A Review of Oxygen Use During Chest Compressions in Newborns—A Meta-Analysis of Animal Data
- 1Faculty of Science, University of Alberta, Edmonton, AB, Canada
- 2Centre for the Studies of Asphyxia and Resuscitation, Neonatal Research Unit, Royal Alexandra Hospital, Edmonton, AB, Canada
- 3Department of Pediatrics, University of Alberta, Edmonton, AB, Canada
- 4Department of Pediatric and Adolescent Medicine, Akershus University Hospital, Lørenskog, Norway
- 5Health Research Centre, University and Polytechnic Hospital La Fe, Valencia, Spain
- 6Division of Neonatology, University and Polytechnic Hospital La Fe, Valencia, Spain
- 7Spanish Maternal and Infant Health and Development Network, National Network, Spain
- 8Department of Pediatric Research, University of Oslo, Oslo, Norway
Background: International consensus statements for resuscitation of newborn infants recommend provision of 100% oxygen once chest compressions are required. However, 100% oxygen exacerbates reperfusion injury and reduces cerebral perfusion in newborn babies.
Objective: We aimed to establish whether resuscitation with air during chest compression is feasible and safe in newborn infants compared with 100% oxygen.
Methods: Systematic search of PubMed, Google Scholar and CINAHL for articles examining variable oxygen concentrations during chest compressions in term newborns.
Results: Overall, no human studies but eight animal studies (n = 323 animals) comparing various oxygen concentrations during chest compression were identified. The pooled analysis showed no difference in mortality rates for animals resuscitated with air vs. 100% oxygen (risk ratio 1.04 [0.35, 3.08], I2 = 0%, p = 0.94). ROSC was also similar between groups with a mean difference of −3.8 [−29.7–22] s, I2 = 0%, p = 0.77. No difference in oxygen damage or adverse events were identified between groups.
Conclusions: Air had similar time to ROSC and mortality as 100% oxygen during neonatal chest compression. A large randomized controlled clinical trial comparing air vs. 100% oxygen during neonatal chest compression is warranted.
Introduction
Approximately, 3% of infants require respiratory assistance at birth and 0.1% require chest compressions (CC) (1, 2). During respiratory support of term and near term newly born infants air (21% oxygen) should be given as trials and meta-analyses reported a significant reduction in mortality in infants resuscitated with air (relative risk 0.71 [95% CI 0.54 to 0.94], risk difference −0.05 [−0.08 to −0.01]) (3, 4), which is also reflected in the neonatal resuscitation guidelines (1, 2). Oxygen (O2) use in the delivery room is associated with potential adverse effects; hyperoxia slows cerebral blood flow, brief periods of 100% O2 causes long-term reductions in cerebral blood flow. High concentrations of O2 lead to generation of oxygen free radicals, which have a role in reperfusion/reoxygenation injury after asphyxia especially to oxyregulator tissues such as myocardium (5–8). Thus, air might be a more appropriate gas than 100% O2.
If starting with air has been unsuccessful, the current resuscitation guidelines suggest to titrate oxygen and increase oxygen to 100% once chest compressions are started (1, 2). However, there is lack of supporting evidence of the beneficial effects of either titrating oxygen or using 100% O2 during cardiopulmonary resuscitation (CPR). The aim of oxygen use during resuscitation is to reactivate mitochondrial activity and energy provision and prevent tissue damage from oxygen deprivation during asphyxia while avoiding adverse effects of oxidative stress on the respiratory system and cerebral circulation; and tissue damage from oxygen free radicals (9).
If air is equally effective as 100% O2 in newborn infants requiring CC, the use of air instead of 100% O2 could reduce morbidity and mortality in asphyxiated infants. The aim of the meta-analysis was to compare the efficacy of air compared to 100% O2 during chest compression in the resuscitation in newborn infants immediately after birth. Further aims included assessment of oxidative stress and inflammatory markers using air vs. 100% O2.
Methods
We searched PubMed, Google Scholar, and CINAHL using the following search terms (last searched on June 6, 2018, Appendix 1): “infant,” “newborn,” “resuscitation,” “chest compression,” “oxygen,” and “delivery room.” Publications were assessed based on title, abstract, and methods. Studies were included if they compared different oxygen concentrations during CC. Studies were excluded when no CC were performed, or if they did not define the infants as newborn. The initial search was aimed to only identify human trials. However, no human trials were identified and therefore the search was expanded to include animal studies. A manual search through the references of the obtained articles was additional performed.
