- 1Section of Surgical Oncology, Department of Surgery, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
- 2Sarcoma Program, Hoag Family Cancer Institute, Hoag Memorial Hospital Presbyterian, Newport Beach, CA, USA
- 3Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
- 4Department of Pathology, Stanford University School of Medicine, Palo Alto, CA, USA
- 5Division of Surgical Oncology, Department of Surgery, The James Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA
Well-differentiated/dedifferentiated (WD/DD) liposarcoma is a rare malignancy of adipocyte origin (“fat cancer”). Tumors may be entirely WD, WD with a DD component, or rarely DD without a clear WD component. WD tumors are low grade and generally indolent, while tumors with a DD component are high grade and behave much more aggressively, with a modest potential for distant metastasis. The presence of cancer progenitor cells in WD/DD liposarcoma is suggested by clinical evidence and reported research findings. In addition, there are emerging data to support the existence of a naturally occurring, antigen-driven, and adaptive immune response within the tumor microenvironment. We hypothesize that the intratumoral immune response is directed against a cancer progenitor cell and that the outcome of this response impacts the development of WD versus DD disease. Further study will likely provide interesting insights into the disease biology of WD/DD liposarcoma that may be readily translated to other more common cancers.
Well-differentiated/dedifferentiated (WD/DD) liposarcoma is a rare form of cancer, which originates from an adipocyte and is therefore a malignancy of fat (1, 2). Tumors most commonly occur in the retroperitoneum, where they can reach impressive size (mean: 30 cm) by the time of initial presentation (3, 4). In addition, unique to this disease, tumors can have distinct low-grade (WD) and high-grade (DD) areas juxtaposed next to one another. In fact, tumors may be entirely WD, WD with a DD component, or rarely DD without a clear WD component. The true relationship between the WD and DD components of tumor is unclear; however, presence of DD dramatically changes the disease biology and clinical outcome (1, 2, 5).
In this article, we will review available data to support the presence of cancer progenitor cells and an intratumoral immune response in this disease. Merging these two areas of ongoing research, we propose a unique hypothesis that may help to elucidate disease biology in WD/DD liposarcoma. We believe that the immune response is directed against a cancer progenitor cell and that the outcome of this response impacts the development of WD versus DD disease.
WD and DD: “A Good Brother and a Bad Brother”
Both WD and DD liposarcoma share a common genetic aberration, amplification of chromosomal region 12q13–15 (1, 2). This results in up to several thousand copies of a number of critical genes, including MDM2 and CDK4. Amplification of this region and/or specific genes found in this region (e.g., MDM2) can be detected by fluorescence in situ hybridization (FISH), and this test is used to establish diagnosis of WD/DD liposarcoma in cases in which diagnosis cannot be made on histology alone.
Despite common genetic features, WD and DD liposarcoma can be thought of as a spectrum of disease with clear differences between WD and DD (5). By histology, WD liposarcoma (low grade) consists of adipocytes of varying size, separated by fibrous bands with hyperchromatic cells (HCs); in contrast, in DD liposarcoma (high grade), the adipocytic areas are replaced by cellular areas, frequently with mitotic figures (Figure 1A). Macroscopically, WD tumors contain mostly fat, whereas DD areas of tumor are more dense, with a white, “fleshy,” and/or vascularized appearance (Figure 1B). These differences can be seen on cross-sectional imaging, which can suggest the presence of DD (often seen arising from or adjacent to WD areas of tumor); however, the final diagnosis of DD is made by histology.
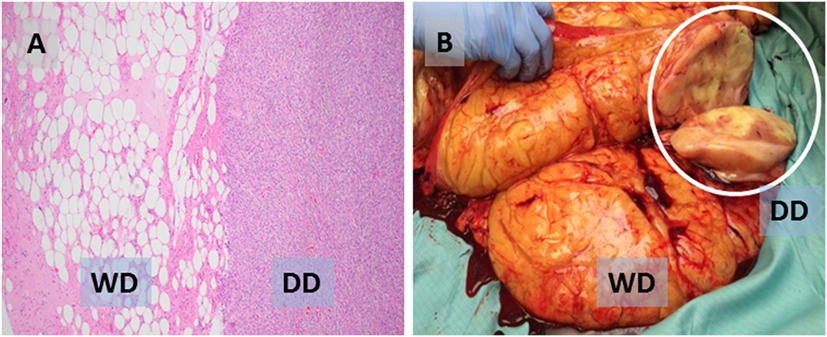
Figure 1. (A) Photomicrograph (H&E) showing characteristic liposarcoma histology with juxtaposed well-differentiated (WD, low grade) and dedifferentiated (DD, high grade) areas of the same tumor. (B) Sectioned WD tumor with a probable focus of DD (white circle).
