- 1Department of Medical Genetics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Men’s Health and Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Department of Critical Care Medicine, Imam Hossein Medical and Educational Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Sprouty RTK signaling antagonist 4-intronic transcript 1 (SPRY4-IT1) is a long non-coding RNA (lncRNA) encoded by a gene located on 5q31.3. This lncRNA has a possible role in the regulation of cell growth, proliferation, and apoptosis. Moreover, since SPRY4-IT1 controls levels of lipin 2, it is also involved in the biosynthesis of lipids. During the process of biogenesis, SPRY4-IT1 is produced as a primary transcript which is then cleaved to generate a mature transcript which is localized in the cytoplasm. SPRY4-IT1 has oncogenic roles in diverse tissues. A possible route of participation of SPRY4-IT1 in the carcinogenesis is through sequestering miRNAs such as miR-101-3p, miR‐6882‐3p and miR-22-3p. The sponging effect of SPRY4-IT1 on miR-101 has been verified in colorectal cancer, osteosarcoma, cervical cancer, bladder cancer, gastric cancer and cholangiocarcinoma. SPRY4-IT1 has functional interactions with HIF-1α, NF-κB/p65, AMPK, ZEB1, MAPK and PI3K/Akt signaling. We explain the role of SPRY4-IT1 in the carcinogenesis according to evidence obtained from cell lines, xenograft models and clinical studies.
Introduction
SPRY4 Intronic Transcript 1 (SPRY4-IT1) is a long non-coding RNA (lncRNA). This transcript is encoded by a gene on the cytogenetic band 5q31.3. During the process of biogenesis, SPRY4-IT1 is produced as a primary transcript which is then cleaved to generate a mature transcript which is localized in the cytoplasm (1). Since the complete size and structure of the primary and cleaved transcripts of SPRY4-IT1 are not clear, it has been speculated that the primary transcript is an alternatively spliced variant of SPRY4 (https://www.ncbi.nlm.nih.gov/gene/100642175).
A pioneer study in this field has suggested that SPRY4-IT1 is originated from an intronic region of the SPRY4 gene. In silico studies have predicted that SPRY4-IT1 has numerous long hairpins in its secondary configuration. Based on the results of RNA-FISH experiments in the melanoma cells, SPRY4-IT1 is mainly localized in the cytoplasm. Since SPRY4-IT1 silencing has altered growth, differentiation, and apoptosis in melanoma cells, it has been suggested that SPRY4-IT1 has a role in the etiology of melanoma (2). Subsequent studies have provided further evidence for participation of SPRY4-IT1 in other types of cancers as well. In normal cells, this lncRNA can regulate cell cycle progression and cell proliferation. In the current review, we explain the role of SPRY4-IT1 in the carcinogenesis based on evidence obtained from cell lines, xenograft models and clinical studies.
