Postural Tachycardia Syndrome in Children and Adolescents: Pathophysiology and Clinical Management
- 1Department of Pediatrics, Peking University First Hospital, Beijing, China
- 2Department of Pediatrics, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
- 3Research Unit of Clinical Diagnosis and Treatment of Pediatric Syncope and Cardiovascular Diseases, Chinese Academy of Medical Sciences, Beijing, China
- 4Key Laboratory of Molecular Cardiovascular Science, The Ministry of Education, Beijing, China
Postural tachycardia syndrome (POTS), characterized by chronic (≥6 months) orthostatic intolerance symptoms with a sustained and excessive heart rate increase while standing without postural hypotension, is common in children and adolescents. Despite the unclear pathogenesis of POTS, the present opinion is that POTS is a heterogeneous and multifactorial disorder that includes altered central blood volume, abnormal autonomic reflexes, “hyperadrenergic” status, damaged skeletal muscle pump activity, abnormal local vascular tension and vasoactive factor release, mast cell activation, iron insufficiency, and autoimmune dysfunction. A number of pediatric POTS patients are affected by more than one of these pathophysiological mechanisms. Therefore, individualized treatment strategies are initiated in the management of POTS, including basal non-pharmacological approaches (e.g., health education, the avoidance of triggers, exercise, or supplementation with water and salt) and special pharmacological therapies (e.g., oral rehydration salts, midodrine hydrochloride, and metoprolol). As such, the recent progress in the pathogenesis, management strategies, and therapeutic response predictors of pediatric POTS are reviewed here.
Introduction
Postural tachycardia syndrome (POTS) is one of the common forms of chronic (at least 6 months) orthostatic intolerance (OI), and most of the cases have presyncope symptoms accompanied by inappropriate sinus tachycardia with normal blood pressure in an upright position (1, 2). The debilitating condition of POTS is often accompanied by orthostatic discomforts including lightheadedness, headache, giddiness, presyncope, momentary “blackout,” blurred vision, cognitive difficulties, sleep disturbances, fatigue, pale complexion, and even sudden syncope. Postural tachycardia syndrome patients sometimes show signs of excessive sympathoexcitation such as chest tightness, palpitations, inappropriate vasomotor skin changes, excessive sweat, and frequent tremulousness (3, 4). For children and adolescents, the diagnostic criteria of POTS include discomforts of OI together with a normal supine heart rate (HR) and HR increase of at least 40 beats per minute (bpm) or a maximum HR over 130 bpm for children aged 6–12 years or >125 bpm for adolescents aged 13–18 years in the first 10 min of an active standing test or during the passive head-up tilt test (HUTT) without orthostatic hypotension shown by a reduction in systolic blood pressure (SBP) of more than 20 mmHg or a reduction in diastolic blood pressure (DBP) by more than 10 mmHg (5). Postural tachycardia syndrome is a heterogeneous disorder with many possible underlying causes, such as fever, anemia, dehydration, hyperthyroidism, myocardial damage, and autonomic neuropathies. Once a specific cause is identified, the POTS label should be discarded in favor of the appropriate disease term (2).
Because of the multisystem discomforts of pediatric POTS patients, there are significant deleterious effects on individual quality of life (5–8). In pediatric POTS, poor health increases the rate of depression and anxiety in young patients and their parents, which has drawn increasing attention in recent years (9, 10). However, in the general pediatric population, the heterogeneous clinical features and the shortage of specific biomarkers have resulted in an underestimated prevalence of POTS (6, 11). Thus, it is quite difficult to establish the exact incidence of POTS in children and adolescents (6, 11, 12). In 2014, our coworkers enrolled 600 Chinese individuals aged 7–18 (11.9 ± 3.0) years to study the incidence of POTS in children and adolescents, and the results indicated that the prevalence of POTS was 6.8% (4). The results also indicated no significant gender difference among the above young patients (4), although some other studies have reported that most patients with POTS are female (2, 6, 11). Regardless, the data on pediatric POTS are insufficient, meriting multicenter studies to obtain the rate of occurrence of POTS in children and teenagers.
To date, although the precise pathophysiology underlying pediatric POTS remains unclear, there have been some significant reports indicating that POTS might be multifactorial and varied in different subpopulations of young POTS patients (3, 6, 12). Thus, the responses of pediatric POTS patients to the same therapeutic intervention are highly variable (12, 13). Therefore, combined individualized treatment should be established according to the different pathophysiological processes and biomedical predictors (3, 5, 13).
Physiological Orthostatic Regulation
Under physiological conditions, the blood flow is redistributed when an individual changes position from lying down to standing. Blood volume shifts from the upper part of the body to the lower part and the splanchnic circulation, as well as from the vascular system into the interstitial space owing to gravity while in the upright position (14). Standing reduces venous return, leading to a temporary decline in both cardiac filling and stroke volume (SV) and even a decrease in arterial blood pressure (1, 14).
To compensate for the changes in orthostatism, the body has a series of regulations to restore venous return and cardiac output (14, 15). Importantly, autonomic reflexes are activated. When tension on the vessel walls decreases corresponding to the reduced SV, the baroreceptors of the carotid sinus and the aortic arch can be suppressed to inhibit the parasympathetic response and stimulate the sympathetic response to cause vasoconstriction, accelerated HR, and increased cardiac output (15). The inhibition of baroreceptors also excites the renin–angiotensin–aldosterone system (RAAS) to promote renal reabsorption of water and sodium, which results in arterial vasoconstriction and an increase in plasma volume (16). The compensatory mechanism increases HR by 10–15 bpm, maintains a negligible change in SBP, and elevates DBP by ~10 mmHg (17). Second, muscle pumps are invoked by movement or by external mechanical compression to increase venous return (18). Third, small veins prevent a large accumulation of blood in the lower body and maintain a relative upward flow of blood. Finally, when hypotension is detected, blood pools can be mobilized to the venous system. Additionally, many bioactive small gas molecules and vasoactive peptides are involved in the mechanisms responsible for regulating local vascular tension and endothelial cell function (19). If autonomic compensatory mechanisms are not sufficient, an excessive increase in HR (e.g., POTS) or hypotension, and even a loss of consciousness (e.g. vasovagal syncope) could happen after standing (2, 14).
Perspectives on the Pathogenesis of Pots
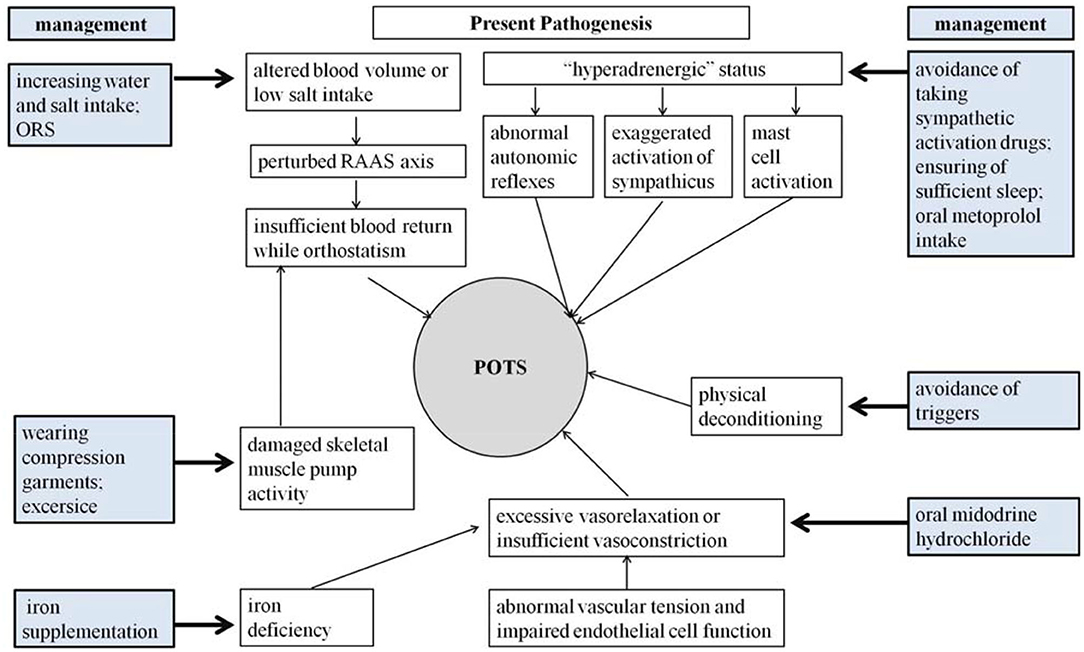
The pathophysiology of POTS is complicated and obscure (3, 12). Current perspectives on the pathogenesis of POTS have been categorized as altered central blood volume, abnormal autonomic reflexes and elevated sympathetic tone, damaged skeletal muscle pump activity, local vascular tension regulation dysfunction, iron insufficiency, mast cell activation (MCA), and autoimmune dysfunction (3, 6, 12).
