- 1Department of Radiation Oncology, University Medical Centre Freiburg, Freiburg, Germany
- 2Medical Physics and Biomedical Engineering, University College London, London, United Kingdom
- 3Department of Radiation Oncology, University of Magdeburg, Magdeburg, Germany
Biliary tract cancers (BTC) are a disease entity comprising diverse epithelial tumors, which are categorized according to their anatomical location as intrahepatic (iCCA), perihilar (pCCA), distal (dCCA) cholangiocarcinomas, and gallbladder carcinomas (GBC), with distinct epidemiology, biology, and prognosis. Complete surgical resection is the mainstay in operable BTC as it is the only potentially curative treatment option. Nevertheless, even after curative (R0) resection, the 5-year survival rate ranges between 20 and 40% and the disease free survival rates (DFS) is approximately 48–65% after one year and 23–35% after three years without adjuvant treatment. Improvements in adjuvant chemotherapy have improved the DFS, but the role of adjuvant radiotherapy is unclear. On the other hand, more than 50% of the patients present with unresectable disease at the time of diagnosis, which limits the prognosis to a few months without treatment. Herein, we review the role of radiotherapy in the treatment of cholangiocarcinoma in the curative and palliative setting.
Introduction
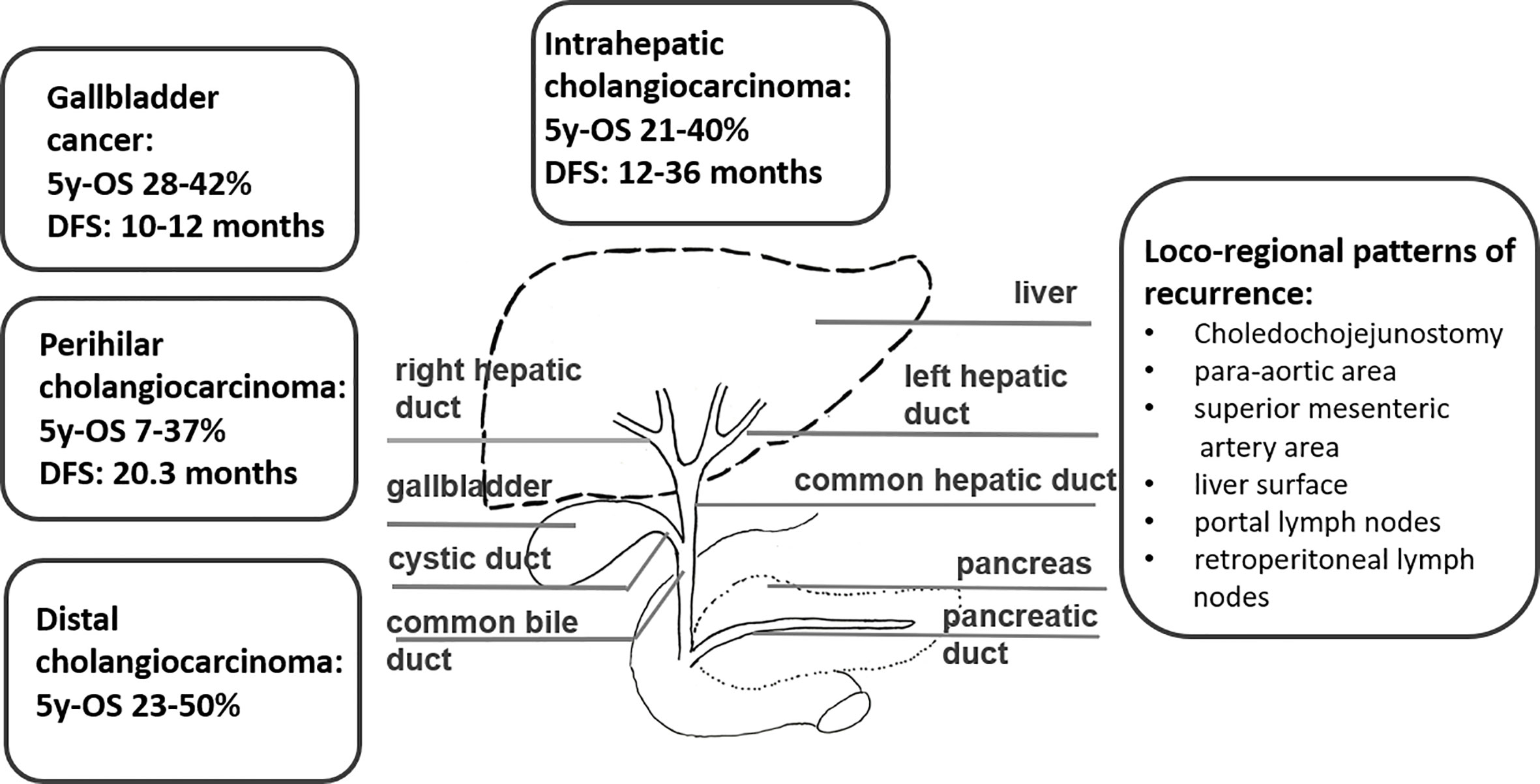
Biliary tract cancers (BTCs) are the second most common hepatic malignancy after hepatocellular carcinomas comprising < 1% of all human cancers (1). They are sub-classified as intrahepatic cholangiocarcinomas (iCCA) originating from the biliary tree within the liver, and extrahepatic cholangiocarcinomas (eCCA) originating from the biliary tree outside the liver, and gallbladder carcinoma (GBC). eCCAs are further subdivided into perihilar (pCCA) and distal (dCCA) cholangiocarcinoma. Their geographical distribution is extremely variable, depending on their localisation, reflecting the difference in risk and genetic factors globally (2–8). BTCs are aggressive tumors and most patients are diagnosed in an advanced stage. More than 50% present with unresectable disease at the time of diagnosis, which limits the prognosis to a few months (9). For patients with operable BTC at diagnosis, complete surgical resection is the mainstay as it is the only potentially curative treatment option (1). Nevertheless, even after curative (R0) resection, the 5-year survival rates range between 20 and 40% (10–12, 13–15). The disease free survival rates (DFS) range between 48 and 65% after 1 year and 23 to 35% after 3 years, without adjuvant treatment (10–12, 13–15). Following surgical resection, both local recurrence and distant metastases occur frequently, with a relapse rate ranging between 56.5 and 88.4% in several prospective trials (16) (Figure 1). Several risk factors for disease recurrence after resection, such as positive margins, positive nodal status and/or vascular invasion, have been identified in several studies (11, 12, 17–21). For muscle invasive gallbladder carcinoma prognosis seems to be even worse than for cholangiocarcinoma (13). Due to the high rates of disease local recurrence and poor survival rates following radical surgery, postoperative treatment modalities, such as chemotherapy, radiotherapy, and chemoradiation, have been considered to improve survival after resection (22). On the other hand, patients with unresectable disease at the time of diagnosis are offered palliative chemotherapy according to guidelines, but radiotherapy could also play an important role. Herein, we review the role of radiotherapy in the treatment of cholangiocarcinoma in the curative and palliative setting (Figure 2).
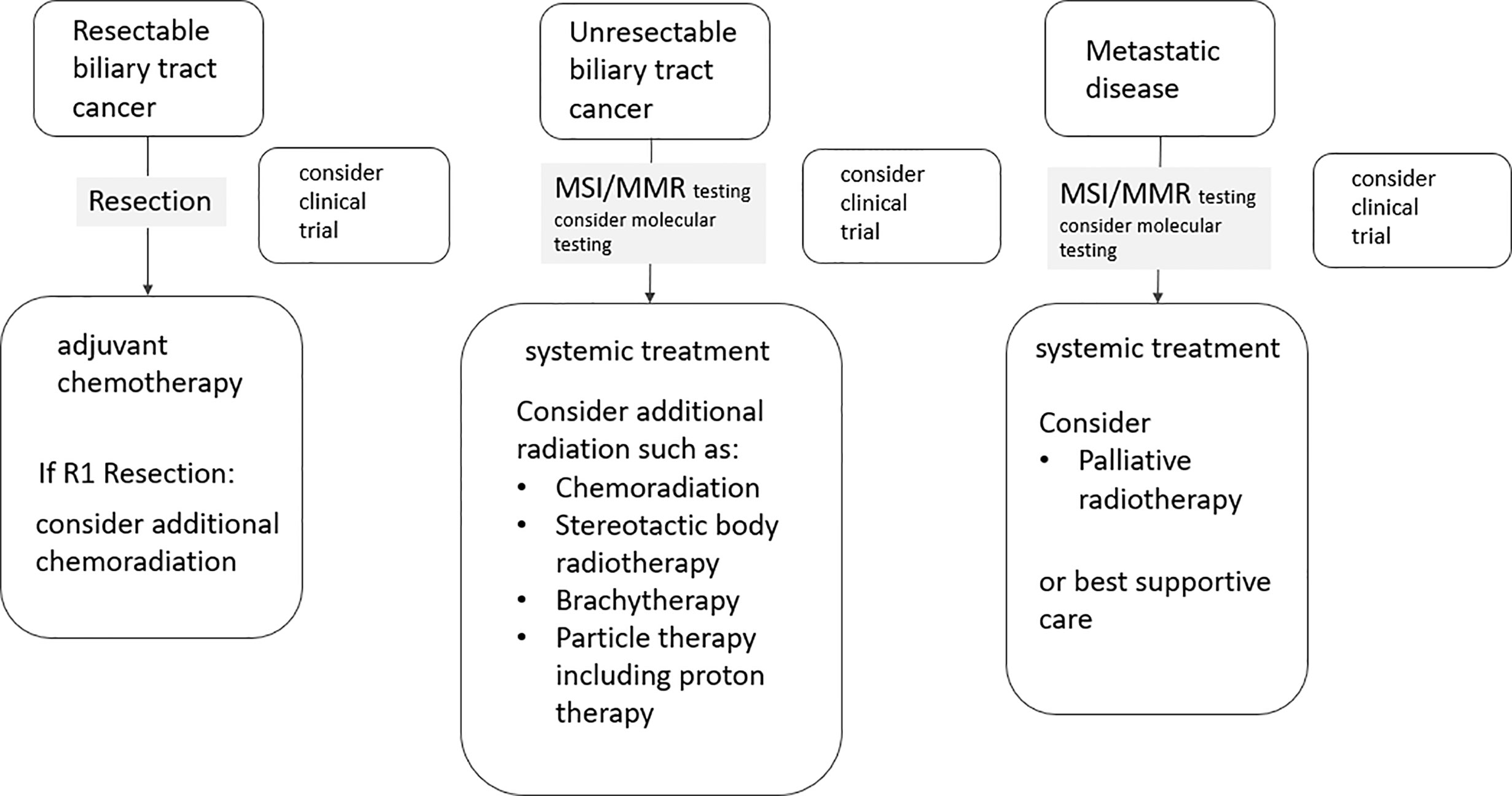
Figure 2 Flow chart illustrating the integration of radiotherapy in the treatment of biliary tract cancers.
Methods
We conducted a systematic search of the PubMed library database published until June 2020 using the following search terms related to cholangiocarcinoma or gallbladder carcinoma and radiotherapy: (cholangiocarcinoma OR bile duct cancer OR Klatskin OR gallbladder) AND (radiotherapy OR chemoradiation OR radiochemotherapy OR chemoradiotherapy OR SBRT OR SABR OR stereotactic body radiotherapy OR stereotactic ablative body radiotherapy OR brachytherapy OR BT OR particle therapy OR proton therapy OR PBT). Additionally, we performed a search for ongoing unpublished trials in clinicaltrials.gov.
Curative Treatment of BTC
In patients with iCCA the DFS ranges between 12 and 36 months in various studies with a 5 year overall survival (OS) between 21 and 40% and median OS as high as 80 months in one cohort after R0 resection (12, 23–26) (Figure 1). Routine lymphadenectomy at the level of the hepato-duodenal ligament is recommended during surgery according to international guidelines (1, 27). Some studies indicate that transplantation might be an effective option in patients with early iCCA. The size of the tumor, the grade, the presence of multiple lesions, vascular and/or perineural invasion, and the lymph node involvement were predictors of short DFS and should be reported by the pathologist to guide decisions regarding adjuvant therapy, although robust evidence for its use is lacking (1, 24).
In patients with pCCA the 5 year OS ranges between 7 and 37% in several studies (25, 26) (Figure 1). The resection often involves lobectomy bile-duct resection, right hemi-hepatectomy, regional lymphadenectomy, and Roux-en-Y hepaticojejunostomy (28). Several surgical advances have facilitated the resection of those tumors in the present years, while liver transplantation offers very good outcomes in selected patients with early disease (29). The presence of regional lymphadenopathy, although not an absolute contraindication for resection, is associated with inferior patient outcomes (28). Lymphadenectomy is at any case a standard part of every curative resection. Liver transplantation in unresectable cases has been explored in study following neoadjuvant chemoradiation, showing a 5-year DFS of 65% (30).
The 5-year OS rates for dCCA rage between 23 and 50% (25, 26) (Figure 1). Patients with dCCA typically undergo partial pancreaticoduodenectomy (Whipple procedure) with extended bile duct resection up to the hilum and dissection of the draining lymph nodes (1). In a large series, R0 resection was achieved in 78%.
GBC has two typical presentations: either (a) incidentally diagnosed in the histological workup of simple cholecystectomies or (b) as a symptomatic right upper quadrant tumor at an advanced stage (1). After R0 resection DFS ranges between 10 and 12 months and OS rates are about 55% after 1 year and about 30% after 3 years in patients with GBC following radical cholecystectomy and partial hepatectomy (10, 13–15) (Figure 1). Over the last 5 years, there is an improvement of the median 5 years OS rate from 28% (between 1995 and 2000) to 42% (between 2015 and 2020) (16). Patients with GBC tend to have higher rates of distant failure compared to CCA (31).
