Intellectual Functioning in Children with Congenital Heart Defects Treated with Surgery or by Catheter Interventions
- 1Psychology Unit, Department of Pediatric Cardiology, The Queen Silvia’s Childrens Hospital, Sahlgrenska University Hospital, Gothenburg, Sweden
- 2Department of Pediatric Cardiology, The Queen Silvia’s Childrens Hospital, Sahlgrenska University Hospital, Gothenburg, Sweden
- 3Department of Psychology, University of Gothenburg, Gothenburg, Sweden
Background: Studies suggest that children with congenital heart defects (CHD) are at risk for adverse intellectual functioning. However, factors related to lower intellectual functioning in this group are largely unknown. This study describes intellectual functioning in children with CHD in relation to severity of the heart defect, the child’s age, and the socioeconomic status of the family (SES).
Methods: Two hundred twenty-eight children treated with surgery or by catheter technique were tested using the Wechsler intelligence scales to determine full scale IQ (FSIQ). FSIQ was then analyzed in relation to age (3-, 5-, 9-, and 15-year olds), severity of the diagnosis (mild, moderate, and severe), and SES (low, medium, and high). The median age was 70 months (5.8 years) with a range of 162 months [30 months (2.5 years) to 192 months (16.0 years)].
Results: The total mean score on FSIQ was 100.8 (SD = 14.5). Children with severe CHD had significantly lower FSIQ than children with mild and moderate CHD, and 9- and 15-year olds had significantly lower FSIQ compared to the 3-year olds. Children from families with low SES had significantly lower FSIQ than children from medium SES and high SES families. No interaction between severity of diagnosis, age, and SES was found for FSIQ.
Conclusion: Eighty-three percent of the children with CHD performed at or above average with respect to FSIQ. SES and severity of diagnosis had significant main effects on FSIQ. These factors should be considered when planning interventions and follow-up programs for children with CHD.
Introduction
Because children with congenital heart defects (CHD) now live longer due to advances in surgical and catheter techniques, their neurodevelopment, including intellectual functioning, has become a major area of concern (1, 2), attracting both clinical and research interest (3).
Severity of the heart diagnosis in children with CHD has been suggested as an important predictor for intellectual functioning: the more severe the heart diagnosis, the higher the risk for lower intellectual functioning (4) and poorer academic performance (5). Results, however, are not consistent. A study comparing univentricular and biventricular patients showed no significant group differences with respect to intellectual functioning; that is, both groups scored within the norms. However, when assessing specific neuropsychological functions within the domains of attention and executive and sensorimotor functioning, only the univentricular patients had lower scores compared to the matched controls (6).
The use of wide age ranges when measuring intellectual functioning in children with CHD have made it difficult to compare studies (5). A meta-analysis described a significant relation between older age and better cognitive functioning. Further analysis disclosed that when excluding patients with the more severe diagnosis, the age effect disappeared, implying that age effects were observed because patients with the more severe diagnosis were often tested at an earlier age (5). Some studies [e.g., Oates et al. (7)], however, have not found correlations between age and intellectual functioning (8).
The relation between SES and intellectual functioning is well investigated in the normal population (9–13) as well as in children with CHD (14–16). Intellectual outcomes in children with CHD are often shown to be related to maternal education (17), comorbidity (i.e., velocardiofacial syndrome), and low SES (18, 19). In a study of cardiac arrest in children with heart disease, higher SES correlated with higher intellectual functioning, lower levels of behavioral problems, and lower levels of parental stress (20). In studies done in healthy populations, intellectual functioning is positively related to SES (21).
Scientific knowledge on neurodevelopment in children with CHD, and specifically, intellectual functioning, is still incomplete. Previous studies have been limited to small groups of patients (22, 23) with a specific diagnosis (17, 24–26), have been overly restricted, specific, or unclear with respect to age groups (27–29), and have been limited to specific surgical procedures (26, 30). Using the intelligence quotient (FSIQ) score, the aim of the present study was to investigate intellectual functioning in children with CHD treated with surgery or by catheter techniques and to investigate if intellectual functioning was related to severity of the heart diagnosis, child age, or socioeconomic status of the families. The following hypotheses were tested:
1. Children with severe CHD have lower intellectual functioning than children with mild and moderate CHD,