Altered Central Blood Volume
Decreased red blood cell volume or low blood volume has been found in many pediatric POTS patients (4, 20, 21). The likelihood of POTS increases 3.9 times if daily water intake is <800 mL, suggesting that hypovolemia is a risk factor in children with POTS (4). In 1996, El-Sayed and Hainsworth (22) confirmed that 24-h urine excretion of sodium was a useful biomarker of the body's volume capacity. In 2014, Zhang et al. (23) showed that there was a negative correlation between 24-h urinary excretion of sodium and symptom severity scores in children and adolescents with POTS. Some investigators have reported that the tachycardic reaction to standing is associated with the seriousness of hypovolemia in pediatric POTS (24) and that symptoms improve following acute (25) or chronic (26) increase in plasma volume. When 24-h urinary sodium excretion is <124 mmol/24 h, salt and water supplementation is efficacious in reducing uncomfortable symptoms in pediatric POTS patients (23). All of the above results indicate that low salt intake compounded by hypovolemia occurs in pediatric POTS. When hypovolemia exists at rest, the body cannot fully compensate for the decrease in blood volume after standing (27), which has to coexist with elevated HR in the upright position (28). Paradoxically, normal plasma volumes in patients with POTS have been reported (29, 30), indicating that hypovolemia is not the unique cause of POTS.
Stewart and Montgomery (31) reported that 37 adolescent patients with POTS, aged 14–21 years, could be classified into a low-flow POTS (LFP) group in 14 patients, a normal-flow POTS (NFP) group in 15 patients, and a high-flow POTS (HFP) group in 8 patients. The blood flow of the lower extremities was measured in the supine position (31). Among these patients, most belonged to the LFP group. The pathogenesis of the NFP group was excessive redistribution to the splanchnic circulation because of inadequate splanchnic vasoconstriction while in an upright posture, leading to thoracic hypovolemia, upright tachycardia, intense peripheral vasoconstriction, and acrocyanosis (32). Interestingly, there were markedly increased microvascular filtration and obviously incompetent peripheral vasoconstriction inducing excessive calf blood flow that was unable to return to the heart accounting for postural tachycardia in the HFP group (33).
In light of the important role of the RAAS in regulating human neurohormones of plasma volume, a perturbed RAAS axis, which is disabled to facilitate the enlargement of blood volume in response to the hypovolemia of POTS patients, might be one of the pathophysiological changes in POTS (34). Several studies have found an inappropriately low plasma renin activity (PRA) in some children and adolescents with POTS, despite the hypovolemia (21, 24, 35), reduced aldosterone levels, and elevated angiotensin (Ang) II levels, with an insensitive receptor response to Ang II (35). Thus, the discordance of Ang II and PRA/aldosterone levels suggested that there might be diminished Ang II degradation in POTS patients (35). Patients with LFP have a decreased body mass index (BMI), which is related to the increased Ang II levels in women aged 14–29 years (36). However, the data on altered RAAS function corresponding to blood volume status in children and adolescents with POTS merit further study.
Abnormal Autonomic Reflexes and a “Hyperadrenergic” Status
Compared to healthy people, patients with POTS demonstrate excessive blood volume accumulation in the lower extremities and visceral circulation, which results in an obvious decrease in blood volume in venous return to the heart and subsequent cardiac ejection during orthostatic stress (14). The above changes bate baroreceptor activity to excite the sympathetic response and inhibit the parasympathetic response to adjust the altered hemodynamics (3). However, some pediatric POTS patients with a “hyperadrenergic” background have an inability to buffer the decrease in baroreceptor activation, leading to persistent sympathetic excitation characterized by sustained tachycardia while in an upright position (3, 37).
An increase in plasma norepinephrine in the standing position is the main biochemical change that occurs in hyperadrenergic POTS patients (38–40). Zhang et al. (38) reported that the plasma norepinephrine level in the upright position was positively associated with the severity of symptoms and the HR increase in the HUTT in pediatric POTS patients, and they speculated that the orthostatic norepinephrine increase might be due to the decreased vasoconstriction mediated by the baroreflex upon standing. Elevated plasma norepinephrine in pediatric patients with POTS suggests an impaired function of the norepinephrine transporter (NET) (39). Studies have shown that inherited or acquired mutations of NET encoded by the solute carrier family 6 neurotransmitter transporter noradrenalin member 2 (SLC6A2) gene impair the removal of norepinephrine in the synaptic cleft and reduce NET-dependent norepinephrine reabsorption (39, 40). The above data suggest that changes in the SLC6A2 gene or NET protein expression eventually lead to increased adrenaline status in “hyperadrenergic” POTS patients.
Sleeping disorders have been considered to be related to a “hyperadrenergic” status in young patients with POTS (4). Sleepiness, unrefreshing sleep, fatigue, frequent arousal from sleep, and reduced quality of life are often described in pediatric patients with POTS (41–44). However, there is limited evidence to evaluate the prevalence of sleep-related symptoms or to elucidate the mechanism for POTS in children and adolescents (4, 43–45). The probability of POTS in children and adolescents increases 5.9 times if the number of hours of sleep is <8 h/d (4). More than 70% of pediatric POTS patients complain that sleep disturbances are their problems (44). High incidences of sleep-related complaints are not the result of primary sleeping disorders specific to POTS, but a comprehensive effect of complaints such as physical fatigue, chronic body pain, and other somatic discomforts (45). Sleeping disorders often trigger ill-defined cognitive impairment or mental fatigue with increased standing HR, disturbing daytime life in adolescents with POTS (46). Serotonin–norepinephrine reuptake inhibitors can worsen “brain fog” in pediatric POTS patients (47). Thus, exaggerated activation of the sympathicus might be an underlying cause of the hyperaroused state and worsen the subjective estimates of sleep quality in POTS patients (48).
Damaged Skeletal Muscle Pump Activity
Normally, contraction of the lower extremity muscles commanded by skeletal muscle pumps provides a guarantee for sufficient venous return to the heart to prevent OI (49). Stewart et al. (50) compared 12 LFP subjects with 10 healthy controls and 7 NFP patients to measure venous volume, peripheral blood capacitance, and calf muscle pump function. They found that the reduced circumferences of the calf were associated with a decreased fraction of emptied calf venous volume during muscle contraction concurrent with general low blood flow in LFP subjects. They proposed that the reduced calf blood capacity might lessen calf muscle size in the LFP group and thus damage the vertical ejection ability of the pumps in the skeletal muscle, further reducing blood flow and finally leading to OI in these patients (50).
