Efficacy of Paracetamol in Closure of Ductus Arteriosus in Infants under 32 Weeks of Gestation
- 1Hospital Reina Sofía de Córdoba, Cordova, Spain
- 2Instituto Maimonides de Investigación Biomédica de Cordoba (IMIBIC), Cordova, Spain
- 3Pediatrics, Hospital Alto Guadalquivir, Andújar, Spain
Background: Standard medical treatment for patent ductus arteriosus (PDA) closure has been indomethacin/ibuprofen or surgical ligation. Up to date, new strategies have been reported with paracetamol. The aim of this study was to present our experience with intravenous paracetamol for closing PDA in preterm neonates presenting contraindication to ibuprofen or ibuprofen had failed and no candidates for surgical ligation because of huge instability.
Materials and methods: We conducted a retrospective case series study in a neonatal intensive care unit from a tertiary hospital. 9 preterm infants ≤32 weeks of gestational age with hemodynamically significant PDA (hsPDA) were enrolled. They received 15 mg/kg/6h intravenous paracetamol for ductal closure. Demographic data and transaminase levels before and after treatment were collected.
Results: 30 preterm babies were diagnosed of hsPDA. 11/30 received ibuprofen with closure in 81.1%. 9 received intravenous paracetamol mainly due to bleeding disorders or thrombocytopenia. Successful closure on paracetamol was achieved in seven of nine babies (77.7%). There was a significant increase in transaminase levels in two patients. They required no treatment for normalization.
Conclusion: Paracetamol is an effective option in closure PDA. It should be a first-line therapeutic option when there are contraindications for ibuprofen treatment. Transaminases must be checked during treatment.
Introduction
Closure of ductus arteriosus after birth is very important for circulation adaptation to the extrauterine life. Patent ductus arteriosus (PDA) in extremely premature infants is associated with morbidities such as necrotizing enterocolitis, bronchopulmonary dysplasia (BPD), and neurodevelopmental disabilities (1). Standard medical treatment for PDA closure has been indometacin/ibuprofen or surgical ligation. Adverse events have been reported with NSAIDs (2), and surgical ligations have been associated with a higher incidence of BPD, retinopathy of prematurity, and neurodevelopmental disorders (3).
Hammerman et al. reported for the first time the use of paracetamol for closing PDA (4). Since then, many studies have reported similar efficiency of paracetamol to COX-inhibitors for closing PDA and less adverse events (5).
The aim of this study was to present our experience with intravenous (iv) paracetamol for closing PDA in preterm neonates presenting contraindication to ibuprofen or ibuprofen had failed and had feeding intolerance.
Materials and Methods
We conducted a retrospective case series study of 30 preterm infants of ≤32 weeks of gestational age (GA) with hemodynamically significant PDA (hsPDA) from May 2015 to January 2017. The medical records were retrospectively evaluated. We collected the percentage of spontaneous closure, the percentage of patients who received ibuprofen versus paracetamol or surgical ligation. Ibuprofen was given intravenously at a regimen of 10, 5, and 5 mg/kg/day [for 3 days, respectively. Pedea (orphan drug) 5 mg/ml]. Nine premature infants received paracetamol (Paracetamol B. Braun 10 mg/ml), 15 mg/kg iv administration every 6 h. All had hsPDA clinically diagnosed and confirmed by means of echo-Doppler cardiography.
