- 1Department of Urology, Seoul National University Hospital, Seoul, South Korea
- 2College of Medicine, Seoul National University, Seoul, South Korea
Urothelial carcinoma (UC) can occur in various parts of the urinary tract and occurs in different stages and grades. The disease recurs frequently and is monitored through a series of invasive tests, such as cystoscopy or ureteroscopy, over the lifetime of an individual. Although many researchers have attempted to stratify the risks of UC, with the majority being based on cancer characteristics and host factors such as performance status, a risk classification system has yet to be fully developed. Cancer affects various parts of the body through the systemic immune response, including changes in hormones, the number and ratio of white blood cells and platelets, and C-reactive protein (CRP) or albumin levels under the influence of neuroendocrine metabolism, hematopoietic function, and protein and energy metabolism, respectively. Herein, we reviewed various systemic inflammatory response markers (SIRs) related to UC, including CRP, albumin-globulin ratio, albumin, Glasgow prognostic score (GPS), modified GPS, neutrophil-lymphocyte ratio, and platelet-lymphocyte ratio. Our aim was to summarize the role of various SIRs in the treatment of patients with UC.
Introduction
Urothelial carcinoma (UC) is the fourth most common cancer (1). The cancer is classified based on the site of occurrence as bladder UC (BC) and upper urinary tract UC (UTUC) (1–4). BC accounts for 90–95% of all UCs, (1–4) and is the fourth most common cancer among men in the United States, ranking eighth in terms of mortality (4). UC can occur in various parts of the urinary tract and in different stages and grades (2–4). Most UCs start in the bladder, and 75% of the patients are initially diagnosed with non-muscle invasive bladder cancer (NMIBC) (3, 4). Endoscopic surgery, intravesical therapy, and immunotherapy can be performed for the treatment of NMIBC (3). A combination of radical cystectomy (RC), radiation therapy (RT), chemotherapy, and immunotherapy among other treatments have been considered in the standard management of MIBC (2). UTUC demonstrates twice as many pyelocalyx as ureters. More than 60% of the patients have been reported to present with invasive cancer at the time of diagnosis and 15–25% have BC (4). Radical nephroureterectomy (RNU) is the standard treatment of UTUC. A 22–47% recurrence rate of the cancer has been reported in the bladder in contrast to 2–6% in the opposite upper tract (4). Local and distant metastases are associated with a poor prognosis despite the availability of various treatment options (2–4). A study reported that the 5-years relative survival rate of patients with BC, diagnosed from 2009 to 2015, was ~77%, with the occurrence of regional and distant metastases in 38 and 5% of the cases, respectively (1). In contrast, the upper tract variant is a relatively rare disease that accounts for 5–10% of all UCs (4). Considering the high relapse rate, the cost of treatment of BC from diagnosis to death, with repeated surgeries and investigations, is the highest among all UCs (2–4). Appropriate risk stratification and prediction of prognosis are important to determine the necessary treatment protocols based on the characteristics of UC (2–4). Accordingly, several studies have assessed the role of systemic inflammatory response markers (SIRs) in the prediction of the progression and prognosis of UC. C-reactive protein (CRP), albumin, albumin-globulin ratio (AGR), Glasgow prognostic score (GPS), modified GPS (mGPS), neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) have been studied as potential prognostic factors. Herein, we reviewed studies on different SIRs for the diagnosis and treatment of UC.
Sir Markers in Cancer
Cancer progression and prognosis are affected by a variety of factors. Representative parameters in the treatment of cancers are the characteristics of the tumor associated with prognosis. These include the pathological variant, stratified grade and stage, and the imaging stage, which are considered in the measurement of disease progression. However, in addition to these characteristics, status of the patient, including performance status, degree of systemic inflammatory response, muscle mass, and weight status (sarcopenia or cachexia) are also important parameters to be considered. Inflammation plays an important role in the development and progression of cancers as well as in the patients' response to therapy (5). Tumors consist of various types of inflammatory and immune cells (6), which can activate immune cells to produce cytokines, chemokines, growth factors, and prostaglandins (6, 7). The inflammatory microenvironment is an essential component of tumorigenesis since most cancers trigger an inflammatory response by building a pro-tumorigenic microenvironment (6, 8). The resultant inflammation affects the host immune response to the tumor (6, 8). Potential SIR-related biomarkers in cancer patients include as CRP, NLR, PLR, albumin and GPS.
Level of CRP increases blood circulation in various immunologically mediated inflammatory conditions, including trauma, infection, surgery, burns, allergic reactions, and cancer (9). CRP is primarily synthesized in the liver and is a major component of the innate immune system. It is involved in the initial immunity during infection and in the process of cell death (10). CRP binds to phosphocholine on the surface of damaged and foreign cells and bacteria, activating phagocytosis by macrophages (10). The normal concentration of CRP in healthy adults is between 0.8 and 3.0 mg/L. However, the level of this protein increases during the acute phase of inflammation (10). Depending on the cause, levels of CRP increase to over 50–100 mg/L in 4–6 h and have also been reported to increase over 1000-fold (10). The concentration of CRP is typically maintained in the range of 2–10 mg/L in individuals with chronic inflammation (10).
It is known that secretion of CRP is primarily regulated by IL-6; however, its mechanism has not been fully elucidated. There is lack of clarity on whether elevation of CRP is related to the inflammatory factors released by the tumor or due to a compromised host immune response. Although there is no clear evidence of the diagnostic or etiological role of CRP in cancer, several studies have reported higher levels of this protein in cancer patients than in healthy individuals (11). Moreover, high baseline levels and specific single nucleotide polymorphisms of CRP have been previously associated with an increased risk of cancer (11). Many recent studies have reported an association between high CRP levels and poor prognosis as well as progressive disease in patients with a variety of cancers, including lung, kidney, colorectal, breast, and ovarian cancers (11).
NLR, another marker of reactions to inflammatory conditions. An absolute NLR ratio has not yet been defined owing to the lack of clarity of the association between high NLR and poor survival in cancer patients. In general, neutrophils provide rapid defense against microorganisms in the infected area during a superficial infection (12). Similarly, in cancers, neutrophils are displaced around tumor cells in response to cytokines and chemical attractants to participate in the initial inflammatory response (12). They recruit CD8+ T cells and promote an anti-tumor response by inducing the production of cytokines, such as TNF-α or IL-12 (12). In contrast, several phenotypes of neutrophil exist, which can promote the formation of tumors via their involvement in immunosuppression mediated by TGF-β (12). Neutrophils stimulated with G-CSF were found to improve the growth of circulating tumor cells in metastatic sites and were involved in angiogenesis caused by cancer (12). Many studies have been published recently on the role of NLR in patients with UC (12). A high NLR was reported to be associated with poor prognosis in cancer patients (12).
Albumin, another marker of reactions to inflammatory conditions. Serum albumin and globulin are the major components of blood plasma (13). Albumin is predominantly produced by the liver. Globulins are produced by the liver as well as the immune system. The main function of albumin is to regulate the volume of blood by maintaining the colloid pressure of blood (13). Infections, burns, liver disease, nephrotic syndrome, and malignant tumors decrease serum albumin levels (13). Normal serum albumin levels range from 3.5 to 5 g/dL and that of serum globulin are 2.6–4.6 g/dL (13). The association between albumin levels and the prognosis of patients has been reported in various cancers, including ovarian, colorectal, and lung cancers, in addition to UC (14). Globulin is composed of various pro-inflammatory proteins such as complement components and immunoglobulins and plays an important role in immunity and inflammation (14). Levels of globulin increase in patients with chronic inflammation or cancer. Albumin and globulin are associated with immune responses in cancer patients as well as the nutritional status (14). Low albumin levels reflect a decline in the status of nutrients, which is common in cancer patients, and is known to interfere with immune mechanisms such as humoral and cellular immunity and phagocytic function (14). Hypoalbuminemia in cancer patients has been reported to result from a reduction in albumin synthesis mediated by secretion of pro-inflammatory cytokines, increased catabolism, and cachexia (14). Increased vascular permeability in these patients also reduces blood albumin levels due to redistribution of the protein from the intravascular to the interstitial areas (14).