Local Vascular Tension Dysfunction and Abnormal Vasoactive Factor Release
Upright stress results in the increased blood filling in the capacitance vessels, followed by reduced venous return (51). Excessive venous pooling has been found in the lower extremities in POTS patients (51, 52), whose resting venous pressures are higher than those of controls (16 vs. 10 mmHg), which causes redistributed blood flow to the lower part of the body even while in a supine position and results in tachycardia via vagal retraction (53). Several studies have attributed such pooling mechanisms to increased venous filling due to arterial inflow (52), increased venous volume owing to mechanical defects in vein (53), blunted arterial vasoconstriction (54–56), altered capillary permeability inducing plasma fluid loss from capillaries to peripheral tissues (33, 57), or impaired venous emptying (58).
To further explore the mechanism for vascular tension dysfunction, some studies have been designed, and the results show that bioactive gaseous regulatory molecules and vasoactive peptides are involved in the development of abnormal partial vascular tension and impaired endothelial cell function in children and adolescents with POTS (5, 35, 59–63). Nitric oxide (NO), an endogenous gas continuously synthesized from L-arginine by nitric oxide synthase (NOS) and constitutively expressed in the endothelium, is known as a vasorelaxant factor and plays a crucial role in regulating the function of vascular endothelial cells (59). Liao et al. (60) reported that there were significantly higher levels of plasma NO and NOS in pediatric POTS patients aged 12 ± 3 years than in age-matched controls. In addition, NOS activity is positively correlated with flow-mediated vasodilation (FMD) of the brachial artery, which indirectly affects vascular dilation function (60). Hydrogen sulfide (H2S), a gasotransmitter alongside NO, exerts a low concentration-produced contraction or high concentration-dependent relaxation of blood vessels (61). Erythrocytic H2S has been detected to be obviously higher in pediatric POTS patients than that in control group (62, 63). Sulfur dioxide (SO2), another gaseous molecule, has recently been found to have vasorelaxant effects (64, 65). Furthermore, plasma SO2 levels were significantly higher in children with POTS than in healthy controls and were positively related to the maximum HR of all the study participants (66). The three increased endogenous gaseous molecules in pediatric POTS support the hypothesis that excessive vasorelaxation might contribute to the pathogenesis of pediatric POTS (19, 60). Additionally, adrenomedullin 2/intermedin (AM2/IMD), one of the members of the calcitonin gene-related peptide (CGRP) family (67), has been found to have a positive correlation with extraordinarily high HR during the HUTT in children with POTS (68). C-type natriuretic peptide (CNP) is generated from the endothelium and acts on adjacent vascular smooth muscle cells serving as a selective endothelium-independent vasodilator (69). The potent systemic cardiovascular actions of CNP reduce cardiac filling pressures and heart output following vasorelaxation and decrease venous return to the heart (70). Upright heart output and total peripheral vascular resistance are significantly lower in pediatric POTS patients than in the same children in the supine position or in healthy children and are positively associated with elevated plasma CNP levels (71). The results suggest that the increased endogenous IMD or CNP levels with similar features of vascular dilation represent the endogenous molecules involved in the development of POTS. Serum resistin, known as a new type of peptide hormone derived from adipocytes (72), has been found to notably enhance vasoconstriction and reduce diastolic function (73). An in vitro experiment showed that resistin could decrease endothelial NOS expression in human coronary artery endothelial cells (74). The serum resistin level in pediatric POTS patients is dramatically higher than that in age-matched healthy controls and is negatively related to the severity of symptoms and changes in HR that occur when changing from a supine to an upright position (74). These findings suggest that resistin may be a protective element in the pathogenesis of pediatric POTS and that the symptoms of POTS can likely be alleviated by raising the resistin level or improving the resistin function in vivo (75). Taken together, these findings confirm that increased local vascular relaxation and the compensatorily increased release of vasorelaxant factors, as well as vascular endothelial cell dysfunction, play important roles in the pathogenesis of POTS in children and adolescents (35, 54).
Iron Insufficiency
As the richest transition metal ion in humans, iron can cause a decrease in vasodilation by inhibiting the biosynthesis, transportation, transformation, and signal transduction of NO (76, 77). Low ferritin and vitamin D levels have been found in adolescents with POTS (78). Low iron storage accompanied by mild anemia is more prevalent in pediatric POTS patients than in healthy children in the United States (79). Additionally, iron-deficiency anemia also increases NO production in children and adolescents (80), suggesting that low iron storage–induced excessive vascular relaxation by increased output of NO might be a potential pathophysiological factor in pediatric POTS (79, 80). In addition, larger mean corpuscular volume and lower mean corpuscular hemoglobin concentration (MCHC) values were found in pediatric POTS patients than those in controls, which might be associated with decreased iron storage (81).
Mast Cell Activation
In an evaluation of young females with POTS, Shibao et al. (82) found that some of them had flushing episodes associated with OI, which suggests that MCA is likely involved in the hyperadrenergic mechanism for POTS (82). Although limited studies are available on the detailed mechanisms of MCA in POTS, the histamine, adenosine, platelet-activating factor, and prostaglandins produced by mast cells might play important roles in adolescents with POTS-related vasodilation and tachycardia (83, 84). A hopeful beneficial treatment would be to block these mediators in pediatric POTS patients with MCA, which merits further research (85).
Autoimmune Dysfunction
Autoimmune disturbance has been considered as one of the mechanisms involved in POTS due to frequent findings of autoantibodies in patients (11, 86, 87). A study from Japan used the luciferase immunoprecipitation system method and detected ~29% anti–ganglionic nicotinic acetylcholine receptor (gAChR) α3 and β4 antibodies from the serum of young POTS population with a median age of 22.2 ± 10.8 years and an onset age of 19.8 ± 10.8 years (88). Dysautonomia may be caused by anti-gAChR antibodies damaging autonomic ganglionic synaptic transmission in POTS patients (89). In addition to gAChR, several adrenergic receptors have been identified in pediatric POTS patients, including Ang II type 1 receptor (AT1R) and β1- and β2-adrenergic receptors (90–92). A study reported that POTS patients had higher autoimmune marker levels [e.g., antinuclear antibody (25 vs. 16%) and antiphospholipid antibody (7 vs. 1%) and increased incidence of comorbid autoimmune disease such as Hashimoto thyroiditis, rheumatoid arthritis, systemic lupus erythematosus, and common variable immunodeficiency (20 vs. 9.4%)] than the healthy population (93). The above findings demonstrate that pediatric POTS has a basis in autoimmune dysfunction; however, the detailed immune mechanisms are unknown and require further evaluation to determine the clinical significance (94).
Management Strategies for Pots in Children and Adolescents
Full recovery is possible for POTS in children and adolescents, but it is difficult to treat patients because of inconsistent and poor therapeutic efficacies (95). Thus, collaborative and multidisciplinary therapies are constantly required (5–8, 95). After the diagnosis of POTS, in general, health education is the fundamental strategy above all, and further daily treatment plans should consist of non-pharmacological and pharmacological interventions (5, 95, 96). To achieve the best therapeutic effects, the detection of distinct neurohumoral biomarkers might predict the specific efficacy of the individualized treatment options in children and adolescents with specific subtypes of POTS (1, 5, 96).