Cell Line Studies
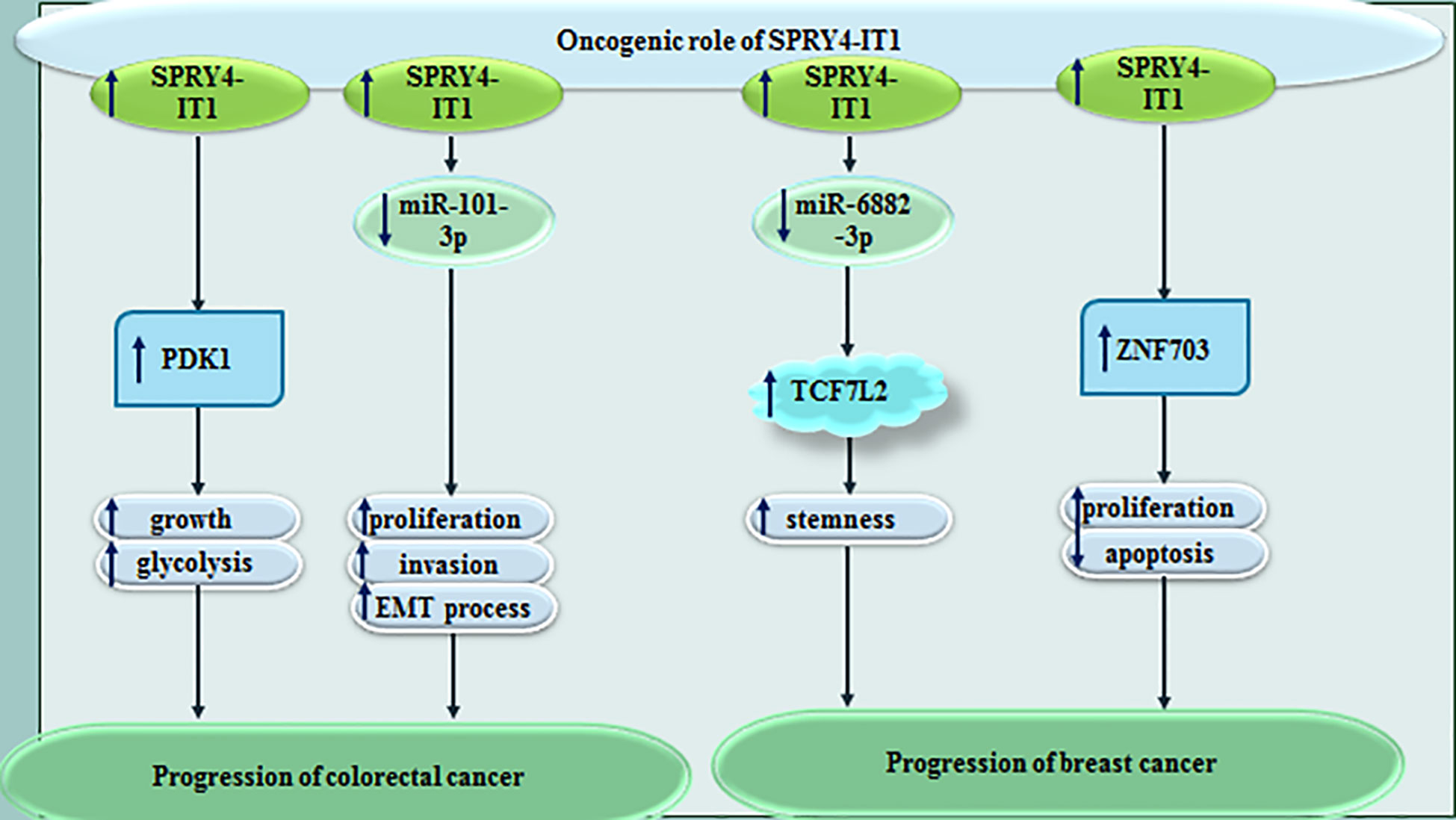
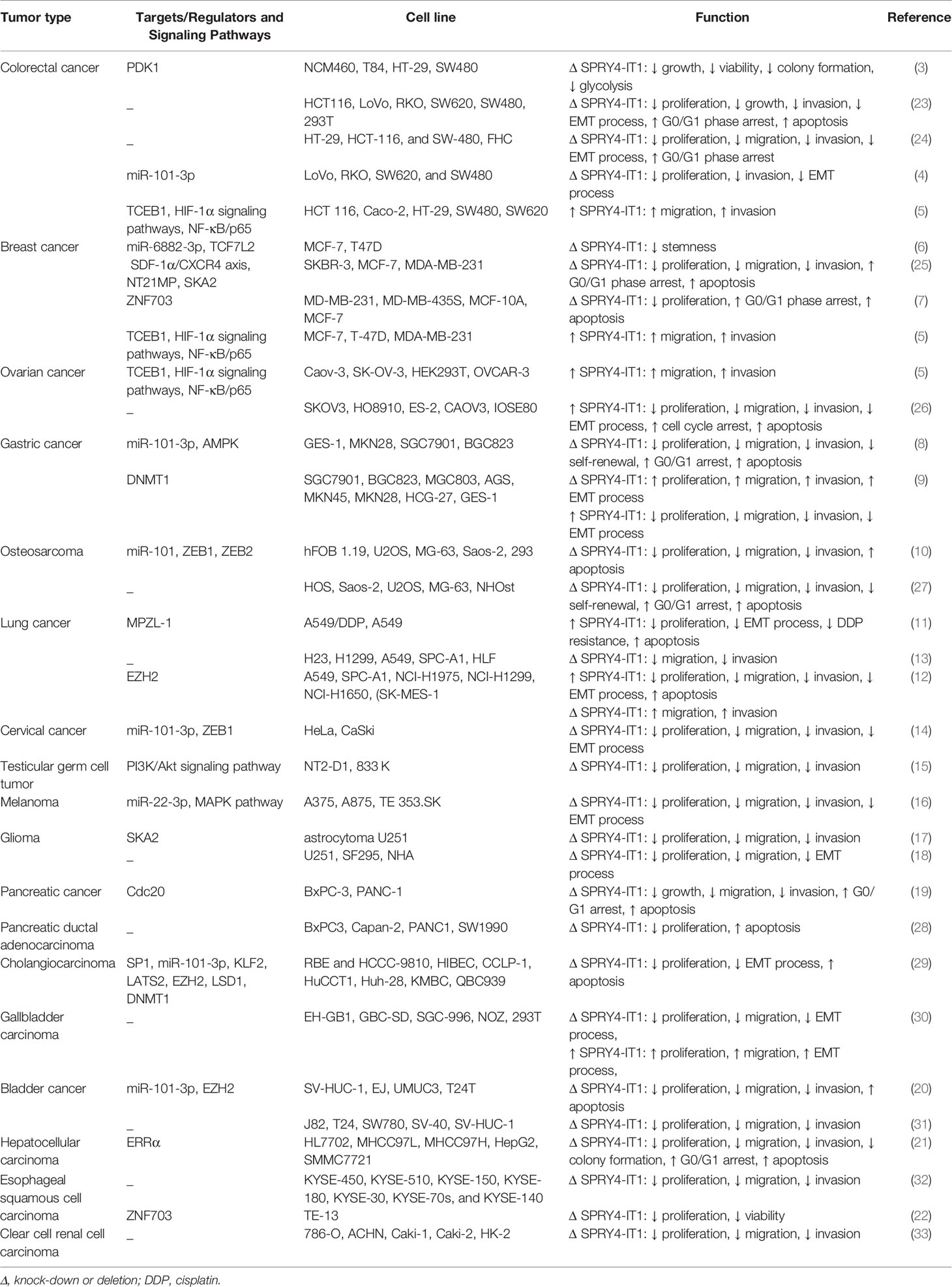
SPRY4-IT1 has been found to up-regulated in colorectal cancer cells. SPRY4-IT1 regulates growth and glycolysis of these cells through enhancing expression of PDK1. SPRY4-IT1 has affected glucose intake, lactic acid synthesis, and levels of ATP in colorectal cancer cells (3). SPRY4-IT1 has also been demonstrated to increase proliferation, migratory potential and invasiveness of colorectal cancer cells. Most notably, SPRY4-IT1 enhances expression of epithelial-mesenchymal transition (EMT)-associated genes. Mechanistically, SPRY4-IT1 negatively regulates expression of miR-101-3p in these cells through binding with this miRNA (4). SPRY4-IT1 up-regulation in a colorectal cancer cell line has resulted in differential expression of several genes among them has been TCEB1. This transcription elongation factor subunit can interact with the Alu element in the 3′untranslated region (UTR) of SPRY4-IT1. Besides, SPRY4-IT1 binds with STAU1 to increase STAU1 recruitment to the 3′-UTR of TCEB1 transcript. It subsequently modulates stability and expression of TCEB1, leading to up-regulation of HIF-1α. STAU1 is attributed to the family of double-stranded RNA-binding proteins. It participates in the transport of transcripts to various subcellular localizations. Expression of SPRY4-IT1 is also activated by NF-κB/p65 (5).
SPRY4-IT1 has also been reported to be over-expressed in MCF-7 cancer stem cells compared with MCF-7 cells. Up-regulation of SPRY4-IT1 has enhanced proliferation and stemness of breast cancer cells. Moreover, SPRY4-IT1 silencing has inhibited renewal capacity of breast cancer stem cells and maintenance of their stemness. Mechanistically, SPRY4-IT1 acts as a sponge for miR-6882-3p to affect expression of TCF7L2 (6). SPRY4-IT1 silencing in breast cancer cells has significantly inhibited their proliferation and prompted cell apoptosis. ZNF703 has been found to be a target of SPRY4-IT1 in these cells (7). The encoded protein by this gene is involved in nucleic acid binding and DNA-binding transcription factor binding. Figure 1 shows the oncogenic effect of SPRY4-IT1 in colorectal and breast cancers.