Patterns of Recurrence and Prognostic Factors for Recurrence After Resection
Despite aggressive resection, at least 50% of patients experience recurrence of tumor with the mean time to recurrence ranging from 10 to 20 months (32), while in the major prospective studies evaluating chemotherapy vs. observation in the adjuvant setting, the incidence of relapse in the adjuvant chemotherapy arms ranged between 53.8 and 79.9% and in the observational arms between 56.5 and 88.4% (16). Some studies report a higher incidence of distant metastases in patients with GBC (31, 33), while in CCA relapse patterns vary significantly between studies. Recurrence most frequently involves intrahepatic metastasis, followed by simultaneous intra- and extrahepatic disease, and extrahepatic recurrence alone being the least common (31, 33, 34). Pathologic data suggest that high recurrence and low survival rates are, in part, a result of frequent and early portal vein, lymphatic, biliary and perineural invasion of tumor (35, 36) supporting a strong role for aggressive multimodal therapy. In a study by Jung et al. (37) relapses were the most frequent in the choledochojejunostomy (17.7%); para-aortic area(16.1%) and superior mesenteric artery area (16.1%); and portal vein area (14.5%). In a further study (38) patients who did not receive adjuvant RT developed loco-regional recurrences in 51%, primarily at biliary anastomosis/liver surface, portal lymph nodes, and retroperitoneal lymph nodes. Concerning the patterns of recurrence after curative resection for GBC many patients developed distant recurrences, although the most common site of recurrence was the liver (n = 22, 34.4%) followed by the peritoneum (n = 10, 15.6%) (39). In a study of 156 patients (80 with GBC and 76 with HCCA) Jarnagin et al. (13) reported that 52 (68%) patients with hCCA and 53 (66%) patients with GBC had disease recurrence at a median follow-up of 24 months. The median time to disease recurrence was shorter for patients with GBC compared with patients with hCCA (11.5 vs. 20.3 months; P = 0.007). Of those who developed disease recurrence, isolated loco-regional disease as the first site of failure occurred in 15% of patients with GBCA compared with 59% of patients with hCCA (P < 0.001).
Factors associated with increased risk of relapse include the presence of R1, high serum carbohydrate antigen (CA) 19–9 and the presence of lymph node metastases (16, 23, 40, 41). In other studies additional factors have been identified such as a tumor size >5 cm, the number of lesions, vascular invasion, tumor grading, obstructive jaundice, a neutrophil to lymphocyte ratio (NLR) < 5, and lack of perineural invasion (12, 17–20, 25, 32, 34, 42–46). In a meta-anlysis by Ke et al. (47) the hazard ratio (HR) was 0.60 (95% CI = 0.51–0.69) in the positive resection margin group, and 0.67 (95% CI = 0.57–0.76) in lymph node metastasis (LNM) group. The effect of adjuvant treatment (AT) on the patients with LNM was evaluated in 4 included cohorts (48–51). Using a random-effect model, the pooled HR for the OS in the AT group was 0.67 (95% CI 0.57–0.76), compared with the non-AT group.
Concerning the resection margins, DeOliveira et al. (24) studied 564 consecutive patients treated between 1973 and 2004 (42% distal, 50% perihilar 8% intrahepatic). Whilst the negative margin rate increased during the period studied, the survival of patients with positive margins was worse and on multivariate analysis patients with R0 and N0 had a statistically significant better survival. Additionally Farges et al. reported that surgical margins less that 1mm had a similar outcome compared to R1 resections and margins greater than 5mm were associated with improved survival (52). In a study by Tamandl et al. (11) the distance between the tumor and resection margins correlated with the median DFS ranging between 11.4 to 9.8 months, while in case of R1 resection, the median disease free survival was 9.9 months. A retrospective study evaluating the results of surgical therapy for intrahepatic CCA showed that the most frequent site of disease recurrence was the liver (53).
For patients diagnosed with eCCA, the presence of postoperative CA19-9 (HR 2.26) and presence of lymph node infiltration (HR 2.33) were associated with worse outcomes. Patients with resected eCCA with high pre-and post-operative CA19-9 were shown to have a higher distant metastasis rate and shorter disease-free interval (40). Involvement of adjacent structures, perineural invasion, and poorly-differentiated histology has also been associated with poor outcomes for resected eCCA (16, 23, 41, 54–56). Five-year survival of N+ versus N0 disease was 0 to 29% versus 32 to 67% in pCCA, and 16 to 21% versus 42 to 61% in dCCA (26).
For iCCA, factors associated with increased relapse rate and poor prognosis include R1 resection, lymphatic invasion, vascular invasion, and peri-ductal infiltrating disease (23, 42, 57–60). Prognostic nomograms have been designed for patients with resected iCCA (61) including serum carcinoembryonic antigen (CEA), CA199), tumor diameter and number, vascular invasion, lymph node metastasis, direct invasion, and local extra-hepatic metastasis, showing a superiority in prognostic discrimination compared to five other staging systems for iCCA (p < 0.001). Five-year survival of N+ versus N0 disease was 0 to 9% versus 36 to 43% in iCCA (26).
For GBM, higher recurrence rates are associated with R1-resection, depth of mural invasion, lymph node metastasis, extramural extension, and perineural invasion (16, 1, 62).
Adjuvant Therapies
The high rates of recurrence following surgery justify the consideration of an adjuvant treatment. In a meta-analysis by Horgan et al. (63), including 6712 patients treated between 1960 and 2010 there was a trend for improved OS with adjuvant treatment compared to resection alone (odds ratio (OR), 0.74; P = 0.06). Chemotherapy regimens, either alone or in combination with radiotherapy, showed a statistically greater impact on survival than radiotherapy alone, especially for patients with lymph node involvement (OR, 0.49; P = 0.004) and involved resection margins (OR, 0.36; P = 0.002). Manterola et al (64). conducted a meta-analysis including 3 systematic reviews and 24 observational studies evaluating the role of adjuvant treatment in GBC concluding that the results do not provide strong evidence that AT is effective in patients who undergo resection for GBC. Subgroups with positive lymph nodes and positive surgical margins may have a survival advantage. Additionally, in the meta-analysis by Ke et al. (47), subgroup analysis showed that the pooled HR for the OS rate in the AT group compared with non-AT group were as follows: chemotherapy group was 0.57 (95% CI = 0.44–0.70), TACE group was 0.56 (95% CI 0.31–0.82), radiotherapy group was 0.71 (95% CI = 0.39–1.03), chemoradiation group was 0.73 (95% CI = 0.57–0.89), positive resection margin group was 0.60 (95% CI = 0.51–0.69), and lymph node metastasis (LNM) group was 0.67 (95% CI = 0.57–0.76). Thus, prospective trials are needed to elaborate the role of adjuvant therapy.
Adjuvant Chemotherapy
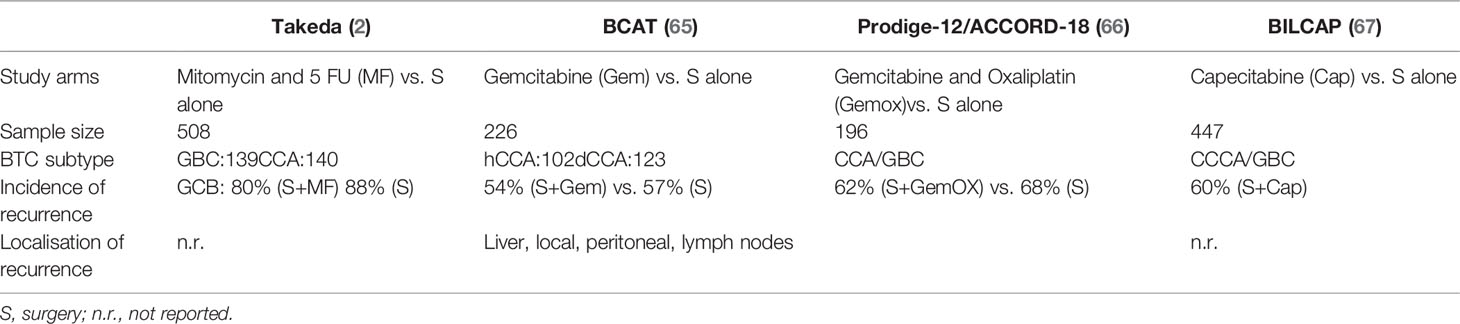
Over the last decades, four randomized phase III clinical trials have evaluated the role of adjuvant chemotherapy in resected BTC (Table 1). In the BCAT study patients with eCCA were randomized between observation alone vs. gemcitabine. Patients with CCA and GBC were randomized between observation vs. gemcitabine and oxaliplatin in the PRODIGE-12/ACCORD-18 trial, observation vs. capecitabine in BILCAP study and observation vs. mitomycin C combined with 5 FU in the study by Takada et al. (10).
In the prospective randomized trial by Takada there was a non-significant benefit for patients with CCA with R0 resection receiving adjuvant therapy, with a DFS at 5 years of 15.8 vs. 32.4% and an OS at 5 years of 28.3 vs. 41.0% (10). The PRODIGE-12/ACCORD-18 trial showed a non-significant improvement in the recurrence-free survival (RFS) for gemcitabine and oxaliplatin compared to observation alone (30.4 vs. 22 months, HR 0.83, 95% CI: 0.58–1.19, p = 0.31) in 196 patients (66), while the BCAT trial has shown that adjuvant gemcitabine is not associated with improved RFS or OS (65). the BILCAP study (68)showed a benefit from adjuvant capecitabine in terms of OS (pre-planned sensitivity analysis in the intention-to-treat population and in the per-protocol analysis), with confirmed benefit in terms of RFS. The treatment was well tolerated without unexpected adverse events or a detriment in quality of life. Based on the BILCAP trial, international guidelines recommend adjuvant capecitabine for a period of 6 months following potentially curative resection of CCA as the current standard of care for resected CCA and GBC (16).
Adjuvant Radiotherapy
Chemoradiation
Several studies have evaluated the role of adjuvant radiotherapy or chemoradiation (CRT) in this setting. A systematic review and meta-analysis on adjuvant radiotherapy in EHCC (69) including 10 studies, demonstrated an improvement in OS with adjuvant radiation therapy or chemoradiation with 5 FU (57% of the studies) (HR, hazards ratio 0.62, 95% CI: 0.48–0.78, p < 0.001) with a low incidence of late radiation toxicity (2–9% of the patients), mainly late obstruction or gastrointestinal bleeding. In a further systematic review and meta-analysis including 20 studies (63, 70), patients receiving CT or CRT derived statistically greater benefit than RT alone (OR, 0.39, 0.61, and 0.98, respectively; P = 0.02), especially patients with LN-positive disease (OR, 0.49; P = 0.004) and R1 disease (OR, 0.36; P = 0.002). While in a further meta-analysis by Ren et al. (71) including 21 studies, with 1465 EHCC and GBC patients, 5-year overall survival (OS) rate was higher in the adjuvant RT group than in the non-RT group (OR = 0.63; 95% CI = 0.50–0.81, p = 0.0002). The 5-year OS rate was significantly higher for those with positive lymph nodes (OR = 0.15; 95% CI = 0.07–0.35; p < 0.00001) and margin-positive resection (OR = 0.40; 95% CI = 0.19–0.85; p = 0.02) in the adjuvant RT group than in the non-RT group. The local recurrence rate was significantly lower in the adjuvant RT group than in the non-RT group (OR = 0.54; 95% CI = 0.38–0.76, p = 0.0004).
Four national cancer database (NCD) analyses and four Surveillance, Epidemiology and End Results (SEER) database analyses have evaluated the role of adjuvant treatment, including radiation. In an analysis from 1998–2013 (72), 2897 patients were identified, R0 status was achieved in 1951 patients (67.3%) and RT was delivered to 525 patients (R0 = 255, R1/R2 = 230, unknown = 43). Following propensity score matching, the OS for R0 versus R1/R2 resection was 31.2 versus 19.5 months (p = .001), respectively. RT was associated with a trend toward improved survival for R1/R2 lymph node negative patients (39.5 vs. 21.1 months; p = 0.052). Patients with a positive resection margin had a higher risk of disease recurrence (HR, 1.61; 95% CI, 1.15–2.27; p = .01) and a shorter overall survival (HR 1.54; 95% CI, 1.12–2.11; p = 0,001). In an additional NCD analysis, from 2004–2012 (73), evaluating the role of surgery and adjuvant therapy in lymph node positive GBC and iCCA, adjuvant treatment, including radiation, was associated with a lower risk of death relative to surgery alone for patients with GBC regardless of margin status (margin-negative resection: HR, 0.66; 95% CI, 0.52–0.84; margin-positive resection: HR, 0.54; 95% CI, 0.39–0.75), while adjuvant chemotherapy alone was not. For patients with iCCA, no survival benefit was detected with adjuvant chemotherapy or radiation for those who underwent either margin-positive or margin-negative resection. In a further NCDB analysis (74), evaluating the benefit of adjuvant therapy following resection for intrahepatic cholangiocarcinoma after adjusting for other prognostic variables, patients were found to significantly benefit from AT if they had positive lymph nodes (chemotherapy: HR, 0.54; p = 0.0365; chemoradiation: HR, 0.50, p = 0.005) or positive margins (chemotherapy: HR, 0.44; p = 0.0016; chemoradiation: HR, 0.57; p = 0.0039). Lastly, in a propensity score matched analysis from a NCD (2004–2014) including extrahepatic bile duct cancers adjuvant therapy was associated with improved median OS for hilar tumors (40.0 vs. 30.6 months; p = 0.025) but not distal tumors (33.0 vs. 30.3 months; p = 0.123), while chemoradiation was associated with superior outcomes compared with chemotherapy alone in the subset of margin-positive resection (HR 0.63; 95% CI, 0.42–0.94) (75).
