- Department of Hematology and Hematologic Malignancies, Tom Baker Cancer Centre and University of Calgary, Calgary, AB, Canada
Although the use of allogeneic hematopoietic cell transplantation (HCT) for chronic lymphocytic leukemia (CLL) has declined with the development of novel targeted agents, it continues to play an important role for eligible patients with high-risk or heavily pretreated CLL who lack other treatment options. CLL is susceptible to a potent graft-versus-leukemia (GVL) effect which produces long-lasting remissions in 30-50% of transplanted patients. While allogeneic HCT is associated with significant risks of graft-versus-host disease (GVHD), infection, and non-relapse mortality (NRM), improvements in patient and donor selection, reduced intensity conditioning (RIC), GVHD prophylaxis, and supportive care have rendered this an increasingly safe and effective procedure in the current era. In this review, we discuss recent advances in allogeneic HCT for CLL, with a focus on the optimal evidence-based strategies to maximize benefit and minimize toxicity of this potentially curative cellular therapy.
Introduction
The advent of novel therapies has revolutionized the treatment of CLL, leading to a persistent decline in the use of allogeneic HCT for this disease (1, 2). However, the powerful GVL effect of allogeneic HCT offers the potential for long-term remissions in an otherwise incurable malignancy. Allogeneic HCT still plays an important role in the treatment of CLL, particularly for eligible patients with high-risk genetic features and those with resistance to Bruton tyrosine kinase (BTK) and/or B-cell lymphoma-2 (BCL2) inhibitors who have limited other therapeutic options (3). In this review, we describe recent advances in allogeneic HCT and the optimal approaches to the application of this cellular therapy in CLL (Table 1).
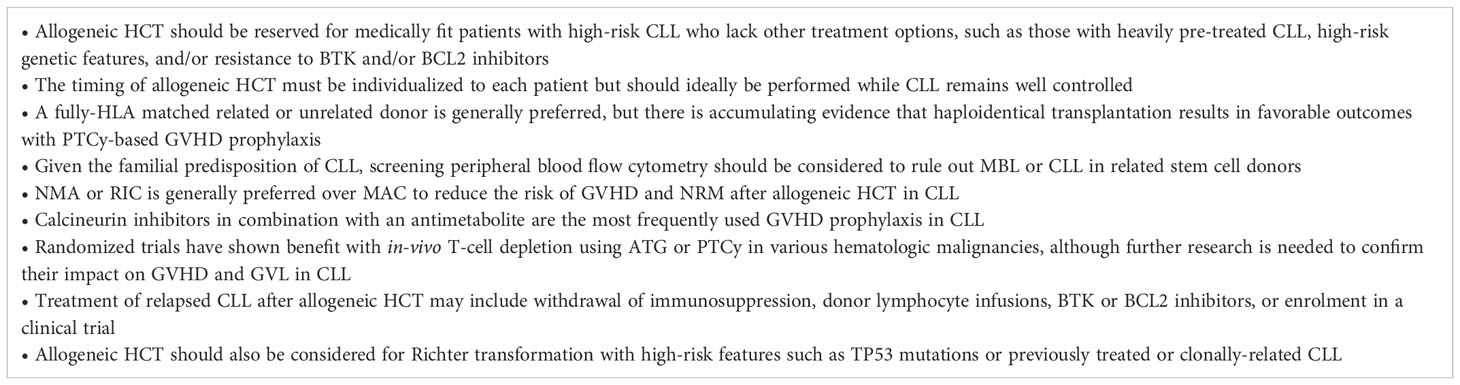
Indications and timing of allogeneic HCT for CLL
CLL is diagnosed at the median age of 70 years and typically carries a favorable prognosis with a 5-year overall survival (OS) rate of 88% (4). Most patients can be successfully managed for many years with active surveillance or low-intensity approaches such as BTK and BCL2 inhibitors alone or in combination with anti-CD20 monoclonal antibodies or alternatively with conventional chemoimmunotherapy (5–9). As a result, most patients with CLL will never require allogeneic HCT during the course of their disease. However, there are important subsets of patients with CLL in whom allogeneic HCT is still warranted, including younger patients who eventually exhaust all available lines of therapy or those with poor prognosis disease characteristics, such as deletion 17p, TP53 aberrations, or complex karyotype (10, 11). Due to the risks of transplant-related morbidity and mortality (TRM), allogeneic HCT is usually reserved for medically fit patients who have limited other treatment options.
Although the benefits of allogeneic HCT in CLL have never been confirmed with a randomized controlled trial, large datasets demonstrate that allogeneic HCT achieves long-lasting remissions in up to 30-50% of patients with heavily pre-treated CLL, which is higher than would be expected with conventional therapies (12–14). Retrospective comparative studies have also suggested that allogeneic HCT is associated with a lower risk of relapse and improved survival compared to non-transplant approaches (15–18). These studies were largely conducted in the pre-BTK and BCL2 inhibitor era and must be interpreted cautiously due to the risk of selection bias, but they do provide some rationale to support the use of allogeneic HCT in high-risk CLL.
The indications for allogeneic HCT have evolved over the years in accordance with the rapidly changing treatment landscape of CLL (2, 5, 19–21). Factors to be taken into consideration when assessing eligibility for allogeneic HCT include patient values, age, performance status, comorbidities, donor availability, deletion 17p and TP53 status, prior treatment history and duration of response, depth of response to current therapy, and alternative treatment options. The 2016 American Society for Transplantation and Cellular Therapy (ASTCT) guidelines recommend allogeneic HCT for eligible patients with relapsed standard-risk CLL who develop BTK inhibitor resistance, and for high-risk patients with deletion 17p, TP53 aberrations, and/or complex karyotype who have relapsed after 2 lines of therapy and/or a BTK or BCL2 inhibitor (2). The 2018 European Society for Blood and Marrow Transplantation (EBMT) guidelines state that allogeneic HCT should be recommended for eligible patients responding to a second targeted agent following resistance to both chemoimmunotherapy and a first targeted agent, and that it may be considered for select patients at low risk of TRM who have TP53 aberrations but are responding to a first targeted agent after previous chemoimmunotherapy failure (19). The current National Comprehensive Cancer Network (NCCN) guidelines recommend allogeneic HCT solely for fit patients with relapsed/refractory CLL after prior treatment with both a BTK and BCL2 inhibitor (22). These patients with “double refractory” CLL have a poor prognosis with median survival of 3.6 months, representing a critical area of unmet need in whom allogeneic HCT can be considered (3, 23). Finally, allogeneic HCT may also be warranted for patients with Richter transformation, as discussed later in this article.
While the optimal timing of allogeneic HCT must be individualized to each patient, it should ideally be performed while the CLL remains well controlled, given that disease status is an important predictor of relapse risk and GVHD (24). For patients with heavily pretreated CLL who prioritize the chance of long-term disease control over potential transplant-related toxicities, it may thus be preferable to proceed to allogeneic HCT while still responding to a second targeted agent, which is usually a BTK or BCL2 inhibitor (19). The median progression-free survival (PFS) of venetoclax is as short as 24 months for patients previously exposed to a BTK inhibitor (25), so it may be reasonable to proceed to allogeneic HCT within the first 1-2 years of commencing venetoclax to avoid risking the loss of remission. A similar timeline can be considered for patients with venetoclax resistance who are responding to a BTK inhibitor, which has a median PFS of 32-34 months in this setting (26, 27). Of note, these data are largely derived from patients receiving targeted agents after prior chemoimmunotherapy, and the timing of transplantation is less clear in the current era of upfront treatment with BTK or BCL inhibitors. It is the opinion of the authors that allogeneic HCT may be reasonably considered for patients with refractory disease and/or short-lived responses to both targeted agents if no clinical trials or other novel therapies are available, given the low probability of achieving a durable response to chemoimmunotherapy following BTK and BCL2 inhibitor failure. In such cases, there is limited data to guide the selection of pre-transplantation bridging therapies for patients who have already exhausted both BTK and BCL2 inhibitors (28). Alternative agents may be used to induce a response prior to allogeneic HCT, including non-covalent BTK inhibitors (29), PI3K inhibitors (30), alemtuzumab (31), anti-CD20 monoclonal antibodies (32), chemoimmunotherapy (33), rechallenging with a BTK inhibitor or venetoclax (27, 34, 35), or enrolment in a clinical trial.
Graft-versus-leukemia effect in CLL
The curative potential of allogeneic HCT in CLL arises from the ability of donor immune effector cells to recognize and eradicate recipient leukemia cells. Evidence to support a strong GVL effect in CLL includes the following: long-term remission of CLL can be achieved with low-intensity non-myeloablative (NMA) conditioning and is more likely to occur for CLL than other hematologic malignancies (14, 36, 37); rapid or complete donor T-cell engraftment is associated with faster and more durable tumor clearance (38–41); the development of chronic GVHD is protective against relapse (42, 43); and responses can be induced by withdrawal of immunosuppression and/or donor lymphocyte infusion (DLI) (41, 44, 45). Of note, the GVL effect is frequently a delayed process in CLL, with a gradual reduction in tumor burden occurring over a period of up to 6-11 months after allogeneic HCT (44, 46)
Donor selection and stem cell source
As with other diseases, HLA matching is an important determinant of outcomes of allogeneic HCT for CLL (47), and a fully HLA-matched related donor (MRD) or unrelated donor (MUD) is generally preferred although a haploidentical related donor is also acceptable (48–50). Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) show that among 1,782 allogeneic HCT recipients with CLL, 3-year OS was 62% with a MRD, 53% with a MUD, 58% with a haploidentical donor, and 49% with a mismatched unrelated donor (MMUD) (50). In addition to HLA matching, other prognostic factors to be considered during donor selection include prioritization of younger donor age (51), preference for cytomegalovirus (CMV) matching (52), and selection of a male donor for a male recipient (53, 54).
The use of a haploidentical donor with post-transplant cyclophosphamide (PTCy)-based GVHD prophylaxis appears to result in lower risks of GVHD and comparable survival outcomes as a MRD or MUD in other lymphoid and myeloid malignancies (55, 56). In CLL, the Baltimore group reported 4-year OS 52% and progression-free survival (PFS) 37% among 64 patients undergoing NMA haploidentical HCT with PTCy prophylaxis, with grade II-IV acute GVHD incidence of 27% and chronic GVHD incidence of 17% (57). An EBMT study of 117 patients who underwent haploidentical HCT for CLL (of whom only 38% received PTCy) reported 2-year OS 48% and PFS 38%, with grade II-IV acute GVHD 32% and chronic GVHD not reported (58).
Umbilical cord blood (UCB) transplantation is performed less frequently in the adult population due to concerns regarding low CD34+ stem cell dose, delayed engraftment, and heightened risks of infection and NRM (59). However, a study of 68 patients with CLL who underwent UCB transplantation reported 3-year OS 54% and PFS 45%, suggesting this may be a viable option for individuals lacking other suitable donors (60). Another emerging alternative is MMUD HCT with PTCy prophylaxis, with a phase II trial including 3 patients with CLL showing encouraging survival outcomes even among recipients with donors matched at 4-6 of 8 HLA alleles (61).
