Pediatric Inflammatory Multisystem Syndrome: Statement by the Pediatric Section of the European Society for Emergency Medicine and European Academy of Pediatrics
- 1Section of Paediatric Infectious Diseases, Department of Infectious Diseases, Faculty of Medicine, Imperial College London, London, United Kingdom
- 2Department of Paediatrics, Ghent University, Ghent, Belgium
- 3Dr. von Hauner Children's Hospital, Ludwig-Maximilians-Universität München, Munich, Germany
- 4Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom
- 5Health Education North East, Newcastle Upon Tyne, United Kingdom
- 6Paediatric Emergency Department, Hopital Universitaire Robert-Debre, Paris, France
- 7Studio Pediatrico, Padova, Italy
- 8Department of Medicine, European University Cyprus, Nicosia, Cyprus
A rise in cases with a new hyperinflammatory disease in children has been reported in Europe and in the Unites States of America, named the Pediatric Inflammatory Multisystem Syndrome—temporally associated with SARS-CoV-2 (PIMS-TS). There appears to be a wide spectrum of signs and symptoms with varying degrees of severity, including a toxic shock like presentation with hypovolaemia and shock, and a Kawasaki-like presentation with involvement of the coronary arteries. Most of these children have evidence of a previous infection with SARS-CoV-2, or a history of significant exposure, but not all. Limited data exist on the incidence of PIMS-TS, but it remains a rare condition. Early recognition and escalation of care is important to prevent the development of serious sequelae, such as coronary artery aneurysms. Clinicians assessing