Tumors that have a DD component are clearly more aggressive, and patients overall do worse. WD tumors are generally indolent and do not have the potential for distant metastasis. In contrast, tumors with DD frequently invade into adjacent organs and structures and up to 20–30% of cases metastasize, most commonly to the lungs (6). After surgery, the rates of locoregional recurrence are higher, and the time of recurrence is faster in DD versus WD tumors (7, 8). Interestingly, although response to radiation therapy and systemic therapy is overall poor for both, for cytotoxic chemotherapy (e.g., doxorubicin), DD has a slightly better response rate compared to WD (12 versus 0%) (9).
Cancer Progenitor Cells in WD/DD Liposarcoma
Cancer progenitor cells or “stem cells” have been reported and characterized in a number of human solid tumors (10–13). This unique subpopulation of cells is defined by the ability to self-renew and the capacity to differentiate into other cell types (pluripotency). Cancer progenitor cells are tumorigenic and, even in small numbers, can form an entire tumor. Clinically, these cells are thought to be more resistant to therapy compared to non-progenitor cells. As a result, cancer progenitor cells may play a fundamental role not only in tumorigenesis but also in recurrence and even distant metastasis. Specific cell surface and functional markers have been identified, which are suggestive of cancer progenitor cells.
There is some clinical evidence to suggest the presence of a cancer progenitor cell in WD/DD liposarcoma. Evans et al. was one of the first to report the observation of heterologous differentiation in this disease (14). By histology, some tumors (~5–10%) may exhibit features consistent with lineage differentiation to bone, muscle, blood vessel, or other tissue, suggesting the existence of a cell within the tumor with pluripotency (15, 16). WD/DD liposarcoma has also been reported to express CD117 or c-kit, also known as “stem cell factor” (17, 18). In addition, some tumors have high expression of CD34, another established stem cell marker (19). Riddle et al. even described a case report of a patient with a CD117 and CD34 double positive, but MDM2-amplified, liposarcoma “masquerading” as a gastrointestinal stromal tumor (20).
Several research investigators have also directly explored the presence of cancer progenitor cells in WD/DD liposarcoma. Stratford et al. used immunohistochemistry (IHC) to show that all (six out of six) cases of WD/DD liposarcoma expressed high levels of aldefluor, a functional stem cell marker (21). Interestingly, all the cases were also negative for CD133, a different stem cell marker. Using a human liposarcoma cell line (SW872)-based xenograft mouse model, the authors were able to identify a progenitor cell subpopulation that could form de novo tumors with as few as 100 cells. Smith et al. studied a xenograft mouse model established by implanting human WD/DD liposarcoma tumors obtained from surgery. Tumors that engrafted successfully appeared to have a gene signature with a “progenitor-like phenotype” (22).
It is important to note that the presence of cancer progenitor cells seems to be more pronounced in DD than WD liposarcoma. Heterologous differentiation is mostly seen in DD. CD117 expression was seen in 30% of DD cases studied by Tayal et al. versus none in WD (17). In liposarcoma cell lines, the majority of those with evidence for stem cell potential are DD or derived from “poorly differentiated” tumors (23). In xenograft mouse models, both Smith et al. and Peng et al. have independently confirmed that DD but not WD tumors have the potential to engraft successfully (22, 24). As a likely explanation, the highest percentage of CD34-positive stem cells is seen in DD tumors compared to WD and normal fat (25).