A question unique to CLL is whether related stem cell donors should undergo screening for CLL and its precursor condition monoclonal B-cell lymphocytosis (MBL). CLL has a strong familial predisposition, and CLL or MBL clones can be identified in up to 13-16% of first-degree relatives of patients with CLL, with increasing incidence with age (62–64). Multiple case reports have documented the transmission of CLL from donor to recipient via allogeneic HCT (65–70). As such, the ASTCT recommends that potential related donors undergo screening with peripheral blood flow cytometry to reduce the risk of transmission of a malignant or premalignant clone to the recipient (2).
The optimal stem cell source for allogeneic HCT is a matter of debate, and there have been no large studies addressing this question in CLL. Multiple randomized trials comparing marrow to peripheral blood stem cells (PBSC) for hematologic malignancies have been conducted (71, 72). A meta-analysis of these studies showed a lower risk of grade 3-4 acute and extensive chronic GVHD but a higher risk of relapse with marrow, with similar OS except in a subgroup of patients with high-risk disease (72). Although the reduced risk of GVHD could potentially make marrow an attractive option for CLL, the lack of survival advantage in most patients and the logistic simplicity of PBSC collection has rendered it the preferred stem cell source used in >90% of allogeneic HCT for CLL (2, 12, 24).
Conditioning regimens
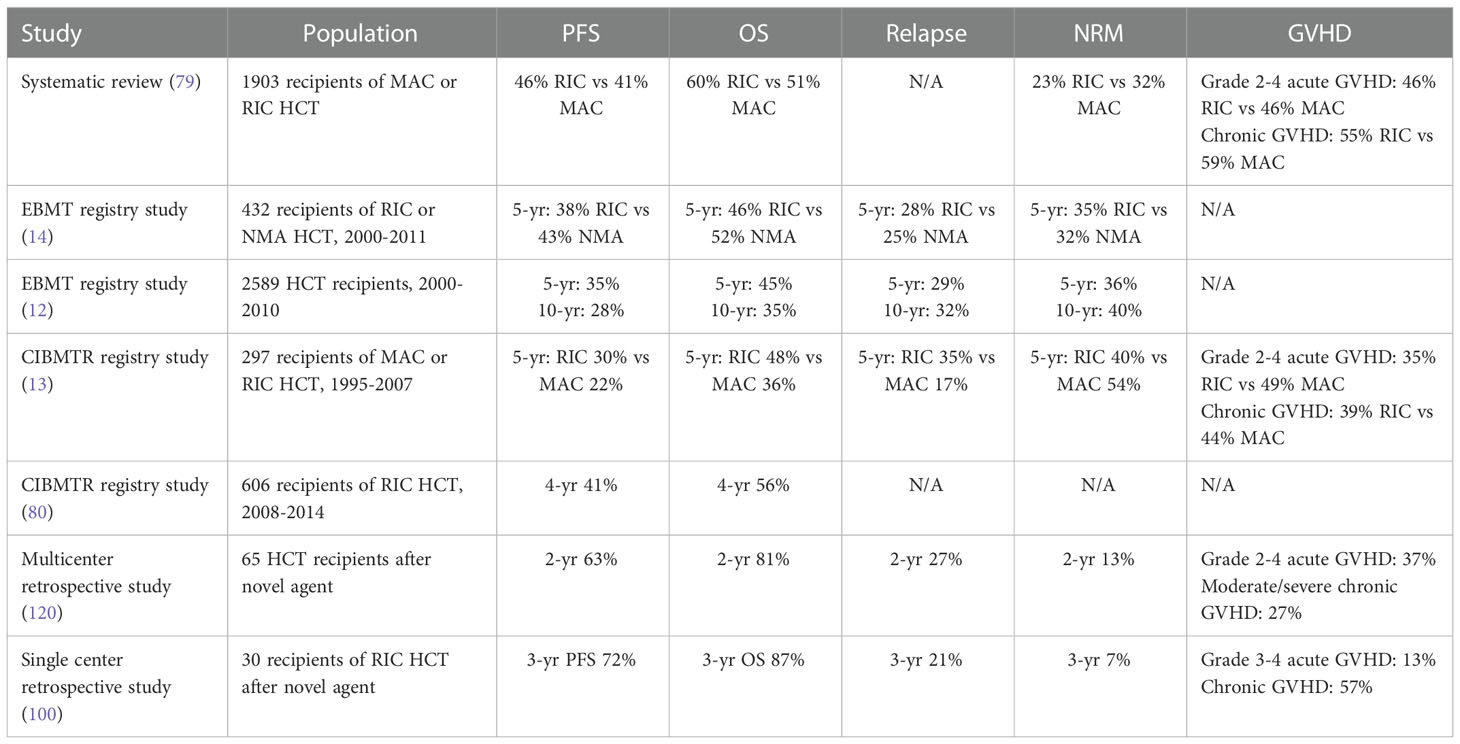
Pre-transplant conditioning plays an important role in cytoreduction and contributes to long-term disease control after allogeneic HCT independently of the GVL effect (66). In a randomized trial enrolling younger patients (age <65 years) with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS), myeloablative conditioning (MAC) was shown to confer a survival advantage over RIC, with the significantly lower relapse risk of MAC offsetting its higher risks of acute and chronic GVHD and NRM (73, 74). However, it is unknown if these results can be extrapolated to CLL, given the generally indolent disease course, older and more comorbid patient population, increased risks of infection from underlying CLL-related immune dysregulation, and potentially greater chemoresistance induced by multiple previous lines of therapy. Retrospective studies have reported that MAC is associated with prohibitively high rates of NRM at 30-50% in CLL, whereas RIC is associated with lower NRM and potentially improved overall survival (13, 24, 75–78). In addition, a systematic review and meta-analysis of 48 studies including 1,903 patients with CLL concluded that RIC regimens are associated with lower NRM and slightly improved OS compared to MAC (79). Of note, these retrospective analyses must be interpreted with caution due to the paucity of randomized data and inherent risk of selection bias. Nevertheless, MAC has largely fallen out of favor for CLL and the majority (>75-80%) of allogeneic HCT performed for this indication employ RIC (12, 80). ASTCT guidelines recommend RIC or NMA for all patients with CLL undergoing allogeneic HCT (2).
The optimal RIC protocol for CLL has not been determined. Drugs with known activity in CLL such as fludarabine are often incorporated (81–83), and the most commonly-used RIC regimens are fludarabine-busulfan 6.4mg/kg (Flu-Bu2) and fludarabine-melphalan (Flu-Mel) (80). Bendamustine-based conditioning has also shown promising results in small prospective trials (84). In addition, the inclusion of anti-CD20 monoclonal antibodies in conditioning may reduce the risk of CLL relapse after allogeneic HCT, although concerns of increased infection and NRM have been raised (85, 86). While comparative data of RIC regimens is lacking in CLL, a CIBMTR analysis of 1,823 patients undergoing RIC allogeneic HCT for non-Hodgkin lymphoma (NHL) found that Flu-Bu2 and fludarabine-cyclophosphamide (Flu-Cy) were associated with lower NRM and favorable OS compared to Flu-Mel140mg/m2 (87). There is also growing interest in the use of treosulfan as a low-toxicity alternative to busulfan in RIC regimens, and a randomized trial in AML/MDS found that fludarabine-treosulfan (Flu-Treo) was associated with improved NRM and OS compared to Flu-Bu2 (88, 89). Preliminary data suggest that Flu-Treo may also be a viable conditioning regimen in NHL (90, 91), but confirmation in larger studies is required.
Given that CLL is particularly susceptibility to the GVL effect, NMA regimens have also been explored to harness the benefits of GVL while minimizing reliance on the cytoreductive but potentially toxic effects of conditioning (37). In a study of 82 patients with fludarabine-refractory CLL, NMA conditioning with 2 Gy TBI ± fludarabine achieved a complete response in 55% with 5-year OS 50%, PFS 39%, and NRM 23%, although high relapse rates were observed among patients with lymphadenopathy ≥5cm (36). Real-world outcomes of NMA and RIC appeared to be generally comparable in an EBMT study of 432 patients with CLL, with a trend to a lower risk of 100-day NRM with NMA at 3% versus 10% (14). NMA regimens can thus be considered a minimal toxicity alternative to RIC and may be favored for older, comorbid, or heavily pretreated patients with low tumor burden CLL.
Total body irradiation (TBI) is often incorporated into conditioning protocols to improve disease eradication, immune clearance, and donor engraftment. However, a CIBMTR analysis study of 897 patients with CLL and other lymphomas reported that the inclusion of low-dose (≤2 Gray [Gy]) TBI in NMA conditioning regimens does not appear to impact OS or PFS (92). Regarding TBI-based versus chemotherapy-only MAC, a CIBMTR study of 180 patients with CLL did not find a significant survival advantage, although there was a trend toward superior OS with TBI-based conditioning (93). As such, any potential role for TBI in allogeneic HCT for CLL is unclear.
GVHD prophylaxis strategies
The cornerstone of GVHD prophylaxis in MRD or MUD HCT is a calcineurin inhibitor (CNI) combined with an antimetabolite such as methotrexate (MTX) or mycophenolate mofetil (MMF) (94). Small numbers of patients with CLL were enrolled in randomized trials demonstrating the efficacy of these combinations (95–97), and they are the most frequently employed GVHD prophylaxis strategies used for CLL in the real-world setting (13, 24). Sirolimus may be an acceptable alternative to methotrexate which is associated with similar risks of GVHD but faster engraftment and less mucositis (98). In addition, a phase III trial including 14 patients with CLL found that the addition of sirolimus to cyclosporine and MMF led to lower risks of acute GVHD and improved NRM and OS following NMA HCT (99).
T-cell depletion is another important prophylaxis strategy which targets the alloreactive donor T-cells believed to be responsible for GVHD. However, T-cell depletion is uncommonly utilized in allogeneic HCT for CLL, likely owing to concerns of potential disease relapse arising from an attenuated GVL effect (1, 13, 14, 100). Ex-vivo T-cell depletion (e.g., with CD34+ selection) is associated with excess risks of infection, NRM, and possibly relapse (101). Conversely, in-vivo T-cell depletion with ATG-Thymoglobulin® has been shown in randomized trials to reduce the risks of acute and chronic GVHD after myeloablative HCT without worsening NRM or relapse (102–107). ATG was also associated with an OS advantage in one randomized study which included 16 patients with CLL and 64 recipients of RIC/NMA regimens (107). Thus, albeit promising, larger studies are needed to definitively confirm a role for ATG in allogeneic HCT for CLL. The anti-CD52 monoclonal antibody alemtuzumab is another intuitively attractive option for in-vivo T-cell depletion in CLL, given its activity as both a therapeutic for CLL and a GVHD prophylaxis agent (108–113). However, alemtuzumab has been linked to increased risks of relapse after allogeneic HCT for CLL (14, 47, 53, 114).
