- 1Biosciences Laboratory, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
- 2Romagna Cancer Registry, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
- 3IRCCS Ospedale Policlinico San Martino, Genetics of Rare Cancers, Genoa, Italy
- 4Genetics of Rare Cancers, Department of Internal Medicine and Medical Specialties, University of Genoa, Genoa, Italy
- 5Scientific Directorate, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
Birt–Hogg–Dubé syndrome (BHDS) is a rare autosomal dominant inherited disorder caused by a mutation in folliculin (FLCN) gene transmitted via germline autosomal dominant pattern. Patients with this syndrome have an increased susceptibility to renal cell carcinoma, lung cysts, spontaneous pneumothorax, and benign skin hamartomas, and its diagnosis is not easy and consequently underestimated. Several mutations have been identified in FLCN gene, among which the majority of alterations are frameshift (insertion/deletion), nonsense, or splice-site mutations that generally produce unfunctional truncated FLCN proteins. Our aim is to present a case of a BHDS family whose proband is a 56-year-old patient who has been experiencing multiple disorders, has an FLCN genetic mutation, and has also been identified to have a pathogenic variant in BRCA2 gene. Our further purpose is to emphasize the importance of the next-generation sequencing (NGS) approach to identify potential multiple germline mutations in complex and rare oncologic disorders, allowing strict and more targeted cancer screening programs.
Introduction
Birt–Hogg–Dubé syndrome (BHDS) is a rare autosomal dominant inherited disorder described firstly in 1977, due to the germline transmission via an autosomal dominant pattern of a mutation in folliculin (FLCN) gene (1). The syndrome is considered to be underdiagnosed due to variable and atypical symptoms, and disease severity can differ significantly even within the same family. Its clinical manifestation is generally associated with multiple pulmonary cysts, frequent spontaneous pneumothoraces (PNXs), benign skin hamartomas, cutaneous fibrofolliculomas, and renal tumors of different histological types (2). FLCN, located on chromosome 17p11.2, is currently the main gene known to be associated with BHDS (3). A broad range of mutations have been identified in FLCN gene, among which the majority of FLCN alterations identified in the germline of BHD patients are frameshift (insertion/deletion), nonsense, or splice-site mutations that generally produce unfunctional truncated FLCN proteins. The most frequently observed mutation is the c.1285dupC/delC, an insertion/deletion of a cytosine in a C8 tract in exon 11 (4, 5). Early and precise diagnosis of BHDS is crucial for clinicians, firstly to better distinguish BHDS patients from others affected by other diseases and secondly to guarantee accurate counseling and screening programs and to ensure targeted treatments, especially in renal cancer patients and their family members. Herein, we present a BHDS family whose proband is a 56-year-old woman suspected to be affected by BHDS and whose diagnosis was confirmed by the mutation of FLCN gene. Furthermore, concomitant with the c.1285dupC; p.His429Profs*27 frameshift mutation in FLCN gene, we reported, as an incidental finding, the pathogenic variant c.7180A>T; p.(Arg2394Ter) in BRCA2 gene that was further observed in some family members.
Case Presentation
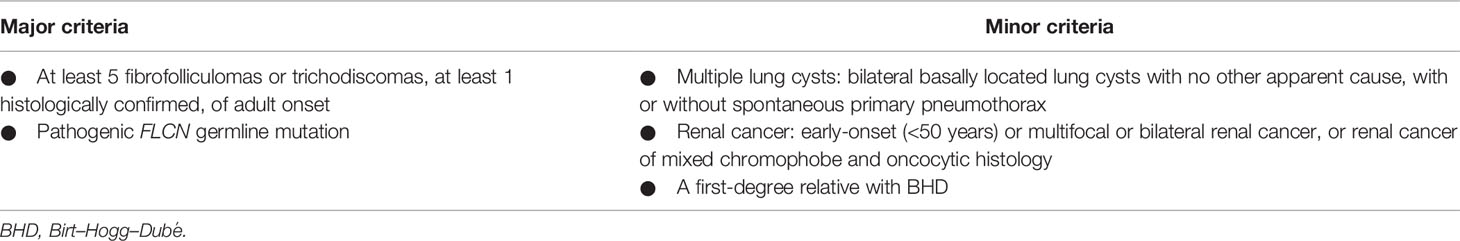
A 56-year-old woman, born from non-consanguineous parents, was recruited by our Oncogenetic Counselling Unit because of her personal multiple tumor history: two melanomas, thyroid, parathyroid, and bilateral kidney cancers. She had four admissions to the hospital for acute dyspnea episodes associated with spontaneous PNX that has been treated with talc, pleurodesis, and apicoectomy. Clinical dermatological examination reported the presence of freckles and the absence of cutaneous fibrofolliculomas and trichodiscomas or other skin lesions.
