Recent insights into breast milk microRNA: their role as functional regulators
- 1Warshel Institute for Computational Biology, The Chinese University of Hong Kong, Shenzhen, Guangdong, China
- 2School of Medicine, The Chinese University of Hong Kong, Shenzhen, Guangdong, China
- 3Institute of Bioinformatics and Systems Biology and Center for Intelligent Drug Systems and Smart Bio-devices (IDS2B), National Yang Ming Chiao Tung University, Hsinchu, Taiwan
- 4Department of Nephrology, Center for Regeneration and Aging Medicine, The Fourth Affiliated Hospital of School of Medicine, and International School of Medicine, International Institutes of Medicine, Zhejiang University, Yiwu, China
- 5Zhejiang-Denmark Joint Laboratory of Regeneration and Aging Medicine, Yiwu, China
Breast milk (BM) is a primary biofluid that plays a crucial role in infant development and the regulation of the immune system. As a class of rich biomolecules in BM, microRNAs (miRNAs) are regarded as active factors contributing to infant growth and development. Surprisingly, these molecules exhibit resilience in harsh conditions, providing an opportunity for infants to absorb them. In addition, many studies have shown that miRNAs in breast milk, when absorbed into the gastrointestinal system, can act as a class of functional regulators to effectively regulate gene expression. Understanding the absorption pattern of BM miRNA may facilitate the creation of formula with a more optimal miRNA balance and pave the way for novel drug delivery techniques. In this review, we initially present evidence of BM miRNA absorption. Subsequently, we compile studies that integrate both in vivo and in vitro findings to illustrate the bioavailability and biodistribution of BM miRNAs post-absorption. In addition, we evaluate the strengths and weaknesses of previous studies and discuss potential variables contributing to discrepancies in their outcomes. This literature review indicates that miRNAs can be absorbed and act as regulatory agents.
1 Introduction
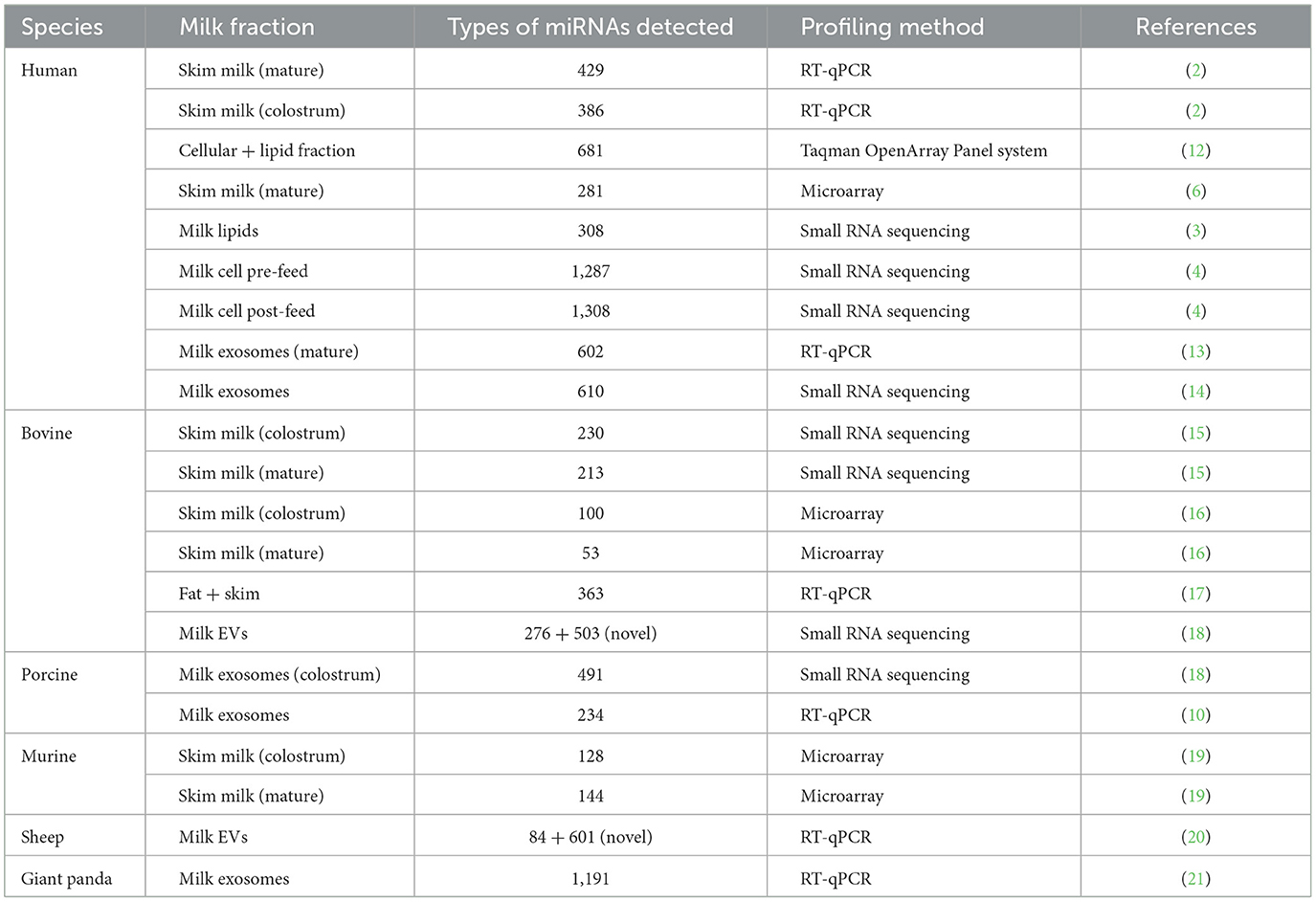
Breast milk (BM) is basically an essential source of nutrients that nourish and support the growth and development of infants. BM can be categorized into cellular, fat, and skim components (1). The number of milk-derived microRNAs (miRNAs) varies significantly among different components and across different species (Table 1). However, some miRNAs are shared in a common (2–4). Various factors could influence miRNA profiles in BM (3), such as lactation periods (4), gestation age (5), sex of infant (6), maternal weight (7), and diet, particularly high-fat diets (8). It's worth noting that milk-derived miRNA profiles undergo significant changes during lactation periods (4). These characteristics remain consistent across a range of species, including human (5), bovine (9), porcine (10), tammar wallaby (11), and more (Table 1). This consistency implies the potential for biological function.