febrile children in primary and secondary care should include PIMS-TS in their differential diagnoses. In children fulfilling the case definition, additional investigations should be undertaken to look for evidence of inflammation and multiorgan involvement. Suspected cases should be discussed with experts in pediatric infectious diseases at an early stage, and advice should be sought from critical care in more severe cases early. There is limited consensus on treatment; but most children have been treated with immunoglobulins or steroids, and with early consideration of biologicals such anti-TNF and anti-IL1 agents. Treatment should ideally be within the context of controlled treatment trials. Clinicians are encouraged to document and share their cases using research registries.
Statement
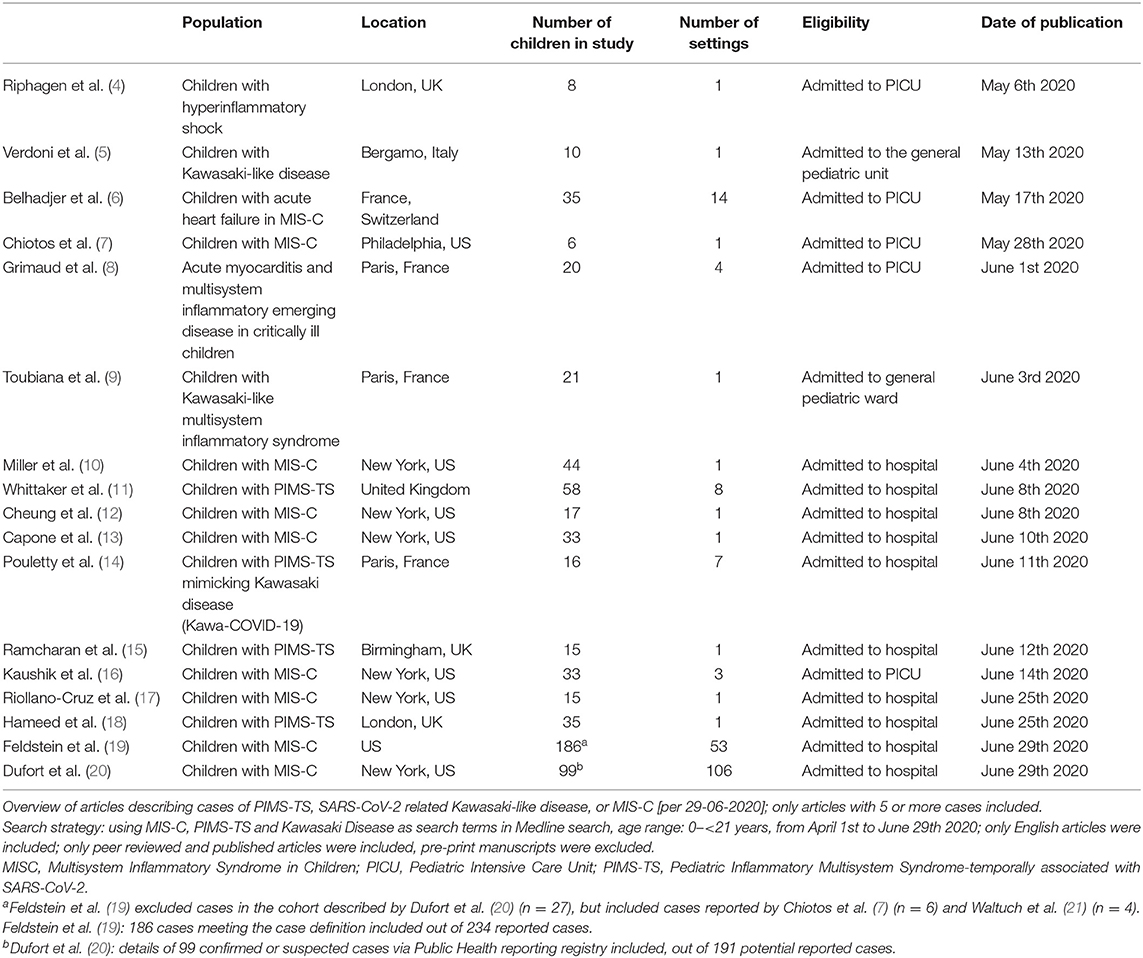
According to the currently available evidence, SARS-CoV-2 infection in children is rarely associated with severe disease (1), children are less likely to be infected compared with adults, and children are likely to be less infectious compared with infective adults (2, 3). However, multiple cases of children with a new hyperinflammatory condition in children subsequent to SARS-CoV-2 infection have been reported in Europe and the United States of America (Tables 1, 2).
This statement has been written on behalf of both the Pediatric Section of the European Society for Emergency Medicine and the European Academy of Pediatrics to provide an update for health care professionals in primary or secondary care undertaking assessments of acutely unwell children. This statement aims to