2. Older children have lower intellectual functioning than younger children with CHD,
3. Children with CHD living in families with low SES have lower intellectual functioning than children in families with higher SES,
4. There is an interaction effect of severity of diagnosis and SES: children with low SES and severe CHD have lower intellectual functioning compared to children with high SES and severe CHD.
Materials and Methods
Participants
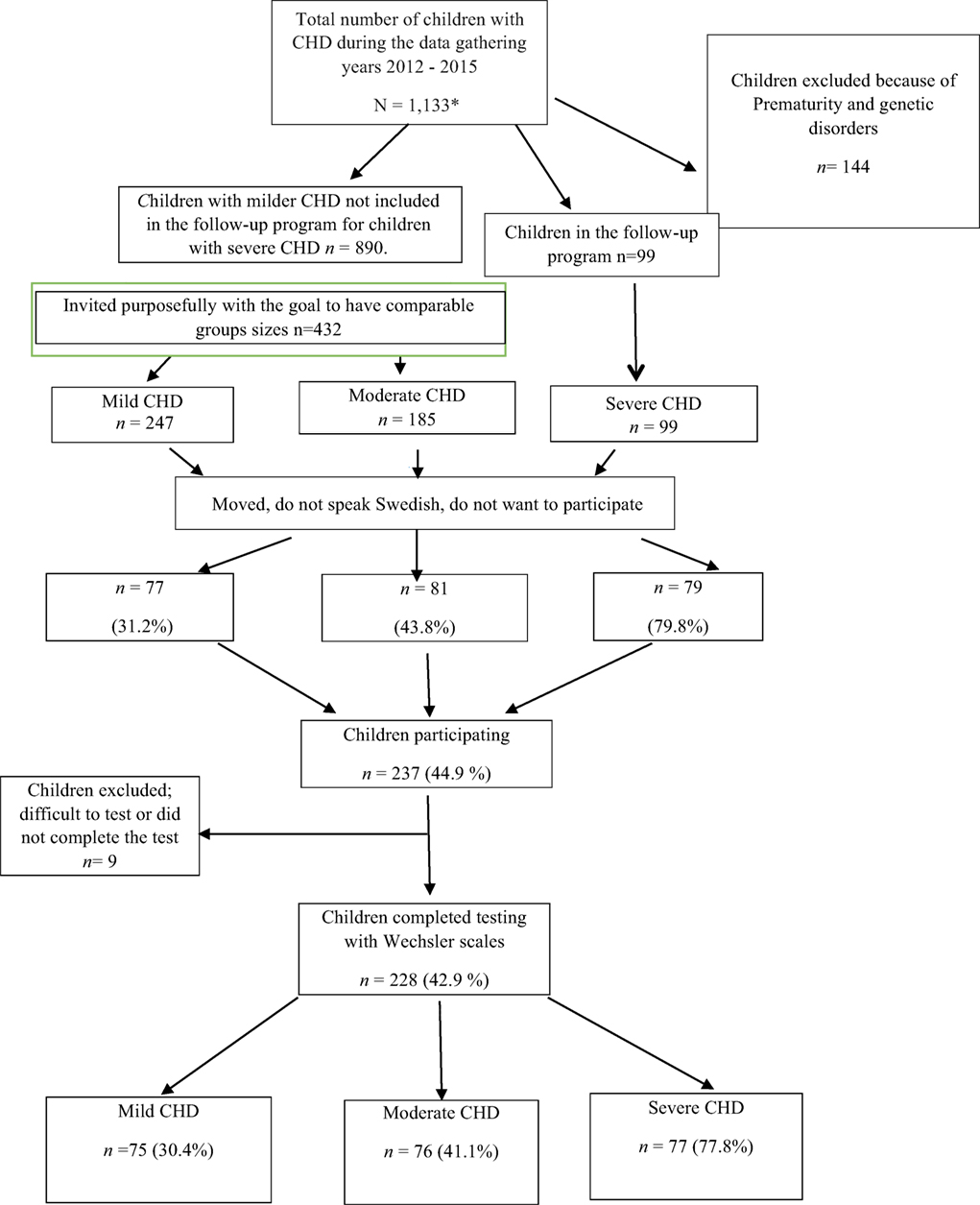
Participants were tested over a 7-year period (2008–2015). In the beginning, only children with severe CHD living in the Gothenburg area were included. As time and financial resources allowed, children with severe CHD were recruited from the whole region of Västra Götaland. Later, children with milder CHD (a larger population) were recruited to obtain comparison groups of comparable sizes. Using the medical records of children living in the Västra Götaland Region (VGR), we know that 1,133 children were treated with surgery or catheter interventions for CHD at Queen Silvia Children’s Hospital in Gothenburg, Sweden during the data collection period. Children with chromosomal defects and disabilities known to influence intellectual functioning (N = 144) were excluded. The invited families were required to speak, read, and write Swedish and to provide a signed consent. All eligible children with severe CHD (N = 99) and 432 (of 890) children with milder CHD were invited. We were unable to locate some patients, and some patients did not want to participate for different reasons (e.g., lack of time, not wanting to worry the child who had a mild CHD and were unaware of any difficulties, and did not speak Swedish). In total, 531 patients were invited (Figure 1); of these, 237 children and their families (44.9%) agreed to participate in the study. Participation rate was higher in the severe group than in the milder groups. All children met with a clinical psychologist at their local hospital for a psychological evaluation.
Four hospitals participated in the gathering of data: The Queen Silvia Children’s Hospital, North Älvsborg County Hospital, Southern Älvsborg Hospital, and Skaraborg Hospital Skövde; most of the testing was done by the same psychologist, however, the groups of psychologists testing the children met regularly to assure test reliability. Nine of the tested children were not included in the analyses: two 3-year olds were unable to complete the tests; two 5-year olds had unreliable test results (one was given a neuropsychiatric diagnosis); and one 15-year old refused to finish the testing. Four 9-year olds were not tested with the Wechsler scales. As a result, 228 children (42.9% of those invited) completed testing with Wechsler scales at age 3, 5, 9, or 15 years (Table 1) and are included in the analyses; 111 (48.7%) were girls and 117 (51.3%) boys. Approval from the ethics committee in Gothenburg, Sweden was obtained on September 20, 2011 (ref. no. 391–11).
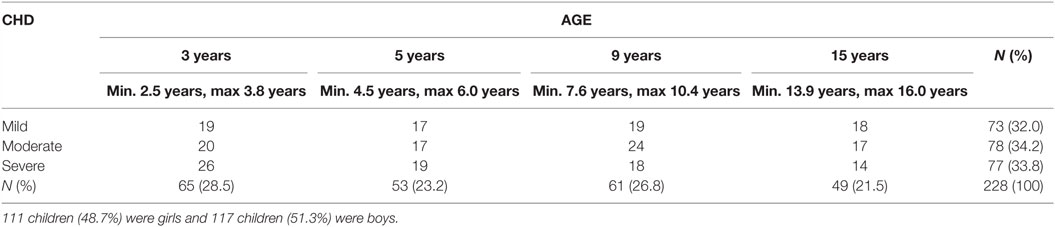
Table 1. Participants grouped by the severity of their cardiac diagnosis and the targeted age for assessment.
Intellectual Assessment
The Swedish versions of the Wechsler Scales of Intelligence (31, 32) were used to assess general intellectual functioning. The Wechsler Preschool and Primary Scale of Intelligence – third edition (WPPSI–III Swedish version) was used to test the 3- and 5-year-old children, and the Wechsler Intelligence Scale for Children – fourth edition (WISC–IV Swedish version) was used to test the 9- and 15-year-old children. Only core subtests were used to compute the full scale IQ (FSIQ). Analysis of verbal IQ (VIQ) and performance IQ (PIQ) did not show significant differences, so we report only FSIQ since it is the most robust measure of intellectual functioning. The children assessed with the WPPSI–III in the 2:6–3:11 age band were administered only five subtests: receptive vocabulary, block design, information, object assembly, and picture naming. For the children in the 4:0–7:3 age band, eight subtests were administered: information, vocabulary, word reasoning, block design, matrix reasoning, picture concepts, coding and symbol search. The children assessed with the WISC–IV, designed for children of 6:0–16:11 age, were assessed using 10 core tests: Similarities, vocabulary, comprehension, block design, matrix reasoning, digit span, coding and symbol search. The Swedish versions of the WPPSI–III and the WISC–IV are based on UK standardization data. Data have shown that previous British and Swedish versions of the Wechsler scales are comparable (31) as both measure similar constructs and their norms are highly consistent (33).
The Wechsler scales of IQ are based on the normal distribution curve with a mean score of 100 (SD 15); 68% of children in a population are expected to have an IQ score between 85 (−1 SD below the mean) and 115 (+1 SD above the mean), 14% are expected to have an IQ score between 116 and 130 (+2 SD), 14% are expected to have an IQ score between 84 and 70 (−2 SD), 2% are expected to have an IQ score between 131 and 145 (+3 SD), and 2% are expected to have an IQ score between 69 and 55 (−3 SD).