It has been shown that milk-derived miRNAs can survive in harsh conditions, including low pH (19, 22) environments (13, 23), such as the freezing-thawing cycle (22, 24) and digestive system. This resilience could be attributed to the protective role of milk exosomes (25), suggesting a potential way for drug delivery. Besides, before internalization, miRNA must cross several physical barriers, including the gastrointestinal (GI) tract and its mucus layer (26). Regarding the protective mechanisms, several studies have proposed different theories. As summarized by Carrillo-Lozano et al., extracellular vesicles, such as exosomes, protect miRNAs from harsh conditions in skim and lipid fractions (27). Other possible molecules, such as fat globules, Argonaute-2, RICS-Complex, and even mammary epithelial cells, may also be involved in BM miRNA protection (23).
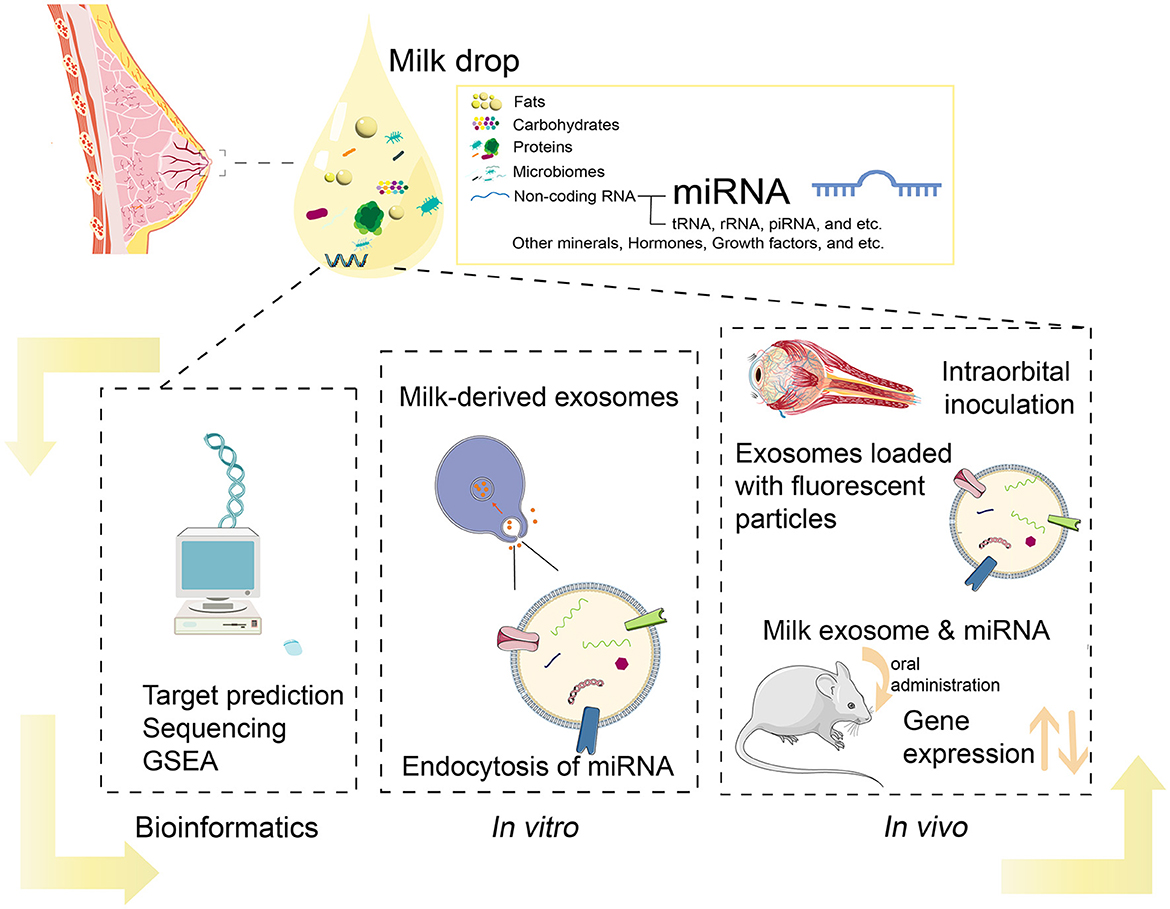
Recent studies indicate that BM miRNAs could act as functional regulators (Figure 1), impacting infant immunity and development (5, 21, 24). For example, miR-223 is a regulator of granulosa lineage cell commitment, and its expression level is not affected by pasteurization, but it is highly expressed in both colostrum and mature human milk (28, 29). Besides, both miR-let-7a-5p and miR-181-5p were found to be highly enriched in BM across species. MiR-let-7a-5p is an important regulator of inflammatory response and cellular phenotype (30), whereas miR-148a-3p plays an essential role in controlling inflammation and affecting cancer development (31). In addition, previous studies have suggested that miRNAs in BM could be involved in infant development, including the regulation of intestinal function (4) and neurogenesis (32–35). Moreover, miRNAs in BM have also been associated with lipid metabolism (36, 37). Taken together, these studies suggest a crucial role of BM miRNAs in infants' immune systems and development.