Study Selection
Two reviewers (CGH and GMS) independently reviewed citations for selection. Studies were included in the review if they met the following criteria: randomized controlled trial; comparing use air vs. 100% O2 during neonatal CPR; and presented the outcomes of either death or ROSC. Our primary outcome measure was mortality during neonatal CPR. Secondary outcomes included time to ROSC, oxygenation, and indicators of organ injury or damage. Full articles for potentially relevant studies were retrieved and independently assessed for their eligibility using a standardized data collection form. We also aimed to identify and if available include multiple publications describing the same study. Authors were contacted for data on return of spontaneous circulation (ROSC) as they were only reported as median (IQR) in the respective articles. No language restrictions were applied. Discrepancies regarding inclusion were resolved with another member of the review team (ALS).
Data Extraction
Data were recorded using a standardized data collection form to record study design and methodological characteristics, patient characteristics, interventions, and outcomes thereof, including their RR (95% CI). Data extraction was independently performed by two investigators (GMS, CGH) and discrepancies were resolved in consultation with another member of the review team (ALS).
Statistical Analysis
The principal summary measures were RR (95% CI) for dichotomous outcomes. Heterogeneity was explored using a chi-square test, and the quantity of heterogeneity was measured using the I2 statistic. We summarized RR estimates using random-effects models. Analyses were performed in RevMan version 5.3 (Cochrane Collaboration, 2014). All p-values are 2-tailed.
Results
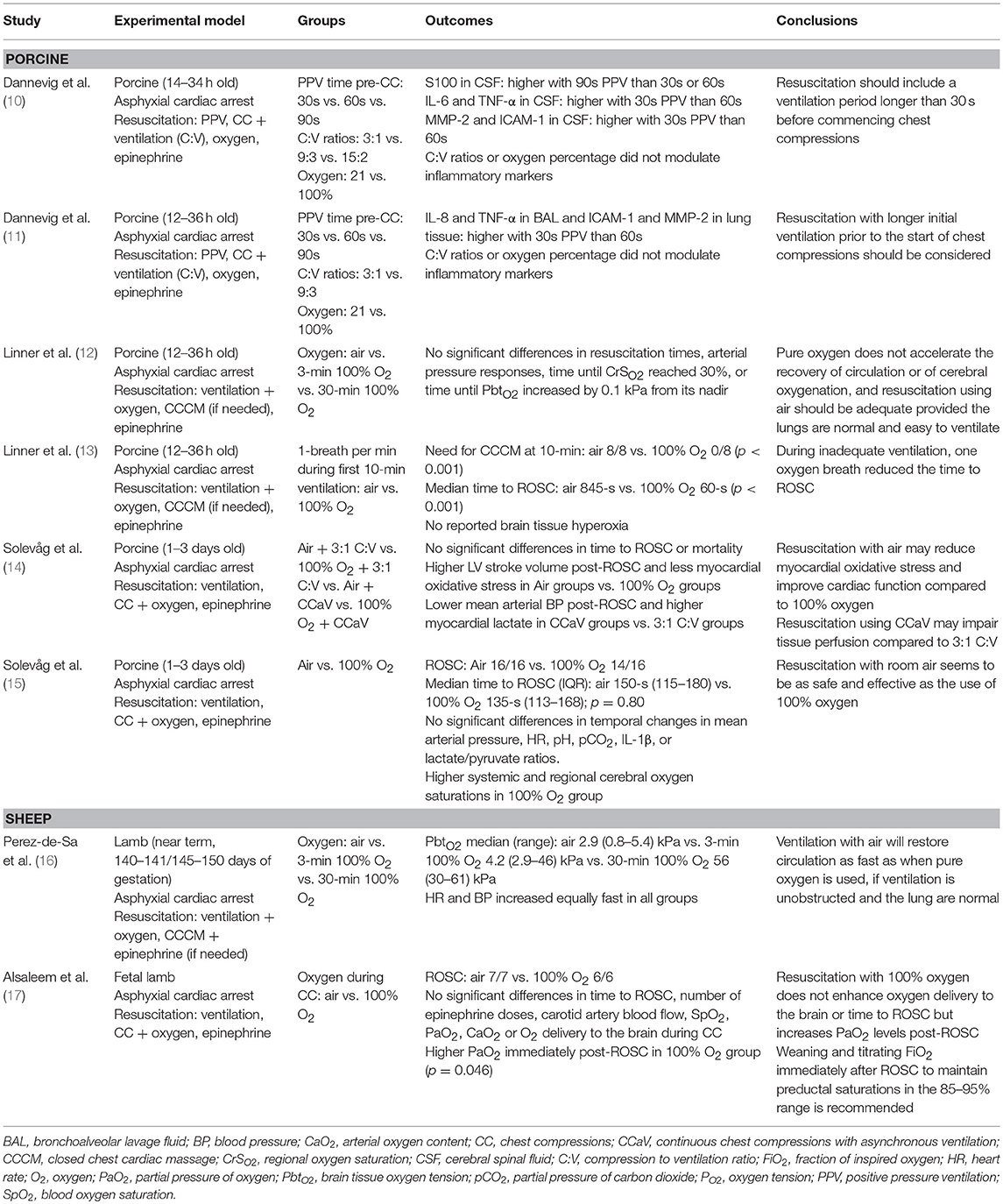