As alluded to earlier, it is anticipated that PTCy-based prophylaxis will play an increasing role in MRD and MUD HCT in the future, given its remarkable ability to overcome the HLA disparity of haploidentical and MMUD transplants (61, 101, 115, 116). In a randomized trial including 10 patients with CLL, the addition of PTCy to CNI plus MMF prophylaxis resulted in significant reductions in acute and chronic GVHD following MRD or MUD NMA HCT, without significantly impacting the risk of relapse or NRM (116). Similarly, the preliminary results from another randomized trial showed that the addition of PTCy to tacrolimus and MMF prophylaxis was associated with lower risks of acute and chronic GVHD in the setting of RIC HCT (117, 118). In light of these promising findings, further research is needed in larger cohorts of patients with CLL to confirm if ATG, PTCy, a combination of ATG and PTCy, or other emerging GVHD prophylaxis agents such as abatacept may improve the outcomes of allogeneic HCT for CLL by providing protection against GVHD while still preserving the GVL effect (119).
Outcomes of allogeneic HCT for CLL
The outcomes of allogeneic HCT for CLL are heterogenous and vary in accordance with patient factors, transplant protocols, and time period (Table 2). In the largest study published to date of 2,589 patients with CLL who underwent allogeneic HCT between 2000-2010, the EBMT reported 5-year OS 45%, PFS 35%, NRM 36%, and relapse 29% (12). Similar findings were observed in a CIBMTR study of 606 patients with CLL who received RIC allogeneic HCT between 2008 and 2014, with 4-year OS 56% and PFS 41% (80). A systemic review and meta-analysis including 1,903 patients with CLL reported pooled rates of OS at 51-60%, PFS at 41-46%, and NRM at 23-32% after allogeneic HCT (79). Outcomes appear to have improved in the current era, possibly thanks to advances in patient/donor selection, conditioning protocols, supportive care, and the introduction of novel CLL therapies (121). As an example, in a report of 108 patients with CLL undergoing allogeneic HCT since 2010, 3-year OS was 73% and PFS was 61%, with cumulative incidences of NRM 14% and relapse 24% (100).
Long-term follow-up is essential to evaluating the curative potential of allogeneic HCT and the impact of late toxicities. In the 10-year follow-up of the CLL3X trial, long-lasting remissions were observed in approximately one-third of patients after RIC allogeneic HCT, with 10-year OS 51%, PFS 34%, relapse 46%, and NRM 20% (42). The EBMT also reported 10-year OS 35%, PFS 28%, relapse 32%, and NRM 40% after allogeneic HCT, with patients reaching the 5-year landmark having a 79% probability of remaining alive and in remission 10 years after transplant (12). It should be noted that the above studies did not have a control group and it is unclear whether the outcomes of allogeneic HCT are better than that of other modalities in the current era. Nevertheless, there appears to be a sizeable minority of allogeneic HCT recipients who are potentially cured of their CLL. Ongoing disease surveillance is still warranted as late relapses have been reported to occur more than 20 years after HCT (122). In addition, long-term survivors remain at persistently elevated risks of late NRM compared to the general population, highlighting the importance of comprehensive survivorship care after allogeneic HCT (12, 123).
While the use of allogeneic HCT has declined with the development of novel targeted agents for CLL, recent publications show it remains a reasonable option in the current era (30, 34, 100, 120). In one report of 30 patients with high-risk CLL previously treated with a BTK and/or BCL2 inhibitor, outcomes were excellent after RIC allogeneic HCT with 3-year OS 87% and PFS 72% (100). A separate study of 65 patients with high-risk, heavily pretreated CLL found similarly favorable outcomes with 2-year OS 81% and PFS 63% following allogeneic HCT after a previous BTK and/or BCL2 inhibitor (120). OS and PFS appeared similar regardless of whether patients had received one versus two or more novel agents or a BTK versus a BCL2 inhibitor as the most recent line of therapy before allogeneic HCT. Encouragingly, both studies also reported low risks of TRM at 7% and 13% with modern transplantation techniques (100, 120). It is conceivable that modern CLL therapies have the potential to improve the outcomes of allogeneic HCT by optimizing pre-transplant disease control or by inducing less toxicity compared to conventional chemotherapy.
Allogeneic HCT also has the potential to overcome the adverse prognosis associated with high-risk tumor biology in CLL (124, 125). While there is conflicting evidence that patients with deletion 17p and complex karyotype may have inferior PFS after allogeneic HCT (54, 80, 126–128), durable remissions are still achieved in a subset of patients with high-risk cytogenetics with reported OS 44-60% and PFS 29-43% (43, 129, 130). Other studies have reported that TP53 mutations may not significantly influence the outcomes of allogeneic HCT, although confirmation in larger datasets is needed (120, 131).
Disease status is another key determinant of outcomes after allogeneic HCT, with multiple studies consistently reporting lower relapse rates and improved PFS among patients in complete or partial response prior to transplantation (13, 14, 46, 128, 132). Nevertheless, up to 30-40% of patients with persistent or progressive CLL at the time of transplantation can still achieve long-term disease control (14, 30). Other factors associated with improved outcomes of allogeneic HCT in CLL include younger patient age, better performance status, lower HCT Comorbidity Index, fewer prior lines of therapy, better HLA matching, donor-recipient sex match, RIC rather than MAC, complete donor engraftment, and higher volume transplant centers (13, 39, 47, 53, 54, 100, 120, 128, 132, 133). Many of these factors have been incorporated into prognostic models proposed to predict survival outcomes after allogeneic HCT for CLL (43, 54, 80).
Complications of allogeneic HCT
GVHD remains a significant complication of allogeneic HCT, and patients with CLL are at particularly elevated risks of both acute and chronic GVHD. In a systematic review of 48 studies evaluating allogeneic HCT for CLL, the pooled rates of grade 2-4 acute GVHD was 46% and chronic GVHD was 55-59% (79). Other studies have reported similar risks of grade 2-4 acute GVHD in 35-49% and chronic GVHD in 27-48% of patients (13, 100, 120). The most well-identified risk factor for acute and chronic GVHD in CLL is the use of MAC as opposed to RIC/NMA (13, 24, 43). Stable or progressive disease at the time of transplantation has also been associated with increased risks of acute GVHD, while poor performance status has been correlated with increased risks of chronic GVHD (13, 24). As with other malignancies, GVHD often represents a dual-edged sword in CLL. On one hand, GVHD is a leading cause of morbidity and mortality after allogeneic HCT (12, 13), while on the other the development of chronic GVHD has been shown to confer protection against relapse in CLL, likely owing to a stronger GVL effect (42, 43). As an example, in one study the risk of relapse was significantly lower among patients with versus without chronic GVHD at 19% versus 53% (43). Nevertheless, appropriate GVHD prophylaxis, monitoring, and treatment is needed to reduce the impact of this complication on the survival and quality of life of patients with CLL (134).
Patients with CLL are predisposed to infection due to humoral and cellular immune defects induced by underlying immune dysregulation and the effects of prior therapies (135), a problem further brought to light by the COVID-19 pandemic (136–138). They are thus particularly vulnerable to the severe immunosuppression induced by allogeneic HCT, and careful monitoring and prevention of infection with prophylactic antimicrobials and revaccinations is essential (139). Although the routine use of intravenous immunoglobulin (IVIg) replacement does not improve survival after allogeneic HCT (140), it can be considered to reduce the risk of infection among patients with CLL who have hypogammaglobulinemia and severe or recurrent infections (141). Patients with CLL are also at heightened risk of secondary malignancies after allogeneic HCT (142, 143), and regular cancer screening including annual skin examination is a critical piece of survivorship care (144).
Monitoring and management of disease relapse after allogeneic HCT
Despite the potent GVL effect in CLL, relapse will ultimately occur in >30% of patients following allogeneic HCT (12). ASTCT guidelines recommend monitoring for measurable residual disease (MRD) with flow cytometry to track disease activity after allogeneic HCT (2). Varying patterns of post-transplant MRD kinetics have been identified which correlate with GVL activity (44, 45, 145), and the absence of MRD 1 year post-transplant is associated with a high likelihood of durable remission (145–148). In addition to its prognostic value, MRD monitoring may enable pre-emptive therapeutic intervention to reduce the risk of relapse after allogeneic HCT (148). In one prospective study, the administration of MRD-guided pre-emptive DLI resulted in achievement of MRD-negativity in 3 of 6 patients (114). This finding prompted a phase II multicenter trial of 42 patients with CLL who received risk-adapted immune intervention with immunosuppression withdrawal +/- DLI in response to serial MRD monitoring with peripheral blood flow cytometry after allogeneic HCT (45). This strategy appeared feasible and effective with 64% of patients achieving MRD negativity at 12 months, yielding a 3-year OS rate of 87% and PFS 63%. Further studies are needed to identify the optimal timepoints for MRD assessment and to confirm the clinical utility of pre-emptive immune intervention in CLL.
Whereas the relapse of acute leukemia after allogeneic HCT is typically associated with a poor prognosis (149), patients with CLL relapsing post-transplant may in fact experience relatively favorable outcomes owing to the availability of multiple rescue therapies (148, 150). In one study of 52 patients with CLL relapsing after allogeneic HCT, the overall response rate to subsequent post-transplant treatment was 45% and 2- and 5-year OS rates were 67% and 38%, respectively (150). Limited data suggest that withdrawal of immunosuppression and/or DLI can induce a GVL effect and achieve complete responses in 30-55% of patients relapsing after allogeneic HCT (41, 151, 152). The addition of rituximab to augment the effects of DLI has been evaluated in two studies with conflicting results; in one report, 20/43 (47%) patients achieved complete responses which tended to be durable, whereas in the second study a sustained remission was achieved in only 2/13 (15%) patients (148, 153). The potential benefit of DLI must also be weighed against the substantial risks of inducing clinically significant GVHD in up to 38-48% of patients (148, 153, 154). BTK and BCL2 inhibitors represent other highly effective treatment options for patients relapsing after allogeneic HCT who have not previously developed resistance to these therapies (155–158). Ibrutinib is a particularly attractive option in the post-transplant setting given its potential role in the treatment of chronic GVHD (159, 160). Finally, patients developing relapse after allogeneic HCT should be strongly considered for enrolment in clinical trials evaluating promising new therapies such as non-covalent BTK inhibitors or CAR-T cells (29, 161).
Allogeneic HCT for histologic transformation of CLL
Richter transformation of CLL to an aggressive diffuse large B-cell lymphoma (DLBCL) occurs in >2-3% of patients and frequently portends a poor prognosis with median survival 3-11 months (162–164). Of note, a subset of patients with Richter transformation may in fact have relatively favorable outcomes akin to de novo DLBCL, particularly if they have previously untreated CLL, TP53 intact and clonally-unrelated Richter transformation, and demonstrate a complete response to R-CHOP (165–167). However, the majority of cases with heavily pre-treated CLL, TP53 mutations, or clonally related Richter transformation are at high risk of treatment failure, with PFS rates <25% after CHOP-based chemoimmunotherapy (168, 169). As a result, allogeneic HCT is recommended for eligible patients with high-risk Richter transformation responding to induction therapy (2, 170). In the largest study of 118 patients undergoing allogeneic HCT for Richter transformation, the CIBMTR reported 4-year OS 52%, PFS 43%, relapse incidence 30%, and NRM 27%. Superior outcomes were noted among patients in complete or partial response at the time of transplant, while the presence of deletion 17p, conditioning intensity, and previous treatment with BTK or BCL2 inhibitors did not have prognostic impact (171). The favorable outcomes of this study and others confirm that allogeneic HCT is the preferred consolidation strategy for eligible patients with high-risk Richter transformation (132, 172, 173). Alternatively, autologous HCT may be considered for selected patients with chemosensitive disease (171), while novel cellular therapies including CAR-T cells have shown promise for small numbers of patients with chemoresistant Richter transformation (174).