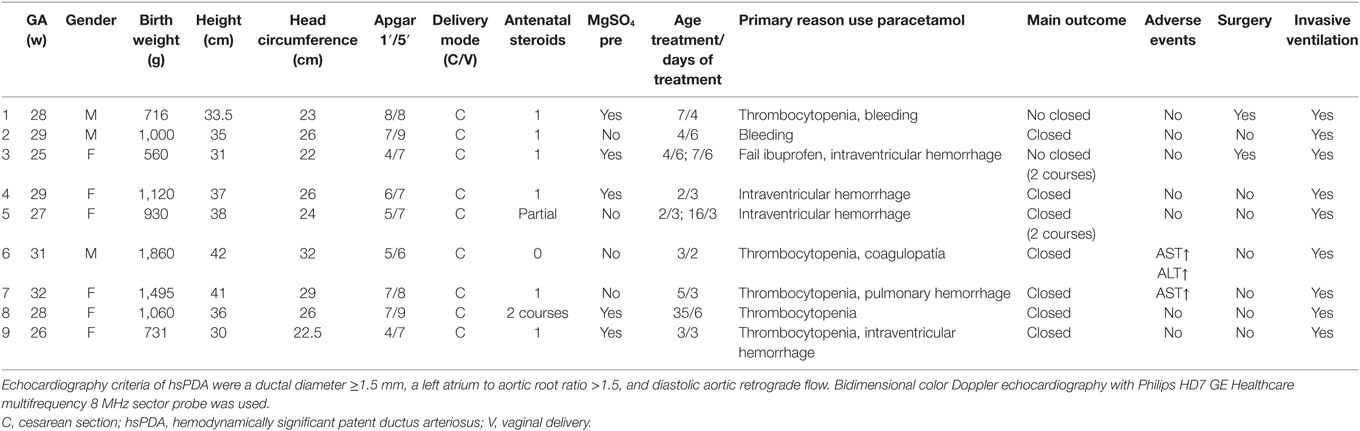
Echocardiography criteria of hsPDA were a ductal diameter ≥1.5 mm, a left atrium to aortic root ratio >1.5, and diastolic aortic retrograde flow. Bidimensional color Doppler echocardiography with Philips HD7 GE Healthcare multifrequency 8 MHz sector probe was used. Daily echocardiographic examination was conducted. If ductus closure was confirmed by echocardiography, treatment was discontinued. The study was carried out in accordance with the recommendations of RSUH Ethics and Research Committee.
Primary reason for using paracetamol was failure to response to ibuprofen administration or the presence of absolute contraindications for ibuprofen (bleeding, platelet count ≤ 60,000, intraventricular hemorrhage, and pulmonary hemorrhage).
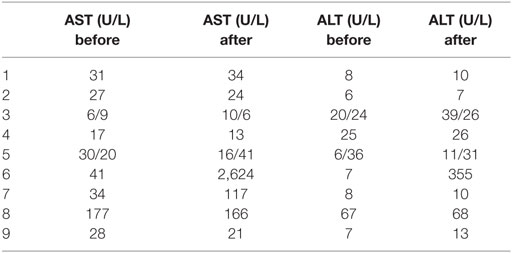
Demographic features (GA, gender, birth weight, height, head circumference, Apgar score, delivery mode, antenatal steroids, MgSO4, age treatment/days of treatment, primary reason to use paracetamol, main outcome, adverse events, surgery, and invasive ventilation), antenatal exposure to steroids and magnesium sulfate, postnatal age at diagnosis, age at first paracetamol dose, duration of treatment, response to treatment, and need of surgical ligation were noted. Before and 24 h after the end of paracetamol treatment, liver function tests were performed in all patients. In all cases, a written informed consent was obtained.
Results
Between May 2015 and January 2017, there were a total of 30 preterm infants who had significant PDA. 11/30 received ibuprofen, and 3/30 had a spontaneous closure (10%) with GA 28, 28, and 29 weeks. 7 died (4 under ibuprofen treatment, two received no treatment, and one underwent PDA ligation), and nine patients received iv paracetamol (5 of them of ≤28 weeks of GA). Results among the nine patients who underwent paracetamol were as follows: mean GA was 28 weeks ranging from 25 to 32 weeks and mean birth weight was 1,052 g ranging from 560 to 1,860 g. 3 preterm were male. All the patients had received antenatal steroids, and five of them have been exposure to antenatal magnesium sulfate as neuroprotection. Table 1 describes main clinical findings among infants who received paracetamol.
In all patients, due to feeding intolerance and clinic instability, iv paracetamol was started after obtaining informed consent signature. Complete closure was observed in 7/9 (77.7%). The mean postnatal age at the first iv paracetamol dose was 4 days, ranging from 2 to 35 days. In eight of nine patients, the treatment was started in the first week of life. In eight infants, ibuprofen was contraindicated, and in one of them, the ibuprofen treatment had failed. One patient was treated for 2 days due to an elevation in liver transaminases, but ductus was closed so treatment was discontinued. Values were normalized 5 days later. Three patients were treated for 3 days, one for 4 days, and 2 for 6 days. Two patients needed two courses; one of them received acetaminophen for 3 days. In that case, ductus persisted opened even though ibuprofen was administered in a second course because there were no contraindications. Finally, baby went to surgical ligation on 16th day of life. One patient was successfully treated on the third day of life, but on the 16th day of life, ductus was reopened and a second course was conducted with definitively ductal closure after 48 h.
