample size might be the source of heterogeneity. Third, although the RRs or ORs were both from the highest category(taking the lowest category as the reference), different studies divided the UPF score range into different intervals. These might cause the heterogeneity. Fourth, the present results were pooled from different countries, including United States, Spain, United Kingdom, Korea, China and Netherlands with different dietary habits, which might result in the observed heterogeneity. Fifth, different confounding factors adjusted in included studies might explain the moderate heterogeneity observed in this study. Sixth, the use of different units of measurement to evaluate UPF intake, such as servings/day, grams/day, might have contributed to the increased heterogeneity in included studies. Finally, considerable heterogeneity remained in subgroup analyses, showing that there may be other unknown confounding factors.
It has been reported that CKD affects 10% of the world’s population, and ranks in the top non-communicable diseases contributing to disability and premature death (37). Given the substantial burden on public health, it is vital to clarify the modifiable factors associated with CKD. In fact, diet has long been advocated as an important and modifiable risk factor for CKD (6). In the past 40 years, diets in most high-income countries, e.g., the United States and the United Kingdom, have shifted toward a dramatic increase in UPF consumption(for example, exceeding 50% of total caloric intake in U.S. adults), which are usually ready-to-eat, hyper-palatable, cheap and high in energy density, and a decline in nutritional quality (11). Thus far, limited epidemiological studies have been conducted to explore the link between UPF consumption and risk of CKD (20–24, 26, 27), but the available results are controversial. The reasons for the discrepant results between published studies are difficult to fully elucidate. However, it is worth noting that differences in the methods used to assess UPF consumption, in the amount and type of UPF consumed in different countries, and duration of study follow-up might explain part of these discrepant results (38). In our study, the results of meta-analysis revealed that high UPF consumption was significantly associated with an increased risk of CKD (RR = 1.18; 95%CI: 1.14–1.23). Even though the evidence associating high UPF consumption to CKD is inconsistent, several possible explanations have been proposed for this detrimental effect, including poor nutritional composition, high energy density and certain food additives. First, UPFs are generally energy density and high in salt, fats and added sugars and low in dietary fiber (11). High consumption of sugar, particularly in the form of sugar-sweetened beverages, has been associated with an increased risk of CKD (39). Also, epidemiological studies also showed that excessive intakes of sugar were significantly related to higher risks of obesity, hypertension, and diabetes (40), all of which are important risk factors for CKD (4). Furthermore, a recent review reported that adequate and appropriate dietary fiber intake is recommended to restore beneficial gut microbiome composition, which will reduce the risks and complications associated with CKD (41). Second, during food processing, especially high temperature heating, UPF may produce some newly formed contaminants, such as advanced glycation end products(AGEs). Snelson et al. found that AGEs could increase the permeability of the intestinal barrier, which in turn leads to local inflammation and CKD in rodents models (42). Meanwhile, higher AGEs consumption also contributes to oxidative stress and inflammation in the body, which can seriously affect kidney function (43). Third, some food additives found in UPFs, e.g., phosphates might play an important role in the progression of CKD. Studies have shown that phosphate is independently associated with eGFR decline, CKD progression and CKD-related mortality (44–46). Apart from phosphates, other food additives, such as artificial sweeteners, have already been reported to be associated with risk of type 2 diabetes and obesity (47), two important risk factors for CKD. Fourth, UPF may be contaminated with contact materials, such as bisphenol A in some plastic packaging, which has been judged as “a substance of very high concern” by the European Chemicals Agency (48). Evidence from experimental studies has indicated that bisphenol A is a ubiquitous environmental toxin, having a deleterious effect on kidney function (49). Finally, the negative impact of high UPF consumption on CKD may be attributed in part to lower consumption of vegetables, fruits, whole grains, and legumes, which are rich in dietary fiber, folate and antioxidants. A previous study by Jankowska et al., has investigated the status of dietary intake of vitamins in patients with CKD, and reported a negative association between vitamin intake and risk of CKD (50). In addition, prior studies have shown that high consumption of dietary fiber is inversely associated with the risk of CKD (9). Altogether, aforementioned these mechanisms may explain why UPF consumption has been associated with an elevated risk of CKD.