In rare cases, CLL may undergo transformation to B-cell prolymphocytic leukemia (B-PLL), which is characterized by the rapid development of B symptoms, marked leukocytosis, and splenomegaly (175, 176). B-PLL was previously defined by the presence of >55% prolymphocytes in the peripheral blood (177). However, the updated World Health Organization classification no longer recognizes B-PLL as a distinct entity; instead, cases of CLL which develop >15% prolymphocytes in the peripheral blood and/or bone marrow are designated as ‘prolymphocytic progression of CLL/SLL’ (178). Although data is sparse, allogeneic HCT may be considered for eligible patients with prolymphocytic progression of CLL due to the poor prognosis of this disease without HCT (179, 180). In one CIBMTR study including 11 patients with B-PLL, 1-year OS was 48% and PFS was 33% after allogeneic HCT (181).
Discussion
Given the risks of transplant-related morbidity and mortality, the decision to proceed to allogeneic HCT for CLL is not one to be taken lightly. Indeed, the emergence of well-tolerated and effective targeted agents for CLL have relegated allogeneic HCT to later lines of therapy, and it is now commonly considered a treatment of last resort for patients who lack other options. However, allogeneic HCT remains an important part of the therapeutic armamentarium for CLL. It is perhaps the only treatment able to alter the natural history of the disease through the GVL effect, which may delay CLL progression and potentially result in cure. With the ability to achieve long-lasting remissions in >30-50% of patients, allogeneic HCT is still a valuable option for patients with heavily pretreated CLL, high-risk genetic features, and resistance to BTK and/or BCL2 inhibitors. In addition, advances in transplantation technique have increased the availability of this therapy to older age groups while still reducing TRM risks to <10-15% in contemporary studies.
While the next generation of CLL treatments such as the non-covalent BTK inhibitor pirtobrutinib have demonstrated encouraging response rates, its median PFS of 18 months among patients previously treated with both BTK and BCL2 inhibitors indicates that additional treatment options will still be needed for CLL, including allogeneic HCT in appropriate candidates (182). CAR-T cell and CAR-NK cell therapies are also in development for CLL, but none have received regulatory approval for this indication and further work is needed to overcome the intrinsic T-cell dysfunction associated with CLL (161, 183, 184). Allogeneic HCT thus remains the best-established cellular therapy for CLL at the present time. Further investigation is needed to determine if the efficacy of allogeneic HCT can be further improved by the addition of novel targeted, immune, and/or cellular therapies as consolidation or maintenance after transplantation. Given that patients with CLL remain at particularly high risk of GVHD, studies on GVHD prophylaxis specifically in this population are needed to confirm if ATG-Thymoglobulin®, PTCy, or other agents may confer protection against GVHD without compromising GVL.
Patients with CLL remain at particularly high risk of GVHD, studies on GVHD prophylaxis specifically in this population are needed to confirm if ATG-Thymoglobulin®, PTCy, or other agents may confer protection against GVHD without compromising GVL. Finally, ongoing research is required to re-evaluate the risks and benefits of allogeneic HCT vis-à-vis the growing number of emerging therapeutic options for multiply relapsed CLL.
In addition to the recent advances in HCT outlined in this article, it is anticipated that further improvements in bridging therapy, donor selection, GVHD prophylaxis, RIC protocols, and supportive care are likely to make allogeneic HCT increasingly safe and effective for the small but deserving group of patients with CLL who are likely to benefit from this procedure in the future.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
RP has received honoraria from Beigene. MS has received honoraria from Janssen, Bei-Gene, AstraZeneca, Kite/Gilead, Novartis, BMS, Incyte, and Roche.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gribben JG. How and when I do allogeneic transplant in CLL. Blood (2018) 132(1):31–9. doi: 10.1182/blood-2018-01-785998
2. Kharfan-Dabaja MA, Kumar A, Hamadani M, Stilgenbauer S, Ghia P, Anasetti C, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia on behalf of the guidelines committee of the American society for blood and marrow transplantation. Biol Blood Marrow Transplant (2016) 22(12):2117–25. doi: 10.1016/j.bbmt.2016.09.013
3. Aronson JH, Skånland SS, Roeker LE, Thompson MC, Mato AR. Approach to a patient with "double refractory" chronic lymphocytic leukemia: "Double, double toil and trouble" (Shakespeare). Am J Hematol (2022) 97(Suppl 2):S19–25. doi: 10.1002/ajh.26682
4. Surveillance Research Program (SRP). Cancer stat facts: Leukemia — chronic lymphocytic leukemia (CLL) national cancer institute surveillance. United States: Epidemiology, and End Results (SEER) Program (2022) Available at: https://seer.cancer.gov/statfacts/html/clyl.html.
5. Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood (2018) 131(25):2745–60. doi: 10.1182/blood-2017-09-806398
6. Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med (2018) 379(26):2517–28. doi: 10.1056/NEJMoa1812836
7. Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet (2020) 395(10232):1278–91. doi: 10.1016/S0140-6736(20)30262-2
8. Shanafelt TD, Wang XV, Kay NE, Hanson CA, O'Brien S, Barrientos J, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med (2019) 381(5):432–43. doi: 10.1056/NEJMoa1817073
9. Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2020) 21(9):1188–200. doi: 10.1016/S1470-2045(20)30443-5
10. Morabito F, Del Poeta G, Mauro FR, Reda G, Sportoletti P, Laurenti L, et al. TP53 disruption as a risk factor in the era of targeted therapies: A multicenter retrospective study of 525 chronic lymphocytic leukemia cases. Am J Hematol (2021) 96(8):E306–E10. doi: 10.1002/ajh.26235
11. Bomben R, Rossi FM, Vit F, Bittolo T, D'Agaro T, Zucchetto A, et al. Mutations with low variant allele frequency predict short survival in chronic lymphocytic leukemia. Clin Cancer Res (2021) 27(20):5566–75. doi: 10.1158/1078-0432.CCR-21-0701
12. van Gelder M, de Wreede LC, Bornhäuser M, Niederwieser D, Karas M, Anderson NS, et al. Long-term survival of patients with CLL after allogeneic transplantation: a report from the European society for blood and marrow transplantation. Bone Marrow Transplant (2017) 52(3):372–80. doi: 10.1038/bmt.2016.282
13. Sobecks RM, Leis JF, Gale RP, Ahn KW, Zhu X, Sabloff M, et al. Outcomes of human leukocyte antigen-matched sibling donor hematopoietic cell transplantation in chronic lymphocytic leukemia: myeloablative versus reduced-intensity conditioning regimens. Biol Blood Marrow Transplant (2014) 20(9):1390–8. doi: 10.1016/j.bbmt.2014.05.020
14. Andersen NS, Bornhäuser M, Gramatzki M, Dreger P, Vitek A, Karas M, et al. Reduced intensity conditioning regimens including alkylating chemotherapy do not alter survival outcomes after allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia compared to low-intensity non-myeloablative conditioning. J Cancer Res Clin Oncol (2019) 145(11):2823–34. doi: 10.1007/s00432-019-03014-x
15. Herth I, Dietrich S, Benner A, Hegenbart U, Rieger M, Stadtherr P, et al. The impact of allogeneic stem cell transplantation on the natural course of poor-risk chronic lymphocytic leukemia as defined by the EBMT consensus criteria: a retrospective donor versus no donor comparison. Ann Oncol (2014) 25(1):200–6. doi: 10.1093/annonc/mdt511
16. Delgado J, Pillai S, Phillips N, Brunet S, Pratt G, Briones J, et al. Does reduced-intensity allogeneic transplantation confer a survival advantage to patients with poor prognosis chronic lymphocytic leukaemia? a case-control retrospective analysis. Ann Oncol (2009) 20(12):2007–12. doi: 10.1093/annonc/mdp259
17. Kharfan-Dabaja MA, Pidala J, Kumar A, Terasawa T, Djulbegovic B. Comparing efficacy of reduced-toxicity allogeneic hematopoietic cell transplantation with conventional chemo-(immuno) therapy in patients with relapsed or refractory CLL: a Markov decision analysis. Bone Marrow Transplant (2012) 47(9):1164–70. doi: 10.1038/bmt.2012.71
18. Poon ML, Fox PS, Samuels BI, O'Brien S, Jabbour E, Hsu Y, et al. Allogeneic stem cell transplant in patients with chronic lymphocytic leukemia with 17p deletion: consult-transplant versus consult- no-transplant analysis. Leuk Lymphoma (2015) 56(3):711–5. doi: 10.3109/10428194.2014.930848
19. Dreger P, Ghia P, Schetelig J, van Gelder M, Kimby E, Michallet M, et al. High-risk chronic lymphocytic leukemia in the era of pathway inhibitors: integrating molecular and cellular therapies. Blood (2018) 132(9):892–902. doi: 10.1182/blood-2018-01-826008
20. Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood (2014) 124(26):3841–9. doi: 10.1182/blood-2014-07-586826
21. Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia (2007) 21(1):12–7. doi: 10.1038/sj.leu.2404441
22. NCCN. Clinical practice guidelines in oncology: Chronic lymphocytic Leukemia/Small lymphocytic lymphoma national comprehensive cancer network (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf.
