- 1Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Breast Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
- 4Department of Orthopedic Surgery, Key Laboratory of Big Data for Spinal Deformities, Beijing Key Laboratory for Genetic Research of Skeletal Deformity, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
- 5Department of Laboratory Medicine, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 6Department of Breast Surgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 7Department of Ultrasound, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 8PET-CT Center, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 9Department of Radiology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
- 10Department of Breast Surgical Oncology, Cancer Hospital of HuanXing, Beijing, China
- 11Department of Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
A proportion of up to 10% of breast cancer resulted from hereditary germline pathogenic variants (GPVs) in cancer predisposition genes (CPGs), which been demonstrated distinct clinical features and imaging manifestations. However, the performance of imaging modalities for breast cancer surveillance in CPG mutation-carriers is still unclear, especially in Asian women. A population of 3002 breast cancer patients who received germline genetic testing of CPGs was enrolled from three hospitals in China. In total, 343 (11.6%) patients were found to harbor GPVs in CPGs, including 137 (4.6%) in BRCA1 and 135 (4.6%) in BRCA2. We compared the performances of ultrasound, mammograms, MRI, and the combining strategies in CPG mutation carriers and non-carriers. As a result, the ultrasound showed a higher detection rate compared with mammograms regardless of the mutation status. However, its detection rate was lower in CPG mutation carriers than in non-carriers (93.2% vs 98.0%, P=2.1×10-4), especially in the BRCA1 mutation carriers (90.9% vs 98.0%, P=2.0×10-4). MRI presented the highest sensitivity (98.5%) and the lowest underestimation rate (14.5%) in CPG mutation carriers among ultrasound, mammograms, and their combination. Supplemental ultrasound or mammograms would add no significant value to MRI for detecting breast cancer (P>0.05). In multivariate logistic regression analysis, the family or personal cancer history could not replace the mutation status as the impact factor for the false-negative result and underestimation. In summary, clinicians and radiologists should be aware of the atypical imaging presentation of breast cancer in patients with GPVs in CPGs.
Introduction
Breast cancer is currently the most common cancer among women both in the West and East (1, 2). A proportion of 5-10% breast cancer resulted from hereditary germline pathogenic variants (GPVs) in cancer predisposition genes (CPGs) such as BRCA1/2, PALB2, etc. (3–5) The BRCA-related breast cancer has demonstrated distinct clinical phenotypes in pathology features and imaging manifestations (6). Thus, special breast cancer screening and diagnosis guidelines with higher sensitivity have been applied in the CPG mutation-carriers in the US and UK (7, 8). However, the performance of imaging modalities in detecting BC in Asian CPG mutation carriers was still unknown.
The mammogram alone is insufficient for young women carrying BRCA1/2 mutations, even in women with low breast density (9). Compared to mammograms, the dynamic contrast-enhanced breast magnetic resonance imaging (MRI) has demonstrated the highest sensitivity in BRCA1/2 mutation-carriers (10, 11). Thus, the National Comprehensive Cancer Network (NCCN) has recommended annual breast MRI combined with an annual mammogram in breast cancer surveillance for women with BRCA1/2 mutations (7); while both the United States Preventive Services Taskforce (USPSTF) and the WHO International Agency for Research on Cancer (IARC) do not provide clear screening recommendations (12). Considering the high cost and high false-positive rate of the MRI, ultrasonography is widely used as a supplemental screening modality in Asian countries (13). It has also significantly increased the detection rate and screening sensitivity (14). A recent meta-analysis of 21 studies showed that supplemental ultrasound shows added value to sensitivity in women with dense breasts compared with mammograms alone (15). However, the clinical utility of ultrasound for detecting breast cancer in CPG mutation-carriers remains unclear (16).
Here, we investigated whether the germline variants could impact the performance of the mammogram, ultrasound, and MRI in a multi-center cohort of 3002 female Chinese breast cancer patients undergoing the multigene testing. This is also the first study to investigate the diagnosis accuracy and the effectiveness of these imaging techniques in screening for breast cancer among Chinese women with CPG mutations.
Methods
Study Participants and Design
This multicenter cohort study recruited consecutive female patients with breast cancer from October 1, 2017, to July 31, 2020, at the Cancer Hospital and Peking Union Medical College Hospital, both of Chinese Academy of Medical Sciences and Peking Union Medical College, and Huanxing Cancer Hospital, all in Beijing, China. Ultrasonography was conducted as the screening modality for all the patients. Digital mammography was provided for patients who were suspected for calcification in the breast or older than 40 years old. The screening MRI was performed according to patients’ willingness. The diagnosis of each patient was based on the pathology results from resection specimens. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (17).
Clinical Evaluation
We collected phenotypic data including the onset age, family history, personal cancer history, imaging evaluation, pathology features, clinical subtype, and clinical stage. Clinical grouping of subtypes was defined by the status of hormone receptor and HER2 according to the St. Gallen 2017 criteria (18). Standard digital mammography, ultrasonography, and MRI techniques were conducted at each center. The images were interpreted and classified according to the fifth edition of the Breast Imaging Reporting and Data System (BI-RADS) standard by two experienced radiologists independently at each center blind to the mutation status and the pathological finding (19). The BI-RADS 0 findings were excluded in the further analysis.
Germline Variants Analysis
Genomic DNA was extracted from peripheral blood or saliva. Germline variants were analyzed by a multiplex amplicon-based library preparation system and targeted a panel covering the coding regions and consensus splice sites of 50 CPGs in DNA-repair pathways for sequencing using an Illumina HiSeq 4000 Platform (20). The cancer predisposition genes included ATM, BARD1, BRIP1, BRCA1, BRCA2, CDH1, PALB2, RAD5IC, RAD51D, CHEK2, NBN, TP53, PTEN, STKI1, APC, MUTYH, MLH1, MSH2, MSH6, PMS2, SMAD4, KIT, PDGFA, HOXB13, RB1, PTCH1, CDK4, CDKN2A, PALLD, WRN, MEN1, RECQL, RET, SDHA, SDHB, SDHC, SDHD, SDHAF2, GNAS, MAX, VHL, MET, FH, FLCN, TSC1, TSC2, PRKAR1A, SMARCA4, SMARCB1, and BRAF. The clinical significance (benign/likely benign/variant of unknown significance/likely pathogenic/pathogenic) of each variant was annotated according to the ACMG/AMP guidelines (21). Pathogenic and likely pathogenic variants were analyzed together as pathogenic variants. Benign and likely benign variants were analyzed together as benign variants. The mutation curation was also conducted by two experienced medical geneticists independently blind to the imaging interpretation.