Inflammatory cytokines or chemokines are responsible for the activation of platelets (15). Activated platelets induce leukocytes to accumulate in inflamed regions (15). Platelets interact with leukocytes and induce a bidirectional cell activation process, wherein the platelets activate leukocytes, and various leukocyte-derived molecules activate platelets (15). Activated platelets stimulate neutrophils, which in turn release chromatin (15). Chromatin further recruits and activates platelets (15). Cancer cells in the bloodstream induce platelet-mediated recognition (16). Platelets are amplified by a variety of immune cells, cell products, cell surface receptors, and extracellular factors (16). Under certain circumstances, the interaction between cancer cells and platelets can suppress the recognition or removal of cancer cells by immune cells (16).
C-Reactive Protein
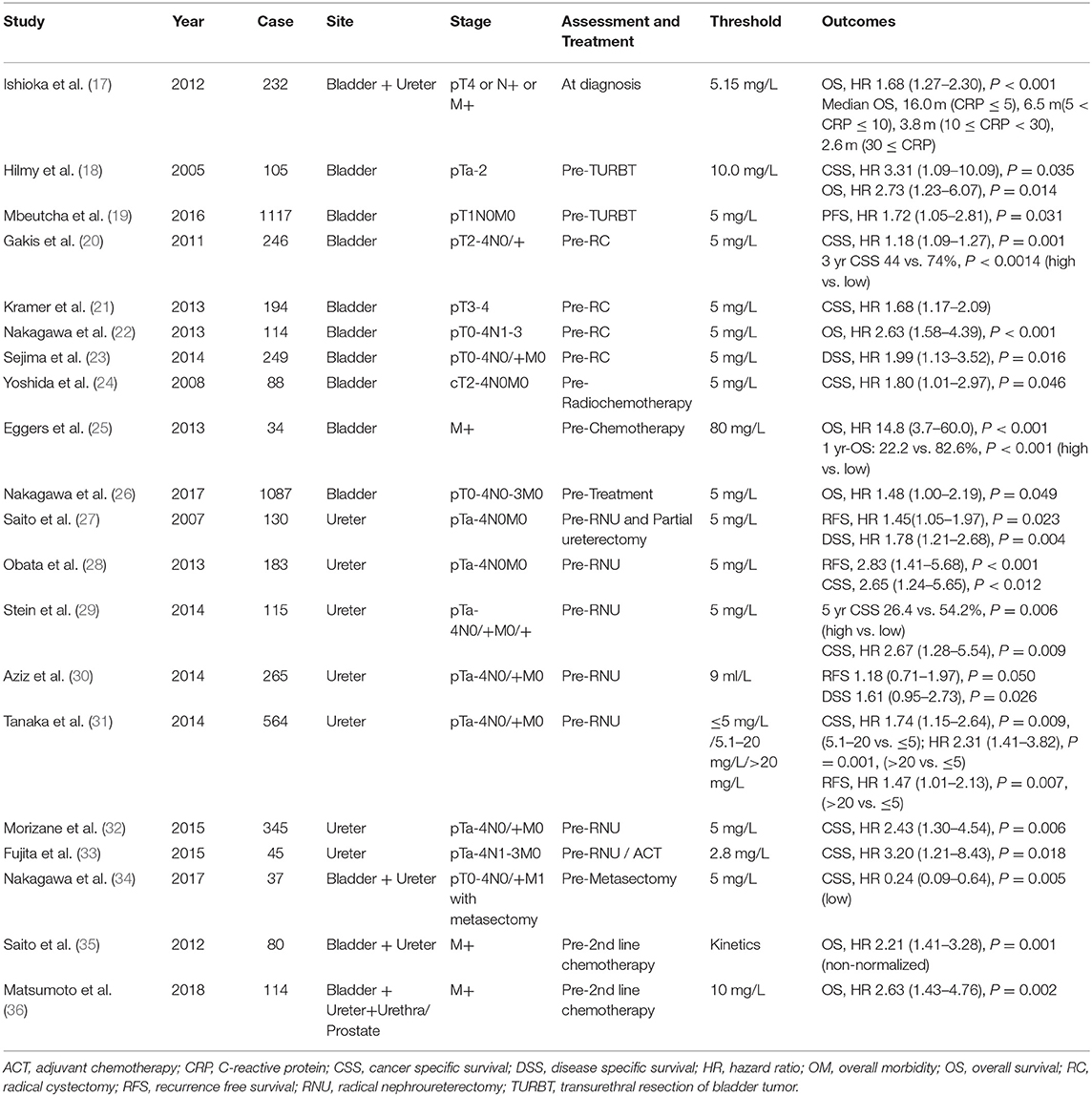
Several studies on UC have reported that elevated CRP levels before surgery or chemotherapy were associated with poor prognosis (Table 1) (17–36). The cutoff value for CRP varies from 3.4 to 10 mg/L. While most studies have compared their results with the prognosis for RNU or RC preoperative evaluation (21–24, 28–34), Yoshida et al. (24), and Egger et al. (25) compared the prognosis with a pre-cancer evaluation. Yoshida et al. included 88 patients with MIBC and reported that a high level of CRP before treatment was a factor in predicting the 5-years cancer specific survival (CSS) after chemoradiotherapy (24). Egger et al. showed that CRP levels before chemotherapy in patients with metastatic BC correlated with patient- and cancer-specific characteristics. The median progression-free survival (PFS) in patients with high CRP levels was extended by 1.5 months compared to patients with low CRP levels. Furthermore, the 1-year overall survival (OS) rate was higher at 60.4% (25). Nakagawa et al. evaluated the prognosis of patients with metastatic UC based on preoperative levels of CRP (34). The study reported that higher pre-metastasectomy levels of CRP was associated with worse prognosis [hazard ratio (HR) 0.24, P = 0.005]. Therefore, the authors suggested evaluation of CRP levels when considering metastasectomy (34).
CRP is considered a clinically significant biomarker based on current evidence and a static variable in predicting prognosis. However, CRP kinetics has been considered a variable to measure prognosis; therefore, its potential as a dynamic variable has also been reported. Saito et al. measured CRP levels before and after second-line chemotherapy and not before surgery (35). The prognosis was evaluated based on the kinetics of CRP. In the umbilical evaluation, patients were divided into groups based on CRP levels greater or lesser than 5 mg/L. Patients with preoperative CRP levels of ≥5 mg/L were categorized as the non-normalized group, and those with levels ≤5 mg/L were considered the normalized group. The study reported that patients in the non-normalized group demonstrated poor prognosis before and after surgery (HR 2.21, P = 0.001). Tanaka et al. also compared the prognosis of individuals with CRP levels ≤ 5, 5.1–20, and ≥20 mg/L. Patients with CRP levels >20 mg/L had poor CSS and recurrence-free survival (RFS) compared to those with values ≤ 5 mg/L (HR 1.74, 1.47, P = 0.0009 and 0.007, respectively) (31).