23. Lew TE, Lin VS, Cliff ER, Blombery P, Thompson ER, Handunnetti SM, et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Adv (2021) 5(20):4054–8. doi: 10.1182/bloodadvances.2021005083
24. Hill BT, Ahn KW, Hu ZH, Aljurf M, Beitinjaneh A, Cahn JY, et al. Assessment of impact of HLA type on outcomes of allogeneic hematopoietic stem cell transplantation for chronic lymphocytic leukemia. Biol Blood Marrow Transplant (2018) 24(3):581–6. doi: 10.1016/j.bbmt.2017.10.015
25. Jones JA, Mato AR, Wierda WG, Davids MS, Choi M, Cheson BD, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol (2018) 19(1):65–75. doi: 10.1016/S1470-2045(17)30909-9
26. Lin VS, Lew TE, Handunnetti SM, Blombery P, Nguyen T, Westerman DA, et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood (2020) 135(25):2266–70. doi: 10.1182/blood.2020004782
27. Mato AR, Roeker LE, Jacobs R, Hill BT, Lamanna N, Brander D, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res (2020) 26(14):3589–96. doi: 10.1158/1078-0432.CCR-19-3815
28. Lew TE, Tam CS, Seymour JF. How I treat chronic lymphocytic leukemia after venetoclax. Blood (2021) 138(5):361–9. doi: 10.1182/blood.2020008502
29. Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory b-cell malignancies (BRUIN): a phase 1/2 study. Lancet (2021) 397(10277):892–901. doi: 10.1016/S0140-6736(21)00224-5
30. Schetelig J, Chevallier P, van Gelder M, Hoek J, Hermine O, Chakraverty R, et al. Idelalisib treatment prior to allogeneic stem cell transplantation for patients with chronic lymphocytic leukemia: a report from the EBMT chronic malignancies working party. Bone Marrow Transplant (2021) 56(3):605–13. doi: 10.1038/s41409-020-01069-w
31. Davids MS, Kim HT, Yu L, De Maeyer G, McDonough M, Vartanov AR, et al. Ofatumumab plus high dose methylprednisolone followed by ofatumumab plus alemtuzumab to achieve maximal cytoreduction prior to allogeneic transplantation for 17p deleted or TP53 mutated chronic lymphocytic leukemia. Leuk Lymphoma (2019) 60(5):1312–5. doi: 10.1080/10428194.2018.1519814
32. Schetelig J, Link CS, Stuhler G, Wagner EM, Hänel M, Kobbe G, et al. Anti-CD20 immunotherapy as a bridge to tolerance, after allogeneic stem cell transplantation for patients with chronic lymphocytic leukaemia: results of the CLLX4 trial. Br J Haematol (2019) 184(5):833–6. doi: 10.1111/bjh.15181
33. van Gelder M, van Oers MH, Alemayehu WG, Abrahamse-Testroote MC, Cornelissen JJ, Chamuleau ME, et al. Efficacy of cisplatin-based immunochemotherapy plus alloSCT in high-risk chronic lymphocytic leukemia: final results of a prospective multicenter phase 2 HOVON study. Bone Marrow Transplant (2016) 51(6):799–806. doi: 10.1038/bmt.2016.9
34. Dreger P, Michallet M, Bosman P, Dietrich S, Sobh M, Boumendil A, et al. Ibrutinib for bridging to allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia or mantle cell lymphoma: a study by the EBMT chronic malignancies and lymphoma working parties. Bone Marrow Transplant (2019) 54(1):44–52. doi: 10.1038/s41409-018-0207-4
35. Thompson MC, Harrup RA, Coombs CC, Roeker LE, Pu JJ, Choi MY, et al. Venetoclax retreatment of patients with chronic lymphocytic leukemia after a previous venetoclax-based regimen. Blood Adv (2022) 6(15):4553–7. doi: 10.1182/bloodadvances.2022007812
36. Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol (2008) 26(30):4912–20. doi: 10.1200/JCO.2007.15.4757
37. Kahl C, Storer BE, Sandmaier BM, Mielcarek M, Maris MB, Blume KG, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood (2007) 110(7):2744–8. doi: 10.1182/blood-2007-03-078592
38. Shaffer BC, Modric M, Stetler-Stevenson M, Arthur DC, Steinberg SM, Liewehr DJ, et al. Rapid complete donor lymphoid chimerism and graft-versus-leukemia effect are important in early control of chronic lymphocytic leukemia. Exp Hematol (2013) 41(9):772–8. doi: 10.1016/j.exphem.2013.04.015
39. Machaczka M, Johansson JE, Remberger M, Hallböök H, Malm C, Lazarevic V, et al. Allogeneic hematopoietic stem cell transplant with reduced-intensity conditioning for chronic lymphocytic leukemia in Sweden: does donor T-cell engraftment 3 months after transplant predict survival? Leuk Lymphoma (2012) 53(9):1699–705. doi: 10.3109/10428194.2012.666661
40. Jones CD, Arai S, Lowsky R, Tyan DB, Zehnder JL, Miklos DB. Complete donor T-cell engraftment 30 days after allogeneic transplantation predicts molecular remission in high-risk chronic lymphocytic leukaemia. Br J Haematol (2010) 150(5):637–9. doi: 10.1111/j.1365-2141.2010.08252.x
41. Thompson PA, Stingo F, Keating MJ, Wierda WG, O'Brien SM, Estrov Z, et al. Long-term follow-up of patients receiving allogeneic stem cell transplant for chronic lymphocytic leukaemia: mixed T-cell chimerism is associated with high relapse risk and inferior survival. Br J Haematol (2017) 177(4):567–77. doi: 10.1111/bjh.14596
42. Krämer I, Stilgenbauer S, Dietrich S, Böttcher S, Zeis M, Stadler M, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood (2017) 130(12):1477–80. doi: 10.1182/blood-2017-04-775841
43. Brown JR, Kim HT, Armand P, Cutler C, Fisher DC, Ho V, et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia (2013) 27(2):362–9. doi: 10.1038/leu.2012.228
44. Ritgen M, Böttcher S, Stilgenbauer S, Bunjes D, Schubert J, Cohen S, et al. Quantitative MRD monitoring identifies distinct GVL response patterns after allogeneic stem cell transplantation for chronic lymphocytic leukemia: results from the GCLLSG CLL3X trial. Leukemia (2008) 22(7):1377–86. doi: 10.1038/leu.2008.96
45. Tournilhac O, Le Garff-Tavernier M, Nguyen Quoc S, Forcade E, Chevallier P, Legrand-Izadifar F, et al. Efficacy of minimal residual disease driven immune-intervention after allogeneic hematopoietic stem cell transplantation for high-risk chronic lymphocytic leukemia: results of a prospective multicenter trial. Haematologica (2021) 106(7):1867–75.
46. Hebenstreit K, Iacobelli S, Leiblein S, Eisfeld AK, Pfrepper C, Heyn S, et al. Low tumor burden is associated with early b-cell reconstitution and is a predictor of favorable outcome after non-myeloablative stem cell transplant for chronic lymphocytic leukemia. Leuk Lymphoma (2014) 55(6):1274–80. doi: 10.3109/10428194.2013.836598
47. Michallet M, Sobh M, Milligan D, Morisset S, Niederwieser D, Koza V, et al. The impact of HLA matching on long-term transplant outcome after allogeneic hematopoietic stem cell transplantation for CLL: a retrospective study from the EBMT registry. Leukemia (2010) 24(10):1725–31. doi: 10.1038/leu.2010.165
48. Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood (2007) 110(13):4576–83. doi: 10.1182/blood-2007-06-097386
49. Dehn J, Spellman S, Hurley CK, Shaw BE, Barker JN, Burns LJ, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood (2019) 134(12):924–34. doi: 10.1182/blood.2019001212
50. Center for International Blood and Marrow Transplant Research. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. United States: Center for International Blood and Marrow Transplant Research (2021) Available at: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx.
51. Kollman C, Spellman SR, Zhang MJ, Hassebroek A, Anasetti C, Antin JH, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood (2016) 127(2):260–7. doi: 10.1182/blood-2015-08-663823
52. Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Blood (2013) 122(19):3359–64. doi: 10.1182/blood-2013-05-499830
53. Schetelig J, de Wreede LC, Andersen NS, Moreno C, van Gelder M, Vitek A, et al. Centre characteristics and procedure-related factors have an impact on outcomes of allogeneic transplantation for patients with CLL: a retrospective analysis from the European society for blood and marrow transplantation (EBMT). Br J Haematol (2017) 178(4):521–33. doi: 10.1111/bjh.14791
54. Schetelig J, de Wreede LC, van Gelder M, Andersen NS, Moreno C, Vitek A, et al. Risk factors for treatment failure after allogeneic transplantation of patients with CLL: a report from the European society for blood and marrow transplantation. Bone Marrow Transplant (2017) 52(4):552–60. doi: 10.1038/bmt.2016.329
55. Kanate AS, Mussetti A, Kharfan-Dabaja MA, Ahn KW, DiGilio A, Beitinjaneh A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood (2016) 127(7):938–47. doi: 10.1182/blood-2015-09-671834
56. Rashidi A, Hamadani M, Zhang MJ, Wang HL, Abdel-Azim H, Aljurf M, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv (2019) 3(12):1826–36. doi: 10.1182/bloodadvances.2019000050
57. Paul S, Tsai HL, Lowery P, Fuchs EJ, Luznik L, Bolaños-Meade J, et al. Allogeneic haploidentical blood or marrow transplantation with post-transplantation cyclophosphamide in chronic lymphocytic leukemia. Biol Blood Marrow Transplant (2020) 26(3):502–8. doi: 10.1016/j.bbmt.2019.11.008
58. van Gorkom G, van Gelder M, Eikema DJ, Blok HJ, van Lint MT, Koc Y, et al. Outcomes of haploidentical stem cell transplantation for chronic lymphocytic leukemia: a retrospective study on behalf of the chronic malignancies working party of the EBMT. Bone Marrow Transplant (2018) 53(3):255–63. doi: 10.1038/s41409-017-0023-2
59. Fuchs EJ, O'Donnell PV, Eapen M, Logan B, Antin JH, Dawson P, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood (2021) 137(3):420–8. doi: 10.1182/blood.2020007535
60. Xavier E, Cornillon J, Ruggeri A, Chevallier P, Cornelissen JJ, Andersen NS, et al. Outcomes of cord blood transplantation using reduced-intensity conditioning for chronic lymphocytic leukemia: A study on behalf of eurocord and cord blood committee of cellular therapy and immunobiology working party, chronic malignancies working party of the European society for blood and marrow transplantation, and the societé française de greffe de moelle et therapie cellulaire. Biol Blood Marrow Transplant (2015) 21(8):1515–23. doi: 10.1016/j.bbmt.2015.04.026
61. Shaw BE, Jimenez-Jimenez AM, Burns LJ, Logan BR, Khimani F, Shaffer BC, et al. National marrow donor program-sponsored multicenter, phase II trial of HLA-mismatched unrelated donor bone marrow transplantation using post-transplant cyclophosphamide. J Clin Oncol (2021) 39(18):1971–82. doi: 10.1200/JCO.20.03502
62. Matos DM, Ismael SJ, Scrideli CA, de Oliveira FM, Rego EM, Falcão RP. Monoclonal b-cell lymphocytosis in first-degree relatives of patients with sporadic (non-familial) chronic lymphocytic leukaemia. Br J Haematol (2009) 147(3):339–46. doi: 10.1111/j.1365-2141.2009.07861.x
63. Del Giudice I, Mauro FR, De Propris MS, Starza ID, Armiento D, Iori AP, et al. Identification of monoclonal b-cell lymphocytosis among sibling transplant donors for chronic lymphocytic leukemia patients. Blood (2009) 114(13):2848–9. doi: 10.1182/blood-2009-06-228395
64. Rawstron AC, Yuille MR, Fuller J, Cullen M, Kennedy B, Richards SJ, et al. Inherited predisposition to CLL is detectable as subclinical monoclonal b-lymphocyte expansion. Blood (2002) 100(7):2289–90. doi: 10.1182/blood-2002-03-0892
65. Ferrand C, Garnache-Ottou F, Collonge-Rame MA, Larosa F, Blanc M, Behar C, et al. Systematic donor blood qualification by flow cytometry would have been able to avoid CLL-type MBL transmission after unrelated hematopoietic stem cell transplantation. Eur J Haematol (2012) 88(3):269–72. doi: 10.1111/j.1600-0609.2011.01741.x
66. Pavletic SZ, Zhou G, Sobocinski K, Marti G, Doney K, DiPersio J, et al. Genetically identical twin transplantation for chronic lymphocytic leukemia. Leukemia (2007) 21(12):2452–5. doi: 10.1038/sj.leu.2404928
67. Flandrin-Gresta P, Callanan M, Nadal N, Jaubert J, Cornillon J, Guyotat D, et al. Transmission of leukemic donor cells by allogeneic stem cell transplantation in a context of familial CLL: should we screen donors for MBL? Blood (2010) 116(23):5077–8. doi: 10.1182/blood-2010-08-300673
68. Perz JB, Ritgen M, Moos M, Ho AD, Kneba M, Dreger P. Occurrence of donor-derived CLL 8 years after sibling donor SCT for CML. Bone Marrow Transplant (2008) 42(10):687–8. doi: 10.1038/bmt.2008.230
69. Herishanu Y, Eshel R, Kay S, Rothman R, Njuguna N, Perry C, et al. Unexpected detection of monoclonal b-cell lymphocytosis in a HLA-matched sibling donor on the day of allogeneic stem cell transplantation for a patient with chronic lymphocytic leukaemia: clinical outcome. Br J Haematol (2010) 149(6):905–7. doi: 10.1111/j.1365-2141.2010.08133.x
70. Nahi H, Jansson M, Sander B, Ljungman P, Hägglund H. Transmission of chronic lymphocytic leukaemia from a blood stem cell sibling donor to the recipient. Br J Haematol (2008) 143(5):751–3. doi: 10.1111/j.1365-2141.2008.07403.x
71. Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med (2012) 367(16):1487–96. doi: 10.1056/NEJMoa1203517