Statistical Analysis
The false-negative rate (FNR) was defined as the proportion of the BI-RADS categories less than 4 (22). The underestimation rate (UR) was defined as the proportion of the estimated malignancy rate of less than 50% (the BI-RADS categories 0-4b). The Student’s t-test was used to analyze the onset age and tumor size. The prevalence of the lymph nodes metastasis, FNRs, and URs were compared using the Pearson χ2 test or the Fisher’s exact test to obtain p values and odds ratios (ORs) with 95% confidence intervals (CIs). We also conducted multivariate logistic regression to evaluate the impact of the characteristics on the diagnostic sensitivity of different imaging techniques. Statistical tests were two-sided, and p values <0.05 were considered significant. Two-side p<0.05 was considered as statistically significant. Statistical analysis was performed using SPSS version 15.0 (SPSS, USA) and R statistical software, version 3.5.1.
Results
Patient Characteristics
In this study, 3002 women who were diagnosed with breast cancer were enrolled from three hospitals in China at a mean ± SD age of 42.8 ± 9.0 years (Figure 1). Thirty-eight patients with advanced breast cancer were excluded. In total, 343 (11.6%, 343/2964) patients were found to harbor GPVs in CPGs, including 137 (4.6%) in BRCA1, 135 (4.6%) in BRCA2, and 71 (2.4%) in other CPGs. Besides, 247 patients with variants of uncertain significance were excluded from further analysis (Figure 1).
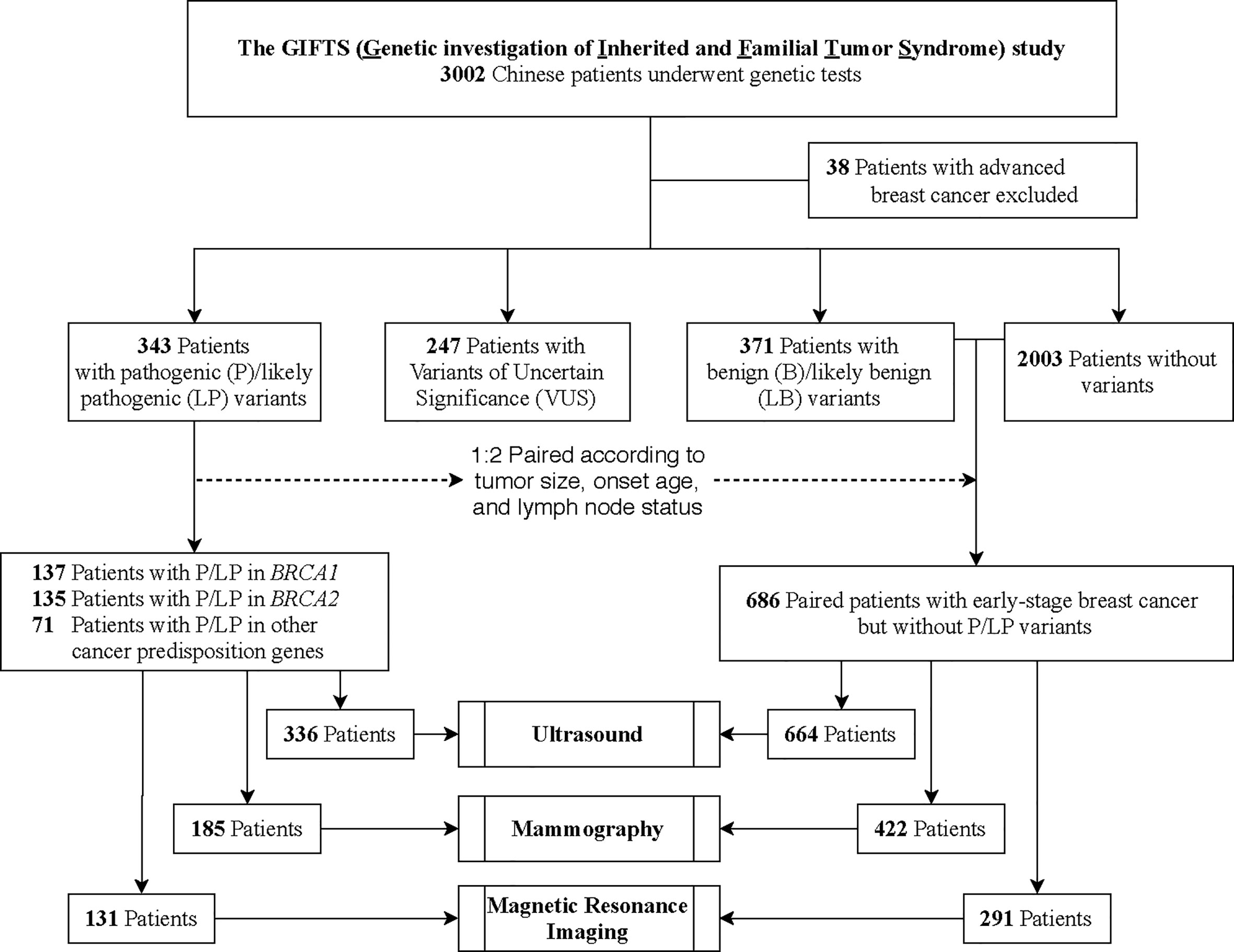
Figure 1 Patient Enrollment and Study Design. In this study, 3002 breast cancer patients were enrolled from three hospitals in China. Thirty-eight patients with advanced breast cancer were excluded. In total, 343 patients were found to harbor germline pathogenic variants (GPVs) in cancer predisposition genes (CPGs). To compare performances of ultrasound, mammograms, Magnetic Resonance Imaging (MRI), and combining strategies between different mutation status, 686 non-carriers were selected as 1:2 paired with the CPG mutation-carriers according to the onset age, tumor size, and lymph node status.