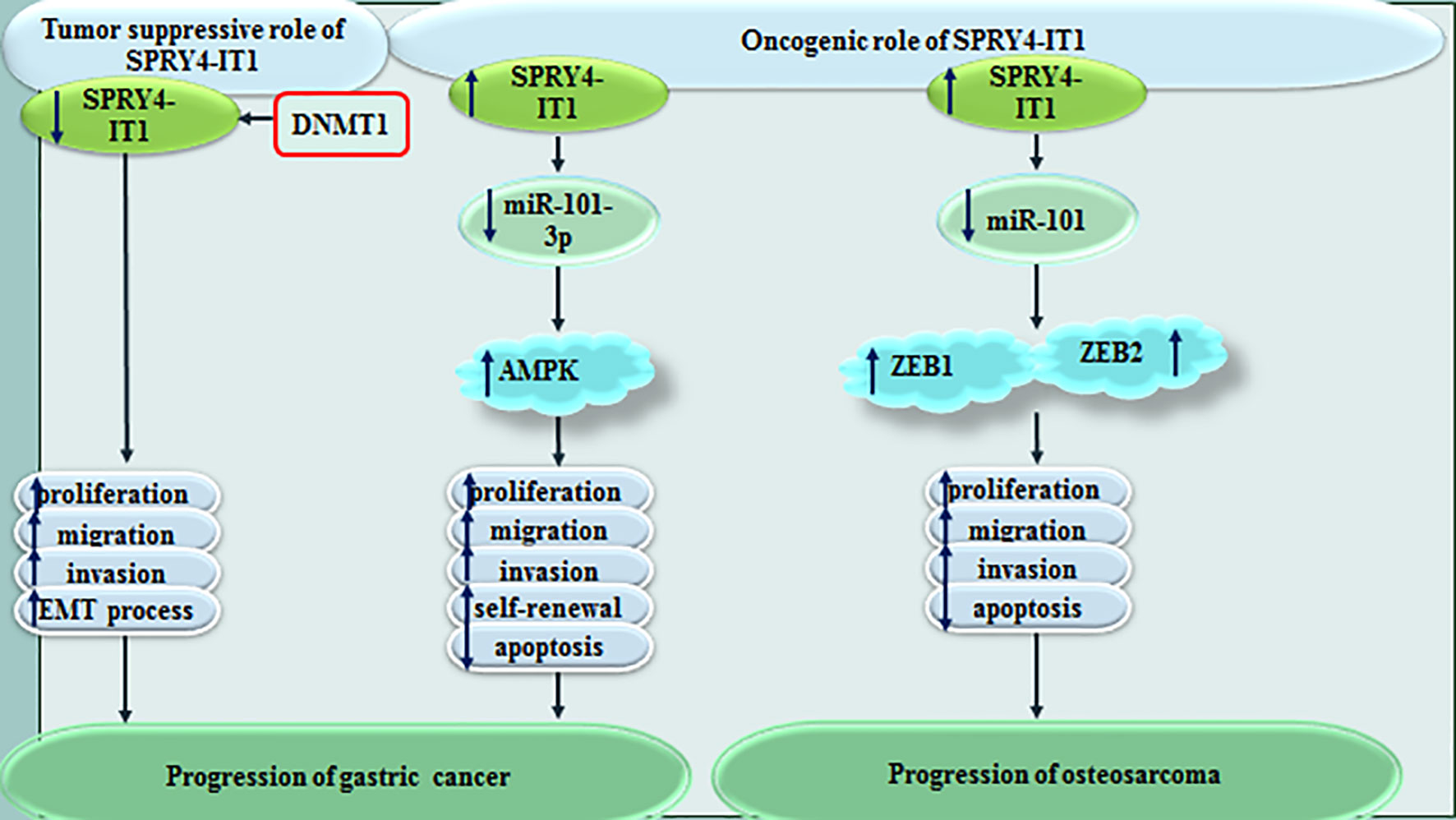
Cao et al. has shown that SPRY4-IT1 silencing significantly constrains proliferation of gastric cancer cells through inducing G1 arrest and enhancing apoptosis. SPRY4-IT1 acts as a sponge for miR-101-3p to increase expression of AMPK (8). On the other hand, Xie et al. have shown tumor suppressor role of SPRY4-IT1 in gastric cancer. DNA methylation has been found to be the main mechanism of control of SPRY4-IT1 expression in these cells. Besides, SPRY4-IT1 has been shown to affect EMT in gastric cancer cells (9). In osteosarcoma cells, SPRY4−IT1 has been shown to promote cancer progression through sequestering miR-101 and enhancing expressions of ZEB1 and ZEB2 (10). Figure 2 shows the effect of SPRY4-IT1 in the pathogenesis of gastric cancer and osteosarcoma.
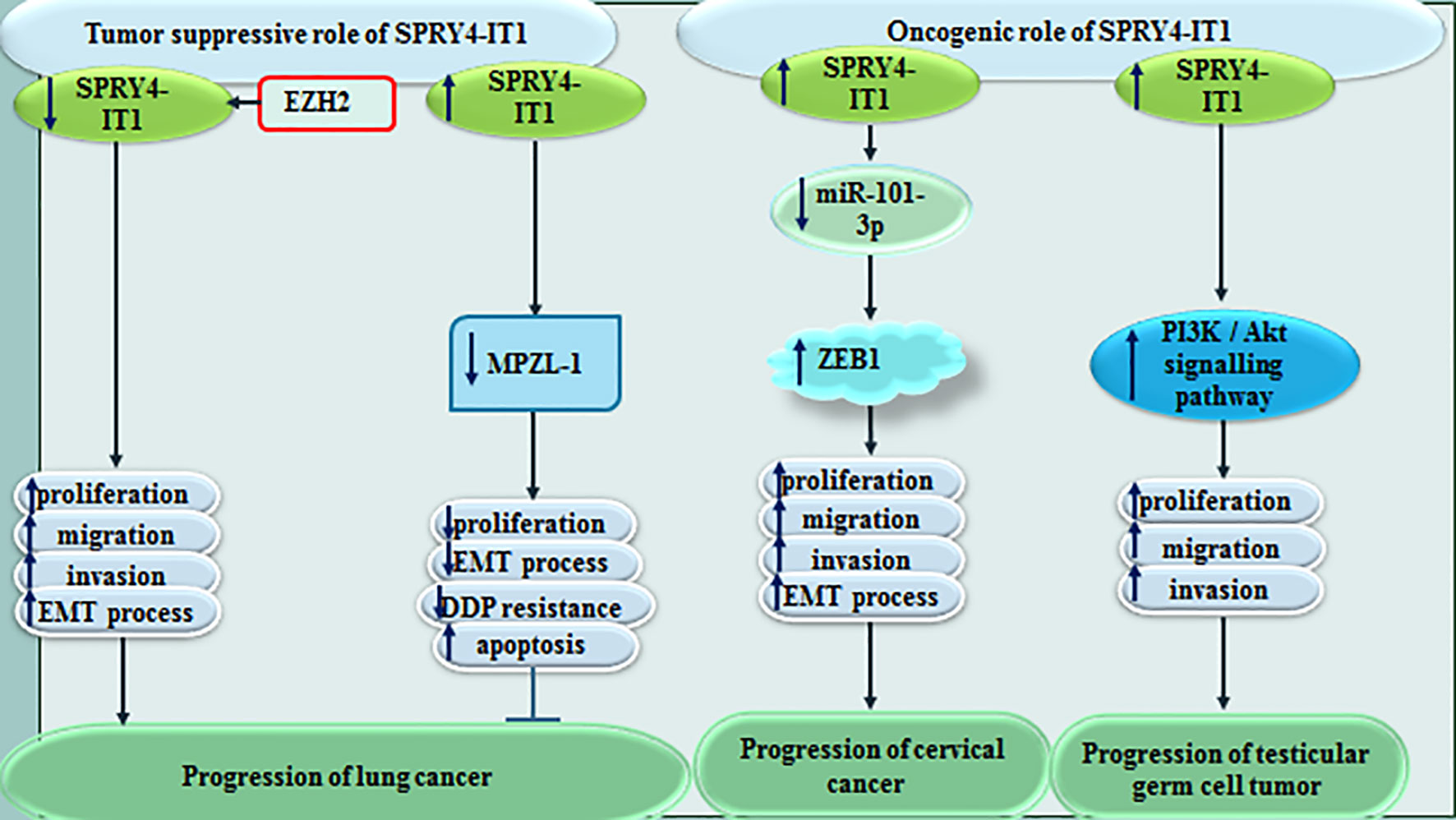
In lung cancer, SPRY4-IT1 has been shown to reverses resistance to cisplatin through decreasing expression of MPZL-1 and suppression of EMT process (11). MPZL-1 is functionally related with tyrosine kinases/adaptors and adhesion. Moreover, EZH2-related epigenetic down-regulation of SPRY4-IT1 has promoted proliferation and metastatic ability of lung cancer cells through influencing EMT (12). Contrary to these studies, Zhang et al. have stated that SPRY4-IT1 increases migration and invasiveness of lung adenocarcinoma cells (13).
In cervical cancer, SPRY4-IT1 can increase EMT influencing activity of the miR-101-3p/ZEB1 axis (14). In testicular germ cell tumors, SPRY4-IT1 has been found to suppress growth of cancer cells and phosphorylation of Akt (15). Figure 3 shows the role of SPRY4-IT1 in the pathogenesis of lung, cervical and testicular cancers.