Neutrophil-to-Lymphocyte Ratio
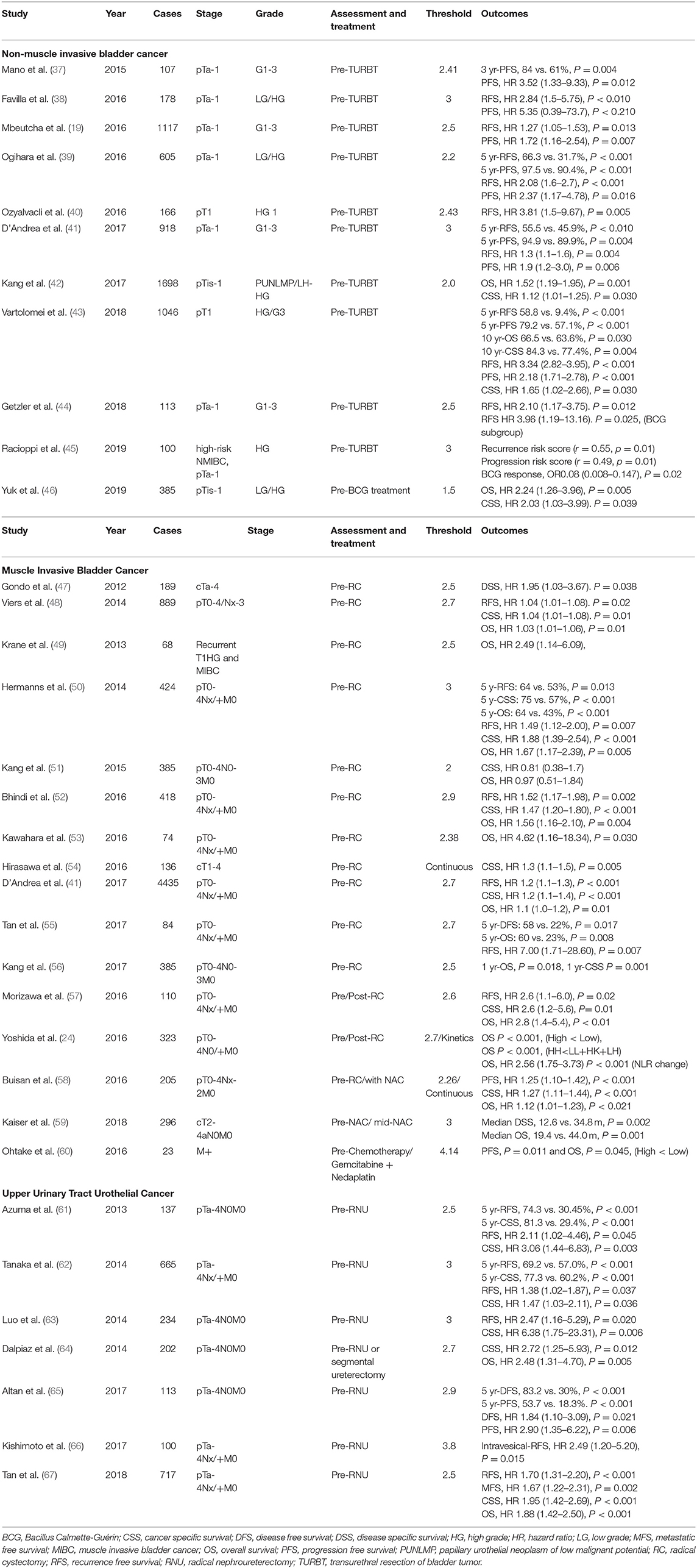
Several papers have reported on the prognostic role of NLR in NMIBC (Table 2) (19, 37–46). Most of these studies have reported on the relationship between NLR, RFS, and PFS before transurethral resection of bladder tumor (TURBT) (19, 37–45). Vartolomei et al. and Mbeutcha et al. analyzed NLR in more than 1,000 patients (19, 43). Mbeutcha et al. reported that a high NLR was associated with RFS (HR 1.27, P = 0.013), which was reported to be related to PFS (HR 1.72, P = 0.007) (19). Similarly, Vartolomei et al. demonstrated high NLR and low NLR (58.8 and 9.4%) for 5-years RFS (P < 0.001), 79.2 and 57.1%) for 5-years PFS (P < 0.001), and 84.3 and 77.4% for 10-years CSS (P = 0.004) (43). A high NLR was associated with RFS (HR 3.34, P < 0.001), PFS (HR 2.18, P < 0.001), and CSS (HR 1.65, P = 0.030) (68). In addition, Yuk et al. reported on the relationship between OS (HR 2.24, P = 0.005) and CSS (HR 2.03. P = 0.039) based on the NLR in patients before BCG treatment (46).
Several studies have reported on the prognostic role of NLR in MIBC and metastatic BC (Table 2) (24, 41, 47–60). Furthermore, several studies have reported on the role of prognostic factors in survival outcomes of MIBC and metastatic UC (24, 41, 47, 48, 50, 52–56, 59, 60), while other studies have suggested that the postoperative stages or disease severity can be predicted (Table 3) (41, 48, 49, 58, 69–73). The majority of the studies reported a positive role of a high NLR on the prediction of worse OS and CSS rates. Furthermore, elevated NLR has been reported as an independent predictor of recurrence in several studies (32, 41, 50, 52, 55), with some reporting higher NLR values in patients with MIBC compared to those with NMIBC (69, 70). Other studies have reported on considering the NLR in predicting disease severity, such as extravesical involvement, upstaging, or LN involvement (48, 49, 55). Furthermore, some studies have considered the NLR to predict the pathologic complete response after chemotherapy or surgery (58, 72, 73). Seah et al. analyzed NLR kinetics before, during, and after NAC and reported differences in the patterns between the CR and non-responder groups (72). Although the role of the preoperative evaluation of NLR has been commonly discussed, some studies have assessed the prognostic value of changes in the ratio through repeated measurements over time (56). Kang et al. reported that postoperative NLR increases were associated with adverse pathological outcomes and were predictors of worse OS and CSS (51). Patients with consistently elevated NLR before and after surgery had worse OS and CSS compared to other patients (51). Yoshida et al. predicted patient prognosis by comparing the NLR before and after surgery (24). Patients with high and low NLRs were categorized based on their preoperative and postoperative values, respectively. Patients were divided into four groups according to the change in NLR before and after surgery. Patients with consistently high NLR before and after surgery demonstrated worse prognosis compared to other patients (24). The degree of change in the NLR before and after surgery was reported to be related to OS (24). In addition, Kiser et al. measured and analyzed the NLR at the beginning of and intermediately during NAC and reported differences in median DSS (12.6 vs. 34.8 m, P = 0.002) and median OS (19.4 vs. 44.0 m, P = 0.001) (59). One study analyzed the differences in the NLR over a period of 1–5 years after surgery and reported significant results of 1-year OS (P = 0.018) and 1-year CSS (P = 0.001) rates; however, there was no difference in the rates due to differences in the NLR from the 2nd year onwards (56). The majority of the studies have reported that the NLR before and after surgery or chemotherapy can be used to predict survival outcomes, pathological findings, and disease progression. Thus, measurement of the NLR before and after treatment is a reliable tool for predicting postoperative survival outcomes in patients with MIBC.
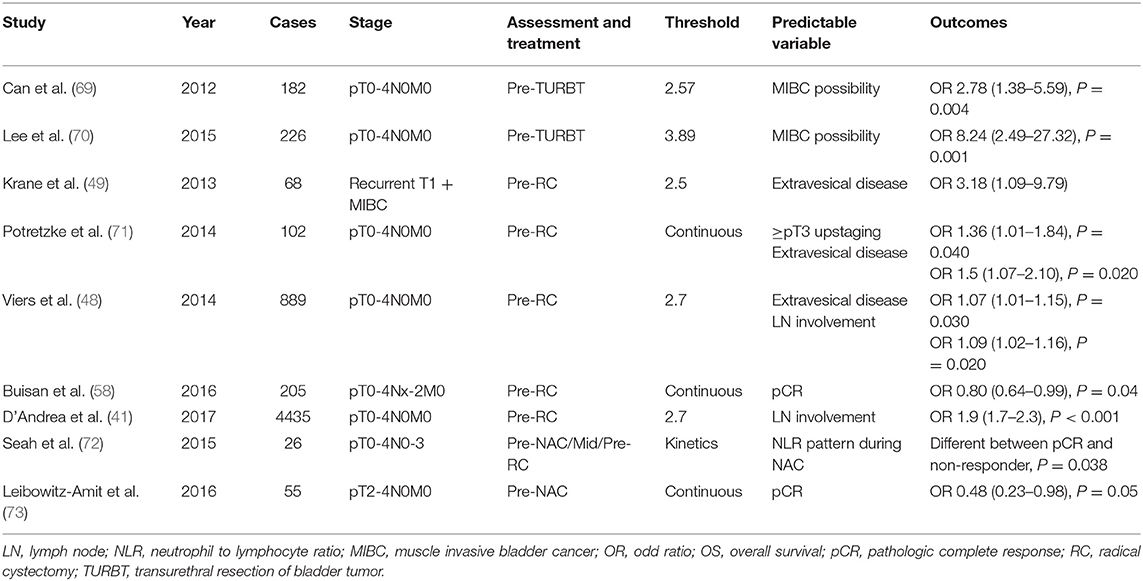
Table 3. The studies evaluating the role of neutrophil to lymphocyte of urothelial cancer pathologic staging.
Several studies have reported that preoperative assessment of the NLR can be considered to predict the survival outcomes in patients with UTUC (Table 2) (61–67), with a cutoff value between 2.5 and 3. Kishimoto et al. evaluated the predictive ability of the NLR before RNU on intra-bladder recurrence (66). The study reported that a high preoperative NLR was associated with an increased risk of recurrence of BC (HR 2.49, P = 0.015). The majority of the studies have reported a link between the CSS and RFS (101, 103, 105, 106). The European Association of Urology proposes the NLR as a prognostic factor for CSS in patients with UTUC (4). However, the evidence supporting prognostic role of NLR in UTUC remains low, and currently there are no guidelines of risk stratification (4).
Studies that evaluated the prognostic value of the NLR before chemotherapy for advanced or metastatic disease, wherein surgery was inappropriate, mainly reported on patients with BC and UTUC (Table 4) (55, 74–77), with a cutoff value of 3. Interestingly, one study evaluated the prognostic value of NLR kinetics before and after chemotherapy. A high NLR before chemotherapy helped predict poor survival outcomes (76).