The Intratumoral Immune Response in WD/DD Liposarcoma
In the late 1990s, Argani et al. and Kraus et al. originally described an “inflammatory” variant of WD liposarcoma, based on the presence of a prominent immune infiltrate (26, 27). Recently, our group has reported a more detailed characterization of the intratumoral immune response that occurs naturally in both WD and DD liposarcoma (28, 29). One noteworthy observation is the presence of organized aggregates of immune cells within the tumor microenvironment, known as tertiary lymphoid structures (TLS) (Figure 2). Our work demonstrated that TLS contain mature dendritic cells situated adjacent to CD4 helper T cells, a feature suggestive of antigen presentation. TLS also contain B cells and, in fact, can have a clear germinal center. CD8 cytotoxic T cells are also found; however, these are typically scattered in the areas of tumor outside of TLS. Taken together, our findings strongly suggest that there is an antigen-driven, adaptive immune response in WD/DD liposarcoma. The key question is: what are the tumor antigen(s) that are being targeted by the intratumoral immune response in WD/DD liposarcoma?
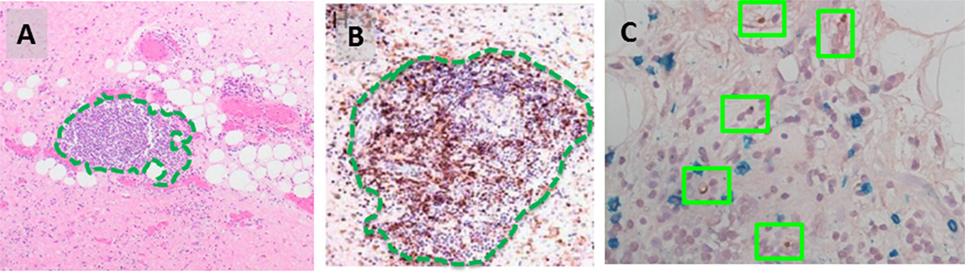
Figure 2. (A) Intratumoral tertiary lymphoid structure (TLS, green outline) found in WD liposarcoma. (B) By immunohistochemistry, TLS contain a dense population of CD4 T cells (brown). (C) DC-LAMP-positive dendritic cells are found adjacent to CD4 T cells (green boxes).
Stromal Hyperchromatic Cells: The “Missing Link”?
Within the tumor stroma of WD liposarcoma, there exists an atypical, spindle-shaped cell with a hyperchromatic nucleus. These HCs are seen often enough by routine histology that they are used by clinical pathologists as part of the criteria for disease diagnosis (30, 31). In support of this, by FISH, HCs demonstrate high, if not the highest levels of amplification, confirming their identity as tumor cells (32). However, the significance of HCs in WD/DD liposarcoma has never been studied to our knowledge.
Our group has preliminary data to suggest that HCs express both CD34 and MHC Class I (Figure 3). CD34 has been reported to be a marker for adipocyte stem cells (ASCs) by several investigators. In addition to liposarcoma (19, 20, 25), CD34-positive cells with stem cell gene expression and functional characteristics have also been reported in normal fat and benign lipomas (33–36). Although further studies are needed, our preliminary data would suggest that HCs may be ASCs in WD/DD liposarcoma. MHC Class I is a cell surface molecule involved in antigen display. Although tumor cells can downregulate MHC Class I to evade immune detection, its expression on HCs suggests that HC antigen can be recognized by the adaptive immune response.
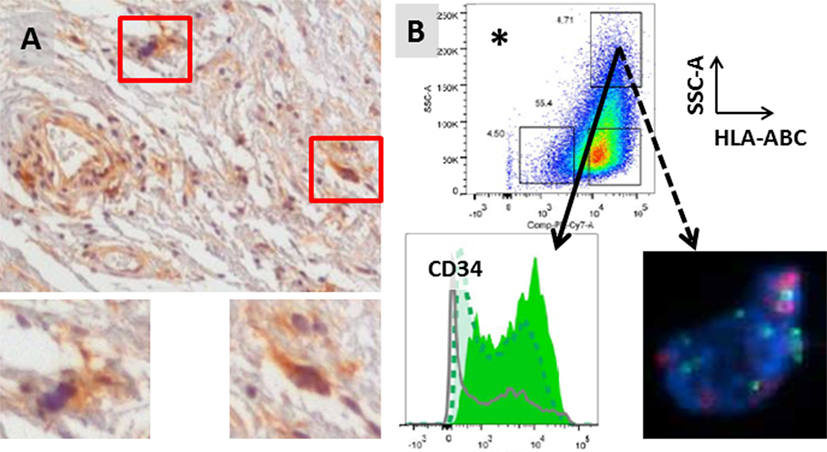
Figure 3. (A) Stromal hyperchromatic cells (HCs) in WD/DD liposarcoma (red boxes). HCs express MHC Class I (brown, insets). (B) HCs also express high levels of CD34 (solid arrow) and have 12q13–15 amplification (dotted arrow; FISH courtesy of KL Bill). *HCs were isolated from fresh tumor tissue after excluding immune cells and endothelial cells and gating on the population with the highest side scatter (SSC-A = internal complexity) and Class I (HLA-ABC).
