- 1Department of Pediatrics, Kobe University Graduate School of Medicine, Kobe, Japan
- 2Department of Hematology and Oncology, Kobe Children's Hospital, Kobe, Japan
Neuroblastoma is a common extracranial solid tumor of neural crest (NC) origin that accounts for up to 15% of all pediatric cancer deaths. The disease arises from a transient population of NC cells that undergo an epithelial-mesenchymal transition (EMT) and generate diverse cell-types and tissues. Patients with neuroblastoma are characterized by their extreme heterogeneity ranging from spontaneous regression to malignant progression. More than half of newly diagnosed patients present highly metastatic tumors and are stratified into a high-risk group with dismal outcome. As many as 20% of high-risk patients have residual disease that is refractory or progressive during induction chemotherapy. Although a majority of high-risk patients achieve remission, larger part of those patients has minimal residual disease (MRD) that causes relapse even after additional consolidation therapy. MRD is composed of drug-resistant tumor cells and dynamically presented as cancer stem cells (CSCs) in residual tumors, circulating tumor cells (CTCs) in peripheral blood (PB), and disseminated tumor cells (DTCs) in bone marrow (BM) and other metastatic sites. EMT appears to be a key mechanism for cancer cells to acquire MRD phenotypes and malignant aggressiveness. Due to the restricted availability of residual tumors, PB and BM have been used to isolate and analyze CTCs and DTCs to evaluate MRD in cancer patients. In addition, recent technical advances make it possible to use circulating tumor DNA (ctDNA) shed from tumor cells into PB for MRD evaluation. Because MRD can be detected by tumor-specific antigens, genetic or epigenetic changes, and mRNAs, numerous assays using different methods and samples have been reported to detect MRD in cancer patients. In contrast to the tumor-specific gene-rearrangement-positive acute lymphoblastic leukemia (ALL) and the oncogenic fusion-gene-positive chronic myelogenous leukemia (CML) and several solid tumors, the clinical significance of MRD remains to be established in neuroblastoma. Given the extreme heterogeneity of neuroblastoma, dynamics of MRD in neuroblastoma patients will hold a key to the clinical validation. In this review, we summarize the biology and detection methods of cancer MRD in general and evaluate the available assays and clinical significance of neuroblastoma MRD to clarify its dynamics in neuroblastoma patients.
Introduction
Neuroblastoma is a common extracranial solid tumor in children and accounts for ~15% of all pediatric cancer-associated mortalities. The disease originates from a transient population of neural crest (NC) cells, especially a subpopulation of NC cells committed to the establishment of the sympathoadrenal tissues (Figure 1). NC cells undergo an epithelial-mesenchymal transition (EMT), and migrate throughout the developing body, and generate diverse cell-types and tissues. Various subpopulations of NC cells adopt specific migratory behaviors that has iterated in highly metastatic neuroblastoma (1, 2). EMT is central to the initiation of NC cell migration and confers on neuroblastoma cells invasive phenotype: increased tumor-initiating and metastatic potential and a greater drug-resistance (3, 4). Indeed, FoxD3, Slug, and Sox9/10 constitute the minimal inducers of EMT in NC cells and have all been shown to be dysregulated in aggressive neuroblastoma (5–7).
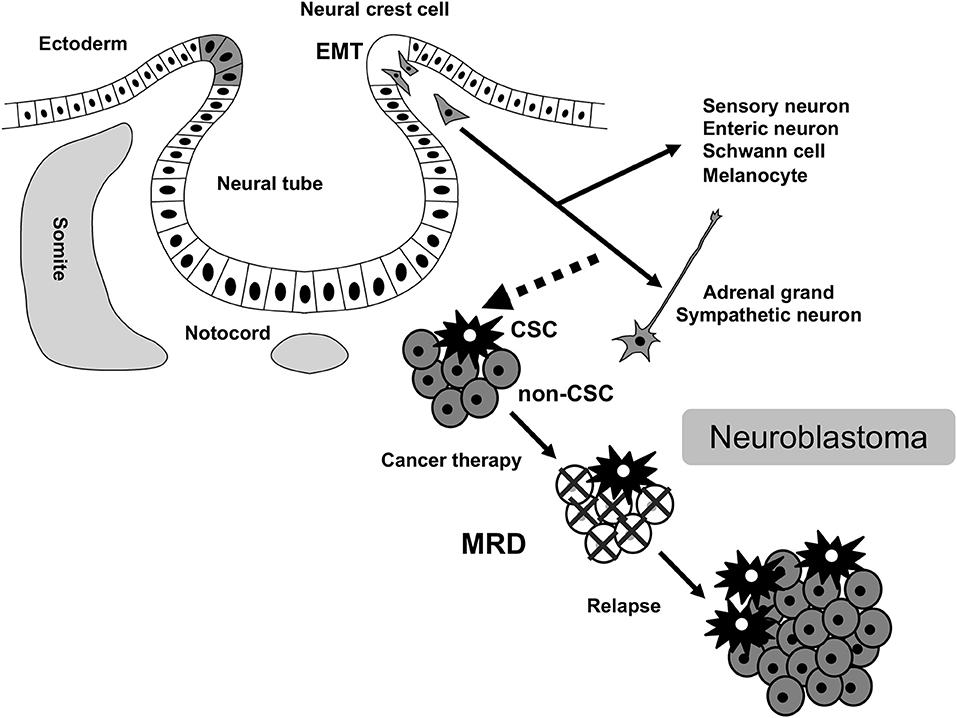
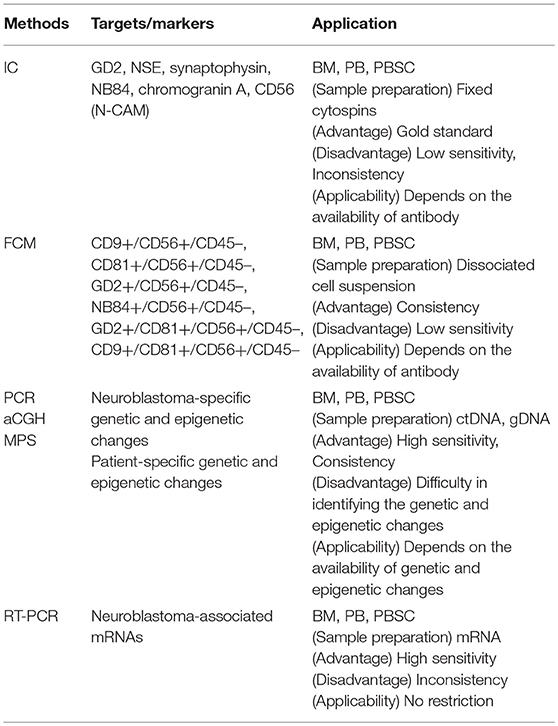
Figure 1. MRD in neuroblastoma. MRD, minimal residual disease; CSC, cancer stem cell; EMT, epithelial-mesenchymal transition.
Patients with neuroblastoma are characterized by their extreme heterogeneity that has been described at multiple levels. These include the anatomical localization of the tumor (8), histology, genomic/molecular profile, and clinical manifestations. Histology of neuroblastoma is mainly defined by the relative proportion of neuroblastic ganglionic cells and reactive stromal Schwann-like cells that determines the tumor's differentiation status. Intratumor heterogeneity of neuroblastoma has been reported recently both at the cellular and molecular levels. These studies conjointly reported two or three types of interconvertible tumor cells with divergent gene expression profiles (undifferentiated mesenchymal and committed adrenergic cell types or NC-like, sympathoadrenal, and mixed cell types) (9, 10). Infants with neuroblastoma frequently experience spontaneous regression, whereas children more than 18-months old with neuroblastoma usually have highly metastatic and aggressive disease (11, 12).
Using a subset of these known clinical and biological prognostic factors, patients with neuroblastoma are classified into three risk groups: low-risk, intermediate-risk, and high-risk. For patients with low-risk and intermediate-risk neuroblastoma, this risk stratification, and subsequent treatment has been successful with over 90% long-term event-free survival rates (13, 14). However, treating high-risk neuroblastoma remains a significant challenge despite intensive multimodal treatment. Children with high-risk neuroblastoma account for approximately half of newly diagnosed patients and are currently treated by the standard regimens containing four main components: induction chemotherapy, local control (surgery and radiation therapy), consolidation therapy, and maintenance therapy (13, 14). Overall survival of high-risk neuroblastoma has improved from 29 to 50% over the past 20 years, mainly due to the intensification of therapy through myeloablative therapy and immunotherapy (15). Furthermore, a >70% of long-term remission is suggested for high-risk patients who received all planned therapy of tandem myeloablative therapy with autologous stem cell transplants and immunotherapy in a recent Children's Oncology Group study ANBL0532 (16). Despite these progress, as many as 20% of high-risk patients have residual disease that is refractory or progressive during induction chemotherapy. The rest of high-risk patients usually can achieve remission, but larger part of those patients has minimal residual disease (MRD) that causes relapse even after additional consolidation therapy. Patients with relapsed neuroblastoma can rarely be cured with < 10% of long-term survival (13, 14).
MRD is defined as drug-resistant persistent tumor cells following cancer therapy. The accurate and sensitive detection of MRD is essential to achieve optimal outcome in neuroblastoma patients (15, 17). Accordingly, numerous MRD assays using different methods and samples have been reported over past two decades but their clinical significance remains to be established. Given the extreme heterogeneity of neuroblastoma, understanding the dynamics of MRD in neuroblastoma patients will be critical to validate these MRD assays. In this review, we will first overview the biology and detection methods of cancer MRD in general and then examine the available assays and clinical significance of neuroblastoma MRD to clarify its dynamics in neuroblastoma patients.