Non-pharmacological Interventions
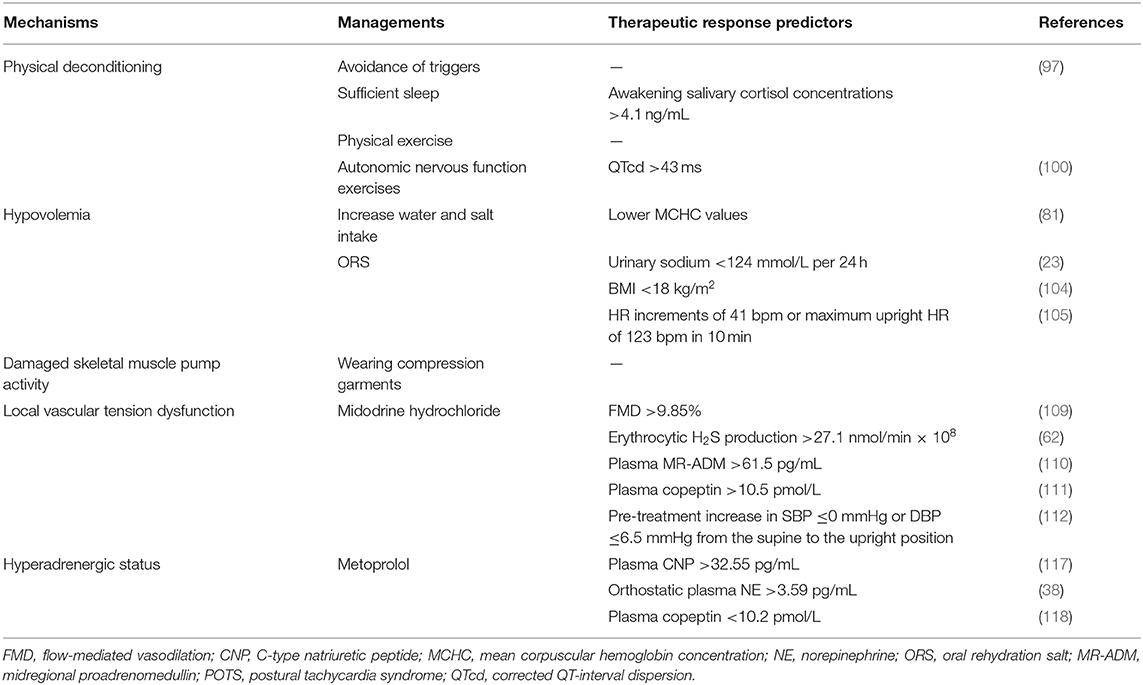
Health education on POTS is needed for young patients and their guardians who should understand the potential precipitating factors and avoid them (5–8, 96). Owing to frequent incapacitating symptoms of POTS that are closely related to an upright posture especially in stifling conditions (2), patients should avoid long periods of standing, sudden head-up postural changes, and high environmental temperatures (5). For POTS patients with a “hyperadrenergic” background, infection, sympathetic activation, and a lack of sleep might aggravate symptoms (4, 38, 39). Thus, patients are prevented from taking drugs such as NET inhibitors or 5-hydroxytryptamine norepinephrine reuptake inhibitors (5). In addition, pediatric POTS patients with sleep disorders should receive a guidance to promote more than 8 h of sleep daily (97). Additionally, it was found that, when the awakening salivary cortisol concentrations were more than 4.1 ng/mL, the sensitivity and specificity of predicting the effect of sleep-promoting therapy were 83.3 and 68.7%, respectively, in patients with POTS (97). Low-flow POTS patients usually suffer from the combination of hypovolemia and muscle pump dysfunction (50), which makes it necessary to drink plenty of water and wear compression garments, which can reduce the venous pooling caused by insufficient peripheral venous reflux and increase peripheral blood return to the heart (98). For those with signs of discomfort, counterpressure actions such as leg crossing and squatting are advised (5).
In addition, appropriate physical exercise to enhance muscle pump function of limbs and autonomic nervous system exercises to improve autonomic tone are also recommended for children with POTS (5, 98). The former refers to regular, organized, progressive exercise plans, which are characterized by aerobic recovery and some resistance training (5). The initial training should be limited to non-upright physical exercises, including the usage of rowing machines, recumbent cycling, and swimming, to reduce standing stress on the heart (5, 98, 99). The latter is especially suitable for pediatric POTS patients whose corrected QT-interval dispersion (QTcd) is more than 43 ms (100). It is recommended that the patients stand against a wall with feet 15 cm from it, starting at 5 min/d, and then gradually increasing to 20 min/d according to patients' tolerance and preference (5, 98). Several training results show that exercise training can improve the symptoms and the quality of life (101–103) and is even better than propranolol in the recovery of upright hemodynamics and the normalization of renal–adrenal reactivity (101).
Children and adolescents with POTS, especially those with 24-h urinary sodium excretion <124 mmol (23) or a BMI <18 kg/m2 (104), are encouraged to drink adequate amounts of water (>800 mL/d) (4) and increase appropriate salt intake for 1–3 months to increase blood volume (5). However, this is not advised for patients suffering from kidney disease, high blood pressure, or heart failure (5, 95).
Pharmacological Treatment
Non-pharmacological interventions should be attempted first in all pediatric POTS patients. If these interventions are ineffective, pharmacological therapies should be applied to solve the specific problems (5, 95, 96). Pediatric patients with POTS who are strongly suspected of having hypovolemia or inadequate salt intake are recommended to use oral rehydration salts (ORSs) for 1–3 months (5, 23, 98, 104) or to accept short-term intravenous saline as rescue treatment for patients with clinical decompensation and significant deterioration of symptoms (25). Saline supplements can alleviate symptoms in “hypovolemic” patients. Many indexes, such as lower MCHC values (81), 24-h urinary sodium excretion <124 mmol (23), and a cutoff of BMI <18 kg/m2 (104), have been shown to predict the therapeutic effect of ORSs on children and adolescents with POTS. Additionally, ORSs are also effective when the increase in HR is >41 bpm or when the maximum upright HR in 10 min is >123 bpm before treatment (105). When both indices are used together, the sensitivity and specificity are higher than for any of the single indices (104). Fludrocortisone is useful to increase sodium retention and amplify the plasma volume in POTS patients by activating the RAAS, but its validity needs to be verified in randomized clinical trials (98). In addition, increased NO generation and elevated NOx concentrations can return to normal after oral iron therapy in adolescents with iron-deficiency anemia (80), suggesting that iron supplementation might be effective for POTS patients with iron deficiency and the subsequent vasorelaxation status.
For children with severe symptoms or a risk of injury with unobvious presyncope that remarkably affect the quality of life and for those who do not demonstrate a good response to health training and salt supplementation therapy, special vasoregulation-related pharmacological interventions should be considered (5, 96).
Midodrine hydrochloride, a drug that is enzymatically hydrolyzed as a selective α1-adrenoceptor agonist desglymidodrine by oral administration (106), constricts veins and arteries to increase venous return, which makes the drug theoretically useful in treating POTS (98). Midodrine hydrochloride significantly reduces both supine and upright tachycardia with better results than the α2-adrenoreceptor agonist clonidine, which decreases supine HR without increasing standing HR, but it is inferior to intravenous infusion of saline in POTS patients (25). Midodrine hydrochloride is indicated to be effective for some subtypes of pediatric POTS (5, 107), and its therapeutic efficacy is greatly increased with the help of biomarkers that are low-cost, non-invasive, and easy to detect (108). A series of important studies have been designed to explore the predictors of the efficacy of midodrine hydrochloride in the treatment of pediatric POTS by using receiver operating characteristic curves (62, 109–113). The results show that the following indications can predict the effectiveness of midodrine hydrochloride in the treatment of POTS in children and adolescents: FMD >9.85%, with high sensitivity (71.6% in 1 month and 74.4% in 3 months of therapy) and specificity (77.8% in 1 month and 80% in 3 months of therapy) (109); H2S production in erythrocytes >27.1 nmol/min × 108 cell, with a sensitivity of 78.9% and a specificity of 77.8% (62); midregional proadrenomedullin plasma levels >61.5 pg/mL, with a sensitivity of 100% and a specificity of 71.6% (110); copeptin plasma levels >10.5 pmol/L, with both high sensitivity (81.3%) and high specificity (76.5%) (111); and SBP drops in changing from a supine to an upright position, with a sensitivity of 72% and a specificity of 88% (112). Additionally, the blood pressure change from a supine to an upright position can well-predict the long-term asymptomatic survival rate of pediatric POTS patients given oral midodrine hydrochloride (113). Midodrine is contraindicated for those with a BP >95% of the average for individuals of the same age and sex or for those with drug allergies (5). Since midodrine hydrochloride has instant and brief effects, the daily dose is suggested to be divided into several administrations (5, 98, 106). The medicine should be taken only during the daytime, no sooner than 4 h before bedtime, because of a risk of hypertension in the supine position (106); therefore, the BP should be monitored during treatment.