However, questions persist regarding the mechanism of BM miRNA absorption, and their bioavailability within the infant's internal environment. So far, two hypotheses exist to explain the function of miRNA in BM: the functional hypothesis, which posits that miRNAs can retain biological activity after absorption; but the nutritional hypothesis, which suggests that miRNAs are degraded before absorption and primarily serve as nutritional components (38).
Several reviews have endeavored to summarize and discuss recent research on the uptake of dietary miRNAs. Some of them focused on bioinformatics analysis on BM miRNA profiles and phenotypic changes after milk meal, revealing many potential benefits of BM on infants, even across species. In this review, we predominantly emphasized experimental studies validated by both in vivo and in vitro studies (Supplementary material). These findings demonstrate the absorption of BM miRNAs, along with their bioavailability and distribution following uptake, showing that BM miRNAs are transferable genetic materials.
2 Absorption of BM-derived miRNAs
Previous studies suggest that the BM miRNAs have the potential to be absorbed by varied cell lines. Wolf et al. employed a fluorescence-based approach to detect the in vitro absorption of bovine milk in both Caco-2 intestinal epithelial cells (IEC) and IEC-6 cells. The uptake of BM exosomal miRNAs could be mediated by endocytosis and appeared to be affected by both cell and exosome surface protein (39, 40). This is indicated by a significant decrease in uptake efficacy when the proteins on the surface of BM exosomes are removed or when Caco-2 cells are treated with proteinase K (39). Wolf et al. also demonstrated that exosomes underwent unidirectional transport across intestinal monolayers, moving from the apical chamber to the basolateral chamber, with minimal reverse transportation (39). The uptake was also found to be dependent on temperature, with more than a 50% decrease in uptake observed when the temperature drops from 37 to 4°C (39).
However, it appears that environmental pH levels may have a limited impact on the absorption of BM-derived miRNAs. Liao et al. conducted an experiment in which fluorescence-labeled human milk exosomes were incubated with human intestinal crypt-like cells (HIEC) (22). They observed an increase in fluorescence density within HIEC after a 2-h incubation at pH 4. However, there was no statistically significant difference in the change of fluorescence density at pH 2, suggesting that variations in environmental pH may not significantly influence the uptake of these miRNAs.
It's worth noting that 10% of the internalized human milk exosomes were identified within the nucleus of HIEC, providing evidence of the potential impact of human milk miRNA on cellular regulation (22). This internalization and nuclear localization can also be seen from confocal microscopy (41). More importantly, both miR-21-5p and miR-30a-5p derived from bovine milk were identified in human plasma following a meal, and these detectable levels persisted for up to 6 h (42). Taken together, these studies have provided compelling evidence that BM miRNA derived from one species can be detected within the cells of another species, strongly supporting the functional hypothesis.
Although the absorption of BM exosomal miRNA is well-studied, it has been reported that non-exosomal BM miRNAs could also be taken up in the digestive system (43). Generally speaking, milk can be separated into different components, including milk fat, whey, casein, cells, and debris, through the process of differential centrifugation. Through employing ultra-centrifugation, it is possible to achieve more refined separation, enabling the isolation of extracellular vesicles (EVs) from the supernatant (44). Lin et al. demonstrated that certain milk-derived miRNAs, such as miR-2291 and miR-7134, displayed unique expression patterns in exosomes compared to the exosome-free supernatant where no such miRNAs are found (43). By examining these exosomal and non-exosomal specific miRNAs in IPEC-J2 cells following incubation with milk exosomes and exosome-free supernatants, their study revealed that both exosomal and non-exosomal miRNAs could be absorbed by the IPEC-J2 cells.
Extracellular vehicles are essential for the uptake of BM miRNAs. They not only deliver miRNAs to target cells, but also significantly enhance miRNAs stability under harsh conditions. Chen et al. suggested that SID-1 transmembrane family member 1 (SIDT1) might act as a transporter for BM miRNA uptake (45). Furthermore, Wei et al. showed that endocytosis of exosomal miRNAs is mediated by caveolae- and lipid raft-dependent pathways (46). Although various mechanisms and sites of uptake have been proposed, such as intestinal epithelial cells and vascular epithelial cells (39, 40), it's widely accepted that BM miRNAs can be absorbed and function as regulators.