Table 2 shows transaminases levels before and after treatment with paracetamol.
Discussion
Hammerman et al. reported for the first time several case reports on premature infants who received paracetamol achieving ductal closure (4). Since then, 24 case reports series have been reported and 6 randomized control trials (RCTs) showing paracetamol utility for ductal closure with similar results comparing to ibuprofen/indomethacin and fewer adverse events.
Prostaglandins are relevant in PDA. Indomethacin and ibuprofen inhibit cyclooxygenase (COX3) in a no selective manner. How paracetamol acts for closing PDA still remains unclear, but it is known that it inhibits prostaglandin synthetase (5). Alternatively, paracetamol has been proposed to selectively inhibit a central isoform of COX3, but the existence of a functional human COX3 has been questioned (6).
Oncel et al. used paracetamol in 10 premature infants under than 30 weeks of GA with a 100% of effectiveness. Nevertheless, other authors did not achieve same striking results (7–9). The most common dosage is 15 mg/k/dose/q6h.
A report on high level of transaminases caused by iv paracetamol treatment for PDA closure in premature infants found that a lower dose of paracetamol also is effective (10, 11). Therefore, the dose and dose interval of iv paracetamol treatment might require revision.
One of our patient received paracetamol on day 35 of life and ductus was closed, even though the most studies state that the earliest beginning of treatment is the most effective. Some studies reported up to a 71.6% of ductal closure when it is administered after 20 days of life (11–17).
Among 13 observational studies published, paracetamol was orally given (18–22) (112 premature infants), and in 12 observational studies, paracetamol was given iv (150 premature infants) with a closing average up to a 72.2% in oral route versus 66% with iv paracetamol. Of the six RCTs, just one compared indomethacin and ibuprofen (9, 23–25) with no statistical significant differences. We have reported a higher average ductal closure probably because paracetamol was given earlier, in the first week of life. Only one patient had received ibuprofen first. Other observational studies show that paracetamol is more effective when there has been no exposure to ibuprofen and less effective when it is administered later (23, 26). In fact, one of our patients with no ductal closure after paracetamol who underwent surgical ligation had received ibuprofen first. Average of spontaneous ductal closure is higher at higher GAs. The small sample size is a limitation for our study, and four of nine patients who received paracetamol were of ≥29 weeks of GA. Nonetheless, the median GA among our 30 premature infants with hsPDA and spontaneous closure was 28 weeks, so we truly believe that the spontaneous closure among our population cannot be due to higher GAs.
The single adverse event we noticed was a transient elevation in liver enzymes in two patients as previously has been reported in literature, and they required no treatment.
Our patients had oral feeding intolerance, so we use iv route. In our opinion, the oral route probably does not represent the optimal choice for ELBW infants. In these patients, gut immaturity together with oral feeding intolerance typical of ELBW can lead to unpredictable and possibly too low intestinal drug absorption.
Since 2012, MgSO4 (magnesium sulfate) have been used as a neuroprotector agent among premature infants under 31 + 6 weeks of GA. 5 out of nine premature infants received MgSO4 as neuroprotection. del Moral et al. (27) related prenatal exposure to MgSO4 with higher incidence of hemodynamically significant persistent ductus arteriosus. Functional closure of the ductus after birth is primarily due to smooth muscle constriction, owing an increase in intracellular calcium concentration (27). Magnesium acts as a calcium antagonist, blocking calcium ion entry into the smooth muscles. More studies are probably needed to investigate the relationship between prenatal magnesium sulfate and PDA in premature infants. Some epidemiological studies suggest a link between early exposure to paracetamol and risk of asthma and other atopic diseases (28). Moreover, one study among 64,322 infants whose mother received acetaminophen during pregnancy reported a higher incidence of attention deficit syndrome and hyperactivity (29).
Conclusion
Our results highlight that paracetamol could become not only an alternative treatment in closing PDA but also the treatment of choice in several scenarios. Nevertheless, one of the main limitations of this study is that it is a case series report with fewer subjects. More studies are needed to know long-term consequences of using paracetamol for closing PDA and to answer important questions about the optimal dose, the best route of administration, safety and the implications for neurodevelopmental, and long-term consequences.