72. Group SCTC. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol (2005) 23(22):5074–87.
73. Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol (2017) 35(11):1154–61. doi: 10.1200/JCO.2016.70.7091
74. Scott BL, Pasquini MC, Fei M, Fraser R, Wu J, Devine SM, et al. Myeloablative versus reduced-intensity conditioning for hematopoietic cell transplantation in acute myelogenous leukemia and myelodysplastic syndromes-Long-Term follow-up of the BMT CTN 0901 clinical trial. Transplant Cell Ther (2021) 27(6):483.e1–.e6. doi: 10.1016/j.jtct.2021.02.031
75. Dreger P, Brand R, Milligan D, Corradini P, Finke J, Lambertenghi Deliliers G, et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia (2005) 19(6):1029–33. doi: 10.1038/sj.leu.2403745
76. Peres E, Braun T, Krijanovski O, Khaled Y, Levine JE, Yanik G, et al. Reduced intensity versus full myeloablative stem cell transplant for advanced CLL. Bone Marrow Transplant (2009) 44(9):579–83. doi: 10.1038/bmt.2009.61
77. Pavletic SZ, Khouri IF, Haagenson M, King RJ, Bierman PJ, Bishop MR, et al. Unrelated donor marrow transplantation for b-cell chronic lymphocytic leukemia after using myeloablative conditioning: results from the center for international blood and marrow transplant research. J Clin Oncol (2005) 23(24):5788–94. doi: 10.1200/JCO.2005.03.962
78. Toze CL, Galal A, Barnett MJ, Shepherd JD, Conneally EA, Hogge DE, et al. Myeloablative allografting for chronic lymphocytic leukemia: evidence for a potent graft-versus-leukemia effect associated with graft-versus-host disease. Bone Marrow Transplant (2005) 36(9):825–30. doi: 10.1038/sj.bmt.1705130
79. Kharfan-Dabaja MA, Moukalled N, Reljic T, El-Asmar J, Kumar A. Reduced intensity is preferred over myeloablative conditioning allogeneic HCT in chronic lymphocytic leukemia whenever indicated: A systematic review/meta-analysis. Hematol Oncol Stem Cell Ther (2018) 11(2):53–64. doi: 10.1016/j.hemonc.2017.11.001
80. Kim HT, Ahn KW, Hu ZH, Davids MS, Volpe VO, Antin JH, et al. Prognostic score and cytogenetic risk classification for chronic lymphocytic leukemia patients: Center for international blood and marrow transplant research report. Clin Cancer Res (2019) 25(16):5143–55. doi: 10.1158/1078-0432.CCR-18-3988
81. Michallet M, Socié G, Mohty M, Sobh M, Bay JO, Morisset S, et al. Rituximab, fludarabine, and total body irradiation as conditioning regimen before allogeneic hematopoietic stem cell transplantation for advanced chronic lymphocytic leukemia: long-term prospective multicenter study. Exp Hematol (2013) 41(2):127–33. doi: 10.1016/j.exphem.2012.10.008
82. Delioukina ML, Palmer JM, Thomas SH, Krishnan A, Stiller T, Forman SJ. Allogeneic hematopoietic cell transplant with fludarabine-based reduced-intensity conditioning as treatment for advanced chronic lymphocytic leukemia. Leuk Lymphoma (2011) 52(4):719–23. doi: 10.3109/10428194.2010.541311
83. Krejci M, Doubek M, Brychtova Y, Stehlikova O, Chovancova J, Tichy B, et al. Fludarabine with cytarabine followed by reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation in patients with poor-risk chronic lymphocytic leukemia. Ann Hematol (2013) 92(2):249–54. doi: 10.1007/s00277-012-1579-y
84. Khouri IF, Sui D, Jabbour EJ, Samuels BI, Turturro F, Alatrash G, et al. Bendamustine added to allogeneic conditioning improves long-term outcomes in patients with CLL. Bone Marrow Transplant (2017) 52(1):28–33. doi: 10.1038/bmt.2016.204
85. Shadman M, Maloney DG, Storer B, Sandmaier BM, Chauncey TR, Smedegaard Andersen N, et al. Rituximab-based allogeneic transplant for chronic lymphocytic leukemia with comparison to historical experience. Bone Marrow Transplant (2020) 55(1):172–81. doi: 10.1038/s41409-019-0660-8
86. Montesinos P, Cabrero M, Valcárcel D, Rovira M, García-Marco JA, Loscertales J, et al. The addition of ofatumumab to the conditioning regimen does not improve the outcome of patients with high-risk CLL undergoing reduced intensity allogeneic haematopoietic cell transplantation: a pilot trial from the GETH and GELLC (CLL4 trial). Bone Marrow Transplant (2016) 51(10):1404–7. doi: 10.1038/bmt.2016.145
87. Ghosh N, Ahmed S, Ahn KW, Khanal M, Litovich C, Aljurf M, et al. Association of reduced-intensity conditioning regimens with overall survival among patients with non-Hodgkin lymphoma undergoing allogeneic transplant. JAMA Oncol (2020) 6(7):1011–8. doi: 10.1001/jamaoncol.2020.1278
88. Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Reményi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol (2020) 7(1):e28–39.
89. Beelen DW, Stelljes M, Reményi P, Wagner-Drouet EM, Dreger P, Bethge W, et al. Treosulfan compared with reduced-intensity busulfan improves allogeneic hematopoietic cell transplantation outcomes of older acute myeloid leukemia and myelodysplastic syndrome patients: Final analysis of a prospective randomized trial. Am J Hematol (2022) 97(8):1023–34. doi: 10.1002/ajh.26620
90. Frietsch JJ, Miethke J, Linke P, Crodel CC, Schnetzke U, Scholl S, et al. Treosulfan plus fludarabine versus TEAM as conditioning treatment before autologous stem cell transplantation for b-cell non-Hodgkin lymphoma. Bone Marrow Transplant (2022) 57(7):1164–70. doi: 10.1038/s41409-022-01701-x
91. Yerushalmi R, Shem-Tov N, Danylesko I, Avigdor A, Nagler A, Shimoni A. Fludarabine and treosulfan compared with other reduced-intensity conditioning regimens for allogeneic stem cell transplantation in patients with lymphoid malignancies. Bone Marrow Transplant (2015) 50(12):1526–35. doi: 10.1038/bmt.2015.174
92. Hong S, Le-Rademacher J, Artz A, McCarthy PL, Logan BR, Pasquini MC. Comparison of non-myeloablative conditioning regimens for lymphoproliferative disorders. Bone Marrow Transplant (2015) 50(3):367–74. doi: 10.1038/bmt.2014.269
93. Sabloff M, Sobecks RM, Ahn KW, Zhu X, de Lima M, Brown JR, et al. Does total body irradiation conditioning improve outcomes of myeloablative human leukocyte antigen-identical sibling transplantations for chronic lymphocytic leukemia? Biol Blood Marrow Transplant (2014) 20(3):421–4. doi: 10.1016/j.bbmt.2013.11.032
94. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European society for blood and marrow transplantation. Lancet Haematol (2020) 7(2):e157–e67. doi: 10.1016/S2352-3026(19)30256-X
95. Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood (1998) 92(7):2303–14.
96. Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant (2004) 34(7):621–5. doi: 10.1038/sj.bmt.1704647
97. Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Fernandez H, Ayala E, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant (2010) 16(7):937–47. doi: 10.1016/j.bbmt.2010.01.010
98. Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood (2014) 124(8):1372–7. doi: 10.1182/blood-2014-04-567164
99. Sandmaier BM, Kornblit B, Storer BE, Olesen G, Maris MB, Langston AA, et al. Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: a multicentre, randomised, phase 3 trial. Lancet Haematol (2019) 6(8):e409–e18. doi: 10.1016/S2352-3026(19)30088-2
100. Kim HT, Shaughnessy CJ, Rai SC, Reynolds C, Ho VT, Cutler C, et al. Allogeneic hematopoietic cell transplantation after prior targeted therapy for high-risk chronic lymphocytic leukemia. Blood Adv (2020) 4(17):4113–23. doi: 10.1182/bloodadvances.2020002184
101. Luznik L, Pasquini MC, Logan B, Soiffer RJ, Wu J, Devine SM, et al. Randomized phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-Versus-Host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol (2022) 40(4):356–68. doi: 10.1200/JCO.21.02293
102. Arai Y, Jo T, Matsui H, Kondo T, Takaori-Kondo A. Efficacy of antithymocyte globulin for allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Leuk Lymphoma (2017) 58(8):1840–8. doi: 10.1080/10428194.2016.1266624
103. Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from gruppo italiano trapianti midollo osseo (GITMO). Blood (2001) 98(10):2942–7. doi: 10.1182/blood.V98.10.2942
104. Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant (2006) 12(5):560–5. doi: 10.1016/j.bbmt.2005.12.034
105. Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant (2014) 49(3):426–33. doi: 10.1038/bmt.2013.191
106. Chang YJ, Wang Y, Mo XD, Zhang XH, Xu LP, Yan CH, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: Long-term outcomes of a prospective randomized trial. Cancer (2017) 123(15):2881–92. doi: 10.1002/cncr.30540
107. Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol (2016) 17(2):164–73. doi: 10.1016/S1470-2045(15)00462-3
108. Waldmann H, Polliak A, Hale G, Or R, Cividalli G, Weiss L, et al. Elimination of graft-versus-host disease by in-vitro depletion of alloreactive lymphocytes with a monoclonal rat anti-human lymphocyte antibody (CAMPATH-1). Lancet (1984) 2(8401):483–6. doi: 10.1016/S0140-6736(84)92564-9
109. Pérez-Simón JA, Kottaridis PD, Martino R, Craddock C, Caballero D, Chopra R, et al. Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood (2002) 100(9):3121–7. doi: 10.1182/blood-2002-03-0701
110. Lozanski G, Heerema NA, Flinn IW, Smith L, Harbison J, Webb J, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood (2004) 103(9):3278–81. doi: 10.1182/blood-2003-10-3729
111. Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood (2002) 99(10):3554–61. doi: 10.1182/blood.V99.10.3554
112. Delgado J, Thomson K, Russell N, Ewing J, Stewart W, Cook G, et al. Results of alemtuzumab-based reduced-intensity allogeneic transplantation for chronic lymphocytic leukemia: a British society of blood and marrow transplantation study. Blood (2006) 107(4):1724–30. doi: 10.1182/blood-2005-08-3372
113. Delgado J, Pillai S, Benjamin R, Caballero D, Martino R, Nathwani A, et al. The effect of in vivo T cell depletion with alemtuzumab on reduced-intensity allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia. Biol Blood Marrow Transplant (2008) 14(11):1288–97. doi: 10.1016/j.bbmt.2008.09.001
114. Dreger P, Döhner H, Ritgen M, Böttcher S, Busch R, Dietrich S, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL study group CLL3X trial. Blood (2010) 116(14):2438–47. doi: 10.1182/blood-2010-03-275420
115. Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol (2019) 6(3):e132–e43.
116. Broers AEC, de Jong CN, Bakunina K, Hazenberg MD, van Marwijk Kooy M, de Groot MR, et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: results of the prospective randomized HOVON-96 trial. Blood Adv (2022) 6(11):3378–85. doi: 10.1182/bloodadvances.2021005847
117. Holtan SG, Hamadani M, Wu J, Malki MM, Runaas L, Elmariah H, et al. Post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil as the new standard for graft-Versus-Host disease (GVHD) prophylaxis in reduced intensity conditioning: Results from phase III BMT CTN 1703. Blood (2022) 140(Supplement 2):LBA–4. doi: 10.1182/blood-2022-171463
118. Zu Y, Li Z, Gui R, Liu Y, Zhang Y, Yu F, et al. Low-dose post-transplant cyclophosphamide with low-dose antithymocyte globulin for prevention of graft-versus-host disease in first complete remission undergoing 10/10 HLA-matched unrelated donor peripheral blood stem cell transplants: a multicentre, randomized controlled trial. Bone Marrow Transplant (2022) 57(10):1573–80. doi: 10.1038/s41409-022-01754-y
119. Watkins B, Qayed M, McCracken C, Bratrude B, Betz K, Suessmuth Y, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol (2021) 39(17):1865–77. doi: 10.1200/JCO.20.01086
120. Roeker LE, Dreger P, Brown JR, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv (2020) 4(16):3977–89. doi: 10.1182/bloodadvances.2020001956
121. Farina L, Barretta F, Scarfò L, Bruno B, Patriarca F, Frustaci AM, et al. Refractory and 17p-deleted chronic lymphocytic leukemia: improving survival with pathway inhibitors and allogeneic stem cell transplantation. Biol Blood Marrow Transplant (2020) 26(10):e256–e62. doi: 10.1016/j.bbmt.2020.06.032
122. Rovira M, Villamor N, Cobo F, Fernández-Aviles F, López-Guerra ML, Guijarro F, et al. Is chronic lymphocytic leukemia curable? a clinical case relapsing 21 years after allogeneic stem-cell transplantation. Bone Marrow Transplant (2020) 55(9):1860–1. doi: 10.1038/s41409-020-0861-1
123. Bhatia S. Caring for the long-term survivor after allogeneic stem cell transplantation. Hematol Am Soc Hematol Educ Program (2014) 2014(1):495–503. doi: 10.1182/asheducation-2014.1.495
124. Ritgen M, Stilgenbauer S, von Neuhoff N, Humpe A, Brüggemann M, Pott C, et al. Graft-versus-leukemia activity may overcome therapeutic resistance of chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene status: implications of minimal residual disease measurement with quantitative PCR. Blood (2004) 104(8):2600–2. doi: 10.1182/blood-2003-12-4321
125. Caballero D, García-Marco JA, Martino R, Mateos V, Ribera JM, Sarrá J, et al. Allogeneic transplant with reduced intensity conditioning regimens may overcome the poor prognosis of b-cell chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene and chromosomal abnormalities (11q- and 17p-). Clin Cancer Res (2005) 11(21):7757–63. doi: 10.1158/1078-0432.CCR-05-0941
126. Jaglowski SM, Ruppert AS, Heerema NA, Bingman A, Flynn JM, Grever MR, et al. Complex karyotype predicts for inferior outcomes following reduced-intensity conditioning allogeneic transplant for chronic lymphocytic leukaemia. Br J Haematol (2012) 159(1):82–7. doi: 10.1111/j.1365-2141.2012.09239.x
127. Chavez JC, Kharfan-Dabaja MA, Kim J, Yue B, Dalia S, Pinilla-Ibarz J, et al. Genomic aberrations deletion 11q and deletion 17p independently predict for worse progression-free and overall survival after allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia. Leuk Res (2014) 38(10):1165–72. doi: 10.1016/j.leukres.2014.04.006
128. van Gelder M, Ziagkos D, de Wreede L, van Biezen A, Dreger P, Gramatzki M, et al. Baseline characteristics predicting very good outcome of allogeneic hematopoietic cell transplantation in young patients with high cytogenetic risk chronic lymphocytic leukemia - a retrospective analysis from the chronic malignancies working party of the EBMT. Clin Lymphoma Myeloma Leuk (2017) 17(10):667–75.e2. doi: 10.1016/j.clml.2017.06.007
129. Schetelig J, Hoek J, Stilgenbauer S, Middeke JM, Andersen NS, Fox CP, et al. Allogeneic hematopoietic cell transplantation for patients with TP53 mutant or deleted chronic lymphocytic leukemia: Results of a prospective observational study. Bone Marrow Transplant (2021) 56(3):692–5. doi: 10.1038/s41409-020-01013-y
130. Schetelig J, van Biezen A, Brand R, Caballero D, Martino R, Itala M, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European group for blood and marrow transplantation analysis. J Clin Oncol (2008) 26(31):5094–100. doi: 10.1200/JCO.2008.16.2982
131. Dreger P, Schnaiter A, Zenz T, Böttcher S, Rossi M, Paschka P, et al. TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: six-year follow-up of the GCLLSG CLL3X trial. Blood (2013) 121(16):3284–8. doi: 10.1182/blood-2012-11-469627
132. Lahoud OB, Devlin SM, Maloy MA, Roeker LE, Dahi PB, Ponce DM, et al. Reduced-intensity conditioning hematopoietic stem cell transplantation for chronic lymphocytic leukemia and richter's transformation. Blood Adv (2021) 5(14):2879–89. doi: 10.1182/bloodadvances.2020003726
133. Bachanova V, Weisdorf DJ, Wang T, Marsh SGE, Cereb N, Haagenson MD, et al. Donor killer cell immunoglobulin-like receptor genotype does not improve graft-versus-Leukemia responses in chronic lymphocytic leukemia after unrelated donor transplant: A center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant (2019) 25(5):949–54. doi: 10.1016/j.bbmt.2018.12.763
134. Fraser CJ, Bhatia S, Ness K, Carter A, Francisco L, Arora M, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the bone marrow transplant survivor study. Blood (2006) 108(8):2867–73. doi: 10.1182/blood-2006-02-003954
135. Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol (2006) 33(2):240–9. doi: 10.1053/j.seminoncol.2005.12.013
136. Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood (2020) 136(10):1134–43. doi: 10.1182/blood.2020006965
137. Roeker LE, Eyre TA, Thompson MC, Lamanna N, Coltoff AR, Davids MS, et al. COVID-19 in patients with CLL: improved survival outcomes and update on management strategies. Blood (2021) 138(18):1768–73. doi: 10.1182/blood.2021011841
138. Scarfò L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European research initiative on CLL, and CLL campus. Leukemia (2020) 34(9):2354–63. doi: 10.1038/s41375-020-0959-x
139. (CIBMTR) CfIBaMTR, (NMDP) NMDP, (EBMT) EBaMTG, (ASBMT) ASoBaMT, (CBMTG) CBaMTG, (IDSA) IDSoA, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant (2009) 44(8):453–558.
140. Ahn H, Tay J, Shea B, Hutton B, Shorr R, Knoll GA, et al. Effectiveness of immunoglobulin prophylaxis in reducing clinical complications of hematopoietic stem cell transplantation: a systematic review and meta-analysis. Transfusion (2018) 58(10):2437–52. doi: 10.1111/trf.14656
141. Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta-analysis. Leuk Lymphoma (2009) 50(5):764–72. doi: 10.1080/10428190902856824
142. Wu PA, Stern RS, Huang V, Liu KX, Chen CA, Tzachanis D, et al. Reduced-intensity conditioning regimens, prior chronic lymphocytic leukemia, and graft-Versus-Host disease are associated with higher rates of skin cancer after allogeneic hematopoietic stem cell transplantation. J Invest Dermatol (2019) 139(3):591–9. doi: 10.1016/j.jid.2018.08.025
143. Fürstenau M, Giza A, Stumpf T, Robrecht S, Maurer C, Linde H, et al. Second primary malignancies in treated and untreated patients with chronic lymphocytic leukemia. Am J Hematol (2021) 96(12):E457–E60.