Strengths and limitations
Our study has several notable strengths. First, to our knowledge, we are the first systematic review and dose-response meta-analysis to ascertain the correlation between UPF consumption and risk of CKD. Our findings reinforce the existing evidence about the negative effect of high UPF consumption on CKD, and support the need to highlight the importance of decreasing UPF consumption in the prevention of CKD. Second, rigorous selection of articles was made according to pre-determined inclusion and exclusion criteria. Third, incident cases of CKD were identified through medical records, avoiding the risk of misdiagnosis. Fourth, the results of quality assessment indicated that six out of included studies were of high quality in the current study. Fifth, there was no obvious signs of publication bias in the funnel plot, and statistical tests for publication bias were not significant. Finally, despite there was moderate heterogeneity, we conducted subgroup and sensitivity analyses to examine the potential sources of heterogeneity. Additionally, dose–response analysis was also conducted to strengthen the association between UPF consumption and risk of CKD. However, it is important to acknowledge some potential limitations of this study. First, due to observational nature of the current study, the possibility of recall and selection biases cannot be precluded. Thus, more prospective cohort studies are required to further confirm the relationship between UPF consumption and CKD risk. Second, the majority of included studies used FFQs to collect dietary information, which might lead to misclassification bias, thereby leading to underestimation or overestimation of UPF consumption. In addition, the FFQs used in included studies were not specifically designed to capture the degree and purpose of food processing or validated to precisely measure UPF intake based on the NOVA food classification. Third, although the various potential confounders have been taken into account, the existence of residual confounders cannot be excluded owing to the undetected or unknown confounders. Also, the adjustment confounders in all the included studies were inconsistent, leading to some degree of difference in the values of OR, RR or HR. Fourth, due to lack of data on gender and disease severity, we could not perform subgroup analysis based on gender or disease severity. While we performed subgroup and sensitivity analyses to examine potential sources of heterogeneity, we could not identify and explain the sources of inter-study heterogeneity adequately. In addition, Finally, six of the eight included studies were performed in Western countries, and only two studies were performed in Asian countries, which might limit the generalizability of the findings to other countries.
Conclusion
To summarize, we found that high consumption of UPF was related to an increased risk of CKD. Our findings added further evidence for a detrimental effect of high UPF consumption on CKD, and emphasized the importance of lowering UPF consumption for the prevention of CKD. Therefore, there is an urgent need for additional well- conducted studies, particularly prospective cohort or intervention trials, to confirm our findings in different countries or regions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XH: Investigation, Writing – original draft. XZ: Data curation, Investigation, Writing – review & editing. CS: Methodology, Writing – review & editing. YF: Methodology, Validation, Writing – review & editing. QZ: Data curation, Formal analysis, Funding acquisition, Methodology, Writing – review & editing. SL: Formal analysis, Writing – review & editing. LS: Conceptualization, Formal analysis, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (grant number: 82004040), Traditional Chinese Medicine Research Project of Zhejiang (Nos. 2020ZB009 and 2021ZB010), and Medical and Health research fund project of Zhejiang Province (No. 2022KY006). The sponsors played no role in the study design, data collection, or analysis, or decision to submit the article for publication.
Acknowledgments
We thank the participants from Department of Digestion and Nutrition, Zhejiang Hospital for their value assistance and support. Moreover, we also acknowledge Lv for his important contributions to data analysis in the current meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1359229/full#supplementary-material
Abbreviations
CKD, Chronic kidney disease; CI, Confidence interval; CNKI, China National Knowledge Infrastructure; CVD, Cardiovascular disease; FFQ, Food frequency questionnaire; HR, Hazard ratio; NOS, Newcastle-Ottawa Quality Scale; OR, Odds ratio; RR, Relative risk; SEs, Standard errors; UPF, Ultra-processed food.