We did not identify any clinical human study on the subject. Therefore, all results come from controlled animal models. Six (75%) studies used a swine model (10–15), and two (25%) an ovine model (16, 17). Included studies assessed mortality (Figure 1), ROSC and/or circulatory recovery, oxygenation, severity of oxygen injury, and adverse events caused by oxygen. Table 1 presents all identified animal studies, intervention groups, and primary outcome, and conclusion (Table 1). Table 2 presents the pH, pCO2, base excess, and lactate prior the start of resuscitation (Table 2). Studies resuscitated animals with air or 100% O2 during CPR. All piglet studies used a post-transitional model (18), while the lamb studies used a fetal-to-neonatal transitional model (18). Dannevig et al. (10, 11) assessed brain and lung inflammation/injury in asphyxiated newborn piglets resuscitated with 21 vs. 100% O2, different compression to ventilation ratios (C:V) and variable durations of initial positive pressure ventilation. Alsaleem et al. (17) resuscitated asphyxiated newborn lambs with 21 or 100% O2 to evaluate cerebral O2 delivery and time to ROSC. The other ovine study, by Perez-de-Sa et al. (16) resuscitated asphyxiated lambs with air, 100% O2 for 3 min or 100% O2 for 30 min and assessed brain tissue oxygen tension. Using a newborn piglet model a study by Linner et al. (12) also tested resuscitation with air vs. 100% O2 for 3 or 30 min after asphyxia to compare time to ROC and cerebral oxygenation. A second study by Linner et al. (13) compared time to ROSC in asphyxiated newborn piglets with air or 100% O2 when ventilation is inadequate. Solevåg et al. compared time to ROSC in asphyxiated newborn piglets resuscitated with air and 100% O2 while an additional study compared 3:1 C:V vs. continuous CC with asynchronous ventilation (CCaV) in addition to air vs. 100% O2 and assessed time to ROSC and oxidative stress(14, 15).
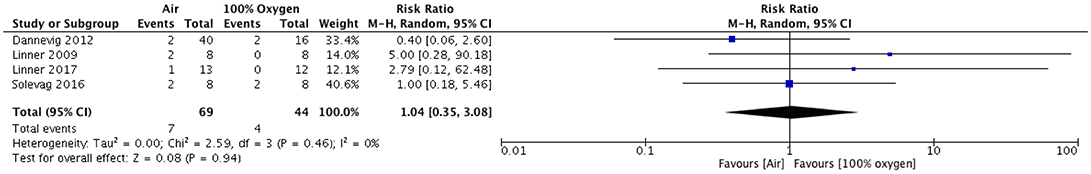
Figure 1. Mortality in piglets resuscitated with either Air or 100% oxygen during neonatal cardio-pulmonary resuscitation.
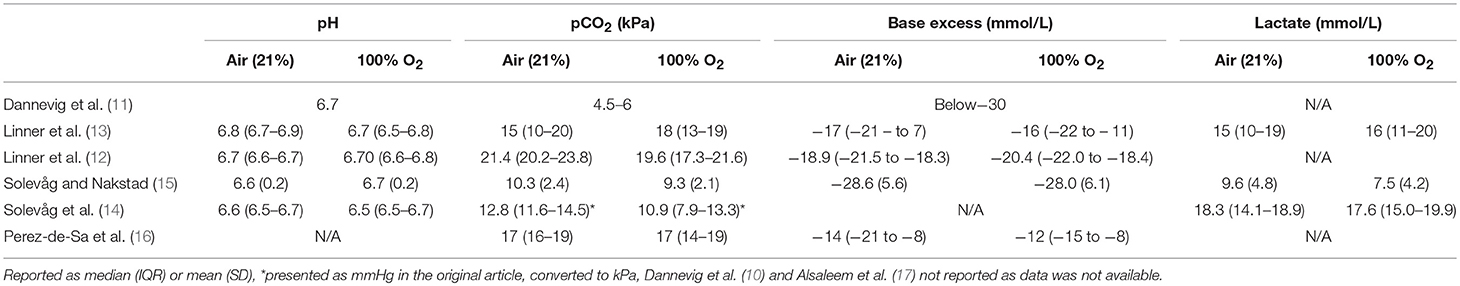
Table 2. Indicators of sickness before resuscitation with variable oxygen concentration during ventilation paired with CC during infant resuscitation.