Minimal Residual Disease (MRD)
Dynamics of MRD
MRD is conceptually defined as residual tumor cells that persistently reside in patients following local and systemic cancer therapy, and its activation causes tumor metastasis and relapse that continue to represent the most difficult challenges for cancer patients (18, 19). Most methods used in the current clinical practices for tumor detection are based on imaging studies and tumor marker assays. Early identification of tumor metastasis and relapse is critical to achieve optimal outcome since therapeutic intervention is more effective and successful in treating smaller tumor. This has led to an intensive research to develop more sensitive and accurate methods for the assessment of MRD status (20).
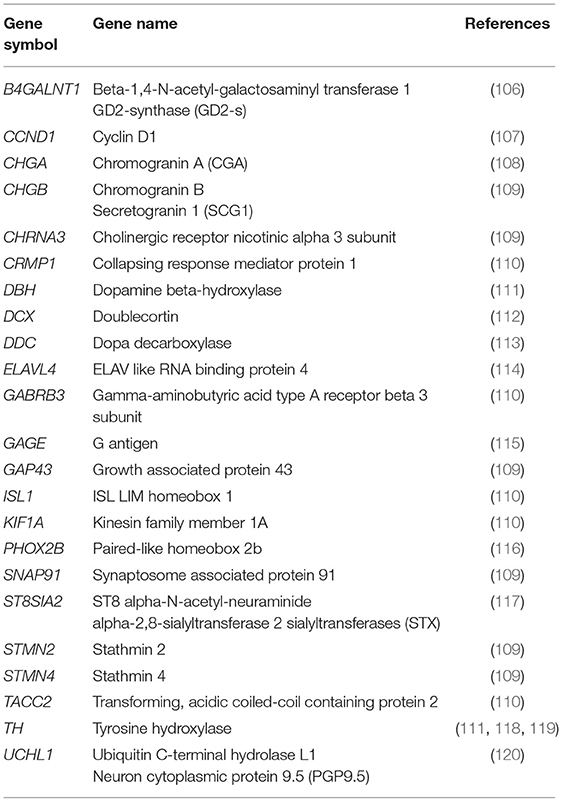
Cancer cells in primary tumor can often gain the ability to invade, migrate, disseminate, and proliferate in distant locations, representing precursors of metastasis and relapse (21). Following local and systemic cancer therapies, residual cancer cells persist as cancer stem cells (CSCs) in primary tumor, circulating tumor cells (CTCs) in peripheral blood (PB), and disseminated tumor cells (DTCs) in bone marrow (BM), lymph node, and micrometastasis in other metastatic tissues. These CSC, CTC, and DTC represent the dynamics of MRD in cancer patients (22) (Figure 2). Since invasive tumor biopsies of primary, metastatic, and recurrent tumors cannot always be performed, the detection and analysis of CTC and DTC by less invasive sampling of PB and BM has been shown to be of clinical relevance in many cancer types, particularly in breast, colon, and prostate cancers (23–25). In addition, circulating tumor DNA (ctDNA), which is shed from tumor cells into PB and isolated from blood serum or plasma, has also been used for diagnostic and prognostic purposes in several cancer types (26, 27). The detection and analysis of CTC, DTC, and ctDNA will serve as complementary methods for the assessment of MRD status in cancer patients (Figure 2).
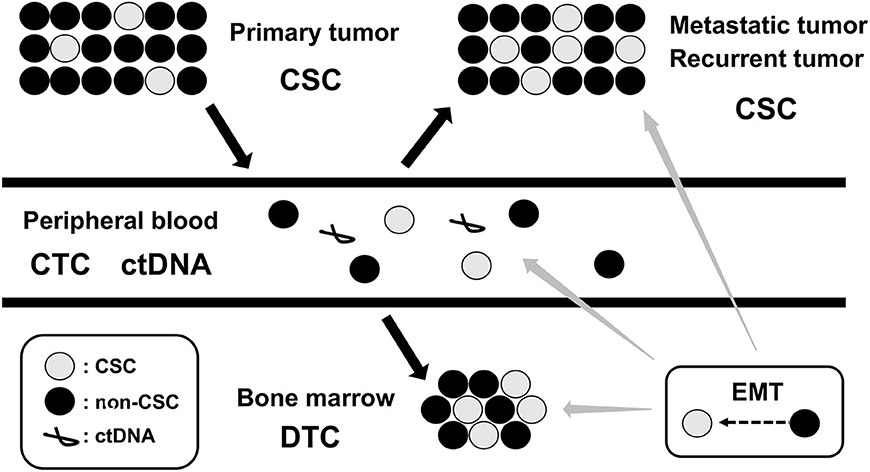
Figure 2. Dynamics of MRD. MRD, minimal residual disease; CSC, cancer stem cell; CTC, circulating tumor cell; DTC, disseminating tumor cell; ctDNA, circulating tumor DNA; EMT, epithelial-mesenchymal transition.
Cancer Stem Cell (CSC)
Tumors are composed of multiple phenotypically distinct subpopulations of cancer cells, which is considered a major driver of the development of resistance to cancer therapy (28, 29). Genome sequencing has delineated the types of genetic changes underling the phenotypic alteration and diversification of cancer cells (30). The ability of cancer cells to frequently interconvert between multiple alternative phenotypic states without genetic changes (epigenetic changes) is also increasingly recognized (31, 32). CSC has provided key insights into how epigenetic mechanisms can contribute to the phenotypic diversity of cancer cells within a tumor (33, 34). Over the past decades, numerous studies have suggested that only a small subpopulation of the cancer cell with tumor-initiating capability, named as CSC, is the core origin of the tumorigenesis. CSCs are defined as cancer cells that have the abilities to self-renew and differentiate into their progeny (non-CSCs). Functionally, CSCs show tumor-initiating potential in vivo, anchorage-independent growth in vitro, and drug-resistance. Methodologically, CSCs can be isolated from tumor samples using flow cytometry employing cell-surface markers such as CD44 and CD133, or functional characteristics such as dye extrusion capability (side population), aldehyde dehydrogenase (ALDH) activity, and sphere formation capability (35). The clinical relevance of CSCs is typically seen in metastatic and recurrent tumors, which are enriched with CSCs and associated with CSC-gene signatures (36).
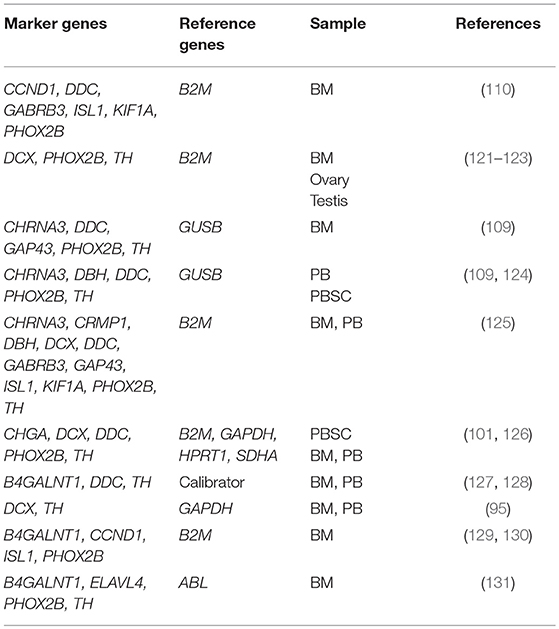
The epigenetic changes between CSCs and non-CSCs seems to be linked to a distinct transcriptional program found during developmental tissue remodeling referred to as EMT, which imparts heritable phenotypic changes to cancer cells through epigenetic modifications without introducing new genetic changes (37) (Figures 2, 3). Upon activation of EMT program, cancer cells lose many of their epithelial characteristics including epithelial cell junctions and apical–basal polarity, and instead acquire mesenchymal attributes such as an increased capacity for migration and invasion (38). In a number of cancer types, only cancer cells within CSC-enriched subpopulations exhibit aspects of activated EMT-program. In addition, EMT-program activation increases the capacity of tumor initiation and confers resistance to various therapeutic agents (3, 4, 39). The CSC phenotypes (CSCs or non-CSCs) interconnected to activated EMT-program likely define a continuum of cellular states, leading to the distinct subpopulations of CSCs (40) (Figures 2, 3).
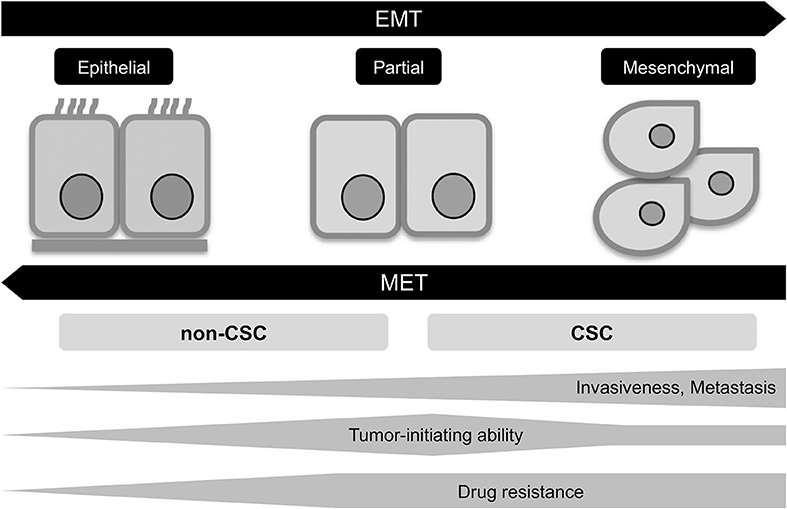
Figure 3. EMT program in tumor progression. EMT, epithelial-mesenchymal transition; MET, mesenchymal-epithelial transition; CSC, cancer stem cell.