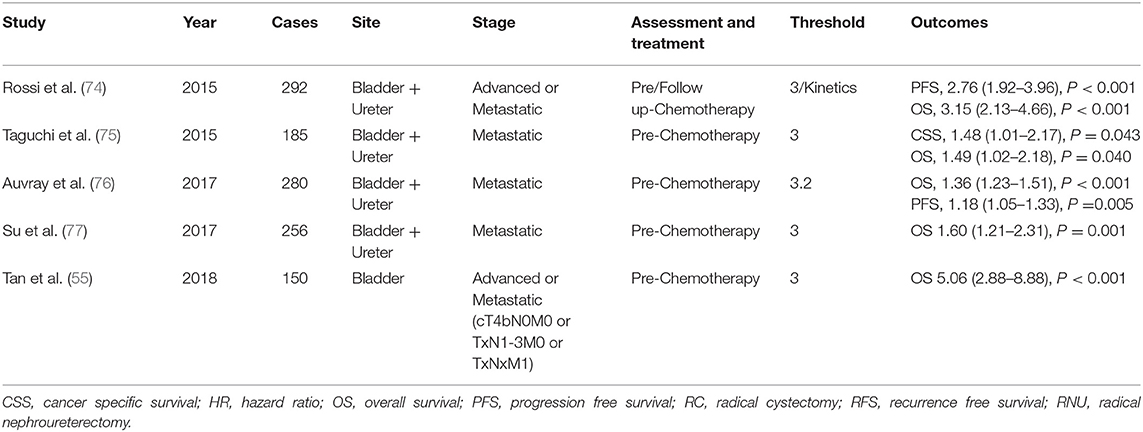
Table 4. The studies evaluating the role of neutrophil to lymphocyte in urothelial cancer on chemotherapy.
Albumin and Albumin-Globulin Ratio
Several studies have reported on the association of hypoalbuminemia with poor prognosis in patients with UC (Table 5) (49, 68, 72, 78–91). The cutoff value of albumin varies from 2 to 4 mg/L, although it is normally around 3.5 mg/L. Many studies have reported on the role of preoperative evaluation of albumin levels on the prognosis of patients. Hwang et al. reported that presence of hypoalbuminemia before chemotherapy was associated with a worse DFS (HR 2.04, P = 0.023) in patients with metastatic BC (83). Laurent et al. reported an increase in the 1-year mortality rate after chemotherapy (HR 3.06, P < 0.001) in patients with hypoalbuminemia before the treatment (82).
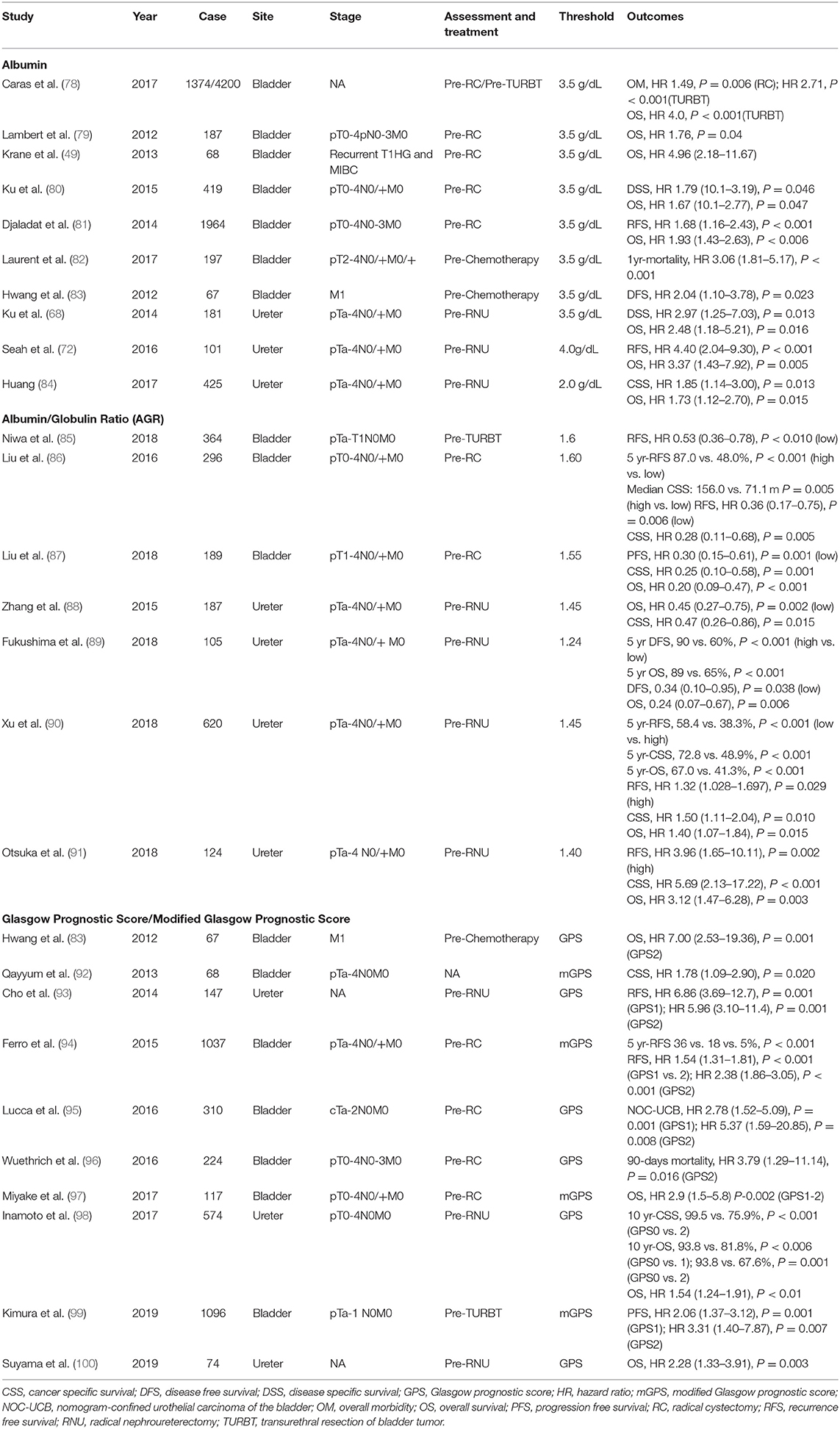
Table 5. The studies evaluating the role of albumin and Glasgow prognostic score in urothelial cancer.
AGR is the ratio of albumin and total proteins to albumin. The assessment of both albumin and globulin is believed to provide a greater prognostic insight than albumin alone. However, considering the limited number of studies, the utility of this combination remains to be established. Recent studies have reported on the prognostic effect of AGR in patients with UC (85–91).
Glasgow Prognostic Score and Modified Glasgow Prognostic Score
The GPS and mGPS are a combination of CRP and albumin scores, representing methods designed to predict the prognosis of cancer patients. The GPS system is an indicator of the nutritional status of an individual based on systemic inflammation. McMillan et al. first introduced GPS for predicting the prognosis of patients with non-small cell lung cancer (101). Since then, GPS has proved to be useful in predicting the prognosis of patients with a variety of cancers, including colorectal, esophageal, lung, gastric, pancreatic, and liver cancers (101). Recently, few studies recorded the aforementioned scores before chemotherapy and surgery in patients with UC (Table 5) (83, 92–100). The majority of the preoperative evaluation studies were performed on patients undergoing RC and RNU. Preoperative GPS or mGPS were reported as factors predicting survival outcomes such as OS, CSS, and RFS. Ferro et al. conducted a study on 1,037 patients undergoing RC and reported longer RFS in the group with higher preoperative mGPS. Furthermore, the group with the highest mGPS demonstrated ~30% longer RFS compared to those with the lowest scores (94). Inamoto et al. conducted a study on 574 patients undergoing RNU and reported that patients with high GPS before surgery demonstrated 23.6% longer 10-years CSS and 12% longer 10-years OS compared to patients with low GPS (98). One study recorded preoperative GPS in patients undergoing TURB. Kimura et al. conducted a study on 1,096 patients undergoing TURB and reported that preoperative mGPS helped in the prediction of PFS after surgery (99). Another study recorded GPS before chemotherapy in patients with M1 BC. Hwang et al. reported that high GPS levels before chemotherapy were indicative of poor survival (HR 7.0, P = 0.001) (83).
