- 1Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2Department of General, Visceral and Transplantation Surgery, University Hospital, LMU Munich, Munich, Germany
- 3Department of Pharmacy, The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, China
- 4Department of Nephrology, Chengdu Integrated TCM and Western Medicine Hospital, Chengdu, China
- 5School of Pharmacy, Southwest Medical University, Luzhou, China
With the rapid development of new technologies, including artificial intelligence and genome sequencing, radiogenomics has emerged as a state-of-the-art science in the field of individualized medicine. Radiogenomics combines a large volume of quantitative data extracted from medical images with individual genomic phenotypes and constructs a prediction model through deep learning to stratify patients, guide therapeutic strategies, and evaluate clinical outcomes. Recent studies of various types of tumors demonstrate the predictive value of radiogenomics. And some of the issues in the radiogenomic analysis and the solutions from prior works are presented. Although the workflow criteria and international agreed guidelines for statistical methods need to be confirmed, radiogenomics represents a repeatable and cost-effective approach for the detection of continuous changes and is a promising surrogate for invasive interventions. Therefore, radiogenomics could facilitate computer-aided diagnosis, treatment, and prediction of the prognosis in patients with tumors in the routine clinical setting. Here, we summarize the integrated process of radiogenomics and introduce the crucial strategies and statistical algorithms involved in current studies.
Background
Advances in genomics and the far-reaching effects of precision medicine have synergistically accelerated research by integrating the individual characteristics of patients (1). Compared with conventional medical treatment, the concept of precision medicine follows a “one-size-fits-one” philosophy and sets out a tailored therapeutic plan according to the genotypic and phenotypic data of individual patients (2).
Cancer is a disease that involves genetic abnormalities caused by hereditary or environmental factors. When genes undergo the error-prone process of replication and alterations, such as nucleotide substitution, insertions, deletions, and chromosomal rearrangements, the activation of oncogenes and loss of tumor suppressor genes may induce oncogenesis (3). Moreover, epigenetic alterations, including histone modification, DNA methylation, and altered expression levels of non-coding RNAs, have also been confirmed to be important contributors to the development of cancer (4). Over recent decades, there have been major advances in our understanding of the genetic alterations involved in oncogenesis. For example, mutations of the Kirsten rat sarcoma viral oncogene (KRAS), epidermal growth factor receptor (EGFR), and anaplastic lymphoma kinase (ALK) genes have been identified to be common oncogenic drivers (5). These abnormalities of specific molecular and signaling pathways can be used as genomic biomarkers that provide personalized information about diagnosis, treatment, and prognosis, and contribute to selection of the optimal therapeutic strategy.
Access to genomic information in conventional clinical procedures is based mainly on biopsy of focal tissue samples and microarray genetic analysis. Histopathological examination is feasible to decipher mutational signatures and genomic information, but these data can only reflect the status of a tumor at the time of biopsy or resection. Moreover, gene expression profiling of only a fraction of the tumor tissue cannot reflect the heterogeneity of the entire tumor. The spatial and temporal variables of gene expression may cause changes in various biological processes in the tumor, including apoptosis, cellular proliferation, growth patterns, and angiogenesis. These alterations occur at the molecular and cellular levels and, to a large extent, are shown as heterogeneous imaging features, which can be transformed into varying degrees of signals in different imaging platforms using radiological technology (6).
Technological progress in microarrays, automated DNA and RNA sequencing, mass spectrometry, and comparative genomic hybridization are essential for exploration of tumor biomarkers and more accurate assessment of disease status in patients, as shown in pancreatic cancer (7). Nowadays, large databases that are suitable for elucidating the relationship between gene expression and clinical features exist. When combined with artificial intelligence, treatment options and survival could be predicted by the performance of individuals in models based on big data (8). Currently, non-invasive detection and monitoring of diseases can be performed repeatedly without causing harm and has become a hotspot in cancer research. The huge diversity of phenotypes can be demonstrated by non-invasive radiological imaging (9), which reveals many characteristics of tumors in both subjective and qualitative ways (10). Recent advances in image acquisition, standardization, and image analysis have allowed identification of objective and precise imaging features, including prognostic and predictive biomarkers (11). Although imaging examinations are often performed repeatedly during treatment, it is still impractical to obtain dynamic genomic or proteomic data. Fortunately, this problem can be solved by analyzing computer-processed images to find underlying predictive and prognostic information (12). Radiomics refers to the qualitative and quantitative extraction of data from clinical images and clinical information as well as the methodology used to convert these features in a way that supports decision-making. Radiogenomics, a new computational discipline, is an emerging area within radiomics and is a combination of the words “radiology” and “genomics” (13). The advent of radiogenomics reflects a shift in the focus of radiology-pathology research from the gross anatomical level to the genetic level. Moreover, radiogenomics aims to investigate the correlation between the integrated hierarchical analysis of a massive number of imaging characteristics and corresponding gene expression profiles and to identify optimal radiomic biomarkers, so as to allow more reliable prediction of prognosis and response to treatment.
Advances in Methodology, Technology, and Workflow in Radiogenomics
The enormous amount of imaging data collected has resulted in a large-scale database that is both diverse and complex. Therefore, advanced frameworks, techniques, algorithms, and analytics are needed to mine significant and valuable radiomic information from the imaging database (8).
The birth and development of radiogenomics relies on high-throughput computing and machine learning, both of which are good methods for managing and analyzing a very large number of variables for different samples and procedures. For example, although the image of a tumor region is typically assessed by a radiologist using functional or morphological features, the imaging actually contains more complicated information that can be extracted and processed effectively using radiogenomic approaches.
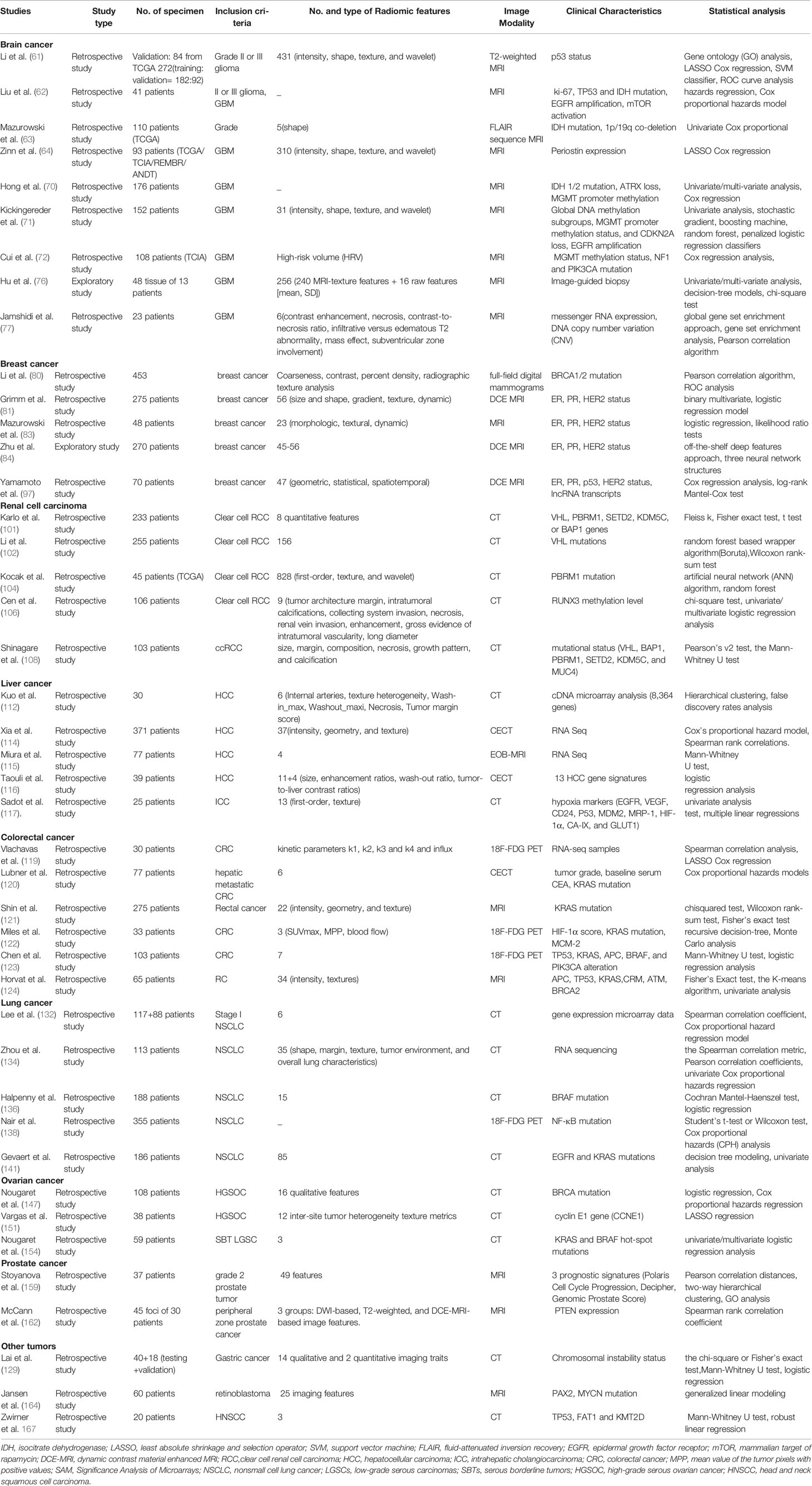
The workflow of radiogenomics mainly includes data acquisition and pre-processing, tumor segmentation, feature extraction, analysis, and modeling (Figure 1). The standardized operating procedure of radiogenomics is a basic assurance of the quality of studies to reduce avoidable errors, particularly in multicenter studies. Along these lines, the Image Biomarker Standardization Initiative consortium, standardized 169 radiomics features in two phases, most of which showed high reproducibility in the third phase and can be applied in different radiomics software (15). Below we provide a brief overview of a feasible imaging protocol for radiogenomics.
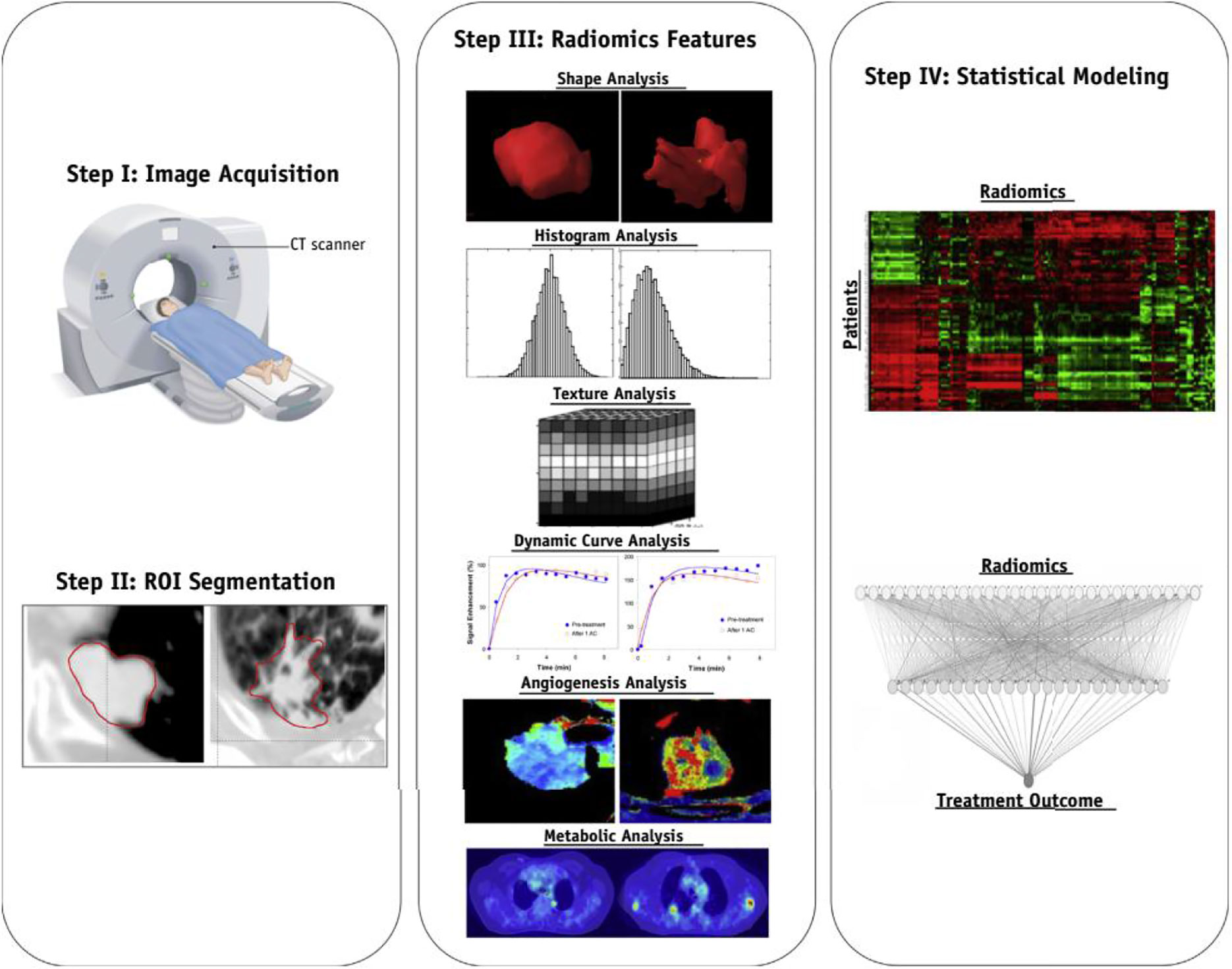
Figure 1 The general radiomics study workflow. Step 1: image acquisition. Step 2: region of interest identification and segmentation. Step 3: quantitative image feature extraction. Step 4: data mining and informatics analysis. The figure was reprinted by ref (14) with permission from the publisher.