Circulating Tumor Cell (CTC)
CTC represents an intermediate of tumor metastasis that requires the acquisition of diverse properties including invasion and migration from the primary tumor, intravasation, survival, extravasation, colonization at a distinct tissues, and growth into a macro metastatic lesion (41, 42). Direct evidences for metastatic precursors of CTCs have recently came from breast and lung cancers that is characterized by early dissemination and dismal prognosis (43, 44). While some CTCs passively enter the bloodstream, CTCs derived from actively invading tumor cells can acquire EMT and CSC phenotypes required for tumor metastasis (45, 46). CTCs can circulate as single cells or clusters, with clusters having increased metastatic potential and a shorter half-life in the circulation (6–10 min for clusters and 25–30 min for single cells) (47). Most CTCs die in the circulation, whereas the surviving CTCs either extravasate into the adjacent tissue or become lodged in the capillary beds of homing tissues (48).
While CTCs are a very rare population of PB sample, they are accessible through simple non-invasive PB sampling. In metastatic tumors, CTCs are derived from both the primary and metastatic tumors, and only from the metastatic tumors when the primary tumor has been resected. Among independent metastatic tumors within a single patient, each tumor can evolve independently and acquire de novo mutations (41, 42). Since surgical resections and biopsies of metastatic tumors are prone to have sampling bias and limited landscape of mutations across all metastatic tumors, CTCs may provide less-biased sampling and more global picture of metastatic tumors. The recent advances in technology for the enrichment and characterization of CTCs have begun to provide new insight into the mechanisms of tumor metastasis and relapse (25, 49).
Disseminated Tumor Cell (DTC)
Although CTCs provide a valuable information about the aggressiveness of tumor, there are additional barriers for CTCs to gain the ability to form metastatic tumors (41, 42). Once CTCs has entered the bloodstream, they must survive, exit the bloodstream, and grow in a foreign microenvironment to cause metastasis. Survived CTCs in the bloodstream disseminate into homing tissues, such as BM, lymph nodes, lung, liver, and brain, and called DTCs (25, 49). Although DTCs and CTCs present similar cytologic and membrane markers, they represent two distinct phenotypes: epithelial-type DTCs and mesenchymal-type CTCs. While a small minority of DTCs generates a proliferating metastatic tumor, a majority of DTCs spread throughout the parenchyma of homing tissues. Depending on cancer types, metastatic tumors develop over a period of months to years (50). A direct contribution of DTCs to metastases formation have shown in breast and esophageal cancers. Presence of DTCs at the time of primary tumor diagnosis or following systemic treatment strongly correlated with metastasis at distant sites in breast cancer patients (51). DTCs established from micrometastatic lymph nodes of esophageal cancer patients have generated tumors in immune-compromised mice (52).
DTCs have been found long after curative treatment of tumor, even in patients without overt metastasis. These DTCs may enter a dormant state but retain the ability to grow into a metastatic tumor or may reach an equilibrium between cellular proliferation and cell death or elimination. Dormant DTCs remain clinically silent and are apparently maintained by an interaction with microenvironment in their homing tissues (53, 54). In breast and prostate cancers, dormant DTCs have acquired additional genetic and epigenetic changes or significant alteration in their microenvironment, and resulted in very late relapses that occur years after treatment (55). BM is the most common homing tissue for blood borne DTCs derived from primary and metastatic tumors. As with CTCs, studies of DTCs in BM have apparent advantages over other homing tissues, which include accessibility, analysis through flow cytometry, and opportunity to gain real-time information about tumors (25, 49).
Circulating Tumor DNA (ctDNA)
Circulating tumor DNA (ctDNA) is a fraction of extracellular nucleic acid (cell-free DNA) that is released into the bloodstream by tumor cells. While CTCs are isolated from the cell pellet containing normal blood cells, ctDNA is isolated from the supernatant. Because the number of CTCs is very low in PB, ctDNA is more sensitive in detecting tumor-associated genetic and epigenetic changes in tumors (26, 27). Although ctDNA can be isolated both from plasma and serum, plasma is recommended as a preferred source for ctDNA because serum-derived ctDNA is contaminated with normal blood cell DNA during the clotting process (56). The release of ctDNA into PB is caused by the apoptosis, necrosis, autophagy, secretion, and other mechanisms, but the exact mechanism of ctDNA release remains unclear (57). In PB, ctDNA circulates predominantly in the form of nucleosomes that retains at least some features of the nuclear chromatin and enables the determination of genetic and epigenetic changes. The size of ctDNA varies between small fragments of 70–200 base pairs and large fragments of ~21 kilobases (26). ctDNA is cleared from the blood by the kidney, liver, and spleen and has a variable half-life ranging from 15 min to several hours (58).
Although cancer patients have higher ctDNA levels than healthy control individuals, the concentrations of overall ctDNA vary considerably in plasma or serum samples in both groups. In cancer patients, ctDNA concentrations are generally lower in localized tumors and higher in metastatic tumors, but also affected by tumor burden, vascularity, and treatment response (56). Tumor-associated genetic and epigenetic changes such as point mutations, genomic rearrangements, copy number variation (CNV), microsatellite instability (MSI), loss of heterozygosity (LOH), and DNA methylation have been detected in ctDNA. These changes warrant a distinction between ctDNA and remaining cell-free DNA, and allow the use of ctDNA as potential biomarkers for determining tumor state, tumor metastasis and relapse, and treatment response (27).
MRD Detection Methods
Cell-Based Assays
While conventional cytology and histology have been accepted as the gold standard for initial diagnosis and staging of tumor patients, these methods are not always possible to detect tumor cells below the level of 1% by morphology alone (59, 60). To achieve more sensitive and quantitative detection of MRD, single-cell-based analytical methods of immunocytology (IC), and flow cytometry (FCM) are applied for quantification of MRD in BM and PB samples (61–63). Both IC and FCM methods rely on the availability of antibodies to tumor-associated cell-surface or intracellular antigens that are ideally expressed on all tumor cells but not normal hematopoietic cells. The sensitivity of IC solely depends on the number of investigated cells and reaches a single tumor cell in 104-105 normal cells in most clinical settings, whereas the sensitivity of FCM is about 10 times lower than IC due to the requirement for at least 10–20 events to call a sample positive (64–66).
gDNA-Based Assays
Tumor DNA can be detected by tracking tumor-specific changes in CTCs, DTCs, and ctDNA samples. In contrast to cell-based assays that are dependent on the availability of antibodies, gDNA-based assays have relied on the existence of tumor-specific genetic and epigenetic changes such as point mutations, genomic rearrangements, CNV, MSI, LOH, and DNA methylation (20, 67). Polymerase chain reaction (PCR) amplification of genomic rearrangements has been particularly powerful in the characterization and detection of hematopoietic tumors. In acute lymphoblastic leukemia (ALL), tumor cells can be detected by PCR amplification of leukemia-specific junctional regions of rearranged immunoglobulin (Ig) genes, and T-cell receptor (TCR) genes (68, 69). Detection of MRD in ALL patients is clinically relevant both in primary and relapsed ALL, and incorporated into patient's risk stratification (70, 71). For solid tumors, DNA sequencing of known oncogene or tumor suppressor mutations has utilized to detect tumor cells. In colorectal cancers, KRAS, APC, and TP53 have a high mutation frequency and their mutation status is correlated with diagnosis, prognosis, and treatment response (72). Recent progress in array comparative genomic hybridization (aCGH) and massively paralleled sequencing (MPS) has begun to realize the detection of patient-specific genetic and epigenetic changes rather than tumor-specific ones (73).
mRNA-Based Assays
Although tumor RNA can be isolated from CTCs and DTCs, the detection of tumor-specific mRNA has been limited to the oncogenic-fusion gene-positive tumors. In chronic myelogenous leukemia (CML), the detection of leukemia-specific BCR-ABL fusion mRNA is used to monitor MRD, and CML patients are currently treated by BCR-ABL fusion kinase inhibitors based on the level of MRD (74, 75). However, the oncogenic-fusion genes have not been identified for most tumors. In melanoma, tumor cells have been identified by the detection of mRNA specific to the tissue of tumor origin (tumor-associated mRNA), tyrosinase mRNA (76). Since the first demonstration in melanoma, the detection of tumor-associated mRNA expression by reverse transcriptase-PCR (RT-PCR) has been applied to detect tumor cells in a number of different tumors. The value of the detection of tumor-associated mRNA expression by RT-PCR has been dependent on the samples evaluated (PB, BM, or lymph nodes), the tumor type, and the clinical stage of tumor (20).