A SEER database comprising patients with EHCC (n = 1569) treated between 1973 and 2005 suggest an early survival advantage for adjuvant radiotherapy (25 vs. 21 months after R1 resection with versus without adjuvant radiotherapy, p < 0.001) whereas survival was almost identical for patients after R0-resection (26 vs. 25 months) (76). In another SEER analysis by Shinohara including 4,758 patients palliative RT prolonged survival, while the benefit associated with surgery and RT was significant on univariate analysis but not after controlling for potential confounders using the propensity score (77). A further SEER database analysis (78) including 3839 patients with IHCC, use of surgery, and adjuvant radiation therapy conferred the greatest benefit on OS (HR = 0.40; 95% CI, 0.34– 0.47), followed by surgery alone (hazard ratio [HR], 0.49; 95% CI, 0.44–0.54) and radiation therapy alone (HR, 0.68; 95% CI, 0.59–0.77) compared with no treatment on multivariate analysis. Finally, in a SEER analysis from 1973–2003, including patients with resected eCCA, adjuvant RT was not associated with an improvement in long-term overall survival in patients with resected extrahepatic bile duct cancer (79). Major limitations of these four SEER database analyses are the lack of information concerning patient and treatment related factors and subsequent treatments. Furthermore, most of these patients were treated without concurrent chemoradiation.
In several studies maintenance chemotherapy after adjuvant concurrent CRT showed promising results (80–82). In a Phase II study in pancreatic cancers and BTCs (83), evaluating the combination of adjuvant chemotherapy with taxane and gemcitabine followed by chemoradiation the treatment was discontinued by 15% of the patients due to adverse events. Grade 3 or greater non-hematological toxicities were observed in 15% of patients. Recently the Phase II SWOG 0809 trial (84) evaluated the role of adjuvant gemcitabine with capecitabine followed by concurrent CRT with capecitabine in patients with BTC mainly EHCC and GBC. The OS was 35 months, similar for both R0 and R1 resected patients (R0, 34 months; R1, 35 months). The trial met the primary endpoint, the treatment incurred toxicity grade 3 to 4 adverse effects such as neutropenia (44%), hand-foot syndrome (11%), diarrhoea (8%), lymphopenia (8%), and leukopenia (6%). This trial establishes the feasibility of conducting national adjuvant trials in EHCC and GBCA and provides baseline data for planning future phase III trials (85).
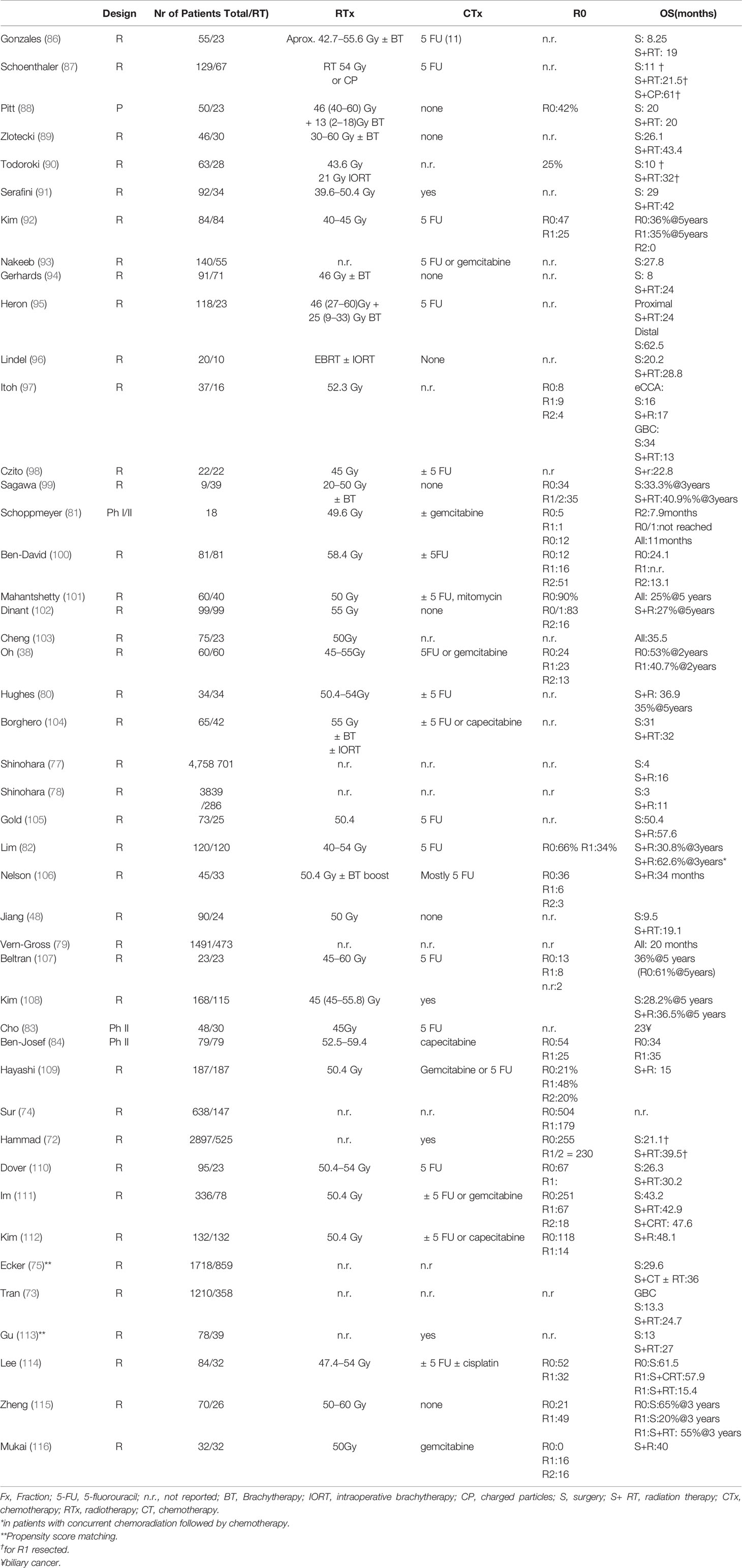
In conclusion, non-randomized phase II trial and meta-analyses support the efficacy chemoradiation in the adjuvant setting. Selected prospective and retrospective studies with subgroups of patients receiving adjuvant radiotherapy or chemoradiation are summarized in Table 2.
Brachytherapy
The role of brachytherapy (BT) mostly as brachytherapy boost after EBRT has been also evaluated in the adjuvant setting mostly in singe-centre retrospective studies. Gerhards (94) et al. reported that the addition of BT to external radiotherapy in the adjuvant setting provided no significant benefit in hCCA, while the incidence of toxicities was higher. In the case of R1 resection, a combination of adjuvant therapy with EBRT plus BT led toa comparable survival as in patients with R0 resection in hCCA with a median survival of 26 months in a small number of selected patients (117). In Table 2, we summarize the results of adjuvant RT with or without brachytherapy boost. In conclusion, the additional advantage through a BT boost in the adjuvant setting is unclear.
Neoadjuvant Chemoradiation
Neoadjuvant chemoradiation has been investigated either before resection or prior to liver transplantation (118–120) as a treatment option in primarily unresectable cholangiocarcinoma.
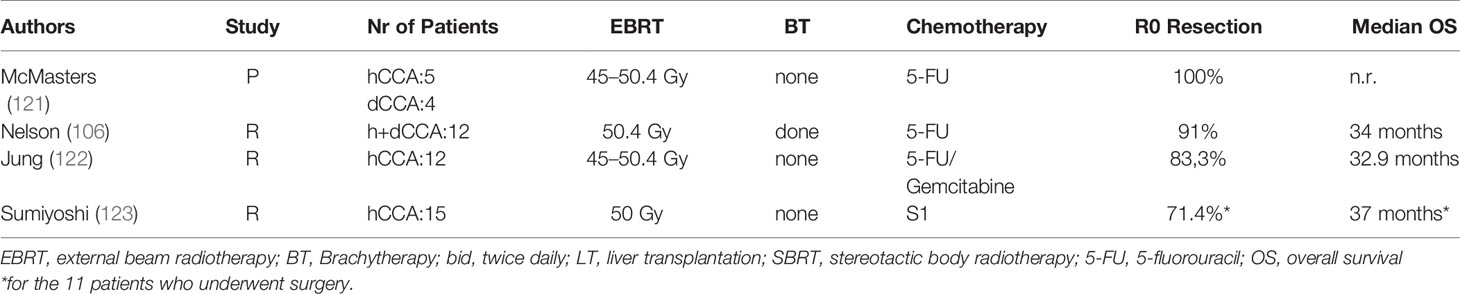
Chemoradiation prior to surgery was evaluated in four studies (Table 3), leading to R0 resection rates between 71.4 and 100%. Nelson et al. (106) compared retrospectively patients treated with adjuvant vs. neoadjuvant chemoradiation showing that neoadjuvant chemoradiation led to a prolonged OS (5-year survival 53 vs. 23%, p = 0.16) and similar rates of Grade 2–3 surgical morbidity (16 vs. 33%, p = 0.24) compared with those treated in the postoperative setting, although the latter presented with more advanced disease at diagnosis. A Phase I trial estimated the maximum tolerated dose of gemcitabine at 600 mg m−2 with 45 Gy in 1.8-Gy daily fraction for neoadjuvant CRT (124).
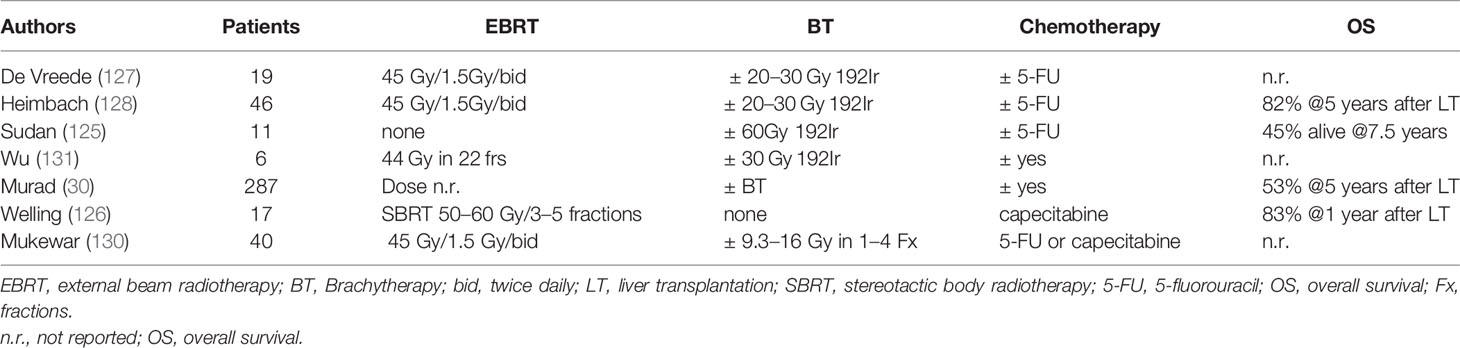
Neoadjuvant chemoradiation was also used in combination with liver transplantation in patients with pCCA in small single centre studies, either as brachytherapy (125), SBRT (126), or concurrent CRT (127–130) (Table 4). In a multi-centre study by Darwish Murad et al. (30) with pCCA treated with neoadjuvant therapy followed up by liver transplantation at 12 US centers, the recurrence-free survival after 5 year was 65% showing this therapy is effective in selected patients. Both concepts should be further evaluated in clinical trials in addition to ongoing trial of Liver Resection versus Radio-chemotherapy-Transplantation for Hilar Cholangiocarcinoma (TRANSPHIL NCT02232932).
Management of Locally Advanced Disease
Systemic Treatment
Systemic treatment is the treatment of choice for unresectable BTC according to guidelines (1). Earlier randomized, controlled studies have shown that chemotherapy improves survival in patients with advanced BTC compared with best supportive care (132–134). In a pooled analysis of 104 studies in advanced BTC, gemcitabine combined with cisplatin or oxaliplatin resulted to the best response rates, however, without significantly improving survival (135). In the phase III UK ABC 02 study Valle et al. (9) reported a median survival close to a year (11.7 months) for cisplatin/gemcitabine, compared with 8.1 months for gemcitabine alone(95% CI: 0.53–0.79; P < 0.001) these results were also confirmed in the BT22 study (136) and in a subsequent meta-analysis (137). Therefore, the combination of cisplatin and gemcitabine is currently regarded as standard of care in metastatic or unresectable BTC. Other treatments tested in randomized trials include the combination of gemcitabine, cisplatin and nab-paclitaxel (138), modified folfirinox vs. cisplatin gemcitabine (PRODIGE38-AMEBICA trial) (139), or nal-IRI, 5 FU, leucovorin vs. cisplatin gemcitabine (NIFE trial) (140).
Concerning the role of targeted therapies, although initial results from a single-arm study using cetuximab in combination with GemOX were promising (141), there was no benefit observed in a subsequent randomized phase II study (142). Similar negative findings were observed with erlotinib or panitumumab, sorafenib, or cedira-nib (an oral VEGFR-1, −2, and−3, PDGF, and c-Kit tyrosine kinase inhibitor) and the cisplatin/gemcitabine combination (1, 143, 144). In a phase II trial, regorafenib showed a disease control rate of 56%, indicating that it might be useful in refractory disease (145). Moreover, recently described gene fusions and mutations are being investigated. Emerging therapies that hold considerable promise include FGFR inhibitors such as pemigatinib and IDH1 and/or IDH2 inhibitors (29, 146, 147), whereas the inhibition of other molecular pathways, including the RAS/RAF/MEK/ERK, the MET, the PI3K/AKT/mTOR and angiogenetic pathways, is unclear (148). Certain tumor genetic aberrations have been associated with a likelihood of response to immune-checkpoint inhibitors, which might relate to the expression of neoantigens capable of eliciting an antitumor T-cell response (29). Several checkpoint inhibitors are currently being evaluated in a large number of clinical trials either as monotherapy or dual checkpoint inhibition but also in combination with chemotherapy or molecular targeted therapies (29). In some studies, it was indicated that tumors with DNA mismatch repair deficiency (dMMR) are sensitive to PD-1 blockade, so that for tumors with microsatellite instability (MSI-high) or dMMR tumors progressing after prior treatment, pembrolizumab is a possible treatment option (149–151).