Development of Radiomics Prediction Models
Acquisition of Raw Images
In oncology, multimodality imaging, such as positron emission tomography (PET)-computed tomography (CT) and single-photon emission CT, can describe both the anatomical and functional features of tumors in great detail. However, recent efforts have focused on a combination of quantitative functional assessments, such as multiple PET tracers, various magnetic resonance imaging (MRI) contrast mechanisms, and PET-MRI, thereby revealing multidimensional features of the tumor phenotype (16–18). For instance, diffusion-weighted MRI is capable of reflecting tumor density and cellularity, and can therefore be used to monitor the response to cytotoxic treatment (19). Furthermore, fluorodeoxyglucose (FDG)-PET is a molecular imaging tool that is frequently used to characterize changes in metabolic activity within a tumor. The rate of uptake, metabolism, and accumulation of FDG can be used to assess the therapeutic effects and disease progression (16, 20, 21). Different parameters can be acquired using different radiological imaging technologies. Therefore, selection of imaging equipment or technology is important for acquisition of desirable parameters.
Pre-Processing of Information
Raw imaging data need to be pre-processed in order to maintain homogenous and reliable traits. One optional step is filtering the imaging signals within the region of interest (ROI). Manual segmentation is the most widely used method but requires clinicians to have sufficient experience to be able to delineate the optimal ROI. If the ROI is too small, it cannot provide sufficient information about voxels for analysis, and if it is too large, it may be easily biased by the heterogeneity of the tumor. However, full manual segmentation may have some limitations, being time-consuming and showing inter-observer variability (22, 23). Although automatic segmentation is superior to manual delineation in terms of precision and efficiency, its performance depends on the accuracy of the algorithm used and its ability to differentiate ROIs from surrounding tissues.
The critical issues of the robustness of quantitative features with respect to imaging variations and inter-institutional variability need to be investigated further. Currently, there are several advanced machines equipped with deep learning-based algorithms aimed at contour functions, including the 3DSlicer (24–26), DeepMind (Google) (27), and Project InnerEye (Microsoft) (https://www.microsoft.com/en-us/research/project/medical-image-analysis/).
An increasing number of studies have proven that the preferred mode for imaging pre-processing is semi-automatic segmentation, which makes use of both manual intervention and software automation (28). Tixier et al. (29) compared the robustness of 108 radiomic features from five categories using a semi-automatic and an interactive segmentation method by two raters. The results demonstrated that the interactive method produced more robust features than the semi-automatic method; however, the robustness of the radiomic features varied by categories. Um et al. (30) used five image pre-processing techniques: 8-bit global rescaling, 8-bit local rescaling, bias field correction, histogram standardization, and isotropic resampling to extract a total of 420 features from 161 cases. Of the examined techniques, histogram standardization was concluded to contribute the most in reducing radiomic feature variability, since it was shown to reduce the covariate shift for three feature categories and to be capable of discriminating patients into groups based on their survival risks. Veeraraghavan et al. (31) developed a novel semi-automatic approach that combines GrowCut (GC) with cancer-specific multiparametric Gaussian Mixture Model (GCGMM) to produce accurate and reproducible segmentations. Segmentation performance using manual and GCGMM segmentations was compared in a sample of 75 patients with invasive breast carcinoma. GCGMM’s segmentations and the texture features computed from those segmentations were shown to be more reproducible than manual delineations and other analyzed segmentation methods.
Extraction of Features
The critical component of radiomics is the extraction of high-dimensional feature sets to quantitatively describe the attributes of oncological phenotypes. These extracted quantitative data reflect the crucial part of the establishment of radiomics prediction models. In practice, 50 to 5,000 radiomic features processed by specific software, including PyRadiomics (32, 33), CERR (34, 35) or IBEX (36, 37), are usually divided into morphological, intensity-based, and dynamic features (14) (Figure 2). Morphology-based features can collect three-dimensional (3D) shape characteristics, including volume, surface area, and sphericity. Intensity-based features can evaluate the gray-level distribution inside the ROI, which can characterize the overall variability in intensity (first-order) and the local distribution (second-order, also referred to as “texture features”). In terms of oncological pathology, both tumors and precancerous lesions have highly heterogeneous cell populations with normal stromal and inflammatory cells. Compared with conventional pathology, which only reveals underlying biological information in subregions, advanced texture analysis is emerging as a novel medical imaging tool for assessment of intratumoral heterogeneity. Texture analysis is used to describe the association between the gray-level intensity of pixels or voxels and their position within ROIs. Texture analysis usually consists of four steps: extraction, texture discrimination, texture classification, and shape reconstruction. Moreover, previous studies have demonstrated that non-uniform staining intensity within tumors may predict more aggressive behavior, poorer response to treatment, and worse prognosis (14, 38).
Figure 2 The classifications and corresponding examples of quantitative radiomics features. The figure was reproduced according to ref (14). with permission from the publisher.
Furthermore, dynamic features derived from dynamic contrast-enhanced CT or MRI and metabolic PET (which can be one or more voxels within the ROI) are widely used to quantify enhancement of or uptake in tumors over time. Evaluating these extracted dynamic features can uncover relationships with molecular subclassifications of tumors and the prognosis (39).
An even more extensive range of features is expected. These radiomics features provide additional data associated with tumor pathophysiology that cannot be achieved by typical radiological interpretation. Therefore, creation of more easily interpretable models will be key to establishing a correlation between radiography and radiomics features in the future. Moreover, the stability and accuracy of features should be validated by test-retest datasets, and any that are volatile or unreliable should be excluded.
Data Analysis
The variables and features collected during extraction are often redundant and may contain unnecessary information that leads to overfitting. Therefore, selection or dimensional limitation of the basic data is essential to maintain the selected significant imaging characteristics that show a strong correlation with clinical events.
The common selection methods can be classified into three categories: filter, wrapper, and embedded methods. Groups of highly correlated radiomics features can be identified via clustering. Filter methods evaluate features without involving the model in a univariate or multivariate way, which means the rank criterion depends only on the relevance of the feature or use of a weighted sum to maximize relevance and minimize redundancy. Features can then be generated and evaluated using the model with wrapper methods. Finally, a feature subset is proposed and evaluated during construction of the model with embedded methods.
Deep Learning and Convolutional Neural Networks
Deep learning is a machine learning algorithm that is characterized by utilization of neural networks with multiple layers (40). It is regarded as a semi-theoretical and semi-empirical modeling method that can be used to construct a holistic architecture on the basis of mathematical knowledge or computing algorithms, correlate training data to large-scale computing ability, adjust internal parameters, and consequently solve target problems. Convolutional neural networks (CNNs) are typically used in deep learning and combine imaging filters with artificial neural networks through a series of successive linear and nonlinear layers (41). CNNs use local connections and weights to analyze the input images, followed by pooling operations to obtain spatially invariant features (42). Furthermore, a fully connected network created at the end of the CNN could convert the final two-dimensional layers into a one-dimensional feature vector (43). After obtaining sufficient training data, deep learning algorithms can determine the optimal feature set and the relative importance of each feature. They can then classify images by utilizing combinations of features. Therefore, machine learning has become a fitting approach for selection and classification of features (44).
Outcome Modeling Through Machine Learning
Once the feature set is obtained, a prediction model is needed to connect the features selected with the genetic information of the diseases in order to prospectively identify subgroups of patients who may benefit from specific treatment. However, without interpretability, these quantitative descriptors are inconvenient and difficult to apply when using radiogenomics in clinical practice. Therefore, interpretable models are required to establish correlations between quantitative formula-derived radiomics features and genetic subtypes. Representative classification methods include conventional logistic regression (45) and advanced machine learning techniques (46), such as decision trees and random forests, support vector machines, and deep neural networks (47), which are able to emulate human intelligence by acquiring knowledge of the surrounding environment from the input data and detect nonlinear complex patterns in the data.
Machine learning can build prediction models in several ways and includes unsupervised, supervised, and semi-supervised approaches. Unsupervised analysis divides the data into subgroups based on the similarity between samples. In the unsupervised model, a distance measurement is used to determine similarity, and similar training samples are stratified into the same group. Moreover, a clinical label is not required to train an unsupervised model that can be applied in more situations. In contrast, supervised learning is used when the endpoints of the treatments such as tumor control or toxicity grades are known, which requires a large amount of training samples to avoid overfitting. Unsupervised methods, such as clustering methods or the use of principal component analysis, provide means to reduce the learning problem curse of dimensionality through feature extraction, and to aid in the visualization of multivariable data and the selection of the optimal learning method parameters for supervised learning methods (48). Each method has its own merits and pitfalls (49). Deep learning is the preferred method when a large amount of data are included in the cohort. Creating a highly complex deep learning model that provides performances similar to simpler statistical tests or machine learning algorithm is redundant (50).
As mentioned earlier, a radiomics model could be validated repeatedly to confirm its potential value for clinical application. Generally, external validation is considered to be a stronger test for a model than an internally validated prediction model because it produces more credible and robust results (51). Many methods have been used successfully to evaluate the performance of radiomics models; the receiver-operating characteristic (ROC) curve is the method most commonly utilized for discrimination analysis and the concordance index is usually used for validation of survival analysis (52).
Radiogenomics Approach
A radiogenomics study may be exploratory or hypothesis-driven. In exploratory studies, a common approach is multiple hypotheses testing, whereby the features extracted are tested against a mass of genomic variables. Accurate conclusions can be reached from exploratory analyses but statistical correction to the significance level is required. The false discovery rate is the optimal metric for controlling the expected proportion of “discovery” that is false when conducting multiple comparisons. Furthermore, hierarchical cluster analysis has proved to be a useful tool for exploratory analysis of gene expression data, which is an algorithm that groups similar objects into clusters that are distinct from each other. Moreover, a type of tree diagram, known as a dendrogram, is usually used to show hierarchical relationships between different clusters. In contrast, when using the hypothesis-driven approach, researchers collect a sufficient number of imaging phenotypes and then investigate them with a specific hypothesis in mind. For example, Konstantinidis et al. used this method in a prospective clinical trial and confirmed a previous hypothesis that MRI can act as an imaging biomarker for prediction of the response to chemotherapy in patients with unresectable intrahepatic cholangiocarcinoma (ICC) (53).
Current Application of Radiogenomics in Oncology
Radiogenomics takes advantage of big data analysis approaches that explore meaningful information for decision-making in the diagnosis and treatment of cancer (54). Furthermore, radiogenomics provides an in-depth understanding of tumor biology and captures imaging biomarkers with relevant implications. These approaches have been validated in a variety of tumors (55). Here we summarize the known and potential imaging features of corresponding genotypes in various types of tumors and their value and feasibility in clinical practice.
Glioblastoma
Glioblastoma multiforme (GBM) is considered to be the most common life-threatening brain cancer, accounting for 45% of primary central nervous system tumors with an average overall survival of only 15 months (56, 57). This dismal prognosis is mainly due to the invasiveness of the tumor, which responds variably to treatment, and the infiltrative ability of tumor cells that cannot be detected with the current imaging technologies. Heterogeneity exists not only at the patient level but also at the level of a single tumor, indicating that GBM includes a wide range of genetic abnormalities and regional transformations in response to microenvironmental cues (58). In general, the most reliable diagnostic imaging method is MRI because of its excellent soft tissue contrast (59). With progress in the genetic understanding of GBM, multiple strategies are being developed to associate the radiological features of GBM with genomic phenotypes, for prediction of the therapeutic response and clinical prognosis.
GBM also shows biological heterogeneity and includes proneural, neural, classical, and mesenchymal subtypes (60). Studies have demonstrated that imaging-based biomarkers not only allow prognostic stratification of individual patients but also have an important role in disease diagnosis (61–63). For example, Zinn et al. (64) identified a causal link between radiomic texture features and periostin expression levels, an important gene involved in GBM invasion and recurrence (65). The results provide evidence for the potential use of non-invasive interventions as predictors of disease prognosis in future clinical trials (66).
IDH Mutation
One of the best known molecular biomarkers in GBM development is the mutation status of isocitrate dehydrogenase (IDH) 1/2 (67). This enzyme is found to regulate the citric acid cycle (68) and increase angiogenesis (69). A retrospective study of 176 patients with GBM conducted in Korea (70) revealed a significant association between the MRI features and corresponding genomic profiles, demonstrating that these imaging characteristics can be used to predict IDH mutation status. Specifically, this study found that a higher proportion of insular involvement, larger tumor volumes, a higher volume ratio on T2-weighted and contrast-enhanced T1-weighted images (solid enhancing portion on the contrast-enhanced T1-weighted volume), and a higher apparent diffusion coefficient (ADC) were more prevalent in patients with IDH mutation. Similarly, Mazurowski et al. (63) analyzed the imaging data of 110 patients with lower-grade gliomas from The Cancer Genome Atlas (TCGA). They found a strong association between a quantitative feature, angular standard deviation (ASD), which measures irregularity of the tumor boundary, and the IDH-1p/19q subtype (p < 0.0017). Higher ASD is generally considered a predictor of poorer outcomes.