Adrenoceptor blockers can be divided into non-selective blockers for β1 and β2 receptors and β1-selective blockers, which have higher selective affinity for β1 receptors than for β2 receptors (114). The latter mainly slow down HR and decrease myocardial contractility when the sympathetic nervous system is activated but have a relatively small effect on the heart at rest (115). In 2009, a single-center retrospective chart analysis and follow-up conducted by the Mayo Clinic showed that adolescents with POTS taking β-blockers were more likely to have an improvement in symptoms than those taking midodrine (116), suggesting that β-blockers were effective for young POTS patients. β1-Selective blockers are useful for “hyperadrenergic” subtypes of POTS patients (96). There is relatively less experience with β1-blocker therapy in pediatric POTS (5–8, 96). Investigators also proposed that metoprolol, as a common β1-blocker, could be used with an initial dose of 0.5 mg/kg per day in treating pediatric POTS (108) and a maximal dose of 2 mg/kg per day in severe cases according to the symptoms (5). They also found several indicators to predict the effect of metoprolol on POTS in children and adolescents (5, 38, 117, 118). The results showed that the sensitivity and specificity were 95.8 and 70%, respectively, when the plasma CNP level was >32.55 pg/mL (117); 76.9 and 91.7%, respectively, with a plasma norepinephrine level >3.59 pg/mL in upright position (38); and 90.5 and 78.6%, respectively, with a plasma copeptin level <10.2 pmol/L in predicting the efficacy of metoprolol in children with POTS (118). Severe sinus bradycardia, atrioventricular block, severe heart failure, hypotension, acute pulmonary edema, bronchial asthma, mental illness, diabetes, and drug allergy are contraindications for the use of β-blockers (5).
Although evidence hints that MCA and autoimmune disorders take part in the pathogenesis of POTS (82, 86, 88), there are no meaningful therapeutic reports about defending against either of them in pediatric POTS patients, and this research gap requires further study.
Conclusion
Although great efforts have been made in exploring the pathogenesis of pediatric POTS, the precise mechanisms for POTS have not yet been totally elucidated (3, 6, 96). Fortunately, a series of effective treatment measures have been designed according to the known pathophysiological mechanisms (Figure 1), and a number of biomedical factors have gradually been discovered to be involved in the POTS pathological process and treatment prediction (35, 96). More and more biomarkers have been confirmed to be effective in predicting treatment efficacy (5–8). Therefore, selecting individual treatment strategies could improve the therapeutic outcomes according to the detailed pathogenesis indicated by the specific biological indexes (Table 1). However, the data of trials in pediatric POTS patients mainly originate from a single center; therefore, it is essential to perform large-sized multicenter studies in the future to optimize the individualized treatment of POTS in children and adolescents.
Author Contributions
GC: literature review, interpretation of data, and first draft of the manuscript. JD: design, organization, and editing of the manuscript. HJ: critical review and editing of studies cited in the manuscript. YH: concept and design of the manuscript, critical review, and editing of the manuscript. All authors: contributed to manuscript revision, read and approved the submitted version.
Funding
This work was supported by Peking University Clinical Scientist Program (BMU2019LCKXJ001), and Fundamental Research Funds for the Central Universities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep. (2015) 15:60. doi: 10.1007/s11910-015-0583-8
2. Stewart JM, Boris JR, Chelimsky G, Fischer PR, Fortunato JE, Grubb BP, et al. Pediatric disorders of orthostatic intolerance. Pediatrics. (2018) 141:e20171673. doi: 10.1542/peds.2017-1673
3. Wells R, Spurrier AJ, Linz D, Gallagher C, Mahajan R, Sanders P, et al. Postural tachycardia syndrome: current perspectives. Vasc Health Risk Manag. (2018) 14:1–11. doi: 10.2147/VHRM.S127393
4. Lin J, Han Z, Li X, Ochs T, Zhao J, Zhang X, et al. Risk factors for postural tachycardia syndrome in children and adolescents. PLoS ONE. (2014) 9:e113625. doi: 10.1371/journal.pone.0113625
5. Wang C, Li Y, Liao Y, Tian H, Huang M, Dong XY, et al. 2018 Chinese Pediatric Cardoilogy Society (CPCS) guideline for diagnosis and treatment of syncope in children and adolescents. Sci Bull. (2018) 63:1558–64. doi: 10.1016/j.scib.2018.09.019
6. Boris JR. Postural orthostatic tachycardia syndrome in children and adolescents. Auton Neurosci. (2018) 215:97–101. doi: 10.1016/j.autneu.2018.05.004
7. Tao C, Liu X, Zhang C, Chen Y, Huang Y. Comments on 2018 CPCS guideline for diagnosis and treatment of syncope in children and adolescents. Sci Bull. (2019) 64:291–2. doi: 10.1016/j.scib.2019.01.008
8. Xu W, Wang T. Diagnosis and treatment of syncope in pediatric patients: a new guideline. Sci Bull. (2019) 64:357. doi: 10.1016/j.scib.2019.01.024
9. Zhao J, Han Z, Zhang X, Du S, Liu AD, Holmberg L, et al. A cross-sectional study on upright heart rate and BP changing characteristics: basic data for establishing diagnosis of postural orthostatic tachycardia syndrome and orthostatic hypertension. BMJ Open. (2015) 5:e007356. doi: 10.1136/bmjopen-2014-007356
10. Zhang Q, Li J, Xie Y, Zhao J, Du J. Orthostatic hypertension in children and adolescents with postural tachycardia syndrome. J Trop Pediatr. (2014) 60:461–6. doi: 10.1093/tropej/fmu055
11. Boris JR, Bernadzik owski T. Demographics of a large paediatric postural orthostatic tachycardia syndrome program. Cardoil Young. (2018) 28:668–74. doi: 10.1017/S1047951117002888
12. Zadourian A, Doherty TA, Swiatkiewicz I, Taub PR. Postural orthostatic tachycardia syndrome: prevalence, pathophysiology, and management. Drugs. (2018) 78:983–94. doi: 10.1007/s40265-018-0931-5
13. Heyer GL. Postural tachycardia syndrome: diagnosis and management in adolescents and young adults. Pediatr Ann. (2017) 46:e145–54. doi: 10.3928/19382359-20170322-01
14. Stewart JM. Update on the theory and management of orthostatic intolerance and related syndromes in adolescents and children. Expert Rev Cardoivasc Ther. (2012) 10:1387–99. doi: 10.1586/erc.12.139
15. Stewart JM. Mechanisms of sympathetic regulation in orthostatic intolerance. J Appl Physiol. (2012) 113:1659–68. doi: 10.1152/japplphysiol.00266.2012
16. Fountain JH, Lappin SL. Physiology, Renin Angiotensin System. StatPearls. Treasure Island, FL: StatPearls Publishing (2019).