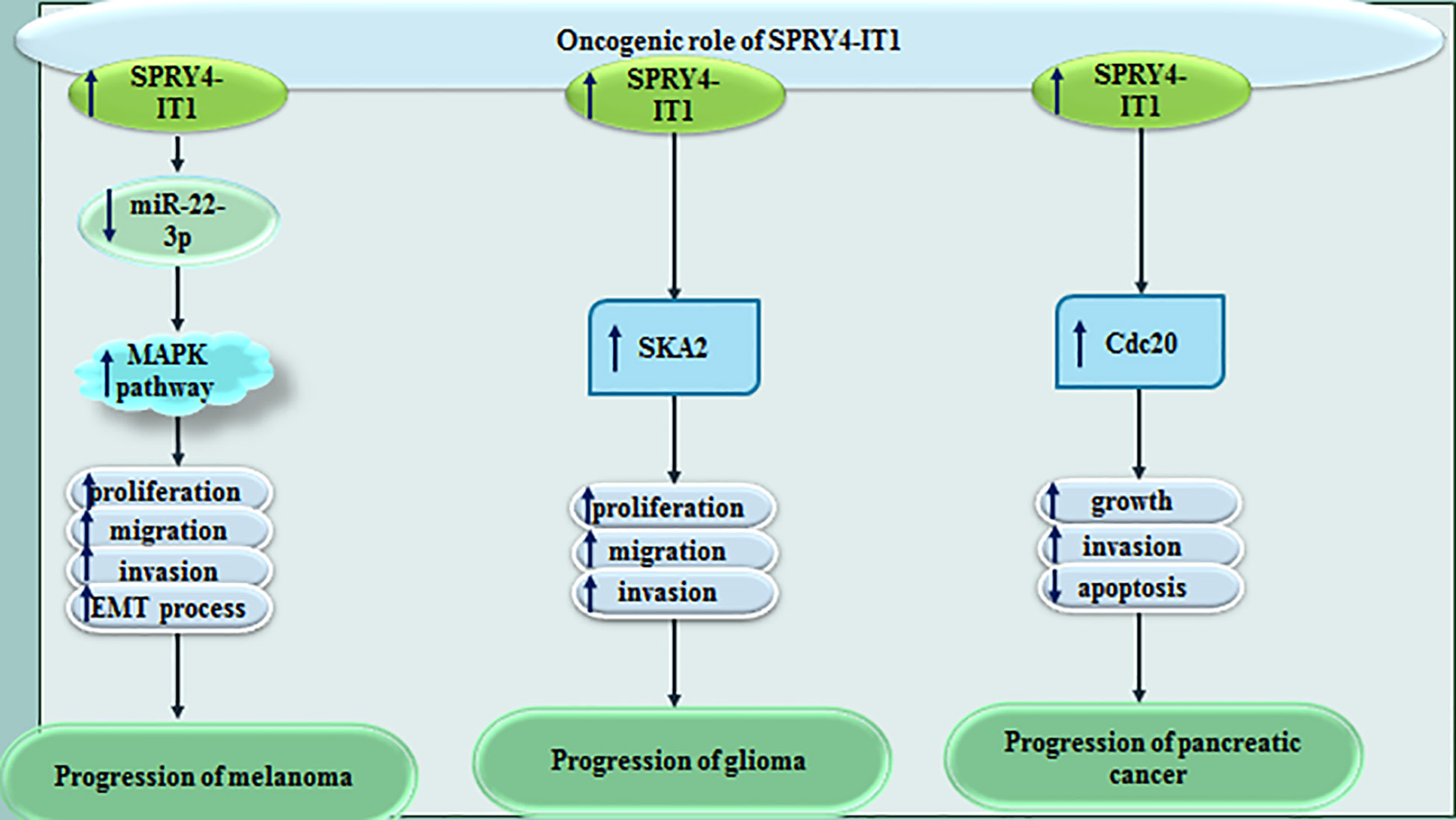
SPRY4-IT1 levels have been found to be elevated in melanoma cells lines when compared to the normal skin cell line. Up-regulation of this lncRNA has been attended by down-regulation of miR-22-3p. Dual luciferase reporter assay has confirmed the interaction between SPRY4-IT1 and miR-22-3p. Under-expression of SPRY4-IT1 has blocked proliferation, invasiveness, migration, and EMT of melanoma cells. Over-expression of miR-22-3p has been shown to decelerate phosphorylation of p38MAPK, MAPKAPK and Hsp27, thus miR-22-3p decreases activity of the p38MAPK/MAPKAPK/Hsp27 signaling (16). In glioma, SPRY4-IT1 has been revealed to stimulate cell proliferation and invasion via up-regulating SKA2 (17). It has a role in enhancement of EMT of glioma cells as well (18). Moreover, SPRY4-IT1 enhances proliferation and invasiveness of pancreatic cancer cells through regulation of Cdc20 (19). Figure 4 shows oncogenic role of SPRY4-IT1 in melanoma, glioma and pancreatic cancer.
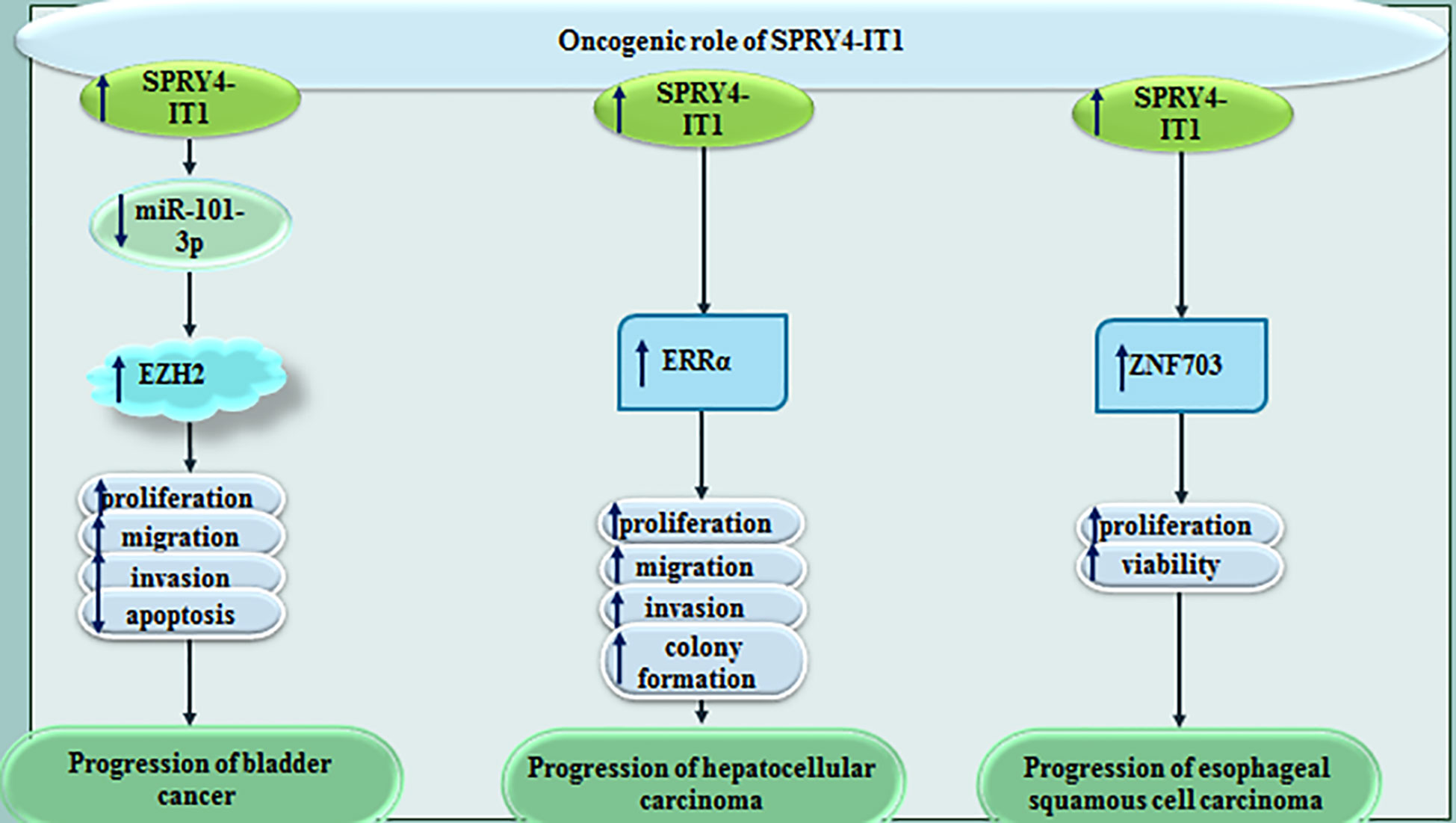
In bladder cancer cells, SPRY4-IT1 sequesters miR-101-3p to increase proliferation and metastatic ability of neoplastic cells via enhancing expression of EZH2 (20). In hepatocellular carcinoma cells, SPRY4-IT1 silencing has attenuated cell proliferation, colony formation, invasiveness and migratory potential. SPRY4-IT1 silencing has led to cell cycle arrest at G0/G1 stage and stimulated cell