MRD Detection in Neuroblastoma Patients
Neuroblastoma-Associated Antigen Detection
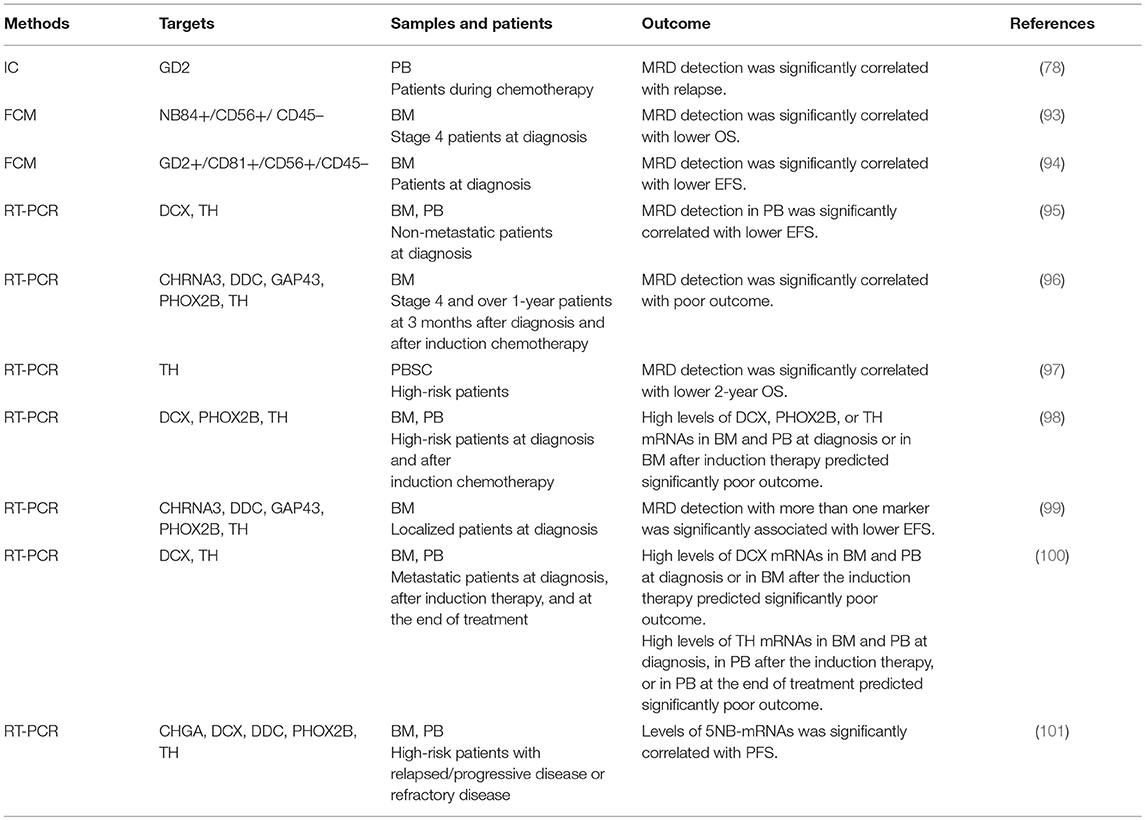
Given the limitation of conventional morphology, single-cell-based IC and FCM with anti-neuroblastoma-associated antigen antibodies have been used to detect residual neuroblastoma cells in BM, PB, and peripheral blood stem cell (PBSC) samples (77–79). A variety of antibodies raised against neuroblastoma cells and established neuronal markers such as neuron specific enolase (NSE) (80), synaptophysin (81), NB84 (82), chromogranin A (83), and CD56 (N-CAM) (84, 85) have been tested for IC. However, many of these antibodies are inappropriate for MRD detection due to their heterogeneous labeling in individual tumors, cross-reactivity with normal hematopoietic cells, or differential labeling on primary vs. metastatic and on undifferentiated vs. differentiated tumor cells (86). Among these neuroblastoma-associated antigens, a cell surface glycosphingolipid, disialoganglioside (GD2), is homogeneously and strongly expressed on neuroblastic tumors but not on normal hematopoietic cells (78, 87, 88). Although the reliability of tumor cell detection and quantification by IC had been controversial, IC detection of neuroblastoma cells was standardized in BM aspirates by using fixed cytospins and anti GD2-antibody (63) and recommended for single-cell based sensitive MRD detection in BM, PB, and PBSC samples by the International Neuroblastoma Risk Group (INRG) (66) (Figures 4, 5, Table 1).
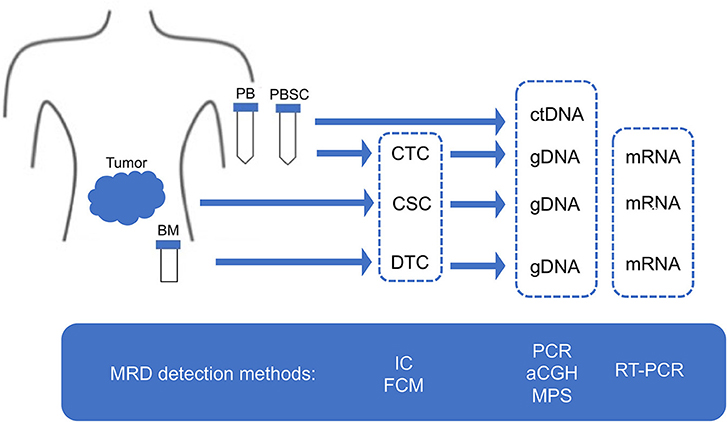
Figure 4. MRD detection methods. MRD, minimal residual disease; CSC, cancer stem cell; CTC, circulating tumor cell; DTC, disseminating tumor cell; ctDNA, circulating tumor DNA; PB, peripheral blood; PBSC, peripheral blood stem cell; BM, bone marrow; IC, immunocytology; FCM, flow cytometry; PCR, polymerase chain reaction; aCGH, array comparative genomic hybridization; MPS, massively paralleled sequencing; RT-PCR, reverse transcriptase-PCR.
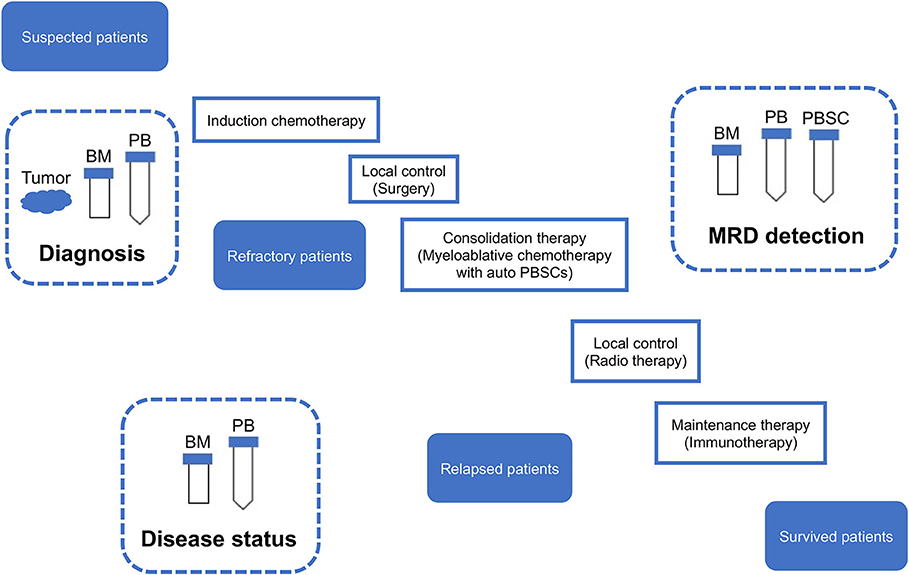
Figure 5. Sampling from neuroblastoma patients. MRD, minimal residual disease; PB, peripheral blood; PBSC, peripheral blood stem cell; BM, bone marrow; IC, immunocytology.
Although immunophenotyping with FCM has proven to be essential for differential diagnosis of most hematopoietic tumors, it remains optional for pediatric solid tumors including neuroblastoma (89). Neuroendocrine tumors were reported to display CD56+/CD45– immunophenotype (90). In addition to CD56, CD9, CD81, GD2, and NB84 antigens were used for FCM. The first multiparameter FCM application for neuroblastoma cell detection was based on CD9+/CD56+/CD45– immunophenotype (61), which was later replaced by CD81+/CD56+/CD45– immunophenotype (91). Subsequently, GD2+/CD56+/CD45–, NB84+/CD56+/CD45–, GD2+/CD81+/CD56+/CD45–, and CD9+/CD81+/CD56+/CD45– immunophenotypes were also applied to FCM-based MRD detection (64, 65, 92, 93) (Figures 4, 5, Table 1). However, a very limited data about prognostic value are currently available for FCM-based MRD detection methods. Detection of NB84+/CD56+/CD45– cells by FCM in initial diagnostic BM samples from metastatic neuroblastoma patients had predicted significantly poor cumulative survival (93). Neuroblastoma patients with GD2+/CD81+/CD56+/CD45– cells in BM by FMC had significantly worse event-free survival and increased cumulative incidence of relapse/progression (94) (Table 4).
Neuroblastoma-Specific gDNA Detection
While tumor DNA can be detected by tracking tumor-specific genetic and epigenetic changes in CTCs, DTCs, and ctDNA samples, neuroblastoma-specific genetic changes such as known oncogene or tumor suppressor mutations and genomic rearrangements have not been identified yet (102). For epigenetic changes specific to neuroblastoma, DNA methylation of tumor suppressor RASSF1A has been applied to detect MRD in DTCs samples (103). In addition to neuroblastoma-specific genetic and epigenetic changes, patient-specific ones have begun to be identified in DTCs and ctDNA sample. Patient-specific DNA breakpoints in eight high-risk neuroblastoma patients have been identified by whole genome sequencing (WGS) of primary tumor and used to detect MRD in DTCs samples (104). Patient-specific genomic mutations in 19 neuroblastoma patients have been detected by whole exome sequencing (WES) of sequentially collected ctDNA samples, revealing a shift of mutational pattern over time and a generation of treatment-resistant clones (105) (Figures 4, 5, Table 1).
Neuroblastoma-Associated mRNA Detection
Neuroblastoma-Associated mRNAs
A number of neuroblastoma-associated mRNAs have been identified initially as a single marker (Table 2) and later as a set of multiple markers (Table 3) for qPCR-based MRD detection in neuroblastoma patients.
In the absence of tumor-specific mRNA, the rate-limiting enzyme in catecholamine biosynthesis, TH, was evaluated as the first neuroblastoma-associated mRNA to detect MRD in neuroblastoma patients (132). While amplification of TH mRNA was shown to detect neuroblastoma cells in BM and PB samples (118, 119), low level of its expression in normal BM cells initially limited its specificity (111). Application of quantitative RT-PCR (qPCR) made it possible to quantify the number of TH mRNA transcripts and allowed to set a threshold of its expression between tumor and normal cells for neuroblastoma MRD detection in BM and PB samples. A number of additional neuroblastoma-associated mRNAs were then reported to detect MRD in PB and BM samples (Table 2). Among these markers, PHOX2B was identified as the most specific and sensitive marker for qPCR-based MRD detection in neuroblastoma patients (116). However, PHOX2B was not highly expressed in all neuroblastoma tumors and its expression considerably varied among different neuroblastoma patients (109).