The age of diagnosis is significantly younger in patients with GPVs as compared with patients without GPVs (40.3 ± 7.9 vs. 43.3 ± 9.3, respectively, p=1.4×10-8), and even younger in patients with GPVs of BRCA1 (39.1 ± 7.7 vs. 43.3 ± 9.3, p=1.5×10-7; Table 1). For pathological characteristics, there was no association between the tumor size and mutation status (p=0.93). A higher proportion of invasive ductal carcinoma was identified in patients with GPVs in BRCA1/2 (94.9% and 86.7% in BRCA1 and BRCA2 mutation-carriers vs. 77.9% in non-carriers, p=1.3×10-7 and 0.02), while less ductal carcinoma in situ (DCIS) was found in patients with GPVs in BRCA1 (0% in BRCA1 mutation-carriers vs. 5.5% in non-carriers, p=1.1×10-3). In patients with GPVs in BRCA1, there was less proportion of histological grade I and II than patients without GPVs (0% in grade I and 19.7% in grade II in BRCA1 mutation-carriers vs. 5.8% and 42.8% in non-carriers, p=6.9×10-4 and 3.4×10-8, respectively), but a higher proportion of grade III (73.0% in BRCA1 mutation-carriers vs. 25.7% in non-carriers, p=4.9×10-29). Compared to the patients without GPVs, less grade I in patients with PGVs in BRCA2 (5.8% vs. 1.5%, p=0.03), while more grade II in patients with PGVs in BRCA2 and other CPGs were identified (42.8% in non-carriers vs. 52.6% in BRCA2 mutation-carriers and 56.3% in other CPG mutation-carriers, both p=0.03, respectively).
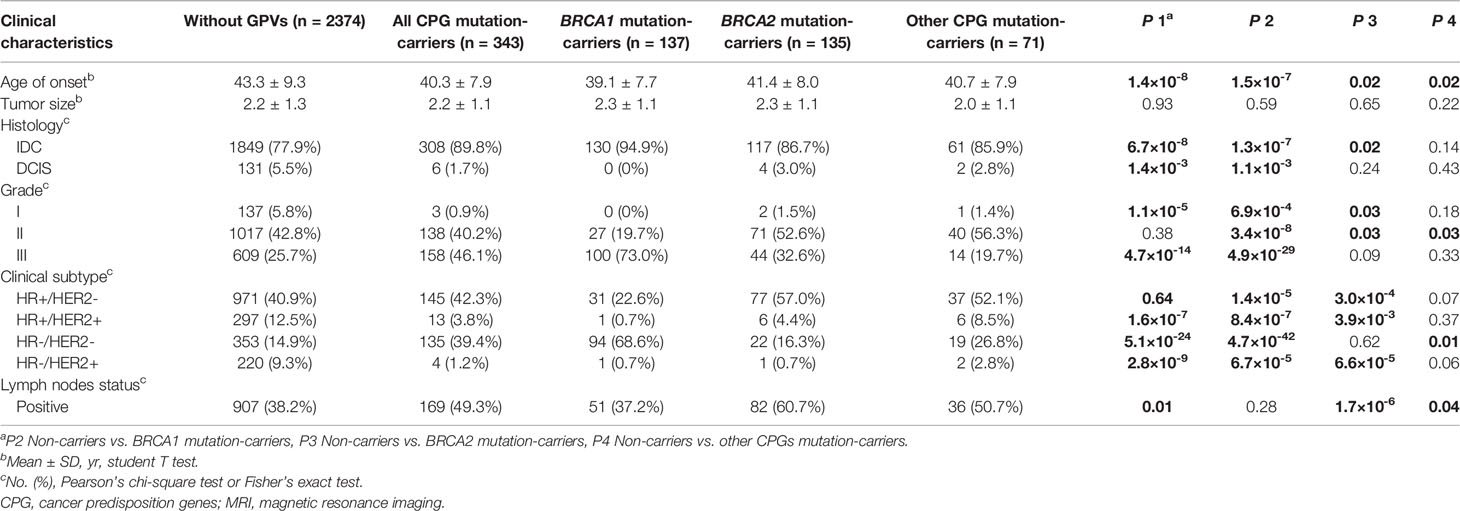
Table 1 Comparison of clinical and pathological characteristics between patients with and without cancer predisposition gene mutations.
For the molecular subtype, more triple-negative breast cancers were found in patients with GPVs in BRCA1 and other CPGs than the non-carriers (68.6% and 26.8% vs. 14.9%, p=4.7×10-42 and 0.01; Table 1). Significantly fewer HER2 positive breast cancers including both HR-/HER2+ and HR+/HER2+ were found in patients with GPVs in BRCA1/2 than the non-carriers (Table 1). However, there were more HR+/HER2- breast cancers in BRCA2 mutation-carriers (57.0%) and fewer in BRCA1 mutation-carriers (22.6%) than the non-carriers (40.9%, p=1.4×10-5 and 3.0×10-4, respectively).
In addition, more patients with lymph node metastasis were found in BRCA2 and other CPGs subgroups than the non-GPVs group (60.7% in BRCA2 mutation-carriers and 50.7% in other CPGs mutation-carriers vs. 38.2% in non-carriers, p=1.7×10-6 and 0.04, respectively).
The Diagnosis Accuracy of the Breast Imaging Modalities
To compare the performances of imaging modalities between different mutation status, 686 non-carriers were selected as 1:2 paired with the CPG mutation-carriers (n=343) according to the onset age, tumor size, and lymph node status (Figure 1). The CPG mutation-carriers were further divided into BRCA1 mutation-carriers (n=137), BRCA2 mutation-carriers (n=135), and other CPGs mutation-carriers (n=71) according to the mutated genes (Table 2). As all the patients underwent ultrasound, 7 patients with mutations and 22 patients without mutations were diagnosed as BI-RADS 0 category. Therefore, the performance of ultrasound was evaluated in 336 patients with mutations and 664 patients without mutations. Meanwhile, the performance of digital mammography and MRI was evaluated in 185 and 131 patients with mutations and 422 and 291 patients without mutations, respectively.