Definitive Chemoradiation
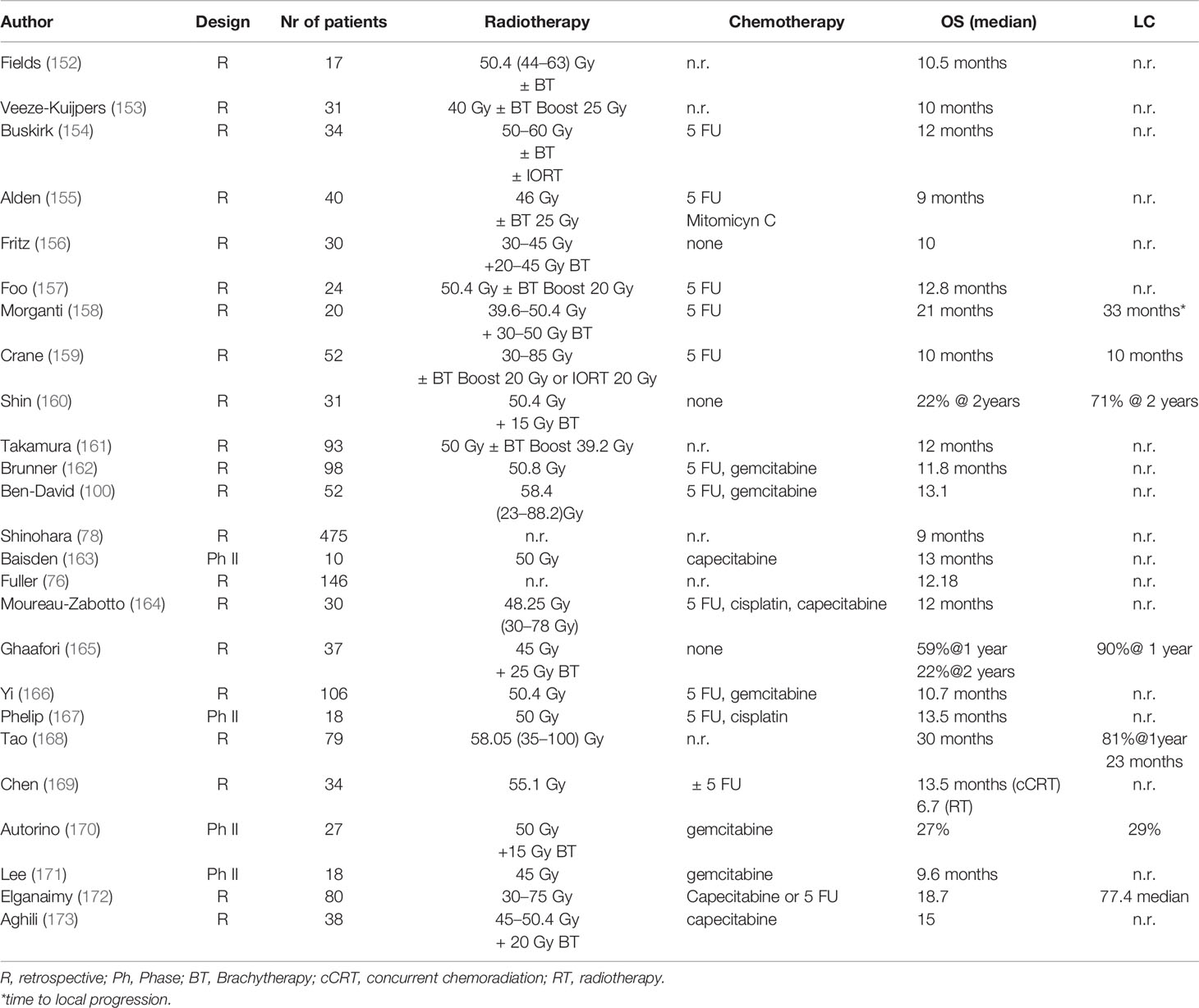
For locally advanced inoperable cholangiocarcinoma definitive chemoradiation in has been evaluated in several prospective and retrospective studies (Table 5). Radiotherapy improved cancer-specific survival in inoperable patients (P <.0001) in a SEER database analysis (174). The French FFCD trial (167) randomized patients with hilar or extrahepatic non-metastatic BTC between chemoradiation (50 Gy with concurrent cisplatin and 5-FU) or chemotherapy with gemcitabine and oxaliplatin (GemOx). The trial was closed before completion due to slow recruitment after 34 patients had been enrolled, showing that GemOx was at least as efficient as chemoradiation. Most studies were conducted in combination with 5 FU, gemcitabine, or cisplatin with a median radiotherapy dose of ca 50 Gy, leading to an actuarial 2-year LC of 29.0% in one study and a PFS between 6.8 and 10.5 months (median: 7.5 months) (163, 164, 166, 167, 169–171), while in other studies, dose escalation led to higher LC rates and improvement of OS (175). Tao et al. (175) reported a median survival of 30 months for all patients (1-, 2-, and 3-year OS was 87, 61, and 44%, respectively). Patients with a higher biological effective dose (BED) of 80.5 Gy had an improved local control (LC: 78 vs. 45% after 3 years, p = 0.03) and overall survival (median OS: not reached vs. 27 months p = 0.02) compared to patients with lower doses. Patients receiving a BT boost had a better LC compared to patients with EBRT without BT (97 vs. 56% at 1 year) (165). OS ranged between 9.6 and 13.5 months (median: 13 months, Table 5), with acceptable toxicity mostly grade ≥ 3 acute hematological and/or gastrointestinal toxicity (163, 164, 166, 167, 169–171), while in some cases, the use of a BT boost resulted to better LC rates (2 year LC 53 vs. 25%) (170).
In conclusion, BTCs might need higher doses in order to achieve a better local control and maybe also a survival benefit. Concepts for safer dose escalation include the use of stereotactic body radiotherapy (SBRT), brachytherapy (BT), or proton beam radiation therapy (PBRT).
Stereotactic Body Radiotherapy
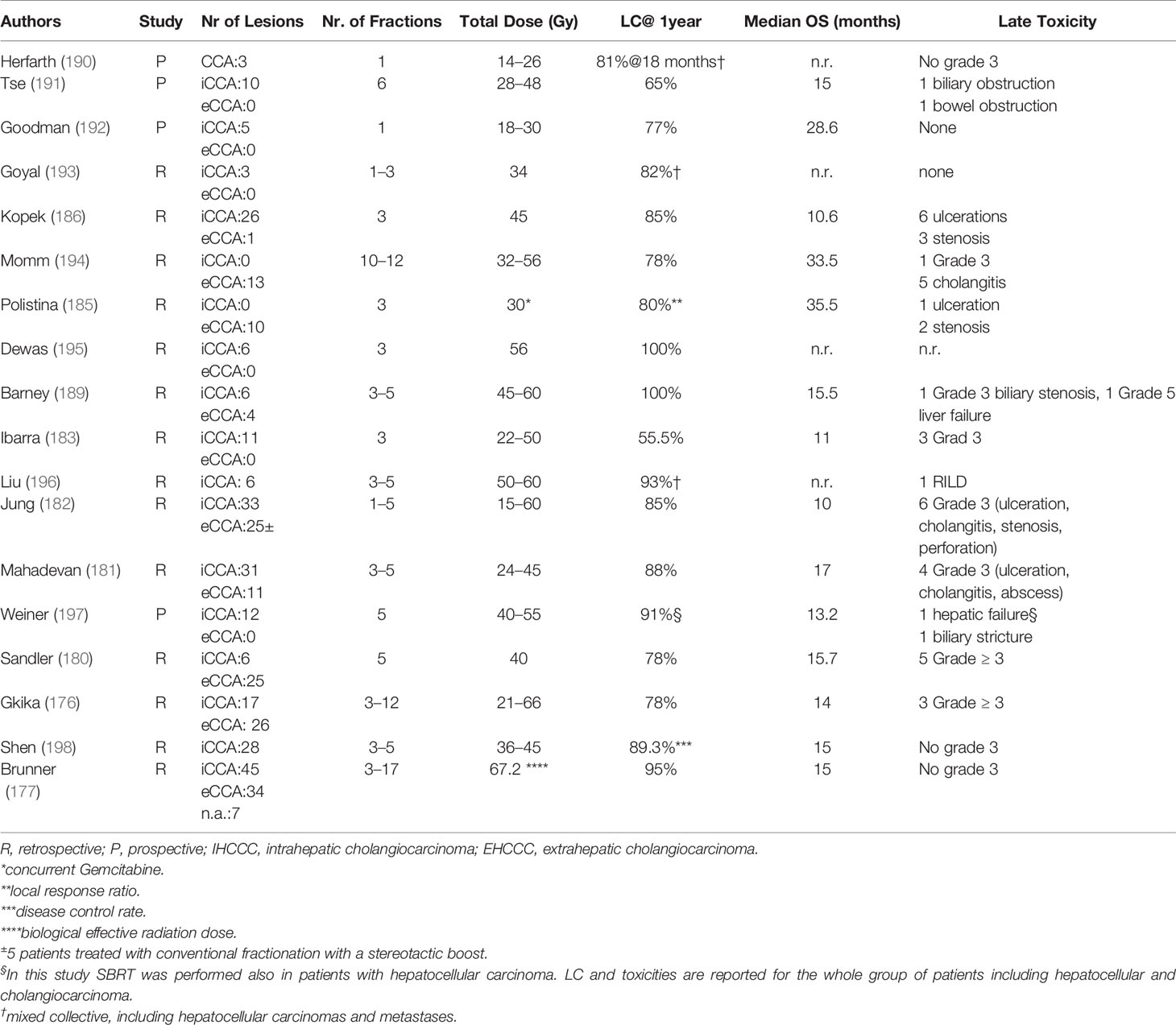
There is emerging evidence concerning the efficacy of stereotactic body radiotherapy (SBRT) in the treatment of inoperable BTC. Several prospective and retrospective studies SBRT led to local control rates ranging between 65 and 100% with a median OS of 11–35.5 years (median 15 months), in selected patients (176–178). In a systematic review, including 10 studies and 231 patients, the pooled 1 year LC was 83.4% (95% CI: 76.5–89.4%) (178). According to the anatomical location of CCA, 1 year OS was 57.1% (range: 45.0–58.0%), 81.5% (range: 80.0–83.0%), and 58.7% (range: 45.0–73.0%) in studies including iCCA, eCCA, and both sites, respectively (126, 178–187).
Furthermore, in several studies dose escalation correlated with prolonged OS and LC (175, 177). In a study by Brunner et al. LC rates at 12 and 24 months were 91% and 80% for BEDmax >91 Gy10 vs. 66 and 39% for lower doses (p = 0.009) (177). Additionally SBRT is a well-tolerated treatment with a low incidence of toxicities <10% (178, 188), while in a meta-analysis (178), only one case of fatal liver failure was reported in one patient despite compliance with dose/volume constraints. Additionally, SBRT has the advantage of being easily incorporated in systemic treatments showing high rates of OS after 1 year (median: 73.0%; range: 58.0–80.0%) (181, 185, 189) or even as neoadjuvant treatment in combination with capecitabine followed by liver transplantation leading to a 1 year OS of 83% as previously reported (126) (Table 6). The addition of stereotactic body radiotherapy to systemic chemotherapy in locally advanced biliary tract cancers is being investigated in a randomized phase II trial (ABC07(ISRCTN10639376) (https://doi.org/10.1186/ISRCTN10639376).
Brachytherapy
Several retrospective studies have evaluated the role EBRT, typically 30–40 Gy in 1.8–2 Gy with a brachytherapy boost. For BT, the dose commonly used is 15–20 Gy prescribed to the BT related PTV, generally over 2–3 treatments (HDR-BT) (130, 199). In a prospective phase I study by Mattiucci (200), investigating three different dose levels (15, 20, and 25 Gy) for HDR BT with 192Ir, recommended a dose of 25 Gy in five fractions (maximum dose level), as no dose limiting toxicities were reported up to this dose level. The median OS for these patients was 12 months. In a propensity score, matched pair analysis comparing patients receiving EBRT vs. EBRT and BT in unresectable BTC the addition of BT to EBRT had no impact on OS or disease specific survival but was associated with a better LC after 2 years (201, 202). Furthermore, BT can be used in the treatment of malignant obstructive jaundice (203). Intraluminal brachytherapy might increase the risk of cholangitis, pain, duodenopathy, and bleeding (130). Late complications such as bile duct stenosis or stricture were also observed (130, 199).
Particle Therapy Including Proton Therapy
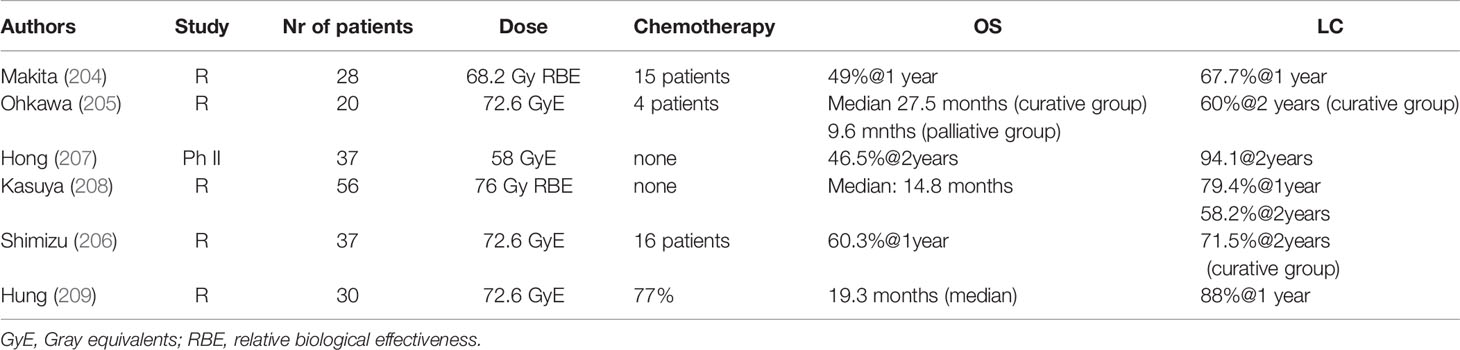
Another treatment option for dose escalation in unresectable cholangiocarcinoma is the use of proton beam radiation (PBT) therapy. Initial studies (204–206) including also patients treated in palliative intent showed promising results (Table 7). In more recent studies, Hung et al. (209) treated 30 patients with a median radiation dose of 72.6 cobalt gray equivalents. The 1 year local control achieved was 88% similar to the SBRT series with a median OS survival of 19.3 months. Three and two patients had grade III-IV toxicities and radiation-induced liver disease. There were no deaths caused by PBT or concurrent chemotherapy. Patients who received concurrent chemotherapy had a better median PFS (12.1 vs. 4.7 months). Furthermore, in a multi-institutional phase II study patients with iCCA treated with high dose hypo-fractionated PBT achieved a LC of 94.1% at 2 years and a 2 years OS of 46.5%, with limited toxicities (4.8% grade 3 toxicity) (209). There were no grade-4 or grade-5 radiation-related toxicities (209). The Japan Carbon Ion Radiation Oncology Study Group (J-CROS) investigated the role of Carbon -ion therapy for 56 patients with intrahepatic (27 patients) and perihilar (29 patients) cholangiocarcinoma (208). Most patients were treated to a total dose of 76 GyE in 20 fractions, with a median survival of 23.8 months for intrahepatic cholangiocarcinoma and 12.6 months in perihilar disease. No patients underwent resection. There was one case of death due to liver injury and one grade 3 bile duct stenosis. Results are summarized in Table 7.