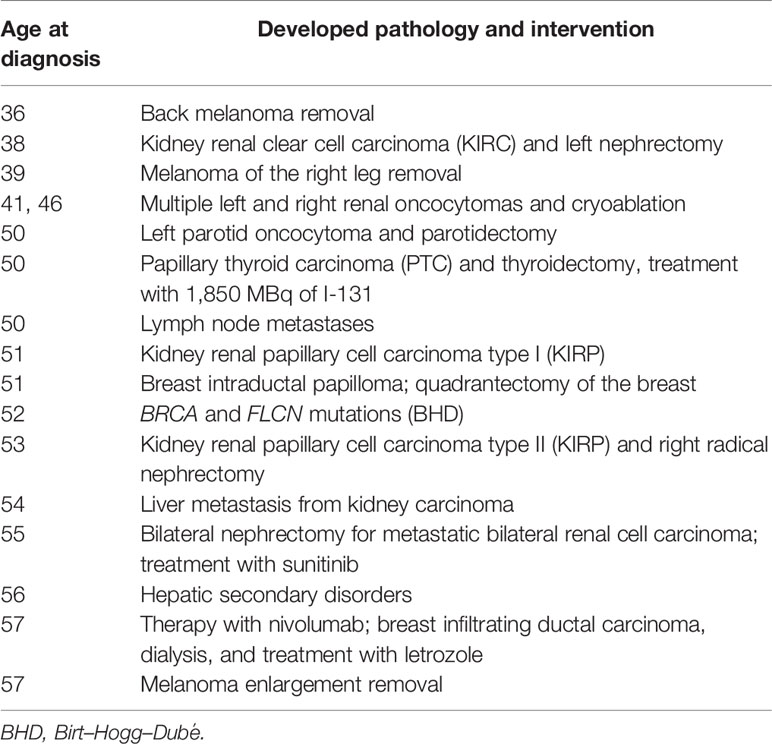
She admitted frequent sunburn during childhood, adolescence, and adulthood. She never made lamps, neither before nor after she was 35 years old. Currently, she does not smoke and does not drink alcohol. She had never used oral contraceptives or hormone replacement therapy. She pointed out having allergies to Augmentin (amoxicillin and clavulanic acid), Valium (diazepam), and Contramal (tramadol). There was no known family history of PNXs, dermatological lesions, or renal cell cancer (RCC). Prior to the PNX episode, she experienced multiple tumors at different sites, firstly developing two melanomas followed by thyroid, parathyroid, and bilateral kidney cancers, despite no previous family cases. Due to the nature of these disorders, she was suspected to be suffering from BHDS according to the diagnostic criteria proposed by European Birt–Hogg–Dubé Consortium (Table 1) (6), and a genetic test was required and performed by our Genetics Unit, confirming the diagnosis. After confirmation of the FLCN pathogenic variant causing the syndrome, the patient further developed multiple disorders, including kidney renal papillary cell carcinoma, liver metastasis, and metastatic bilateral renal cell carcinoma and she underwent some interventions, as summarized in Table 2. She was recently diagnosed with stage 1 breast invasive ductal carcinoma (IDC) according to the American Joint Committee on Cancer [AJCC] Cancer Staging Manual (7): the tumor was characterized by neoplastic cells positive for the expression of estrogen receptors (80%), progesterone receptors (30%), and Ki67 (5%) and negative for the expression of HER2/neu. Since she is firstly on dialysis and undergoing chemotherapy following secondary liver disorders, she cannot be subjected to surgery. In consideration of the size of the lesion, she started aromatase inhibitor (AI) therapy with letrozole. At present, the patient has been in a stable condition with tolerable life quality and is still in continuous dialysis treatment and nivolumab administration at a 240-mg dose for kidney metastases, although she is still not suitable for operation.