Author Contributions
IT: patient recruitment after informed consent was signed up, review of the charts, review of the literature, and writing of the manuscript. MR: review literature and patient recruitment and made corrections. MC: establish the protocol and writing of the special informed consent for an off-label indication treatment. AP: review of the charts. RR: patient recruitment. MP: corrections.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Allegaert K, Anderson B, Simons S, van Overmeire B. Paracetamol to induce ductus arteriosus closure: is it valid? Arch Dis Child (2013) 98:462–6. doi:10.1136/archdischild-2013-303688
2. Brunner B, Hoeck M, Schermer E, Streif W, Kiechl-Kohlendorfer U. Patent ductus arteriosus low platelets. Cyclooxygenase inhibitors and intraventricular hemorrhage in very low birth weight preterm infants. J Pediatr (2013) 163:23–8. doi:10.1016/j.jpeds.2012.12.035
3. Weisz DE, More K, McNamara PJ, Shah PS. PDA ligation and health outcomes: a meta-analysis. Pediatrics (2014) 133(4):e1024–46. doi:10.1542/peds.2013-3431
4. Hammerman C, Bin-Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D. Ductal clorure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics (2011) 128:e1618–21. doi:10.1542/peds.2011-0359
5. Dani C, Poggi CH, Mosca F, Schena F, Lista G, Ramenghi L, et al. Efficacy and safety of intravenous paracetamol in comparison to ibuprofen for the treatment of patent ductus arteriosus in preterm infants: study protocol for a randomized control trial. Trials (2016) 17:182. doi:10.1186/s13063-016-1294-4
6. El-Khuffash A, Amish J, Corcoran D, Shah P, Hooper CHW, Brown N, et al. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatr Res (2014) 76:238–44. doi:10.1038/pr.2014.82
7. Oncel MY, Yurttutan S, Uras N, Altug N, Ozdemir R, Ekmen S, et al. An alternative drug (paracetamol) in the management of patent ductus arteriosus in ibuprofen-resistant or contraindicated preterm infants. Arch Dis Child Fetal Neonatal Ed (2013) 98:F94. doi:10.1136/archdischild-2012-302044
8. Oncel MY, Yurttutan S, Degirmencioglu H, Uras N, Altug N, Erdeve O, et al. Intravenous paracetamol treatment in the management of patent ductus arteriosus in extremely low birthweight infants. Neonatology (2013) 103:166–9. doi:10.1159/000345337
9. Roofthooft DW, van Beynum IM, Helbing WA, ReissI K, Simons SH. Paracetamol for ductus arteriosus closure: not always a success story. Neonatology (2013) 104:170. doi:10.1159/000353451
10. Tekgündüz KS, Ceviz N, Caner I, Olgun H, Demirelli Y, Yolcu C, et al. Intravenous paracetamol with a lower dose is also effective for the treatment of patent ductus arteriosus in pretermin fants. Cardiol Young (2015) 25(6):1060–4. doi:10.1017/S1047951114001577
11. Sinha R, Negi V, Dalal SS. An interesting observation of PDA closure with oral paracetamol in preterm neonates. J Clin Neonatol (2013) 2:30–2. doi:10.4103/2249-4847.109245
12. Bardanzellu F, Neroni P, Dessi A, Fanos V. Paracetamol in patent ductus arteriosus treatment: efficacious and safe? Biomed Res Int (2017) 2017:1438038. doi:10.1155/2017/1438038
13. Kessel I, Waisman D, Lavie-Nevo K, Golzman M, Lorber A, Rotschild A. Paracetamol effectiveness, safety and blood level monitoring during patent ductus arteriosus closure: a case series. J Matern Fetal Neonatal Med (2014) 27:1719–21. doi:10.3109/14767058.2013.871630
14. Nadir E, Kassem E, Foldi S, Hochberg A, Feldman M. Paracetamol treatment of patent ductus arteriosus in preterm infants. J Perinatol (2014) 34:748–9. doi:10.1038/jp.2014.96
15. Dash SK, Kabra NS, Avasthi BK, Sharma SR, Padhi P, Ahmed J. Enteral paracetamol or intravenous indomethacin for closure of patent ductus arteriosus in preterm neonates: a randomized controlled trial. Indian Pediatr (2015) 52:573–8. doi:10.1007/s13312-015-0677-z