3 Bioavailability of BM-derived miRNA
Considering the uptake of BM miRNA, questions have emerged regarding whether miRNAs absorbed from BM can reach their target sites and directly impact gene expression. So far, several studies have given positive answers to the questions by cooperating in vitro and in vivo experiments. The core methodology of these studies is consistent: they track changes in specific miRNA attributes or biomarkers in vivo and then explore the resulting shifts in cellular metabolism caused by these altered miRNA traits in a controlled in vitro setting. Therefore, in conducting in vitro experiments, it's crucial to carefully consider confounding factors such as the duration miRNAs remain stable in the in vivo environment before degrading, and the real concentration of miRNAs that are absorbed into the system.
Chen et al. not only showed that porcine milk-derived miRNAs, including miR-7134, miR-1343, miR-2320, miR-181a, miR-769-3p, and miR-128, were absorbed by porcine intestinal cell line (IPEC-J2), but also observed a down-regulation of FAS and SERPINE in the cell line after the uptake of miRNAs (47). These genes are targets of the aforementioned miRNAs.
Similarly, Baier et al. showed a significant absorption of miR-29b and miR-200c through the administration of varying milk doses to individuals in a randomized crossover design study. In addition, they also observed significant changes in the expression levels of miRNAs' target genes (48). To investigate the bioactivity of the absorbed miRNAs further, they performed a luciferase reporter gene assay. These reporter genes incorporated 3′UTR regions containing specific binding sites for these miRNAs (48). The assay revealed a substantial decrease in the activity of reporter genes when HEK-293 cells were cultured with milk exosomes containing miR-29b and miR-200c that mimicked postprandial concentrations, suggesting the function of milk-derived miRNAs after absorption (48). The detection of miR-1 is also involved in this study, as it was not detectable in milk and served as a negative control, which is one of the key points for the experiment. Another notable aspect is that they added the milk exosomes to the cell culture media mimicking the postprandial concentrations of miR-29b and miR-200c (48). However, Auerbach et al. failed to replicate these results and pointed out that this contradiction might be attributed to technique variations, such as the differences in RNA purification, qPCR assay design and other factors (49).
In another study, researchers revealed the influence of BM miR-26a on the development of offspring's adipose tissue (50). Their findings demonstrated that changes in the level of miR-26a, delivered through milk, can alter the expression of target genes in the offspring, subsequently affecting adipose tissue development (50). The key aspect of this discovery relied on the selection of miR-26a, which is among the 10 most abundant miRNAs in breast milk, as indicated by several studies (8, 22, 41, 51, 52). Nevertheless, it is also essential for future studies to consider the in vivo metabolism and circulation of BM miR-26a.
Bioinformatics analysis also offered indirect support for the functional hypothesis. For example, upregulated miRNAs in moderate/very preterm compared to term mature milk tended to be enriched in the neuro-related GO pathways (53). This result may indicate that BM miRNAs play a crucial role in infants' neurodevelopment (54). In addition, the target genes of the most abundant miRNAs in BM were found to be enriched in immune-related pathways, such as TGF-beta signaling, T-cell receptor signaling, Toll-like receptor signaling, Jak-STAT signaling, and Th1 and Th2 cell differentiation (55). Other studies employing comparable methodologies have supplied evidence suggesting that miRNAs may function as regulators in various domains, including neurogenesis, gut maturation, epigenetics, and infant metabolism and development (56). Collectively, these studies suggest the functional hypothesis of BM miRNAs.
4 Biodistribution of milk-derived miRNA
Current studies show that the milk exosomes were predominantly concentrated in the liver and spleen following uptake (40, 57). Kusuma et al. observed that the majority of exosomes were cleared from the circulation and distributed in a region near the liver within 18 h after intra-orbital injection of DiR-labeled milk exosomes (40). Manca et al. also reported that the liver and spleen were the primary organs enriched with milk-derived exosomes following a milk meal (57). Small extracellular vesicles were also estimated to accumulate in the intestinal mucosa, liver, brain, bone, and thymus, with a bioavailability of up to 45% after oral administration (58).
The distribution of exosomal miRNA exhibited variations may vary depending on different administration methods (such as intravenous injection or oral administration) and the types of miRNA (57). Munagala et al. (48) showed that the presence of milk exosomes could also be detected in other organs that were not reported by the previous studies, regardless of administration methods (59). This inconsistency could be attributed to the absence of controls (free DiR or unlabeled exosomes) and the notable difference in oral miRNA doses, which were four times higher compared to the previous study (57).
Although these studies point to the biodistribution of exosome uptake, their function in particular organs remains unknown. Considering that macrophages in the liver and spleen are responsible for clearing foreign exosomes administered to mice (57), it suggests a likelihood that milk-derived exosomal miRNAs could exert their effects before undergoing clearance by macrophages. Moreover, the failure of bovine milk exosomes to rescue Drosha knockout mice—genetically modified mice with a loss of microRNA maturation—indicates the need for further investigation to comprehend the biological efficacy and absorption levels of milk-derived miRNAs (57).