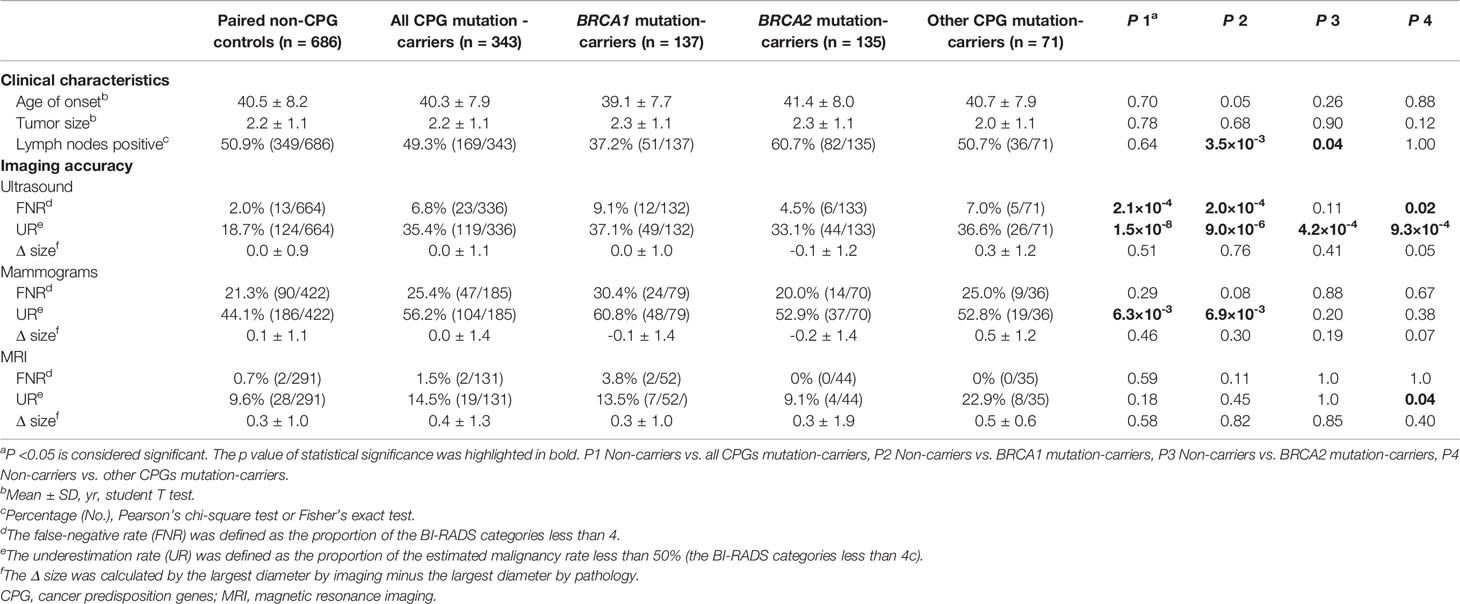
Table 2 The performance of imaging modalities in patients with cancer predisposition gene mutations and pair non-mutation controls.
The mammography performed poorly in both the CPG mutation-carriers and non-carriers (FNR=25.4% and 21.3%, p=0.29). The UR of the mammography was still higher in evaluating the CPG mutation-carriers than the non-carriers (56.2% vs. 44.1%, p=6.3×10-3), especially in the BRCA1 mutation-carriers (60.8% vs. 44.1%, p=6.9×10-3; Table 2 and Figure S1). Intriguingly, the ultrasound showed a higher detection rate compared with mammograms regardless of the mutation status (Table 2). However, the FNR of ultrasound was significantly higher in patients with GPVs in CPGs than the non-carriers (6.8% vs. 2.0%, p=2.1×10-4; Table 2 and Figure S2), especially in BRCA1 mutation-carriers (9.1% vs. 2.0%, p=2.0×10-4) and other CPGs mutation-carriers (7.0% vs. 2.0%, p=0.02). The UR of ultrasound was also higher in patients with GPVs in all CPGs than the non-carriers (35.4% vs. 18.7%, p=1.5×10-8; Table 2 and Figure S1). We also investigated the performance of imaging modalities in patients with mutations in non-BRCA1/2 cancer predisposition genes which affected no less than 5 patients. As a result, the RAD51D mutation carriers showed the highest FNR and UR by ultrasound when comparing with CHEK2, PALB2, and TP53 mutation carriers (Table S1). The FNRs of MRI were consistently low among different mutation status (0.7% in non-carriers, 1.5% in all CPG mutation-carriers, 3.8% in BRCA1 mutation-carriers, 0% in BRCA2 mutation carriers, and 0% in other CPG mutation-carriers); while the UR of MRI was significantly higher in patients with GPVs in CPGs other than BRCA1/2 than the non-carriers (22.9% vs. 9.6%, p=0.04; Table 2 and Figure S1). Three modalities showed similar performances measuring the tumor diameters among different mutation status. However, the estimated sizes according to MRI were larger than the tumor sizes (Table 2).
The Accuracy of Combined Imaging Strategies
To evaluate the combined strategies, we also assessed the FNR and UR by combining two imaging techniques. Similar to the performance of ultrasound, the FNR of combining the ultrasound and mammograms was higher in CPG mutation-carriers than the non-carriers (2.8% vs. 0.5%, p=0.03), especially in BRCA1 mutation-carriers (5.3% vs. 0.5%, p=6.7×10-3; Table 3 and Figure S2). Its URs were also higher in all CPG mutation-carriers than the non-carriers (27.2% in all CPG mutation-carriers, 27.6% in BRCA1 mutation-carriers, 25.0% in BRCA2 mutation carriers, and 30.6% in other CPG mutation-carriers vs. 13.8% in non-carriers, p=1.6×10-4, 5.6×10-3, 0.03, and 0.02, respectively; Table 3 and Figure S1). The combination of ultrasound and mammograms performed superior than the ultrasound or the mammograms separately with lower URs in the non-carriers (OR [95%CI] =0.7 [0.5-1.0] and 0.2 [0.1-0.3], p=0.04 and 2.3×10-22; Figure S3). While this combination only showed lower FNR than the mammograms in the non-carriers (OR [95%CI] =0.0 [0.0-0.1], p=2.9×10-26; Figure S4). In CPG mutation-carriers, this combination also showed lower UR (OR [95%CI] =0.3 [0.2-0.5], p=2.7×10-8; Figure S3) and lower FNR (OR [95%CI] =0.1 [0.0-0.2], p=9.0×10-11; Figure S4) than the mammograms. However, the combination of ultrasound and mammograms still showed significantly higher URs than the MRI (OR [95%CI] =2.2 [1.2-4.2], p= 8.2×10-3; Figure S3).