Materials and Methods
Patient Sample Collection
Patients with a history of BHD and referred for genetic counseling of the Genetics Unit of IRST IRCCS were included in the study and enrolled between 2015 and 2020. The study was approved by the institutional review board (Ethics Committee IRST IRCCS-AVR, 2207/2012) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects before the study. Information about possible tumors and malignancies related to family history of first- and second-degree relatives was also collected.
Blood Collection and DNA Extraction
Peripheral blood samples were collected and stored at −80°C at the Biosciences Laboratory of the IRCCS Istituto Romagnolo per lo Studio dei Tumori “Dino Amadori.” Genomic DNA was extracted using the QIAamp DNA mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA was quantified by Qubit fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) with Qubit dsDNA BR Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA).
Next-Generation Sequencing
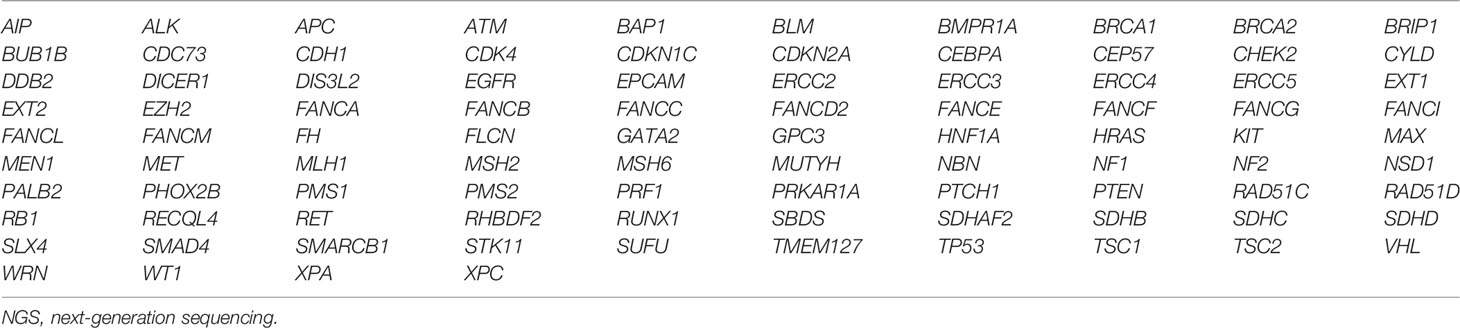
Two panels containing targeted genes, FLCN and BRCA2, were used for next-generation sequencing (NGS). Genetic analysis of the proband and a part of the relatives was performed using the enrichment protocol TruSight Cancer (Illumina, San Diego, CA, USA), which is an enrichment protocol for the simultaneous sequencing of 94 genes involved in the main hereditary cancer syndromes, starting at 50 ng of genomic DNA to create sequencing libraries (Table 3). The panel covers a total of 355 kb and includes the entire coding regions of the 94 genes and the flanking introns (50 bp upstream and downstream of each exon). Raw de-multiplexed reads from MiSeq sequencer were aligned to the reference human genome (UCSC-Build37/hg19) using the Burrows–Wheeler algorithm, running in paired-end mode. To ensure good call quality and to reduce the number of false positives, samples underwent Base Quality Score Recalibration (BQSR), through the Genome Analysis Toolkit GATK, version 3.2.2. After BQSR, sequences around regions with insertions and deletions (indels) were realigned locally with GATK. The MarkDuplicates tool was used to remove duplicate read-pairs that have arisen as artifacts during either PCR amplification or sequencing. For variant analysis, UnifiedGenotyper of GATK was used to search for single-nucleotide variants (SNVs) and indel. Genomic and functional annotations of detected variants were made by Annovar. Coverage statistics was performed by DepthOfCoverage utility of GATK. BASH and R custom scripts were used to obtain the list of low coverage (50×) regions per sample. The regions under this threshold were considered not evaluable. The potential impact of amino acid changes (MAPP p-value) was assessed with PolyPhen-2 HVAR and SIFT. A further part of the subjects was analyzed using the enrichment protocol of SOPHiA Hereditary Cancer Solution (HCS) v1.1 by SOPHiA GENETICS (Saint-Sulpice, Switzerland), which investigates 