References
1. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
2. Zhang, F, Hu, N, Li, J, Pu, M, Li, X, Li, Y, et al. The correlation of urinary strontium with the risk of chronic kidney disease among the general United States population. Front Public Health. (2023) 11:1251232. doi: 10.3389/fpubh.2023.1251232
3. Wang, L, Xu, X, Zhang, M, Hu, C, Zhang, X, Li, C, et al. Prevalence of chronic kidney disease in China: results from the sixth China chronic disease and risk factor surveillance. JAMA Intern Med. (2023) 183:298–310. doi: 10.1001/jamainternmed.2022.6817
4. Jha, V, Garcia-Garcia, G, Iseki, K, Li, Z, Naicker, S, Plattner, B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. (2013) 382:260–72. doi: 10.1016/S0140-6736(13)60687-X
5. Rahimlu, M, Shab-Bidar, S, and Djafarian, K. Body mass index and all-cause mortality in chronic kidney disease: a dose-response meta-analysis of observational studies. J Ren Nutr. (2017) 27:225–32. doi: 10.1053/j.jrn.2017.01.016
6. Yin, T, Chen, Y, Tang, L, Yuan, H, Zeng, X, and Fu, P. Relationship between modifiable lifestyle factors and chronic kidney disease: a bibliometric analysis of top-cited publications from 2011 to 2020. BMC Nephrol. (2022) 23:120. doi: 10.1186/s12882-022-02745-3
7. Shi, Z, Taylor, AW, Riley, M, Byles, J, Liu, J, and Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin Nutr. (2018) 37:276–84. doi: 10.1016/j.clnu.2016.12.025
8. Strippoli, GF, Craig, JC, Rochtchina, E, Flood, VM, Wang, JJ, and Mitchell, P. Fluid and nutrient intake and risk of chronic kidney disease. Nephrology (Carlton). (2011) 16:326–34. doi: 10.1111/j.1440-1797.2010.01415.x
9. He, LQ, Wu, XH, Huang, YQ, Zhang, XY, and Shu, L. Dietary patterns and chronic kidney disease risk: a systematic review and updated meta-analysis of observational studies. Nutr J. (2021) 20:4. doi: 10.1186/s12937-020-00661-6
10. Monteiro, CA, Levy, RB, Claro, RM, de Castro, IR, and Cannon, G. Increasing consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. (2011) 14:5–13. doi: 10.1017/S1368980010003241
11. Baker, P, Machado, P, Santos, T, Sievert, K, Backholer, K, Hadjikakou, M, et al. Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes Rev. (2020) 21:e13126. doi: 10.1111/obr.13126
12. Fiolet, T, Srour, B, Sellem, L, Kesse-Guyot, E, Allès, B, Méjean, C, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ. (2018) 360:k322. doi: 10.1136/bmj.k322
13. Shu, L, Huang, Y, Si, C, Zhu, Q, Zheng, P, and Zhang, X. Association between ultra-processed food intake and risk of colorectal cancer: a systematic review and meta-analysis. Front Nutr. (2023) 10:1170992. doi: 10.3389/fnut.2023.1170992
14. Beslay, M, Srour, B, Méjean, C, Allès, B, Fiolet, T, Debras, C, et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Santé cohort. PLoS Med. (2020) 17:e1003256. doi: 10.1371/journal.pmed.1003256
15. Chen, Z, Khandpur, N, Desjardins, C, Wang, L, Monteiro, CA, Rossato, SL, et al. Ultra-processed food consumption and risk of type 2 diabetes: three large prospective U.S. cohort studies. Diabetes Care. (2023) 46:1335–44. doi: 10.2337/dc22-1993
16. Srour, B, Fezeu, LK, Kesse-Guyot, E, Allès, B, Méjean, C, Andrianasolo, RM, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ. (2019) 365:l1451. doi: 10.1136/bmj.l1451
17. Romaguera, D, Fernández-Barrés, S, Gracia-Lavedán, E, Vendrell, E, Azpiri, M, Ruiz-Moreno, E, et al. Consumption of ultra-processed foods and drinks and colorectal, breast, and prostate cancer. Clin Nutr. (2021) 40:1537–45. doi: 10.1016/j.clnu.2021.02.033