apoptosis. Moreover, SPRY4-IT1 silencing has inhibited expression of estrogen-related receptor α (ERRα) at transcript and protein level (21). Upregulation of SPRY4-IT1 has also been shown to increase viability of esophageal squamous cell carcinoma cells through inducing expression of zinc finger 703 (22). Figure 5 shows impact of SPRY4-IT1 in the pathoetiology of bladder, liver and esophageal cancers.
Table 1 summarizes the effect of SPRY4-IT1 in cancers based on cell line studies.
Animal Studies
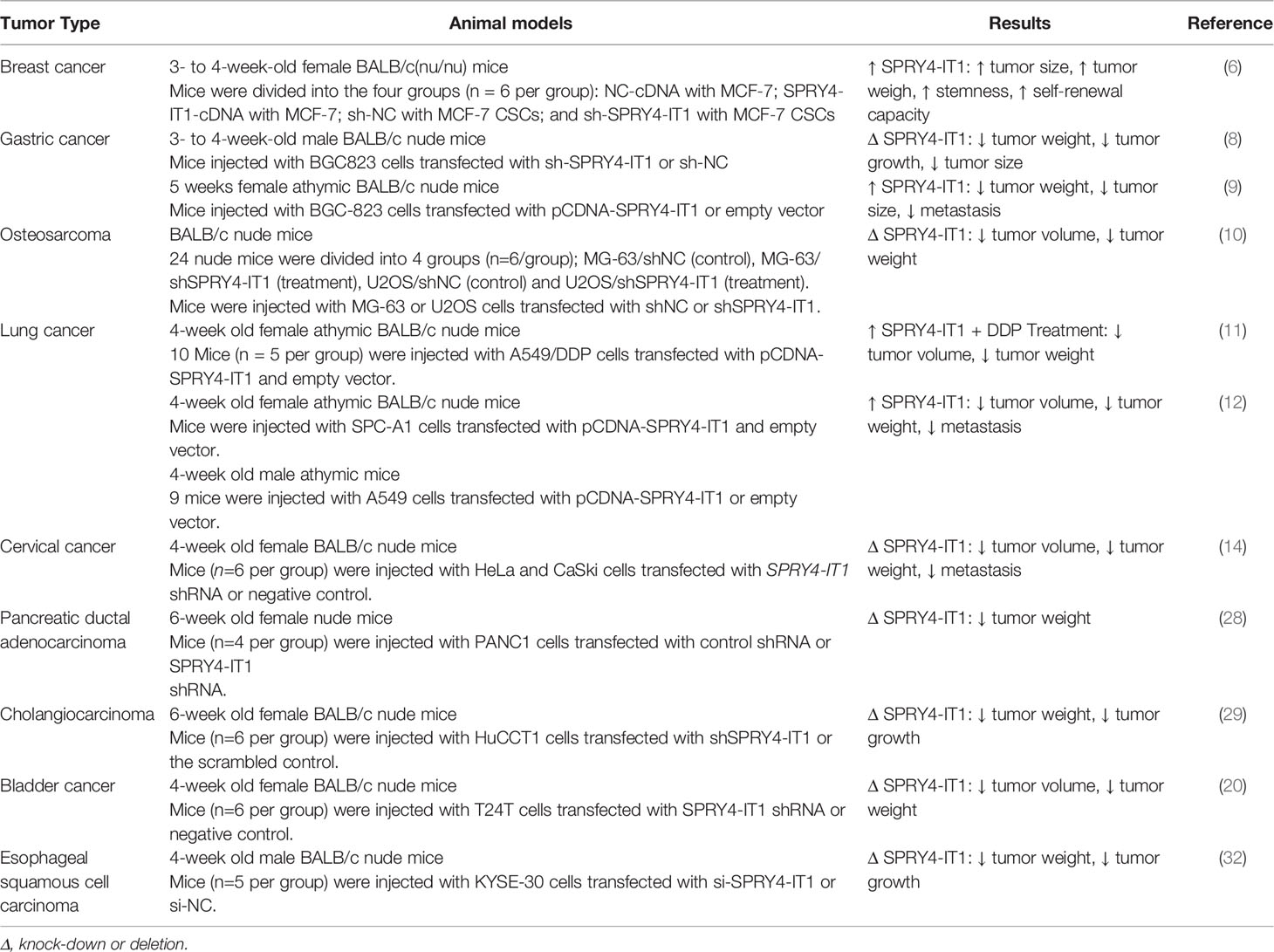
Experiments in animal models of cancers have verified the influence of SPRY4-IT1 in the carcinogenesis. For instance, up-regulation of SPRY4-IT1 has enhanced proliferation and stemness of breast cancer cells in animal models. Besides, investigations in animal models have shown that SPRY4-IT1 silencing inhibits renewal capacity of breast cancer stem cells and reduces their stemness (6). In xenograft models of gastric cancer, two different studies have reported conflicting results. While in BALB/c nude mice, SPRY4-IT1 silencing has decreased malignant behavior of neoplastic cells (8), another study in male athymic mice has shown the reverse results (9). In animal models of lung cancer, concomitant up-regulation of SPRY4-IT1 and cisplatin treatment has attenuated tumor growth and metastasis (11). However, in other types of cancers, xenograft models have shown oncogenic roles of SPRY4-IT1 (Table 2).