26 cancer predisposition genes (ABRAXAS1, APC, ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MRE11, MSH2, MSH6, MUTYH, NBN, PALB2, PIK3CA, PMS2, PTEN, RAD50, RAD51C, RAD51D, STK11, TP53, and XRCC2) and pseudogene PMS2CL (Table 4). Sequencing libraries were created from 200 ng of genomic DNA. Sequences were mapped to the human reference genome GRCh37/hg19. Data output files (FASTQ) were uploaded on the SOPHiA DDM Platform v5.5.0 (SOPHiA GENETICS, Saint-Sulpice, Switzerland) for analysis. The multigene panel (MGP) testing targets a total of 105 kb of the human genome and their flanking regions (on average 25 bp upstream and downstream of each exon). The sequencing was performed using the MiSeq sequencer platform (Illumina) and MiSeq Reagent Kit v2 or MiSeq Reagent Kit v3 600 cycles, configured 2 × 151 cycles in accordance with the manufacturer’s instructions. The bioinformatics analysis of NGS results was performed through Miseq Reporter software (Illumina) and processed with a customized pipeline for TruSight analysis. Furthermore, to validate the FLCN variant c.1285dupC; p.(H429fs), specific intronic primer pairs of exon 11 of FLCN (LRG_325, NG_008001, NM_144997.5) were designed, using the Primer3 algorithm (https://primer3plus.com), a primer designing tool. Purified PCR products were sequenced, with the same primer of the PCR amplification, using the BigDye Terminator v1.1 cycle sequencing kit (Life Technologies, Carlsbad, CA, USA) and a 3130xL Genetic Analyzer (Life Technologies) according to the manufacturer’s instructions.
Variant Classification
Genetic variants identified were classified according to the International Agency for Research on Cancer (IARC) recommendations (8), and pathogenic (PV; class 5) and likely pathogenic (LPV; class 4) were taken into consideration. The classification of BRCA1/2 and FLCN variants was performed through the main mutation databases and tool prediction software—BRCA Shar (formerly Universal Mutation Database), Leiden Open Variation Database (LOVD), BRCA Exchange, ClinVar, dbSNP, HCI Cancer Susceptibility Genes Prior Probabilities of Pathogenicity, and Varsome (9)—and were categorized according to the available clinical interpretation (10). Variants automatically annotated by the platform were manually checked on the main human genomic databases.
Results
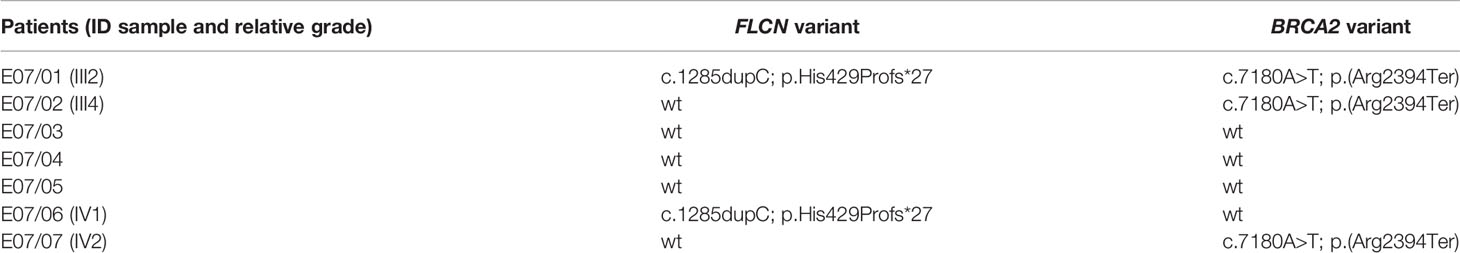
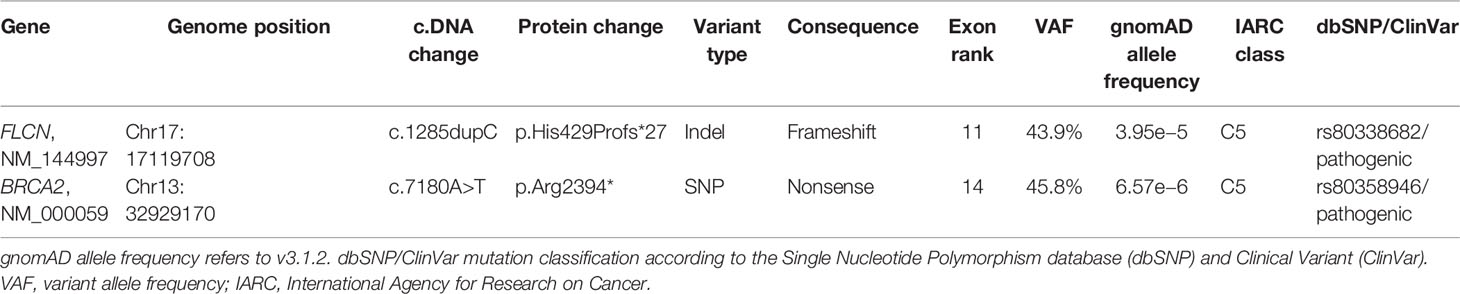
FLCN variant c.1285dupC; p.His429Profs*27, a frameshift mutation in heterozygosity predicted to be a disease-causing mutation, was detected in the proband patient and further observed in one family member (Table 5), confirming the BHDS diagnosis. After confirmation of the diagnosis, the whole family underwent a specific analysis program. None of the two brothers and sister was found to be carriers of the same pathogenic variant in FLCN, so they were assigned an equal population cancer risk and were monitored periodically, in accordance with the screening program. FLCN