5 Discussion
In this review, we follow the logic of “absorption-bioavailability-biodistribution” to provide evidence supporting the functional hypothesis. The discussion of bioavailability and biodistribution is based on the fact that miRNAs in breast milk are indeed absorbed by infants, and aims to discuss how and where they have biological effects on gene expression. Based on these studies, we conclude that miRNAs can be absorbed and exert biological function.
However, some confounding factors should be carefully assessed. The selection of miRNA as evidence of absorption demands careful consideration due to its inherent properties. For example, internal miRNA profile could influence the results. The disparities in milk-derived miRNA biodistribution between mice after oral administration and intravenous injection suggest that oral milk meals may indeed induce a series of changes in miRNA profiles (57). Therefore, using labeled exogenous miRNAs that cannot be naturally produced by the host would be a preferable method for detecting the absorption of BM miRNA. This approach helps eliminate the potential confounding factors associated with endogenous miRNA changes and provides a more accurate assessment of the specific miRNAs introduced through the diet.
Transgenic mice are also used to validate the absorption of BM-derived miRNAs by examining changes in endogenous miRNA expression after in vitro incubation or in vivo uptake. However, this approach may include unknown variables that could affect the experiment results. For example, the miRNAs being uptaken may not exclusively originate from the milk meal and could also come from other gastric contents (60).
In addition, Wang et al. (42) suggested that using heparin tubes for blood collection can entirely eliminate the miRNAs in the sample, while the hemolysis of human red blood cells can significantly increase miR-16 levels in the sample (42). This underscores the importance of employing the correct method for collecting blood samples when detecting miRNA after a milk meal.
Studies of the absorption and bioavailability of milk-derived miRNAs have significant importance. Understanding this process could facilitate the development of artificially modified milk compositions containing a more balanced array of miRNAs, thus potentially enhancing infant development. it provides a natural solution that surpasses the traditional use of artificial nanoparticles as carriers for RNA interference (RNAi) drugs (26, 57). Once it is confirmed that BM miRNAs are functional regulators, more comprehensive testing of commercial milk may need to be considered to ensure the quality of commercial milk at the molecular level (61). Therefore, several issues or questions should be considered in future studies: (1) Although the results from in vivo experiments consistently demonstrate the absorption of milk-derived miRNA from the digestive system, there is a disparity in the conclusion derived from in vivo experiments. More careful selection of miRNAs as biomarkers is needed. (2) Further investigation is required to ascertain the bioavailability and adequacy of milk-derived miRNAs in altering gene expression after absorption (57). To assess the bioavailability of milk-derived miRNAs, it is imperative to investigate the metabolism of these miRNAs after a milk meal. (3) Understanding the mechanism of miRNA absorption is essential, including whether the gastrointestinal tract possesses specific sites for the utilization of milk-derived miRNA and the potential role of miRNAs in the gastrointestinal tract (62). This is necessary as it may explain the lack of observed evidence regarding the absorption of milk-derived miRNA into organs or circulation. (4) Apart from cellular uptake in the digestive tract itself, it is also plausible that the microbiome could involve the process of milk-derived miRNA absorption.
Author contributions
Y-RX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JZ: Investigation, Visualization, Writing – review & editing. H-YH: Writing – review & editing, Methodology. Y-C-DL: Writing – review & editing, Methodology. T-YL: Writing – review & editing, Methodology. H-DH: Writing – review & editing, Methodology. YY: Writing – review & editing, Funding acquisition, Resources, Supervision. Y-FW: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Shenzhen-Hong Kong Jointly Funded Project (Category A) from Shenzhen Science and Technology Innovation Committee (SGDX20230116093201002), Research Start-Up Fund from the Chinese University of Hong Kong, Shenzhen (CUHK-Shenzhen; K10120220256 and UDF01002831/UF02002831), Ganghong Young Scholar Development Fund (E10120210019 and E10120220220), Zhejiang Provincial Nature Science Foundation of China (LZ24H050001), National Natural Science Foundation of China (Grant Nos. 32070659 and 82201893), Yushan Young Fellow Program (112C1N084C) by the Ministry of Education (MOE), and National Science and Technology Council (NSTC 112-2321-B-A49-016 and 112-2740-B-400-005) from Taiwan.