ATRX Loss
The alpha thalassemia/mental retardation X-linked gene (ATRX) is involved in chromatin remodeling and maintenance of telomeres. ATRX mutations are mainly associated with diffuse astrocytomas and gliomas with higher sensitivity to treatment. Tumors with loss of ATRX have been shown to a great extent to harbor a sharper hypersignal intensity area margin and a higher ADC value of the T2 hyperintense lesion compared with tumors that contain wild-type ATRX, which suggests a better prognosis in patients with this GBM subtype (70).
TP53 Mutations
TP53 is an important gene that suppresses tumorigenesis by inducing cell cycle arrest and is frequently altered in diffuse gliomas and particularly in astrocytomas. Mutation of p53 results in proliferation and invasion of tumor cells, which is a prognostic marker for diffuse glioma. Preoperative MRI examinations found a specific correlation of p53 with the tumor location and enhancement pattern in lower-grade glioma. Li et al. (61) indicated that Maximum_6 and Median_6 values (signals of microvessel counts on T2-weighted images) are higher in tumors with mutant than in those with wild-type p53. Furthermore, they showed that Uniformity_4, a radiological parameter indicating the consistency of the image, could predict the mutation status of p53 (61). This observation may reflect the fact that p53 mutation increases the aggressiveness and heterogeneity of a tumor, leading to disparity of uniformity.
O6-Methylguanine-DNA-Methyltransferase Methylation
The association between epigenomic clusters and MRI traits was also uncovered by research that created predictive machine learning-based classification models. The status of DNA methylation using O6-methylguanine-DNA-methyltransferase (MGMT) promoter methylation status and the tumor’s copy number variation profile can be used to classify glioblastoma in various subgroups (71). Due to the function of MGMT in promoting DNA repair and reducing the efficacy of alkylating events, epigenetic silencing of the MGMT DNA repair gene through promoter methylation leads to irreparable DNA damage and cell death and increased sensitivity to alkylating chemotherapy.
In a study, MGMT methylation was mainly observed in tumors with a higher percentage of contrast-enhancing tumor volume to complete tumor volume, higher Gaussian-normalized relative cerebral blood volume (nrCBV) and nrCBV in the contrast-enhanced and total tumor volumes (72). The indicator relative cerebral blood volume (rCBV) is widely utilized and can reflect tumor hypoxia and angiogenesis, which can be evaluated more precisely by imaging of vessel size. The methylated MGMT promoter is also related to the presence of pseudoprogression. Therefore, increases in enhancement within 3 months after completion of radiotherapy in patients with MGMT methylation are regarded as treatment-related effects (pseudoprogression) rather than progressive disease. Tixier et al. (73) investigated the combination of the MGMT status with radiomics and found that a feature named edge descriptor was significantly correlated with MGMT methylation and predicted better survival of GBM patients.
Phosphatidylinositol 3-Kinase -Akt-Mammalian Target of Rapamycin Pathway
Identification of a marked correlation between expression of the mammalian target of rapamycin (mTOR) and the maximum rCBV in the enhanced GBM (62) has paved the way for prediction of mTOR status from images. Given that mTOR inhibitors can improve the response of GBM to temozolomide, this prediction model may facilitate identification of a suitable patient population.
Furthermore, Cui el at has shown that the high-risk volume (HRV) was higher in GBMs with mutations in either Nuclear Factor I (NF1) or PIK3CA than in those that were wild type (72). These two genes play a critical role in the progression of GBM. It has been shown that mutations of NF1, a tumor suppressor gene, are quite common in the mesenchymal molecular subtype, which has a very poor prognosis due to aggressive biological behavior (60, 74). Patients with GBM who have an activated phosphatidylinositol 3-kinase (PI3K) signaling pathway also have poorer outcomes than those who do not (75). Inhibitors targeting the PI3K pathway are under active development and offer hope for patients with GBM.
Epidermal Growth Factor Receptor Amplification
Another study (76) identified compelling imaging connections for six oncogenes or tumor suppressor genes (EGFR, PDGFRA, PTEN, CDKN2A, RB1, and TP53) in 48 biopsies collected from 13 tumors. By establishing multivariate predictive models for each gene, the investigators found a significant association between amplification of EGFR and local binary patterns texture on rCBV maps.
Apart from a single gene mutation, advanced high-throughput measurement of, for example, a change in mRNA expression and DNA copy number variation could also enable identification of correlations between individual genes or loci and particular imaging features. Jamshidi et al. (77) created a multilevel radiogenomics association map to highlight genes that showed concordant mRNA expression and gene dose changes and their links with MRI features. That study identified 34 gene loci, including LTBP1, RUNX3, and KLK3, as biomarkers of GBM.
Breast Cancer
Breast cancer is the most common malignancy in women and is regarded as a heterogeneous and complex disease. Breast cancer can be classified into luminal A, luminal B, human epidermal growth factor receptor 2 (HER2), and basal molecular subtypes (78). Specific molecular subtypes are shown to have different patterns of initial disease presentation, different relapse-free survival rates, and distinct variations in response to treatment. Conventional imaging techniques, including mammography, ultrasound, and MRI, are used to detect malignant lesions and monitor disease progression.
Gene Expression
Women with BRCA1/2 gene mutation are considered as being at a higher risk of developing breast and/or ovarian cancer (79). Li et al. (80) found that computerized mammographic assessment of breast density and parenchymal patterns (phenotypes of coarseness and contrast) from radiographic texture analysis could together be used to distinguish BRCA1/2 gene-mutation carriers from low-risk women.
Molecular Subclassification
Several studies have attempted to delineate the correlation between findings on breast MRI and molecular subtype. For example, Grimm et al. (81) identified two dynamic imaging features that were independent predictors of the luminal A and luminal B subtypes: 1) the ratio of enhancement of the tumor to that of the fibroglandular tissue at two time points; 2) the sequence number at which peak enhancement occurs. Meanwhile, Blaschke et al. (82) found that HER2-positive cancers showed more rapid early uptake of contrast compared to other subtypes, and Mazurowski et al. (83) demonstrated that the imaging features of luminal B had a higher tumor enhancement ratio. Moreover, Zhu et al. (84) developed three deep learning models to distinguish between breast cancer subtypes by analyzing dynamic contrast-enhanced MRI scans. However, they found that the best area under the curve of the models was only 0.65, indicating that deep learning can help the research of radiogenomic correlations, but is still limited.
In another study, the MRI phenotype with a heterogeneous enhancement pattern was proven to be significantly associated with immune-related genes characterizing the interferon-rich subtype, most of which is part of triple-negative breast cancer (85). In 10 patients with breast cancer, radiogenomics analysis showed that 12 dynamic contrast-enhanced MRI-specific traits were significantly associated with high expression of immune-related genes, including STAT1, CXCL9, and IFIT1 (85).
Signaling Pathways
The tumor necrosis factor-alpha (TNF-α)/NF-kappaB/Snail pathway is one of the critical molecular pathways in breast cancer and is involved in many activities related to the tumor cell, including epithelial-mesenchymal transition (86), proliferation (87), angiogenesis (88, 89), invasion, and metastasis (90). Wu et al. analyzed 10 quantitative imaging characteristics related to enhancement patterns in the tumor-adjacent region and found an association between the TNF signaling pathway and parenchymal imaging features in breast cancer, which are of prognostic value (91).
Janus kinases (JAK) belong to the family of non-receptor tyrosine kinases and centrally involved in activation of signal transducer and activator of transcription proteins (STAT) proteins in breast cancer (92). The JAK/STAT pathway is a rapid cytoplasmic to nuclear signaling pathway and leads to the activation of genes through a process called transcription (93). Disrupted JAK-STAT signaling could induce carcinogenesis. Yeh et al. (94) intended to perform quantitative radiomic analysis on 47 invasive breast cancers and obtained gene expression data on corresponding fresh frozen tissue samples. Gene set enrichment analysis was used to identify significant associations between the 186 gene pathways and the 38 image-based features. As a result, they found that tumors with higher expression of JAK/STAT and VEGF pathways appeared to have positive correlation with contrast, difference variance, and entropy, and negative correlation with homogeneity and image linearity.
RNA Sequencing
The Oncotype Dx Recurrence Score (ODxRS), which incorporates the mRNA expression of 21 genes, is used clinically to predict the prognosis of early-stage invasive breast cancer (95). Sutton et al. (96) aimed to illustrate the relationship between ODxRS and morphological and texture-based image features extracted from MRI imaging of 95 breast cancer patients. Two MRI-derived image features, kurtosis and histologic nuclear grade, were found to be significantly correlated with the ODxRS.
Long noncoding RNAs, defined as the noncoding transcript, are a crucial group of regulatory RNAs that have been implicated in the development of numerous types of solid tumors. The emergence of next-generation sequencing technologies has provided a good opportunity for increasingly rapid development of RNA sequencing, shedding light on novel and undiscovered transcriptional and epigenetic regulators in breast cancer. Yamamoto et al. (97) performed a radiogenomics analysis to determine the association between the enhancing rim fraction and the expression of 14,880 long noncoding RNAs. Interestingly, the enhancing rim fraction score was found to be correlated with a known predictor of tumor metastasis, HOTAIR (homeobox transcript antisense intergenic RNA). These findings prompted the development of radiogenomics in breast cancer, which has the potential to become an alternative to genetic testing.
Renal Cell Carcinoma
Renal cell carcinoma (RCC) constitutes 2%–3% of all cancers in adults worldwide and has an increasing incidence (98). Clear cell RCC (ccRCC) is the most common subtype and accounts for 70%–80% of all RCCs, followed by papillary RCC and chromophobe RCC (99). Percutaneous biopsy is widely used for preoperative diagnosis of RCC; however, its use is controversial because of potential complications and sampling errors. Recently, radiomics techniques that focus on changes at the molecular level have become an effective way of screening quantitative features for accurate diagnosis of these tumors and prognostic assessment.
Von Hippel–Lindau Mutation
Previous studies have shown that loss or mutation of Von Hippel-Lindau tumor suppressor (VHL) is a critical driver of ccRCC and is believed to occur at an early stage in renal cancer (100). According to our current understanding of the tumorigenesis of ccRCC, alteration of VHL promotes the expression of hypoxia-inducible factors, which are believed to be the central event in upregulation of angiogenesis-related factors. There is mounting evidence of radiogenomic associations between subtype-discriminative CT features and VHL mutation status, possibly arising from a previous finding of significant associations between ccRCC with VHL mutation and clear tumor margins, nodular enhancement, and an intratumoral vasculature on enhanced CT images (101). Li et al. (102) developed a radiomics model with all eight minimum redundancy maximum relevance features from CT images of 170 RCC patients and found it to be significantly associated with VHL mutation.
Polybromo 1 Mutation
The second most frequent mutation in ccRCC is in Polybromo 1 (PBRM1), which, like VHL mutation, becomes mutated in the early stage of tumorigenesis. A meta-analysis that included 2942 patients reported that a lower PBRM1 expression level is correlated with a dismal prognosis, advanced clinical stage, and a higher Fuhrman nuclear grade in ccRCC as well as responsiveness to immune checkpoint inhibitors (103). Kocak et al. found that high-dimensional quantitative CT texture analysis was potentially able to identify ccRCC with and without PBRM1 mutations using the artificial neural network (ANN) and random forest (RF) algorithms as machine learning classifiers; RF outperformed ANN in that study (95.0% vs 88.2%) (104).
Runt-Related Transcription Factor-3 Methylation
The runt-related transcription factor-3 (RUNX3) gene, a noted tumor suppressor gene, regulates gene expression in some dominant developmental pathways and has antitumor activity in various types of tumors (105). RUNX3 is reportedly inactivated in numerous types of tumors and is involved in various biological tumor processes, including epithelial-mesenchymal transition, adhesion, migration, and invasion. Therefore, the methylation level of RUNX3 can also influence tumor cell phenotype. For example, Cen et al. (105) reported a significant relationship between a high level of RUNX3 methylation and shorter survival. Meanwhile, high intratumoral vascularity, unclear margins, and a left-sided tumor can be used to predict high RUNX3 methylation level (106).
BRCA1-Associated Protein 1 Mutation
The BAP1 (BRCA1-associated protein 1) gene mutation, which is present in 15% of ccRCCs, has been associated with Fuhrman grade 3 or 4 tumors and poor survival, as well as greater sensitivity to radiotherapy and mTOR blockade (107). Shinagare et al. (108) identified ill-defined margins and the presence of calcification to be critical predictors of BAP1 mutation in patients with ccRCC.
Liver Cancer
One of the most aggressive malignancies is primary liver cancer, the most common types of which are hepatocellular carcinoma (HCC) and ICC (109). HCC is the most clinically prevalent subtype and is characterized by high morbidity and mortality rates worldwide (110). Over the past several decades, there is strong evidence of a link between HCC and chronic hepatitis B virus (HBV) infection (111). At present, several imaging modalities for HCC screening/surveillance and diagnosis are endorsed by the international guidelines, including ultrasonography, CT, and MRI, which can provide essential information about tumor staging and are used to assess the treatment response. So far, there have been few radiogenomics studies in HCC.