Human Studies
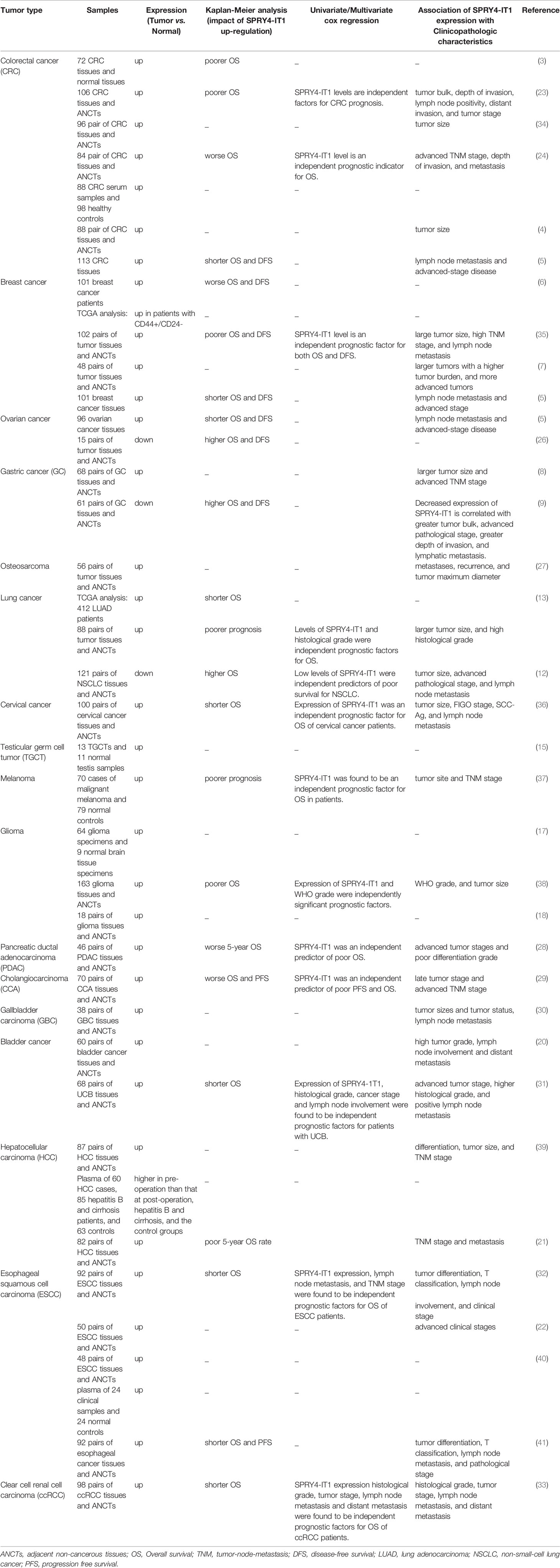
Using a panel of colon, breast, and ovarian cancer tissues, Zhao et al. have found that elevation of SPRY4-IT1 expression is associated with aggressive behavior and poor clinical outcome of patients (5). Another study has shown that SPRY4-IT1 overexpression in breast cancer tissues is associated with a larger neoplasm bulk and higher pathological stage (7).
SPRY4-IT1 has also been reported to be increased in gastric cancer tissues and serum exosomes. Notably, up-regulation of SPRY4-IT1 in serum exosomes has been correlated with metastatic ability of this cancer (8). On the other hand, Xie et al. have reported down-regulation of SPRY4-IT1 in gastric cancer tissues in association with greater tumor dimension, higher pathological stage, higher depth of tumor invasion and lymphatic metastasis. Down-regulation of SPRY4-IT1 has been associated with poor prognosis of gastric cancer patients in this cohort (9).
Elevation of SPRY4-IT1 in patients with hepatocellular carcinoma has been associated with poor five year survival of patients. Besides, expression of SPRY4-IT1 in these patients has been correlated with TNM stage (21). Table 3 summarizes the role of SPRY4-IT1 in cancers based on clinical studies.
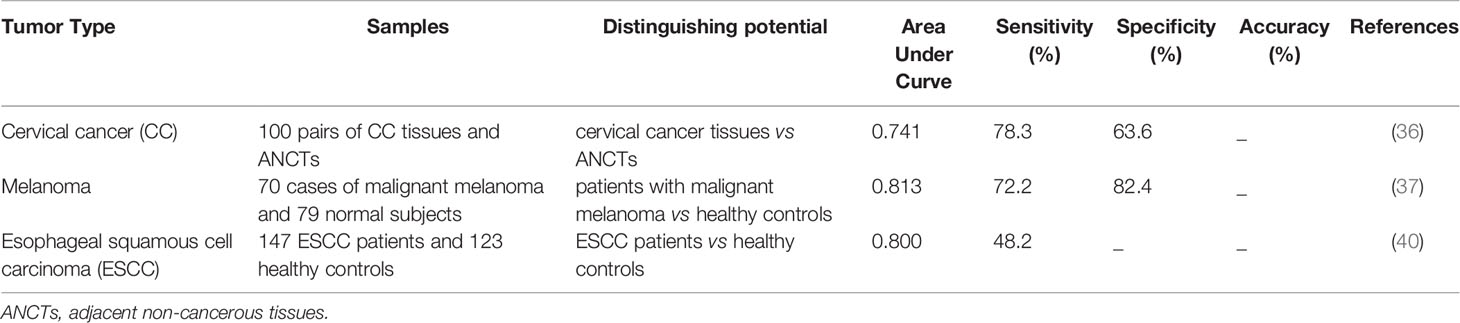
Expression of SPRY4-IT1 in tissues and peripheral blood might be used for separation of healthy tissues/blood samples from those obtained from patients with neoplastic conditions (Table 4).
Discussion
SPRY4-IT1 has oncogenic roles in diverse tissues. A possible path of participation of SPRY4-IT1 in the carcinogenesis is through decreasing bioavailability of miRNAs such as miR-101-3p, miR‐6882‐3p and miR-22-3p. The sponging effect of SPRY4-IT1 on miR-101 has been verified in colorectal cancer, osteosarcoma, cervical cancer, bladder cancer, gastric cancer and cholangiocarcinoma. Thus, this miRNA is the main target of SPRY4-IT1 in the carcinogenesis process. In spite of the bulk of evidence pointing to the oncogenic roles of SPRY4-IT1 in diverse tissues, single studies in lung, ovarian and gastric cancers have reported a tumor suppressor role for this lncRNA. Notably, in gastric cancer, animal studies have also shown contradictory results. The number of passages of the cancer cell lines and other in vitro and in vivo conditions should be compared between these studies to find the underlying causes of such inconsistent results.
SPRY4-IT1 has functional interactions with HIF-1α, NF-κB/p65, AMPK, ZEB1, MAPK and PI3K/Akt signaling, thus it can influence the carcinogenesis from different aspects.
Diagnostic value of SPRY4-IT1 has been assessed in cervical malignancy, melanoma and esophageal squamous cell carcinoma, with the best values being reported in the melanoma. Since this lncRNA has been identified in serum exosomes of patients with cancer, it represents a possible candidate in non-invasive diagnostic strategies. Yet, these results should be confirmed in large cohorts of patients with different stages of cancers to appraise this potential application.
Except for three types of cancers, namely lung, ovarian and gastric cancers which have contradictory results, elevation of SPRY4-IT1 in other types of cancers has been associated with poor prognosis of patients.