- provide information about the Pediatric Inflammatory Multisystem Syndrome temporally associated with SARS-CoV-2 (PIMS-TS);
- give initial guidance on the clinical assessment and management of children suspected of this new condition for health care professionals dealing with acutely unwell children;
- point out useful resources on the recognition and management of these children.
This statement does not address the management of children with PIMS-TS admitted under specialist care in the fields of infectious diseases, cardiology, or critical care.
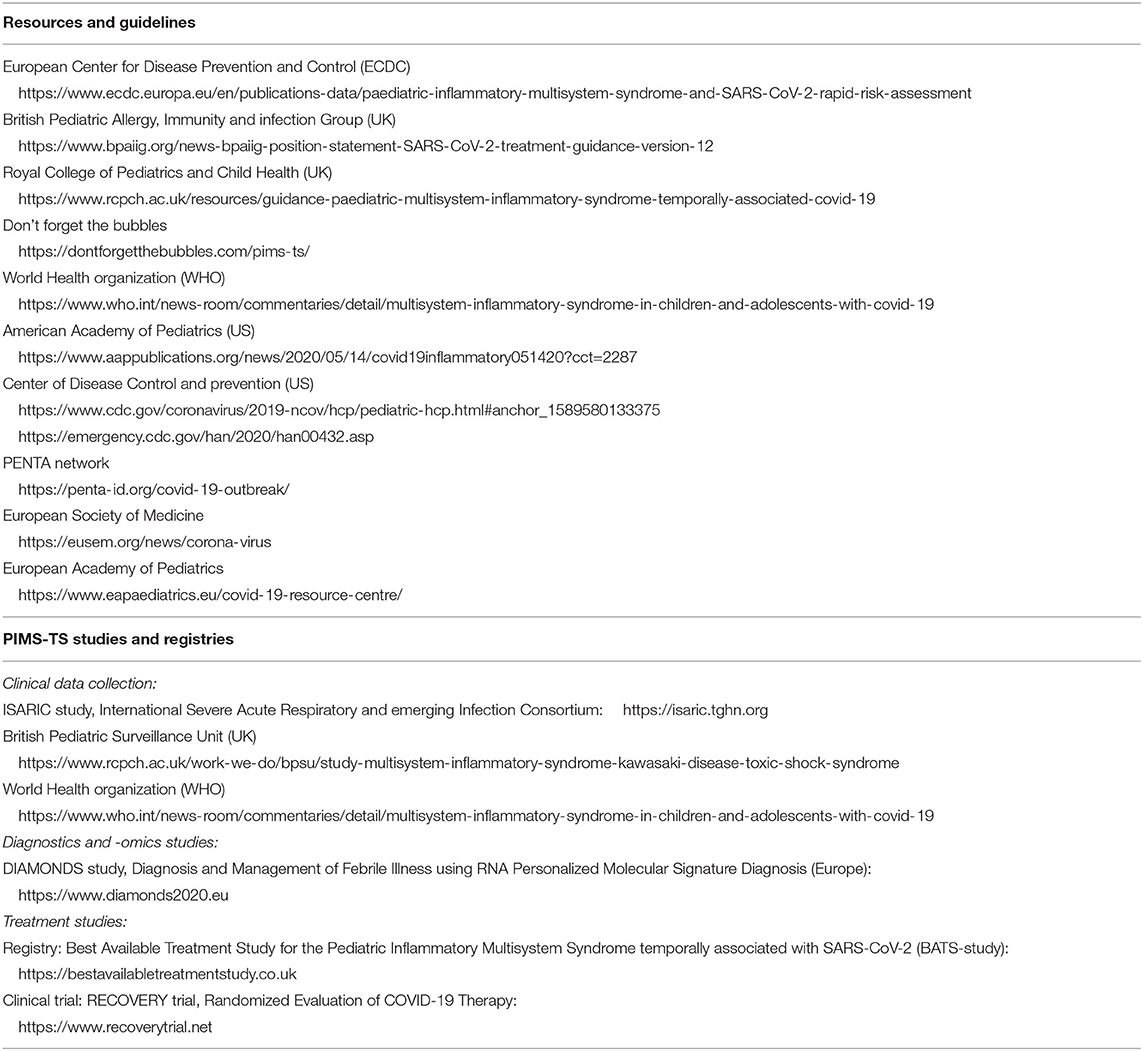
Historically, a link between coronaviruses and Kawasaki syndrome was first proposed in 2005 (23). However, a causal relation has thus far not been proven, and the cause of Kawasaki Disease is currently unknown (24, 25). Awareness of the new hyperinflammatory condition affecting children was raised by clinicians in the UK, leading to an alert by the UK National Health Service, on April 25th 2020. Since then many other countries have issued similar alerts with guidance on management of these children. The first case report of a child with Kawasaki-like disease was reported in the international literature on April 22nd (26). The first case series with cases from the UK was reported on May 7th (5), followed by similar case series from Italy on May 13th [9]. Cases have now been reported throughout Europe and North America, with the European Center for Disease Control providing an up to date overview (27). The European Society for Medicine and the European Academy of Pediatrics hosted informative webinars (links in Table 3).