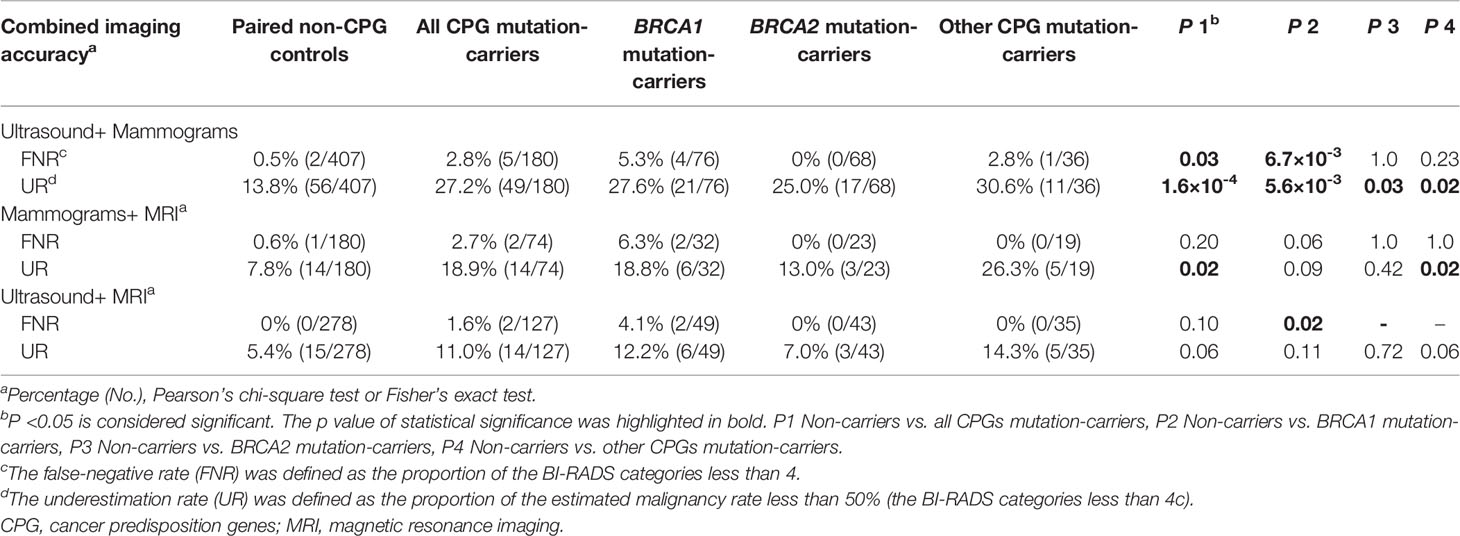
Table 3 The performance of combined imaging modalities in patients with cancer predisposition gene mutations and pair non-mutation controls.
With the combination of mammograms and MRI, the FNR showed no difference among different subgroups (p>0.05; Figure S2), while the UR was higher in CPG mutation-carriers than the non-carriers (18.9% vs. 7.8%, p=0.02), especially in other CPG mutation-carriers (26.3% vs. 7.8%, p=0.02; Table 3 and Figure S1). The combination of mammograms and MRI showed lower URs (OR [95%CI] =0.2 [0.1-0.4] and 0.1 [0.1-0.2], p=4.1×10-8 and 2.1×10-20, respectively; Figure S3) and lower FNRs (OR [95%CI] =0.1 [0.0-0.3] and 0.0 [0.0-0.1], p=5.6×10-6 and 2.5×10-14, respectively; Figure S4) than mammograms in both CPG mutation-carriers and non-carriers. However, the mammograms didn’t benefit the accuracy of MRI in this combination (p>0.05; Figures S3 and S4).
Combining the ultrasound and MRI, the FNR was only found higher in BRCA1 mutation-carriers than the non-carriers (4.1% vs. 0%, p=0.02; Figures S2) but not in BRCA2 mutation-carriers and other CPG mutation-carriers, and the URs were consistently low among different subgroups (Table 3 and Figures S1). The combination of ultrasound and MRI showed lower URs than the ultrasound alone in both CPG mutation-carriers and non-carriers (OR [95%CI] =0.2 [0.1-0.4] and 0.3 [0.1-0.4], p=7.4×10-8 and 3.1×10-8, respectively; Figure S3). Similarly, the ultrasound also didn’t benefit the accuracy of MRI in this combination (p>0.05; Figures S3 and S4).
Furthermore, 174 patients without GPVs and 72 patients with GPVs in CPGs have conducted all three imaging modalities. In patients without GPVs, all the patients can be detected by the combination of these three modalities. However, one patient (0.6%) can only be detected by ultrasound, while none patient can only be detected by mammograms or MRI (Table S2). In patients with GPVs in BRCA1, two patients (6.5%) were only detected by MRI and two patients (6.5%) cannot be detected by the combination of these three modalities. Intriguingly, both the two undetectable lesions were triple-negative breast cancers which were suspected as fibroadenoma. All three combinations of the two imaging modalities showed a satisfactory detection rate in patients with GPVs in BRCA2. In patients with GPVs in other CPGs, one 37-years-old patient (5.3%) with a stop-gained variant in RAD51D was only detected by MRI (Table S2). One of the three lesions missed by both ultrasound and mammograms was triple-negative breast cancer; while two were ER-positive breast cancers.
The Characteristics Impact the Diagnostic Sensitivity
To identify the characteristics that might impact the diagnostic sensitivity of different imaging techniques, multivariable logistic regression was conducted. The CPG mutation status, age of onset, lymph nodes status, and tumor size measured by ultrasound significantly impacted the FNR in ultrasound (p=8.1×10-4, 0.01, 6.7×10-4, and 0.03, respectively; Table S3). However, the family history or the personal history of breast or ovarian cancer showed no impact on the FNR (p=0.89 and 0.41, respectively; Table S3). The CPG mutation status and the lymph nodes status also significantly impacted the UR in ultrasound (p=2.3×10-9 and 5.7×10-7; Table S4).
For the mammograms, only the tumor size measured by mammograms, rather than CPG mutation status and the family history or the personal history, significantly impacted the FNR (p=3.0×10-6; Table S5). Only the lymph nodes status significantly impacted the UR (p=0.04; Table S6). The FNR of MRI was not relevant to these characteristics (Table S7), while the UR of the MRI was only impacted by the lymph nodes status (p=6.5×10-4; Table S8).
Discussion
In this study, 343 patients (11.5%) with PGVs in CPGs were identified in a consecutive multicenter cohort of female patients with breast cancer. Compared to patients without CPGs, distinct clinical phenotypes including the onset age, family and personal cancer history, and pathological features have been found in patients with BRCA1, BRCA2, and other CPGs. The diagnosis accuracies of ultrasound, mammograms, MRI, and the combinations of these modalities were investigated in breast cancer patients with or without CPG mutations by calculating the FNRs and URs. Furthermore, the impacts of each characteristic on the diagnostic performance of different imaging techniques were evaluated.
Although mammography has shown satisfactory detection accuracy in Western countries (23), it demonstrated the highest FNRs and URs regardless of the mutation status in this study (Table 2), which might result from the high breast density in Chinese women. It has been reported that most breast cancers detected by ultrasound were not detectable at mammography, even in retrospect (24). Compared with the non-carriers, the BRCA1 mutation-carriers showed more benign morphologic features in mammograms, which resulted in the highest FNR and UR. The less proportion of DCIS (0%) and the presentation of calcifications (25) in BRCA1 mutation-carriers might also limit the application of mammograms.