Early in 2007, Kuo et al. (112) performed a radiogenomics analysis to mine the relationship between imaging features in HCC and expression of 313 liver-specific genes. CYP27A1 and CYP4V2, which belong to the cytochrome p450 superfamily, were found to be responsible for drug metabolism and detoxification and to be significantly associated with the tumor margin score. In a series of studies, Banerjee et al. identified a CT biomarker called radiogenomic venous invasion, which was found to be a strong predictor of microvascular invasion in HCC (113). Moreover, the presence of radiogenomic venous invasion has been associated with tumor recurrence and a shorter survival time (113). Xia et al. (114) reported several methodological benefits of the association between CT imaging features and gene expression profiles when deciphering non-invasive surrogate biomarkers for HCC. They constructed different gene modules according to their prognostic significance and identified enrichment of the MEred gene module in the biological functions and pathways involved in virus-related RNA transcription that were significantly associated with the determined prognostic geometry features. For example, hepatitis B can greatly increase the risk of liver cirrhosis and HCC. Furthermore, functional enrichment of the MEyellow gene module promotes lipid metabolism and complement activation. Interestingly, an earlier study demonstrated alterations in fatty metabolism in HCC that could promote dedifferentiation of tumor cells. Miura et al. (115) retrospectively performed clinicopathological and global gene expression analyses and found that SLCO1B3 was upregulated in HCC cases with a higher intensity lesion in the hepatobiliary phase on gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI.
Taouli et al. (116) analyzed dozens of qualitative and quantitative imaging traits seen in preoperative CT or MRI data and found some to be correlated with aggressive genomic signatures of HCC. For example, the “infiltrative pattern” showed the strongest associations with gene signatures representing enhanced cellular proliferation and vascular invasion while “presence of macrovascular invasion” was also an important imaging feature that showed a significant correlation with the molecular signatures of vascular invasion, distant metastases, and TNM staging in HCC.
ICC is a relatively rare but lethal primary liver cancer originating from the intrahepatic bile duct epithelium. ICC has high expression levels of EGFR and vascular endothelial growth factor gene (VEGF) as well of pro-angiogenic and hypoxia markers. Sadot et al. (117) investigated the relationship between imaging phenotypes and molecular profiling of ICC by visually analyzing imaging features and performing texture analysis with immunohistochemical assessment of molecular markers in 25 patients with ICC. Linear regression analysis showed that the correlation texture feature was significantly associated with expression of VEGF, whereas correlation and entropy texture features were significantly related to expression of EGFR.
Colorectal Cancer
Colorectal cancer (CRC) is the third most common cancer worldwide and is characterized by substantial spatial phenotypic and genotypic variations (118). The development of colon cancer involves multiple steps with a continuous cumulative effect of genetic mutation in tumor suppressors and oncogenes. CT and MRI, as well as 18F-FDG-PET imaging, are widely used for the diagnosis, monitoring of therapeutic response, and prognosis of CRC (119). Recently, there has been an increasing number of investigations on whether or not conventional imaging techniques can predict critical gene mutations in CRC without the need for an invasive procedure.
KRAS Mutation
Mutation of the KRAS gene is found in nearly two fifths of CRCs and is regarded as an independent prognostic factor for survival and a downstream marker of tumor resistance to anti-EGFR-targeted therapy. Lubner et al. (120) found that skewness, a texture parameter that can measure asymmetry of the pixel histogram on CT, showed a negative correlation with KRAS mutation status. Furthermore, Shin et al. (121) demonstrated that a higher prevalence of KRAS mutations was significantly associated with a more advanced nodal stage and the presence of polypoid tumors. Rectal cancers with KRAS mutations have a higher axial tumor length and a larger ratio of axial to longitudinal tumor dimensions on rectal MRI. Miles et al. (122) analyzed multiparametric PET-CT imaging phenotypes using a recursive decision-tree to integrate measurements of 18F-FDG uptake, CT texture, and perfusion. This methodology identified KRAS mutations with high accuracy and a low false-positive rate. However, Chen et al. (123) found that an increased accumulation of FDG measured using a 40% threshold level for maximal uptake of CT-based tumor width was an independent predictor of KRAS mutations.
Other Gene Mutations
A preliminary study (124) that sought to identify other frequent gene mutations in CRC found a significant correlation of tumor location with APC and RASA1 mutation, a significant association of absence of lymph node metastasis with BRCA2 mutation, and a correlation of tumor size with FLT4 mutation, as well as a higher frequency of ATM mutation in patients with a positive circumferential resection margin. However, the results of multiple comparisons were not significant. Therefore, large-scale studies are needed for additional evaluation and to validate these early observations.
Gastric Cancer
Gastric cancer is one of the most common and aggressive solid tumors worldwide and has its highest incidence and mortality rate in Eastern Asia (125). Approximately 20%–40% of patients who receive standard treatment develop recurrent disease (126). Based on the gene expression profile of gastric cancer, there are four molecular subtypes: Epstein-Barr virus-positive, microsatellite unstable, chromosomal instability (CIN), and genomically stable (127). Previous studies have shown that the CIN subtype of gastric cancer has a distinct prognosis. For example, Sohn et al. found that patients with the CIN subtype obtained the greatest benefit from adjuvant chemotherapy (128). CT is regarded as the routine preoperative evaluation modality. Furthermore, Lai et al. (129) investigated the relationship between CT imaging features and CIN status and found that an acute tumor transition angle was the most accurate imaging feature of the CIN subtype of gastric cancer, which provides additional prognosis-related information.
Lung Cancer
Lung cancer is another common cancer with a high mortality rate and accounts for 13% of all newly diagnosed cancers (98). Histologically, lung cancer can be divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Nearly 85% of patients with lung cancer have NSCLC (130).
NSCLC is a group of distinct diseases with genetic and cellular heterogeneity due to different mutations in oncogenic signaling pathways. Conventional imaging methods include radiography and CT, which can provide valuable information for diagnosis, clinical staging, and treatment decisions. Invasive biopsy plays a central role in the pathological diagnosis; however, only a small portion of tissue is generally obtained and cannot completely reflect the properties of the whole tumor. Therefore, radiogenomics mapping is being increasingly used to solve the growing demand for prognostic image-based biomarkers.
There is an urgent need to identify high-risk patients who are more likely to relapse and require more extensive follow-up or aggressive treatment. Invasion of lung cancer into the visceral pleura is a frequent pathological phenomenon (131). Tumors with visceral invasion are classified as T2a and have a bleak prognosis. Lee et al. (132) defined a quantitative pleural contact index, which is the ratio of the tumor-pleura contact length to the maximum tumor length on CT images, and investigated its prognostic value as well as the molecular background of pleural invasion. They found that the pleural contact index was associated with remodeling of the extracellular matrix and that related genes also acted as independent predictors of overall survival in patients with NSCLC. Fave et al. (133) calculated the radiomic features from images of 107 patients with stage III NSCLC and found that texture-strength measured at the end of treatment significantly predicted the risk of local recurrence.
Zhou et al. (134) established a radiogenomics map that integrated CT imaging features and gene expression profiles in patients with NSCLC. By summarizing gene functional enrichment and CT characteristics, they identified a cluster of co-expressed genes involved in the epidermal growth factor pathway that had a significant relationship with the degree of ground-glass opacity and irregular nodules or nodules with poorly defined margins.
BRAF Mutation
BRAF is a serine/threonine-protein kinase that belongs to the Ras/mitogen-activated signaling pathway family. BRAF mutations occur in 2%–5% of NSCLCs; they are responsible for phosphorylation of MEK and ultimately promote cell proliferation and survival (135). Halpenny et al. compared the CT features of BRAF-mutated lung carcinomas with those of lung carcinomas with wild-type BRAF and found no significant difference between BRAF lesions and non-BRAF lesions (136).
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B-CellActivation
Nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-ĸB) is an important transcription factor that regulates multiple signaling cascades and is a positive mediator of cell growth and proliferation (137). Therefore, NF-ĸB and its target genes are involved in the process of tumorigenesis. Nair et al. (138) found a relationship between NF-ĸB expression and tumor glucose metabolism on FDG-PET that could be a potential prognostic biomarker.
Epidermal Growth Factor Receptor Mutation
EGFR belongs to a family of receptor tyrosine kinases and is expressed in more than 60% of NSCLCs (139). Most EGFR mutations involving exons 18, 19, and 21 are considered to predict a favorable response to treatment with an EGFR tyrosine kinase inhibitor (140). Gevaert et al. (141) confirmed that ground-glass opacities and nodule margins are indicative of the presence of EGFR mutations. Aerts et al. (142) collected high-resolution CT imaging of 47 patients with early-stage NSCLC before and after gefitinib therapy to investigate if radiomics can identify a gefitinib response-phenotype. Changes in features between two scans, delta volume and delta maximum diameter, were strongly predictive of EGFR mutation status and of the associated gefitinib response.
Anaplastic Lymphoma Kinase Rearrangement
The ALK gene encodes a tyrosine kinase transmembrane protein, a member of the superfamily of insulin receptors, which are responsible for 3%–7% of NSCLCs and often undergo fusion with echinoderm microtubule-associated protein-like 4 (143). Crizotinib and two other ATP-competitive ALK inhibitors, ceritinib and alectinib, have achieved improved outcomes in this subset of patients. A meta-analysis (144) that summarized the imaging features from 12 studies that included 2,210 patients with NSCLC found that the presence of ALK rearrangement in a primary tumor showed distinct imaging characteristics, including a likelihood of being solid and being less likely to show cavitation and air bronchograms.
Ovarian Cancer
Ovarian cancer is the deadliest malignancy of the female genital tract and has five major histopathological subtypes. Ninety percent of ovarian cancers are high-grade serous ovarian cancer (HGSOC), which has the least favorable prognosis (145). Previous studies have demonstrated the genomic complexity and heterogeneity of ovarian cancer (146). Genetic heterogeneity, including copy number variant, transcriptome analysis, and methylation array, has been discovered in HGSOC, which may explain its drug resistance and open up new avenues for targeted molecular-based treatment. CT is an indispensable imaging examination for patients with HGSOC and can allow staging evaluation for preoperative planning and determination of surgical resectability. Several studies have shown that the CT features can predict critical molecular alteration events in HGSOC, which may have substantial prognostic and therapeutic implications at the time of diagnosis (147).
BRCA Mutation
Approximately 15%–20% of HGSOC cases are inherited. BRCA1 and BRCA2 mutations are the most commonly identified germline mutations, whereas 6% of these tumors harbor somatic BRCA mutations (148). Previous cohort studies have determined that BRCA1/2 mutations are associated with improved long-term survival in patients with ovarian cancer (149, 150). A retrospective study assessed the preoperative CT scans of 108 patients with HGSOC and found qualitative CT features that could distinguish the BRCA mutation status of HGSOC. Multiple regression analysis showed that the pattern of peritoneal disease, presence of peritoneal disease in the gastrohepatic ligament, and supradiaphragmatic lymphadenopathy were associated with HGSOC harboring BRCA mutation, whereas the presence of peritoneal disease in the lesser sac and left upper quadrant, mesenteric involvement, and lymphadenopathy in the supradiaphragmatic and suprarenal para-aortic regions were correlated with wild-type BRCA (147).
Inter-Site Heterogeneity
Inter-site heterogeneity describes a phenomenon whereby tumor cells from different metastatic sites in the same patients can show distinct morphological and phenotypic characteristics. This phenomenon is found in ovarian cancer and is informative for the prognosis and treatment decisions. Vargas et al. (151) provided valuable data indicating that CT texture-based measures can be utilized to evaluate spatial heterogeneity across multiple metastatic lesions and to predict clinical outcomes in patients with HGSOC. In particular, patients with amplification of the cyclin E1 gene exhibited more inter-site texture heterogeneity on CT imaging.
BRAF Mutation
Recent studies have demonstrated mutations of BRAF or KRAS in approximately 60% of serous borderline tumors and low-grade serous carcinomas (152). The presence of BRAF mutation in a serous borderline tumor is a favorable prognostic factor and may inhibit progression to low-grade serous cancer (153). A retrospective study by Nougaret et al. showed that the presence of bilateral ovarian masses, peritoneal lesions, and higher solid tumor volumes was significantly associated with wild-type BRAF (154).
Prostate Cancer
Prostate cancer is the most prevalent malignancy in men in the United States. An epidemiological investigation in 2015 showed that 1.6 million men were diagnosed with prostate cancer and that there had been a 66% increase in its incidence over the previous decade (155).
Currently, the National Comprehensive Cancer Network risk stratification system, which is mainly based on pathological grading from a biopsy sample, prostate serum antigen levels, and T staging, is widely used (156). Even though its prognostic precision has been reproduced in various settings, numerous studies have shown that the impact of adverse pathology is unavoidably underestimated in about 38%–46% of patients (157), partly because of the spatial heterogeneity in tumor growth patterns. Imaging examination can overcome the sampling bias resulting from prostate biopsy; therefore, the properties of the entire tumor can be assessed using a non-invasive platform. Multiparametric MRI is the most accurate imaging modality for detection and localization of prostate cancer lesions and provides both functional tissue information and anatomical information (158). Stoyanova et al. (159) first identified a significant association between quantitative multiparametric MRI features and gene expression in multiparametric MRI-guided biopsy samples. The identified gene clusters related to radiomic features were used for gene ontology analysis and were correlated with distinct biological processes, including immune response, metabolism, cell, and biological adhesion.
PTEN Deletion
The PTEN gene encodes for the phosphatase and tensin homolog and is a tumor suppressor gene on chromosome 10 in region 10q23 that is mutated or deleted throughout the human cancer spectrum (160). Deletion of PTEN has been confirmed to be an important event in prostate carcinogenesis due to activation of the PI3K/Akt signaling pathway. Furthermore, loss of PTEN has been shown to confer a seven-fold increased mortality risk from prostate cancer (161). McCann et al. (162) analyzed the preoperative multiparametric MRI scans of 45 peripheral zone cancer foci and found weak correlations of the reverse reflux rate constant between the extracellular space and the plasma and of the Gleason score with PTEN expression in prostate cancer. However, further investigation and validation of this finding is needed.