Hypothesis and Further Areas of Study
We hypothesize that, in WD/DD liposarcoma, the intratumoral immune response (TLS) is directed against a candidate cancer progenitor cell (HC). We further hypothesize that differential immune responses against this unique tumor cell ultimately leads to the development of WD versus DD disease (Figure 4). Initially, HCs undergo a low level of proliferation that results in a WD tumor. On a cellular level, HCs (CD34-positive, likely ASC) differentiate along an adipocytic lineage, and the histology is low grade. In at least some HCs, antigen is presented on the cell surface (via MHC Class I) and recognized by the adaptive immune cells, which, in response, form an intratumoral TLS. An attempt is made by the immune cells to eliminate HCs; however, this is unsuccessful. HCs are able to evade the adaptive immune response or alternatively, TLS may even become an “immune-privileged” site in direct support of HCs. The net result is much more rapid proliferation of HCs, forming the cellular areas characteristic of high-grade disease (=DD). The tumor microenvironment(s) in WD/DD liposarcoma may therefore represent a dynamic form of cancer immunoediting (“elimination, equilibrium, and escape”), similar to that which has been proposed by others (37–39). In WD/DD liposarcoma, immunoediting occurs across the spectrum of low- to high-grade disease and is directed against a cancer progenitor cell.
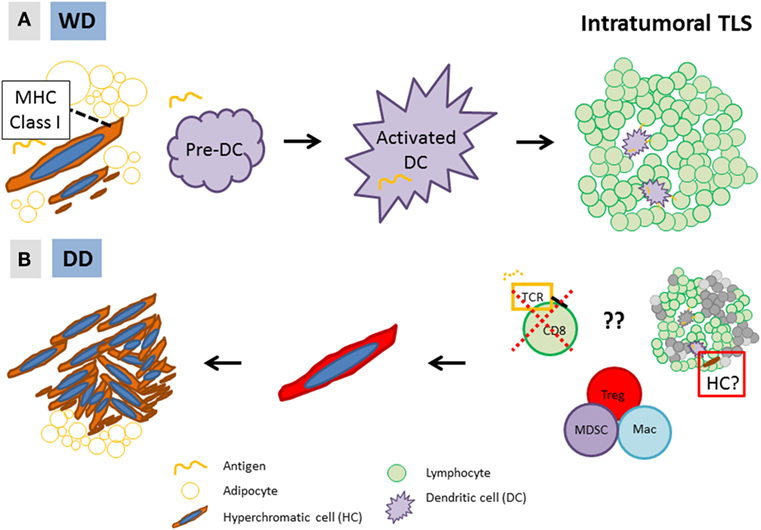
Figure 4. Hypothesis: (A) in low-grade, well-differentiated (WD) disease, MHC Class I-expressing stromal hyperchromatic cells (HCs) differentiate along an adipocytic lineage and undergo slow proliferation. Some HCs are recognized by dendritic cells (DC), which form an intratumoral tertiary lymphoid structure (TLS); (B) however, through yet undefined mechanisms, HCs are able to evade the immune response and rapidly proliferate, resulting in high-grade, dedifferentiated (DD) disease. Alternatively, TLS may be an “immune-privileged” site that directly supports the growth of HCs (right, red box).