Mortality
For the pooled analysis, the study by Solevåg et al. (15) was excluded because the study by Dannevig et al. (10) included the same piglets reported by Solevåg et al. (15). A total of 323 animals were included with an overall mortality of 17(5%). All animals in the ovine studies survived and therefore no pooled analysis was possible. For the outcome of mortality, the pooled analysis of the porcine studies showed no difference in mortality for piglets resuscitated with air vs. 100% oxygen (odds ratio 0.84 [0.26, 2.72], p = 0.77, I2 = 0%; Figure 1).
Return of Spontaneous Circulation and Circulatory Recovery
Overall, the individual studies and the pooled analysis showed no difference in the time to ROSC with air vs. 100% O2 with a mean difference of −3.8 [−29.7–22] s, I2 = 0%, p = 0.77 (Figure 2). Solevåg et al. (15) randomly assigned piglets to receive air or 100% O2 during CPR after asphyxia induced asystole and reported similar median [interquartile range (IQR)] time to ROSC of 135 (113–168) s vs. 150 (115–180) s in the air and 100% group, respectively. A second study by Solevåg et al. (14) randomized newborn piglets to air vs. 100% O2 and 3:1 C:V vs. CCaV. Overall, no difference in time to ROSC between the groups were observed. Circulatory recovery including left ventricular stroke volume with air and 100% O2 was comparable 1.4 vs. 1.0 mL/kg and 0.8 vs. 0.5 mL/kg 30 min and 4 h after ROSC, respectively. Studies by Linner et al in 2009 and 2017 (12, 13) randomized piglets to air for the duration of resuscitation, 100% O2 for 3 min followed by air, or 100% O2 for 30 min followed by air after apnea induced cardiac arrest. Time until ROSC (heart rate >150 bpm) were similar among groups with median (IQR) times of 67 (60–76) s, 88 (76–126) s, and 68 (56–81) s in the air, 100% for three min group, or 100% for 30 min group, respectively.
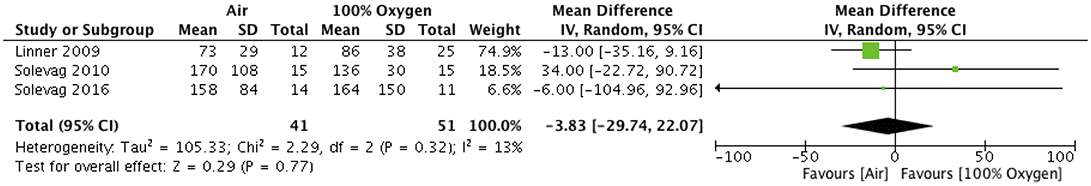
Figure 2. Time to return of Spontaneous Circulation in piglets resuscitated with either Air or 100% oxygen during neonatal cardio-pulmonary resuscitation.
Alsaleem et al. (17) induced cardiac arrest in fetal lambs by umbilical cord occlusion. The lambs were delivered and resuscitated with air then randomized to continue to receive air at the onset of CC or the O2 to be increased to 100%. There was no difference in mean (±SD) time to ROSC 211 (±145) s and 306 (±270) s in the air and 100% O2 group, respectively. Perez-de-Sa et al. (16), induced cardiac arrest in fetal lambs and randomized them to either air, 100% for the first 3 min, or 100% O2 during the first 30 min. If there was no sign of ROSC CC were initiated. No difference in time to a heart rate of 150 bpm was reported in all groups [68 (6–150) s, 107 (5–182) s and 58 (23–368) s]. These studies demonstrate that air is as effective as 100% O2 to achieve ROSC and circulatory recovery. However, recently Linner et al. demonstrated that when PPV is ineffective ROSC can be faster achieved using 100% O2 compared to air with 60 (11–120) s vs. 845 (611–death) s (p < 0.001) (13).