Cumulatively, SPRY4-IT1 is a potential cancer-related lncRNA which can be used as a possible therapeutic target for diverse malignancies. Several issues should be solved before application of SPRY4-IT1-targeting strategies in the clinical setting the most important one being the possible tissue-specific effect of this lncRNA in the carcinogenesis. Moreover, the impact of genetic variants within SPRY4-IT1 coding gene on susceptibility to cancer and response to therapeutic options should be appraised in future investigations.
Author Contributions
SG-F wrote the draft and revised it. MT designed and supervised the study. SS and TK collected the data and designed the figures and tables. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mazar J, Zhao W, Khalil AM, Lee B, Shelley J, Govindarajan SS, et al. The Functional Characterization of Long Noncoding RNA SPRY4-IT1 in Human Melanoma Cells. Oncotarget (2014) 5:8959–69. doi: 10.18632/oncotarget.1863
2. Khaitan D, Dinger ME, Mazar J, Crawford J, Smith MA, Mattick JS, et al. The Melanoma-Upregulated Long Noncoding RNA SPRY4-IT1 Modulates Apoptosis and Invasion. Cancer Res (2011) 71:3852–62. doi: 10.1158/0008-5472.CAN-10-4460
3. Liu S, Huang F, Ye Q, Li Y, Chen J, Huang H. SPRY4-IT1 Promotes Survival of Colorectal Cancer Cells Through Regulating PDK1-Mediated Glycolysis. Anim Cells Syst (2020) 24:220–7. doi: 10.1080/19768354.2020.1784274
4. Jin J, Chu Z, Ma P, Meng Y, Yang Y. Long Non-Coding RNA SPRY4-IT1 Promotes Proliferation and Invasion by Acting as a CeRNA of miR-101-3p in Colorectal Cancer Cells. Tumor Biol (2017) 39:1010428317716250. doi: 10.1177/1010428317716250
5. Zhao L, Jiang L, Zhang M, Zhang Q, Guan Q, Li Y, et al. Nf-κb-Activated SPRY4-IT1 Promotes Cancer Cell Metastasis by Downregulating TCEB1 mRNA via Staufen1-Mediated mRNA Decay. Oncogene (2021), 1–11. doi: 10.1038/s41388-021-01900-8
6. Song X, Zhang X, Wang X, Chen L, Jiang L, Zheng A, et al. Lncrna SPRY4-IT1 Regulates Breast Cancer Cell Stemness Through Competitively Binding Mir-6882-3p With TCF7L2. J Cell Mol Med (2020) 24:772–84. doi: 10.1111/jcmm.14786
7. Shi Y, Li J, Liu Y, Ding J, Fan Y, Tian Y, et al. The Long Noncoding RNA SPRY4-IT1 Increases the Proliferation of Human Breast Cancer Cells by Upregulating ZNF703 Expression. Mol Cancer (2015) 14:1–13. doi: 10.1186/s12943-015-0318-0
8. Cao S, Lin L, Xia X, Wu H. Lncrna SPRY4-IT1 Regulates Cell Proliferation and Migration by Sponging Mir-101-3p and Regulating AMPK Expression in Gastric Cancer. Mol Therapy-Nucleic Acids (2019) 17:455–64. doi: 10.1016/j.omtn.2019.04.030
9. Xie M, . Nie F-q, Sun M, Xia R, Liu Y-w, Zhou P, et al. Decreased Long Noncoding RNA SPRY4-IT1 Contributing to Gastric Cancer Cell Metastasis Partly via Affecting Epithelial–Mesenchymal Transition. J Trans Med (2015) 13:1–11. doi: 10.1186/s12967-015-0595-9
10. Yao H, Hou G, Wang QY, Xu WB, Zhao HQ, Xu YC. LncRNA SPRY4−IT1 Promotes Progression of Osteosarcoma by Regulating ZEB1 and ZEB2 Expression Through Sponging of Mir−101 Activity. Int J Oncol (2020) 56:85–100. doi: 10.3892/ijo.2019.4910
11. Ye Y, Gu J, Liu P, Wang H, Jiang L, Lei T, et al. Long Non-Coding RNA SPRY4-IT1 Reverses Cisplatin Resistance by Downregulating MPZL-1 via Suppressing EMT in NSCLC. OncoTargets Ther (2020) 13:2783. doi: 10.2147/OTT.S232769
12. Sun M, Liu X, Lu K, Nie F, Xia R, Kong R, et al. EZH2-Mediated Epigenetic Suppression of Long Noncoding RNA SPRY4-IT1 Promote s NSCLC Cell Proliferation and Metastasis by Affecting the Epithelial–Mesenchymal Transition. Cell Death Dis (2014) 5:e1298–8. doi: 10.1038/cddis.2014.256
13. Zhang X, Chi Q, Zhao Z. Up-Regulation of Long non-Coding RNA SPRY4-IT1 Promotes Tumor Cell Migration and Invasion in Lung Adenocarcinoma. Oncotarget (2017) 8:51058. doi: 10.18632/oncotarget.16918
14. Fan M-J, Zou Y-H, He P-J, Zhang S, Sun X-M, Li C-Z. Long Non-Coding RNA SPRY4-IT1 Promotes Epithelial–Mesenchymal Transition of Cervical Cancer by Regulating the Mir-101-3p/ZEB1 Axis. Biosci Rep (2019) 39:BSR20181339. doi: 10.1042/BSR20181339
15. Das MK, Furu K, Evensen HF, Haugen Ø.P., Haugen TB. Knockdown of SPRY4 and SPRY4-IT1 Inhibits Cell Growth and Phosphorylation of Akt in Human Testicular Germ Cell Tumours. Sci Rep (2018) 8:1–8. doi: 10.1038/s41598-018-20846-8
16. Li Z, Tang X, Duan S. Interference From Lncrna SPRY4-IT1 Restrains the Proliferation, Migration, and Invasion of Melanoma Cells Through Inactivating MAPK Pathway by Up-Regulating Mir-22-3p. Int J Clin Exp Pathol (2019) 12:477–87.
17. He XJ, Bian EB, Ma CC, Wang C, Wang HL, Zhao B. Long Non-Coding RNA SPRY4-IT1 Promotes the Proliferation and Invasion of U251 Cells Through Upregulation of SKA2. Oncol Lett (2018) 15:3977–84. doi: 10.3892/ol.2018.7776
18. Liu H, Lv Z, Guo E. Knockdown of Long Noncoding RNA SPRY4-IT1 Suppresses Glioma Cell Proliferation, Metastasis and Epithelial-Mesenchymal Transition. Int J Clin Exp Pathol (2015) 8:9140.