Tertiary lymphoid structures have recently been suggested to serve as a microniche to promote the growth of cancer progenitor cells in hepatocellular carcinoma (HCC) (40). In the precancerous liver of a genetically engineered mouse model of HCC, TLS form around a progenitor cell, which proliferates and ultimately “overtakes” the TLS, resulting in frank HCC. The concept of progenitor cells existing in an immune-privileged niche has been reported in other cancers as well (41, 42). Re-examination of WD/DD liposarcoma demonstrates that, in fact, hyperchromatic and strongly MDM2-positive cells are found within TLS (Figure 5). Moreover, we have observed unique patterns of TLS organization that could be interpreted as a progressive “overtaking” of TLS by a developing cellular/DD area of tumor (Figure 6). Coincidentally, although TLS have been reported in a number of other cancers and shown to be associated with better prognosis, the opposite is true in both HCC and WD/DD liposarcoma (29, 40).
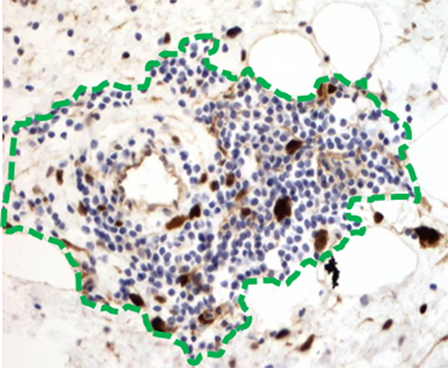
Figure 5. Hyperchromatic and MDM2-positive tumor cells (brown) are found within and just adjacent to TLS (green outline).
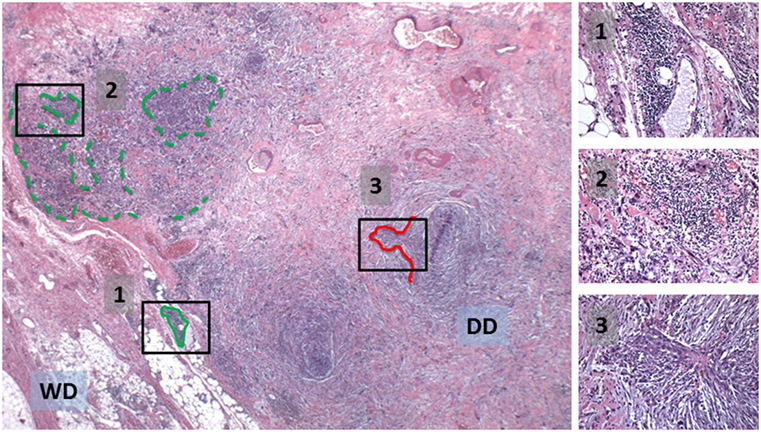
Figure 6. Potential natural progression from a formed TLS (1, green outline) to disrupted TLS with an adjacent, developing cellular/DD area of tumor (2) to a locally-invasive, cellular/DD area (red outline) without TLS (3). See also insets.
Further study to support or refute our hypothesis is clearly needed. From an immunology standpoint, this should first occur broadly, looking at the differences in the immune response across the spectrum of disease, with tumors that are entirely WD, WD with a DD component, and DD without a WD component. If our hypothesis is true, the overall immune bias (cellular composition, function, and cytokine production) should differ vastly from WD to DD, even regionally within the same tumor. From a tumor standpoint, HCs should be isolated and further characterized. If HCs are the targets of TLS via antigen display on MHC Class I, it is unclear why they are selectively targeted. CD34-positive ASCs have been reported to undergo proliferation in benign lipomas (35, 36). In WD liposarcoma, it is possible that in some proliferating HCs low levels of mutated antigen(s) arise, which are then recognized by the adaptive immune cells. There should also be focused investigation into the initial cell-to-cell interaction between HCs and the dendritic cell precursors that will result in antigen presentation to naive T and B cells within TLS. Importantly, is there an ultimate failure of HC-antigen presentation or is the deficiency at the downstream effector cells targeting HCs? In support of the latter, we have previously reported that in WD/DD liposarcoma, intratumoral CD8 T cells, which are typically outside of TLS, have high expression of PD-1, an immune checkpoint molecule and natural “stop signal” for these effector cells (29). Alternatively, do the HCs recruit suppressive immune cell types or do they themselves directly suppress the mounting immune response? In support of the former, we recently described a case report of multicystic DD liposarcoma with a prominent monocytic immune cell subpopulation that is likely comprised of myeloid-derived suppressor cells and tumor-associated macrophages (43).