Oxygenation
All studies confirmed the significant arterial hyperoxemia in the group of animals resuscitated with 100% oxygen. Using cerebral near-infrared spectroscopy, studies measured speed of recovery of brain oxygenation by how fast cerebral regional oxygen saturation reached 30% and when brain tissue oxygenation (PbtO2) had increased 0.1 kPa from its nadir. Studies in near-term lambs reported higher PbtO2 with 100% O2, and significantly increased partial pressure of arterial oxygen levels immediately after ROSC compared to lambs who remained in air (165 ± 145 vs. 41 ± 16 mmHg), (p = 0.046) (17). However, throughout CC, no significant difference between blood oxygen saturation, partial pressure of arterial oxygen, arterial oxygen content, or O2 delivery to the brain between groups was observed (17). Perez-de-Sa et al. reported brain tissue oxygenation as measured by the partial pressure of oxygen in extracellular fluid (PbtO2) reaching a maximum of 56 kPa (420 mmHg) in the group initially ventilated with 100% O2 for 30 min, a level only previously seen in hyperbaric conditions(16). The groups receiving 100% O2 for 3 min had significantly lower PbtO2 peaking at 4.2 kPa (31.5 mmHg) while those receiving air peaked at 2.9 kPa (19.5 mmHg) (p = 0.002). The 2009 study by Linner et al. found no difference in time to recovery of brain oxygenation but observed arterial hyperoxemia 34 (30–41) kPa [255 (225–308) mmHg] by 2.5 min and peak PbtO2 values during resuscitation were higher in groups ventilated with 100% O2 [4.2 (3.3–5.4) kPa (31.5 (24.8–40.5) mmHg], 12 (6.4–15) kPa [90 (48–112) mmHg], and 25 (15–36) kPa [187.5 (112.5–270) mmHg], respectively) (12).
Indicators of Organ Injury or Damage
There were two studies that also examined the tissue oxidative stress or damage when 100% O2 vs. air was used in the resuscitation after asphyxia induced cardiac arrest in newborn piglets. Dannevig et al. (10, 11) found no difference in brain or lung inflammatory markers (e.g., lactate, lactate/pyruvate, or IL-1) in the groups with 100% O2 or air. Dannevig et al. also examined damage associated with different C:V ratios and duration of initial PPV (10, 11), however these results were excluded as they were not within the scope of this review.
Damage caused by oxidative stress was measured in the piglet study assessing 100% O2 and air with 3:1 C:V or CCaV (14). The oxidized glutathione to glutathione ratio, a marker for oxidative stress, was significantly higher with 100% O2 than air, 0.14 (0.11–0.22) and 0.10 (0.08–0.11) (p = 0.005), respectively.
Discussion
Current neonatal resuscitation guidelines recommend 100% O2 during CPR, however the most effective oxygen concentration in newborn infants remains controversial (2, 19, 20). Oxygen has been used in neonatal resuscitation for over 200 years (21). Its use spread rapidly in response to reports of brain damage in infants who had survived birth asphyxia (22). The inclusion of skin color in the Apgar score further contributed to an increased use of oxygen in the delivery room. The use of 100% oxygen was accepted based on experts' opinion despite a lack of experimental evidence. However, over the last decades the use of 100% O2 has been questioned as even a brief exposure to 100% O2 may be detrimental and several studies reported that air is as effective as 100% O2 (4, 23–27); Indeed, 21% O2 resulted in a significant reduction in mortality (28/284 vs. 60/321 [relative risk (RR) 0.71 (95% Confidence Interval (CI) 0.54 to 0.94), risk difference −0.05 (−0.08 to −0.01)] (3, 4), decreased time to first breath >3 min (102/321 vs. 71/288 [RR (95%CI) 0.78 (0.6–1.0)], and less Apgar scores < 7 at 5 min (107/659 vs. 70/616 [RR (95%CI) 0.71 (0.54–0.94)] when compared to 100% O2 (4, 4). This has led to a change in the 2010 neonatal resuscitation guidelines to start respiratory support with air and oxygen delivery titrated according to target oxygen saturations in term and near-term newborn infants (28). While these studies have examined oxygen use during respiratory support of term and near-term newborn infants no human study has compared air vs. 100% O2 during neonatal chest compression.
Our meta-analysis identified several animal studies comparing air vs. 100% O2 during chest compression in newborn animal models. Overall, air was as effective as 100% O2 during neonatal CPR to achieve ROSC [mean difference of −3.8 (−29.7–22) s, I2 = 0%, p = 0.77 (Figure 2)] and also had similar mortality rates between groups [(odds ratio 0.84 [0.26, 2.72], p = 0.77, I2 = 0%; Figure 1)].