17. Sahni M, Lowenthal DT, Meuleman J. A clinical, physiology and pharmacology evaluation of orthostatic hypotension in the elderly. Int Urol Nephrol. (2005) 37:669–74. doi: 10.1007/s11255-005-7663-7
18. Platts SH, Tuxhorn JA, Ribeiro LC, Stenger MB, Lee SM, Meck JV. Compression garments as countermeasures to orthostatic intolerance. Aviat Space Environ Med. (2009) 80:437–42. doi: 10.3357/ASEM.2473.2009
19. Bai W, Chen SY, Jin HF, Du JB. Vascular dysfunction of postural tachycardia syndrome in children. World J Pediatr. (2018) 14:13–7. doi: 10.1007/s12519-017-0104-8
20. Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci. (2007) 334:57–60. doi: 10.1097/MAJ.0b013e318063c6c0
21. Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. (2005) 111:1574–82. doi: 10.1161/01.CIR.0000160356.97313.5D
22. El-Sayed H, Hainsworth R. Salt supplement increase plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart. (1996) 75:134–40. doi: 10.1136/hrt.75.2.134
23. Zhang Q, Liao Y, Tang C, Du J, Jin H. Twenty-four-hour urinary sodium excretion and postural orthostatic tachycardia syndrome. J Pediatr. (2012) 16:281–4. doi: 10.1016/j.jpeds.2012.01.054
24. Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. (1997) 103:128–33. doi: 10.1016/S0002-9343(97)00133-2
25. Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, et al. Effects of volume loading and pressor agents in idoipathic orthostatic tachycardia. Circulation. (1997) 96:575–80. doi: 10.1161/01.CIR.96.2.575
26. Moak JP, Leong D, Fabian R, Freedenberg V, Jarosz E, Toney C, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardoil. (2016) 37:278–82. doi: 10.1007/s00246-015-1274-6
27. Medow MS, Stewart JM. The postural tachycardia syndrome. Cardoil Rev. (2007) 15:67–75. doi: 10.1097/01.crd.0000233768.68421.40
28. Jarjour IT. Postural tachycardia syndrome in children and adolescents. Semin Pediatr Neurol. (2013) 20:18–26. doi: 10.1016/j.spen.2013.01.001
29. Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol. (2006) 291:H904–13. doi: 10.1152/ajpheart.01359.2005
30. Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. (2006) 290:H665–73. doi: 10.1152/ajpheart.00784.2005
31. Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. (2004) 287:H1319–27. doi: 10.1152/ajpheart.00086.2004
32. Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. (2003) 285:H2749–56. doi: 10.1152/ajpheart.00429.2003
33. Stewart JM. Microvascular filtration is increased in postural tachycardia syndrome. Circulation. (2003) 107:2816–22. doi: 10.1161/01.CIR.0000070951.93566.FC
34. Kumar V, Usmani S, Alam S, Fraz M, Patel D, Goparaju P, et al. MON-LB009 renin angiotensin aldosterone system inactivation in postural orthostatic tachycardia syndrome. J Endocr Soc. (2019) 3(Suppl. 1):MON–LB009. doi: 10.1210/js.2019-MON-LB009
35. Zheng X, Chen Y, Du J. Recent advances in the understanding of the mechanisms underlying postural tachycardia syndrome in children: practical implications for treatment. Cardoil Young. (2017) 27:413–7. doi: 10.1017/S1047951116002559
36. Stewart JM, Taneja I, Medow MS. Reduced body mass index is associated with increased angiotensin II in young women with postural tachycardia syndrome. Clin Sci. (2007) 113:449–57. doi: 10.1042/CS20070104
37. Li H, Liao Y, Wang Y, Liu P, Sun C, Chen Y, et al. Baroreflex sensitivity predicts short-term outcome of postural tachycardia syndrome in children. PLoS ONE. (2016) 11:e0167525. doi: 10.1371/journal.pone.0167525
38. Zhang Q, Chen X, Li J, Du J. Orthostatic plasma norepinephrine level as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. J Transl Med. (2014) 12:249. doi: 10.1186/s12967-014-0249-3
39. Khan AW, Corcoran SJ, Esler M, El-Osta A. Epigenomic changes associated with impaired norepinephrine transporter function in postural tachycardia syndrome. Neurosci Biobehav Rev. (2017) 74:342–55. doi: 10.1016/j.neubiorev.2016.06.015
40. Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. (2000) 342:541–9. doi: 10.1056/NEJM200002243420803
41. Ojha A, Chelimsky TC, Chelimsky G. Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr. (2011) 158:20–3. doi: 10.1016/j.jpeds.2010.07.005
42. Deb A, Morgenshtern K, Culbertson CJ, Wang LB, Hohler AD. A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. Proc (Bayl Univ Med Cent). (2015) 28:157–9. doi: 10.1080/08998280.2015.11929217
43. Bagai K, Song Y, Ling JF, Malow B, Black BK, Biaggioni I, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. (2011) 7:204–10. doi: 10.5664/jcsm.28110
44. Chelimsky G, Kovacic K, Nugent M, Mueller A, Simpson P, Chelimsky TC. Comorbid conditions do not differ in children and young adults with functional disorders with or without postural tachycardia syndrome. J Pediatr. (2015) 167:120–4. doi: 10.1016/j.jpeds.2015.03.039
45. Miglis MG, Muppidi S, Feakins C, Fong L, Prieto T, Jaradeh S. Sleep disorders in patients with postural tachycardia syndrome. Clin Auton Res. (2016) 26:67–73. doi: 10.1007/s10286-015-0331-9
46. Miglis MG, Barwick F. Sleep disorders in patients with postural tachycardia syndrome: a review of the literature and guide for clinicians. Auton Neurosci. (2018) 215:62–9. doi: 10.1016/j.autneu.2018.05.002
47. Ross AJ, Medow MS, Rowe PC, Stewart JM. What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res. (2013) 23:305–11. doi: 10.1007/s10286-013-0212-z
48. Bagai K, Peltier AC, Malow BA, Diedrich A, Shibao CA, Black BK, et al. Objective sleep assessments in patients with postural tachycardia syndrome using overnight polysomnograms. J Clin Sleep Med. (2016) 12:727–33. doi: 10.5664/jcsm.5806
49. Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics. (2013) 131:968–80. doi: 10.1542/peds.2012-2610
50. Stewart JM, Medow MS, Montgomery LD, McLeod K. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am J Physiol Heart Circ Physiol. (2004) 286:H1216–22. doi: 10.1152/ajpheart.00738.2003
51. Hainsworth R, Al-Shamma YM. Cardoivascular responses to upright tilting in healthy subjects. Clin Sci. (1988) 74:17–22. doi: 10.1042/cs0740017
52. Streeten DH, Scullard TF. Excessive gravitational blood pooling caused by impaired venous tone is the predominant non-cardiac mechanism of orthostatic intolerance. Clin Sci. (1996) 90:277–85. doi: 10.1042/cs0900277
53. Stewart JM, Weldon A. Vascular perturbations in the chronic orthostatic intolerance of the postural orthostatic tachycardia syndrome. J Appl Physiol. (2000) 89:1505–12. doi: 10.1152/jappl.2000.89.4.1505
54. Stewart JM, Weldon A. Reflex vascular defects in the orthostatic tachycardia syndrome of adolescents. J Appl Physiol. (2001) 90:2025–31. doi: 10.1152/jappl.2001.90.6.2025
55. Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. (2002) 105:2274–81. doi: 10.1161/01.CIR.0000016348.55378.C4