Retinoblastoma
Retinoblastoma originates from immature retinal cells. It is the most prevalent intraocular malignancy in children, with 95% of cases diagnosed by the age of 5 years. Most bilateral tumors are caused by germline mutations in the RB1 gene whereas the majority of unilateral retinoblastomas are associated with the presence of somatic RB1 mutations (163). Furthermore, amplification of MYCN was identified in wild-type RB1 retinoblastomas, suggesting that amplification of this gene can trigger tumorigenesis in the background of a functional retinoblastoma protein. Jansen et al. (164) assessed the association between imaging features and the genome-wide mRNA expression profiles of 60 patients with retinoblastoma and found a correlation between a lower photoreceptor gene signature and advanced-stage imaging features, including multiple lesions and a large eye size. Moreover, expression of MYCN was associated with subretinal seeding, while differential expression of SERTAD3 was significantly associated with diffuse growth, a plaque-shape, and multifocality.
Head and Neck Squamous Cell Cancer
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide (165). The Cancer Genome Atlas (TCGA) has revealed that human papillomavirus-associated tumors are accompanied by PIK3CA mutations, loss of TRAF3, and amplification of E2F1, whereas smoking-related HNSCCs exhibit a higher frequency of TP53 mutations and CDKN2A copy number alterations. Furthermore, mutations of the chromatin modifier NSD1 and the Wnt pathway genes AJUBA and FAT1 were also detected in a subgroup of HNSCCs (166). Zwirner et al. (167) followed a hypothesis-driven approach for finding associations between radiomic heterogeneity and genetic aberrations and found that FAT1 somatic mutations were associated with reduced radiomic measures of tumor heterogeneity, possibly clarifying the reason for the previously described better prognosis of patients with human papillomavirus-negative, FAT1-mutated HNSCC.
Unresolved Issues/Limitations
Convincing evidence has emerged showing that there is a moderate association between imaging characteristics and genomic or related characteristics of various kinds of cancers (Table 1). However, adoption of this work into common clinical practice needs to overcome significant challenges.
The foremost limitation of current radiogenomics models is their repeatability and reproducibility (168). Researchers should not overlook the variability arising from use of different equipment and different software or that arising at different clinics. These problems lead to results that are difficult to reproduce, which has largely impeded the progress of radiogenomics models. Therefore, implementation of standard practice guidelines is a critical step to ensure the accuracy and reliability of analytic results in radiogenomics studies (169).
First, differences in acquisition radiomics parameters and variations in contrast enhancement protocols due to different machines and patient conditions are major issues during image acquisition and reconstruction. Therefore, establishment of standardized protocols for each modality is an essential step to avoid this situation. Second, reproducibility and reliability are crucial in the tumor segmentation process. The variability among different readers in delineation of ROIs depends on the methods of segmentation used. It has been shown that semi-automatic delineation not only has machine-like precision, but also can be manually corrected. With regard to feature extraction, a wide range of voxel intensities and image noise needs to be filtered to preserve the desirable signal and reduce unwanted noise; variation in discretization methods also leads to different results. A suitable solution would be to adopt absolute discretization with fixed bin sizes, which have better repeatability and stability (170). Finally, feature nomenclature, algorithms, methodology, and software have also varied between the different studies, which can jeopardize the accuracy and performance of the models (52). Therefore, the lack of conformity between the above aspects must be elucidated and unified to eliminate differences as much as possible in the process of feature extraction.
On the other hand, there are still some shortcomings in the design and construction of radiogenomics studies. Firstly, most studies are retrospective with small sample sizes and a lack of prospective validation cohorts. The main restriction for deep learning radiogenomics is the limited size of the available datasets. The insufficiency of the required volume of data can lead to inadequate stratification (171–173) among training, validation and testing datasets, compromising the model adaptation, optimization, and evaluation process, respectively. In addition, quantitative descriptors with interpretability are also important in clinical practice. Therefore, interpretable models combined with open-access, curated and high-quality public benchmark databases with complete genomic and imaging data across disease types are urgently needed. Only in this way can we perform better investigations to address tumor heterogeneity. All these deficiencies are prone to produce statistical issues related to overfitted data and multiple testing.
Another drawback is the lack of multicenter studies, which raises doubts that the findings to date would be reproducible by difference in readers, imaging equipment, and radiologists in different fields. Due to the technological imperfections, there is a significant mismatch between the perceived capabilities and the actual capabilities of artificial intelligence in current studies.
Future Direction
To eliminate the inconsistency and uncertainty of different studies, the most critical matter is to formulate a standardized workflow and internationally agreed methods to guide the implementation of efficient studies. Several consortia have been developed worldwide for this purpose. One is the Image Biomarker Standardization Initiative (IBSI) (35), which was established to reach consensus and provide a standard for calculation of the frequently used radiomics features and for the image processing needed before extraction of the radiomics features. It shall also provide guidelines for summarizing comprehensive information on radiomics experiments (174). The TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) statement is a guideline for reporting studies with development or validation of a multivariable prediction model (175). Generally, the standardization of radiomics methods is a prerequisite for subsequent clinical translation, and the workflow and benchmarked values defined by the noted consortia, represent advancement to calibrate future investigations of radiogenomics.
Some additional details on the quality of radiomics studies are being developed, notably for standardization of radiogenomics protocols and quality assurance. High-quality research is key to progress in this field. Researchers should insist on following the “FAIR (findability, accessibility, interoperability, and reusability) guiding principles” by ensuring that all research objectives are traceable, accessible, interoperable, and recyclable, thus enabling independent validation and quality assurance (176). In the near future, international cooperative efforts will be required to confirm the added value of promising quantitative models compared to existing methods (177). Concerted efforts are required to provide a thorough understanding of the relationship between dataset sizes, possible confounders, and the performance of outcome prediction. Consequently, large-scale multicenter prospective studies are needed to generate machine learning-based models.
Fusing imaging modalities (24) with no a priori knowledge or evidence about their optimal combination for the targeted therapy can lead to unnecessary, redundant analysis with a negative effect on the final decision. Thus, clinicians should provide insight and participate in cooperation with the data science engineers regarding specific lesion attributes concerning the followed diagnosis protocols. Other types of clinical information, including laboratory exam results, anthropometricorphic (height and weight), demographic (age and sex) and supplementary imaging modalities can introduce diversity and complementarity toward achieving better problem formulation, improved predictive power, and a robust decision support process (178).
Moreover, future advances in imaging technology, post-processing techniques, and computer-aided diagnostic technology, including sophisticated functional imaging techniques such as 23Na-MR imaging, chemical exchange saturation transfer (CEST), blood oxygen level-dependent (BOLD) MRI, and hybrid PET-MRI, may reinforce the role of radiogenomics in tumor classification and treatment. Development of a pool of labeled metabolites has triggered further insight into cellular activity and provides a potential tool for identification of correlations between imaging features and tumor genotype.
Conclusion
In conclusion, radiogenomics is an inevitable outcome following the trend of precision medicine nowadays. With three main advantages, including lower cost than conventional genome sequencing, availability of whole tumor information as opposed to a limited biopsy specimen, and increased spatial resolution, a comprehensive radiomics-based approach may reflect the spatial variation and heterogeneity of voxel intensities within a tumor and generate predictive and prognostic information (179). Applying a mass of automatic extraction of characterized data algorithms and combining them with clinical information into open databases, radiogenomics has emerged as a bridge between the phenotype and genotype of tumors (180).
It is expected that the role of radiogenomics will extend to every aspect of cancer management, from calibrating detection and diagnosis to predicting the therapeutic response, to risk surveillance. Furthermore, input of imaging data directly into the discovery engine rather than using radiomics feature sets previously developed or recognized and constructing a customized sequence through deep learning architecture has encouraged further exploratory radiogenomics studies (14).
In the future, it is expected that the data acquired from imaging examinations will be transformed into quantitative data and interfaced with existing databases to offer diagnostic and prognostic evidence for supporting clinical decision-making.
Authors’ Contributions
LS and HR contributed to the writing of the paper. XY, JL, and ZC provided essential assistance and designed the constructure of this review. YC, HZ, and PS supervised the manuscript and revised the paper. The authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We owe thanks to all the members participating in this work.
Abbreviations
ADC, apparent diffusion coefficient; ALK, anaplastic lymphoma kinase; ATRX, alpha thalassemia/mental retardation X-linked gene; BAP1, BRCA1-associated protein 1; BOLD-MRI, blood oxygen level-dependent MRI; CBV, cerebral blood volume; ccRCC, clear cell renal cell carcinoma; CEST, chemical exchange saturation transfer; CIN, chromosomal instability; CNNs, convolutional neural networks; CRC, colorectal cancer; EGFR, epidermal growth factor receptor; EML4, echinoderm microtubule-associated protein-like 4; FDG, fluorodeoxyglucose; GBM, glioblastoma multiforme; GCGMM, GrowCut with cancer-specific multiparametric Gaussian Mixture Model; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HGSOC, high-grade serous ovarian cancer; HNSCCs, head and neck squamous cell cancers; HOTAIR, homeobox transcript antisense intergenic RNA; HRV, high-risk volume; IBSI, Image Biomarker Standardization Initiative; ICC, intrahepatic cholangiocarcinoma; IDH, isocitrate dehydrogenase; KRAS, Kristen rat sarcoma viral oncogene; MGMT, O6-methylguanine-DNA-methyltransferase; MRI, magnetic resonance imaging; NF-ĸB, nuclear factor kappa-light-chain-enhancer of activated B-cells; NSCLC, non-small cell lung cancer; PET-CT, positron emission tomography-computed tomography; RCC, renal cell carcinoma; ROC, receiver-operating characteristic; ROI, region of interest; RUNX3, runt-related transcription factor-3 gene; SCLC, small cell lung cancer; SPECT, single-photon emission-computed tomography; TNF-α, tumor necrosis factor-alpha; TRIPOD, Transparent Reporting of a multivariable prediction model for Individual; Prognosis Or Diagnosis.
References
1. Nakagawa H, Fujita M, Fujimoto A. Genome sequencing analysis of liver cancer for precision medicine. Semin Cancer Biol (2019) 55:120–7. doi: 10.1016/j.semcancer.2018.03.004
2. Ramaswami R, Bayer R, Galea S. Precision Medicine from a Public Health Perspective. Annu Rev Public Health (2018) 39:153–68. doi: 10.1146/annurev-publhealth-040617-014158
3. Ding L, Bailey MH, Porta-Pardo E, Thorsson V, Colaprico A, Bertrand D, et al. Perspective on Oncogenic Processes at the End of the Beginning of Cancer Genomics. Cell (2018) 173(2):305–320.e10. doi: 10.1016/j.cell.2018.03.033
4. Zhou S, Treloar AE, Lupien M. Emergence of the Noncoding Cancer Genome: A Target of Genetic and Epigenetic Alterations. Cancer Discovery (2016) 6(11):1215–29. doi: 10.1158/2159-8290.CD-16-0745
5. Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer (2019) 19(9):495–509. doi: 10.1038/s41568-019-0179-8
6. Kamel HFM, Al-Amodi HSAB. Exploitation of Gene Expression and Cancer Biomarkers in Paving the Path to Era of Personalized Medicine. Genomics Proteomics Bioinf (2017) 15(4):220–35. doi: 10.1016/j.gpb.2016.11.005
7. Dimitrakopoulos C, Vrugt B, Flury R, Schraml P, Knippschild U, Wild P, et al. Identification and Validation of a Biomarker Signature in Patients With Resectable Pancreatic Cancer via Genome-Wide Screening for Functional Genetic Variants. JAMA Surg (2019) 154(6):e190484. doi: 10.1001/jamasurg.2019.0484
8. Panayides AS, Pattichis MS, Leandrou S, Pitris C, Constantinidou A, Pattichis CS. Radiogenomics for Precision Medicine With a Big Data Analytics Perspective. IEEE J BioMed Health Inform (2019) 23(5):2063–79. doi: 10.1109/JBHI.2018.2879381
9. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun (2014) 5:4644. doi: 10.1038/ncomms5006
10. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology (2016) 278(2):563–77. doi: 10.1148/radiol.2015151169
11. Larue RT, Defraene G, De Ruysscher D, Lambin P, van Elmpt W. Quantitative radiomics studies for tissue characterization: a review of technology and methodological procedures. Br J Radiol (2017) 90(1070):20160665. doi: 10.1259/bjr.20160665
12. Aerts HJ. The Potential of Radiomic-Based Phenotyping in Precision Medicine: A Review. JAMA Oncol (2016) 2(12):1636–42. doi: 10.1001/jamaoncol.2016.2631
13. Rosenstein BS, West CM, Bentzen SM, Alsner J, Andreassen CN, Azria D, et al. Radiogenomics: radiobiology enters the era of big data and team science. Int J Radiat Oncol Biol Phys (2014) 89(4):709–13. doi: 10.1016/j.ijrobp.2014.03.009
14. Nie K, Al-Hallaq H, Li XA, Benedict SH, Sohn JW, Moran JM. NCTN Assessment on Current Applications of Radiomics in Oncology. Int J Radiat Oncol Biol Phys (2019) 104(2):302–15. doi: 10.1016/j.ijrobp.2019.01.087
15. Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology (2020) 295(2):328–38. doi: 10.1148/radiol.2020191145
16. Yankeelov TE, Abramson RG, Quarles CC. Quantitative multimodality imaging in cancer research and therapy. Nat Rev Clin Oncol (2014) 11(11):670–80. doi: 10.1038/nrclinonc.2014.134
17. Nyflot MJ, Yang F, Byrd D, Bowen SR, Sandison GA, Kinahan PE. Quantitative radiomics: impact of stochastic effects on textural feature analysis implies the need for standards. J Med Imaging (Bellingham) (2015) 2(4):41002. doi: 10.1117/1.JMI.2.4.041002
18. Tixier F, Cheze-le-Rest C, Schick U, Simon B, Dufour X, Key S, et al. Transcriptomics in cancer revealed by Positron Emission Tomography radiomics. Sci Rep (2020) 10(1):5660. doi: 10.1038/s41598-020-62414-z
19. Anderson AW, Xie J, Pizzonia J, Bronen RA, Spencer DD, Gore JC. Effects of cell volume fraction changes on apparent diffusion in human cells. Magn Reson Imaging (2000) 18(6):689–95. doi: 10.1016/s0730-725x(00)00147-8
20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
21. Mu W, Tunali I, Gray JE, Qi J, Schabath MB, Gillies RJ. Radiomics of 18F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur J Nucl Med Mol Imaging (2020) 47(5):1168–82. doi: 10.1007/s00259-019-04625-9
22. Parmar C, Rios Velazquez E, Leijenaar R, Jermoumi M, Carvalho S, Mak RH, et al. Robust Radiomics feature quantification using semiautomatic volumetric segmentation. PloS One (2014) 9(7):e102107. doi: 10.1371/journal.pone.0102107.