Given the extreme heterogeneity of neuroblastoma, an increasing number of multiple marker sets for qPCR-based MRD detection have been reported (Table 3). A set of six markers (CCND1, DDC, GABRB3, ISL1, KIF1A, PHOX2B) was identified by genome-wide gene expression microarray analyses of 48 neuroblastoma tumors and nine remission BM samples followed by selecting genes with higher tumor-to-BM expression ratios (110). A set of three markers (DCX, PHOX2B, TH) was identified by genome-wide gene expression microarray analyses of 32 neuroblastoma tumors and pooled PB sample from 24 healthy volunteers followed by selecting genes not expressed in pooled PB samples (121–123). Two sets of five markers (CHRNA3, DDC, GAP43, PHOX2B, TH) for BM samples and five markers (CHRNA3, DBH, DDC, PHOX2B, TH) for PB samples were identified by comparing serial analysis of gene expression (SAGE) libraries of neuroblastoma and healthy tissues followed by qPCR analysis of 56 neuroblastoma tumors, 51 control BM samples, and 37 control PB samples (109, 124). A set of 11 markers (CHRNA3, CRMP1, DBH, DCX, DDC, GABRB3, GAP43, ISL1, KIF1A, PHOX2B, TH) was identified by validating known 14 makers expression in CSC-enriched spheres of neuroblastoma BE(2)-C cells (125). A set of five markers (CHGA, DCX, DDC, PHOX2B, TH) normalized by four references (B2M, GAPDH, HPRT1, SDHA) was also developed (101, 126). In addition, several amended sets of two markers (DCX, TH) for BM and PB samples (95), three markers (B4GALNT1, DDC, TH) for BM and PB samples (127), four markers (B4GALNT1, CCND1, ISL1, PHOX2B) for BM sample (129, 130), and four markers (B4GALNT1, ELAVL4, PHOX2B, TH) for BM sample were reported (131).
Clinical Significance of qPCR-Based MRD Detection
A growing number of qPCR-based MRD detection assays using different methods (MRD markers) and samples have been reported over past two decades. These assays use three types of samples (BM, PB, and PBSC). BM is the most common site of infiltration in metastatic neuroblastoma patients at the time of diagnosis, and is a frequent site of tumor relapse (133). Neuroblastoma cells have been detected in PB of metastatic neuroblastoma patients at diagnosis and during therapy (78). High-risk neuroblastoma patients are treated with the standard regimens containing high-dose myeloablative chemotherapy rescued with autologous stem cells (auto PBSCs) and have naturally expected to cause relapse if PBSC contaminated with neuroblastoma cells are transplanted (126). These assays are also targeted for different groups of neuroblastoma patients (localized and metastatic neuroblastoma) (Figures 4, 5, Table 1).
Accordingly, qPCR-based MRD detection assays can be classified into the following five groups: (1) Localized neuroblastoma-PB samples, (2) Localized neuroblastoma-BM samples, (3) Metastatic neuroblastoma-PBSC samples, (4) Metastatic neuroblastoma-PB samples, (5) Metastatic neuroblastoma-BM samples. At the moment, the clinical significance of all five groups remains to be established. However, the promising data has been reported in two groups (Metastatic neuroblastoma-PB and BM samples).
Localized neuroblastoma-PB and BM samples
In localized neuroblastoma, the clinical significance of qPCR-based MRD detection is still not clear in both PB and BM samples.
Expression of B4GALNT1, DCX, DDC, ELAVL4, PHOX2B, ST8SIA2, and TH mRNAs in BM and PB samples at diagnosis was not associated with clinical events in localized neuroblastoma patients (134). MRD detection evaluated by DCX or TH mRNA expression in PB samples at diagnosis predicted worse event-free survival (EFS) of non-metastatic neuroblastoma patients (95). When BM samples are evaluated by a set of five markers (CHRNA3, DDC, GAP43, PHOX2B, and TH), unfavorable outcome in localized neuroblastoma patients is associated with the detection of more than one positive-marker (99) (Table 4).
Metastatic neuroblastoma-PBSC samples
In metastatic neuroblastoma, the clinical significance of qPCR-based MRD detection is still not clear in PBSC samples.
There are conflicts between the previous reports. TH mRNA expression in PBSC samples was not statistically associated with unfavorable outcome in high-risk metastatic neuroblastoma patients (135). qPCR-based MRD detection by B4GALNT1 or TH mRNAs in PBSC samples did not affect survival of stage 4 neuroblastoma patients (136). High-risk neuroblastoma patients received unpurged PBSCs did not have more relapses (126). In contrast to these reports, positive TH mRNA in PBSC samples predicted a lower 2-year OS in high-risk neuroblastoma patients (97). High levels of CHGA, DCX, DDC, PHOX2B, or TH mRNA expression in PBSC samples was associated with worse outcome (126) (Table 4).
Metastatic neuroblastoma-PB and BM samples
In metastatic neuroblastoma, the clinical significance of qPCR-based MRD detection remains to be established in both PB and BM samples.
While the independent study groups reported the promising data, they used different methods (MRD markers) and their data remained controversial. MRD detection in BM samples evaluated by a set of five markers (CHRNA3, DDC, GAP43, PHOX2B, and TH) was associated with poor outcome in patients aged over 1 year with stage 4 neuroblastoma (96). High levels of DCX, PHOX2B, or TH mRNAs in BM and PB samples at diagnosis or BM samples after induction therapy predicted poor outcome in children with stage 4 neuroblastoma (98). High levels of DCX and TH mRNAs in BM and PB samples at diagnosis was associated with poor outcome in metastatic neuroblastoma (100). Expression levels of B4GALNT1, CCND1, ISL1, or PHOX2B mRNAs in BM samples predicted progression-free survival (PFS) and overall survival (OS) for anti-GD2 antibody-treated patients in first or second remission or with refractory disease (130). The combined signature of five markers (CHGA, DCX, DDC, PHOX2B, and TH) expression in BM and PB was correlated with PFS of relapsed/refractory neuroblastoma (101) (Table 4).
Conclusion
Drug-resistant MRD is principally responsible for dismal outcome for aggressive cancers. Current intensive multimodal therapies eliminate the bulk population of tumor cells while sparing the minor subpopulations of MRD that is dynamically presented as CSCs, CTCs, and DTCs in cancer patients. EMT appears to be a key mechanism operated in cancer cells to acquire these MRD phenotypes. Although qPCR of tumor-associated mRNAs in BM and PB samples have detected clinically relevant tumor cells in many hematopoietic and solid tumors, current qPCR-based assays for neuroblastoma MRD detection use different neuroblastoma-associated mRNAs (alone or in combination) and will require the prospective quality-controlled clinical studies that evaluate the clinical significance of these qPCR-based assays. Given the extreme heterogeneity of neuroblastoma, the normal variations, which can arise from sampling of the different patients or sequential sampling of the same patient, hold a key to interpret the data from these clinical studies.
Recent advances in techniques to isolate and detect rare DNA changes in a complex mixture of DNA with high sensitivity have made it possible to track tumor-specific genetic and epigenetic changes in CTCs, DTCs, and ctDNA. Because neuroblastoma relapses often occur at multiple anatomic sites and distinct DNA aberrations can be unique to each metastatic site, the assessment of ctDNA would provide unprecedented insights into the metastatic and recurrent processes of neuroblastoma. The complementary assessment of MRD status in CTCs, DTCs, and ctDNA collected from the same patient during entire course of treatment will provide our foundation to understand the dynamics of MRD in neuroblastoma patients. Toward the optimal stratification and outcome of neuroblastoma patients, there is an urgent need for these clinical studies realizing a solid evaluation of neuroblastoma MRD.
Author Contributions
SU, DH, YK, KI, and NoN conceptualized the manuscript. SU and NoN wrote the draft. SU, TI, KT, NY, AT, KK, NaN, KL, ST, and TM reviewed and edited the manuscript. DH, YK, KI, and NoN revised the manuscript.
Funding
This work was supported in part by an institutional research funding from the Sysmex Corporation.