Acknowledgments
Y-FW, H-YH, Y-C-DL, and H-DH sincerely appreciate the supports from Warshel Institute for Computational Biology funding from Shenzhen City and Longgang District. T-YL thanks the supports from the Center for Intelligent Drug Systems and Smart Biodevices (IDS2B) from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1366435/full#supplementary-material
References
1. Alsaweed M, Hartmann PE, Geddes DT, Kakulas F. MicroRNAs in breastmilk and the lactating breast: potential immunoprotectors and developmental regulators for the infant and the mother. Int J Environ Res Public Health. (2015) 12:13981–4020. doi: 10.3390/ijerph121113981
2. Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. (2010) 56:1733–41. doi: 10.1373/clinchem.2010.147405
3. Munch EM, Harris RA, Mohammad M, Benham AL, Pejerrey SM, Showalter L, et al. Transcriptome profiling of microRNA by next-gen deep sequencing reveals known and novel miRNA species in the lipid fraction of human breast milk. PLoS ONE. (2013) 8:e50564. doi: 10.1371/journal.pone.0050564
4. Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F. Human milk cells contain numerous miRNAs that may change with milk removal and regulate multiple physiological processes. Int J Mol Sci. (2016) 17:956. doi: 10.3390/ijms17060956
5. Tingö L, Ahlberg E, Johansson L, Pedersen SA, Chawla K, Sætrom P, et al. Non-coding RNAs in human breast milk: a systematic review. Front Immunol. (2021) 12:725323. doi: 10.3389/fimmu.2021.725323
6. Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. (2010) 1:7. doi: 10.1186/1758-907X-1-7
7. Carney MC, Tarasiuk A, DiAngelo SL, Silveyra P, Podany A, Birch LL, et al. Metabolism-related microRNAs in maternal breast milk are influenced by premature delivery. Pediatr Res. (2017) 82:226–36. doi: 10.1038/pr.2017.54
8. Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Alshaer W, Hasan H, Albakri KA, et al. Immunomodulatory properties of human breast milk: microRNA contents and potential epigenetic effects. Biomedicines. (2022) 10:1219. doi: 10.3390/biomedicines10061219
9. Hese IV, Goossens K, Vandaele L, Opsomer G. Invited review: microRNAs in bovine colostrum-Focus on their origin and potential health benefits for the calf. J Dairy Sci. (2020) 103:1–15. doi: 10.3168/jds.2019-16959
10. Gu Y, Li M, Wang T, Liang Y, Zhong Z, Wang X, et al. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PLoS ONE. (2012) 7:e43691. doi: 10.1371/journal.pone.0043691
11. Modepalli V, Kumar A, Hinds LA, Sharp JA, Nicholas KR, Lefevre C. Differential temporal expression of milk miRNA during the lactation cycle of the marsupial tammar wallaby (Macropus eugenii). BMC Genomics. (2014) 15:1012. doi: 10.1186/1471-2164-15-1012
12. Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci Rep. (2016) 6:20680. doi: 10.1038/srep20680
13. Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, et al. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. (2011) 8:118–23. doi: 10.7150/ijbs.8.118
14. Xi Y, Jiang X, Li R, Chen M, Song W, Li X. The levels of human milk microRNAs and their association with maternal weight characteristics. Eur J Clin Nutr. (2016) 70:455–60. doi: 10.1038/ejcn.2015.168
15. Chen X, Gao C, Li H, Huang L, Sun Q, Dong Y, et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. (2010) 20:1128–37. doi: 10.1038/cr.2010.80
16. Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci. (2012) 95:4831–41. doi: 10.3168/jds.2012-5489
17. Golan-Gerstl R, Elbaum Shiff Y, Moshayoff V, Schecter D, Leshkowitz D, Reif S. Characterization and biological function of milk-derived miRNAs. Mol Nutr Food Res. (2017) 61:1700009. doi: 10.1002/mnfr.201700009
18. Chen T, Xi QY, Ye RS, Cheng X, Qi QE, Wang SB, et al. Exploration of microRNAs in porcine milk exosomes. BMC Genomics. (2014) 15:100. doi: 10.1186/1471-2164-15-100
19. Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Time-dependent expression profiles of microRNAs and mRNAs in rat milk whey. PLoS ONE. (2014) 9:e88843. doi: 10.1371/journal.pone.0088843
20. Quan S, Nan X, Wang K, Jiang L, Yao J, Xiong B. Characterization of sheep milk extracellular vesicle-miRNA by sequencing and comparison with cow milk. Anim Open Access J MDPI. (2020) 10:331. doi: 10.3390/ani10020331
21. Ma J, Wang C, Long K, Zhang H, Zhang J, Jin L, et al. Exosomal microRNAs in giant panda (Ailuropoda melanoleuca) breast milk: potential maternal regulators for the development of newborn cubs. Sci Rep. (2017) 7:3507. doi: 10.1038/s41598-017-03707-8
22. Liao Y, Du X, Li J, Lönnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res. (2017) 61:1700082. doi: 10.1002/mnfr.201700082