Discussion and Future Perspectives
High loco-regional disease recurrence rates after R1 resection provide a rationale for using adjuvant radiotherapy with chemotherapy. Evidence from the Phase II SWOG S0809 (85)trial have demonstrated efficacy of gemcitabine and capecitabine followed by concurrent capecitabine and radiotherapy. The 2-year OS of 65% (67% and 60% in R0 and R1, respectively) and LR rates at 2 years of 11% (95% CI, 4 to18%) overall, 9% (95% CI, 2 to 17%) for R0, and 16% (95% CI, 2 to 30%) for R1 were significantly higher than the rates expected based on historical controls (84) with low toxicity rates. Currently there are no published randomized data testing the efficacy of adjuvant chemoradiation after R1 resection, as these trials are ongoing. In the phase III ACTICCA-1 trial adjuvant chemotherapy with gemcitabine and cisplatin compared to standard of care after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma has recently embedded a radiotherapy sub-study (NCT02170090 randomizing between adjuvant CRT vs. chemotherapy in EHCC and GBC (NCT02798510).
In the locally advanced inoperable cholangiocarcinoma neoadjuvant chemoradiation might confer a benefit in terms of downsizing with consecutive assessment of resectability, but this concept should be further evaluated within clinical trials. A prospective registry study is evaluating induction gemcitabine followed by 5-FU-based CCRT and maintenance capecitabine prior to LT in unresectable CCA (NCT00301379). Another randomized prospective multi -centre study is ongoing with an aim to compare 5-year OS and 3-year RFS between resection vs. CCRT followed by LT in hCCA (NCT02232932), while in a further trial the role of neoadjuvant chemoradiotherapy with concomitant oral capecitabine followed by gemcitabine in the treatment of unresectable hCCA is being evaluated prior to LT (NCT04378023).
In patients with inoperable disease, several studies have shown that dose escalation might lead to a survival benefit (175, 177). A Phase III trial from India is ongoing comparing intensity-modulated radiation therapy with weekly gemcitabine and systemic chemotherapy vs. systemic chemotherapy alone in unresectable cholangiocarcinoma (NCT02773485). Forms of safe dose escalation might include the use of a simultaneous integrated boost (SIB) or the use of brachytherapy after external radiotherapy or proton therapy with encouraging results. The use of hypo-fractionation or SBRT leads to high rates of disease control with reduced toxicity and is currently being prospectively evaluated in several trials such as the STRONG trial (NCT03307538) or the LAPIS trial (DRKS00011266). Due to the short treatment time, hypo-fractionation including SBRT can be easily incorporated into systemic treatments. Moreover, ionizing radiation, beside cytotoxicity, has been shown to additionally induce immune-modulatory effects, which trigger anti-tumor immune responses (210–215). The potentiation of anti-tumor immune responses can cause immunogenic cell death of cancer cells, change the tumor immune microenvironment, and alter antigen presentation of the tumor cells, thus enhancing immunogenicity of the tumor (216, 217). SBRT, by applying a high single dose with a few but more than one fractions, seems to have the potential to lead to an activation of specific T-cell response in the tumor (218–220). Thus, SBRT might be particularly attractive for combinations with checkpoint inhibitors. Furthermore, the short treatment interval seems to be favourable for a T-cell response. The immunomodulatory effects of SBRT are currently evaluated of LAPIS trial (DRKS00011266). In a Phase I/II Study (NCT04068194) patients with advanced/metastatic solid tumors and hepatobiliary malignancies including cholangiocarcinomas are treated with hypo-fractionated radiation in combination with M3814 and avelumab another trial is investigating the combination of hypo-fractionated RT with modified Immune Cells (Autologous Dendritic Cells) and a Vaccine (Prevnar) in patients with liver tumos (NCT03942328) including CCAs. The combination of chemotherapy with normo-fractionated RT or SBRT and the anti-PD-1 Antibody Camrelizumab is currently investigated in another prospective trial (NCT03898895). However, the mode of cell death and the systemic effects induced by ionizing irradiation are not uniform, and it clearly depends on the irradiation dose, the fractionation regimen, and the genetic repertoire of the irradiated cells (221).
In the past decade, the genetic landscape of cholangiocarcinoma subtypes has evolved and promising molecular targets for precision medicine have been identified. As the molecular classification and liquid biopsies are being gradually integrated in the treatment of solid tumors, efforts should be focused in identifying biomarkers to aid patients’ selection for radiotherapy or combined treatments such as SBRT with checkpoint inhibitors.
Author Contributions
All authors contributed to the article and approved the submitted version.
Funding
MH is supported by funding from the NIHR Biomedical Research Centre at University College London Hospitals NHS Foundation Trust.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27(suppl 5):v28–37. doi: 10.1093/annonc/mdw324
2. Seehofer D, Kamphues C, Neuhaus P. Management of bile duct tumors. Expert Opin Pharmacother (2008) 9(16):2843–56. doi: 10.1517/14656566.9.16.2843
3. Yang J, Yan LN. Current status of intrahepatic cholangiocarcinoma. World J Gastroenterol (2008) 14(41):6289–97. doi: 10.3748/wjg.14.6289
4. Ustundag Y, Bayraktar Y. Cholangiocarcinoma: a compact review of the literature. World J Gastroenterol (2008) 14(42):6458–66. doi: 10.3748/wjg.14.6458
5. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer (2010) 127(12):2893–917. doi: 10.1002/ijc.25516
6. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol (2014) 6:99–109. doi: 10.2147/CLEP.S37357
7. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology (2013) 145(6):1215–29. doi: 10.1053/j.gastro.2013.10.013
8. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet (Lond Engl) (2014) 383(9935):2168–79. doi: 10.1016/S0140-6736(13)61903-0
9. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl J Med (2010) 362(14):1273–81. doi: 10.1056/NEJMoa0908721
10. Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer (2002) 95(8):1685–95. doi: 10.1002/cncr.10831
11. Tamandl D, Herberger B, Gruenberger B, Puhalla H, Klinger M, Gruenberger T. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol (2008) 15(10):2787–94. doi: 10.1245/s10434-008-0081-1
12. Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol (2009) 16(11):3048–56. doi: 10.1245/s10434-009-0631-1
13. Jarnagin WR, Ruo L, Little SA, Klimstra D, D’Angelica M, DeMatteo RP, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer (2003) 98(8):1689–700. doi: 10.1002/cncr.11699
14. Mayo SC, Shore AD, Nathan H, Edil B, Wolfgang CL, Hirose K, et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg (2010) 14(10):1578–91. doi: 10.1007/s11605-010-1335-3
15. Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol (2008) 98(7):485–9. doi: 10.1002/jso.21141
16. Lamarca A, Edeline J, McNamara MG, Hubner RA, Nagino M, Bridgewater J, et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat Rev (2020) 84:101936. doi: 10.1016/j.ctrv.2019.101936
17. Saxena A, Chua TC, Sarkar A, Chu F, Morris DL. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unit. J Gastrointest Surg (2010) 14(7):1128–38. doi: 10.1007/s11605-010-1203-1
18. Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg (2009) 33(6):1247–54. doi: 10.1007/s00268-009-9970-0
19. Nuzzo G, Giuliante F, Ardito F, De Rose AM, Vellone M, Clemente G, et al. Intrahepatic cholangiocarcinoma: prognostic factors after liver resection. Updates Surg (2010) 62(1):11–9. doi: 10.1007/s13304-010-0007-x
20. Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Prognostic Factors After Surgical Resection for Intrahepatic, Hilar, and Distal Cholangiocarcinoma. Ann Surg Oncol (2010) 250(6):950–6. doi: 10.1245/s10434-010-1325-4
21. Li SQ, Liang LJ, Hua YP, Peng BG, He Q, Lu MD, et al. Long-term outcome and prognostic factors of intrahepatic cholangiocarcinoma. Chin Med J (Engl) (2009) 122(19):2286–91. doi: 10.3760/cma.j.issn.0366-6999.2009.19.018
22. Anderson C, Kim R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer Treat Rev (2009) 35(4):322–7. doi: 10.1016/j.ctrv.2008.11.009
23. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg (2007) 245(5):755–62. doi: 10.1097/01.sla.0000251366.62632.d3
24. Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg (2008) 248(1):84–96. doi: 10.1097/SLA.0b013e318176c4d3
25. Brunner TB, Seufferlein T. Radiation therapy in cholangiocellular carcinomas. Best Pract Res Clin Gastroenterol (2016) 30(4):593–602. doi: 10.1016/j.bpg.2016.08.003
26. Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver (2017) 11(1):13–26. doi: 10.5009/gnl15568
27. Matsumoto N, Ebata T, Yokoyama Y, Igami T, Sugawara G, Shimoyama Y, et al. Role of anatomical right hepatic trisectionectomy for perihilar cholangiocarcinoma. Br J Surg (2014) 101(3):261–8. doi: 10.1002/bjs.9383
28. Nagorney DM, Kendrick ML. Hepatic Resection in the Treatment of Hilar Cholangiocarcinoma. Adv Surg (2006) 40:159–71. doi: 10.1016/j.yasu.2006.05.009
29. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol (2018) 15(2):95–111. doi: 10.1038/nrclinonc.2017.157
30. Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology (2012) 143(1):88–98.e3; quiz e14. doi: 10.1053/j.gastro.2012.04.008
31. Jarnagin WR, Ruo L, Little SA. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer (2003) 98(8):1689–700. doi: 10.1002/cncr.11699
32. Simo KA, Halpin LE, McBrier NM, Hessey JA, Baker E, Ross S, et al. Multimodality treatment of intrahepatic cholangiocarcinoma: A review. J Surg Oncol (2016) 113(1):62–83. doi: 10.1002/jso.24093
33. Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. BJS (Br J Surg) (2018) 105(3):192–202. doi: 10.1002/bjs.10776
34. Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery (2013) 153(6):811–8. doi: 10.1016/j.surg.2012.12.005
35. Nakajima T, Kondo Y, Miyazaki M, Okui K. A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: histologic classification and modes of spreading. Hum Pathol (1988) 19(10):1228–34. doi: 10.1016/s0046-8177(88)80156-4
36. Weinbren K, Mutum SS. Pathological aspects of cholangiocarcinoma. J Pathol (1983) 139: (2):217–38. doi: 10.1002/path.1711390210
37. Jung W, Kim K, Min SK, Nam EM, Lee JK. Mapping of local recurrence after pancreaticoduodenectomy for distal extrahepatic cholangiocarcinoma: implications for adjuvant radiotherapy. Br J Radiol (2019) 92(1100):20190285. doi: 10.1259/bjr.20190285
38. Oh D, Lim DH, Heo JS, Choi SH, Choi DW, Ahn YC, et al. The Role of Adjuvant Radiotherapy in Microscopic Tumor Control After Extrahepatic Bile Duct Cancer Surgery. Am J Clin Oncol (2007) 30(1):21–5. doi: 10.1097/01.coc.0000245467.97180.78
39. Margonis GA, Gani F, Buettner S, Amini N, Sasaki K, Andreatos N, et al. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford) (2016) 18(11):872–8. doi: 10.1016/j.hpb.2016.05.016
40. Kim BH, Kim E, Kim K, Jang JY, Kim SW, Oh DY, et al. The impact of perioperative CA19-9 change on the survival and recurrence patterns after adjuvant chemoradiotherapy in resectable extrahepatic cholangiocarcinoma. J Surg Oncol (2018) 117(3):380–8. doi: 10.1002/jso.24856
41. Wade TP, Prasad CN, Virgo KS, Johnson FE. Experience with distal bile duct cancers in U.S. Veterans Affairs hospitals: 1987–1991. J Surg Oncol (1997) 64(3):242–5. doi: 10.1002/(sici)1096-9098(199703)64:3<242::Aid-jso12>3.0.Co;2-6
42. Hanazaki K, Kajikawa S, Shimozawa N, Shimada K, Hiraguri M, Koide N, et al. Prognostic factors of intrahepatic cholangiocarcinoma after hepatic resection: Univariate and multivariate analysis. Hepato-gastroenterology (2002) 49:311–6.