Platelet-to-Lymphocyte Ratio
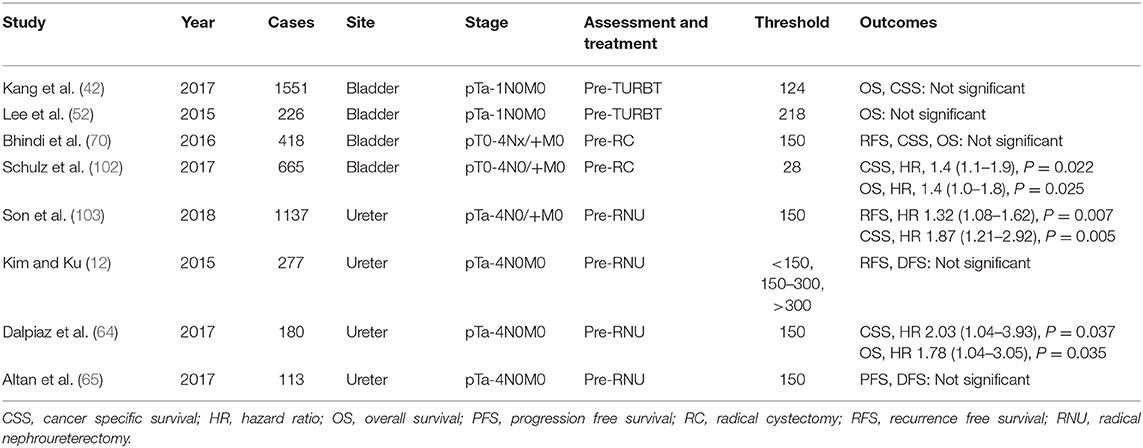
Several studies have reported on the prognostic value of the preoperative assessment of the PLR (Table 6) (12, 42, 52, 64, 65, 70, 102, 103), wherein a high ratio has been reported to be associated with poor OS, CSS, and RFS. The cutoff value for PLR varies from 123 to 218. Interestingly, Kim and Ku categorized the PLR into three sections with two values, 150 and 300, but did not observe any relationship with the OS of the patients (12).
Discussion
Since hematologic tests in cancer patients are basic and frequently repeated, SIR biomarkers using them can be easily obtained in cancer patients and used as economic and objective parameters. In the UC, in the system for predicting the existing prognosis or in nomogram and risk stratification studies, hematologic factors predicting pathological prognoses such as the number of tumors, tumor diameter, tumor grade, T stage, CIS, and LIV rather than biomarkers I mainly used them (12, 17, 26, 47, 95). However, in the case of clinical staging, the upstaging rate in pathological results after RC reaches 50%, and its accuracy is still low (12). Therefore, there is a need for other biomarkers to predict the prognosis of patients before treatment and to stratify risk, and for this purpose, SIR-related hematologic biomarkers can be a potential and effective factor. In the UC, the SIR biomarker is known to play an essential role in the progression and various oncologic outcomes, even though the inflammatory process develops cancer. In particular, CRP and NLR seem to be particularly useful for these oncologic outcomes and as predictors. And Albumin, AGP GPS, mGPS, PLR, etc. are potential factors, but they still lack data. The standard treatment for NMIBC is TURBT (2). However, due to frequent relapses and progression to MIBC after TURBT, regular examinations, and long-term follow-up are required (2). This repeated and long-term follow-up is putting a high economic burden on the patient (2). In addition, cystoscopy, the most representative test method, is an invasive test that causes significant pain and discomfort to the patient. Based on previous study results, patients with high CRP before surgery in NMIBC have a significant correlation with adverse outcomes in treatment outcomes and survival (18, 19). NLR had a high rate of pathologic upstaging in patients with increased NLR before TURBT (37–45), and the risk of progression to MIBC was also high (69, 70). SIR biomarkers can also be of great help in predicting the prognosis of NMIBC patients and determining the duration or method of follow-up and re-TURB. For MIBC, the standard treatment is RC. There are also various supplementary treatments such as chemotherapy, RT, and immunotherapy. In MIBC (3), patients with high CRP before surgery have a significant correlation with adverse outcomes in treatment outcomes and survival. And NLR was relatively low in complete response and pathologic T0 in patients with high NLR before RC or before NAC (47–60). And increased NLR levels during 1–3 months post RC were associated with adverse survival outcomes (12). The SIR biomarker may be helpful in MIBC patients considering the sequence and timing of treatment such as immediate RC and NAC after prognosis. And the patterns of change in NLRs before and after surgery can also provide useful information for determining additional target groups, such as adjuvant chemotherapy, radiation therapy, and immunotherapy.
In addition to the systemic inflammatory response, CRP is a biomarker that predicts the outcome of IL-2 or IFN-α immunotherapy and has been considered in the evaluation of patients with renal cancer (104). Patients with normal CRP have been reported to demonstrate good tolerance and adherence to immunotherapy (104). The scope of immunotherapy is expanding in patients with UC, and CRP could be considered a factor in predicting the prognosis and compliance to treatment.
The limitations of SIR biomarker's research at UC are: First, there is no objective and clear cut off value. Second, unlike pathological prognostic factors, SIR biomarkers may be affected by other inflammatory diseases or physical conditions other than the patient's cancer. And third, due to the nature of the research, most studies are retrospective and lack of large-scale prospective research results.
Conclusion
In this review, we discussed the applicability of CRP, AGR, albumin, GPS, mGPS, NLR, and PLR as SIRs in patients with UC. These SIRs have proven to be useful during preoperative evaluations in the treatment and risk assessment of patients with UC. However, there is a need to develop uniform classification criteria with a consensus regarding the use of these SIRs in clinical settings. We believe that large prospective studies are needed on this subject based on observations of this retrospective review.
Author Contributions
HY and JK contributed to the concept, project planning, and contributed significantly to the writing of the manuscript. JK contributed significantly in the editing of the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1F1A1050507).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. (2017) 71:462–75. doi: 10.1016/j.eururo.2016.06.020
3. Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European association of urology guidelines on non-muscle-invasive bladder cancer. (TaT1 and carcinoma in situ) - 2019 update. Eur Urol. (2019) 76:639–57. doi: 10.1016/j.eururo.2019.08.016
4. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. (2018) 73:111–22. doi: 10.1016/j.eururo.2017.07.036
5. Liccardi G, Pentimalli F. Cancer, immunity and inflammation. report from the CDD cambridge conferences 2018 and 2019. Cell Death Dis. (2019) 10:798. doi: 10.1038/s41419-019-2032-0
6. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
7. Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. (2008) 43:374–9. doi: 10.1016/j.cyto.2008.07.014
8. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
9. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. (1999) 340:448–54. doi: 10.1056/NEJM199902113400607
10. Bray C, Bell LN, Liang H, Haykal R, Kaiksow F, Mazza JJ, et al. Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine. WMJ. (2016) 115:317–21.
11. Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-Reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS ONE. (2015) 10:e0143080. doi: 10.1371/journal.pone.0143080
12. Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Dis Markers. (2016) 2016:8345286. doi: 10.1155/2016/8345286
13. Farrugia A. Albumin usage in clinical medicine: tradition or therapeutic? Transfus Med Rev. (2010) 24:53–63. doi: 10.1016/j.tmrv.2009.09.005
14. Moujaess E, Fakhoury M, Assi T, Elias H, El Karak F, Ghosn M, et al. The therapeutic use of human albumin in cancer patients' management. Crit Rev Oncol Hematol. (2017) 120:203–9. doi: 10.1016/j.critrevonc.2017.11.008
15. Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. (2015) 114:449–58. doi: 10.1160/TH14-12-1067
16. Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. (2014) 33:231–69. doi: 10.1007/s10555-014-9498-0
17. Ishioka J, Saito K, Sakura M, Yokoyama M, Matsuoka Y, Numao N, et al. Development of a nomogram incorporating serum C-reactive protein level to predict overall survival of patients with advanced urothelial carcinoma and its evaluation by decision curve analysis. Br J Cancer. (2012) 107:1031–6. doi: 10.1038/bjc.2012.254
18. Hilmy M, Bartlett JM, Underwood MA, McMillan DC. The relationship between the systemic inflammatory response and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. (2005) 92:625–7. doi: 10.1038/sj.bjc.6602406
19. Mbeutcha A, Shariat SF, Rieken M, Rink M, Xylinas E, Seitz C, et al. Prognostic significance of markers of systemic inflammatory response in patients with non-muscle-invasive bladder cancer. Urol Oncol. (2016) 34:483.e17–24. doi: 10.1016/j.urolonc.2016.05.013
20. Gakis G, Todenhofer T, Renninger M, Schilling D, Sievert KD, Schwentner C, et al. Development of a new outcome prediction model in carcinoma invading the bladder based on preoperative serum C-reactive protein and standard pathological risk factors: the TNR-C score. BJU Int. (2011) 108:1800–5. doi: 10.1111/j.1464-410X.2011.10234.x
21. Kramer MW, Heinisch A, Wegener G, Abbas M, von Klot C, Peters I, et al. [C-reactive protein prior to radical cystectomy: preoperative determination of CRP]. Urologe A. (2014) 53:222–7. doi: 10.1007/s00120-013-3299-x
22. Nakagawa T, Hara T, Kawahara T, Ogata Y, Nakanishi H, Komiyama M, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol. (2013) 189:1275–81. doi: 10.1016/j.juro.2012.10.065
23. Sejima T, Morizane S, Yao A, Isoyama T, Saito M, Amisaki T, et al. Prognostic impact of preoperative hematological disorders and a risk stratification model in bladder cancer patients treated with radical cystectomy. Int J Urol. (2014) 21:52–7. doi: 10.1111/iju.12161
24. Yoshida S, Saito K, Koga F, Yokoyama M, Kageyama Y, Masuda H, et al. C-reactive protein level predicts prognosis in patients with muscle-invasive bladder cancer treated with chemoradiotherapy. BJU Int. (2008) 101:978–81. doi: 10.1111/j.1464-410X.2007.07408.x
25. Eggers H, Seidel C, Schrader AJ, Lehmann R, Wegener G, Kuczyk MA, et al. Serum C-reactive protein: a prognostic factor in metastatic urothelial cancer of the bladder. Med Oncol. (2013) 30:705. doi: 10.1007/s12032-013-0705-6
26. Nakagawa T, Taguchi S, Uemura Y, Kanatani A, Ikeda M, Matsumoto A, et al. Nomogram for predicting survival of postcystectomy recurrent urothelial carcinoma of the bladder. Urol Oncol. (2017) 35:457.e15–21. doi: 10.1016/j.urolonc.2016.12.010
27. Saito K, Kawakami S, Ohtsuka Y, Fujii Y, Masuda H, Kumagai J, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with upper urinary tract urothelial carcinoma treated surgically. BJU Int. (2007) 100:269–73. doi: 10.1111/j.1464-410X.2007.06934.x
28. Obata J, Kikuchi E, Tanaka N, Matsumoto K, Hayakawa N, Ide H, et al. C-reactive protein: a biomarker of survival in patients with localized upper tract urothelial carcinoma treated with radical nephroureterectomy. Urol Oncol. (2013) 31:1725–30. doi: 10.1016/j.urolonc.2012.05.008
29. Stein B, Schrader AJ, Wegener G, Seidel C, Kuczyk MA, Steffens S. Preoperative serum C- reactive protein: a prognostic marker in patients with upper urinary tract urothelial carcinoma. BMC Cancer. (2013) 13:101. doi: 10.1186/1471-2407-13-101
30. Aziz A, Rink M, Gakis G, Kluth LA, Dechet C, Miller F, et al. Preoperative C-reactive protein in the serum: a prognostic biomarker for upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Urol Int. (2014) 93:352–60. doi: 10.1159/000362248
31. Tanaka N, Kikuchi E, Shirotake S, Kanao K, Matsumoto K, Kobayashi H, et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: a multi-institutional study. Eur Urol. (2014) 65:227–34. doi: 10.1016/j.eururo.2012.11.050
32. Morizane S, Yumioka T, Yamaguchi N, Masago T, Honda M, Sejima T, et al. Risk stratification model, including preoperative serum C-reactive protein and estimated glomerular filtration rate levels, in patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Int Urol Nephrol. (2015) 47:1335–41. doi: 10.1007/s11255-015-1033-x
33. Fujita K, Inamoto T, Yamamoto Y, Tanigawa G, Nakayama M, Mori N, et al. Role of adjuvant chemotherapy for lymph node-positive upper tract urothelial carcinoma and the prognostic significance of C-reactive protein: a multi-institutional, retrospective study. Int J Urol. (2015) 22:1006–12. doi: 10.1111/iju.12868
34. Nakagawa T, Taguchi S, Kanatani A, Kawai T, Ikeda M, Urakami S, et al. Oncologic outcome of metastasectomy for urothelial carcinoma: who is the best candidate? Ann Surg Oncol. (2017) 24:2794–800. doi: 10.1245/s10434-017-5970-8
35. Saito K, Urakami S, Komai Y, Yasuda Y, Kubo Y, Kitsukawa S, et al. Impact of C-reactive protein kinetics on survival of patients with advanced urothelial carcinoma treated by second-line chemotherapy with gemcitabine, etoposide and cisplatin. BJU Int. (2012) 110:1478–84. doi: 10.1111/j.1464-410X.2012.11153.x
36. Matsumoto R, Abe T, Ishizaki J, Kikuchi H, Harabayashi T, Minami K, et al. Outcome and prognostic factors in metastatic urothelial carcinoma patients receiving second-line chemotherapy: an analysis of real-world clinical practice data in Japan. Jpn J Clin Oncol. (2018) 48:771–6. doi: 10.1093/jjco/hyy094
37. Mano R, Baniel J, Shoshany O, Margel D, Bar-On T, Nativ O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. (2015) 33:67 e1–7. doi: 10.1016/j.urolonc.2014.06.010
38. Favilla V, Castelli T, Urzi D, Reale G, Privitera S, Salici A, et al. Neutrophil to lymphocyte ratio, a biomarker in non-muscle invasive bladder cancer: a single-institutional longitudinal study. Int Braz J Urol. (2016) 42:685–93. doi: 10.1590/S1677-5538.IBJU.2015.0243
39. Ogihara K, Kikuchi E, Yuge K, Yanai Y, Matsumoto K, Miyajima A, et al. The preoperative neutrophil-to-lymphocyte ratio is a novel biomarker for predicting worse clinical outcomes in non-muscle invasive bladder cancer patients with a previous history of smoking. Ann Surg Oncol. (2016) 23(Suppl.5):1039–47. doi: 10.1245/s10434-016-5578-4
40. Ozyalvacli ME, Ozyalvacli G, Kocaaslan R, Cecen K, Uyeturk U, Kemahli E, et al. Neutrophil-lymphocyte ratio as a predictor of recurrence and progression in patients with high-grade pT1 bladder cancer. Can Urol Assoc J. (2015) 9:E126–31. doi: 10.5489/cuaj.2523
41. D'Andrea D, Moschini M, Gust K, Abufaraj M, Ozsoy M, Mathieu R, et al. Prognostic role of neutrophil-to-lymphocyte ratio in primary non-muscle-invasive bladder cancer. Clin Genitourin Cancer. (2017) 15:e755–64. doi: 10.1016/j.clgc.2017.03.007
42. Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. Preoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget. (2017) 8:12891–901. doi: 10.18632/oncotarget.14179
43. Vartolomei MD, Ferro M, Cantiello F, Lucarelli G, Di Stasi S, Hurle R, et al. Validation of neutrophil-to-lymphocyte ratio in a multi-institutional cohort of patients with T1G3 non-muscle-invasive bladder cancer. Clin Genitourin Cancer. (2018) 16:445–52. doi: 10.1016/j.clgc.2018.07.003