Cardiac Diagnosis and Severity
The children in the study had a wide range of cardiac diagnoses and were divided into three groups. Recruitment of children with moderate and mild CHD was done with the aim of having approximately equal numbers of children as in the severe group (see Table 1 for distribution of these groups). The three severity groups were based on the risk the child had for further complications and their cardiac diagnosis: (1) the mild group – children usually treated once with surgery or by catheter intervention with little risk for further complications and who were, in most cases, no longer followed up, e.g., atrial septal defect, ventricular septal defect, persistent ductus arteriosus, isolated coarctation of the aorta, and pulmonary stenosis; (2) the moderate group – children who had been treated with surgery and/or catheter intervention and were followed up regularly since there were risks for further complications, e.g., transposition of the great arteries, tetralogy of Fallot, complete AV defect, total anomalous pulmonary venous drainage, and aortic stenosis; and (3) the severe group – children with complex heart defects for whom long-term prognosis was uncertain and serious complications were not uncommon, e.g., univentricular heart lesions, pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals, and heart transplantation.
Age
Testing was performed between 2008 and 2015. Families of children who met the inclusion criteria were invited when children were at the right age for testing. Children were tested as toddlers (3 years), pre-schoolers (5 years), school aged (9 years), or teenagers (15 years) (±6 months from their birthday), recruitment of patients from the moderate and mild CHD-group was done purposefully to get equal number of children in each age group to match the severe group. Ages for data gathering were chosen to the match the follow-up program for children with CHD at Queen Silvia Children’s Hospital in Gothenburg.
Socioeconomic Status
The Hollingshead “Four Factor Index of Social Status” was used to determine socioeconomic status index (SES) for each family. This index weighs education, occupation, and employment status to determine a composite score of social status.1 From our sample of 228 children, 366 parents (202 mothers and 164 fathers) answered the questionnaire. In 17 families, none of the parents provided data on SES and for 154 families, both parents answered the questionnaire. When both parents provided data, we followed the Hollingshead manual’s requirements and divided the sum of their scores by two to create a SES index for the family (see text footnote 1). Based on a Z-transformation, three SES groups were formed: low (−1 SD), medium (±1 SD), and high (+1 SD). The scores on the index ranged from 3 to 66; the mean score for our sample was 43.6 (SD = 12.5), indicating that, on average, our patients came from medium to high SES families. Previous studies have shown an average SES of 37.0 (SD = 11.7) in the Swedish population (13).
Statistical Analysis
SPSS 20.0 was used for the statistical analyses. The dependent variable – FSIQ – was normally distributed and therefore parametric tests were used. For between-group comparisons of intellectual functioning (FSIQ) in relation to age (3-, 5-, 9-, and 15-year olds), severity levels (mild, moderate, and severe) and SES (low, medium, and high), we used analysis of variance (ANOVA) with eta-square to analyze effect size, interpreting 0.02 as small, 0.13 as medium, and 0.26 as large effect size (34). For the post hoc tests, Bonferroni was used. All variables were check for normality. For non-parametric statistics, we used cross tabs and chi-square test (p ≤ 0.05 statistically significant).
Results
Intellectual Functioning
For the 228 children, the total mean score for FSIQ was 100.8 (SD = 14.5), displayed in Figures 3–6 with a horizontal line. No significant differences were found between boys and girls. Figure 2 shows the distribution of children performing within the normal range and ±1 and ±2 SD compared to norms.
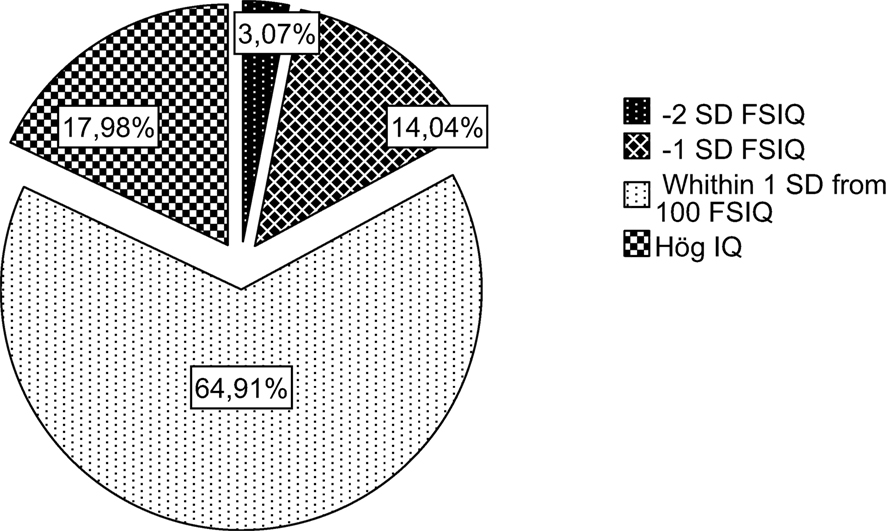
Figure 2. Proportion of children performing in the normal range (±1 SD) and over (+1 and SD) and under (−1 and −2 SD).
In the following sections, FSIQ will be presented in relation to severity of diagnosis, age, and SES. We begin each section by presenting FSIQ as a continuous variable, followed by presenting FSIQ as a categorical variable [i.e., the proportion of children performing within the normal range, ±1 SD, +1 SD (no child over +2 SD), and −1 and −2 SD in relation to severity of diagnosis, age, and SES]. See Table 2 for descriptive data.