In WD/DD liposarcoma, further study of the intratumoral immune response against a candidate cancer progenitor cell will likely provide meaningful insights into the biology of this disease. However, one important caveat is that the clonal origin and true relationship between WD and DD is still unknown. Nonetheless, this work may also result in identification of clinical biomarkers for disease progression and novel targets for therapy (e.g., immunotherapy) (44). There may be a specific gene or cytokine signature in TLS that is indicative of pure WD versus early (subclinical) development of DD disease. One interesting observation is that DD occurs much less frequently in tumors that arise in the extremity and trunk compared to the retroperitoneum (15, 45, 46). Could this be driven or, at least, influenced by the intratumoral immune response (e.g., frequency or type of TLS formed)? In terms of therapy, appropriate manipulation of TLS may potentially prevent DD and maintain WD, which would improve prognosis and is overall a more clinically manageable disease.
Finally, further hypothesis-driven study in WD/DD liposarcoma may uncover findings that can be readily translated into other, more common cancers. There is a plethora of literature about cancer progenitor cells in a variety of cancers; there is now also an increasing body of work characterizing TLS in non-small cell lung cancer, melanoma, colorectal cancer, and others (47). To our knowledge, the true “targets” of the adaptive immune response represented by TLS in these cancers is still yet undefined. Therefore, although rare, with its unique disease characteristics, WD/DD liposarcoma may be an ideal “cancer model” to study the tumor–immune microenvironment and, specifically, from the standpoint of the immune response against cancer progenitor cells.
Author Contributions
WWT and SC conceived of the hypothesis and reviewed the literature. EGE and REP provided critical discussion and input to help develop the hypothesis. WWT wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Janet Stallman, Department of Pathology at Hoag Hospital, for providing some of the images used for our figures and Geok Choo Sim, Department of Immunology, Moffitt Cancer Center, for careful review of this manuscript.
This publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U54CA168512. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Coindre JM, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch (2010) 456:167–79. doi:10.1007/s00428-009-0815-x
2. Dodd LG. Update on liposarcoma: a review for cytopathologists. Diagn Cytopathol (2012) 40:1122–31. doi:10.1002/dc.21794
3. Matthyssens LE, Creytens D, Ceelen WP. Retroperitoneal liposarcoma: current insights in diagnosis and treatment. Front Surg (2015) 2:4. doi:10.3389/fsurg.2015.00004
4. Olimpiadi Y, Song S, Hu JS, Matcuk GR, Chopra S, Eisenberg BL, et al. Contemporary management of retroperitoneal soft tissue sarcomas. Curr Oncol Rep (2015) 17:39. doi:10.1007/s11912-015-0462-0
5. Thway K, Jones RL, Noujaim J, Zaidi S, Miah AB, Fisher C. Dedifferentiated liposarcoma: updates on morphology, genetics, and therapeutic strategies. Adv Anat Pathol (2016) 23:30–40. doi:10.1097/PAP.0000000000000101
6. Tirumani SH, Tirumani H, Jagannathan JP, Shinagare AB, Hornick JL, Ramaiya NH, et al. Metastasis in dedifferentiated liposarcoma: predictors and outcome in 148 patients. Eur J Surg Oncol (2015) 41:899–904. doi:10.1016/j.ejso.2015.01.012
7. Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol (2008) 15:1585–93. doi:10.1245/s10434-007-9805-x
8. Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg (2016) 263(5):1002–9. doi:10.1097/SLA.0000000000001447
9. Italiano A, Toulmonde M, Cioffi A, Penel N, Isambert N, Bompas E, et al. Advanced well-differentiated/dedifferentiated liposarcomas: role of chemotherapy and survival. Ann Oncol (2012) 23:1601–7. doi:10.1093/annonc/mdr485
10. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A (2003) 100:3983–8. doi:10.1073/pnas.0530291100
11. Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res (2003) 63:5821–8.