High concentrations of O2 delivery during CPR generates O2-free radicals, which play major role in reperfusion/reoxygenation injury after asphyxia, especially to oxyregulatory tissues (e.g., myocardium) (28). During CPR 100% O2 causes significant arterial hyperoxemia and increased partial pressure of arterial oxygen levels immediately after ROSC (165 ± 145 vs. 41 ± 16 mmHg, p = 0.046) compared to resuscitation with air (17). However, this did not result in higher oxygenation at the brain. Furthermore, tissue oxygenation, and tissue/organ damage were not significantly different between air and 100% O2. Indeed, only the study by Solevåg et al. reported damage caused by oxidative stress in piglets resuscitated with 100% O2 compared to air. In the study by Solevåg et al. the oxidized glutathione to glutathione ratio, a marker for oxidative stress, was significantly higher with 100% O2 than air, 0.14 (0.11–0.22) and 0.10 (0.08–0.11) (p = 0.005), respectively (14). However, other studies did not report any difference in brain or lung inflammatory markers (e.g., lactate, lactate/pyruvate, or IL-1) in the groups with 100% O2 or air (10, 11). Based on these findings pure oxygen is not associated with damage to the nervous system or lungs any more than air but is a cause of oxidative stress. In addition, 100% O2 exposure at birth has been associated with increased risk of neonatal mortality and childhood cancer (29, 30).
Limitations
Limitations of the current review among others include (i) only data from animal studies were included, (ii) the data should not be directly extrapolated to clinical practice, (iii) different animal models (e.g., piglets and sheep), (iv) transitional model (lambs delivered via cesarean section) vs. post-delivery model (piglets 1–3 days old), (v) induction of cardiac arrest by asphyxia or potassium chloride, or (vi) all animals were intubated with a tightly sealed endotracheal tube (except the latest by Linner et al.) to prevent any endotracheal tube leak (10–18, 31). These variations might have influenced the results; however, subgroup analysis would have been impossible given the small sample size of each study. In addition, results in preterm infants might differ due to their immature antioxidant defense system and increased likelihood to need resuscitation (32–34). Furthermore, not all studies were randomized and only the study by Solevåg et al. (14) adhered to the ARRIVE guidelines (35), which would have been a strength and could reduce potential bias.
Future directions
Our results indicate that air during CC is safe and human trials are urgently needed. Human studies should compare air vs. 100% and examine effects of both oxygen concentrations to reduce exposure to hypoxia and hyperoxia (36). Alternatively, attempting to mimic the gradual rise in oxygen saturation of healthy term babies in the first 10 min after birth by titrating the concentration to the baby's saturation or using any intermediate options should be assessed in regards of benefits and harms (1, 2). Furthermore, any human trial should include long-term neurodevelopmental follow-up (29, 30).
Conclusion
No human studies were identified and the results obtained are from animal models. The data suggest that using air instead of 100% oxygen during neonatal chest compression had comparable outcomes including time to return of spontaneous circulating and mortality. Hyperoxia and oxidative stress were significantly higher with 100% O2. Human trials comparing air vs. 100% and/or oxygen titration as an alternative to air or 100% oxygen during neonatal chest compression are urgently needed.
Author Contributions
GS, P-YC, MO, and AS Conception and design; GS, CG-H, MO, MV, P-YC, AS, and OS Collection and assembly of data, analysis and interpretation of the data; GS, CG-H, MO, P-YC, AS, MV, and OS Drafting of the article; GS, CG-H, MO, P-YC, MV, AS, and OS; Critical revision of the article for important intellectual content, final approval of the article.
Funding
We would like to thank the public for donating money to our funding agencies: CG-H is a recipient of the Office of the Provost and VP (Academic) Summer Student Award, Faculty of Medicine and Dentistry, University of Alberta. GS is a recipient of the Heart and Stroke Foundation/University of Alberta Professorship of Neonatal Resuscitation, a National New Investigator of the Heart and Stroke Foundation Canada and an Alberta New Investigator of the Heart and Stroke Foundation Alberta. This research has been facilitated by the Women and Children's Health Research Institute through the generous support of the Stollery Children's Hospital Foundation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2018.00400/full#supplementary-material
Abbreviations
3:1 C:V, Compression to Ventilation ratio; CPR, Cardiopulmonary resuscitation; CC, Chest compression; O2, Oxygen; PPV, Positive pressure ventilation; ROSC, Return of spontaneous circulation; IQR, Interquartile range; PbtO2, Brain tissue oxygenation; CCaV, Continuous CC with asynchronous ventilation.