Intellectual Functioning in Relation to Severity of Diagnosis
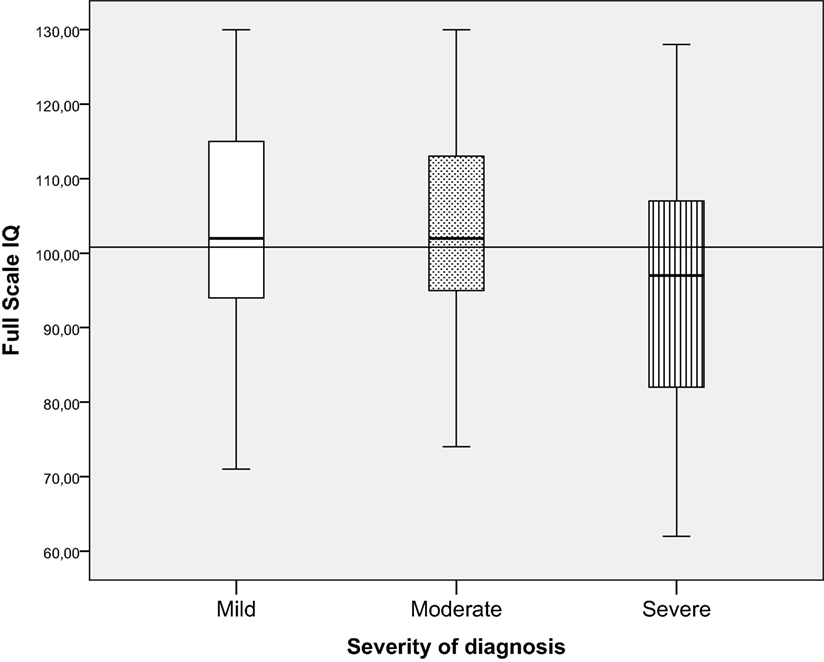
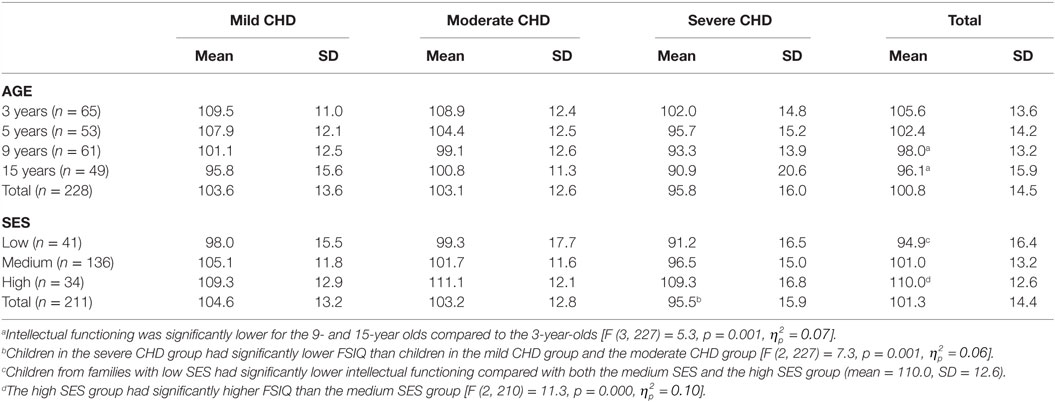
Children in the severe CHD group had significantly lower FSIQ (mean = 95.8, SD = 16.0) than children in the mild CHD group (mean = 103.6, SD = 13.6) and the moderate CHD group (mean = 103.2, SD = 12.6) [F (2, 227) = 7.3, p = 0.001, ], see Figure 3. The effect size was small for distribution of children from the different severity groups performing within the normal range and ±1–2 SD. More children with severe CHD had FSIQ below −1 SD (32.5%) than children with milder forms of CHD (9.3%) [λ2 (df 6) = 23.7 p < 0.001]. Table 3.
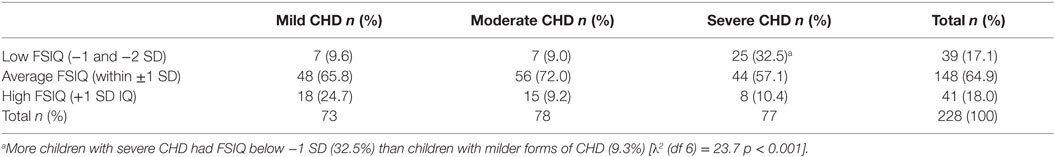
Table 3. Proportion of children with low, average, or high intellectual functioning in relation to severity of diagnosis.
Intellectual Functioning in Relation to Age
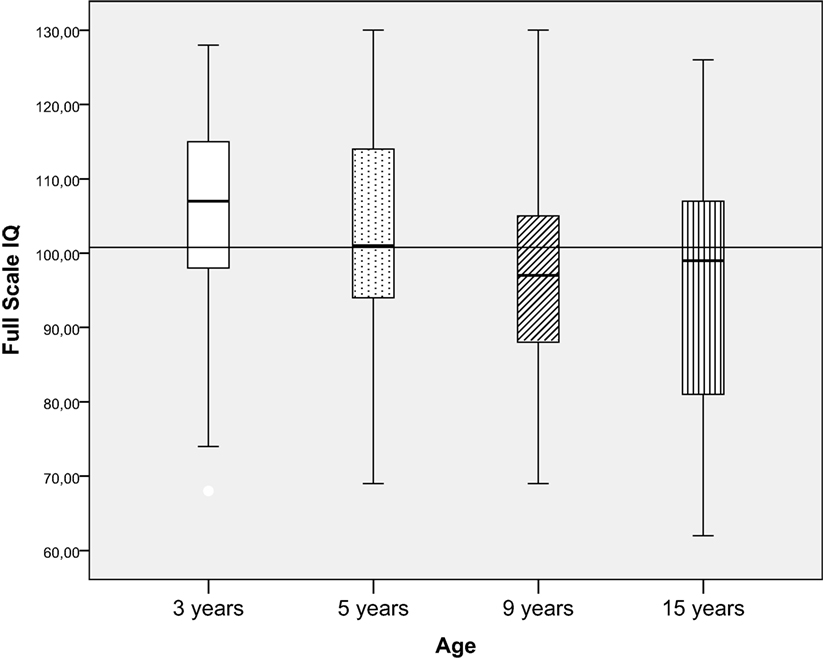
Intellectual functioning was significantly lower for the 9-year olds (mean = 98.0, SD = 13.1) and 15-year olds (mean = 96.1, SD = 15.9) compared to the 3-year olds (mean = 105.6, SD = 13.6) [F (3, 227) = 5.3, p = 0.001, ], and the effect size was small. However, when children were divided into categorical IQ-groups (low, average, and high intellectual functioning), there were no significant differences in the proportion of children in the groups in relation to age (Figure 4; Table 2).