Compared with mammograms, ultrasound has advantages including higher sensitivity in women with dense or small breasts, no radiation exposure, lower cost, and easier access in China (12). For ultrasound, the FNRs were significantly higher in patients with GPVs in CPGs except for BRCA2, and the URs were higher in all the CPG mutation-carriers. The BRCA-associated breast cancers were commonly assessed as benign lesions by the ultrasound according to the fibroadenoma-like appearance and morphologic features of round or oval masses with circumscribed margins (25, 26). Meanwhile, aggressive pathologic features in BRCA1 mutation-carriers, such as the higher proportion of grade III tumors (73.0%), resulted in the rapid tumor growth, which has been also suggested as one of the most important underlying factors contributing to the FNR at imaging test (6). Similarly, the high FNR and UR by ultrasound in RAD51D mutation carriers might result from their BRCA1-like phenotypes, i.e. higher proportion of triple-negative breast cancer (5/8) and higher Ki67 proliferation fractions (6/8 higher than 30%) in this study.
Although some studies showed the ultrasound was comparable with mammograms among women at high risk of breast cancer, the adjunctive ultrasonography could increase the sensitivity of mammograms (14). Consistent with a previous study (27), the addition of the ultrasound to the mammograms would significantly increase the detection rate and diagnostic accuracy regardless of mutation status (Figures S3 and S4). In the Chinese multi-modality independent screening trial (MIST) (28), the supplementary ultrasound after negative mammography result additionally identified 11.9% breast cancer patients (12, 28). However, the FNR in ultrasound alone demonstrated no significant difference from the combination of ultrasound and mammograms in all the patients; the UR was lower through the combination strategy in breast cancer without GPVs.
The MRI has shown the most sensitivity in detecting breast cancer in CPG mutation-carriers even comparing with the combination of ultrasound and mammograms, which was consistent with the previous studies (10, 29, 30). In CPG mutation-carriers, the MRI detected three breast cancers (4.2%) in which the ultrasound and mammograms were undetectable (Table S2). In a Japanese case series, two in five primary breast cancers in patients with BRCA1/2 mutation were only detectable on MRI in a 48-month breast cancer surveillance program including biannual ultrasonography, annual mammography, and MRI (31). A prospective multicenter MRI screening study in Dutch has demonstrated that the supplemental screening MRI would benefit the early cancer detection and the prognosis in women with BRCA1/2 mutations after an over 9-year follow-up (32). Additionally, MRI has shown better performance than mammograms and ultrasound in dense breasts (33, 34), which are more common in Asian women (2). Therefore, the CPG mutation-carriers were recommended to undergo the MRI for breast cancer surveillance, which might not be replaced by the combination of the ultrasound and the mammograms.
In combination with MRI, the mammograms or the ultrasound seem to have no added value to the sensitivity in both CPG mutation-carriers and non-carriers in this study. Although mammograms have been proved to add value to MRI in older patients (35), whose benefit was limited in young patients especially in BRCA1/2 mutation-carriers (36, 37). Thus, mammograms might be omitted in younger women who have undergone MRI. However, the BRCA2 mutation-carriers have a higher proportion of DCIS, which were sometimes only detected as mammographic calcifications (35). While the supplemental mammograms were only proposed in BRCA2 mutation-carriers to at least age 40 (38). Additionally, the ultrasound was considered as a supplemental screening tool for MRI in BRCA1/2 mutation-carriers (39), but it has shown no benefit to the MRI in this study.
In the current clinical practice, fewer than 10% of the CPG mutation-carriers are identified (40), which significantly limited the mutation-based breast cancer surveillance. Thus, we testified the impact of the family history and personal history on the detection sensitivities of the three imaging techniques instead of the mutation status. As a result, the family or personal cancer history showed no impact on the FNRs or URs in all three modalities; while the CPG mutation status significantly impacted the FNR and UR in ultrasound but not in mammograms or MRI. Therefore, the genetic test of CPGs should be performed when ultrasound-based surveillance is conducted.
Limitation
There were several limitations in this study. First, this was a retrospective case-control study to investigate the detection performance of imaging techniques in Chinese CPG mutation-carriers. Second, only patients with breast cancer were enrolled in this study. Thus, specificity was not evaluated in this study. Third, a limited number of patients underwent all three imaging modalities, especially the MRI, which resulted from the accessibility and waiting period of each technique. Meanwhile, the number of patients with mutations in other CPGs except for BRCA1/2 was also limited. As there was no long-term follow-up in this study, it cannot evaluate the performance of detecting interval cancers. Therefore, double-blind, long-term, randomized prospective clinical trials involving all imaging modalities are needed to verify the diagnostic accuracy, cost-effectiveness, and long-term survival benefits in the future.
Conclusion
In summary, the genetic etiology of breast cancers is closely correlated with distinct clinical and pathological phenotypes and imaging manifestations. For Chinese breast cancer patients, the ultrasound showed a higher detection rate than mammograms regardless of the mutation status, while its accuracies were lower in CPG mutation-carriers. MRI presented the highest sensitivity, even higher than the combination of ultrasound and mammograms. Additionally, ultrasound and mammograms would add no significant value to MRI for detecting breast cancer in CPG mutation-carriers. Furthermore, the family or personal cancer history cannot replace the mutation status as the impact factor for the false-negative result and underestimation. Clinicians and radiologists should be aware of the atypical imaging presentation of breast cancer in patients with GPVs in CPGs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The study was reviewed and approved by the ethics committee of the Cancer Hospital and Peking Union Medical College Hospital, both of Chinese Academy of Medical Sciences and Peking Union Medical College, and Huanxing Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JQL, XinW, and XiaW conceived the study. JQL, XinW, LD, XH, JXL, SH, WW, ZX, ZJ, LQ, GL, JW, YL, MZ, and KF enrolled the patients and collected the data. JQL, LD, PY, and JY conducted the genetic tests. JQL and HZ designed the computational framework and analyzed the data. JW, YM, and XL interpreted the medical images. JQL and XiaW supervised the findings of this study. JQL wrote the manuscript. JQL, XinW, LD, and XH contributed equally to this study. All authors contributed to the article and approved the submitted version.
Funding
This study was funded in part by the National Natural Science Foundation of China (81802669 to JQL), the CAMS Innovation Fund for Medical Sciences (2020-I2M-C&T-B-068 to JQL), the Beijing Hope Run Special Fund (LC2020B05 to JQL), and the CAMS Initiative Fund for Medical Sciences (2016-I2M-1-001 to XiaW).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all the individuals, families, and physicians involved in the study for their participation. We thank Ms. Jiayi Li for editing the language.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.710156/full#supplementary-material
Abbreviations
GPV, Germline pathogenic variant; CPG, Cancer predisposition gene; MRI, Magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; USPSTF, United States Preventive Services Taskforce; IARC, International Agency for Research on Cancer; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; BI-RADS, Breast Imaging Reporting and Data System; FNR, False-negative rate; UR, Underestimation rate; OR, Odds ratio; CI, Confidence interval; MIST, Multi-modality independent screening trial.