18. Delpino, FM, Figueiredo, LM, Bielemann, RM, da Silva, BGC, Dos Santos, FS, Mintem, GC, et al. Ultra-processed food and risk of type 2 diabetes: a systematic review and meta-analysis of longitudinal studies. Int J Epidemiol. (2022) 51:1120–41. doi: 10.1093/ije/dyab247
19. Askari, M, Heshmati, J, Shahinfar, H, Tripathi, N, and Daneshzad, E. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes. (2020) 44:2080–91. doi: 10.1038/s41366-020-00650-z
20. Liu, M, Yang, S, Ye, Z, Zhang, Y, Zhang, Y, He, P, et al. Relationship of ultra-processed food consumption and new-onset chronic kidney diseases among participants with or without diabetes. Diabetes Metab. (2023) 49:101456. doi: 10.1016/j.diabet.2023.101456
21. Kityo, A, and Lee, SA. The intake of ultra-processed foods and prevalence of chronic kidney disease: the health examinees study. Nutrients. (2022) 14:3548. doi: 10.3390/nu14173548
22. Du, S, Kim, H, Crews, DC, White, K, and Rebholz, CM. Association between Ultraprocessed food consumption and risk of incident CKD: a prospective cohort study. Am J Kidney Dis. (2022) 80:589–598.e1. doi: 10.1053/j.ajkd.2022.03.016
23. Sullivan, VK, Appel, LJ, Anderson, CAM, Kim, H, Unruh, ML, Lash, JP, et al. Ultraprocessed foods and kidney disease progression, mortality, and cardiovascular disease risk in the CRIC study. Am J Kidney Dis. (2023) 82:202–12. doi: 10.1053/j.ajkd.2023.01.452
24. Cai, Q, Duan, MJ, Dekker, LH, Carrero, JJ, Avesani, CM, Bakker, SJL, et al. Ultraprocessed food consumption and kidney function decline in a population-based cohort in the Netherlands. Am J Clin Nutr. (2022) 116:263–73. doi: 10.1093/ajcn/nqac073
25. Rey-García, J, Donat-Vargas, C, Sandoval-Insausti, H, Bayan-Bravo, A, Moreno-Franco, B, Banegas, JR, et al. Ultra-processed food consumption is associated with renal function decline in older adults: a prospective cohort study. Nutrients. (2021) 13:428. doi: 10.3390/nu13020428
26. Gu, Y, Li, H, Ma, H, Zhang, S, Meng, G, Zhang, Q, et al. Consumption of ultraprocessed food and development of chronic kidney disease: the Tianjin chronic low-grade systemic inflammation and health and UK biobank cohort studies. Am J Clin Nutr. (2023) 117:373–82. doi: 10.1016/j.ajcnut.2022.11.005
27. Valle-Hita, C, Díaz-López, A, Becerra-Tomás, N, Toledo, E, Cornejo-Pareja, I, Abete, I, et al. Associations between ultra-processed food consumption and kidney function in an older adult population with metabolic syndrome. Clin Nutr. (2023) 42:2302–10. doi: 10.1016/j.clnu.2023.09.028
28. Xiao, B, Huang, J, Chen, L, Lin, Y, Luo, J, Chen, H, et al. Ultra-processed food consumption and the risk of incident chronic kidney disease: a systematic review and meta-analysis of cohort studies. Ren Fail. (2024) 46:2306224. doi: 10.1080/0886022X.2024.2306224
29. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
30. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
31. Schwingshackl, L, Knüppel, S, Schwedhelm, C, Hoffmann, G, Missbach, B, Stelmach-Mardas, M, et al. Perspective: NutriGrade: a scoring system to assess and judge the Meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. (2016) 7:994–1004. doi: 10.3945/an.116.013052
32. Symons, MJ, and Moore, DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. (2002) 55:893–9. doi: 10.1016/s0895-4356(02)00443-2
33. Grant, RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. (2014) 348:f7450. doi: 10.1136/bmj.f7450
34. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
35. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
36. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
37. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
38. Shu, L, Zhang, X, Zhu, Q, Lv, X, and Si, C. Association between ultra-processed food consumption and risk of breast cancer: a systematic review and dose-response meta-analysis of observational studies. Front Nutr. (2023) 10:1250361. doi: 10.3389/fnut.2023.1250361
39. Rebholz, CM, Young, BA, Katz, R, Tucker, KL, Carithers, TC, Norwood, AF, et al. Patterns of beverages consumed and risk of incident kidney disease. Clin J Am Soc Nephrol. (2019) 14:49–56. doi: 10.2215/CJN.06380518
40. Johnson, RJ, Nakagawa, T, Sanchez-Lozada, LG, Shafiu, M, Sundaram, S, Le, M, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. (2013) 62:3307–15. doi: 10.2337/db12-1814
41. Ranganathan, N, and Anteyi, E. The role of dietary Fiber and gut microbiome modulation in progression of chronic kidney disease. Toxins (Basel). (2022) 14:183. doi: 10.3390/toxins14030183
42. Snelson, M, Tan, SM, Clarke, RE, de Pasquale, C, Thallas-Bonke, V, Nguyen, TV, et al. Processed foods drive intestinal barrier permeability and microvascular diseases. Sci Adv. (2021) 7:eabe4841. doi: 10.1126/sciadv.abe4841
43. Kellow, NJ, and Coughlan, MT. Effect of diet-derived advanced glycation end products on inflammation. Nutr Rev. (2015) 73:737–59. doi: 10.1093/nutrit/nuv030
44. Vervloet, MG, Sezer, S, Massy, ZA, Johansson, L, Cozzolino, M, and Fouque, D. ERA–EDTA working group on chronic kidney disease–mineral and bone disorders and the European renal nutrition working group. The role of phosphate in kidney disease. Nat Rev Nephrol. (2017) 13:27–38. doi: 10.1038/nrneph.2016.164
45. Kestenbaum, B, Sampson, JN, Rudser, KD, Patterson, DJ, Seliger, SL, Young, B, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. (2005) 16:520–8. doi: 10.1681/ASN.2004070602
46. O'Seaghdha, CM, Hwang, SJ, Muntner, P, Melamed, ML, and Fox, CS. Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant. (2011) 26:2885–90. doi: 10.1093/ndt/gfq808
47. Nettleton, JA, Lutsey, PL, Wang, Y, Lima, JA, Michos, ED, and Jacobs, DR Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. (2009) 32:688–94. doi: 10.2337/dc08-1799
48. European Chemical Agency (ECHA). Member State Committee support document for identification of 4,4-isopropylidenediphenol (bisphenol a) as a substance of very high concern because of its toxic for reproduction (Article 57 c) properties. (2016) Adopted on 2 December 2016. Available at: https://echa.europa.eu/documents/10162/b10d6a00-8e47-9b14-4f61-c779a8dc8450.
49. Priego, AR, Parra, EG, Mas, S, Morgado-Pascual, JL, Ruiz-Ortega, M, and Rayego-Mateos, S. Bisphenol a modulates autophagy and exacerbates chronic kidney damage in mice. Int J Mol Sci. (2021) 22:7189. doi: 10.3390/ijms22137189
Keywords: ultra-processed food, chronic kidney disease, systematic review, dose–response meta-analysis, observational study
Citation: He X, Zhang X, Si C, Feng Y, Zhu Q, Li S and Shu L (2024) Ultra-processed food consumption and chronic kidney disease risk: a systematic review and dose–response meta-analysis. Front. Nutr. 11:1359229. doi: 10.3389/fnut.2024.1359229
Edited by:
Marjolein Bonthuis, Academic Medical Center, NetherlandsReviewed by:
Rianne Boenink, Academic Medical Center, NetherlandsMehran Rahimlou, Zanjan University of Medical Sciences, Iran
Copyright © 2024 He, Zhang, Si, Feng, Zhu, Li and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Shu, shulong19880920@126.com
 Xingzhen He
Xingzhen He Xiaoyan Zhang
Xiaoyan Zhang Caijuan Si
Caijuan Si Yuliang Feng
Yuliang Feng Qin Zhu
Qin Zhu Songtao Li3
Songtao Li3  Long Shu
Long Shu