Intellectual Functioning in Relation to SES
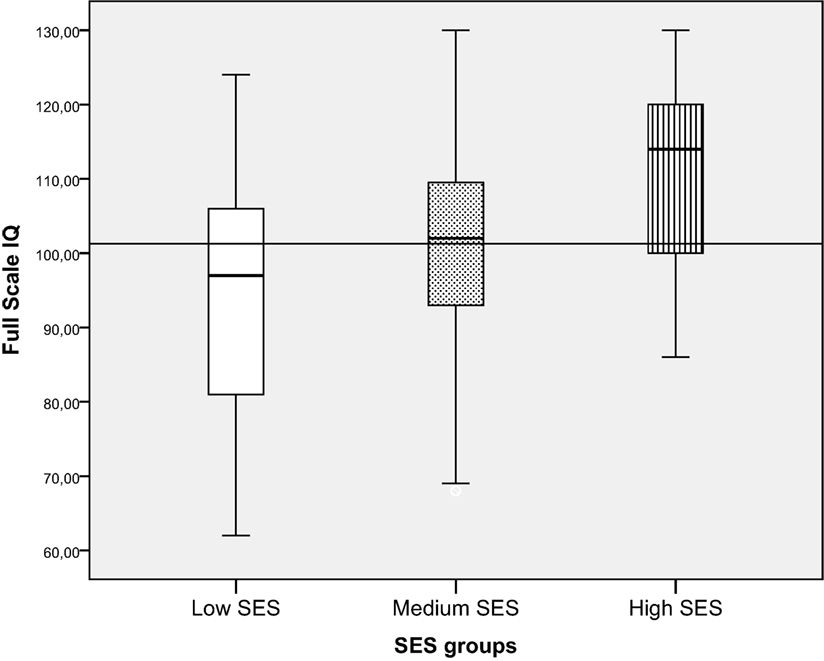
Children from families with low SES had significantly lower intellectual functioning (mean = 94.9, SD = 16.4) compared with both the medium SES (mean = 101.0, SD = 13.2) and the high SES group (mean = 110.0, SD = 12.6). The high SES group also had significantly higher FSIQ than the medium SES group [F (2, 210) = 11.3, p = 0.000, ]. The effect size was of medium size. When children were divided into categorical IQ-groups (low, average, and high intellectual functioning), a higher proportion of children from families with low SES had FSIQ below −1 SD (34.1%) compared to children from families with average (14.7%) or high SES (2.9%) [λ2 (df 6) = 33.0 p < 0.01] (Figure 5; Table 4).

Table 4. Proportion of children with low, average, or high intellectual functioning in relation to SES.
Interaction Effects
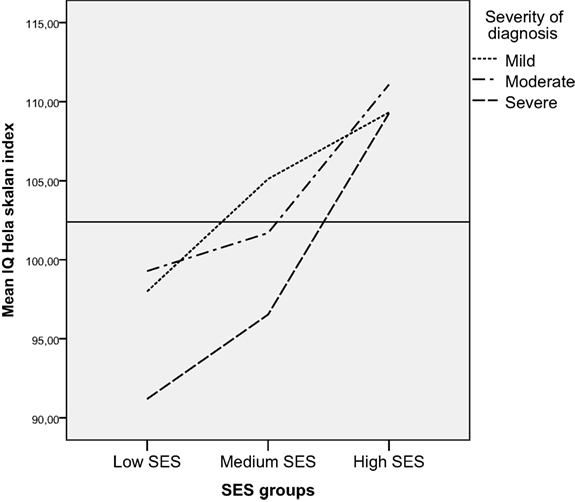
We found no interaction effect between severity of diagnosis and SES for FSIQ. Using ANOVA to control for the effect of SES on FSIQ, we found that the effect size for severity of diagnosis on FSIQ decreased from.06 to.05 after controlling for SES, but the differences in FSIQ between the severity groups remained significant. Both SES and severity of CHD diagnosis had significant main effects on FSIQ. When children were divided into categorical IQ-groups (low, average, and high intellectual functioning), a larger proportion of children both diagnosed with severe CHD and living in families with low SES performed in the low range of intellectual functioning more often than children diagnosed with severe CHD living in families with medium and high SES (Figure 6) [Pearson chi-square (df 6) = 25.6, p < 0.01]. Among those children having low SES and a severe CHD 8 of 20 (40%) had low IQ (−1 or 2 SD); those having medium SES and severe CHD 13 of 43 (30%) had low IQ, and those with high SES severe CHD, 1 (of 4) had low IQ.
Discussion
In our study of 228 Swedish children with CHD treated with surgery or by catheter technique, the mean score on FSIQ in Wechsler Scales of Intelligence was 100.8 (SD = 14.5). This finding indicates that children with CHD treated with surgery or by catheter interventions as a group performed within the normal range on overall intellectual functioning, a result that contradicts some early studies such as the ones presented in a literature review by Amianto, et al. (8). In this literature review, some studies reported that CHD in children was related to lower intellectual functioning; however, other studies found normal performance in these children not only with respect to general intelligence but also with respect to academic results, learning abilities, and visuospatial abilities (8).
Although 65% of children in the present study performed within the normal range, 17% had scores −1 or −2 SD below the mean, and 18% had scores +1 SD above the mean. When comparing subgroups of children with CHD, some children are clearly more at risk than others in terms of intellectual functioning. As hypothesized, intellectual functioning in children with CHD was related to severity of diagnosis, age, and SES.
In this study, we created similarly sized diagnosis groups with diverse severity levels to avoid focus on specific heart defects, a limitation of many other studies (17, 24–26). The results show that children with severe CHD had significantly lower intellectual functioning than children with mild or moderate CHD. This finding agrees with studies reporting that children with milder forms of CHD, such as ventricular septal defect, present lower incidence of neurodevelopmental problems than children with more severe forms of CHD (6, 35). The fact that children with more severe forms of CHD present higher risk for neurodevelopmental problems suggests that cognitive problems could be related to intraoperative factors and to the surgery procedures themselves (36). Although some studies have evaluated the risks that specific surgery techniques confer on children’s intellectual functioning, no clear relation has been proven (29). However, genetic comorbidities and neurological status before surgery are shown to be significant (37). Brain development during fetal and early postnatal life has shown to be influenced by environmental conditions such as maternal stress, and this psychosocial strain in turn influences intellectual functioning (38, 39). A study measuring brain size in infants with CHD found that although brain size in these children was smaller than in healthy term infants, cerebral grow rates were comparable with the cerebral growth rates of the controls (4), and there were no significant differences in neurodevelopmental outcomes in pre-term-born infants with CHD compared to term-born infants (40, 41). Studies have highlighted the long-term effects of preoperative status (42, 43) and of the surgical procedures, which are determined by the severity of the heart defect (1, 2, 23). In the present study, the SD in the severe group was shown to be wider than in the mild and moderate groups, i.e., the difference in intellectual functioning between the lowest and highest performance in the severe group was larger than in the mild or moderate groups. This finding has also been observed in previous studies (25, 44, 45).
Because using unrestricted or very restricted age groups is a well-known problem in many previous studies (27–29), we created specific comparable age groups. Results showed that children in the older groups (9- and 15-year olds) had significantly lower intellectual functioning compared to the 3-year olds. Three-year olds were assessed with WPPSI–III, and the 9- and 15-year olds were assessed with WISC–IV, a choice that probably influenced the results. Although good correlations have been established between these instruments, the WPPSI–III produces slightly higher scores than the WISC–IV. In addition, as children become older and progress through school, the demands on their intellectual functioning increase. Even though intellectual functioning is one of the best predictors of school performance, there are specific cognitive functions that influence learning (e.g., attention and memory). These specific cognitive factors are not targeted in the FSIQ of the Wechsler scales. Deficits in specific cognitive factors were shown in one study of children with CHD tested at age 5 and 10 years; despite stable intelligence scores, the risk for cognitive deficits increased with age (42). Difficulties with specific cognitive functions – e.g., attention, working memory, and processing speed – may not impact general performance in a test situation such as the Wechsler scales of intelligence but may become evident in everyday situations as demands in school increase with age. The clinical experience is that children with CHD more often require special education and learning interventions when they are older. Future research should target specific cognitive domains such as attention, working memory, and executive function in children with CHD in relation to FSIQ and school performance.