23. Jiang M, Sang X, Hong Z. Beyond nutrients: food-derived microRNAs provide cross-kingdom regulation. BioEssays News Rev Mol Cell Dev Biol. (2012) 34:280–4. doi: 10.1002/bies.201100181
24. Rani P, Vashisht M, Golla N, Shandilya S, Onteru S, Singh D. Milk miRNAs encapsulated in exosomes are stable to human digestion and permeable to intestinal barrier in vitro. J Funct Foods. (2017) 34:431–9. doi: 10.1016/j.jff.2017.05.009
25. Shandilya S, Rani P, Onteru SK, Singh D. Small interfering RNA in milk exosomes is resistant to digestion and crosses the intestinal barrier in vitro. J Agric Food Chem. (2017) 65:9506–13. doi: 10.1021/acs.jafc.7b03123
26. Afrin H, Geetha Bai R, Kumar R, Ahmad SS, Agarwal SK, Nurunnabi M. Oral delivery of RNAi for cancer therapy. Cancer Metastasis Rev. (2023) 23:1–26. doi: 10.1007/s10555-023-10099-x
27. Carrillo-Lozano E, Sebastián-Valles F, Knott-Torcal C. Circulating microRNAs in breast milk and their potential impact on the infant. Nutrients. (2020) 12:3066. doi: 10.3390/nu12103066
28. Perri M, Lucente M, Cannataro R, De Luca IF, Gallelli L, Moro G, et al. Variation in immune-related microRNAs profile in human milk amongst lactating women. MicroRNA Shariqah United Arab Emir. (2018) 7:107–14. doi: 10.2174/2211536607666180206150503
29. Río P, Agirre X, Garate L, Baños R, Álvarez L, San José-Enériz E, et al. Down-regulated expression of hsa-miR-181c in Fanconi anemia patients: implications in TNFα regulation and proliferation of hematopoietic progenitor cells. Blood. (2012) 119:3042–9. doi: 10.1182/blood-2011-01-331017
30. Wen K, Ni K, Guo J, Bu B, Liu L, Pan Y, et al. MircroRNA Let-7a-5p in airway smooth muscle cells is most responsive to high stretch in association with cell mechanics modulation. Front Physiol. (2022) 13:830406. doi: 10.3389/fphys.2022.830406
31. Yin H, He H, Cao X, Shen X, Han S, Cui C, et al. MiR-148a-3p regulates skeletal muscle satellite cell differentiation and apoptosis via the PI3K/AKT signaling pathway by targeting Meox2. Front Genet. (2020) 11:512. doi: 10.3389/fgene.2020.00512
32. Zhang W, Thevapriya S, Kim PJ, Yu WP, Shawn Je H, King Tan E, et al. Amyloid precursor protein regulates neurogenesis by antagonizing miR-574-5p in the developing cerebral cortex. Nat Commun. (2014) 5:3330. doi: 10.1038/ncomms4330
33. Lv X, Jiang H, Liu Y, Lei X, Jiao J. MicroRNA-15b promotes neurogenesis and inhibits neural progenitor proliferation by directly repressing TET3 during early neocortical development. EMBO Rep. (2014) 15:1305–14. doi: 10.15252/embr.201438923
34. Voloboueva LA, Sun X, Xu L, Ouyang YB, Giffard RG. Distinct effects of miR-210 reduction on neurogenesis: increased neuronal survival of inflammation but reduced proliferation associated with mitochondrial enhancement. J Neurosci. (2017) 37:3072–84. doi: 10.1523/JNEUROSCI.1777-16.2017
35. Roshan R, Shridhar S, Sarangdhar MA, Banik A, Chawla M, Garg M, et al. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA N Y N. (2014) 20:1287–97. doi: 10.1261/rna.044008.113
36. Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F. Human milk cells and lipids conserve numerous known and novel miRNAs, some of which are differentially expressed during lactation. PLoS ONE. (2016) 11:e0152610. doi: 10.1371/journal.pone.0152610
37. Fu X, Dong B, Tian Y, Lefebvre P, Meng Z, Wang X, et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J Clin Invest. (2015) 125:2497–509. doi: 10.1172/JCI75438
38. Melnik BC, Kakulas F, Geddes DT, Hartmann PE, John SM, Carrera-Bastos P, et al. Milk miRNAs: simple nutrients or systemic functional regulators? Nutr Metab. (2016) 13:42. doi: 10.1186/s12986-016-0101-2
39. Wolf T, Baier SR, Zempleni J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma CacO2 cells and rat small intestinal IEC-6 cells. J Nutr. (2015) 145:2201–6. doi: 10.3945/jn.115.218586
40. Kusuma RJ, Manca S, Friemel T, Sukreet S, Nguyen C, Zempleni J. Human vascular endothelial cells transport foreign exosomes from cow's milk by endocytosis. Am J Physiol Cell Physiol. (2016) 310:C800–7. doi: 10.1152/ajpcell.00169.2015
41. Kahn S, Liao Y, Du X, Xu W, Li J, Lönnerdal B. Exosomal microRNAs in milk from mothers delivering preterm infants survive in vitro digestion and are taken up by human intestinal cells. Mol Nutr Food Res. (2018) 62:e1701050. doi: 10.1002/mnfr.201701050
42. Wang L, Sadri M, Giraud D, Zempleni J. RNase H2-dependent polymerase chain reaction and elimination of confounders in sample collection, storage, and analysis strengthen evidence that microRNAs in bovine milk are bioavailable in humans. J Nutr. (2018) 148:153–9. doi: 10.1093/jn/nxx024