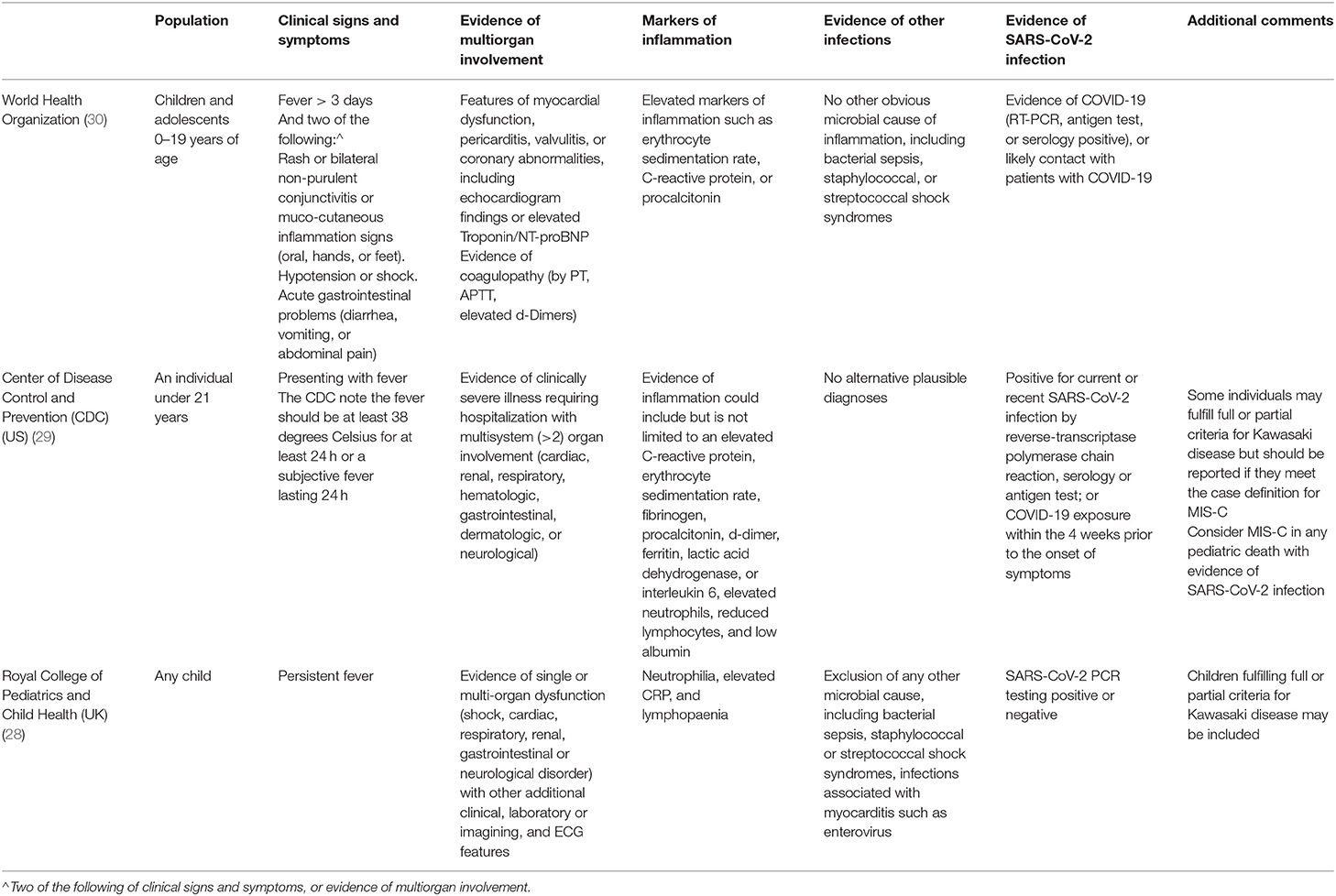
The initial guidance from the Royal College of Pediatrics and Child Health in the United Kingdom provided a case definition and called this emerging disease entity the Pediatric Inflammatory Multisystem Syndrome—temporally associated with SARS-CoV-2 (PIMS-TS) (28). In the United States this disorder is referred to as the multisystem inflammatory syndrome in children (MIS-C) (29). By now, three case definitions are in use (Table 4). We recommend adhering to the case definition by the World Health Organization, as at present this definition appears to capture the spectrum of presenting signs and symptoms best (30). All current bodies proposing case definitions acknowledge the rapidly developing evidence and the need for continued evolvement of the case definitions.