56. Mustafa HI, Raj SR, Diedrich A, Black BK, Paranjape SY, Dupont WD, et al. Altered systemic hemodynamic and baroreflex response to angiotensin II in postural tachycardia syndrome. Circ Arrhythm Electrophysiol. (2012) 5:173–80. doi: 10.1161/CIRCEP.111.965343
57. Brown CM, Hainsworth R. Assessment of capillary fluid shifts during orthostatic stress in normal subjects and subjectswith orthostatic intolerance. Clin Auton Res. (1999) 9:69–73. doi: 10.1007/BF02311762
58. Stewart JM, Taneja I, Medow MS. Reduced central blood volume and cardiac output and increased vascular resistance during static handgrip exercise in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. (2007) 293:H1908–17. doi: 10.1152/ajpheart.00439.2007
59. Lüscher TF. Endothelium-derived nitric oxide: the endogenous nitrovasodilator in the human cardoivascular system. Eur Heart J. (1991) 12(Suppl. E):2–11. doi: 10.1093/eurheartj/12.suppl_E.2
60. Liao Y, Chen S, Liu X, Zhang Q, Ai Y, Wang Y, et al. Flow-mediated vasodilation and endothelium function in children with postural orthostatic tachycardia syndrome. Am J Cardoil. (2010) 106:378–82. doi: 10.1016/j.amjcard.2010.03.034
61. d'Emmanuele di Villa Bianca R, Sorrentino R, Coletta C, Mitidieri E, Rossi A, Vellecco V, et al. Hydrogen sulfide-induced dual vascular effect involves arachidonic acid cascade in rat mesenteric arterial bed. J Pharmacol Exp Ther. (2011) 337:59–64. doi: 10.1124/jpet.110.176016
62. Yang J, Zhao J, Du S, Liu D, Fu C, Li X, et al. Postural orthostatic tachycardia syndrome with increased erythrocytic hydrogen sulfide and reponse to midodrine hydrochloride. J Pediatr. (2013) 163:1169–73. doi: 10.1016/j.jpeds.2013.04.039
63. Zhang F, Li X, Stella C, Chen L, Liao Y, Tang C, et al. Plasma hydrogen sulfide in differential diagnosis between vasovagal syncope and postural orthostatic tachycardia syndrome in children. J Pediatr. (2012) 160:227–31. doi: 10.1016/j.jpeds.2011.08.008
64. Liu D, Jin H, Tang C, Du J. Sulfur doixide: a novel gaseous signal in the regulation of cardoivascular functions. Mini Rev Med Chem. (2010) 10:1039–45. doi: 10.2174/1389557511009011039
65. Du SX, Jin HF, Bu DF, Zhao X, Geng B, Tang CS, et al. Endogenously generated sulfur doixide and its vasorelaxant effect in rats. Acta Pharmacol Sin. (2008) 29:923–30. doi: 10.1111/j.1745-7254.2008.00845.x
66. Li HX, Zheng XC, Chen SY, Liao Y, Han ZH, Huang P, et al. Increased endogenous sulfur doixide involved in the pathogenesis of postural tachycardia syndrome in children: a case-control study. Chin Med J. (2018) 131:435–9. doi: 10.4103/0366-6999.225051
67. Telli G, Erac Y, Tel BC, Gumusel B. Mechanism of adrenomedullin 2/ intermedin mediated vasorelaxation in rat main pulmonary artery. Peptides. (2018) 103:65–71. doi: 10.1016/j.peptides.2018.03.015
68. Li H, Liao Y, Han Z, Wang Y, Liu P, Zhang Q, et al. Changes of plasma intermedin during head-up tilt test in children with postural tachycardia syndrome and its significance. Zhonghua Er Ke Za Zhi. (2015) 53:375–8. doi: 10.3760/cma.j.issn.0578-1310.2015.05.013
69. Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. (2009) 191:341–66. doi: 10.1007/978-3-540-68964-5_15
70. Chen HH, Burnett JC Jr. C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. J Cardoivasc Pharmacol. (1998) 32(Suppl 3):S22–8.
71. Li H, Han Z, Chen S, Liao Y, Wang Y, Liu P, et al. Total peripheral vascular resistance, cardiac output, and plasma C-type natriuretic peptide level in children with postural tachycardia syndrome. J Pediatr. (2015) 166:1385–9. doi: 10.1016/j.jpeds.2015.03.032
72. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. (2001) 409:307–12. doi: 10.1038/35053000
73. Oriowo MA. Perivascular adipose tissue, vascular reactivity and hypertension. Med Princ Pract. (2015) 24(Suppl. 1):29–37. doi: 10.1159/000356380
74. Chen C, Jiang J, Lü JM, Chai H, Wang X, Lin PH, et al. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. (2010) 299:H193–201. doi: 10.1152/ajpheart.00431.2009
75. Bai W, Han Z, Chen S, Li H, Song J, Qi J, et al. Serum resistin negatively correlates with clinical severity of postural tachycardia syndrome in children. Pediatr Cardoil. (2017) 38:1639–44. doi: 10.1007/s00246-017-1708-4
76. Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. (2017) 21(Suppl. 1):S6–20. doi: 10.1111/hdi.12542
77. Hsiao HY, Chung CW, Santos JH, Villaflores OB, Lu TT. Fe in biosynthesis, translocation, and signal transduction of NO: toward bioinorganic engineering of dinitrosyl iron complexes into NO-delivery scaffolds for tissue engineering. Dalton Trans. (2019) 48:9431–53. doi: 10.1039/C9DT00777F
78. Antiel RM, Caudill JS, Burkhardt BE, Brands CK, Fisher PR. Iron insufficiency and hypovitaminosis D in adolescents with chronic fatigue and orthostatic intolerance. South Med J. (2011) 104:609–11. doi: 10.1097/SMJ.0b013e3182246809
79. Jarjour IT, Jarjour LK. Low iron storage and mild anemia in postural tachycardia syndrome in adolescents. Clin Auton Res. (2013) 23:175–9. doi: 10.1007/s10286-013-0198-6
80. Choi JW, Pai SH, Kim SK, Ito M, Park CS, Cha YN. Iron deficiency anemia increases nitric oxide production in healthy adolescents. Ann Hematol. (2002) 81:1–6. doi: 10.1007/s00277-001-0409-4
81. Lu W, Yan H, Wu S, Xu W, Jin H, Du J. Hemocytometric measures predict the efficacy of oral rehydration for children with postural tachycardia syndrome. J Pediatr. (2017) 187:220–4. doi: 10.1016/j.jpeds.2017.04.034
82. Shibao C, Arzubiaga C, Roberts LJ 2nd, Raj S, Black B, Harris P, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. (2005) 45:385–90. doi: 10.1161/01.HYP.0000158259.68614.40
83. Romero SA, McCord JL, Ely MR, Sieck DC, Buck TM, Luttrell MJ, et al. Mast cell degranulation and de novo histamine formation contribute to sustained post exercise vasodilation in humans. J Appl Physiol. (2017) 122:603–10. doi: 10.1152/japplphysiol.00633.2016
84. Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet- activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. (2008) 358:28–35. doi: 10.1056/NEJMoa070030
85. Doherty TA, White AA. Postural orthostatic tachycardia syndrome and the potential role of mast cell activation. Auton Neurosci. (2018) 215:83–8. doi: 10.1016/j.autneu.2018.05.001
86. Dahan S, Tomljenovic L, Shoenfeld Y. Postural orthostatic tachycardia syndrome (POTS) – a novel member of the autoimmune family. Lupus. (2016) 25:339–42. doi: 10.1177/0961203316629558
87. Li J, Zhang Q, Hao H, Jin H, Du J. Clinical features and management of postural tachycardia syndrome in children: a single-center experience. Chin Med J. (2014) 127:3684–9. doi: 10.3760/cma.j.issn.0366-6999.20140244