12. O’brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature (2007) 445:106–10. doi:10.1038/nature05372
13. Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature (2008) 451:345–9. doi:10.1038/nature06489
14. Evans HL, Khurana KK, Kemp BL, Ayala AG. Heterologous elements in the dedifferentiated component of dedifferentiated liposarcoma. Am J Surg Pathol (1994) 18:1150–7. doi:10.1097/00000478-199411000-00009
15. Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol (1997) 21:271–81. doi:10.1097/00000478-199703000-00002
16. Gronchi A, Collini P, Miceli R, Valeri B, Renne SL, Dagrada G, et al. Myogenic differentiation and histologic grading are major prognostic determinants in retroperitoneal liposarcoma. Am J Surg Pathol (2015) 39:383–93. doi:10.1097/PAS.0000000000000366
17. Tayal S, Classen E, Bemis L, Robinson WA. C-kit expression in dedifferentiated and well-differentiated liposarcomas; immunohistochemistry and genetic analysis. Anticancer Res (2005) 25:2215–20.
18. Kopf B, Bernardeschi P, Turrisi G, Fiorentini G, Zago S, Lanzanova G. Lack of activity of imatinib in two cases of KIT+ retroperitoneal liposarcoma. Hepatogastroenterology (2007) 54:2.
19. Barreto MM, Fortes HR, Valiante PM, Zanetti G, Marchiori E. Lung and chest-wall metastasis of liposarcoma. Lung (2015) 193:1047–9. doi:10.1007/s00408-015-9779-6
20. Riddle ND, Gonzalez RJ, Bridge JA, Antonia S, Bui MM. A CD117 and CD34 immunoreactive sarcoma masquerading as a gastrointestinal stromal tumor: diagnostic pitfalls of ancillary studies in sarcoma. Cancer Control (2011) 18:152–9.
21. Stratford EW, Castro R, Wennerstrom A, Holm R, Munthe E, Lauvrak S, et al. Liposarcoma cells with aldefluor and CD133 activity have a cancer stem cell potential. Clin Sarcoma Res (2011) 1:8. doi:10.1186/2045-3329-1-8
22. Smith KB, Tran LM, Tam BM, Shurell EM, Li Y, Braas D, et al. Novel dedifferentiated liposarcoma xenograft models reveal PTEN down-regulation as a malignant signature and response to PI3K pathway inhibition. Am J Pathol (2013) 182:1400–11. doi:10.1016/j.ajpath.2013.01.002
23. Stratford EW, Castro R, Daffinrud J, Skårn M, Lauvrak S, Munthe E, et al. Characterization of liposarcoma cell lines for preclinical and biological studies. Sarcoma (2012) 2012:148614. doi:10.1155/2012/148614
24. Peng T, Zhang P, Liu J, Nguyen T, Bolshakov S, Belousov R, et al. An experimental model for the study of well-differentiated and dedifferentiated liposarcoma; deregulation of targetable tyrosine kinase receptors. Lab Invest (2011) 91:392–403. doi:10.1038/labinvest.2010.185
25. Zhang Y, Young ED, Bill K, Belousov R, Peng T, Lazar AJ, et al. Heterogeneity and immunophenotypic plasticity of malignant cells in human liposarcomas. Stem Cell Res (2013) 11:772–81. doi:10.1016/j.scr.2013.04.011
26. Argani P, Facchetti F, Inghirami G, Rosai J. Lymphocyte-rich well-differentiated liposarcoma: report of nine cases. Am J Surg Pathol (1997) 21:884–95. doi:10.1097/00000478-199708000-00013
27. Kraus MD, Guillou L, Fletcher CD. Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma. Am J Surg Pathol (1997) 21:518–27. doi:10.1097/00000478-199705000-00003
28. Tseng WW, Demicco EG, Lazar AJ, Lev DC, Pollock RE. Lymphocyte composition and distribution in inflammatory, well-differentiated retroperitoneal liposarcoma: clues to a potential adaptive immune response and therapeutic implications. Am J Surg Pathol (2012) 36:941–4. doi:10.1097/PAS.0b013e31824f2594
29. Tseng WW, Malu S, Zhang M, Chen J, Sim GC, Wei W, et al. Analysis of the intratumoral adaptive immune response in well differentiated and dedifferentiated retroperitoneal liposarcoma. Sarcoma (2015) 2015:547460. doi:10.1155/2015/547460
31. Evans HL. Atypical lipomatous tumor, its variants, and its combined forms: a study of 61 cases, with a minimum follow-up of 10 years. Am J Surg Pathol (2007) 31:1–14. doi:10.1097/01.pas.0000213406.95440.7a