43. Lin D, Chen T, Xie M, Li M, Zeng B, Sun R, et al. Oral administration of bovine and porcine milk exosome alter miRNAs profiles in piglet serum. Sci Rep. (2020) 10:6983. doi: 10.1038/s41598-020-63485-8
44. Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol Baltim. (2007) 179:1969–78. doi: 10.4049/jimmunol.179.3.1969
45. Chen Q, Zhang F, Dong L, Wu H, Xu J, Li H, et al. SIDT1-dependent absorption in the stomach mediates host uptake of dietary and orally administered microRNAs. Cell Res. (2021) 31:247–58. doi: 10.1038/s41422-020-0389-3
46. Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L, et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. (2017) 16:132. doi: 10.1186/s12943-017-0694-8
47. Chen T, Xie MY, Sun JJ, Ye RS, Cheng X, Sun RP, et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci Rep. (2016) 6:33862. doi: 10.1038/srep33862
48. Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr. (2014) 144:1495–500. doi: 10.3945/jn.114.196436
49. Auerbach A, Vyas G, Li A, Halushka M, Witwer K. Uptake of dietary milk miRNAs by adult humans: a validation study. F1000Research. (2016) 5:721. doi: 10.12688/f1000research.8548.1
50. Pomar CA, Serra F, Palou A, Sánchez J. Lower miR-26a levels in breastmilk affect gene expression in adipose tissue of offspring. FASEB J. (2021) 35:e21924. doi: 10.1096/fj.202100623R
51. Smyczynska U, Bartlomiejczyk MA, Stanczak MM, Sztromwasser P, Wesolowska A, Barbarska O, et al. Impact of processing method on donated human breast milk microRNA content. PLoS ONE. (2020) 15:e0236126. doi: 10.1371/journal.pone.0236126
52. Cai M, He H, Jia X, Chen S, Wang J, Shi Y, et al. Genome-wide microRNA profiling of bovine milk-derived exosomes infected with Staphylococcus aureus. Cell Stress Chaperones. (2018) 23:663–72. doi: 10.1007/s12192-018-0876-3
53. Freiría-Martínez L, Iglesias-Martínez-Almeida M, Rodríguez-Jamardo C, Rivera-Baltanás T, Comís-Tuche M, Rodrígues-Amorím D, et al. Human breast milk microRNAs, potential players in the regulation of nervous system. Nutrients. (2023) 15:3284. doi: 10.3390/nu15143284
54. Batalle D, Hughes EJ, Zhang H, Tournier JD, Tusor N, Aljabar P, et al. Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage. (2017) 149:379–92. doi: 10.1016/j.neuroimage.2017.01.065
55. Ahlberg E, Al-Kaabawi A, Thune R, Simpson MR, Pedersen SA, Cione E, et al. Breast milk microRNAs: potential players in oral tolerance development. Front Immunol. (2023) 14:1154211. doi: 10.3389/fimmu.2023.1154211
56. Leroux C, Chervet ML, German JB. Perspective: milk microRNAs as important players in infant physiology and development. Adv Nutr. (2021) 12:1625–35. doi: 10.1093/advances/nmab059
57. Manca S, Upadhyaya B, Mutai E, Desaulniers AT, Cederberg RA, White BR, et al. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep. (2018) 8:11321. doi: 10.1038/s41598-018-29780-1
58. Khanam A, Ngu A, Zempleni J. Bioavailability of orally administered small extracellular vesicles from bovine milk in C57BL/6J mice. Int J Pharm. (2023) 639:122974. doi: 10.1016/j.ijpharm.2023.122974
59. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. (2016) 371:48–61. doi: 10.1016/j.canlet.2015.10.020
60. Kakimoto Y, Matsushima Y, Tanaka M, Hayashi H, Wang T, Yokoyama K, et al. MicroRNA profiling of gastric content from breast-fed and formula-fed infants to estimate last feeding: a pilot study. Int J Legal Med. (2020) 134:903–9. doi: 10.1007/s00414-019-02226-7
61. Wang Y, Fang J, Zeng HF, Zhong JF, Li HX, Chen KL. Identification and bioinformatics analysis of differentially expressed milk exosomal microRNAs in milk exosomes of heat-stressed Holstein cows. Funct Integr Genomics. (2022) 22:77–87. doi: 10.1007/s10142-021-00814-8
Keywords: miRNA, breast milk, infant development, immune system, digestive system
Citation: Xu Y-R, Zhao J, Huang H-Y, Lin Y-C-D, Lee T-Y, Huang H-D, Yang Y and Wang Y-F (2024) Recent insights into breast milk microRNA: their role as functional regulators. Front. Nutr. 11:1366435. doi: 10.3389/fnut.2024.1366435
Received: 10 January 2024; Accepted: 02 April 2024;
Published: 16 April 2024.
Edited by:
Erika Cione, University of Calabria, ItalyReviewed by:
Maria Cristina Caroleo, University Magna Graecia of Catanzaro, ItalyBertrand Kaeffer, Institut National de recherche pour l'agriculture, l'alimentation et l'environnement (INRAE), France
Diana Marisol Abrego-Guandique, Magna Græcia University, Italy
Copyright © 2024 Xu, Zhao, Huang, Lin, Lee, Huang, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Fei Wang, yfwang@cuhk.edu.cn; Yi Yang, yangyixk@zju.edu.cn
 Yi-Ran Xu
Yi-Ran Xu Jinglu Zhao1,2
Jinglu Zhao1,2  Hsi-Yuan Huang
Hsi-Yuan Huang Yang-Chi-Dung Lin
Yang-Chi-Dung Lin Hsien-Da Huang
Hsien-Da Huang Yi Yang
Yi Yang Yong-Fei Wang
Yong-Fei Wang