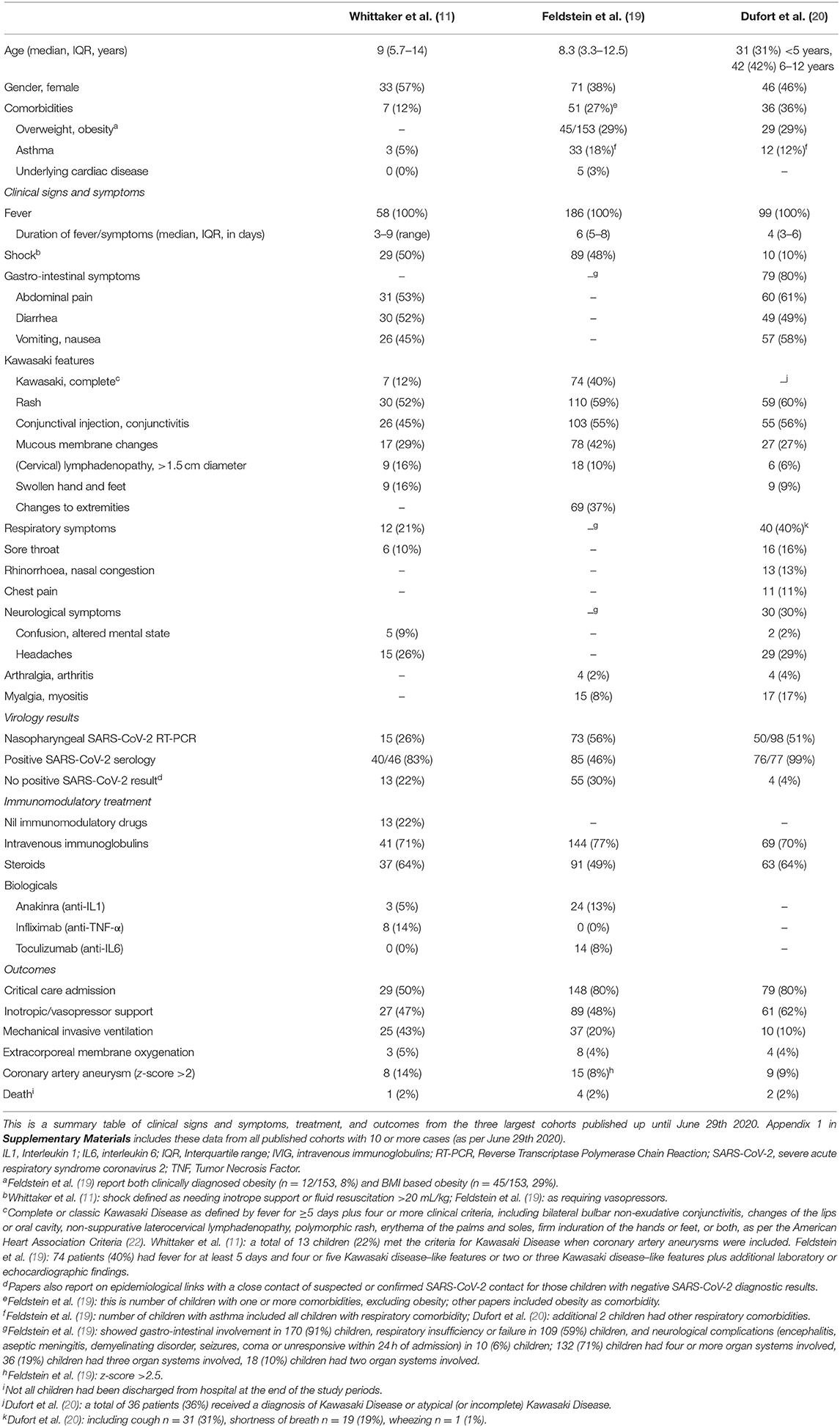
The three case definitions have in common that they describe a population of children at risk with (1) persistent fever, (2) clinical signs and biochemical profiles reflecting ongoing inflammation, (3) the potential of multiorgan involvement (and most importantly the risk of cardiac involvement), (4) the absence of other reasonable explanations of the acute illness, and (5) evidence of a preceding SARS-CoV-2 infection or exposure to a suspected or confirmed case. Notably, children of all ages appear affected, but it has been more commonly reported in the adolescent age group, which is distinctly different from the children with classic Kawasaki Disease and children with toxic shock syndrome (5, 11). Also, boys and girls appear affected similarly (Table 2, Appendix 1 in Supplementary Materials), whereas children of black and minority ethnicity backgrounds appear affected more often (4, 9, 11).