Conflict of Interest Statement
NN received an institutional research funding from the Sysmex Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Tomolonis JA, Agarwal S, Shohet JM. Neuroblastoma pathogenesis: deregulation of embryonic neural crest development. Cell Tissue Res. (2018) 372:245–62. doi: 10.1007/s00441-017-2747-0
2. Delloye-Bourgeois C, Castellani V. Hijacking of embryonic programs by neural crest-derived neuroblastoma: from physiological migration to metastatic dissemination. Front Mol Neurosci. (2019) 12:366. doi: 10.3389/fnmol.2019.00052
3. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. (2019) 20:69–84. doi: 10.1038/s41580-018-0080-4
4. Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. (2017) 14:611–629. doi: 10.1038/nrclinonc.2017.44
5. Tanno B, Sesti F, Cesi V, Bossi G, Ferrari-Amorotti G, Bussolari R, et al. Expression of Slug is regulated by c-Myb and is required for invasion and bone marrow homing of cancer cells of different origin. J Biol Chem. (2010) 285:29434–45. doi: 10.1074/jbc.M109.089045
6. Ferronha T, Rabadán MA, Gil-Guiñon E, Le Dréau G, de Torres C, Martí E. LMO4 is an essential cofactor in the Snail2-mediated epithelial-to-mesenchymal transition of neuroblastoma and neural crest cells. J Neurosci. (2013) 33:2773–83. doi: 10.1523/JNEUROSCI.4511-12.2013
7. Li D, Mei H, Qi M, Yang D, Zhao X, Xiang X, et al. FOXD3 is a novel tumor suppressor that affects growth, invasion, metastasis and angiogenesis of neuroblastoma. Oncotarget. (2013) 4:2021–44. doi: 10.18632/oncotarget.1579
8. Vo KT, Matthay KK, Neuhaus J, London WB, Hero B, Ambros PF, et al. Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol. (2014) 32:3169–76. doi: 10.1200/JCO.2014.56.1621
9. Boeva V, Louis-Brennetot C, Peltier A, Durand S, Pierre-Eugène C, Raynal V, et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat Genet. (2017) 49:1408–13. doi: 10.1038/ng.3921
10. van Groningen T, Koster J, Valentijn LJ, Zwijnenburg DA, Akogul N, Hasselt NE, et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat Genet. (2017) 49:1261–6. doi: 10.1038/ng.3899
11. Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. (2003) 3:203–16. doi: 10.1038/nrc1014
12. Nakagawara A, Li Y, Izumi H, Muramori K, Inada H, Nishi M. Neuroblastoma. Jpn J Clin Oncol. (2018) 48:214–41. doi: 10.1093/jjco/hyx176
13. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. (2007) 369:2106–20. doi: 10.1016/S0140-6736(07)60983-0
14. Maris JM. Recent advances in neuroblastoma. N Engl J Med. (2010) 362:2202–11. doi: 10.1056/NEJMra0804577
15. Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. (2015) 33:3008–17. doi: 10.1200/JCO.2014.59.4648
16. Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children's Oncology Group (COG) study. J Clin Oncol. (2016) 34:LBA3. doi: 10.1200/JCO.2016.34.18_suppl.LBA3
17. Tolbert VP, Matthay KK. Neuroblastoma: clinical and biological approach to risk stratification and treatment. Cell Tissue Res. (2018) 372:195–209. doi: 10.1007/s00441-018-2821-2
18. Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. (2013) 19:1450–64. doi: 10.1038/nm.3391
19. Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. (2016) 352:169–75. doi: 10.1126/science.aaf2784
20. Burchill SA, Selby PJ. Molecular detection of low-level disease in patients with cancer. J Pathol. (2000) 190:6–14. doi: 10.1002/(SICI)1096-9896(200001)190:1<6::AID-PATH486>3.0.CO;2-M
21. Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. (2004) 4:448–56. doi: 10.1038/nrc1370
22. Mordant P, Loriot Y, Lahon B, Castier Y, Lesèche G, Soria J-C, et al. Minimal residual disease in solid neoplasia: new frontier or red-herring? Cancer Treat Rev. (2012) 38:101–10. doi: 10.1016/j.ctrv.2011.04.014
23. Pantel K, Alix-Panabières C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep. (2014) 3:584. doi: 10.1038/bonekey.2014.79
24. Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. (2016) 35:1216–24. doi: 10.1038/onc.2015.192
25. Wang H, Stoecklein NH, Lin PP, Gires O. Circulating and disseminated tumor cells: diagnostic tools and therapeutic targets in motion. Oncotarget. (2017) 8:1884–912. doi: 10.18632/oncotarget.12242
26. Alix-Panabières C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. (2012) 63:199–215. doi: 10.1146/annurev-med-062310-094219
27. Sumbal S, Javed A, Afroze B, Zulfiqar HF, Javed F, Noreen S, et al. Circulating tumor DNA in blood: future genomic biomarkers for cancer detection. Exp Hematol. (2018) 65:17–28. doi: 10.1016/j.exphem.2018.06.003
29. Welch DR. Tumor heterogeneity—A “contemporary concept” founded on historical insights and predictions. Cancer Res. (2016) 76:4–6. doi: 10.1158/0008-5472.CAN-15-3024
30. Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. (2013) 501:338–45. doi: 10.1038/nature12625
31. Meacham CE, Morrison SJ. Tumor heterogeneity and cancer cell plasticity. Nature. (2013) 501:328–37. doi: 10.1038/nature12624
32. De Sousa E, Melo F, Vermeulen L, Fessler E, Medema JP. Cancer heterogeneity-a multifaceted view. EMBO Rep. (2013) 14:686–695. doi: 10.1038/embor.2013.92
33. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. (2006) 355:1253–61. doi: 10.1056/NEJMra061808
34. Vermeulen L, de Sousa e, Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. (2012) 13:e83–9. doi: 10.1016/S1470-2045(11)70257-1
35. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumors: accumulating evidence and unresolved questions. Nat Rev Cancer. (2008) 8:755–68. doi: 10.1038/nrc2499
36. Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. (2013) 501:355–64. doi: 10.1038/nature12627
37. Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. (2008) 133:704–15. doi: 10.1016/j.cell.2008.03.027
38. Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. (2013) 342:1234850. doi: 10.1126/science.1234850
39. Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. (2015) 25:675–86. doi: 10.1016/j.tcb.2015.07.012
40. Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, et al. Identification of the tumour transition states occurring during EMT. Nature. (2018) 556:463–8. doi: 10.1038/s41586-018-0040-3
41. Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. (2016) 529:298–306. doi: 10.1038/nature17038
42. Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev. (2017) 31:1827–40. doi: 10.1101/gad.305805.117
43. Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. (2013) 31:539–544. doi: 10.1038/nbt.2576
44. Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. (2014) 20:897–903. doi: 10.1038/nm.3600
45. Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. (2013) 339:580–4. doi: 10.1126/science.1228522
46. Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, et al. Acquisition of epithelial–mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. (2013) 4:e875. doi: 10.1038/cddis.2013.407
47. Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. (2014) 158:1110–22. doi: 10.1016/j.cell.2014.07.013
48. Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. (2015) 7:1–11. doi: 10.15252/emmm.201303698
49. Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. (2015) 6:10697–711. doi: 10.18632/oncotarget.4037
50. Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proc Natl Acad Sci USA. (2011) 108:12396–400. doi: 10.1073/pnas.1106613108
51. Riethdorf S, Pantel K. Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization. Pathobiology. (2008) 75:140–8. doi: 10.1159/000123852
52. Hosch S, Kraus J, Scheunemann P, Izbicki JR, Schneider C, Schumacher U, et al. Malignant potential and cytogenetic characteristics of occult disseminated tumor cells in esophageal cancer. Cancer Res. (2000) 60:6836–40.
53. Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. (2007) 7:834–46. doi: 10.1038/nrc2256
54. Yeh AC, Ramaswamy S. Mechanisms of cancer cell dormancy—another hallmark of cancer? Cancer Res. (2015) 75:5014–22. doi: 10.1158/0008-5472.CAN-15-1370
55. Dittmer J. Mechanisms governing metastatic dormancy in breast cancer. Semin Cancer Biol. (2017) 44:72–82. doi: 10.1016/j.semcancer.2017.03.006
56. Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. (2015) 61:112–23. doi: 10.1373/clinchem.2014.222679
57. Jung K, Fleischhacker M, Rabien A. Cell-free DNA in the blood as a solid tumor biomarker—a critical appraisal of the literature. Clin Chim Acta. (2010) 411:1611–24. doi: 10.1016/j.cca.2010.07.032
58. Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. (2011) 11:426–37. doi: 10.1038/nrc3066
59. Moss TJ, Reynolds CP, Sather HN, Romansky SG, Hammond GD, Seeger RC. Prognostic value of immunocytologic detection of bone marrow metastases in neuroblastoma. N Engl J Med. (1991) 324:219–26. doi: 10.1056/NEJM199101243240403
60. Cheung NK, Heller G, Kushner BH, Liu C, Cheung IY. Detection of metastatic neuroblastoma in bone marrow: when is routine marrow histology insensitive? J Clin Oncol. (1997) 15:2807–17. doi: 10.1200/JCO.1997.15.8.2807
61. Komada Y, Zhang XL, Zhou YW, Inaba H, Deguchi T, Azuma E, et al. Flow cytometric analysis of peripheral blood and bone marrow for tumor cells in patients with neuroblastoma. Cancer. (1998) 82:591–9.
62. Seeger RC, Reynolds CP, Gallego R, Stram DO, Gerbing RB, Matthay KK. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: a Children's Cancer Group Study. J Clin Oncol. (2000) 18:4067–76. doi: 10.1200/JCO.2000.18.24.4067
63. Swerts K, Ambros PF, Brouzes C, Navarro JMF, Gross N, Rampling D, et al. Standardization of the immunocytochemical detection of neuroblastoma cells in bone marrow. J Histochem Cytochem. (2005) 53:1433–40. doi: 10.1369/jhc.5C6661.2005
64. Swerts K, De Moerloose B, Dhooge C, Brichard B, Benoit Y, Laureys G, et al. Detection of residual neuroblastoma cells in bone marrow: comparison of flow cytometry with immunocytochemistry. Cytometry B Clin Cytom. (2004) 61:9–19. doi: 10.1002/cyto.b.20019
65. Beiske K, Ambros PF, Burchill SA, Cheung IY, Swerts K. Detecting minimal residual disease in neuroblastoma patients-the present state of the art. Cancer Lett. (2005) 228:229–40. doi: 10.1016/j.canlet.2005.02.053
66. Beiske K, Burchill SA, Cheung IY, Hiyama E, Seeger RC, Cohn SL, et al. Consensus criteria for sensitive detection of minimal neuroblastoma cells in bone marrow, blood and stem cell preparations by immunocytology and QRT-PCR: recommendations by the international neuroblastoma risk group task force. Br J Cancer. (2009) 100:1627–37. doi: 10.1038/sj.bjc.6605029
67. Wang L, Wheeler DA. Genomic sequencing for cancer diagnosis and therapy. Annu Rev Med. (2014) 65:33–48. doi: 10.1146/annurev-med-120811-171056
68. van der Velden VHJ, Panzer-Grümayer ER, Cazzaniga G, Flohr T, Sutton R, Schrauder A, et al. Optimization of PCR-based minimal residual disease diagnostics for childhood acute lymphoblastic leukemia in a multi-center setting. Leukemia. (2007) 21:706–13. doi: 10.1038/sj.leu.2404535
69. van der Velden VHJ, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grümayer ER, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. (2007) 21:604–11. doi: 10.1038/sj.leu.2404586
70. Flohr T, Schrauder A, Cazzaniga G, Panzer-Grümayer R, van der Velden V, Fischer S, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. (2008) 22:771–82. doi: 10.1038/leu.2008.5
71. Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. (2017) 3:e170580. doi: 10.1001/jamaoncol.2017.0580
72. Huang D, Sun W, Zhou Y, Li P, Chen F, Chen H, et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. (2018) 37:173–87. doi: 10.1007/s10555-017-9726-5
73. Leary RJ, Kinde I, Diehl F, Schmidt K, Clouser C, Duncan C, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. (2010) 2:20ra14. doi: 10.1126/scitranslmed.3000702
74. Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. (2006) 108:28–37. doi: 10.1182/blood-2006-01-0092
75. Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. (2010) 362:2260–70. doi: 10.1056/NEJMoa1002315
76. Smith B, Selby P, Southgate J, Pittman K, Bradley C, Blair GE. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. (1991) 338:1227–9.