23. Yip SSF, Parmar C, Blezek D, Estepar RSJ, Pieper S, Kim J, et al. Application of the 3D slicer chest imaging platform segmentation algorithm for large lung nodule delineation. PloS One (2017) 12(6):e0178944. doi: 10.1371/journal.pone.0178944.
24. Dorador J, Rodríguez-Tovar FJ. CroSSED sequence, a new tool for 3D processing in geosciences using the free software 3DSlicer. Sci Data (2020) 7(1):270. doi: 10.1038/s41597-020-00614-y.
25. Mouawad M, Biernaski H, Brackstone M, Lock M, Kornecki A, Shmuilovich O, et al. The Effect of Registration on Voxel-Wise Tofts Model Parameters and Uncertainties from DCE-MRI of Early-Stage Breast Cancer Patients Using 3DSlicer. J Digit Imaging (2020) 33(5):1065–72. doi: 10.1007/s10278-020-00374-6
26. Velazquez ER, Parmar C, Jermoumi M, Mak RH, van Baardwijk A, Fennessy FM, et al. Volumetric CT-based segmentation of NSCLC using 3D-Slicer. Sci Rep (2013) 3:3529. doi: 10.1038/srep03529
27. Powles J, Hodson H. Google DeepMind and healthcare in an age of algorithms. Health Technol (Berl) (2017) 7(4):351–67. doi: 10.1007/s12553-017-0179-1
28. Sensakovic WF, Armato SG, Straus C, Roberts RY, Caligiuri P, Starkey A, et al. Computerized segmentation and measurement of malignant pleural mesothelioma. Med Phys (2011) 38(1):238–44. doi: 10.1118/1.3525836
29. Tixier F, Um H, Young RJ, Veeraraghavan H. Reliability of tumor segmentation in glioblastoma: Impact on the robustness of MRI-radiomic features. Med Phys (2019) 46(8):3582–91. doi: 10.1002/mp.13624
30. Um H, Tixier F, Bermudez D, Deasy JO, Young RJ, Veeraraghavan H. Impact of image preprocessing on the scanner dependence of multi-parametric MRI radiomic features and covariate shift in multi-institutional glioblastoma datasets. Phys Med Biol (2019) 64(16):165011. doi: 10.1088/1361-6560/ab2f44
31. Veeraraghavan H, Dashevsky BZ, Onishi N, Sadinski M, Morris E, Deasy JO, et al. Appearance Constrained Semi-Automatic Segmentation from DCE-MRI is Reproducible and Feasible for Breast Cancer Radiomics: A Feasibility Study. Sci Rep (2018) 8(1):4838. doi: 10.1038/s41598-018-22980-9
32. Liu Q, Jiang P, Jiang Y, Ge H, Li S, Jin H, et al. Prediction of Aneurysm Stability Using a Machine Learning Model Based on PyRadiomics-Derived Morphological Features. Stroke (2019) 50(9):2314–21. doi: 10.1161/STROKEAHA.119.025777
33. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging (2012) 30(9):1323–41. doi: 10.1016/j.mri.2012.05.001
34. Chen Y, Chang W, Ren L, Chen J, Tang W, Liu T, et al. Comprehensive Evaluation of Relapse Risk (CERR) Score for Colorectal Liver Metastases: Development and Validation. Oncologist (2020) 25(7):e1031–41. doi: 10.1634/theoncologist.2019-0797
35. Apte AP, Iyer A, Crispin-Ortuzar M, Pandya R, van Dijk LV, Spezi E, et al. Technical Note: Extension of CERR for computational radiomics: A comprehensive MATLAB platform for reproducible radiomics research. Med Phys (2018) 10.1002/mp.13046. doi: 10.1002/mp.13046
36. Bettinelli A, Branchini M, De Monte F, Scaggion A, Paiusco M. Technical Note: An IBEX adaption toward image biomarker standardization. Med Phys (2020) 47(3):1167–73. doi: 10.1002/mp.13956
37. Ger RB, Cardenas CE, Anderson BM, Yang J, Mackin DS, Zhang L, et al. Guidelines and Experience Using Imaging Biomarker Explorer (IBEX) for Radiomics. J Vis Exp (2018) 131):57132. doi: 10.3791/57132
38. Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA. Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology (2013) 266:326–36. doi: 10.1148/radiol.12112428
39. Sibille L, Seifert R, Avramovic N, Vehren T, Spottiswoode B, Zuehlsdorff S, et al. 18F-FDG PET/CT Uptake Classification in Lymphoma and Lung Cancer by Using Deep Convolutional Neural Networks. Radiology (2020) 294(2):445–52. doi: 10.1148/radiol.2019191114
40. El Naqa I, Kerns SL, Coates J, Luo Y, Speers C, West CML, et al. Radiogenomics and radiotherapy response modeling. Phys Med Biol (2017) 62(16):R179–206. doi: 10.1088/1361-6560/aa7c55
41. Gao J, Jiang Q, Zhou B, Chen D. Convolutional neural networks for computer-aided detection or diagnosis in medical image analysis: An overview. Math Biosci Eng (2019) 16(6):6536–61. doi: 10.3934/mbe.2019326
42. Liu B, Chi W, Li X, Li P, Liang W, Liu H, et al. Evolving the pulmonary nodules diagnosis from classical approaches to deep learning-aided decision support: three decades’ development course and future prospect. J Cancer Res Clin Oncol (2020) 146(1):153–85. doi: 10.1007/s00432-019-03098-5
43. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature (2015) 521(7553):436–44. doi: 10.1038/nature14539
44. Xu Y, Hosny A, Zeleznik R, Parmar C, Coroller T, Franco I, et al. Deep Learning Predicts Lung Cancer Treatment Response from Serial Medical Imaging. Clin Cancer Res (2019) 25(11):3266–75. doi: 10.1158/1078-0432.CCR-18-2495
45. Levy JJ, O’Malley AJ. Don’t dismiss logistic regression: the case for sensible extraction of interactions in the era of machine learning. BMC Med Res Methodol (2020) 20(1):171. doi: 10.1186/s12874-020-01046-3
46. Deist TM, Dankers FJWM, Valdes G, Wijsman R, Hsu IC, Oberije C, et al. Machine learning algorithms for outcome prediction in (chemo)radiotherapy: An empirical comparison of classifiers. Med Phys (2019) Feb46(2):1080–7. doi: 10.1002/mp.12967
47. Luo Y, Chen S, Valdes G. Machine learning for radiation outcome modeling and prediction. Med Phys (2020) 47(5):e178–84. doi: 10.1002/mp.13570
48. Kang J, Schwartz R, Flickinger J, Beriwal S. Machine Learning Approaches for Predicting Radiation Therapy Outcomes: A Clinician’s Perspective. Int J Radiat Oncol Biol Phys (2015) 93(5):1127–35. doi: 10.1016/j.ijrobp.2015.07.2286
49. Bibault JE, Giraud P, Burgun A. Big Data and machine learning in radiation oncology: State of the art and future prospects. Cancer Lett (2016) 382(1):110–7. doi: 10.1016/j.canlet.2016.05.033
50. Bibault JE, Xing L, Giraud P, El Ayachy R, Giraud N, Decazes P, et al. Radiomics: A primer for the radiation oncologist. Cancer Radiother (2020) 24(5):403–10. doi: 10.1016/j.canrad.2020.01.011
51. Nieboer D, van der Ploeg T, Steyerberg EW. Assessing Discriminative Performance at External Validation of Clinical Prediction Models. PloS One (2016) 11(2):e0148820. doi: 10.1371/journal.pone.0148820
52. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol (2017) 14(12):749–62. doi: 10.1038/nrclinonc.2017.141
53. Konstantinidis IT, Do RK, Gultekin DH, Gönen M, Schwartz LH, Fong Y, et al. Regional chemotherapy for unresectable intrahepatic cholangiocarcinoma: a potential role for dynamic magnetic resonance imaging as an imaging biomarker and a survival update from two prospective clinical trials. Ann Surg Oncol (2014) 21(8):2675–83. doi: 10.1245/s10434-014-3649-y
54. Incoronato M, Aiello M, Infante T, Cavaliere C, Grimaldi AM, Mirabelli P, et al. Radiogenomic Analysis of Oncological Data: A Technical Survey. Int J Mol Sci (2017) 18(4):805. doi: 10.3390/ijms18040805
55. Pinker K, Shitano F, Sala E, Do RK, Young RJ, Wibmer AG, et al. Background, current role, and potential applications of radiogenomics. J Magn Reson Imaging (2018) 47(3):604–20. doi: 10.1002/jmri.25870
56. Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol (2014) 16(5):760. doi: 10.1093/neuonc/not151
57. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
58. Rybinski B, Yun K. Addressing intra-tumoral heterogeneity and therapy resistance. Oncotarget (2016) 7(44):72322–42. doi: 10.18632/oncotarget.11875
59. Just M, Thelen M. Tissue characterization with T1, T2, and proton density values: results in 160 patients with brain tumors. Radiology (1988) 169(3):779–85. doi: 10.1148/radiology.169.3.3187000
60. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. ntegrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. I Cancer Cell (2010) 17(1):98–110. doi: 10.1016/j.ccr.2009.12.020
61. Li Y, Qian Z, Xu K, Wang K, Fan X, Li S, et al. MRI features predict p53 status in lower-grade gliomas via a machine-learning approach. NeuroImage Clin (2017) 17:306–11. doi: 10.1016/j.nicl.2017.10.030
62. Liu X, Mangla R, Tian W, Qiu X, Li D, Walter KA, et al. The preliminary radiogenomics association between MR perfusion imaging parameters and genomic biomarkers, and their predictive performance of overall survival in patients with glioblastoma. J Neurooncol (2017) 135(3):553–60. doi: 10.1007/s11060-017-2602-x
63. Mazurowski MA, Clark K, Czarnek NM, Shamsesfandabadi P, Peters KB, Saha A. Radiogenomics of lower-grade glioma: algorithmically-assessed tumor shape is associated with tumor genomic subtypes and patient outcomes in a multi-institutional study with The Cancer Genome Atlas data. J Neurooncol (2017) 133(1):27–35. doi: 10.1007/s11060-017-2420-1
64. Zinn PO, Singh SK, Kotrotsou A, Hassan I, Thomas G, Luedi MM, et al. A Coclinical Radiogenomic Validation Study: Conserved Magnetic Resonance Radiomic Appearance of Periostin-Expressing Glioblastoma in Patients and Xenograft Models. Clin Cancer Res (2018) 24(24):6288–99. doi: 10.11.58/1078-0432.CCR-17-3420
65. Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol (2015) 17(2):170–82. doi: 10.1038/ncb3090
66. Park SY, Piao Y, Jeong KJ, Dong J, de Groot JF. Periostin (POSTN) Regulates Tumor Resistance to Antiangiogenic Therapy in Glioma Models. Mol Cancer Ther (2016) 15(9):2187–97. doi: 10.1158/1535-7163.MCT-15-0427
67. Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol (2009) 118(4):469–74. doi: 10.1007/s00401-009-0561-9
68. Parker SJ, Metallo CM. Metabolic consequences of oncogenic IDH mutations. Pharmacol Ther (2015) 152:54–62. doi: 10.1016/j.pharmthera.2015.05.003
69. Kickingereder P, Sahm F, Radbruch A, Wick W, Heiland S, Deimling Av, et al. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci Rep (2015) 5:16238. doi: 10.1038/srep16238
70. Hong EK, Choi SH, Shin DJ, Jo SW, Yoo RE, Kang KM, et al. Radiogenomics correlation between MR imaging features and major genetic profiles in glioblastoma. Eur Radiol (2018) 28(10):4350–61. doi: 10.1007/s00330-018-5400-8
71. Kickingereder P, Bonekamp D, Nowosielski M, Kratz A, Sill M, Burth S, et al. Radiogenomics of Glioblastoma: Machine Learning-based Classification of Molecular Characteristics by Using Multiparametric and Multiregional MR Imaging Features. Radiology (2016) 281(3):907–18. doi: 10.1148/radiol.2016161382
72. Cui Y, Ren S, Tha KK, Wu J, Shirato H, Li R. Volume of high-risk intratumoral subregions at multi-parametric MR imaging predicts overall survival and complements molecular analysis of glioblastoma. Eur Radiol (2017) 27(9):3583–92. doi: 10.1007/s00330-017-4751-x
73. Tixier F, Um H, Bermudez D, Iyer A, Apte A, Graham MS, et al. Preoperative MRI-radiomics features improve prediction of survival in glioblastoma patients over MGMT methylation status alone. Oncotarget (2019) 10(6):660–72. doi: 10.18632/oncotarget.26578
74. Razis E, Kotoula V, Koliou GA, Papadopoulou K, Vrettou E, Giannoulatou E, et al. Is There an Independent Role of TERT and NF1 in High Grade Gliomas? Transl Oncol (2020) 13(2):346–54. doi: 10.1016/j.tranon.2019.10.016
75. Koul D. PTEN signaling pathways in glioblastoma. Cancer Biol Ther (2008) 7(9):1321–5. doi: 10.4161/cbt.7.9.6954