It is important to stress that the incidence of PIMS-TS is rare with an estimated incidence at 2 in 100,000 persons <21 years of age (20). Also, although some deaths in children with PIMS-TS have been reported, as well as children requiring extra-corporeal membrane oxygenation (27), many affected children don't require critical care and have a full, and rapid clinical recovery (Table 2, Appendix 1 in Supplementary Materials).
There appears to be a wide spectrum of signs and symptoms with varying degree of severity (Table 2, Appendix 1 in Supplementary Materials). All children present with persistent fever. Typically, a substantial proportion of children present with abdominal symptoms, with some having symptoms of such severity that they had US and CT imaging, and some undergoing surgical procedures (10, 11, 31). The PIMS-TS spectrum includes disease entities that need urgent recognition and treatment, such as a toxic shock like presentation with hypovolaemia and shock, as well as Kawasaki-like disease with involvement of the coronary arteries (4–6). Children with Kawasaki-like disease will have all or some of the typical features, such as a rash and skin changes, conjunctival injection, mucous membrane changes, unilateral lymphadenopathy, and swollen hands and feet (6, 11). A majority of the reported children with PIMS-TS had evidence of multiorgan involvement (19). A number of these children will subsequently need critical care with (multiorgan) supportive care, whereas others can be managed safely on normal pediatric wards. Almost exclusively, these children appear ill and present in a manner that warrants enough concern to admit them to hospital and to perform additional diagnostic tests. By now, it appears that the biochemical profile of children with PIMS-TS is distinct from that of children with classic Kawasaki Disease and Kawasaki Disease with shock, with children with PIMS-TS having noticeably higher markers of inflammation (e.g., CRP, ferritin), marked lymfopaenia, greater elevation of troponin, and higher levels of fibrinogen; it is less clear how the biochemical profiles differ from children with toxic shock syndrome (11). Lastly, it is important to note, however, that the cases reported in the literature are likely to represent the more severe spectrum of disease.
Some of these children test positive for SARS-CoV-2 on PCR, and most have evidence of a previous infection with positive SARS-CoV-2 serology (Table 2, Appendix 1 in Supplementary Materials); however, some cases do not have a history of exposure to SARS-CoV-2 and do not have clear evidence of a (previous) SARS-CoV-2 infection (11). It remains important to perform (serial) tests to confirm the presence of active or previous infection with SARS-CoV-2, and to further our understanding of the relationship between and the underlying mechanisms of infection with SARS-CoV-2 and PIMS-TS (32).
After a reduction in the number of presentations to emergency departments during the lockdown periods across Europe (33), the raised awareness of PIMS-TS in the media might lead to an increase of presentations of children with fever and other infectious symptoms to acute care facilities. Public health bodies should communicate clearly with the general public about the warning signs of PIMS-TS and about when to seek care as to prevent delayed presentations (34, 35). Moreover, an increase in incidence of PIMS-TS has a 4–6 weeks delay after the onset of a COVID-19 outbreak (19, 20, 36).