44. Getzler I, Bahouth Z, Nativ O, Rubinstein J, Halachmi S. Preoperative neutrophil to lymphocyte ratio improves recurrence prediction of non-muscle invasive bladder cancer. BMC Urol. (2018) 18:90. doi: 10.1186/s12894-018-0404-x
45. Racioppi M, Di Gianfrancesco L, Ragonese M, Palermo G, Sacco E, Bassi PF. Can neutrophil-to-Lymphocyte ratio predict the response to BCG in high-risk non muscle invasive bladder cancer? Int Braz J Urol. (2019) 45:315–24. doi: 10.1590/s1677-5538.ibju.2018.0249
46. Yuk HD, Jeong CW, Kwak C, Kim HH, Ku JH. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in non-muscle invasive bladder cancer patients: initial intravesical bacillus calmette-guerin treatment after transurethral resection of bladder tumor setting. Front Oncol. (2018) 8:642. doi: 10.3389/fonc.2018.00642
47. Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. (2012) 79:1085–91. doi: 10.1016/j.urology.2011.11.070
48. Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. (2014) 66:1157–64. doi: 10.1016/j.eururo.2014.02.042
49. Krane LS, Richards KA, Kader AK, Davis R, Balaji KC, Hemal AK. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol. (2013) 27:1046–50. doi: 10.1089/end.2012.0606
50. Hermanns T, Bhindi B, Wei Y, Yu J, Noon AP, Richard PO, et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer. (2014) 111:444–51. doi: 10.1038/bjc.2014.305
51. Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. The prognostic significance of the early postoperative neutrophil-to-lymphocyte ratio in patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Ann Surg Oncol. (2016) 23:335–42. doi: 10.1245/s10434-015-4708-8
52. Bhindi B, Hermanns T, Wei Y, Yu J, Richard PO, Wettstein MS, et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. Br J Cancer. (2016) 114:207–12. doi: 10.1038/bjc.2015.432
53. Kawahara T, Furuya K, Nakamura M, Sakamaki K, Osaka K, Ito H, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in bladder cancer patients after radical cystectomy. BMC Cancer. (2016) 16:185. doi: 10.1186/s12885-016-2219-z
54. Hirasawa Y, Nakashima J, Yunaiyama D, Sugihara T, Gondo T, Nakagami Y, et al. Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. (2016) 23(Suppl.5):1048–54. doi: 10.1245/s10434-016-5606-4
55. Tan YG, Eu EWC, Huang HH, Lau WKO. High neutrophil-to-lymphocyte ratio predicts worse overall survival in patients with advanced/metastatic urothelial bladder cancer. Int J Urol. (2018) 25:232–8. doi: 10.1111/iju.13480
56. Kang M, Balpukov UJ, Jeong CW, Kwak C, Kim HH, Ku JH. Can the preoperative neutrophil-to-lymphocyte ratio significantly predict the conditional survival probability in muscle-invasive bladder cancer patients undergoing radical cystectomy? Clin Genitourin Cancer. (2017) 15:e411–20. doi: 10.1016/j.clgc.2016.10.015
57. Morizawa Y, Miyake M, Shimada K, Hori S, Tatsumi Y, Nakai Y, et al. Neutrophil-to-lymphocyte ratio as a detection marker of tumor recurrence in patients with muscle-invasive bladder cancer after radical cystectomy. Urol Oncol. (2016) 34:257 e11–7. doi: 10.1016/j.urolonc.2016.02.012
58. Buisan O, Orsola A, Areal J, Font A, Oliveira M, Martinez R, et al. Low pretreatment neutrophil-to-lymphocyte ratio predicts for good outcomes in patients receiving neoadjuvant chemotherapy before radical cystectomy for muscle invasive bladder cancer. Clin Genitourin Cancer. (2017) 15:145–51 e2. doi: 10.1016/j.clgc.2016.05.004
59. Kaiser J, Li H, North SA, Leibowitz-Amit R, Seah JA, Morshed N, et al. The prognostic role of the change in neutrophil-to-lymphocyte ratio during neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: a retrospective, multi-institutional study. Bladder Cancer. (2018) 4:185–94. doi: 10.3233/BLC-170133
60. Ohtake S, Kawahara T, Kasahara R, Ito H, Osaka K, Hattori Y, et al. Pretreatment neutrophil-to-lymphocyte ratio can predict the prognosis in bladder cancer patients who receive gemcitabine and nedaplatin therapy. Biomed Res Int. (2016) 2016:9846823. doi: 10.1155/2016/9846823
61. Azuma T, Matayoshi Y, Odani K, Sato Y, Sato Y, Nagase Y, et al. Preoperative neutrophil-lymphocyte ratio as an independent prognostic marker for patients with upper urinary tract urothelial carcinoma. Clin Genitourin Cancer. (2013) 11:337–41. doi: 10.1016/j.clgc.2013.04.003
62. Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Shirotake S, Miyazaki Y, et al. A multi-institutional validation of the prognostic value of the neutrophil-to-lymphocyte ratio for upper tract urothelial carcinoma treated with radical nephroureterectomy. Ann Surg Oncol. (2014) 21:4041–8. doi: 10.1245/s10434-014-3830-3
63. Luo HL, Chen YT, Chuang YC, Cheng YT, Lee WC, Kang CH, et al. Subclassification of upper urinary tract urothelial carcinoma by the neutrophil-to-lymphocyte ratio. (NLR) improves prediction of oncological outcome. BJU Int. (2014) 113:E144–9. doi: 10.1111/bju.12582
64. Dalpiaz O, Ehrlich GC, Mannweiler S, Hernandez JM, Gerger A, Stojakovic T, et al. Validation of pretreatment neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. BJU Int. (2014) 114:334–9. doi: 10.1111/bju.12441
65. Altan M, Haberal HB, Akdogan B, Ozen H. A critical prognostic analysis of neutrophil-lymphocyte ratio for patients undergoing nephroureterectomy due to upper urinary tract urothelial carcinoma. Int J Clin Oncol. (2017) 22:964–71. doi: 10.1007/s10147-017-1150-x
66. Kishimoto N, Takao T, Kuribayashi S, Yamamichi G, Nakano K, Kawamura M, et al. The neutrophil-to-lymphocyte ratio as a predictor of intravesical recurrence in patients with upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Int J Clin Oncol. (2017) 22:153–8. doi: 10.1007/s10147-016-1040-7
67. Tan P, Xu H, Liu L, Ai J, Xu H, Jiang Y, et al. The prognostic value of preoperative neutrophil-to-lymphocyte ratio in patients with upper tract urothelial carcinoma. Clin Chim Acta. (2018) 485:26–32. doi: 10.1016/j.cca.2018.06.019
68. Ku JH, Kang M, Kim HS, Jeong CW, Kwak C, Kim HH. The prognostic value of pretreatment of systemic inflammatory responses in patients with urothelial carcinoma undergoing radical cystectomy. Br J Cancer. (2015) 112:461–7. doi: 10.1038/bjc.2014.631
69. Can C, Baseskioglu B, Yilmaz M, Colak E, Ozen A, Yenilmez A. Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol Int. (2012) 89:468–72. doi: 10.1159/000343278
70. Lee SM, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores. (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol. (2015) 56:749–55. doi: 10.4111/kju.2015.56.11.749
71. Potretzke A, Hillman L, Wong K, Shi F, Brower R, Mai S, et al. NLR is predictive of upstaging at the time of radical cystectomy for patients with urothelial carcinoma of the bladder. Urol Oncol. (2014) 32:631–6. doi: 10.1016/j.urolonc.2013.12.009
72. Seah JA, Leibowitz-Amit R, Atenafu EG, Alimohamed N, Knox JJ, Joshua AM, et al. Neutrophil-Lymphocyte ratio and pathological response to neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer. Clin Genitourin Cancer. (2015) 13:e229–33. doi: 10.1016/j.clgc.2015.02.001
73. Leibowitz-Amit R, Israel A, Gal M, Atenafu EA, Symon Z, Portnoy O, et al. Association between the absolute baseline lymphocyte count and response to neoadjuvant platinum-based chemotherapy in muscle-invasive bladder cancer. Clin Oncol. (2016) 28:790–6. doi: 10.1016/j.clon.2016.07.007
74. Rossi L, Santoni M, Crabb SJ, Scarpi E, Burattini L, Chau C, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol. (2015) 22:1377–84. doi: 10.1245/s10434-014-4097-4
75. Taguchi S, Nakagawa T, Matsumoto A, Nagase Y, Kawai T, Tanaka Y, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of survival in patients with metastatic urothelial carcinoma: a multi-institutional study. Int J Urol. (2015) 22:638–43. doi: 10.1111/iju.12766