gene was found to be normally expressed in the younger son, while the oldest one was found to be a carrier. For this reason, the oldest son was subjected to a very careful and closely monitored screening program. Furthermore, as an incidental finding, the MGP analysis highlighted the presence of a pathogenic variant also in BRCA2 gene in 3 out of 7 members of the family (Table 5). For that reason, other than the indicated screening of BHDS, we recommended close screening for increased risk of breast and ovarian cancers, according to the high-risk program. More in detail, the identified variant BRCA2 referred to the mutation c.7180A>T; p.(Arg2394Ter), and to our knowledge, no other cases were observed so far to be carrying mutations in both FLCN and BRCA2 genes. The details about pathogenic FLCN and BRCA2 variants detected are listed in Table 6. The pedigree of family members harboring pathogenic FLCN and BRCA2 variants is shown in Figure 1. Insights on the BRCA analysis performed on relatives pointed out one of two brothers as the carrier of the same pathogenic variant in BRCA2. He was therefore subjected to periodic controls, particularly about prostate and breast. In addition, the younger proband’s son was found to be a BRCA2 mutation carrier. The two proband’s nephews, and the son and daughter of BRCA2 mutation carrier brother are actually too young to undergo genetic analysis to identify their eventual carrier status. Later, a first paternal cousin developed breast cancer (BC) at 50 years old but have wild-type BRCA2 and FLCN. It was not possible to perform the same analysis for the parents, as they have died, so it cannot be established whether the origin of the two mutations was paternal or maternal. Furthermore, a maternal cousin who experienced BC at 67 years old declined to undergo the analysis.
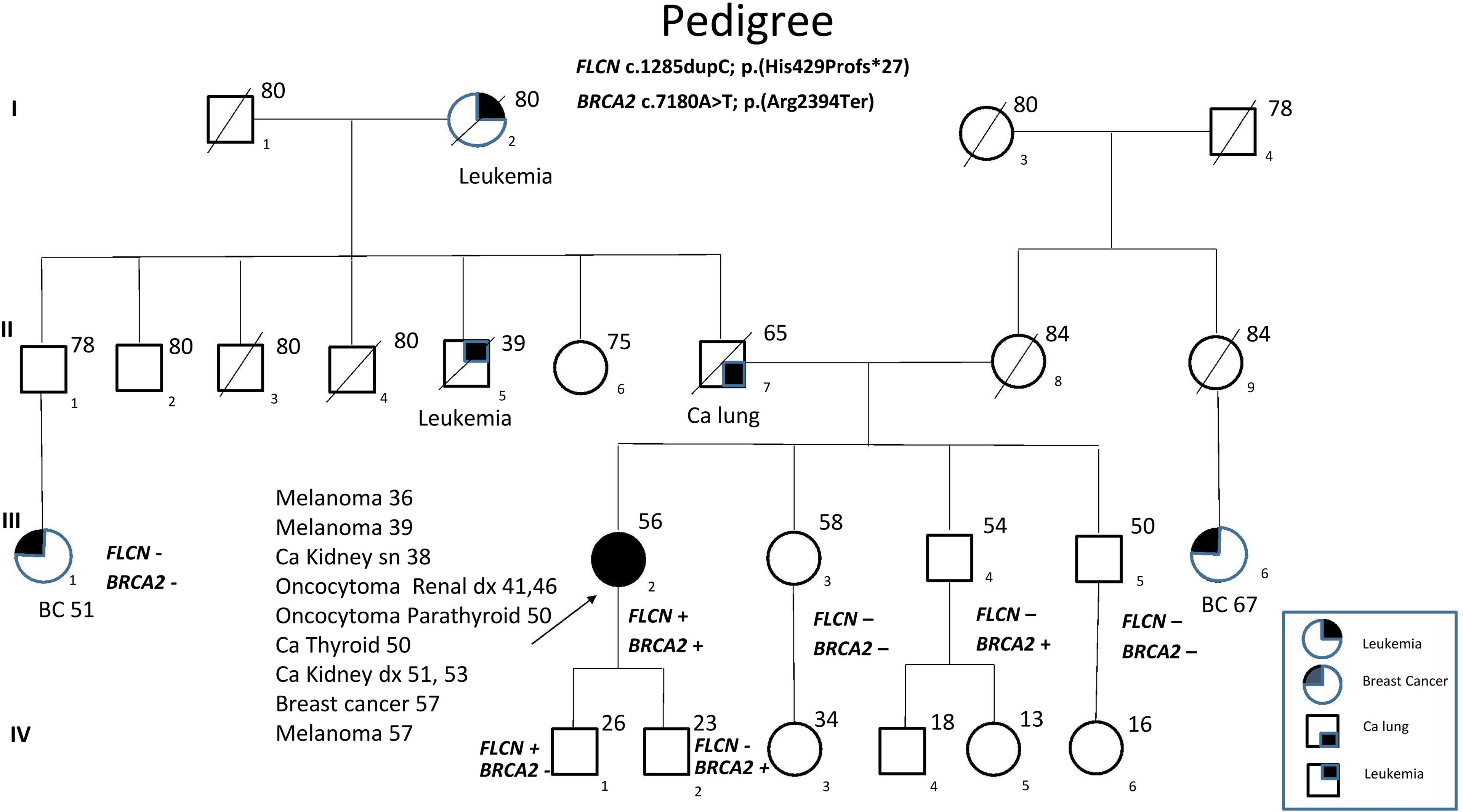
Figure 1 Pedigree of the family with disease-associated FLCN/BRCA2 mutations. Circles represent females, and squares represent males. Symbols with a quarter represent cancer patients. Symbols with a slash indicate deceased individuals. The arrow points to the proband.
Discussion
BHDS is a rare hereditary syndrome with high exposure to recurrent PNX correlated to multiple lung cysts and an increased risk of experiencing renal cancer and fibrofolliculomas in the skin. Multiple lung cysts are observed in approximately 67%–90% of patients with BHDS; and in about 40% of subjects, the onset of PNX is typical (11). Furthermore, a notably considerable proportion of BHDS families developed colorectal cancer (CRC) before the age of 50 years, or more than two family members are affected by CRC (12). Management of BHDS depends upon the clinical manifestation of the phenotype. It is extremely important to identify this rare syndrome at early stages, both for patients and also for subjects with a positive family history of RCC and PNX. FLCN sequencing should be taken into account in patients and their families since