References
1. Zhang S, Sun K, Zheng R, Zheng H, Wang S, Chen R, et al. Cancer Incidence and Mortality in China, 2015. J Natl Cancer Center (2021) 1:2–11. doi: 10.1016/j.jncc.2020.12.001
2. Yap Y-S, Lu Y-S, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights Into Breast Cancer in the East vs the Wes. JAMA Oncol (2019) 5:1489–96. doi: 10.1001/jamaoncol.2019.0620
3. Turner NC. Signatures of DNA-Repair Deficiencies in Breast Cancer. N Engl J Med (2017) 377(25):2490–2. doi: 10.1056/NEJMcibr1710161
4. Sun J, Meng H, Yao L, Lv M, Bai J, Zhang J, et al. Germline Mutations in Cancer Susceptibility Genes in a Large Series of Unselected Breast Cancer Patients. Clin Cancer Res (2017) 23(20):6113–9. doi: 10.1158/1078-0432.CCR-16-3227
5. Li L, Han J, Mo H, Ma F. Expert Consensus on Diagnosis, Treatment and Fertility Management of Young Breast Cancer Patients. J Natl Cancer Center (2021) 1: 23–30. doi: 10.1016/j.jncc.2021.02.001
6. Ha SM, Chae EY, Cha JH, Kim HH, Shin HJ, Choi WJ. Association of BRCA Mutation Types, Imaging Features, and Pathologic Findings in Patients With Breast Cancer With BRCA1 and BRCA2 Mutations. AJR Am J Roentgenol (2017) 209(4):920–8. doi: 10.2214/AJR.16.16957
7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncolog. In: Genetic/familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Version 1. (2020) Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf.
8. NICE. Familial Breast Cancer: Classification and Care Ofpeople at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People With a Family History of Breast Cancer. In: NICE Clinical Guideline. London, UK: National Institute for Health and Care Excellence (2013). CG164 ed.
9. Bigenwald RZ, Warner E, Gunasekara A, Hill KA, Causer PA, Messner SJ, et al. Is Mammography Adequate for Screening Women With Inherited BRCA Mutations and Low Breast Density? Cancer Epidemiol Biomarkers Prev (2008) 17(3):706–11. doi: 10.1158/1055-9965.EPI-07-0509
10. Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea MK, et al. Triple-Modality Screening Trial for Familial Breast Cancer Underlines the Importance of Magnetic Resonance Imaging and Questions the Role of Mammography and Ultrasound Regardless of Patient Mutation Status, Age, and Breast Density. J Clin Oncol (2015) 33(10):1128–35. doi: 10.1200/JCO.2014.56.8626
11. Passaperuma K, Warner E, Causer PA, Hill KA, Messner S, Wong JW, et al. Long-Term Results of Screening With Magnetic Resonance Imaging in Women With BRCA Mutations. Br J Cancer (2012) 107(1):24–30. doi: 10.1038/bjc.2012.204
12. Huang Y, Tong Z, Chen K, Wang Y, Liu P, Gu L, et al. Interpretation of Breast Cancer Screening Guideline for Chinese Women. Cancer Biol Med (2019) 16(4):825–35. doi: 10.20892/j.issn.2095-3941.2019.0322
13. Shen S, Zhou Y, Xu Y, Zhang B, Duan X, Huang R, et al. A Multi-Centre Randomised Trial Comparing Ultrasound vs Mammography for Screening Breast Cancer in High-Risk Chinese Women. Br J Cancer (2015) 112(6):998–1004. doi: 10.1038/bjc.2015.33
14. Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng Y-F, et al. Sensitivity and Specificity of Mammography and Adjunctive Ultrasonography to Screen for Breast Cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): A Randomised Controlled Trial. Lancet (2016) 387(10016):341–8. doi: 10.1016/s0140-6736(15)00774-6
15. Yuan WH, Hsu HC, Chen YY, Wu CH. Supplemental Breast Cancer-Screening Ultrasonography in Women With Dense Breasts: A Systematic Review and Meta-Analysis. Br J Cancer (2020) 123: 673–88. doi: 10.1038/s41416-020-0928-1
16. Elezaby M, Lees B, Maturen KE, Barroilhet L, Wisinski KB, Schrager S, et al. BRCA Mutation Carriers: Breast and Ovarian Cancer Screening Guidelines and Imaging Considerations. Radiology (2019) 291(3):554–69. doi: 10.1148/radiol.2019181814
17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med (2007) 147(8):573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
18. Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, et al. De-Escalating and Escalating Treatments for Early-Stage Breast Cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol (2017) 28(8):1700–12. doi: 10.1093/annonc/mdx308
19. Spak DA, Plaxco JS, Santiago L, Dryden MJ, Dogan BE. BI-RADS((R)) Fifth Edition: A Summary of Changes. Diagn Interv Imaging (2017) 98(3):179–90. doi: 10.1016/j.diii.2017.01.001
20. Zhao S, Zhang Y, Chen W, Li W, Wang S, Wang L, et al. Diagnostic Yield and Clinical Impact of Exome Sequencing in Early-Onset Scoliosis (EO). J Med Genet (2021) 58(1):41–7. doi: 10.1136/jmedgenet-2019-106823
21. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Patholog. Genet Med (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
22. Lo G, Scaranelo AM, Aboras H, Ghai S, Kulkarni S, Fleming R, et al. Evaluation of the Utility of Screening Mammography for High-Risk Women Undergoing Screening Breast MR Imaging. Radiology (2017) 285(1):36–43. doi: 10.1148/radiol.2017161103
23. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic Density and the Risk and Detection of Breast Cancer. N Engl J Med (2007) 356(3):227–36. doi: 10.1056/NEJMoa062790
24. Bae MS, Moon WK, Chang JM, Koo HR, Kim WH, Cho N, et al. Breast Cancer Detected With Screening US: Reasons for Nondetection at Mammography. Radiology (2014) 270(2):369–77. doi: 10.1148/radiol.13130724