As with any emerging disease, it might not immediately be straightforward deciding who is at risk of PIMS-TS, and in whom to perform additional investigations. This could adversely lead to false positive test results creating clinical uncertainty, erroneous clinical decision making, and heightened parental anxiety. Similarly, there is a risk that children will be classified as having sepsis, exacerbated by the risk of delayed presentations during a pandemic (34), and that they will be managed as such in their local hospital, without appropriate investigations and escalation of care. There is also a risk that many children with other, more common, childhood infections will be classified as suspected PIMS-TS and will undergo unnecessary diagnostic tests and treatment. For children with abdominal symptoms, an early diagnosis of PIMS-TS may inadvertently lead to a delay in surgical review, and concerns of surgical abdominal pathology could inversely lead to a delay in recognition of a diagnosis of PIMS-TS. Involvement of senior clinical decision makers and adherence to up-to-date case definitions and guidance are advisable.
Altogether, the emergence of PIMS-TS dictates careful clinical decision making when dealing with febrile children presenting to acute care facilities across Europe:
1. The need for additional investigations should be based on the initial clinical assessment of a febrile child by a clinician experienced in pediatric care.
2. The management of febrile children who appear clinically well, with a clear focus of infection, and who would previously have been deemed well enough for discharge from our care without any interventions should generally not change; this is likely to reflect the management of the vast majority of febrile children presenting to acute care facilities.
3. Ensure that the presence or absence of all Kawasaki-like features are documented for all febrile children; ensure that a blood pressure and a full set of vital parameters are recorded.
4. Clinicians should be aware of and follow published guidance on PIMS-TS by (inter)national societies (Table 3).
5. Perform additional investigations at an early stage in unwell appearing febrile children with sufficient heightened clinical concern as based on the case definition, or with clinical signs of inflammation and/or shock.
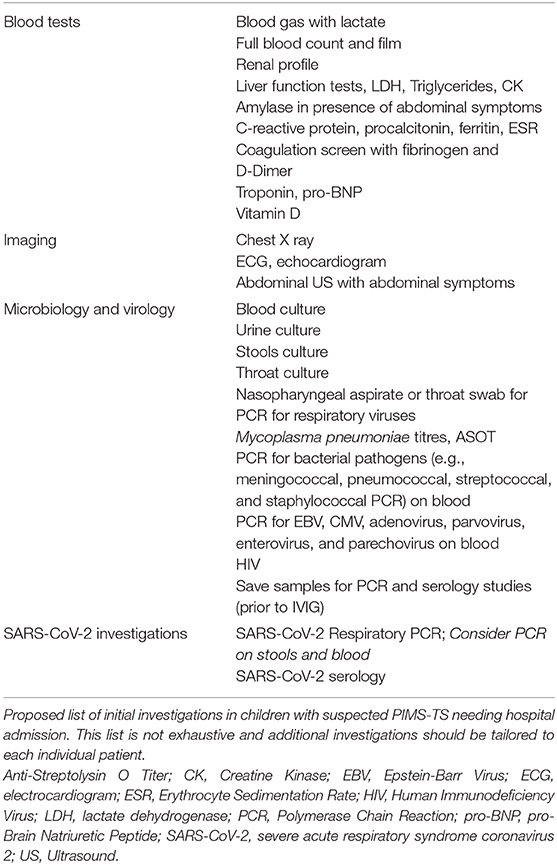
6. Additional laboratory investigations should include markers of myocardial involvement (such as troponin, pro-BNP), hypercoagulation (including APTT, PT, fibrinogen, D-Dimer), markers of inflammation (such as C-reactive protein, procalcitonin, ferritin, IL-6, erythrocyte sedimentation rate), creatine kinase, lactate dehydrogenase, full blood count, renal profile, vitamin D level, and liver function tests, including triglycerides; include amylase in the presence of abdominal symptoms (Table 5).
7. Request appropriate microbiology, including a blood culture, and virology tests to rule out any other infectious cause of the illness (Table 5). We also suggest PCR of oropharyngeal swab for SARS-CoV-2 in first instance. Save an EDTA and serum sample for PCR and serology studies prior to giving intravenous immunoglobulins.