144. Inamoto Y, Shah NN, Savani BN, Shaw BE, Abraham AA, Ahmed IA, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant (2015) 50(8):1013–23. doi: 10.1038/bmt.2015.63
145. Böttcher S, Ritgen M, Dreger P. Allogeneic stem cell transplantation for chronic lymphocytic leukemia: lessons to be learned from minimal residual disease studies. Blood Rev (2011) 25(2):91–6. doi: 10.1016/j.blre.2011.01.001
146. Algrin C, Golmard JL, Michallet M, Reman O, Huynh A, Perrot A, et al. Flow cytometry minimal residual disease after allogeneic transplant for chronic lymphocytic leukemia. Eur J Haematol (2017) 98(4):363–70. doi: 10.1111/ejh.12836
147. Farina L, Carniti C, Dodero A, Vendramin A, Raganato A, Spina F, et al. Qualitative and quantitative polymerase chain reaction monitoring of minimal residual disease in relapsed chronic lymphocytic leukemia: early assessment can predict long-term outcome after reduced intensity allogeneic transplantation. Haematologica (2009) 94(5):654–62. doi: 10.3324/haematol.2008.000273
148. Hahn M, Böttcher S, Dietrich S, Hegenbart U, Rieger M, Stadtherr P, et al. Allogeneic hematopoietic stem cell transplantation for poor-risk CLL: dissecting immune-modulating strategies for disease eradication and treatment of relapse. Bone Marrow Transplant (2015) 50(10):1279–85. doi: 10.1038/bmt.2015.150
149. Hong S, Rybicki L, Corrigan D, Hamilton BK, Sobecks R, Kalaycio M, et al. Survival following relapse after allogeneic hematopoietic cell transplantation for acute leukemia and myelodysplastic syndromes in the contemporary era. Hematol Oncol Stem Cell Ther (2021) 14(4):318–26. doi: 10.1016/j.hemonc.2020.11.006
150. Rozovski U, Benjamini O, Jain P, Thompson PA, Wierda WG, O'Brien S, et al. Outcomes of patients with chronic lymphocytic leukemia and richter's transformation after transplantation failure. J Clin Oncol (2015) 33(14):1557–63. doi: 10.1200/JCO.2014.58.6750
151. El-Jurdi N, Reljic T, Kumar A, Pidala J, Bazarbachi A, Djulbegovic B, et al. Efficacy of adoptive immunotherapy with donor lymphocyte infusion in relapsed lymphoid malignancies. Immunotherapy (2013) 5(5):457–66. doi: 10.2217/imt.13.31
152. Richardson SE, Khan I, Rawstron A, Sudak J, Edwards N, Verfuerth S, et al. Risk-stratified adoptive cellular therapy following allogeneic hematopoietic stem cell transplantation for advanced chronic lymphocytic leukaemia. Br J Haematol (2013) 160(5):640–8. doi: 10.1111/bjh.12197
153. Khouri IF, Bassett R, Poindexter N, O'Brien S, Bueso-Ramos CE, Hsu Y, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer (2011) 117(20):4679–88. doi: 10.1002/cncr.26091
154. Brierley CK, Jones FM, Hanlon K, Peniket AJ, Hatton C, Collins GP, et al. Impact of graft-versus-lymphoma effect on outcomes after reduced intensity conditioned-alemtuzumab allogeneic haematopoietic stem cell transplantation for patients with mature lymphoid malignancies. Br J Haematol (2019) 184(4):547–57. doi: 10.1111/bjh.15685
155. Michallet M, Dreger P, Sobh M, Koster L, Hoek J, Boumendil A, et al. Ibrutinib as a salvage therapy after allogeneic HCT for chronic lymphocytic leukemia. Bone Marrow Transplant (2020) 55(5):884–90. doi: 10.1038/s41409-019-0742-7
156. Ryan CE, Sahaf B, Logan AC, O'Brien S, Byrd JC, Hillmen P, et al. Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood (2016) 128(25):2899–908. doi: 10.1182/blood-2016-06-715284
157. Link CS, Teipel R, Heidenreich F, Rücker-Braun E, Schmiedgen M, Reinhardt J, et al. Durable responses to ibrutinib in patients with relapsed CLL after allogeneic stem cell transplantation. Bone Marrow Transplant (2016) 51(6):793–8. doi: 10.1038/bmt.2015.339
158. Al-Sawaf O, Herling CD, Holtick U, Scheid C, Cramer P, Sasse S, et al. Venetoclax plus rituximab or obinutuzumab after allogeneic hematopoietic stem cell transplantation in chronic lymphocytic leukemia. Haematologica (2019) 104(5):e224–e6. doi: 10.3324/haematol.2018.212837
159. Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood (2017) 130(21):2243–50. doi: 10.1182/blood-2017-07-793786
160. Waller EK, Miklos D, Cutler C, Arora M, Jagasia MH, Pusic I, et al. Ibrutinib for chronic graft-versus-Host disease after failure of prior therapy: 1-year update of a phase 1b/2 study. Biol Blood Marrow Transplant (2019) 25(10):2002–7. doi: 10.1016/j.bbmt.2019.06.023
161. Siddiqi T, Soumerai JD, Dorritie KA, Stephens DM, Riedell PA, Arnason J, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood (2022) 139(12):1794–806. doi: 10.1182/blood.2021011895
162. Al-Sawaf O, Robrecht S, Bahlo J, Fink AM, Cramer P, V Tresckow J, et al. Richter Transformation in chronic lymphocytic leukemia (CLL)-a pooled analysis of German CLL study group (GCLLSG) front line treatment trials. Leukemia (2021) 35(1):169–76. doi: 10.1038/s41375-020-0797-x
163. Ding W. Richter Transformation in the era of novel agents. Hematol Am Soc Hematol Educ Program (2018) 2018(1):256–63. doi: 10.1182/asheducation-2018.1.256
164. Ben-Dali Y, Hleuhel MH, da Cunha-Bang C, Brieghel C, Poulsen CB, Clasen-Linde E, et al. Richter's transformation in patients with chronic lymphocytic leukaemia: a nationwide epidemiological study. Leuk Lymphoma (2020) 61(6):1435–44. doi: 10.1080/10428194.2020.1719092
165. Rossi D, Spina V, Deambrogi C, Rasi S, Laurenti L, Stamatopoulos K, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood (2011) 117(12):3391–401. doi: 10.1182/blood-2010-09-302174
166. Abrisqueta P, Delgado J, Alcoceba M, Oliveira AC, Loscertales J, Hernández-Rivas JA, et al. Clinical outcome and prognostic factors of patients with Richter syndrome: real-world study of the Spanish chronic lymphocytic leukemia study group (GELLC). Br J Haematol (2020) 190(6):854–63. doi: 10.1111/bjh.16748
167. Wang Y, Tschautscher MA, Rabe KG, Call TG, Leis JF, Kenderian SS, et al. Clinical characteristics and outcomes of Richter transformation: experience of 204 patients from a single center. Haematologica (2020) 105(3):765–73. doi: 10.3324/haematol.2019.224121
168. Langerbeins P, Busch R, Anheier N, Dürig J, Bergmann M, Goebeler ME, et al. Poor efficacy and tolerability of r-CHOP in relapsed/refractory chronic lymphocytic leukemia and Richter transformation. Am J Hematol (2014) 89(12):E239–43. doi: 10.1002/ajh.23841
169. Eyre TA, Clifford R, Bloor A, Boyle L, Roberts C, Cabes M, et al. NCRI phase II study of CHOP in combination with ofatumumab in induction and maintenance in newly diagnosed Richter syndrome. Br J Haematol (2016) 175(1):43–54. doi: 10.1111/bjh.14177
170. Eyre TA, Riches JC, Patten PEM, Walewska R, Marr H, Follows G, et al. Richter Transformation of chronic lymphocytic leukaemia: a British society for haematology good practice paper. Br J Haematol (2022) 196(4):864–70. doi: 10.1111/bjh.17882
171. Herrera AF, Ahn KW, Litovich C, Chen Y, Assal A, Bashir Q, et al. Autologous and allogeneic hematopoietic cell transplantation for diffuse large b-cell lymphoma-type Richter syndrome. Blood Adv (2021) 5(18):3528–39. doi: 10.1182/bloodadvances.2021004865
172. Cwynarski K, van Biezen A, de Wreede L, Stilgenbauer S, Bunjes D, Metzner B, et al. Autologous and allogeneic stem-cell transplantation for transformed chronic lymphocytic leukemia (Richter's syndrome): A retrospective analysis from the chronic lymphocytic leukemia subcommittee of the chronic leukemia working party and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol (2012) 30(18):2211–7. doi: 10.1200/JCO.2011.37.4108
173. Aulakh S, Reljic T, Yassine F, Ayala E, Chavez JC, Chanan-Khan A, et al. Allogeneic hematopoietic cell transplantation is an effective treatment for patients with Richter syndrome: A systematic review and meta-analysis. Hematol Oncol Stem Cell Ther (2021) 14(1):33–40. doi: 10.1016/j.hemonc.2020.05.002
174. Kittai AS, Bond DA, William B, Saad A, Penza S, Efebera Y, et al. Clinical activity of axicabtagene ciloleucel in adult patients with Richter syndrome. Blood Adv (2020) 4(19):4648–52. doi: 10.1182/bloodadvances.2020002783
175. Enno A, Catovsky D, O'Brien M, Cherchi M, Kumaran TO, Galton DA. 'Prolymphocytoid' transformation of chronic lymphocytic leukaemia. Br J Haematol (1979) 41(1):9–18.
176. Melo JV, Catovsky D, Gregory WM, Galton DA. The relationship between chronic lymphocytic leukaemia and prolymphocytic leukaemia. IV. analysis of survival and prognostic features. Br J Haematol (1987) 65(1):23–9. doi: 10.1111/j.1365-2141.1987.tb06130.x
177. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
178. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia (2022) 36(7):1720–48. doi: 10.1038/s41375-022-01620-2
179. Dearden C. How I treat prolymphocytic leukemia. Blood (2012) 120(3):538–51. doi: 10.1182/blood-2012-01-380139
180. Cross M, Dearden C. B and T cell prolymphocytic leukaemia. Best Pract Res Clin Haematol (2019) 32(3):217–28. doi: 10.1016/j.beha.2019.06.001
181. Kalaycio ME, Kukreja M, Woolfrey AE, Szer J, Cortes J, Maziarz RT, et al. Allogeneic hematopoietic cell transplant for prolymphocytic leukemia. Biol Blood Marrow Transplant (2010) 16(4):543–7. doi: 10.1016/j.bbmt.2009.11.021
182. Mato AR, Pagel JM, Coombs CC, Shah NN, Lamanna N, Munir T, et al. S147: Pirtobrutinib, a highly selective, non-covalent (Reversible) btk inhibitor in previously treated Cll/Sll: Updated results from the phase 1/2 bruin study. Hemasphere (2022) 6:48–9. doi: 10.1097/01.HS9.0000843480.04890.cc
183. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med (2020) 382(6):545–53. doi: 10.1056/NEJMoa1910607
Keywords: chronic lymphocytic leukemia (CLL), allogeneic hematopoietic cell transplant, graft-versus-hose disease (GvHD), Conditioning, graft-versus-leukemia (GvL)
Citation: Puckrin R, Shafey M and Storek J (2023) The role of allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia: A review. Front. Oncol. 12:1105779. doi: 10.3389/fonc.2022.1105779
Received: 23 November 2022; Accepted: 30 December 2022;
Published: 18 January 2023.
Edited by:
Lindsey Roeker, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Evgeny Klyuchnikov, University Medical Center Hamburg-Eppendorf, GermanyAngelo Michele Carella, Home for Relief of Suffering (IRCCS), Italy
Copyright © 2023 Puckrin, Shafey and Storek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Storek, jstorek@ucalgary.ca
 Robert Puckrin
Robert Puckrin Mona Shafey
Mona Shafey Jan Storek
Jan Storek