32. Thway K, Wang J, Swansbury J, Min T, Fisher C. Fluorescence in situ hybridization for MDM2 amplification as a routine ancillary diagnostic tool for suspected well-differentiated and dedifferentiated liposarcomas: experience at a tertiary center. Sarcoma (2015) 2015:812089. doi:10.1155/2015/812089
33. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell (2008) 135:240–9. doi:10.1016/j.cell.2008.09.036
34. Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science (2008) 322:583–6. doi:10.1126/science.1156232
35. Suga H, Eto H, Inoue K, Aoi N, Kato H, Araki J, et al. Cellular and molecular features of lipoma tissue: comparison with normal adipose tissue. Br J Dermatol (2009) 161:819–25. doi:10.1111/j.1365-2133.2009.09272.x
36. Zavan B, De Francesco F, D’andrea F, Ferroni L, Gardin C, Salzillo R, et al. Persistence of CD34 stem marker in human lipoma: searching for cancer stem cells. Int J Biol Sci (2015) 11:1127–39. doi:10.7150/ijbs.11946
37. Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology (2007) 121:1–14. doi:10.1111/j.1365-2567.2007.02587.x
38. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (2011) 331:1565–70. doi:10.1126/science.1203486
39. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases – elimination, equilibrium and escape. Curr Opin Immunol (2014) 27:16–25. doi:10.1016/j.coi.2014.01.004
40. Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol (2015) 16:1235–44. doi:10.1038/ni.3290
41. Bruttel VS, Wischhusen J. Cancer stem cell immunology: key to understanding tumorigenesis and tumor immune escape? Front Immunol (2014) 5:360. doi:10.3389/fimmu.2014.00360
42. Maccalli C, Volontè A, Cimminiello C, Parmiani G. Immunology of cancer stem cells in solid tumours. A review. Eur J Cancer (2014) 50:649–55. doi:10.1016/j.ejca.2013.11.014
43. Khoury M, Sim GC, Harao M, Radvanyi L, Amini B, Benjamin RS, et al. Multicystic dedifferentiated retroperitoneal liposarcoma: tumour cyst fluid analysis and implications for management. BMJ Case Rep (2015). doi:10.1136/bcr-2015-211218
44. Tseng W, Somaiah N, Engleman E. Potential for immunotherapy in soft tissue sarcoma. Hum Vaccin Immunother (2014) 10(11):3117–24. doi:10.4161/21645515.2014.983003
45. Okada K, Hasegawa T, Kawai A, Ogose A, Nishida J, Yanagisawa M, et al. Primary (de novo) dedifferentiated liposarcoma in the extremities: a multi-institution Tohoku Musculoskeletal Tumor Society study of 18 cases in Northern Japan. Jpn J Clin Oncol (2011) 41:1094–100. doi:10.1093/jjco/hyr098
46. Le Guellec S, Chibon F, Ouali M, Perot G, Decouvelaere AV, Robin YM, et al. Are peripheral purely undifferentiated pleomorphic sarcomas with MDM2 amplification dedifferentiated liposarcomas? Am J Surg Pathol (2014) 38:293–304. doi:10.1097/PAS.0000000000000131
Keywords: liposarcoma, dedifferentiation, tertiary lymphoid structures, ectopic lymph node, cancer stem cells, tumor-initiating cells
Citation: Tseng WW, Chopra S, Engleman EG and Pollock RE (2016) Hypothesis: The Intratumoral Immune Response against a Cancer Progenitor Cell Impacts the Development of Well-Differentiated versus Dedifferentiated Disease in Liposarcoma. Front. Oncol. 6:134. doi: 10.3389/fonc.2016.00134
Received: 07 April 2016; Accepted: 23 May 2016;
Published: 10 June 2016
Edited by:
Zongbing You, Tulane University, USAReviewed by:
Francesco De Francesco, Second University of Naples, ItalyRobert J. Canter, UC Davis School of Medicine, USA
Copyright: © 2016 Tseng, Chopra, Engleman and Pollock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William W. Tseng, william.tseng@med.usc.edu
 William W. Tseng
William W. Tseng Shefali Chopra
Shefali Chopra Edgar G. Engleman4
Edgar G. Engleman4 Raphael E. Pollock
Raphael E. Pollock