References
1. Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Simon WM, et al. Part 13: Neonatal resuscitation 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care (Reprint). Pediatrics (2015) 136:S196–218. doi: 10.1542/peds.2015-3373G
2. Wyllie JP, Wyckoff MH, Aziz K, Kim HS, Liley HG, Mildenhall LFJ, et al. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Pediatrics 136(Suppl. 2):S120–66. doi: 10.1161/CIR.0000000000000276
3. Tan A, Schulze AA, O'Donnell CPF. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev (2005) CD002273. doi: 10.1002/14651858.CD002273.pub3
4. Davis PG, Tan A, O'Donnell CPF, Schulze AA. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet (2004) 364:1329–33. doi: 10.1016/S0140-6736(04)17189-4
5. Lundstrøm KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Arch Dis Child Fetal Neonatal Ed (1995) 73:F81–6.
6. Vento M, Sastre J, Lloret A, García-Sala F, Miñana JB, Viña J. Hyperoxemia caused by resuscitation with pure oxygen may alter intracellular redox status by increasing oxidized glutathione in asphyxiated newly born infants. Semin Perinatol. (2002) 26:406–10. doi: 10.1053/sper.2002.37312
7. Saugstad OD. Hyperoxia in the term newborn: more evidence is still needed for optimal oxygen therapy. Acta Paediatr Suppl (2012) 101:34–38. doi: 10.1111/j.1651-2227.2011.02546.x
8. Mutinati M, Pantaleo M, Roncetti M, Piccinno M, Rizzo A, Sciorsci RL. Oxidative stress in neonatology. a review. Reprod Dom Anim. (2013) 49:7–16. doi: 10.1111/rda.12230
9. Kuligowski J, Torres-Cuevas I, Quintás G, van Goudoever JB, Cubells E, Asensi M, et al. Assessment of oxidative damage to proteins and DNA in urine of newborn infants by a validated UPLC-MS/MS approach. PLoS ONE (2014) 9:e93703. doi: 10.1371/journal.pone.0093703
10. Dannevig I, Solevåg AL, Sonerud T, Saugstad OD, Nakstad B. Brain inflammation induced by severe asphyxia in newborn pigs and the impact of alternative resuscitation strategies on the newborn central nervous system. Pediatr Res. (2013) 73:163–70. doi: 10.1038/pr.2012.167
11. Dannevig I, Solevåg AL, Saugstad OD, Nakstad B. Lung injury in asphyxiated newborn pigs resuscitated from cardiac arrest - the impact of supplementary oxygen, longer ventilation intervals and chest compressions at different compression-to-ventilation ratios. Open Respir Med J. (2012) 6:89–96. doi: 10.2174/1874306401206010089
12. Linner R, Werner O, Perez-de-Sa V, Cunha-Goncalves D. Circulatory recovery is as fast with air ventilation as with 100% oxygen after asphyxia-induced cardiac arrest in piglets. Pediatr Res. (2009) 66:391–4. doi: 10.1203/PDR.0b013e3181b3b110
13. Linner R, Cunha-Goncalves D, Perez-de-Sa V. One oxygen breath shortened the time to return of spontaneous circulation in severely asphyxiated piglets. Acta Paediatrica (2017) 106:1556–63. doi: 10.1111/apa.13920
14. Solevåg AL, Schmölzer GM, OReilly M, Lu M, Lee T-F, Hornberger LK, et al. Myocardial perfusion and oxidative stress after 21% vs. 100% oxygen ventilation and uninterrupted chest compressions in severely asphyxiated piglets. Resuscitation (2016) 106:7–13. doi: 10.1016/j.resuscitation.2016.06.014
15. Solevåg AL, Nakstad B. Resuscitation of severely asphyctic newborn pigs with cardiac arrest by using 21% or 100% oxygen. Neonatology (2010) 98:64–72. doi: 10.1159/000275560
16. Perez-de-Sa V, Cunha-Goncalves D, Nordh A, Hansson S, Larsson A, Ley D, et al. High brain tissue oxygen tension during ventilation with 100% oxygen after fetal asphyxia in newborn sheep. Pediatr Res. (2009) 65:57–61. doi: 10.1203/PDR.0b013e31818a01a4
17. Alsaleem M, Rawat M, Chandrasekharan P, Nair J, Koenigsknecht C, Gugino SF, et al. Optimal inspired oxygen concentration during chest compressions in term lambs with perinatal asphyxia -induced cardiac arrest. In: Pediatric Academic Societies Meeting. Toronto, ON (2018).