43. Dhanasekaran R, Hemming AW, Zendejas I, George T, Nelson DR, Soldevila-Pico C, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep (2013) 29(4):1259–67. doi: 10.3892/or.2013.2290
44. Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg (2009) 208(2):218–28. doi: 10.1016/j.jamcollsurg.2008.10.017
45. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol (2011) 29(23):3140–5. doi: 10.1200/jco.2011.35.6519
46. Chen L-P, Li C, Wang C, Wen T-F, Yan L-N, Li B. Predictive Factors of Recurrence for Patients with Intrahepatic Cholangiocarcinoma after Hepatectomy. Hepato-gastroenterology (2012) 59:1765–8. doi: 10.5754/hge11820
47. Ke Q, Lin N, Deng M, Wang L, Zeng Y, Liu J. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: A systematic review and meta-analysis. PLoS One (2020) 15(2):e0229292. doi: 10.1371/journal.pone.0229292
48. Jiang W, Zeng Z-C, Tang Z-Y, Fan J, Zhou J, Zeng M-S, et al. Benefit of radiotherapy for 90 patients with resected intrahepatic cholangiocarcinoma and concurrent lymph node metastases. J Cancer Res Clin Oncol (2010) 136(9):1323–31. doi: 10.1007/s00432-010-0783-1
49. Roayaie S, Guarrera JV, Ye MQ, Thung SN, Emre S, Fishbein TM, et al. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Surg (1998) 187(4):365–72. doi: 10.1016/s1072-7515(98)00203-8
50. Reames BN, Bagante F, Ejaz A, Spolverato G, Ruzzenente A, Weiss M, et al. Impact of adjuvant chemotherapy on survival in patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis. HPB (2017) 19(10):901–9. doi: 10.1016/j.hpb.2017.06.008
51. Lee GC, Ferrone CR, Tanabe KK, Lillemoe KD, Blaszkowsky LS, Zhu AX, et al. Predictors of adjuvant treatment and survival in patients with intrahepatic cholangiocarcinoma who undergo resection. Am J Surg (2019) 218(5):959–66. doi: 10.1016/j.amjsurg.2019.02.036
52. Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg (2011) 254(5):824–29; discussion 30. doi: 10.1097/SLA.0b013e318236c21d
53. Ercolani G, Vetrone G, Grazi GL, Aramaki O, Cescon M, Ravaioli M, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg (2010) 252(1):107–14. doi: 10.1097/SLA.0b013e3181e462e6
54. Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma–part 3: update on 5-year survival. J Gastrointestinal Surg (2005) 9(9):1191–204; discussion 204-6. doi: 10.1016/j.gassur.2005.08.034
55. Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg (2005) 92(5):547–56. doi: 10.1002/bjs.4881
56. Chen CY, Shiesh SC, Tsao HC, Lin XZ. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma–the influence of sampling time and hepatolithiasis. Hepato-gastroenterology (2002) 49(45):616–20.
57. Miwa S, Miyagawa S, Kobayashi A, Akahane Y, Nakata T, Mihara M, et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol (2006) 41(9):893–900. doi: 10.1007/s00535-006-1877-z
58. Jan YY, Yeh CN, Yeh TS, Hwang TL, Chen MF. Clinicopathological factors predicting long-term overall survival after hepatectomy for peripheral cholangiocarcinoma. World J Surg (2005) 29(7):894–8. doi: 10.1007/s00268-005-7763-7
59. Madariaga JR, Iwatsuki S, Todo S, Lee RG, Irish W, Starzl TE. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg (1998) 227(1):70–9. doi: 10.1097/00000658-199801000-00011
60. Hirohashi K, Uenishi T, Kubo S, Yamamoto T, Tanaka H, Shuto T, et al. Macroscopic types of intrahepatic cholangiocarcinoma: clinicopathologic features and surgical outcomes. Hepato-gastroenterology (2002) 49(44):326–9.
61. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol (2013) 31(9):1188–95. doi: 10.1200/jco.2012.41.5984
62. Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg (2002) 235(3):392–9. doi: 10.1097/00000658-200203000-00011
63. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol (2012) 30(16):1934–40. doi: 10.1200/JCO.2011.40.5381
64. Manterola C, Duque G, Grande L, de Aretxabala X, Conejeros R, Otzen T, et al. A systematic review of the effectiveness of adjuvant therapy for patients with gallbladder cancer. HPB (Oxford) (2019) 21(11):1427–35. doi: 10.1016/j.hpb.2019.02.019
65. Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg (2018) 105(3):192–202. doi: 10.1002/bjs.10776
66. Edeline J, Bonnetain F, Phelip JM. Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol (2017) 35(no 4 suppl):abstr 225. doi: 10.1200/JCO.2017.35.4_suppl.225
67. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol (2019) 20(5):663–73. doi: 10.1016/S1470-2045(18)30915-X
68. Primrose J, Fox R, Palmer DH. Adjuvant capecitabine for biliary tract cancer: The BILCAP randomized study. J Clin Oncol (2017) 35(suppl):abstr 4006. doi: 10.1200/JCO.2017.35.15_suppl.4006
69. Bonet Beltran M, Allal AS, Gich I, Sole JM, Carrio I. Is adjuvant radiotherapy needed after curative resection of extrahepatic biliary tract cancers? A systematic review with a meta-analysis of observational studies. Cancer Treat Rev (2012) 38(2):111–9. doi: 10.1016/j.ctrv.2011.05.003
70. Horgan AM, Amir E. Adjuvant therapy in the treatment of biliary tract cancer (BTC): A systematic review and meta-analysis. J Clin Oncol (2011) 29(suppl):abstr 4050. doi: 10.1200/jco.2011.29.15_suppl.4050
71. Ren B, Guo Q, Yang Y, Liu L, Wei S, Chen W, et al. A meta-analysis of the efficacy of postoperative adjuvant radiotherapy versus no radiotherapy for extrahepatic cholangiocarcinoma and gallbladder carcinoma. Radiat Oncol (2020) 15(1):15. doi: 10.1186/s13014-020-1459-x
72. Hammad AY, Berger NG, Eastwood D, Tsai S, Turaga KK, Christian KK, et al. Is Radiotherapy Warranted Following Intrahepatic Cholangiocarcinoma Resection? The Impact of Surgical Margins and Lymph Node Status on Survival. Ann Surg Oncol (2016) 23(Suppl 5):912–20. doi: 10.1245/s10434-016-5560-1
73. Tran Cao HS, Zhang Q, Sada YH, Chai C, Curley SA, Massarweh NN. The role of surgery and adjuvant therapy in lymph node–positive cancers of the gallbladder and intrahepatic bile ducts. Cancer (2018) 124(1):74–83. doi: 10.1002/cncr.30968
74. Sur MD, In H, Sharpe SM, Baker MS, Weichselbaum RR, Talamonti MS, et al. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol (2015) 22(7):2209–17. doi: 10.1245/s10434-014-4275-4
75. Ecker BL, Vining CC, Roses RE, Maggino L, Lee MK, Drebin JA, et al. Identification of Patients for Adjuvant Therapy After Resection of Carcinoma of the Extrahepatic Bile Ducts: A Propensity Score-Matched Analysis. Ann Surg Oncol (2017) 24(13):3926–33. doi: 10.1245/s10434-017-6095-9
76. Fuller CD, Wang SJ, Choi M, Czito BG, Cornell J, Welzel TM, et al. Multimodality therapy for locoregional extrahepatic cholangiocarcinoma: a population-based analysis. Cancer (2009) 115(22):5175–83. doi: 10.1002/cncr.24572
77. Shinohara ET, Mitra N, Guo M, Metz JM. Radiotherapy is associated with improved survival in adjuvant and palliative treatment of extrahepatic cholangiocarcinomas. Int J Radiat Oncol Biol Phys (2009) 74(4):1191–8. doi: 10.1016/j.ijrobp.2008.09.017
78. Shinohara ET, Mitra N, Guo M, Metz JM. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys (2008) 72(5):1495–501. doi: 10.1016/j.ijrobp.2008.03.018
79. Vern-Gross TZ, Shivnani AT, Chen K, Lee CM, Tward JD, MacDonald OK, et al. Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys (2011) 81(1):189–98. doi: 10.1016/j.ijrobp.2010.05.001
80. Hughes MA, Frassica DA, Yeo CJ, Riall TS, Lillemoe KD, Cameron JL, et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys (2007) 68(1):178–82. doi: 10.1016/j.ijrobp.2006.11.048
81. Schoppmeyer K, Miethe S, Wiedmann M, Liebmann A, Hauss J, Mossner J, et al. Radiochemotherapy followed by gemcitabine and capecitabine in extrahepatic bile duct cancer: a phase I/II trial. Am J Clin Oncol (2006) 29(6):576–82. doi: 10.1097/01.coc.0000239167.17922.82
82. Lim KH, Oh DY, Chie EK, Jang JY, Im SA, Kim TY, et al. Adjuvant concurrent chemoradiation therapy (CCRT) alone versus CCRT followed by adjuvant chemotherapy: which is better in patients with radically resected extrahepatic biliary tract cancer?: a non-randomized, single center study. BMC Cancer (2009) 9:345. doi: 10.1186/1471-2407-9-345
83. Cho M, Wang-Gillam A, Myerson R, Gao F, Strasberg S, Picus J, et al. A phase II study of adjuvant gemcitabine plus docetaxel followed by concurrent chemoradation in resected pancreaticobiliary carcinoma. HPB (2015) 17(7):587–93. doi: 10.1111/hpb.12413
84. Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol (2015) 33(24):2617–22. doi: 10.1200/JCO.2014.60.2219
85. Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol (2005) 23(34):8739–47. doi: 10.1200/JCO.2005.01.5354
86. González González D, Gerard JP, Maners AW, De la Lande-Guyaux B, Van Dijk-Milatz A, Meerwaldt JH, et al. Results of radiation therapy in carcinoma of the proximal bile duct (Klatskin tumor). Semin Liver Dis (1990) 10(2):131–41. doi: 10.1055/s-2008-1040466
87. Schoenthaler R, Phillips TL, Castro J, Efird JT, Better A, Way LW. Carcinoma of the extrahepatic bile ducts. The University of California at San Francisco experience. Ann Surg (1994) 219(3):267–74. doi: 10.1097/00000658-199403000-00006
88. Pitt HA, Nakeeb A, Abrams RA, Coleman J, Piantadosi S, Yeo CJ, et al. Perihilar Cholangiocarcinoma Postoperative Radiotherapy Does Not Improve Survival. Ann Surg (1995) 221(6):788–802. doi: 10.1097/00000658-199506000-00017
89. Zlotecki RA, Jung LA, Vauthey JN, Vogel SB, Mendenhall WM. Carcinoma of the extrahepatic biliary tract: Surgery and radiotherapy for curative and palliative intent. Radiat Oncol Investig (1998) 6(5):240–7. doi: 10.1002/(sici)1520-6823(1998)6:5<240::Aid-roi6>3.0.Co;2-r
90. Todoroki T, Ohara K, Kawamoto T, Koike N, Yoshida S, Kashiwagi H, et al. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys (2000) 46(3):581–7. doi: 10.1016/S0360-3016(99)00472-1
91. Serafini FM, Sachs D, Bloomston M, Carey LC, Karl RC, Murr MM, et al. Location, not staging, of cholangiocarcinoma determines the role for adjuvant chemoradiation therapy. Am Surg (2001) 67(9):839–43; discussion 43-4.
92. Kim S, Kim SW, Bang YJ, Heo DS, Ha SW. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys (2002) 54(2):414–9. doi: 10.1016/s0360-3016(02)02952-8
93. Nakeeb A, Tran KQ, Black MJ, Erickson BA, Ritch PS, Quebbeman EJ, et al. Improved survival in resected biliary malignancies. Surgery (2002) 132(4):555–63; discission 63-4. doi: 10.1067/msy.2002.127555
94. Gerhards MF, van Gulik TM, González González D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg (2003) 27(2):173–9. doi: 10.1007/s00268-002-6434-1
95. Heron DE, Stein DE, Eschelman DJ, Topham AK, Waterman FM, Rosato EL, et al. Cholangiocarcinoma: The Impact of Tumor Location and Treatment Strategy on Outcome. Radiat Oncol Investig (2003) 26(4):422–8. doi: 10.1097/01.Coc.0000026833.73428.1f
96. Lindell G, Holmin T, Ewers SB, Tranberg KG, Stenram U, Ihse I. Extended operation with or without intraoperative (IORT) and external (EBRT) radiotherapy for gallbladder carcinoma. Hepato-gastroenterology (2003) 50(50):310–4.
97. Itoh H, Nishijima K, Kurosaka Y, Takegawa S, Kiriyama M, Dohba S, et al. Magnitude of Combination Therapy of Radical Resection and External Beam Radiotherapy for Patients With Carcinomas of the Extrahepatic Bile Duct and Gallbladder. Dig Dis Sci (2005) 50(12):2231–42. doi: 10.1007/s10620-005-3040-8
98. Czito BG, Hurwitz HI, Clough RW, Tyler DS, Morse MA, Clary BM, et al. Adjuvant external-beam radiotherapy with concurrent chemotherapy after resection of primary gallbladder carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys (2005) 62(4):1030–4. doi: 10.1016/j.ijrobp.2004.12.059
99. Sagawa N, Kondo S, Morikawa T, Okushiba S, Katoh H. Effectiveness of radiation therapy after surgery for hilar cholangiocarcinoma. Surg Today (2005) 35(7):548–52. doi: 10.1007/s00595-005-2989-4
100. Ben-David MA, Griffith KA, Abu-Isa E, Lawrence TS, Knol J, Zalupski M, et al. External-beam radiotherapy for localized extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys (2006) 66(3):772–9. doi: 10.1016/j.ijrobp.2006.05.061
101. Mahantshetty UM, Palled SR, Engineer R, Homkar G, Shrivastava SK, Shukla PJ. Adjuvant radiation therapy in gall bladder cancers: 10 years experience at Tata Memorial Hospital. J Cancer Res Ther (2006) 2(2):52–6. doi: 10.4103/0973-1482.25850
102. Dinant S, Gerhards MF, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol (2006) 13(6):872–80. doi: 10.1245/aso.2006.05.053
103. Cheng Q, Luo X, Zhang B, Jiang X, Yi B, Wu M. Predictive factors for prognosis of hilar cholangiocarcinoma: Postresection radiotherapy improves survival. Eur J Surg Oncol (EJSO) (2007) 33(2):202–7. doi: 10.1016/j.ejso.2006.09.033
104. Borghero Y, Crane CH, Szklaruk J, Oyarzo M, Curley S, Pisters PW, et al. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol (2008) 15(11):3147–56. doi: 10.1245/s10434-008-9998-7
105. Gold DG, Miller RC, Haddock MG, Gunderson LL, Quevedo F, Donohue JH, et al. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys (2009) 75(1):150–5. doi: 10.1016/j.ijrobp.2008.10.052
106. Nelson JW, Ghafoori AP, Willett CG, Tyler DS, Pappas TN, Clary BM, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys (2009) 73(1):148–53. doi: 10.1016/j.ijrobp.2008.07.008
107. Bonet Beltrán M, Roth AD, Mentha G, Allal AS. Adjuvant radio-chemotherapy for extrahepatic biliary tract cancers. BMC Cancer (2011) 11:267. doi: 10.1186/1471-2407-11-267
108. Kim TH, Han S-S, Park S-J, Lee WJ, Woo SM, Moon SH, et al. Role of Adjuvant Chemoradiotherapy for Resected Extrahepatic Biliary Tract Cancer. Int J Radiat Oncol Biol Phys (2011) 81(5):e853–e9. doi: 10.1016/j.ijrobp.2010.12.019
109. Hayashi K, Isohashi F, Ogawa K, Oikawa H, Onishi H, Ito Y, et al. Postoperative External Irradiation of Patients with Primary Biliary Tract Cancer: A Multicenter Retrospective Study. Anticancer Res (2015) 35(11):6231–7.