the incidence of renal cancer in BHD patients is very high, and detection at early stages can prevent its metastasis. Interestingly, it has been suggested that certain FLCN variants lead to a form of BHDS with PNXs but no renal carcinomas, although the risk of developing fatal renal cancers with crucial consequences is high (13). In this report, we present the case of a patient who was diagnosed with BHD syndrome after the manifestation of multiple serious symptoms and in whom a c.1285dupC; p.His429Profs*27 mutation on exon 11 of FLCN gene was identified. This variant is among the most frequent BHD mutations, and exon 11 represents the most common mutation site for this rare pathology (14). At once, we could not think of a disorder consistent with hereditary breast and ovarian cancer (HBOC), as the patient had neither breast nor ovarian tumors, and BC cases in paternal and maternal lines were not sufficient to consider a hereditary–familial form. The symptoms presented by the patient (PNX and oncocytoma) were suspicious for BHDS. Curiously, as an incidental finding, we also detected a c.7180A>T; p.(Arg2394Ter) nonsense mutation on exon 14 of BRCA2 gene, a type of variant already identified in families at risk for HBOC (15), but without previous correlations with BHDS. The diagnosis of BHDS, carried out primarily thanks to the genetic counseling service and the confirmation of FLCN mutation, was able to guarantee life-extending treatments for the patient and surely an improved long-term survival. To date, the patient is currently in stable condition, on dialysis, and under letrozole treatment for BC and nivolumab for kidney cancer metastasis. Certainly, in most cases, prognosis depends upon the occurrence of histologic type of renal cancer, and the majority of deaths results from metastatic diseases due to clear cell carcinoma (16). Subsequently, of extreme importance, it was possible to allow family members to undergo a surveillance screening program for the possible detection of the same genetic disorder or correlated symptoms. A periodic screening was recommended to family members, and it is noteworthy that three individuals were identified to be carriers of FLCN or BRCA2 mutations through family tracing; therefore, they are more likely not to develop complications, as they are being strictly monitored. This case supports the importance of early diagnosis of the syndrome and highlights the relevance of MGPs in identifying patients with the co-existence of pathogenic germline mutations that could be unfavorable for the risk of cancer development. In our case report, we describe how these analyses allowed unaffected carriers to have a guaranteed specific surveillance strategy. Although it was not possible to recover DNA from all family members, especially from the proband’s parents, the genetic analysis of FLCN and BRCA2 genes involved in hereditary cancers, combined with the family history, ensured to outline a more accurate diagnosis and