18. Solevåg AL, Cheung PY, Lie H, OReilly M, Aziz K, Nakstad B, et al. Chest compressions in newborn animal models: a review. Resuscitation (2015) 96:151–5. doi: 10.1016/j.resuscitation.2015.08.001
19. Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Simon WM. Part 13: Neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation (2015) 132:S543–60. doi: 10.1161/CIR.0000000000000267
20. Wyllie JP, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, Kim HS, et al. Part 7: neonatal resuscitation. 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation (2015) 95:e169–201. doi: 10.1016/j.resuscitation.2015.07.045
21. Obladen M. History of neonatal resuscitation–part 2: oxygen and other drugs. Neonatology (2009) 95:91–6. doi: 10.1159/000151761
22. Perlman JM, Wyllie JP, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 122(16 Suppl. 2):S516–38. doi: 10.1161/CIRCULATIONAHA.110.971127
23. Saugstad OD, Ramji S, Soll R, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology (2008) 94:176–82. doi: 10.1159/000143397
24. Ramji S, Saugstad OD. Use of 100% oxygen or room air in neonatal resuscitation. NeoReviews (2005) 6:e172–6. doi: 10.1542/neo.6-4-e172
25. Saugstad OD. Optimal oxygen therapy in the newborn period. Pediatr Pulmonol. (2004) 37:112–3. doi: 10.1002/ppul.70073
26. Saugstad OD. Optimal oxygenation at birth and in the neonatal period. Neonatology (2007) 91:319–22. doi: 10.1159/000101349
27. Saugstad OD, Vento M, Ramji S. Oxygen for newborn resuscitation: how much is enough? Pediatrics (2006) 118:789–92. doi: 10.1542/peds.2006-0832
28. Vento M, Sastre J, Asensi MA, Viña J. Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med. (2005) 172:1393–8. doi: 10.1164/rccm.200412-1740OC
29. Spector LG, Klebanoff MA, Feusner JH, Georgieff MK, Ross JA. Childhood cancer following neonatal oxygen supplementation. J Pediatr. (2005) 147:27–31. doi: 10.1016/j.jpeds.2005.03.008
30. Naumburg E, Bellocco R, Cnattingius S, Jonzon A, Ekbom A. Supplementary oxygen and risk of childhood lymphatic leukaemia. Acta Paediatr. (2002) 91:1328–33. doi: 10.1111/j.1651-2227.2002.tb02829.x
31. Hassan MA, Mendler M, Maurer M, Waitz M, Huang L, Hummler HD. Reliability of pulse oximetry during cardiopulmonary resuscitation in a piglet model of neonatal cardiac arrest. Neonatology (2015) 107:113–9. doi: 10.1159/000368178
32. Kapadia VS, Chalak LF, Chalak LF, Sparks JE, Allen JR, Savani RC, et al. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics (2013) 132:e1488–96. doi: 10.1542/peds.2013-0978
33. Oei JL, Saugstad OD, Vento M. Oxygen and preterm infant resuscitation. Curr Opin Pediatr. (2018) 30:192–8. doi: 10.1097/MOP.0000000000000610
34. Escrig R, Arruza L, Izquierdo I, Villar G, Saenz P, Gimeno A, et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics (2008) 121:875–81. doi: 10.1542/peds.2007-1984
35. Kilkenny C, Altman DG, Browne WJ, Cuthill IC, Emerson M. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol (2010) 8:e1000412. doi: 10.1371/journal.pbio.1000412
Keywords: infants, newborn, neonatal resuscitation, chest compressions, oxygen, asphyxia
Citation: Garcia-Hidalgo C, Cheung P-Y, Solevåg AL, Vento M, O'Reilly M, Saugstad O and Schmölzer GM (2018) A Review of Oxygen Use During Chest Compressions in Newborns—A Meta-Analysis of Animal Data. Front. Pediatr. 6:400. doi: 10.3389/fped.2018.00400
Received: 26 October 2018; Accepted: 03 December 2018;
Published: 18 December 2018.
Edited by:
Karel Allegaert, University Hospitals Leuven, BelgiumReviewed by:
Antonio Rodriguez-Nunez, University of Santiago de Compostela, SpainJeroen van Vonderen, Leiden University Medical Center, Netherlands
Copyright © 2018 Garcia-Hidalgo, Cheung, Solevåg, Vento, O'Reilly, Saugstad and Schmölzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georg M. Schmölzer, georg.schmoelzer@me.com
 Catalina Garcia-Hidalgo
Catalina Garcia-Hidalgo Po-Yin Cheung
Po-Yin Cheung Anne Lee Solevåg
Anne Lee Solevåg Maximo Vento
Maximo Vento Megan O'Reilly2,3
Megan O'Reilly2,3  Georg M. Schmölzer
Georg M. Schmölzer