110. Dover LL, Oster RA, McDonald AM, DuBay DA, Wang TN, Jacob R. Impact of adjuvant chemoradiation on survival in patients with resectable cholangiocarcinoma. HPB (Oxford) (2016) 18(10):843–50. doi: 10.1016/j.hpb.2016.07.008
111. Im JH, Seong J, Lee IJ, Park JS, Yoon DS, Kim KS, et al. Surgery Alone Versus Surgery Followed by Chemotherapy and Radiotherapy in Resected Extrahepatic Bile Duct Cancer: Treatment Outcome Analysis of 336 Patients. Cancer Res Treat (2016) 48(2):583–95. doi: 10.4143/crt.2015.091
112. Kim BH, Kim K, Chie EK, Kwon J, Jang JY, Kim SW, et al. Long-Term Outcome of Distal Cholangiocarcinoma after Pancreaticoduodenectomy Followed by Adjuvant Chemoradiotherapy: A 15-Year Experience in a Single Institution. Cancer Res Treat (2017) 49(2):473–83. doi: 10.4143/crt.2016.166
113. Gu B, Qian L, Yu H, Hu J, Wang Q, Shan J, et al. Concurrent Chemoradiotherapy in Curatively Resected Gallbladder Carcinoma: A Propensity Score–Matched Analysis. Int J Radiat Oncol Biol Phys (2018) 100(1):138–45. doi: 10.1016/j.ijrobp.2017.09.029
114. Lee J, Kang SH, Noh OK, Chun M, Oh YT, Kim BW, et al. Adjuvant concurrent chemoradiation therapy in patients with microscopic residual tumor after curative resection for extrahepatic cholangiocarcinoma. Clin Transl Oncol (2018) 20(8):1011–7. doi: 10.1007/s12094-017-1815-y
115. Zheng X, Chen B, Wu JX, Jia AY, Rong WQ, Wang LM, et al. Benefit of adjuvant radiotherapy following narrow-margin hepatectomy in patients with intrahepatic cholangiocarcinoma that adhere to major vessels. Cancer Manag Res (2018) 10:3973–81. doi: 10.2147/CMAR.S172940
116. Mukai Y, Matsuyama R, Koike I, Kumamoto T, Kaizu H, Homma Y, et al. Outcome of postoperative radiation therapy for cholangiocarcinoma and analysis of dose-volume histogram of remnant liver. Med (Baltimore) (2019) 98(31):e16673. doi: 10.1097/MD.0000000000016673
117. Stein DE, Heron DE, Rosato EL, Anné PR, Topham AK. Positive microscopic margins alter outcome in lymph node-negative cholangiocarcinoma when resection is combined with adjuvant radiotherapy. Am J Clin Oncol (2005) 28(1):21–3. doi: 10.1097/01.coc.0000139017.90599.f5
118. Frosio F, Mocchegiani F, Conte G, Bona ED, Vecchi A, Nicolini D, et al. Neoadjuvant therapy in the treatment of hilar cholangiocarcinoma: Review of the literature. World J Gastrointest Surg (2019) 11(6):279–86. doi: 10.4240/wjgs.v11.i6.279
119. Sahai P, Kumar S. External radiotherapy and brachytherapy in the management of extrahepatic and intrahepatic cholangiocarcinoma: available evidence. Br J Radiol (2017) 90:20170061. doi: 10.1259/bjr.20170061
120. Grendar J, Grendarova P, Sinha R, Dixon E. Neoadjuvant therapy for downstaging of locally advanced hilar cholangiocarcinoma: a systematic review. HPB (2014) 16(4):297–303. doi: 10.1111/hpb.12150
121. McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, et al. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg (1997) 174(6):605–8; discussion 8-9. doi: 10.1016/s0002-9610(97)00203-1
122. Jung JH, Lee HJ, Lee HS, Jo JH, Cho IR, Chung MJ, et al. Benefit of neoadjuvant concurrent chemoradiotherapy for locally advanced perihilar cholangiocarcinoma. World J Gastroenterol (2017) 23(18):3301–8. doi: 10.3748/wjg.v23.i18.3301
123. Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J, et al. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J Surg (2018) 42(9):2910–8. doi: 10.1007/s00268-018-4558-1
124. Katayose Y, Rikiyama T, Motoi F, Yamamoto K, Yoshida H, Morikawa T, et al. Phase I trial of neoadjuvant chemoradiation with gemcitabine and surgical resection for cholangiocarcinoma patients (NACRAC study). Hepato-gastroenterology (2011) 58(112):1866–72. doi: 10.5754/hge10106
125. Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw J, McCashland T, et al. Radiochemotherapy and Transplantation Allow Long-Term Survival For Nonresectable Hilar Cholangiocarcinoma. Am J Transplant (2002) 2: (8):774–9. doi: 10.1034/j.1600-6143.2002.20812.x
126. Welling TH, Feng M, Wan S, Hwang SY, Volk ML, Lawrence TS, et al. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transplant (2014) 20(1):81–8. doi: 10.1002/lt.23757
127. De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transplant (2000) 6(3):309–16. doi: 10.1053/lv.2000.6143
128. Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis (2004) 24(2):201–7. doi: 10.1055/s-2004-828896
129. Wu DH, Liu L, Chen LH. Therapeutic effects and prognostic factors in three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol (2004) 10(15):2184–9. doi: 10.3748/wjg.v10.i15.2184
130. Mukewar S, Gupta A, Baron TH, Gores G, Furutani K, Haddock MG, et al. Endoscopically inserted nasobiliary catheters for high dose-rate brachytherapy as part of neoadjuvant therapy for perihilar cholangiocarcinoma. Endoscopy (2015) 47(10):878–83. doi: 10.1055/s-0034-1392044
131. Wu Y, Johlin FC, Rayhill SC, Jensen CS, Xie J, Cohen MB, et al. Long-term, tumor-free survival after radiotherapy combining hepatectomy-Whipple en bloc and orthotopic liver transplantation for early-stage hilar cholangiocarcinoma. Liver Transplant (2008) 14(3):279–86. doi: 10.1002/lt.21287
132. Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. Journal of clinical oncology: official journal of the. Am Soc Clin Oncol (2010) 28(30):4581–6. doi: 10.1200/jco.2010.29.3605
133. Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol (1996) 7(6):593–600. doi: 10.1093/oxfordjournals.annonc.a010676
134. Koeberle D, Saletti P, Borner M, Gerber D, Dietrich D, Caspar CB, et al. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol (2008) 26(22):3702–8. doi: 10.1200/JCO.2008.16.5704
135. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer (2007) 96(6):896–902. doi: 10.1038/sj.bjc.6603648
136. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer (2010) 103(4):469–74. doi: 10.1038/sj.bjc.6605779
137. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol (2014) 25(2):391–8. doi: 10.1093/annonc/mdt540
138. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol (2019) 5(6):824–30. doi: 10.1001/jamaoncol.2019.0270
139. Phelip J-M, Edeline J, Blanc J-F, Barbier E, Michel P, Bourgeois V, et al. Modified FOLFIRINOX versus CisGem first-line chemotherapy for locally advanced non resectable or metastatic biliary tract cancer (AMEBICA)-PRODIGE 38: Study protocol for a randomized controlled multicenter phase II/III study. Dig Liver Dis (2019) 51(2):318–20. doi: 10.1016/j.dld.2018.11.018
140. Perkhofer L, Berger AW, Beutel AK, Gallmeier E, Angermeier S, Fischer von Weikersthal L, et al. Nal-IRI with 5-fluorouracil (5-FU) and leucovorin or gemcitabine plus cisplatin in advanced biliary tract cancer - the NIFE trial (AIO-YMO HEP-0315) an open label, non-comparative, randomized, multicenter phase II study. BMC Cancer (2019) 19(1):990. doi: 10.1186/s12885-019-6142-y
141. Gruenberger B, Schueller J, Heubrandtner U, Wrba F, Tamandl D, Kaczirek K, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol (2010) 11(12):1142–8. doi: 10.1016/S1470-2045(10)70247-3
142. Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol (2014) 15(8):819–28. doi: 10.1016/s1470-2045(14)70212-8
143. Valle JW, Wasan H, Lopes A, Backen AC, Palmer DH, Morris K, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol (2015) 16(8):967–78. doi: 10.1016/s1470-2045(15)00139-4
144. Moehler M, Maderer A, Schimanski C, Kanzler S, Denzer U, Kolligs FT, et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer (2014) 50(18):3125–35. doi: 10.1016/j.ejca.2014.09.013
145. Sun W, Patel A, Normolle D, Patel K, Ohr J, Lee JJ, et al. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer (2019) 125(6):902–9. doi: 10.1002/cncr.31872
146. Goyal L, Shi L, Liu LY, Fece de la Cruz F, Lennerz JK, Raghavan S, et al. TAS-120 Overcomes Resistance to ATP-Competitive FGFR Inhibitors in Patients with FGFR2 Fusion-Positive Intrahepatic Cholangiocarcinoma. Cancer Discov (2019) 9(8):1064–79. doi: 10.1158/2159-8290.Cd-19-0182
147. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol (2020) 21(5):671–84. doi: 10.1016/S1470-2045(20)30109-1
148. Ntanasis-Stathopoulos I, Tsilimigras DI, Gavriatopoulou M, Schizas D, Pawlik TM. Cholangiocarcinoma: investigations into pathway-targeted therapies. Expert Rev Anticancer Ther (2020) 20(9):765–73. doi: 10.1080/14737140.2020.1807333
149. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (N Y NY) (2017) 357(6349):409–13. doi: 10.1126/science.aan6733
150. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
151. Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med (2019) 25(5):744–50. doi: 10.1038/s41591-019-0407-5
152. Fields JN, Emami B. Carcinoma of the extrahepatic biliary system—results of primary and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys (1987) 13(3):331–8. doi: 10.1016/0360-3016(87)90006-X
153. Veeze-Kuijpers B, Meerwaldt JH, Lameris JS, Van Blankenstein M, Van Putten WLJ, Terpstra OT. The role of radiotherapy in the treatment of bile duct carcinoma. Int J Radiat Oncol Biol Phys (1990) 18(1):63–7. doi: 10.1016/0360-3016(90)90268-O
154. Buskirk SJ, Gunderson LL, Schild SE, Bender CE, Williams HJJ, Mcilrath DC, et al. Analysis of Failure After Curative Irradiation of Extrahepatic Bile Duct Carcinoma. Ann Surg (1992) 215(2):125–31. doi: 10.1097/00000658-199202000-00006
155. Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys (1994) 28(4):945–51. doi: 10.1016/0360-3016(94)90115-5
156. Fritz P, Brambs H-J, Schraube P, Freund U, Berns C, Wannenmacher M. Combined external beam radiotherapy and intraluminal high dose rate brachytherapy on bile duct carcinomas. Int J Radiat Oncol Biol Phys (1994) 29(4):855–61. doi: 10.1016/0360-3016(94)90576-2
157. Foo ML, Gunderson LL, Bender CE, Buskirk SJ. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys (1997) 39(4):929–35. doi: 10.1016/S0360-3016(97)00299-X
158. Morganti AG, Trodella L, Valentini V, Montemaggi P, Costamagna G, Smaniotto D, et al. Combined modality treatment in unresectable extrahepatic biliary carcinoma. Int J Radiat Oncol Biol Phys (2000) 46(4):913–9. doi: 10.1016/S0360-3016(99)00487-3
159. Crane CH, Macdonald KO, Vauthey JN. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys (2002) 53(4):969–74. doi: 10.1016/s0360-3016(02)02845-6
160. Shin HS, Seong J, Kim WC, Lee HS, Moon SR, Lee IJ, et al. Combination of external beam irradiation and high-dose-rate intraluminal brachytherapy for inoperable carcinoma of the extrahepatic bile ducts. Int J Radiat Oncol Biol Phys (2003) 57(1):105–12. doi: 10.1016/S0360-3016(03)00410-3
161. Takamura A, Saito H, Kamada T, Hiramatsu K, Takeuchi S, Hasegawa M, et al. Intraluminal low-dose-rate 192Ir brachytherapy combined with external beam radiotherapy and biliary stenting for unresectable extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys (2003) 57(5):1357–65. doi: 10.1016/S0360-3016(03)00770-3
162. Brunner TB, Schwab D, Meyer T, Sauer R. Chemoradiation may prolong survival of patients with non-bulky unresectable extrahepatic biliary carcinoma. A retrospective analysis. Strahlenther und Onkol Organ der Deutschen Rontgengesellschaft [et al] (2004) 180(12):751–7. doi: 10.1007/s00066-004-1315-1
163. Baisden JM, Kahaleh M, Weiss GR, Sanfey H, Moskaluk CA, Yeaton P, et al. Multimodality Treatment With Helical Tomotherapy Intensity Modulated Radiotherapy, Capecitabine, and Photodynamic Therapy is Feasible and Well Tolerated in Patients With Hilar Cholangiocarcinoma. Gastrointest Cancer Res (2008) 2(5):219–24.