19. Guo W, Zhong K, Wei H, Nie C, Yuan Z. Long Non-Coding RNA SPRY4-IT1 Promotes Cell Proliferation and Invasion by Regulation of Cdc20 in Pancreatic Cancer Cells. PloS One (2018) 13:e0193483. doi: 10.1371/journal.pone.0193483
20. Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X, et al. Lncrna SPRY4-IT1 Sponges Mir-101-3p to Promote Proliferation and Metastasis of Bladder Cancer Cells Through Up-Regulating EZH2. Cancer Lett (2017) 388:281–91. doi: 10.1016/j.canlet.2016.12.005
21. Yu G, Lin J, Liu C, Hou K, Liang M, Shi B. Long Non-Coding RNA SPRY4-IT1 Promotes Development of Hepatic Cellular Carcinoma by Interacting With Errα and Predicts Poor Prognosis. Sci Rep (2017) 7:1–9. doi: 10.1038/s41598-017-16781-9
22. Xue-Liang J, Ming-Dong W, Ya-Bi Z, Wang-Yue W. Upregulated Long Noncoding RNA SPRY4-IT1 Contributes to Increased Cell Viability by Activating Zinc Finger 703 Expression in Esophageal Squamous Cell Carcinoma. Indian J Cancer (2015) 52:164. doi: 10.4103/0019-509X.186566
23. Tan W, Z.-z. Song, Xu Q, Qu X, Li Z, Wang Y, et al. Up-Regulated Expression of SPRY4-IT1 Predicts Poor Prognosis in Colorectal Cancer. Med Sci monitor: Int Med J Exp Clin Res (2017) 23:309. doi: 10.12659/MSM.898369
24. Cao D, Ding Q, Yu W, Gao M, Wang Y. Long Noncoding RNA SPRY4-IT1 Promotes Malignant Development of Colorectal Cancer by Targeting Epithelial–Mesenchymal Transition. OncoTargets Ther (2016) 9:5417. doi: 10.2147/OTT.S111794
25. Wu H, Wang Y, Chen T, Li Y, Wang H, Zhang L, et al. The N-Terminal Polypeptide Derived From Vmip-II Exerts its Anti-Tumor Activity in Human Breast Cancer by Regulating LncRNA SPRY4-IT1. Biosci Rep (2018) 31–8. doi: 10.1042/BSR20180411
26. Yu J, Han Q, Cui Y. Decreased Long Non-Coding RNA SPRY4-IT1 Contributes to Ovarian Cancer Cell Metastasis Partly via Affecting Epithelial–Mesenchymal Transition. Tumor Biol (2017) 39:1010428317709129. doi: 10.1177/1010428317709129
27. Xu J, Ding R, Xu Y. Effects of Long Non-Coding RNA SPRY4-IT1 on Osteosarcoma Cell Biological Behavior. Am J Trans Res (2016) 8:5330.
28. Yao Y, Gao P, Chen L, Wang W, Zhang J, Li Q, et al. Upregulated Long Non-Coding RNA SPRY4-IT1 Predicts Dismal Prognosis for Pancreatic Ductal Adenocarcinoma and Regulates Cell Proliferation and Apoptosis. Gene (2018) 659:52–8. doi: 10.1016/j.gene.2018.03.048
29. Xu Y, Yao Y, Jiang X, Zhong X, Wang Z, Li C, et al. SP1-Induced Upregulation of Lncrna SPRY4-IT1 Exerts Oncogenic Properties by Scaffolding EZH2/LSD1/DNMT1 and Sponging Mir-101-3p in Cholangiocarcinoma. J Exp Clin Cancer Res (2018) 37:1–13. doi: 10.1186/s13046-018-0747-x
30. Yang L, Cheng X, Ge N, Guo W, Feng F, Wan F. Long Non-Coding RNA SPRY4-IT1 Promotes Gallbladder Carcinoma Progression. Oncotarget (2017) 8:3104. doi: 10.18632/oncotarget.13621
31. Zhao X-L, Zhao Z-H, Xu W-C, Hou J-Q, Du X-Y. Increased Expression of SPRY4-IT1 Predicts Poor Prognosis and Promotes Tumor Growth and Metastasis in Bladder Cancer. Int J Clin Exp Pathol (2015) 8:1954.
32. Xie H-W, Wu Q-Q, Zhu B, Chen F-J, Ji L, Li S-Q, et al. Long Noncoding RNA SPRY4-IT1 Is Upregulated in Esophageal Squamous Cell Carcinoma and Associated With Poor Prognosis. Tumor Biol (2014) 35:7743–54. doi: 10.1007/s13277-014-2013-y
33. Zhang H-M, Yang F-Q, Yan Y, Che J-P, Zheng J-H. High Expression of Long Non-Coding RNA SPRY4-IT1 Predicts Poor Prognosis of Clear Cell Renal Cell Carcinoma. Int J Clin Exp Pathol (2014) 7:5801.
34. Shen F, Cai W-S, Feng Z, Chen J-w, Feng J-h, Liu Q-c, et al. Long Non-Coding RNA SPRY4-IT1 Pormotes Colorectal Cancer Metastasis by Regulate Epithelial-Mesenchymal Transition. Oncotarget (2017) 8:14479. doi: 10.18632/oncotarget.10407
35. Xiang Y, Chen Y, Shi Y, Wu X, Hao R, Li Q, et al. Upregulation of the Long Non-Coding RNA SPRY4-IT1 Predicts Poor Prognosis in Breast Cancer. Int J Clin Exp Pathol (2019) 12:1003.
36. Cao Y, Liu Y, Lu X, Wang Y, Qiao H, Liu M. Upregulation of Long Noncoding RNA SPRY4-IT1 Correlates With Tumor Progression and Poor Prognosis in Cervical Cancer. FEBS Open Bio (2016) 6:954–60. doi: 10.1002/2211-5463.12102
37. Liu T, Shen SK, Xiong JG, Xu Y, Zhang HQ, Liu HJ, et al. Clinical Significance of Long Noncoding RNA SPRY4-IT1 in Melanoma Patients. FEBS Open Bio (2016) 6:147–54. doi: 10.1002/2211-5463.12030
38. Zhou Y, Wang D, Pang Q. Long Noncoding RNA SPRY4-IT1 Is a Prognostic Factor for Poor Overall Survival and has an Oncogenic Role in Glioma. Eur Rev Med Pharmacol Sci (2016) 20:3035–9.
39. Jing W, Gao S, Zhu M, Luo P, Jing X, Chai H, et al. Potential Diagnostic Value of Lncrna SPRY4-IT1 in Hepatocellular Carcinoma. Oncol Rep (2016) 36:1085–92. doi: 10.3892/or.2016.4859
40. Tong Y-S, Wang X-W, Zhou X-L, Liu Z-H, Yang T-X, Shi W-H, et al. Identification of the Long Non-Coding RNA POU3F3 in Plasma as a Novel Biomarker for Diagnosis of Esophageal Squamous Cell Carcinoma. Mol Cancer (2015) 14:1–13. doi: 10.1186/1476-4598-14-3
Keywords: SPRY4-IT1, cancer, biomarker, expression, carcinogenesis
Citation: Ghafouri-Fard S, Khoshbakht T, Taheri M and Shojaei S (2022) A Review on the Role of SPRY4-IT1 in the Carcinogenesis. Front. Oncol. 11:779483. doi: 10.3389/fonc.2021.779483
Received: 18 September 2021; Accepted: 23 December 2021;
Published: 13 January 2022.
Edited by:
Jian-ye Zhang, Guangzhou Medical University, ChinaReviewed by:
N. Sanjib Banerjee, University of Alabama at Birmingham, United StatesCopyright © 2022 Ghafouri-Fard, Khoshbakht, Taheri and Shojaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, mohammad_823@yahoo.com; Seyedpouzhia Shojaei, psh1182002@yahoo.com
 Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Tayyebeh Khoshbakht
Tayyebeh Khoshbakht Mohammad Taheri
Mohammad Taheri Seyedpouzhia Shojaei4*
Seyedpouzhia Shojaei4*