Our results showed a significant relation between SES and intellectual functioning in children with CHD. Children from families with low SES had significantly lower intellectual functioning compared with both the medium SES and the high SES groups. This goes in line with studies carried out in healthy populations in which intellectual functioning is believed to be determined by socioeconomic status (21, 46). Results in our study showed that the high SES group also had significantly higher FSIQ compared to the medium SES group. Larger proportion of the children from families with low SES had FSIQ below −1 SD compared to children from families with average or high SES. Yet, it is important to interpret these results cautiously since our sample had very small subgroups. This finding, however, corresponds with previous studies showing that parental education (28), environmental processes (15, 47), and parental stress (48) are related to intellectual functioning in children with CHD (14). Furthermore, studies reporting results of interventions aimed to promote intellectual functioning, development, emotional adjustment, and resilience in children with CHD have shown that reduced levels of anxiety in mothers, good mental health in parents, and good family functioning are significant advantages not only for intellectual functioning (and fewer missed school days) but also for self-perceived health (49, 50).
In the ANOVA, no interaction effect was found between severity of diagnosis, age, and SES, probably because of the relatively small sample size. However, when using non-parametric test, children simultaneously exposed to severe CHD diagnosis and to low SES were found to more often perform in the low range of intellectual functioning. This finding also corresponds with previous studies showing that children with severe heart defects and lower SES are at greater risk for problems related to intellectual functioning (14, 51).
In sum, children with CHD as a group performed well on FSIQ, although we identified severity of the heart diagnosis and SES as factors related to increased risk for lower FSIQ in children with CHD. We believe that providing parents with specific and accurate information on the risks of lower intellectual functioning, supporting schools with psychoeducational advice, and introducing follow-up and intervention programs for families as early as possible are important steps to improving the outcomes for children with CHD. Therefore, children with severe CHD and children from low SES families should be assessed for further interventions and included in follow-up and intervention programs.
Limitations
This study is limited by the absence of a control group. Although believed to be reliable, norm data have limitations since we do not know if the groups are comparable on important background variables. Another limitation of this study is the use of only one dependent variable, FSIQ. Although intellectual functioning is one of the best predictors of school performance, it does not fully capture children’s learning problems or behavioral difficulties. Therefore, we believe that future studies should address specific cognitive functions (e.g., executive function, attention, and memory functions) as often these functions are impaired in children seeking help for school problems in this group. Analysis of these functions together with FSIQ could give a more complete understanding of the problems presented in children with CHD.
The response rate in the severe group was much higher than the response rate in the mild group. We do not have background data on the non-responders, and there is a risk of response-bias. It is common that parents with higher education more often agree to participate in scientific studies compared to parents with lower education. It might also be the case that parents of children with more difficulties at school were more inclined to participate because they wanted a cognitive evaluation of their child and more support from the school. We know that FSIQ in our mild CHD group was normally distributed, and the FSIQ scores in the severe CHD group were skewed with lower values on FSIQ. Looking closer at the severity of diagnosis- and SES-relation, we could see that 23% of children in the mild group had high SES while only 7% of children in the severe group had high SES. FSIQ in the mild group could be systematically higher because parents with high IQ and higher education more often agreed to participate or FSIQ in the mild could be lower than expected because parents who were worried about their child more often agreed to participate. The moderate and severe groups’ participation level was much higher, and the risk for systematic response bias lower, although it is possible that the most fragile families, such as families with very ill children and very low socioeconomic status, did not participate. More studies on larger samples are needed to confirm the results of the present study.
Because this is a cross-sectional study, effects should be interpreted with caution. The differences between age groups could be due to many factors. Factors such as older and less effective surgical techniques used in the older children and the fact that different tests were used in younger and in older children contribute to the uncertainty of the results. The WIPPSI is usually considered to produce higher FSIQ scores than the WISC, which may account for the higher scores among the 3-year olds. Also, cognitive assessment in younger children is more problematic and unstable than in older ones (52). Therefore, longitudinal studies of children with CHD are needed to develop an accurate picture of cognitive development.
Author Contributions
CR has substantially contributed to the design of the work, the acquisition, analysis, and interpretation of the data. JS has substantially contributed to the design of the work, retrieval of patients, classification of severity of diagnosis, and interpretation of the data. MT has substantially contributed to the acquisition, analysis, and interpretation of the data. MB has substantially contributed to the design of the work, analysis, and interpretation of the data. All authors – CR, JS, MT, and MB – have revised the work critically, approved of the final version, and agreed to it.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Contributor: Petter Silfverskiölds Minnesfond, contribution period: 2011–2012. Contributor: Hjärtebarnsfonden, contribution period: 2011–2012. Contributor: Stiftelsen Sven Jerrings Fond, contribution period: 2011–2012. Contributor: T&H Manheime Fond, SEB, contribution period: 2011–2012. Contributor: Stiftelsen Samariten, contribution period: 2011–2012. Contributor: Stiftelsen Radio Hjälpen, contribution period: 2011–2012. Contributor: Drottning Silvias barn och ungdomssjukhus forskningsfond, contribution period: 2011–2012. Contributor: Stiftelsen Emelle fond, contribution period: 2012–2013. Contributor: Stiftelsen Kempe-Carlgrenska Fonden, contribution period: 2012–2013. Contributor: Stiftelsen Sigurd och Elsa Goljes Minne, contribution period: 2012–2013. Contributor: Stiftelsen Drottning Silvias Jubileumsfond, contribution period: 2013–2014. Contributor: Regionala FoU-medel Västra Götalandsregionen (VGFOUREG-307701), contribution period: 2013–2014. Contributor: Regionala FoU-medel Västra Götalandsregionen (VGFOUREG-458741), contribution period: 2015–2016. Contributor: Stiftelsen Solstickan, contribution period: 2015–2016. Contributor: Stiftelsen HjärtLung, Contribution period: 2015–2016. Contributor: Stiftelsen Emelle fond, contribution period: 2016–2017. Contributor: Sahlgrenska Universitetssjukhuset, contribution period: 2011–2016.
Footnote
- ^Adams J, Weakliem DL. August B. Hollingshead’s “four factor index of social status”: from unpublished paper to citation classic. Yale J Sociol (2011) 8:11–20.