to assess the risk of specific cancers of the family. The MGP represents an important tool to gain insight into the mechanisms that lead to high susceptibility to certain tumors and the interactions between causative mutations. Moreover, background mutations, as for BRCA in this family, increase the knowledge of the connections between the genotype and the phenotype of the family. Overall, the findings of the case we presented, combined with the BRCA variant identified as the incidental report, have enhanced our understanding of BHDS, raising the surveillance threshold for this syndrome also for any other future cases and allowing strict monitoring programs for family members involved, which could ensure long-term benefits.
Data Availability Statement
The datasets presented in this article are not readily available because they are part of genetic data obtained from analyzes of patients. Requests to access the datasets should be directed to corresponding author erika.bandini@irst.emr.it.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee IRST IRCCS-AVR, 2207/2012. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
VZ and RD conceived the study. IC, VAr, VAn, GP, LP, and VZ performed the experiments and analyzed and interpreted the data. VAr, MR, GM, FF, DC, and RD were responsible for clinical resources and management. EB wrote the manuscript. RD was responsible for the supervision of the project. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the patient and her family for participating in this study.
References
1. Adhikari N, Karki A. A Case Report of Recurrent Pneumothoraces as a Presentation of Birt Hogg Dube Syndrome. Respir Med Case Rep (2021) 32:101340. doi: 10.1016/j.rmcr.2021.101340
2. Daccord C, Good J-M, Morren M-A, Bonny O, Hohl D, Lazor R. Birt–Hogg–Dubé Syndrome. Eur Respir Rev (2020) 29:200042. doi: 10.1183/16000617.0042-2020
3. Cai M, Zhang X, Fan L, Cheng S, Kiram A, Cen S, et al. A Novel FLCN Intragenic Deletion Identified by NGS in a BHDS Family and Literature Review. Front Genet (2021) 12:636900. doi: 10.3389/fgene.2021.636900
4. Ramirez Reyes JMJ, Cuesta R, Pause A. Folliculin: A Regulator of Transcription Through AMPK and mTOR Signaling Pathways. Front Cell Dev Biol (2021) 9:667311. doi: 10.3389/fcell.2021.667311
5. Schmidt LS, Linehan WM. FLCN : The Causative Gene for Birt-Hogg-Dubé Syndrome. Gene (2018) 640:28–42. doi: 10.1016/j.gene.2017.09.044
6. Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, et al. Birt-Hogg-Dubé Syndrome: Diagnosis and Management. Lancet Oncol (2009) 10:1199–206. doi: 10.1016/S1470-2045(09)70188-3
7. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
8. Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, et al. Sequence Variant Classification and Reporting: Recommendations for Improving the Interpretation of Cancer Susceptibility Genetic Test Results. Hum Mutat (2008) 29:1282–91. doi: 10.1002/humu.20880