88. Watari M, Nakane S, Mukaino A, Nakajima M, Mori Y, Maeda Y, et al. Autoimmune postural orthostatic tachycardia syndrome. Ann Clin Transl Neurol. (2018) 5:486–92. doi: 10.1002/acn3.524
89. Wang Z, Low PA, Vernino S. Antibody-mediated impairment and homeostatic plasticity of autonomic ganglionic synaptic transmission. Exp Neurol. (2010) 222:114–9. doi: 10.1016/j.expneurol.2009.12.016
90. Fedorowski A, Li H, Yu X, Koelsch KA, Harris VM, Liles C, et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. (2017) 19:1211–9. doi: 10.1093/europace/euw154
91. Yu XC, Li HL, Murphy TA, Nuss Z, Liles J, Liles C, et al. Angiotensin II type 1 receptor autoantibodies in postural tachycardia syndrome. J Am Heart Assoc. (2018) 7:e008351. doi: 10.1161/JAHA.117.008351
92. Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. (2014) 3:e000755. doi: 10.1161/JAHA.113.000755
93. Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus. (2015) 24:1364–9. doi: 10.1177/0961203315587566
94. Vernino S, Stiles LE. Autoimmunity in postural orthostatic tachycardic syndrome: current understanding. Auton Neurosci. (2018) 215:78–82. doi: 10.1016/j.autneu.2018.04.005
95. Kizilbash SJ, Ahrens SP, Bruce BK, Chelimsky G, Driscoll SW, Harbeck-Weber C, et al. Adolescent fatigue, POTS, and recovery: a guide for clinicians. Curr Probl Pediatr Adolesc Health Care. (2014) 44:108–33. doi: 10.1016/j.cppeds.2013.12.014
96. Xu WR, Jin HF, Du JB. Pathogenesis and individualized treatment for postural tachycardia syndrome in children. Chin Med J. (2016) 129:2241–5. doi: 10.4103/0366-6999.189915
97. Lin J, Zhao H, Shen J, Jiao F. Salivary cortisol levels predict therapeutic response to a sleep-promoting method in children with postural tachycardia syndrome. J Pediatr. (2017) 191:91–5. doi: 10.1016/j.jpeds.2017.08.039
98. Sheldon RS, Grubb BP, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. (2015) 12:e41–63. doi: 10.1016/j.hrthm.2015.03.029
99. Winker R, Barth A, Bidmon D, Ponocny I, Weber M, Mayr O, et al. Endurance exercise training in orthostatic intolerance: a randomized, controlled trial. Hypertension. (2005) 45:391–8. doi: 10.1161/01.HYP.0000156540.25707.af
100. Lu W, Yan H, Wu S, Chen S, Xu W, Jin H, et al. Electrocardoigraphy-derived predictors for therapeutic response to treatment in children with postural tachycardia syndrome. J Pediatr. (2016) 176:128–33. doi: 10.1016/j.jpeds.2016.05.030
101. Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. (2011) 58:167–75. doi: 10.1161/HYPERTENSIONAHA.111.172262
102. Fu Q, Levine BD. Exercise in the postural orthostatic tachycardia syndrome. Auton Neurosci. (2015) 188:86–9. doi: 10.1016/j.autneu.2014.11.008
103. Shibata S, Fu Q, Bivens TB, Hastings JL, Wang W, Levine BD. Short-term exercise training improves the cardoivascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol. (2012) 590:3495–505. doi: 10.1113/jphysiol.2012.233858
104. Li H, Wang Y, Liu P, Chen Y, Feng X, Tang C, et al. Body mass index (BMI) is associated with the therapeutic response to oral rehydration solution in children with postural tachycardia syndrome. Pediatr Cardoil. (2016) 37:1313–8. doi: 10.1007/s00246-016-1436-1
105. Lin J, Liu P, Wang Y, Li H, Li X, Zhao J, et al. Evaluation of the changes in heart rate during head-up test predicting the efficacy of oral rehydration salts on postural tachycardia syndrome in children. Zhonghua Er Ke Za Zhi. (2015) 53:25–9. doi: 10.2106/JBJS.O.00099
106. McClellan KJ, Wiseman LR, Wilde MI. Midodrine. A review of its therapeutic use in the management of orthostatic hypotension. Drugs Aging. (1998) 12:76–86. doi: 10.2165/00002512-199812010-00007
107. Chen L, Wang L, Sun J, Qin J, Tang C, Jin H, et al. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ J. (2011) 75:927–31. doi: 10.1253/circj.CJ-10-0514
108. Lin J, Jin H, Du J. Assessment of therapeutic biomarkers in the treatment of children with postural tachycardia syndrome and vasovagal syncope. Cardoil Young. (2014) 24:792–6. doi: 10.1017/S1047951114000316
109. Liao Y, Yang J, Zhang F, Chen S, Liu X, Zhang Q, et al. Flow-mediated vasodilation as a predictor of therapeutic response to midodrine hydrochloride in children with postural orthostatic tachycardia syndrome. Am J Cardoil. (2013) 112:816–20. doi: 10.1016/j.amjcard.2013.05.008
110. Zhang F, Li X, Ochs T, Chen L, Liao Y, Tang C, et al. Midregional pro-adrenomedullin as a predictor for therapeutic response to midodrine hydrochloride in children with postural orthostatic tachycardia syndrome. J Am Coll Cardoil. (2012) 60:315–20. doi: 10.1016/j.jacc.2012.04.025
111. Zhao J, Tang C, Jin H, Du J. Plasma copeptin and therapeutic effectiveness of midodrine hydrochloride on postural tachycardia syndrome in children. J Pediatr. (2014) 165:290–4. doi: 10.1016/j.jpeds.2014.04.032
112. Deng W, Liu Y, Liu AD, Holmberg L, Ochs T, Li X, et al. Difference between supine and upright blood pressure associates to the efficacy of midodrine on postural orthostatic tachycardia syndrome (POTS) in children. Pediatr Cardoil. (2014) 35:719–25. doi: 10.1007/s00246-013-0843-9
113. Li HX, Deng WJ, Zhang CY, Jin HF, Du JB. Predictive value of upright blood pressure change for long-term prognosis of children with postural tachycardia syndrome treated with midodrine hydrochloride. Zhonghua Er Ke Za Zhi. (2016) 54:519–22. doi: 10.3760/cma.j.issn.0578-1310.2016.07.009
114. Ogrodowczyk M, Dettlaff K, Jelinska A. Beta-blockers: current state of knowledge and perspectives. Mini Rev Med Chem. (2016) 16:40–54. doi: 10.2174/1389557515666151016125948
115. López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. (2004) 25:1341–62. doi: 10.1016/j.ehj.2004.06.002
116. Lai CC, Fischer PR, Brands CK, Fisher JL, Porter CB, Driscoll SW, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol. (2009) 32:234–8. doi: 10.1111/j.1540-8159.2008.02207.x
117. Lin J, Han Z, Li H, Chen SY, Li X, Liu P, et al. Plasma C-type natriuretic peptide as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. PLoS ONE. (2015) 10:e0121913. doi: 10.1371/journal.pone.0121913
Keywords: postural tachycardia syndrome, pathogenesis, management, children, adolescents
Citation: Chen G, Du J, Jin H and Huang Y (2020) Postural Tachycardia Syndrome in Children and Adolescents: Pathophysiology and Clinical Management. Front. Pediatr. 8:474. doi: 10.3389/fped.2020.00474
Received: 01 March 2020; Accepted: 06 July 2020;
Published: 20 August 2020.
Edited by:
Giovanni Biglino, University of Bristol, United KingdomReviewed by:
Ju Liu, Shandong University, ChinaFederico Gutierrez-Larraya, University Hospital La Paz, Spain
Copyright © 2020 Chen, Du, Jin and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaqian Huang, yaqianhuang@126.com
 Guozhen Chen
Guozhen Chen Junbao Du
Junbao Du Hongfang Jin
Hongfang Jin Yaqian Huang
Yaqian Huang