164. Moureau-Zabotto L, Turrini O, Resbeut M, Raoul J-L, Giovannini M, Poizat F, et al. Impact of radiotherapy in the management of locally advanced extrahepatic cholangiocarcinoma. BMC Cancer (2013) 13:568–. doi: 10.1186/1471-2407-13-568
165. Ghafoori AP, Nelson JW, Willett CG, Chino J, Tyler DS, Hurwitz HI, et al. Radiotherapy in the Treatment of Patients With Unresectable Extrahepatic Cholangiocarcinoma. Int J Radiat Oncol Biol Phys (2011) 81(3):654–9. doi: 10.1016/j.ijrobp.2010.06.018
166. Yi SW, Kang DR, Kim KS, Park MS, Seong J, Park JY, et al. Efficacy of concurrent chemoradiotherapy with 5-fluorouracil or gemcitabine in locally advanced biliary tract cancer. Cancer Chemother Pharmacol (2014) 73(1):191–8. doi: 10.1007/s00280-013-2340-5
167. Phelip J-M, Vendrely V, Rostain F, Subtil F, Jouve J-L, Gasmi M, et al. Gemcitabine plus cisplatin versus chemoradiotherapy in locally advanced biliary tract cancer: Fédération Francophone de Cancérologie Digestive 9902 phase II randomised study. Eur J Cancer (2014) 50(17):2975–82. doi: 10.1016/j.ejca.2014.08.013
168. Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol (2015) 34(3):219–26. doi: 10.1200/JCO.2015.61.3778
169. Chen SC, Chen MH, Li CP, Chen MH, Chang PM, Liu CY, et al. External beam radiation therapy with or without concurrent chemotherapy for patients with unresectable locally advanced hilar cholangiocarcinoma. Hepato-gastroenterology (2015) 62(137):102–7.
170. Autorino R, Mattiucci GC, Ardito F, Balducci M, Deodato F, Macchia G, et al. Radiochemotherapy with Gemcitabine in Unresectable Extrahepatic Cholangiocarcinoma: Long-term Results of a Phase II Study. Anticancer Res (2016) 36(2):737–40.
171. Lee KJ, Yi SW, Cha J, Seong J, Bang S, Song SY, et al. A pilot study of concurrent chemoradiotherapy with gemcitabine and cisplatin in patients with locally advanced biliary tract cancer. Cancer Chemother Pharmacol (2016) 78(4):841–6. doi: 10.1007/s00280-016-3143-2
172. Elganainy D, Holliday EB, Taniguchi CM, Smith GL, Shroff R, Javle M, et al. Dose escalation of radiotherapy in unresectable extrahepatic cholangiocarcinoma. Cancer Med (2018) 7(10):4880–92. doi: 10.1002/cam4.1734
173. Aghili M, Saberi H, Zadeh MM, Jafari F, Mehrabinejad MM, Dashti H, et al. Multimodality treatment in unresectable cholangiocarcinoma. J Contemp Brachyther (2020) 12(2):131–8. doi: 10.5114/jcb.2020.94582
174. Liu J, Zhong M, Feng Y, Zeng S, Wang Y, Xu H, et al. Prognostic Factors and Treatment Strategies for Intrahepatic Cholangiocarcinoma from 2004 to 2013: Population-Based SEER Analysis. Trans Oncol (2019) 12(11):1496–503. doi: 10.1016/j.tranon.2019.05.020
175. Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol (2016) 34(3):219–26. doi: 10.1200/jco.2015.61.3778
176. Gkika E, Hallauer L, Kirste S, Adebahr S, Bartl N, Neeff HP, et al. Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer (2017) 17(1):781. doi: 10.1186/s12885-017-3788-1
177. Brunner TB, Blanck O, Lewitzki V, Abbasi-Senger N, Momm F, Riesterer O, et al. Stereotactic body radiotherapy dose and its impact on local control and overall survival of patients for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. Radiother Oncol (2019) 132:42–7. doi: 10.1016/j.radonc.2018.11.015
178. Frakulli R, Buwenge M, Macchia G, Cammelli S, Deodato F, Cilla S, et al. Stereotactic body radiation therapy in cholangiocarcinoma: a systematic review. Br J Radiol (2019) 92(1097):20180688. doi: 10.1259/bjr.20180688
179. Shen ZT, Zhou H, Li AM, Li B, Shen JS, Zhu XX. Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma. Oncotarget (2017) 8(55):93541–50. doi: 10.18632/oncotarget.19972
180. Sandler KA, Veruttipong D, Agopian VG, Finn RS, Hong JC, Kaldas FM, et al. Stereotactic Body Radiotherapy (SBRT) for Locally Advanced Extrahepatic and Intrahepatic Cholangiocarcinoma. Adv Radiat Oncol (2016) 1(4):237–43. doi: 10.1016/j.adro.2016.10.008
181. Mahadevan A, Dagoglu N, Mancias J, Raven K, Khwaja K, Tseng JF, et al. Stereotactic Body Radiotherapy (SBRT) for Intrahepatic and Hilar Cholangiocarcinoma. J Cancer (2015) 6(11):1099–104. doi: 10.7150/jca.13032
182. Jung DH, Kim MS, Cho CK, Yoo HJ, Jang WI, Seo YS, et al. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat Oncol J (2014) 32(3):163–9. doi: 10.3857/roj.2014.32.3.163
183. Ibarra RA, Rojas D, Snyder L, Yao M, Fabien J, Milano M, et al. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol (2012) 51(5):575–83. doi: 10.3109/0284186x.2011.652736
184. Barney BM, Olivier KR, Miller RC, Haddock MG. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol (2012) 7:67. doi: 10.1186/1748-717x-7-67
185. Polistina FA, Guglielmi R, Baiocchi C, Francescon P, Scalchi P, Febbraro A, et al. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother Oncol (2011) 99(2):120–3. doi: 10.1016/j.radonc.2011.05.016
186. Kopek N, Holt MI, Hansen AT, Hoyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol (2010) 94(1):47–52. doi: 10.1016/j.radonc.2009.11.004
187. Tse RV, Hawkins M, Lockwood G. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol (2008) 26(4):657–64. doi: 10.1200/jco.2007.14.3529
188. Lee J, Yoon WS, Koom WS, Rim CH. Efficacy of stereotactic body radiotherapy for unresectable or recurrent cholangiocarcinoma: a meta-analysis and systematic review. Strahlenther und Onkol Organ der Deutschen Rontgengesellschaft [et al] (2019) 195(2):93–102. doi: 10.1007/s00066-018-1367-2
189. Barney BM, Olivier KR, Miller RC, Haddock MG. Clinical outcomes and toxicity using Stereotactic Body Radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol (2012) 7(1):67. doi: 10.1186/1748-717x-7-67
190. Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol (2001) 19(1):164–70. doi: 10.1200/jco.2001.19.1.164
191. Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol (2008) 26(4):657–64. doi: 10.1200/JCO.2007.14.3529
192. Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys (2010) 78(2):486–93. doi: 10.1016/j.ijrobp.2009.08.020
193. Goyal K, Einstein D, Yao M, Kunos C, Barton F, Singh D, et al. Cyberknife stereotactic body radiation therapy for nonresectable tumors of the liver: preliminary results. HPB Surg (2010) 2010. doi: 10.1155/2010/309780
194. Momm F, Schubert E, Henne K, Hodapp N, Frommhold H, Harder J, et al. Stereotactic fractionated radiotherapy for Klatskin tumors. Radiother Oncol (2010) 95(1):99–102. doi: 10.1016/j.radonc.2010.03.013
195. Dewas S, Bibault JE, Mirabel X, Fumagalli I, Kramar A, Jarraya H, et al. Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy. Radiat Oncol (2012) 7:166. doi: 10.1186/1748-717x-7-166
196. Liu E, Stenmark MH, Schipper MJ, Balter JM, Kessler ML, Caoili EM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors. Trans Oncol (2013) 6(4):442–6. doi: 10.1593/tlo.12448
197. Weiner AA, Olsen J, Ma D, Dyk P, DeWees T, Myerson RJ, et al. Stereotactic body radiotherapy for primary hepatic malignancies - Report of a phase I/II institutional study. Radiother Oncol (2016) 121(1):79–85. doi: 10.1016/j.radonc.2016.07.020
198. Shen P-C, Chang W-C, Lo C-H, Yang J-F, Lee M-S, Dai Y-H, et al. Comparison of Stereotactic Body Radiation Therapy and Transarterial Chemoembolization for Unresectable Medium-Sized Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys (2019) 105(2):307–18. doi: 10.1016/j.ijrobp.2019.05.066
199. Skowronek J, Zwierzchowski G. Brachytherapy in the treatment of bile duct cancer - a tough challenge. J Contemp Brachyther (2017) 9(2):187–95. doi: 10.5114/jcb.2017.66893
200. Mattiucci GC, Autorino R, Tringali A, Perri V, Balducci M, Deodato F, et al. A Phase I study of high-dose-rate intraluminal brachytherapy as palliative treatment in extrahepatic biliary tract cancer. Brachytherapy (2015) 14(3):401–4. doi: 10.1016/j.brachy.2014.12.002
201. Yoshioka Y, Ogawa K, Oikawa H, Onishi H, Kanesaka N, Tamamoto T, et al. Impact of intraluminal brachytherapy on survival outcome for radiation therapy for unresectable biliary tract cancer: a propensity-score matched-pair analysis. Int J Radiat Oncol Biol Phys (2014) 89(4):822–9. doi: 10.1016/j.ijrobp.2014.04.020
202. Aggarwal R, Patel FD, Kapoor R, Kang M, Kumar P, Chander Sharma S. Evaluation of high-dose-rate intraluminal brachytherapy by percutaneous transhepatic biliary drainage in the palliative management of malignant biliary obstruction–a pilot study. Brachytherapy (2013) 12(2):162–70. doi: 10.1016/j.brachy.2012.06.002
203. Chen Y, Wang XL, Yan ZP, Cheng JM, Wang JH, Gong GQ, et al. HDR-192Ir intraluminal brachytherapy in treatment of malignant obstructive jaundice. World J Gastroenterol (2004) 10(23):3506–10. doi: 10.3748/wjg.v10.i23.3506
204. Makita C, Nakamura T, Takada A, Takayama K, Suzuki M, Ishikawa Y, et al. Clinical outcomes and toxicity of proton beam therapy for advanced cholangiocarcinoma. Radiat Oncol (Lond Engl) (2014) 9:26–. doi: 10.1186/1748-717X-9-26
205. Ohkawa A, Mizumoto M, Ishikawa H, Abei M, Fukuda K, Hashimoto T, et al. Proton beam therapy for unresectable intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol (2015) 30(5):957–63. doi: 10.1111/jgh.12843
206. Shimizu S, Okumura T, Oshiro Y, Fukumitsu N, Fukuda K, Ishige K, et al. Clinical outcomes of previously untreated patients with unresectable intrahepatic cholangiocarcinoma following proton beam therapy. Radiat Oncol (2019) 14(1):241. doi: 10.1186/s13014-019-1451-5
207. Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol (2016) 34(5):460–8. doi: 10.1200/JCO.2015.64.2710
208. Kasuya G, Terashima K, Shibuya K, Toyama S, Ebner DK, Tsuji H, et al. Carbon-ion radiotherapy for cholangiocarcinoma: a multi-institutional study by and the Japan carbon-ion radiation oncology study group (J-CROS). Oncotarget (2019) 10(43):4369–79. doi: 10.18632/oncotarget.27028
209. Hung S-P, Huang B-S, Hsieh C-E, Lee C-H, Tsang N-M, Chang JT-C, et al. Clinical Outcomes of Patients With Unresectable Cholangiocarcinoma Treated With Proton Beam Therapy. Am J Clin Oncol (2020) 43(3):180–6. doi: 10.1097/coc.0000000000000646
210. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature (2011) 480(7378):480–9. doi: 10.1038/nature10673
211. Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys (2012) 84(4):879–80. doi: 10.1016/j.ijrobp.2012.06.020
212. Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol (2014) 4:325. doi: 10.3389/fonc.2014.00325
213. Eckert F, Gaipl US, Niedermann G, Hettich M, Schilbach K, Huber SM, et al. Beyond checkpoint inhibition - Immunotherapeutical strategies in combination with radiation. Clin Transl Radiat Oncol (2017) 2:29–35. doi: 10.1016/j.ctro.2016.12.006
214. Ko EC, Raben D, Formenti SC. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Clin Cancer Res (2018) 24(23):5792–806. doi: 10.1158/1078-0432.CCR-17-3620
215. Rödel F, Frey B, Multhoff G, Gaipl U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett (2015) 356(1):105–13. doi: 10.1016/j.canlet.2013.09.015
216. Golden EB, Formenti SC. Is tumor (R)ejection by the immune system the “5th R” of radiobiology? Oncoimmunology (2014) 3(1):e28133. doi: 10.4161/onci.28133
217. Yoshimura M, Itasaka S, Harada H, Hiraoka M. Microenvironment and radiation therapy. BioMed Res Int (2013) 2013:685308. doi: 10.1155/2013/685308
218. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med (2012) 366(10):925–31. doi: 10.1056/NEJMoa1112824
219. Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res (2013) 1(6):365–72. doi: 10.1158/2326-6066.CIR-13-0115
220. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res (2009) 15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265
Keywords: cholangiocarcinoma, biliary tract cancer, stereotactic body radiotherapy, chemoradiation, brachytherapy
Citation: Gkika E, Hawkins MA, Grosu A-L and Brunner TB (2020) The Evolving Role of Radiation Therapy in the Treatment of Biliary Tract Cancer. Front. Oncol. 10:604387. doi: 10.3389/fonc.2020.604387
Received: 09 September 2020; Accepted: 04 November 2020;
Published: 14 December 2020.
Edited by:
Susanne Rogers, Aarau Cantonal Hospital, SwitzerlandReviewed by:
Irene Chong, Royal Marsden Hospital, United KingdomMilly Buwenge, University of Bologna, Italy
Copyright © 2020 Gkika, Hawkins, Grosu and Brunner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleni Gkika, eleni.gkika@uniklinik-freiburg.de
 Eleni Gkika
Eleni Gkika Maria A. Hawkins
Maria A. Hawkins Anca-Ligia Grosu1
Anca-Ligia Grosu1