25. Tilanus-Linthorst M, Verhoog L, Obdeijn IM, Bartels K, Menke-Pluymers M, Eggermont A, et al. A BRCA1/2 Mutation, High Breast Density and Prominent Pushing Margins of a Tumor Independently Contribute to a Frequent False-Negative Mammography. Int J Cancer (2002) 102(1):91–5. doi: 10.1002/ijc.10666
26. Schrading S, Kuhl CK. Mammographic, US, and MR Imaging Phenotypes of Familial Breast Cancer. Radiology (2008) 246(1):58–70. doi: 10.1148/radiol.2461062173
27. Cho N, Han W, Han BK, Bae MS, Ko ES, Nam SJ, et al. Breast Cancer Screening With Mammography Plus Ultrasonography or Magnetic Resonance Imaging in Women 50 Years or Younger at Diagnosis and Treated With Breast Conservation Therapy. JAMA Oncol (2017) 3(11):1495–502. doi: 10.1001/jamaoncol.2017.1256
28. Dong H, Huang Y, Song F, Dai H, Liu P, Zhu Y, et al. Improved Performance of Adjunctive Ultrasonography After Mammography Screening for Breast Cancer Among Chinese Females. Clin Breast Cancer (2018) 18(3):e353–61. doi: 10.1016/j.clbc.2017.07.014
29. Phi XA, Houssami N, Obdeijn IM, Warner E, Sardanelli F, Leach MO, et al. Magnetic Resonance Imaging Improves Breast Screening Sensitivity in BRCA Mutation Carriers Age >/= 50 Years: Evidence From an Individual Patient Data Meta-Analysis. J Clin Oncol (2015) 33(4):349–56. doi: 10.1200/JCO.2014.56.6232
30. van Zelst JCM, Mus RDM, Woldringh G, Rutten M, Bult P, Vreemann S, et al. Surveillance of Women With the BRCA1 or BRCA2 Mutation by Using Biannual Automated Breast US, MR Imaging, and Mammography. Radiology (2017) 285(2):376–88. doi: 10.1148/radiol.2017161218
31. Shimada S, Yoshida R, Nakashima E, Kitagawa D, Gomi N, Horii R, et al. Five Screening-Detected Breast Cancer Cases in Initially Disease-Free BRCA1 or BRCA2 Mutation Carriers. Breast Cancer (2019) 26(6):846–51. doi: 10.1007/s12282-019-00971-6
32. Saadatmand S, Obdeijn IM, Rutgers EJ, Oosterwijk JC, Tollenaar RA, Woldringh GH, et al. Survival Benefit in Women With BRCA1 Mutation or Familial Risk in the MRI Screening Study (MRIS). Int J Cancer (2015) 137(7):1729–38. doi: 10.1002/ijc.29534
33. Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of Breast Cancer With Addition of Annual Screening Ultrasound or a Single Screening MRI to Mammography in Women With Elevated Breast Cancer Risk. JAMA (2012) 307(13):1394–404. doi: 10.1001/jama.2012.388
34. Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated Breast Magnetic Resonance Imaging (MRI): First Postcontrast Subtracted Images and Maximum-Intensity Projection-a Novel Approach to Breast Cancer Screening With MR. J Clin Oncol (2014) 32(22):2304–10. doi: 10.1200/JCO.2013.52.5386
35. Vreemann S, van Zelst JCM, Schlooz-Vries M, Bult P, Hoogerbrugge N, Karssemeijer N, et al. The Added Value of Mammography in Different Age-Groups of Women With and Without BRCA Mutation Screened With Breast MR. Breast Cancer Res (2018) 20(1):84. doi: 10.1186/s13058-018-1019-6
36. Phi XA, Saadatmand S, De Bock GH, Warner E, Sardanelli F, Leach MO, et al. Contribution of Mammography to MRI Screening in BRCA Mutation Carriers by BRCA Status and Age: Individual Patient Data Meta-Analysis. Br J Cancer (2016) 114(6):631–7. doi: 10.1038/bjc.2016.32
37. Cortesi L, Canossi B, Battista R, Pecchi A, Drago A, Dal Molin C, et al. Breast Ultrasonography (BU) in the Screening Protocol for Women at Hereditary-Familial Risk of Breast Cancer: Has the Time Come to Rethink the Role of BU According to Different Risk Categories? Int J Cancer (2019) 144(5):1001–9. doi: 10.1002/ijc.31794
38. Chen L, Fu F, Huang M, Lv J, Zhang W, Wang C. The Spectrum of BRCA1 and BRCA2 Mutations and Clinicopathological Characteristics in Chinese Women With Early-Onset Breast Cancer. Breast Cancer Res Treat (2020) 180(3):759–66. doi: 10.1007/s10549-020-05573-x
39. Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, Monticciolo D, et al. Breast Cancer Screening With Imaging: Recommendations From the Society of Breast Imaging and the ACR on the Use of Mammography, Breast MRI, Breast Ultrasound, and Other Technologies for the Detection of Clinically Occult Breast Cancer. J Am Coll Radiol (2010) 7(1):18–27. doi: 10.1016/j.jacr.2009.09.022
Keywords: hereditary breast cancer, BRCA1/2, mammography, ultrasonography, magnetic resonance imaging
Citation: Liu J, Wang X, Dong L, Huang X, Zhao H, Li J, Huang S, Yuan P, Wang W, Wang J, Xing Z, Jia Z, Ming Y, Li X, Qin L, Liu G, Wu J, Li Y, Zhang M, Feng K, Ying J and Wang X (2021) The Distinct Performances of Ultrasound, Mammograms, and MRI in Detecting Breast Cancer in Patients With Germline Pathogenic Variants in Cancer Predisposition Genes. Front. Oncol. 11:710156. doi: 10.3389/fonc.2021.710156
Received: 15 May 2021; Accepted: 21 June 2021;
Published: 13 July 2021.
Edited by:
Daniela Turchetti, University of Bologna, ItalyReviewed by:
Laura Cortesi, University Hospital of Modena, ItalyYonglan Zheng, University of Chicago, United States
Copyright © 2021 Liu, Wang, Dong, Huang, Zhao, Li, Huang, Yuan, Wang, Wang, Xing, Jia, Ming, Li, Qin, Liu, Wu, Li, Zhang, Feng, Ying and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Wang, xiangw@vip.sina.com; Jianming Ying, jmying@cicams.ac.cn
†These authors have contributed equally to this work
 Jiaqi Liu
Jiaqi Liu Xin Wang
Xin Wang Lin Dong
Lin Dong Xin Huang
Xin Huang Hengqiang Zhao4
Hengqiang Zhao4 Jiaxin Li
Jiaxin Li Shengkai Huang
Shengkai Huang Ziqi Jia
Ziqi Jia Yue Ming
Yue Ming