16. Weisz DE, Martins FF, Nield LE, El-Khuffash A, Jain A, McNamara PJ. Acetaminophen to avoid surgical ligation in extremely low gestational age neonates with persistent hemodynamically significant patent ductus arteriosus. J Perinatol (2016) 36:649–53. doi:10.1038/jp.2016.60
17. Ozdemir OM, Dogan M, Kucuktasci K, Ergin H, Sabin O. Paracetamol therapy for patent ductus arteriosus in premature infants: a chance before surgical ligation. Pediatr Cardiol (2014) 35:236–9. doi:10.1007/s00246-013-0770-9
18. Dang D, Wang D, Zhang CH, Zhou W, Zhou O, Wu H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLoS One (2013) 8:e77888. doi:10.1371/journal.pone.0077888
19. Jasani B, Kabra N, Nanavati RN. Oral paracetamol in treatment of closure of patent ductus arteriosus in preterm neonates. J Postgrad Med (2013) 59:312–4. doi:10.4103/0022-3859.123164
20. Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr (2014) 164:510–4.e1. doi:10.1016/j.jpeds.2013.11.008
21. Yang B, Gao X, Ren Y, Wang Y, Zhang Q. Oral paracetamol vs. oral ibuprofen in the treatment of symptomatic patent ductus arteriosus in premature infants: a randomized controlled trial. Exp Ther Med (2016) 12:2531–6. doi:10.3892/etm.2016.3676
22. Bagheri MM, Niknafs P, Sabsevari F, Torabi MH, Bahman Bijari B, Noroozi E, et al. Comparison of oral acetaminophen versus ibuprofen in premature infants with patent ductus arteriosus. Iran J Pediatr (2016) 26:e3975. doi:10.5812/ijp.3975
23. El-Mashad AE, El-Mahdy H, El Amrousy D, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr (2017) 176:233–40. doi:10.1007/s00431-016-2830-7
24. Roofthooft D, van Beynum IM, Klerk JC, van Dijk M, van den Anker JN, Reiss IK, et al. Limited effects of intravenous paracetamol on patent ductus arteriosus in very low birth weight infants with contraindications for ibuprofen or after ibuprofen failure. Eur J Pediatr (2015) 174(11):1433–40. doi:10.1007/s00431-015-2541-5
25. Memisoglu A, Alp Ünkar Z, Cetiner N, Akalın F, Ozdemir H, Bilgen HS, et al. Ductal closure with intravenous paracetamol: a new approach to patent ductus arteriosus treatment. J Matern Fetal Neonatal Med (2016) 29:987–90. doi:10.3109/14767058.2015.1029912
26. Valerio E, Valente MR, Salvadori S, Frigo AC, Baraldi E, Lago P. Intravenous paracetamol for PDA closure in the preterm: a single-center experience. Eur J Pediatr (2016) 175:953–66. doi:10.1007/s00431-016-2731-9
27. del Moral T, Gonzalez Quintero VH, Claure N, Vanbuskirk S, Bancalari E. Antenatal exposure to magnesium sulphate and the incidence of patent ductus arteriosus in extremely low birth weight infants. J Perinatol (2007) 27:154–7. doi:10.1038/sj.jp.7211663
28. Cheelo M, Lodge CJ, Dharmage SC, Simpson JA, Matheson M, Heinrich J, et al. Paracetamol exposure in pregnancy and early childhood and development of childhood asthma: a systematic review and meta-analysis. Arch Dis Child (2015) 100:81–9. doi:10.1136/archdischild-2012-303043
Keywords: patent ductus arteriosus, paracetamol, preterm, treatment, ibuprofen
Citation: Tofe I, Ruiz-González MD, Cañete MD, Pino A, Rueda RL, Parraga MJ and Perez-Navero JL (2018) Efficacy of Paracetamol in Closure of Ductus Arteriosus in Infants under 32 Weeks of Gestation. Front. Pediatr. 6:25. doi: 10.3389/fped.2018.00025
Received: 06 October 2017; Accepted: 25 January 2018;
Published: 14 February 2018
Edited by:
Giovanni Biglino, University of Bristol, United KingdomReviewed by:
Yogen Singh, Cambridge University Hospitals NHS Foundation Trust, United KingdomHopewell Nkosipendule Ntsinjana, University of the Witwatersrand, South Africa
Copyright: © 2018 Tofe, Ruiz-González, Cañete, Pino, Rueda, Parraga and Perez-Navero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Dolores Ruiz-González, maesrugo17@hotmail.com
 Ines Tofe
Ines Tofe Maria Dolores Ruiz-González1*
Maria Dolores Ruiz-González1*
 Maria Dolores Cañete
Maria Dolores Cañete Rosa Lorena Rueda
Rosa Lorena Rueda Maria Jose Parraga
Maria Jose Parraga