76. Hu LS, Ning S, Eschbacher JM, Baxter LC, Gaw N, Ranjbar S, et al. Radiogenomics to characterize regional genetic heterogeneity in glioblastoma. Neuro Oncol (2017) 19(1):128–37. doi: 10.1093/neuonc/now135
77. Jamshidi N, Diehn M, Bredel M, Kuo MD. Illuminating radiogenomic characteristics of glioblastoma multiforme through integration of MR imaging, messenger RNA expression, and DNA copy number variation. Radiology (2014) 270(1):1–2. doi: 10.1148/radiol.13130078
78. Huber KE, Carey LA, Wazer DE. Breast cancer molecular subtypes in patients with locally advanced disease: impact on prognosis, patterns of recurrence, and response to therapy. Semin Radiat Oncol (2009) 19(4):204–10. doi: 10.1016/j.semradonc.2009.05.004
79. Tung NM, Garber JE. BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer (2018) 119(2):141–52. doi: 10.1038/s41416-018-0127-5
80. Li H, Giger ML, Lan L, Janardanan J, Sennett CA. Comparative analysis of image-based phenotypes of mammographic density and parenchymal patterns in distinguishing between BRCA1/2 cases, unilateral cancer cases, and controls. J Med Imaging (Bellingham) (2014) 1(3):31009. doi: 10.1117/1.JMI.1.3.031009
81. Grimm LJ, Zhang J, Mazurowski MA. Computational approach to radiogenomics of breast cancer: Luminal A and luminal B molecular subtypes are associated with imaging features on routine breast MRI extracted using computer vision algorithms. J Magn Reson Imaging (2015) 42(4):902–7. doi: 10.1002/jmri.24879
82. Blaschke E, Abe H. MRI phenotype of breast cancer: Kinetic assessment for molecular subtypes. J Magn Reson Imaging (2015) 42(4):920–4. doi: 10.1002/jmri.24884
83. Mazurowski MA, Zhang J, Grimm LJ, Yoon SC, Silber JI. Radiogenomic analysis of breast cancer: luminal B molecular subtype is associated with enhancement dynamics at MR imaging. Radiology (2014) 273(2):365–72. doi: 10.1148/radiol.14132641
84. Zhu Z, Albadawy E, Saha A, Zhang J, Harowicz MR, Mazurowski MA. Deep learning for identifying radiogenomic associations in breast cancer. Comput Biol Med (2019) 109:85–90. doi: 10.1016/j.compbiomed.2019.04.018
85. Yamamoto S, Maki DD, Korn RL, Kuo MD. Radiogenomic analysis of breast cancer using MRI: a preliminary study to define the landscape. AJR Am J Roentgenol (2012) 199(3):654–63. doi: 10.2214/AJR.11.7824
86. Bates RC, Mercurio AM. Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell (2003) 14(5):1790–800. doi: 10.1091/mbc.e02-09-0583
87. Montesano R, Soulié P, Eble JA, Carrozzino F. Tumour necrosis factor alpha confers an invasive, transformed phenotype on mammary epithelial cells. J Cell Sci (2005) 118(Pt 15):3487–500. doi: 10.1242/jcs.02467
88. Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature (1987) 329(6140):630–2. doi: 10.1038/329630a0
89. Orosz P, Echtenacher B, Falk W, Rüschoff J, Weber D, Männel DN. Enhancement of experimental metastasis by tumor necrosis factor. J Exp Med (1993) 177(5):1391–8. doi: 10.1084/jem.177.5.1391
90. Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev (2006) 25(3):409–16. doi: 10.1007/s10555-006-9005-3
91. Wu J, Li B, Sun X, Cao G, Rubin DL, Napel S, et al. Heterogeneous Enhancement Patterns of Tumor-adjacent Parenchyma at MR Imaging Are Associated with Dysregulated Signaling Pathways and Poor Survival in Breast Cancer. Radiology (2017) 285(2):401–13. doi: 10.1148/radiol.2017162823
92. Tabassum S, Abbasi R, Ahmad N, Farooqi AA. Targeting of JAK-STAT Signaling in Breast Cancer: Therapeutic Strategies to Overcome Drug Resistance. Adv Exp Med Biol (2019) 1152:271–81. doi: 10.1007/978-3-030-20301-6_14
93. Quintás-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res (2013) 19(8):1933–40. doi: 10.1158/1078-0432.CCR-12-0284
94. Yeh AC, Li H, Zhu Y, Zhang J, Khramtsova G, Drukker K, et al. Radiogenomics of breast cancer using dynamic contrast enhanced MRI and gene expression profiling. Cancer Imaging (2019) 19(1):48. doi: 10.1186/s40644-019-0233-5
95. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med (2004) 351(27):2817–26. doi: 10.1056/NEJMoa041588
96. Sutton EJ, Oh JH, Dashevsky BZ, Veeraraghavan H, Apte AP, Thakur SB, et al. Breast cancer subtype intertumor heterogeneity: MRI-based features predict results of a genomic assay. J Magn Reson Imaging (2015) 42(5):1398–406. doi: 10.1002/jmri.24890
97. Yamamoto S, Han W, Kim Y, Du L, Jamshidi N, Huang D, et al. Breast Cancer: Radiogenomic Biomarker Reveals Associations among Dynamic Contrast-enhanced MR Imaging, Long Noncoding RNA, and Metastasis. Radiology (2015) 275:384–92. doi: 10.1148/radiol.15142698
98. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
99. Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ (2014) 349:g4797. doi: 10.1136/bmj.g4797
100. Hakimi AA, Pham CG, Hsieh JJ. A clear picture of renal cell carcinoma. Nat Genet (2013) 45(8):849–50. doi: 10.1038/ng.2708
101. Karlo CA, Di Paolo PL, Chaim J, Hakimi AA, Ostrovnaya I, Russo P, et al. Radiogenomics of clear cell renal cell carcinoma: associations between CT imaging features and mutations. Radiology (2014) 270(2):464–71. doi: 10.1148/radiol.13130663
102. Li ZC, Zhai G, Zhang J, Wang Z, Liu G, Wu GY, et al. Differentiation of clear cell and non-clear cell renal cell carcinomas by all-relevant radiomics features from multiphase CT: a VHL mutation perspective. Eur Radiol (2019) 29(8):3996–4007. doi: 10.1007/s00330-018-5872-6
103. Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science (2018) 359(6377):801–6. doi: 10.1126/science.aan5951
104. Kocak B, Durmaz ES, Ates E, Ulusan MB. Radiogenomics in Clear Cell Renal Cell Carcinoma: Machine Learning-Based High-Dimensional Quantitative CT Texture Analysis in Predicting PBRM1 Mutation Status. AJR Am J Roentgenol (2019) 212(3):W55–63. doi: 10.2214/AJR.18.20443
105. Chuang LS, Ito Y. RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene (2010) 29(18):2605–15. doi: 10.1038/onc.2010.88
106. Cen D, Xu L, Zhang S, et al. Renal cell carcinoma: predicting RUNX3 methylation level and its consequences on survival with CT features. Eur Radiol (2019) 29(10):5415–22. doi: 10.1007/s00330-019-06049-3
107. Kapur P, Peña-Llopis S, Christie A, Zhrebker L, Pavía-Jiménez A, Rathmell WK, et al. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol (2013) 14(2):159–67. doi: 10.1016/S1470-2045(12)70584-3
108. Shinagare AB, Vikram R, Jaffe C, Akin O, Kirby J, Huang E, et al. Radiogenomics of clear cell renal cell carcinoma: preliminary findings of The Cancer Genome Atlas-Renal Cell Carcinoma (TCGA-RCC) Imaging Research Group. Abdom Imaging (2015) 40(6):1684–92. doi: 10.1007/s00261-015-0386-z
109. Connell LC, Harding JJ, Shia J, Abou-Alfa GK. Combined intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Chin Clin Oncol (2016) 5(5):66. doi: 10.21037/cco.2016.10.02
110. Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist (2010) 15 Suppl 4:5–13. doi: 10.1634/theoncologist.2010-S4-05
111. Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology (2016) 150(4):835–53. doi: 10.1053/j.gastro.2015.12.041
112. Kuo MD, Gollub J, Sirlin CB, Ooi C, Chen X. Radiogenomic analysis to identify imaging phenotypes associated with drug response gene expression programs in hepatocellular carcinoma. J Vasc Interv Radiol (2007) 18(7):821–31. doi: 10.1016/j.jvir.2007.04.031
113. Banerjee S, Wang DS, Kim HJ, Sirlin CB, Chan MG, Korn RL, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology (2015) 62(3):792–800. doi: 10.1002/hep.27877
114. Xia W, Chen Y, Zhang R, Yan Z, Zhou X, Zhang B, et al. Radiogenomics of hepatocellular carcinoma: multiregion analysis-based identification of prognostic imaging biomarkers by integrating gene data-a preliminary study. Phys Med Biol (2018) 63(3):035044. doi: 10.1088/1361-6560/aaa609
115. Miura T, Ban D, Tanaka S, Mogushi K, Kudo A, Matsumura S, et al. Distinct clinicopathological phenotype of hepatocellular carcinoma with ethoxybenzyl-magnetic resonance imaging hyperintensity: association with gene expression signature. Am J Surg (2015) 210(3):561–9. doi: 10.1016/j.amjsurg.2015.03.027
116. Taouli B, Hoshida Y, Kakite S, Chen X, Tan PS, Sun X, et al. Imaging-based surrogate markers of transcriptome subclasses and signatures in hepatocellular carcinoma: preliminary results. Eur Radiol (2017) 27(11):4472–81. doi: 10.1007/s00330-017-4844-6
117. Sadot E, Simpson AL, Do RK, Gonen M, Shia J, Allen PJ, et al. Cholangiocarcinoma: Correlation between Molecular Profiling and Imaging Phenotypes. PloS One (2015) 10(7):e0132953. doi: 10.1371/journal.pone.0132953
118. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The Global Burden of Cancer 2013. JAMA Oncol (2015) Aug1(5):690. doi: 10.1001/jamaoncol.2015.0735
119. Vlachavas EI, Pilalis E, Papadodima O, Koczan D, Willis S, Klippel S, et al. Radiogenomic Analysis of F-18-Fluorodeoxyglucose Positron Emission Tomography and Gene Expression Data Elucidates the Epidemiological Complexity of Colorectal Cancer Landscape. Comput Struct Biotechnol J (2019) 17:177–85. doi: 10.1016/j.csbj.2019.01.007
120. Lubner MG, Stabo N, Lubner SJ, del Rio AM, Song C, Halberg RB, et al. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging (2015) 40(7):2331–7. doi: 10.1007/s00261-015-0438-4
121. Shin YR, Kim KA, Im S, Hwang SS, Kim K. Prediction of KRAS Mutation in Rectal Cancer Using MRI. Anticancer Res (2016) 36(9):4799–804. doi: 10.21873/anticanres.11039
122. Miles KA, Ganeshan B, Rodriguez-Justo M, Goh VJ, Ziauddin Z, Engledow A, et al. Multifunctional imaging signature for V-KI-RAS2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations in colorectal cancer. J Nucl Med (2014) 55(3):386–91. doi: 10.2967/jnumed.113.120485
123. Chen SW, Lin CY, Ho CM, Chang YS, Yang SF, Kao CH, et al. Genetic Alterations in Colorectal Cancer Have Different Patterns on 18F-FDG PET/CT. Clin Nucl Med (2015) 40(8):621–6. doi: 10.1097/RLU.0000000000000830
124. Horvat N, Veeraraghavan H, Pelossof RA, Fernandes MC, Arora A, Khan M, et al. Radiogenomics of rectal adenocarcinoma in the era of precision medicine: A pilot study of associations between qualitative and quantitative MRI imaging features and genetic mutations. Eur J Radiol (2019) 113:174–81. doi: 10.1016/j.ejrad.2019.02.022
125. Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol (2012) 4(7):156–69. doi: 10.4251/wjgo.v4.i7.156
126. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med (2001) 345(10):725–30. doi: 10.1056/NEJMoa010187
127. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature (2014) 513(7517):202–9. doi: 10.1038/nature13480
128. Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, et al. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clin Cancer Res (2017) 10.1158/1078-0432.CCR-16-2211. doi: 10.1158/1078-0432.CCR-16-2211
129. Lai YC, Yeh TS, Wu RC, Tsai CK, Yang LY, Lin G, et al. Acute Tumor Transition Angle on Computed Tomography Predicts Chromosomal Instability Status of Primary Gastric Cancer: Radiogenomics Analysis from TCGA and Independent Validation. Cancers (Basel) (2019) 11(5):641. doi: 10.3390/cancers11050641
130. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc (2008) 83(5):584–94. doi: 10.4065/83.5.584
131. Neri S, Yoshida J, Ishii G, Matsumura Y, Aokage K, Hishida T, et al. Prognostic impact of microscopic vessel invasion and visceral pleural invasion in non-small cell lung cancer: a retrospective analysis of 2657 patients. Ann Surg (2014) 260(2):383–8. doi: 10.1097/SLA.0000000000000617
132. Lee J, Cui Y, Sun X, Li B, Wu J, Li D, et al. Prognostic value and molecular correlates of a CT image-based quantitative pleural contact index in early stage NSCLC. Eur Radiol (2018) 28(2):736–46. doi: 10.1007/s00330-017-4996-4
133. Fave X, Zhang L, Yang J, Mackin D, Balter P, Gomez D, et al. Delta-radiomics features for the prediction of patient outcomes in non-small cell lung cancer. Sci Rep (2017) 7(1):588. doi: 10.1038/s41598-017-00665-z
134. Zhou M, Leung A, Echegaray S, Gentles A, Shrager JB, Jensen KC, et al. Non-Small Cell Lung Cancer Radiogenomics Map Identifies Relationships between Molecular and Imaging Phenotypes with Prognostic Implications. Radiology (2018) 286(1):307–15. doi: 10.1148/radiol.2017161845
135. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature (2002) 417(6892):949–54. doi: 10.1038/nature00766
136. Halpenny DF, Plodkowski A, Riely G, Zheng J, Litvak A, Moscowitz C, et al. Radiogenomic evaluation of lung cancer - Are there imaging characteristics associated with lung adenocarcinomas harboring BRAF mutations? Clin Imaging (2017) 42:147–51. doi: 10.1016/j.clinimag.2016.11.015
137. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol (2003) 3(9):745–56. doi: 10.1038/nri1184
138. Nair VS, Gevaert O, Davidzon G, Plevritis SK, West R. NF-κB protein expression associates with (18)F-FDG PET tumor uptake in non-small cell lung cancer: a radiogenomics validation study to understand tumor metabolism. Lung Cancer (2014) 83(2):189–96. doi: 10.1016/j.lungcan.2013.11.001
139. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol (2011) 6:49–69. doi: 10.1146/annurev-pathol-011110-130206
140. Ellison G, Zhu G, Moulis A, Dearden S, Speake G, McCormack R. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol (2013) 66(2):79–89. doi: 10.1136/jclinpath-2012-201194
141. Gevaert O, Echegaray S, Khuong A, Hoang CD, Shrager JB, Jensen KC, et al. Predictive radiogenomics modeling of EGFR mutation status in lung cancer. Sci Rep (2017) 7:41674. doi: 10.1038/srep41674
142. Aerts HJ, Grossmann P, Tan Y, Oxnard GR, Rizvi N, Schwartz LH, et al. Defining a Radiomic Response Phenotype: A Pilot Study using targeted therapy in NSCLC. Sci Rep (2017) Feb 177:41197. doi: 10.1038/srep33860
143. Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer (2018) 9(4):423–30. doi: 10.1111/1759-7714.12613
144. Mendoza DP, Stowell J, Muzikansky A, Shepard JO, Shaw AT, Digumarthy SR. Computed Tomography Imaging Characteristics of Non-Small-Cell Lung Cancer With Anaplastic Lymphoma Kinase Rearrangements: A Systematic Review and Meta-Analysis. Clin Lung Cancer (2019) 20(5):339–49. doi: 10.1016/j.cllc.2019.05.006
145. Engel J, Eckel R, Schubert-Fritschle G, Kerr J, Kuhn W, Diebold J, et al. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer (2002) 38(18):2435–45. doi: 10.1016/s0959-8049(02)00495-1
146. Cooke SL, Ng CK, Melnyk N, Garcia MJ, Hardcastle T, Temple J, et al. Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene (2010) 29(35):4905–13. doi: 10.1038/onc.2010.245
147. Nougaret S, Lakhman Y, Gönen M, Goldman DA, Miccò M, D’Anastasi M, et al. High-Grade Serous Ovarian Cancer: Associations between BRCA Mutation Status, CT Imaging Phenotypes, and Clinical Outcomes. Radiology (2017) 285(2):472–81. doi: 10.1148/radiol.2017161697
148. Neff RT, Senter L, Salani R. BRCA mutation in ovarian cancer: testing, implisations and treatment considerations. Ther Adv Med Oncol (2017) 9(8):519–31. doi: 10.1177/1758834017714993
149. Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol (2008) 26(1):20–5. doi: 10.1200/JCO.2007.11.6905
150. Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer [published correction appears in JAMA. 2012 Jan 25;307(4):363]. JAMA (2011) 306(14):1557–65. doi: 10.1001/jama.2011.1456
151. Vargas HA, Veeraraghavan H, Micco M, Nougaret S, Lakhman Y, Meier AA, et al. A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. Eur Radiol (2017) 27(9):3991–4001. doi: 10.1007/s00330-017-4779-y
152. Shih I, Kurman RJ. Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res (2005) 11(20):7273–9. doi: 10.1158/1078-0432.CCR-05-0755
153. Kaldawy A, Segev Y, Lavie O, Auslender R, Sopik V, Narod SA. Low-grade serous ovarian cancer: A review. Gynecol Oncol (2016) 143(2):433–8. doi: 10.1016/j.ygyno.2016.08.320
154. Nougaret S, Lakhman Y, Molinari N, Feier D, Scelzo C, Vargas HA, et al. CT Features of Ovarian Tumors: Defining Key Differences Between Serous Borderline Tumors and Low-Grade Serous Carcinomas. AJR Am J Roentgenol (2018) 210(4):918–26. doi: 10.2214/AJR.17.18254
155. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol (2017) 3(3):418. doi: 10.1001/jamaoncol.2016.5688
156. Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(5):479–505. doi: 10.6004/jnccn.2019.0023
157. Smith CP, Czarniecki M, Mehralivand S, Stoyanova R, Choyke PL, Harmon S, et al. Radiomics and radiogenomics of prostate cancer. Abdom Radiol (NY) (2019) 44(6):2021–9. doi: 10.1007/s00261-018-1660-7
158. Fütterer JJ. Multiparametric MRI in the Detection of Clinically Significant Prostate Cancer. Korean J Radiol (2017) 18(4):597–606. doi: 10.3348/kjr.2017.18.4.597
159. Stoyanova R, Pollack A, Takhar M, Lynne C, Parra N, Lam LL, et al. Association of multiparametric MRI quantitative imaging features with prostate cancer gene expression in MRI-targeted prostate biopsies. Oncotarget (2016) 7(33):53362–76. doi: 10.18632/oncotarget.10523
160. Poluri RTK, Audet-Walsh É. Genomic Deletion at 10q23 in Prostate Cancer: More Than PTEN Loss? Front Oncol (2018) 8:246. doi: 10.3389/fonc.2018.00246
161. Liu W, Xie CC, Thomas CY, Kim ST, Lindberg J, Egevad L, et al. Genetic markers associated with early cancer-specific mortality following prostatectomy. Cancer (2013) 119(13):2405–12. doi: 10.1002/cncr.27954
162. McCann SM, Jiang Y, Fan X, Wang J, Antic T, Prior F, et al. Quantitative Multiparametric MRI Features and PTEN Expression of Peripheral Zone Prostate Cancer: A Pilot Study. AJR Am J Roentgenol (2016) 206(3):559–65. doi: 10.2214/AJR.15.14967
163. Dimaras H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL, et al. Retinoblastoma. Nat Rev Dis Primers (2015) 1:15021. doi: 10.1038/nrdp.2015.21
164. Jansen RW, de Jong MC, Kooi IE, Sirin S, Göricke S, Brisse HJ, et al. MR Imaging Features of Retinoblastoma: Association with Gene Expression Profiles. Radiology (2018) 288(2):506–15. doi: 10.1148/radiol.2018172000
165. Jou A, Hess J. Epidemiology and Molecular Biology of Head and Neck Cancer. Oncol Res Treat (2017) 40(6):328–32. doi: 10.1159/000477127
166. Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature (2015) 517(7536):576–82. doi: 10.1038/nature14129
167. Zwirner K, Hilke FJ, Demidov G, Socarras Fernandez J, Ossowski S, Gani C, et al. Radiogenomics in head and neck cancer: correlation of radiomic heterogeneity and somatic mutations in TP53, FAT1 and KMT2D. Radiogenomics bei Kopf-Hals-Tumoren: Korrelation von bildgebender Heterogenität und somatischen Mutationen in TP53, FAT1 und KMT2D. Strahlenther Onkol (2019) 195(9):771–9. doi: 10.1007/s00066-019-01478-x
168. Kang J, Rancati T, Lee S, Oh JH, Kerns SL, Scott JG, et al. Machine Learning and Radiogenomics: Lessons Learned and Future Directions. Front Oncol (2018) 8:228. doi: 10.3389/fonc.2018.00228
169. Andreassen CN, Schack LM, Laursen LV, Alsner J. Radiogenomics - current status, challenges and future directions. Cancer Lett (2016) 382(1):127–36. doi: 10.1016/j.canlet.2016.01.035
170. van Velden FH, Kramer GM, Frings V, Nissen IA, Mulder ER, de Langen AJ, et al. Repeatability of Radiomic Features in Non-Small-Cell Lung Cancer [(18)F]FDG-PET/CT Studies: Impact of Reconstruction and Delineation. Mol Imaging Biol (2016) 18(5):788–95. doi: 10.1007/s11307-016-0940-2
171. Chang P, Grinband J, Weinberg BD, Bardis M, Khy M, Cadena G, et al. Deep-Learning Convolutional Neural Networks Accurately Classify Genetic Mutations in Gliomas. AJNR Am J Neuroradiol (2018) 39(7):1201–7. doi: 10.3174/ajnr.A5667
172. Ha R, Mutasa S, Karcich J, Gupta N, Pascual Van Sant E, Nemer J, et al. Predicting Breast Cancer Molecular Subtype with MRI Dataset Utilizing Convolutional Neural Network Algorithm. J Digit Imaging (2019) 32(2):276–82. doi: 10.1007/s10278-019-00179-2
173. Zhu Z, Harowicz M, Zhang J, Saha A, Grimm LJ, Hwang ES, et al. Deep learning analysis of breast MRIs for prediction of occult invasive disease in ductal carcinoma in situ. Comput Biol Med (2019) 115:103498. doi: 10.1016/j.compbiomed.2019.103498
174. Morin O, Vallières M, Jochems A, Woodruff HC, Valdes G, Braunstein SE, et al. A Deep Look Into the Future of Quantitative Imaging in Oncology: A Statement of Working Principles and Proposal for Change. Int J Radiat Oncol Biol Phys (2018) 102(4):1074–82. doi: 10.1016/j.ijrobp.2018.08.032
175. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ (2015) 350:g7594. doi: 10.1136/bmj.g7594
176. Boeckhout M, Zielhuis GA, Bredenoord AL. The FAIR guiding principles for data stewardship: fair enough? Eur J Hum Genet (2018) 26(7):931–6. doi: 10.1038/s41431-018-0160-0
177. Fornacon-Wood I, Mistry H, Ackermann CJ, Blackhall F, McPartlin A, Faivre-Finn C, et al. Reliability and prognostic value of radiomic features are highly dependent on choice of feature extraction platform. Eur Radiol (2020) 30(11):6241–50. doi: 10.1007/s00330-020-06957-9
178. Trivizakis E, Papadakis GZ, Souglakos I, Papanikolaou N, Koumakis L, Spandidos DA, et al. Artificial intelligence radiogenomics for advancing precision and effectiveness in oncologic care (Review). Int J Oncol (2020) 57(1):43–53. doi: 10.3892/ijo.2020.5063
179. Chen S, Ning Z, He X, Qi X. Radiogenomics Map: A Novel Approach for Noninvasive Identification of Molecular Properties? Radiology (2017) 285(3):1060–1. doi: 10.1148/radiol.2017171819
Keywords: precision medicine, deep learning, artificial intelligence, radiogenomics, radiological imaging
Citation: Shui L, Ren H, Yang X, Li J, Chen Z, Yi C, Zhu H and Shui P (2021) The Era of Radiogenomics in Precision Medicine: An Emerging Approach to Support Diagnosis, Treatment Decisions, and Prognostication in Oncology. Front. Oncol. 10:570465. doi: 10.3389/fonc.2020.570465
Received: 08 June 2020; Accepted: 08 December 2020;
Published: 26 January 2021.
Edited by:
Sebastian Cerdan, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Harini Veeraraghavan, Cornell University, United StatesRui Vasco Simoes, Champalimaud Foundation, Portugal
Copyright © 2021 Shui, Ren, Yang, Li, Chen, Yi, Zhu and Shui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Zhu, 441695131@qq.com; Pixian Shui, spx6702@163.com
†These authors have contributed equally to this work
 Lin Shui
Lin Shui Haoyu Ren2†
Haoyu Ren2† Xi Yang
Xi Yang Jian Li
Jian Li Cheng Yi
Cheng Yi Hong Zhu
Hong Zhu