References
1. von Rhein M, Dimitropoulos A, Valsangiacomo Buechel ER, Landolt MA, Latal B. Risk factors for neurodevelopmental impairments in school-age children after cardiac surgery with full-flow cardiopulmonary bypass. J Thorac Cardiovasc Surg (2012) 144(3):577–83. doi:10.1016/j.jtcvs.2012.02.005
2. Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young (2006) 16(S1):92–104. doi:10.1017/S1047951105002398
3. Schultz AH, Jarvik GP, Wernovsky G, Bernbaum J, Clancy RR, D’Agostino JA, et al. Effect of congenital heart disease on neurodevelopmental outcomes within multiple-gestation births. J Thorac Cardiovasc Surg (2005) 130(6):1511–6. doi:10.1016/j.jtcvs.2005.07.040
4. Ortinau C, Inder T, Lamberth J, Wallendorf M, Finucane K, Beca J. Congenital heart disease affects cerebral size but not brain growth. Pediatr Cardiol (2012) 33(7):1138–46. doi:10.1007/s00246-012-0269-9
5. Karsdorp PA, Everaerd W, Kindt M, Barbara JM. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol (2007) 32(5):527–41. doi:10.1093/jpepsy/jsl047
6. Sarrechia I, Miatton M, De Wolf D, Francois K, Gewillig M, Meyns B, et al. Neurocognitive development and behaviour in school-aged children after surgery for univentricular or biventricular congenital heart disease. Eur J Cardiothorac Surg (2016) 49(1):167–74. doi:10.1093/ejcts/ezv029
7. Oates RK, Simpson JM, Cartmill TB, Turnbull JAB. Intellectual function and age of repair in cyanotic congenital heart disease. Arch Dis Child (1995) 72(4):298–301. doi:10.1136/adc.72.4.298
8. Amianto F, Bergui G, Abbate-Daga G, Bellicanta A, Munno D, Fassino S. Growing up with a congenital heart disease: neuro-cognitive, psychopathological and quality of life outcomes. Panminerva Med (2011) 53(2):109–27.
9. Camargo-Figuera FA, Barros AJ, Santos IS, Matijasevich A, Barros FC. Early life determinants of low IQ at age 6 in children from the 2004 Pelotas Birth Cohort: a predictive approach. BMC Pediatr (2014) 14:308. doi:10.1186/s12887-014-0308-1
10. Hart CL, Taylor MD, Smith GD, Whalley LJ, Starr JM, Hole DJ, et al. Childhood IQ and all-cause mortality before and after age 65: prospective observational study linking the Scottish Mental Survey 1932 and the Midspan studies. Br J Health Psychol (2005) 10(Pt 2):153–65. doi:10.1348/135910704X14591
11. Julvez J, Guxens M, Carsin AE, Forns J, Mendez M, Turner MC, et al. A cohort study on full breastfeeding and child neuropsychological development: the role of maternal social, psychological, and nutritional factors. Dev Med Child Neurol (2014) 56(2):148–56. doi:10.1111/dmcn.12282
12. Erdem O, Van Lenthe FJ, Prins RG, Voorham TA, Burdorf A. Socioeconomic inequalities in psychological distress among urban adults: the moderating role of neighborhood social cohesion. PLoS One (2016) 11(6):e0157119. doi:10.1371/journal.pone.0157119
13. Olsson MB, Hwang PC. Influence of macrostructure of society on the life situation of families with a child with intellectual disability: Sweden as an example. J Intellect Disabil Res (2003) 47(Pt 4–5):328–41. doi:10.1046/j.1365-2788.2003.00494.x
14. Limbers CA, Emery K, Uzark K. Factors associated with perceived cognitive problems in children and adolescents with congenital heart disease. J Clin Psychol Med Settings (2013) 20(2):192–8. doi:10.1007/s10880-012-9326-z
15. Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics (2015) 135(5):816–25. doi:10.1542/peds.2014-3825
16. Bergvall N, Cnattingius S. Familial (shared environmental and genetic) factors and the foetal origins of cardiovascular diseases and type 2 diabetes: a review of the literature. J Intern Med (2008) 264(3):205–23. doi:10.1111/j.1365-2796.2008.01974.x
17. Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation (2012) 125(17):2081–91. doi:10.1161/CIRCULATIONAHA.111.064113
18. Forbess JM, Visconti KJ, Hancock-Friesen C, Howe RC, Bellinger DC, Jonas RA. Neurodevelopmental outcome after congenital heart surgery: results from an Institutional registry. Circulation (2002) 106(12 Suppl 1):I95–102.
19. Spijkerboer AW, Utens EMWJ, Borgers AJJC, Verhulst FC, Helbing WA. Long-term intellectual functioning and school behavioral outcomes in children and adolescents after invasive treatment for congenital heart disease. Br J Dev Psychol (2008) 26:457–70. doi:10.1348/026151007X253323
20. Bloom AA, Wright JA, Morris RD, Campbell RM, Krawiecki NS. Additive impact of in-hospital cardiac arrest on the functioning of children with heart disease. Pediatrics (1997) 99(3):390–8. doi:10.1542/peds.99.3.390
21. Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci (2003) 14(6):623–8. doi:10.1046/j.0956-7976.2003.psci_1475.x
22. Calderon J, Bonnet D, Courtin C, Concordet S, Plumet MH, Angeard N. Executive function and theory of mind in school-aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Dev Med Child Neurol (2010) 52(12):1139–44. doi:10.1111/j.1469-8749.2010.03735.x
23. Sarajuuri A, Jokinen E, Mildh L, Tujulin AM, Mattila I, Valanne L, et al. Neurodevelopmental burden at age 5 years in patients with univentricular heart. Pediatrics (2012) 130(6):e1636–46. doi:10.1542/peds.2012-0486
24. Andropoulos DB, Easley RB, Brady K, McKenzie ED, Heinle JS, Dickerson HA, et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg (2012) 94(4):1250–5; discussion 5–6. doi:10.1016/j.athoracsur.2012.04.050
25. Bergemann A, Hansen JH, Rotermann I, Voges I, Scheewe J, Otto-Morris C, et al. Neuropsychological performance of school-aged children after staged surgical palliation of hypoplastic left heart syndrome. Eur J Cardiothorac Surg (2015) 47(5):803–11. doi:10.1093/ejcts/ezu299
26. Knirsch W, Liamlahi R, Hug MI, Hoop R, von Rhein M, Prêtre R, et al. Mortality and neurodevelopmental outcome at 1 year of age comparing hybrid and Norwood procedures. Eur J Cardiothorac Surg (2012) 42(1):33–9. doi:10.1093/ejcts/ezr286
27. Mackie AS, Alton GY, Dinu IA, Joffe AR, Roth SJ, Newburger JW, et al. Clinical outcome score predicts the need for neurodevelopmental intervention after infant heart surgery. J Thorac Cardiovasc Surg (2013) 145(5):1248.e–1254.e. doi:10.1016/j.jtcvs.2012.04.029
28. McCusker CG, Doherty NN, Molloy B, Casey F, Rooney N, Mulholland C, et al. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Arch Dis Child (2007) 92(2):137–41. doi:10.1136/adc.2005.092320
29. Pizarro C, Sood ED, Kerins P, Duncan D, Davies RR, Woodford E. Neurodevelopmental outcomes after infant cardiac surgery with circulatory arrest and intermittent perfusion. Ann Thorac Surg (2014) 98(1):119–24. doi:10.1016/j.athoracsur.2014.02.042
30. Sarrechia I, Miatton M, De Wolf D, François K, Vingerhoets G. Neurobehavioural functioning in school-aged children with a corrected septal heart defect. Acta Cardiol (2013) 68(1):23–30. doi:10.2143/AC.68.1.2959628
31. Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. Stockholm: Harcourt Assessment, Inc. (2007).
32. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3rd ed. Stockholm: NCS Pearson Inc. (2005).
33. Wechsler D. WISC-IV. Technical and Interpretative Manual. Stockholm: Harcourt Assessment, Inc. (2003). 187 p.
34. Pierce CA, Block RA, Aguinis H. Cautionary note on reporting Eta-squared values from multifactor ANOVA designs. Educ Psychol Meas (2004) 64(6):916–24. doi:10.1177/0013164404264848
35. Gaynor JW, Gerdes M, Nord AS, Bernbaum J, Zackai E, Wernovsky G, et al. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? J Thorac Cardiovasc Surg (2010) 140(6):1230–7. doi:10.1016/j.jtcvs.2010.07.069
36. Massaro AN, El-Dib M, Glass P, Aly H. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain Dev (2008) 30(7):437–46. doi:10.1016/j.braindev.2007.12.013
37. Knirsch W, Zingg W, Bernet V, Balmer C, Dimitropoulos A, Pretre R, et al. Determinants of body weight gain and association with neurodevelopmental outcome in infants operated for congenital heart disease. Interact Cardiovasc Thorac Surg (2010) 10(3):377–82. doi:10.1510/icvts.2009.216135
38. Nilsson PM, Nilsson JA, Ostergren PO, Rasmussen F. Fetal growth predicts stress susceptibility independent of parental education in 161991 adolescent Swedish male conscripts. J Epidemiol Community Health (2004) 58(7):571–3. doi:10.1136/jech.2003.015495
39. Grizenko N, Fortier MÈ, Gaudreau-Simard M, Jolicoeur C, Joober R. The Effect of maternal stress during pregnancy on IQ and ADHD symptomatology. J Can Acad Child Adolesc Psychiatry (2015) 24(2):92–9.
40. Ortinau C, Inder T, Lambeth J, Wallendorf M, Finucane K, Beca J. Congenital heart disease affects cerebral size but not brain growth. Pediatr Cardiol (2012) 33(7):1138–46. doi:10.1007/s00246-012-0269-9
41. von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain (2014) 137(Pt 1):268–76. doi:10.1093/brain/awt322
42. Heinrichs AK, Holschen A, Krings T, Messmer BJ, Schnitker R, Minkenberg R, et al. Neurologic and psycho-intellectual outcome related to structural brain imaging in adolescents and young adults after neonatal arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg (2014) 148(5):2190–9. doi:10.1016/j.jtcvs.2013.10.087
43. Majnemer A, Limperopoulos C, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C. A new look at outcomes of infants with congenital heart disease. Pediatr Neurol (2009) 40(3):197–204. doi:10.1016/j.pediatrneurol.2008.09.014
44. Bellinger DC, Newburger JW, Wypij D, Kuban KC, duPlesssis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: the Boston Circulatory Arrest Trial. Cardiol Young (2009) 19(1):86–97. doi:10.1017/S1047951108003454
45. von Rhein M, Kugler J, Liamlahi R, Knirsch W, Latal B, Kaufmann L. Persistence of visuo-constructional and executive deficits in adolescents after open-heart surgery. Res Dev Disabil (2015) 36:303–10. doi:10.1016/j.ridd.2014.10.027
46. Buckingham J. Why poor children are more likely to become poor readers: the school years. Aust J Educ (2013) 57(3):190–213. doi:10.1177/0004944113495500
47. Alton GY, Taghados S, Joffe AR, Robertson CM, Dinu I; Western Canadian Pediatric Therapies Follow-Up Group. Prediction of preschool functional abilities after early complex cardiac surgery. Cardiol Young (2015) 25(4):655–62. doi:10.1017/S1047951114000535
48. Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Developmental and functional outcomes at school entry in children with congenital heart defects. J Pediatr (2008) 153(1):55–60. doi:10.1016/j.jpeds.2007.12.019
49. McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A controlled trial of early interventions to promote maternal adjustments and development in infants born with severe congenital heart disease. Child Care Health Dev (2009) 36(1):110–7. doi:10.1111/j.1365-2214.2009.01026.x
50. McCusker CG, Doherty NN, Molloy B, Casey F, Rooney N, Mulholland C, et al. A randomized controlled trial of interventions to promote adjustment in children with congenital heart disease. J Pediatr Psychol (2012) 37(10):1089–103. doi:10.1093/jpepsy/jss092
51. Sarrechia I, De Wolf D, Miatton M, Francois K, Gewillig M, Meyns B, et al. Neurodevelopment and behavior after transcatheter versus surgical closure of secundum type atrial septal defect. J Pediatr (2015) 166(1):31–8. doi:10.1016/j.jpeds.2014.08.039
Keywords: intellectual functioning, neurodevelopment, congenital heart defects, cardiac treatment by surgery or by catheter interventions
Citation: Ryberg C, Sunnegårdh J, Thorson M and Broberg M (2016) Intellectual Functioning in Children with Congenital Heart Defects Treated with Surgery or by Catheter Interventions. Front. Pediatr. 4:113. doi: 10.3389/fped.2016.00113
Received: 09 July 2016; Accepted: 03 October 2016;
Published: 17 November 2016
Edited by:
Antonio Francesco Corno, Glenfield Hospital, UKReviewed by:
Karolijn Dulfer, Erasmus MC – Sophia, NetherlandsKatya Els De Groote, Ghent University Hospital, Belgium
Copyright: © 2016 Ryberg, Sunnegårdh, Thorson and Broberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmen Ryberg, carmen.ryberg@vgregion.se
 Carmen Ryberg
Carmen Ryberg Jan Sunnegårdh
Jan Sunnegårdh Maria Thorson
Maria Thorson Malin Broberg
Malin Broberg