77. Carey PJ, Thomas L, Buckle G, Reid MM. Immunocytochemical examination of bone marrow in disseminated neuroblastoma. J Clin Pathol. (1990) 43:9–12. doi: 10.1136/jcp.43.1.9
78. Moss TJ, Sanders DG. Detection of neuroblastoma cells in blood. J Clin Oncol. (1990) 8:736–40. doi: 10.1200/JCO.1990.8.4.736
79. Moss TJ, Cairo M, Santana VM, Weinthal J, Hurvitz C, Bostrom B. Clonogenicity of circulating neuroblastoma cells: implications regarding peripheral blood stem cell transplantation. Blood. (1994) 83:3085–9. doi: 10.1182/pgash-83-10-3085
80. Dhillon A, Roder J, Leathem A. Neurone specific enolase: an aid to the diagnosis of melanoma and neuroblastoma. Histopathology. (1982) 6:81–92. doi: 10.1111/j.1365-2559.1982.tb02704.x
81. Gould VE, Wiedenmann B, Lee I, Schwechheimer K, Dockhorn-Dworniczak B, Radosevich JA, et al. Synaptophysin expression in neuroendocrine neoplasms as determined by immunocytochemistry. Am J Pathol. (1987) 126:243–57.
82. Thomas JO, Nijjar J, Turley H, Micklem K, Gatter KC. NB84: a new monoclonal antibody for the recognition of neuroblastoma in routinely processed material. J Pathol. (1991) 163:69–75. doi: 10.1002/path.1711630112
83. Helman LJ, Gazdar AF, Park JG, Cohen PS, Cotelingam JD, Israel MA. Chromogranin A expression in normal and malignant human tissues. J Clin Invest. (1988) 82:686–90. doi: 10.1172/JCI113648
84. Goldman A, Vivian G, Gordon I, Pritchard J, Kemshead J. Immunolocalization of neuroblastoma using radiolabeled monoclonal antibody UJ13A. J Pediatr. (1984) 105:252–6. doi: 10.1016/S0022-3476(84)80122-5
85. Patel K, Moore SE, Dickson G, Rossell RJ, Beverley PC, Kemshead JT, et al. Neural cell adhesion molecule (NCAM) is the antigen recognized by monoclonal antibodies of similar specificity in small-cell lung carcinoma and neuroblastoma. Int J Cancer. (1989) 44:573–8. doi: 10.1002/ijc.2910440402
86. Sugimoto T, Sawada T, Matsumura T, Kemshead JT, Horii Y, Saida T, et al. Diagnosis of neuroblastoma metastasis in bone marrow with a panel of monoclonal antibodies. Med Pediatr Oncol. (1988) 16:190–6. doi: 10.1002/mpo.2950160308
87. Cheung NK, Von Hoff DD, Strandjord SE, Coccia PF. Detection of neuroblastoma cells in bone marrow using GD2 specific monoclonal antibodies. J Clin Oncol. (1986) 4:363–9. doi: 10.1200/JCO.1986.4.3.363
88. Sariola H, Terävä H, Rapola J, Saarinen UM. Cell-surface ganglioside GD2 in the immunohistochemical detection and differential diagnosis of neuroblastoma. Am J Clin Pathol. (1991) 96:248–52.
89. Ferreira-Facio CS, Milito C, Botafogo V, Fontana M, Thiago LS, Oliveira E, et al. Contribution of multiparameter flow cytometry immunophenotyping to the diagnostic screening and classification of pediatric cancer. PLoS ONE. (2013) 8:e55534. doi: 10.1371/journal.pone.0055534
90. Bryson GJ, Lear D, Williamson R, Wong RCW. Detection of the CD56+/CD45- immunophenotype by flow cytometry in neuroendocrine malignancies. J Clin Pathol. (2002) 55:535–7. doi: 10.1136/jcp.55.7.535
91. Nagai J, Ishida Y, Koga N, Tanaka Y, Ohnuma K, Toyoda Y, et al. A new sensitive and specific combination of CD81/CD56/CD45 monoclonal antibodies for detecting circulating neuroblastoma cells in peripheral blood using flow cytometry. J Pediatr Hematol Oncol. (2000) 22:20–6.
92. Warzynski MJ, Graham DM, Axtell RA, Higgins JV, Hammers YA. Flow cytometric immunophenotyping test for staging/monitoring neuroblastoma patients. Cytometry. (2002) 50:298–304. doi: 10.1002/cyto.10159
93. Bozzi F, Gambirasio F, Luksch R, Collini P, Brando B, Fossati-Bellani F. Detecting CD56+/NB84+/CD45- immunophenotype in the bone marrow of patients with metastatic neuroblastoma using flow cytometry. Anticancer Res. (2006) 26:3281–7.
94. Popov A, Druy A, Shorikov E, Verzhbitskaya T, Solodovnikov A, Saveliev L, et al. Prognostic value of initial bone marrow disease detection by multiparameter flow cytometry in children with neuroblastoma. J Cancer Res Clin Oncol. (2019) 145:535–42. doi: 10.1007/s00432-018-02831-w
95. Yáñez Y, Grau E, Oltra S, Cañete A, Martínez F, Orellana C, et al. Minimal disease detection in peripheral blood and bone marrow from patients with non-metastatic neuroblastoma. J Cancer Res Clin Oncol. (2011) 137:1263–72. doi: 10.1007/s00432-011-0997-x
96. Stutterheim J, Zappeij-Kannegieter L, Versteeg R, Caron HN, van der Schoot CE, Tytgat GAM. The prognostic value of fast molecular response of marrow disease in patients aged over 1 year with stage 4 neuroblastoma. Eur J Cancer. (2011) 47:1193–202. doi: 10.1016/j.ejca.2011.02.003
97. Chambon F, Tchirkov A, Pereira B, Rochette E, Deméocq F, Kanold J. Molecular assessment of minimal residual disease in PBSC harvests provides prognostic information in neuroblastoma. Pediatr Blood Cancer. (2013) 60:E109–12. doi: 10.1002/pbc.24538
98. Viprey VF, Gregory WM, Corrias MV, Tchirkov A, Swerts K, Vicha A, et al. Neuroblastoma mRNAs predict outcome in children with stage 4 neuroblastoma: a European HR-NBL1/SIOPEN study. J Clin Oncol. (2014) 32:1074–83. doi: 10.1200/JCO.2013.53.3604
99. van Wezel EM, Decarolis B, Stutterheim J, Zappeij-Kannegieter L, Berthold F, Schumacher Kuckelkorn R, et al. Neuroblastoma messenger RNA is frequently detected in bone marrow at diagnosis of localised neuroblastoma patients. Eur J Cancer. (2016) 54:149–58. doi: 10.1016/j.ejca.2015.11.007
100. Yáñez Y, Hervás D, Grau E, Oltra S, Pérez G, Palanca S, et al. TH and DCX mRNAs in peripheral blood and bone marrow predict outcome in metastatic neuroblastoma patients. J Cancer Res Clin Oncol. (2016) 142:573–80. doi: 10.1007/s00432-015-2054-7
101. Marachelian A, Villablanca JG, Liu CW, Liu B, Goodarzian F, Lai HA, et al. Expression of five neuroblastoma genes in bone marrow or blood of patients with relapsed/refractory neuroblastoma provides a new biomarker for disease and prognosis. Clin Cancer Res. (2017) 23:5374–83. doi: 10.1158/1078-0432.CCR-16-2647
102. Bosse KR, Maris JM. Advances in the translational genomics of neuroblastoma: from improving risk stratification and revealing novel biology to identifying actionable genomic alterations. Cancer. (2016) 122:20–33. doi: 10.1002/cncr.29706
103. Stutterheim J, Ichou FA, den Ouden E, Versteeg R, Caron HN, Tytgat GAM, et al. Methylated RASSF1a is the first specific DNA marker for minimal residual disease testing in neuroblastoma. Clin Cancer Res. (2012) 18:808–14. doi: 10.1158/1078-0432.CCR-11-0849
104. van Wezel EM, Zwijnenburg D, Zappeij-Kannegieter L, Bus E, van Noesel MM, Molenaar JJ, et al. Whole-genome sequencing identifies patient-specific DNA minimal residual disease markers in neuroblastoma. J Mol Diagn. (2015) 17:43–52. doi: 10.1016/j.jmoldx.2014.09.005
105. Chicard M, Colmet Daage L, Clement N, Danzon A, Bohec M, Bernard V, et al. Whole-exome sequencing of cell-free DNA reveals temporo-spatial heterogeneity and identifies treatment-resistant clones in neuroblastoma. Clin Cancer Res. (2018) 24:939–49. doi: 10.1158/1078-0432.CCR-17-1586
106. Cheung IY, Cheung NK. Quantitation of marrow disease in neuroblastoma by real-time reverse transcription-PCR. Clin Cancer Res. (2001) 7:1698–705.
107. Cheung IY, Feng Y, Vickers A, Gerald W, Cheung N-KV. Cyclin D1, a novel molecular marker of minimal residual disease, in metastatic neuroblastoma. J Mol Diagn. (2007) 9:237–241. doi: 10.2353/jmoldx.2007.060130
108. Pagani A, Macri L, Faulknerf LB, Tintori V, Paoli A, Garaventa A, et al. Detection procedures for neuroblastoma cells metastatic to blood and bone marrow: blinded comparison of chromogranin A heminested reverse transcription polymerase chain reaction to tyrosine hydroxylase nested reverse transcription polymerase chain reaction and to anti-GD2 immunocytology. Diagn Mol Pathol. (2002) 11:98–106.
109. Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, Yalcin B, Dee R, van Noesel MM, et al. Detecting minimal residual disease in neuroblastoma: the superiority of a panel of real-time quantitative PCR markers. Clin Chem. (2009) 55:1316–26. doi: 10.1373/clinchem.2008.117945
110. Cheung IY, Feng Y, Gerald W, Cheung N-KV. Exploiting gene expression profiling to identify novel minimal residual disease markers of neuroblastoma. Clin Cancer Res. (2008) 14:7020–7. doi: 10.1158/1078-0432.CCR-08-0541
111. Kuci Z, Seitz G, Kuci S, Kreyenberg H, Schumm M, Lang P, et al. Pitfalls in detection of contaminating neuroblastoma cells by tyrosine hydroxylase RT-PCR due to catecholamine-producing hematopoietic cells. Anticancer Res. (2006) 26:2075–80.
112. Oltra S, Martínez F, Orellana C, Grau E, Fernandez JM, Cañete A, et al. The doublecortin gene, a new molecular marker to detect minimal residual disease in neuroblastoma. Diagn Mol Pathol. (2005) 14:53–7. doi: 10.1097/01.pas.0000149876.32376.c0
113. Bozzi F, Luksch R, Collini P, Gambirasio F, Barzanò E, Polastri D, et al. Molecular detection of dopamine decarboxylase expression by means of reverse transcriptase and polymerase chain reaction in bone marrow and peripheral blood: utility as a tumor marker for neuroblastoma. Diagn Mol Pathol. (2004) 13:135–143.
114. Swerts K. Potential application of ELAVL4 real-time quantitative reverse transcription-PCR for detection of disseminated neuroblastoma cells. Clin Chem. (2006) 52:438–45. doi: 10.1373/clinchem.2005.059485
115. Cheung IY, Cheung NK. Molecular detection of GAGE expression in peripheral blood and bone marrow: utility as a tumor marker for neuroblastoma. Clin Cancer Res. (1997) 3:821–6.
116. Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, Kleijn I, Dee R, Hooft L, et al. PHOX2B is a novel and specific marker for minimal residual disease testing in neuroblastoma. J Clin Oncol. (2008) 26:5443–9. doi: 10.1200/JCO.2007.13.6531
117. Cheung IY, Vickers A, Cheung N-KV. Sialyltransferase STX (ST8SiaII): a novel molecular marker of metastatic neuroblastoma. Int J Cancer. (2006) 119:152–6. doi: 10.1002/ijc.21789
118. Naito H, Kuzumaki N, Uchino J, Kobayashi R, Shikano T, Ishikawa Y, et al. Detection of tyrosine hydroxylase mRNA and minimal neuroblastoma cells by the reverse transcription-polymerase chain reaction. Eur J Cancer. (1991) 27:762–5.
119. Burchill SA, Bradbury FM, Smith B, Lewis IJ, Selby P. Neuroblastoma cell detection by reverse transcriptase-polymerase chain reaction (RT-PCR) for tyrosine hydroxylase mRNA. Int J Cancer. (1994) 57:671–5. doi: 10.1002/ijc.2910570510
120. Wang Y, Einhorn P, Triche TJ, Seeger RC, Reynolds CP. Expression of protein gene product 9.5 and tyrosine hydroxylase in childhood small round cell tumors. Clin Cancer Res. (2000) 6:551–8.
121. Viprey VF, Lastowska MA, Corrias MV, Swerts K, Jackson MS, Burchill SA. Minimal disease monitoring by QRT-PCR: guidelines for identification and systematic validation of molecular markers prior to evaluation in prospective clinical trials. J Pathol. (2008) 216:245–52. doi: 10.1002/path.2406
122. Grèze V, Brugnon F, Chambon F, Halle P, Canis M, Amiot C, et al. Highly sensitive assessment of neuroblastoma minimal residual disease in ovarian tissue using RT-qPCR-A strategy for improving the safety of fertility restoration. Pediatr Blood Cancer. (2017) 64:e26287. doi: 10.1002/pbc.26287
123. Grèze V, Kanold J, Chambon F, Halle P, Gremeau A-S, Rives N, et al. RT-qPCR for PHOX2B mRNA is a highly specific and sensitive method to assess neuroblastoma minimal residual disease in testicular tissue. Oncol Lett. (2017) 14:860–6. doi: 10.3892/ol.2017.6238
124. van Wezel EM, Stutterheim J, Vree F, Zappeij-Kannegieter L, Decarolis B, Hero B, et al. Minimal residual disease detection in autologous stem cell grafts from patients with high risk neuroblastoma. Pediatr Blood Cancer. (2015) 62:1368–73. doi: 10.1002/pbc.25507
125. Hartomo TB, Kozaki A, Hasegawa D, Pham TVH, Yamamoto N, Saitoh A, et al. Minimal residual disease monitoring in neuroblastoma patients based on the expression of a set of real-time RT-PCR markers in tumor-initiating cells. Oncol Rep. (2013) 29:1629–36. doi: 10.3892/or.2013.2286
126. Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. (2013) 14:999–1008. doi: 10.1016/S1470-2045(13)70309-7
127. Träger C, Vernby A, Kullman A, Øra I, Kogner P, Kågedal B. mRNAs of tyrosine hydroxylase and dopa decarboxylase but not of GD2 synthase are specific for neuroblastoma minimal disease and predicts outcome for children with high-risk disease when measured at diagnosis. Int J Cancer. (2008) 123:2849–55. doi: 10.1002/ijc.23846
128. Kågedal B, Kullman A, Lenner L, Träger C, Kogner P, Farnebäck M. Pterin-dependent tyrosine hydroxylase mRNA is not expressed in human melanocytes or melanoma cells. Pigment Cell Res. (2004) 17:346–51. doi: 10.1111/j.1600-0749.2004.00153.x
129. Cheung N-KV, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. (2012) 30:3264–70. doi: 10.1200/JCO.2011.41.3807
130. Cheung N-KV, Ostrovnaya I, Kuk D, Cheung IY. Bone marrow minimal residual disease was an early response marker and a consistent independent predictor of survival after anti-GD2 immunotherapy. J Clin Oncol. (2015) 33:755–63. doi: 10.1200/JCO.2014.57.6777
131. Druy AE, Shorikov EV, Tsaur GA, Popov AM, Zaychikov AN, Tuponogov SN, et al. Prospective investigation of applicability and the prognostic significance of bone marrow involvement in patients with neuroblastoma detected by quantitative reverse transcription PCR. Pediatr Blood Cancer. (2018) 369:e27354. doi: 10.1002/pbc.27354
132. Miyajima Y, Kato K, Numata SI, Kudo K, Horibe K. Detection of neuroblastoma cells in bone marrow and peripheral blood at diagnosis by the reverse transcriptase-polymerase chain reaction for tyrosine hydroxylase mRNA. Cancer. (1995) 75:2757–61.
133. Morandi F, Corrias MV, Pistoia V. Evaluation of bone marrow as a metastatic site of human neuroblastoma. Ann N Y Acad Sci. (2015) 1335:23–31. doi: 10.1111/nyas.12554
134. Corrias MV, Haupt R, Carlini B, Cappelli E, Giardino S, Tripodi G, et al. Multiple target molecular monitoring of bone marrow and peripheral blood samples from patients with localized neuroblastoma and healthy donors. Pediatr Blood Cancer. (2012) 58:43–9. doi: 10.1002/pbc.22960
135. Burchill SA, Kinsey SE, Picton S, Roberts P, Pinkerton CR, Selby P, et al. Minimal residual disease at the time of peripheral blood stem cell harvest in patients with advanced neuroblastoma. Med Pediatr Oncol. (2001) 36:213–9. doi: 10.1002/1096-911X(20010101)36:1<213::AID-MPO1052>3.0.CO;2-9
Keywords: neuroblastoma, minimal residual disease (MRD), cancer stem cell (CSC), circulating tumor cell (CTC), disseminating tumor cell (DTC), circulating tumor DNA (ctDNA), epithelial-mesenchymal transition (EMT)
Citation: Uemura S, Ishida T, Thwin KKM, Yamamoto N, Tamura A, Kishimoto K, Hasegawa D, Kosaka Y, Nino N, Lin KS, Takafuji S, Mori T, Iijima K and Nishimura N (2019) Dynamics of Minimal Residual Disease in Neuroblastoma Patients. Front. Oncol. 9:455. doi: 10.3389/fonc.2019.00455
Received: 10 March 2019; Accepted: 14 May 2019;
Published: 04 June 2019.
Edited by:
Rimas J. Orentas, Seattle Children's Research Institute, United StatesReviewed by:
Mark Applebaum, University of Chicago, United StatesAlexander Popov, Dmitry Rogachev National Research Center of Pediatric Hematology, Oncology and Immunology, Russia
Copyright © 2019 Uemura, Ishida, Thwin, Yamamoto, Tamura, Kishimoto, Hasegawa, Kosaka, Nino, Lin, Takafuji, Mori, Iijima and Nishimura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noriyuki Nishimura, nnishi@med.kobe-u.ac.jp
 Suguru Uemura
Suguru Uemura Toshiaki Ishida
Toshiaki Ishida Khin Kyae Mon Thwin
Khin Kyae Mon Thwin Nobuyuki Yamamoto1
Nobuyuki Yamamoto1 Kyaw San Lin
Kyaw San Lin Satoru Takafuji
Satoru Takafuji Takeshi Mori
Takeshi Mori Kazumoto Iijima
Kazumoto Iijima Noriyuki Nishimura
Noriyuki Nishimura