9. Rapposelli IG, Zampiga V, Cangini I, Arcangeli V, Ravegnani M, Valgiusti M, et al. Comprehensive Analysis of DNA Damage Repair Genes Reveals Pathogenic Variants Beyond BRCA and Suggests the Need for Extensive Genetic Testing in Pancreatic Cancer. BMC Cancer (2021) 21:611. doi: 10.1186/s12885-021-08368-5
10. Ramírez-Calvo M, García-Casado Z, Fernández-Serra A, de Juan I, Palanca S, Oltra S, et al. Implementation of Massive Sequencing in the Genetic Diagnosis of Hereditary Cancer Syndromes: Diagnostic Performance in the Hereditary Cancer Programme of the Valencia Community (FamCan-NGS). Hered Cancer Clin Pract (2019) 17:3. doi: 10.1186/s13053-019-0104-x
11. Ather H, Zahid N. Recurrent Renal Cancer in Birt–Hogg–Dubé Syndrome: A Case Report. Int J Surg Case Rep (2018) 42:75–8. doi: 10.1016/j.ijscr.2017.11.032
12. Sattler EC, Syunyaeva Z, Reithmair M, Dempke W, Steinlein OK. Colorectal Cancer Risk in Families With Birt-Hogg-Dubé Syndrome Increased. Eur J Cancer (2021) 151:168–74. doi: 10.1016/j.ejca.2021.04.013
13. Matsumoto K, Lim D, Pharoah PD, Maher ER, Marciniak SJ. A Systematic Review Assessing the Existence of Pneumothorax-Only Variants of FLCN. Implications for Lifelong Surveillance of Renal Tumours. Eur J Hum Genet (2021) 29:1595–600. doi: 10.1038/s41431-021-00921-x
14. Guo T, Shen Q, Ouyang R, Song M, Zong D, Shi Z, et al. The Clinical Characteristics of East Asian Patients With Birt-Hogg-Dubé Syndrome. Ann Transl Med (2020) 8:1436–6. doi: 10.21037/atm-20-1129
15. Fernandes GC, Michelli RAD, Galvão HCR, Paula AE, Pereira R, Andrade CE, et al. Prevalence of BRCA1/BRCA2 Mutations in a Brazilian Population Sample at-Risk for Hereditary Breast Cancer and Characterization of Its Genetic Ancestry. Oncotarget (2016) 7:80465–81. doi: 10.18632/oncotarget.12610
Keywords: case report, Birt–Hogg–Dubé, FLCN, BRCA2, NGS
Citation: Bandini E, Cangini I, Arcangeli V, Ravegnani M, Andreotti V, Prisinzano G, Pastorino L, Martinelli G, Falcini F, Calistri D, Zampiga V and Danesi R (2022) Case Report: A BRCA2 Mutation Identified Through Next-Generation Sequencing in a Birt–Hogg–Dubè Syndrome Family. Front. Oncol. 12:835346. doi: 10.3389/fonc.2022.835346
Received: 14 December 2021; Accepted: 19 January 2022;
Published: 14 February 2022.
Edited by:
Giovana Tardin Torrezan, A. C. Camargo Cancer Center, BrazilReviewed by:
Anna Maria Pinto, Azienda Osedaliera Universitaria Senese, ItalyJoshi Stephen, Baylor College of Medicine, United States
Copyright © 2022 Bandini, Cangini, Arcangeli, Ravegnani, Andreotti, Prisinzano, Pastorino, Martinelli, Falcini, Calistri, Zampiga and Danesi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erika Bandini, erika.bandini@irst.emr.it
†These authors have contributed equally to this work
 Erika Bandini
Erika Bandini Ilaria Cangini1†
Ilaria Cangini1† Valentina Arcangeli
Valentina Arcangeli Virginia Andreotti
Virginia Andreotti Giovanna Prisinzano
Giovanna Prisinzano Lorenza Pastorino
Lorenza Pastorino Giovanni Martinelli
Giovanni Martinelli Valentina Zampiga
Valentina Zampiga Rita Danesi
Rita Danesi