76. Auvray M, Elaidi R, Ozguroglu M, Guven S, Gauthier H, Culine S, et al. Prognostic value of baseline neutrophil-to-lymphocyte ratio in metastatic urothelial carcinoma patients treated with first-line chemotherapy: a large multicenter study. Clin Genitourin Cancer. (2017) 15:e469–e76. doi: 10.1016/j.clgc.2016.10.013
77. Su YL, Hsieh MC, Chiang PH, Sung MT, Lan J, Luo HL, et al. Novel inflammation-based prognostic score for predicting survival in patients with metastatic urothelial carcinoma. PLoS ONE. (2017) 12:e0169657. doi: 10.1371/journal.pone.0169657
78. Caras RJ, Lustik MB, Kern SQ, McMann LP, Sterbis JR. Preoperative albumin is predictive of early postoperative morbidity and mortality in common urologic oncologic surgeries. Clin Genitourin Cancer. (2017) 15:e255–62. doi: 10.1016/j.clgc.2016.09.008
79. Lambert JW, Ingham M, Gibbs BB, Given RW, Lance RS, Riggs SB. Using preoperative albumin levels as a surrogate marker for outcomes after radical cystectomy for bladder cancer. Urology. (2013) 81:587–92. doi: 10.1016/j.urology.2012.10.055
80. Ku JH, Kim M, Choi WS, Kwak C, Kim HH. Preoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. (2014) 40:753–62. doi: 10.1590/S1677-5538.IBJU.2014.06.06
81. Djaladat H, Bruins HM, Miranda G, Cai J, Skinner EC, Daneshmand S. The association of preoperative serum albumin level and American Society of Anesthesiologists. (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int. (2014) 113:887–93. doi: 10.1111/bju.12240
82. Laurent M, Brureau L, Demery ME, Flechon A, Thuaut AL, Carvahlo-Verlinde M, et al. Early chemotherapy discontinuation and mortality in older patients with metastatic bladder cancer: the AGEVIM multicenter cohort study. Urol Oncol. (2017) 35:34 e9–16. doi: 10.1016/j.urolonc.2016.08.003
83. Hwang EC, Hwang IS, Yu HS, Kim SO, Jung SI, Hwang JE. Utility of inflammation-based prognostic scoring in patients given systemic chemotherapy first-line for advanced inoperable bladder cancer. Jpn J Clin Oncol. (2012) 42:955–60. doi: 10.1093/jjco/hys124
84. Huang J, Wang Y, Yuan Y, Chen Y, Kong W, Chen H, et al. Preoperative serum pre-albumin as an independent prognostic indicator in patients with localized upper tract urothelial carcinoma after radical nephroureterectomy. Oncotarget. (2017) 8:36772–9. doi: 10.18632/oncotarget.13694
85. Niwa N, Matsumoto K, Ide H, Nagata H, Oya M. Prognostic value of pretreatment albumin-to-globulin ratio in patients with non-muscle-invasive bladder cancer. Clin Genitourin Cancer. (2018) 16:e655–e61. doi: 10.1016/j.clgc.2017.12.013
86. Liu J, Dai Y, Zhou F, Long Z, Li Y, Liu B, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. (2016) 34:484 e1–8. doi: 10.1016/j.urolonc.2016.05.024
87. Zhang B, Yu W, Zhou LQ, He ZS, Shen C, He Q, et al. Prognostic significance of preoperative albumin-globulin ratio in patients with upper tract urothelial carcinoma. PLoS ONE. (2015) 10:e0144961. doi: 10.1371/journal.pone.0144961
88. Fukushima H, Kobayashi M, Kawano K, Morimoto S. Prognostic value of albumin/globulin ratio in patients with upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Anticancer Res. (2018) 38:2329–34. doi: 10.21873/anticanres.12478
89. Xu H, Tan P, Ai J, Huang Y, Lin T, Yang L, et al. Prognostic impact of preoperative albumin-globulin ratio on oncologic outcomes in upper tract urothelial carcinoma treated with radical nephroureterectomy. Clin Genitourin Cancer. (2018) 16:e1059–68. doi: 10.1016/j.clgc.2018.06.003
90. Otsuka M, Kamasako T, Uemura T, Takeshita N, Shinozaki T, Kobayashi M, et al. Prognostic role of the preoperative serum albumin : globulin ratio after radical nephroureterectomy for upper tract urothelial carcinoma. Int J Urol. (2018) 25:871–8. doi: 10.1111/iju.13767
91. Liu J, Wang F, Li S, Huang W, Jia Y, Wei C. The prognostic significance of preoperative serum albumin in urothelial carcinoma: a systematic review and meta-analysis. Biosci Rep. (2018) 38(4) doi: 10.1042/BSR20180214
92. Qayyum T, McArdle P, Hilmy M, Going J, Orange C, Seywright M, et al. A prospective study of the role of inflammation in bladder cancer. Curr Urol. (2013) 6:189–93. doi: 10.1159/000343537
93. Cho YH, Seo YH, Chung SJ, Hwang I, Yu HS, Kim SO, et al. Predictors of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: an inflammation-based prognostic score. Korean J Urol. (2014) 55:453–9. doi: 10.4111/kju.2014.55.7.453
94. Ferro M, De Cobelli O, Buonerba C, Di Lorenzo G, Capece M, Bruzzese D, et al. Modified glasgow prognostic score is associated with risk of recurrence in bladder cancer patients after radical cystectomy: a multicenter experience. Medicine. (2015) 94:e1861. doi: 10.1097/MD.0000000000001861
95. Lucca I, Hofbauer SL, Leitner CV, de Martino M, Ozsoy M, Susani M, et al. Development of a preoperative nomogram incorporating biomarkers of systemic inflammatory response to predict nonorgan-confined urothelial carcinoma of the bladder at radical cystectomy. Urology. (2016) 95:132–8. doi: 10.1016/j.urology.2016.06.007
96. Wuethrich PY, Vidal A, Burkhard FC. There is a place for radical cystectomy and urinary diversion, including orthotopic bladder substitution, in patients aged 75 and older: results of a retrospective observational analysis from a high-volume center. Urol Oncol. (2016) 34:58 e19–27. doi: 10.1016/j.urolonc.2015.08.011
97. Miyake M, Morizawa Y, Hori S, Marugami N, Iida K, Ohnishi K, et al. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology. (2017) 93:259–69. doi: 10.1159/000477405
98. Inamoto T, Matsuyama H, Sakano S, Ibuki N, Takahara K, Komura K, et al. The systemic inflammation-based glasgow prognostic score as a powerful prognostic factor in patients with upper tract urothelial carcinoma. Oncotarget. (2017) 8:113248–57. doi: 10.18632/oncotarget.22641
99. Kimura S, D DA, Soria F, Foerster B, Abufaraj M, Vartolomei MD, et al. Prognostic value of modified glasgow prognostic score in non-muscle-invasive bladder cancer. Urol Oncol. (2019) 37:179 e19–28. doi: 10.1016/j.urolonc.2018.11.005
100. Suyama T, Kanbe S, Maegawa M, Shimizu H, Nakajima K. Prognostic significance of inflammation-based prognostic scoring in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. (2019) 45:541–8. doi: 10.1590/s1677-5538.ibju.2018.0251
101. McMillan DC. The systemic inflammation-based glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. (2013) 39:534–40. doi: 10.1016/j.ctrv.2012.08.003
102. Schulz GB, Grimm T, Buchner A, Jokisch F, Grabbert M, Schneevoigt BS, et al. Prognostic value of the preoperative platelet-to-leukocyte ratio for oncologic outcomes in patients undergoing radical cystectomy for bladder cancer. Clin Genitourin Cancer. (2017) 15:e915–21. doi: 10.1016/j.clgc.2017.05.009
103. Son S, Hwang EC, Jung SI, Kwon DD, Choi SH, Kwon TG, et al. Prognostic value of preoperative systemic inflammation markers in localized upper tract urothelial cell carcinoma: a large, multicenter cohort analysis. Minerva Urol Nefrol. (2018) 70:300–9.
104. Casamassima A, Picciariello M, Quaranta M, Berardino R, Ranieri C, Paradiso A, et al. C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. J Urol. (2005) 173:52–5. doi: 10.1097/01.ju.0000146713.50673.e5
Keywords: biomarker, C-reactive protein, neutrophil to lymphocyte ratio, systemic inflammation response, urothelial cancer
Citation: Yuk HD and Ku JH (2020) Role of Systemic Inflammatory Response Markers in Urothelial Carcinoma. Front. Oncol. 10:1473. doi: 10.3389/fonc.2020.01473
Received: 31 January 2020; Accepted: 10 July 2020;
Published: 21 August 2020.
Edited by:
Ronald M. Bukowski, Cleveland Clinic, United StatesReviewed by:
Ewa Czeslawa Izycka-Swieszewska, Medical University of Gdansk, PolandRetnagowri Rajandram, University of Malaya, Malaysia
Copyright © 2020 Yuk and Ku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ja Hyeon Ku, kuuro70@snu.ac.kr
 Hyeong Dong Yuk1,2
Hyeong Dong Yuk1,2 Ja Hyeon Ku
Ja Hyeon Ku