8. An ECG and echocardiogram should be part of the diagnostic work-up of all children with suspected PIMS-TS.
9. We recommend early discussion with a specialist pediatric infectious diseases team if there is clinical and biochemical evidence of inflammation and suspicion of PIMS-TS. We also recommend early discussion with a pediatric critical care team in children in need for single or multiple organ support. Any evidence of myocardial involvement (for example, raised troponin or pro-BNP, or concerning ECG or echocardiogram) warrants early escalation to a center with pediatric cardiology expertise.
This statement does not cover the specific treatment of individual children with PIMS-TS; all these children should be discussed with a pediatric expertise center. At present, there is no high-level evidence to support any best care recommendations about the treatment of these children outside providing optimal supportive care. For children with Kawasaki-like disease, the mainstay of treatment consists of intravenous immunoglobulins, steroids and possibly additional biologicals, such as anti-TNF, anti-IL1, or anti-IL6, and aspirin. For children presenting with a toxic shock like picture, treatment should focus on early cardiovascular support, treatment and reversal of shock, and intravenous immunoglobulins. Fluid boluses should be titrated carefully in view of the risk of myocardial impairment; inotropes and vasopressors should be started early as per established advanced life support guidelines (37). Another clinical picture describing patients with both Kawasaki-like disease plus shock will need aggressive treatment for both. Standards of antimicrobial stewardship should be upheld, but it will be difficult to withhold broad spectrum antibiotics as part of initial emergency care in most instances, and secondary bacterial infections have been reported. Additionally, many of these children will have evidence of hypercoagulation, and prophylactic or therapeutic anti-coagulation should be considered early in all children with PIMS-TS. It is crucial to highlight that treatment is time critical and should not be delayed. Immunomodulatory treatments should ideally be given in the context of a controlled trial, but it might prove difficult to set up and enroll children in randomized controlled trials for the treatment of PIMS-TS in the near future. The UK RECOVERY trial (Randomized Evaluation of COVID-19 Therapy) is currently the largest randomized controlled trial including children (38). Other international registries are aiming to capture clinical data (e.g. ISARIC study) and the use of different treatments (e.g. BATS study) around the world (Table 3).
A concern of the treatment with immunoglobulins, or convalescent plasma, is the unexplored potential to trigger an antibody mediated response, similar to the one that potentially underlies this hyperinflammatory syndrome.
Recommendations for the intermediate and long-term follow-up of children with PIMS-TS after hospitals discharge should be guided by the tertiary specialty teams. Special consideration should be given to the need of performing post-discharge echocardiograms to exclude late cardiac complications (15).
Recommendations by the Pediatric Section of the European Society of Emergency Medicine and the European Academy of Pediatrics
1. Parents should seek medical attention if their child is unwell, develops any warning signs of fever as described by the traffic light system by the National Institute of Health and Care Excellence, or has a persistent fever for more than 5 days, to avoid delayed presentation (39).
2. Health care professionals in primary and secondary care looking after acutely unwell children are urged to take notice of the emerging PIMS-TS (Pediatric Inflammatory Multisystem Syndrome—temporally associated with SARS-CoV-2) and to be vigilant, as serious sequelae might occur if not recognized and treated in a timely manner.
3. We recommend performing additional investigations as per published guidelines (Table 5) and to refer patients with severe manifestations to specialist centers.
4. Children with PIMS-TS appear to respond well to treatment if recognized promptly with no delay in treatment, and most of the children will have a full and quick recovery. Thus, we urge all primary and secondary care health care professionals to include the diagnosis of PIMS-TS in the differential diagnosis of children with persistent fever for more than 5 days.
5. We recommend that clinicians document and share the data of their patients using research registries (Table 3) to study the full clinical spectrum of presenting signs and symptoms, ascertaining biochemical profiles of these children, validate predictors of disease progression, and to monitor treatment response (40).
Public and Patient Involvement
This statement was derived without input from patients or representatives of the general public.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
RN was funded by an Academic Clinical Lecturer award by the National Institute of Health Research (ACL-2018-21-007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.00490/full#supplementary-material
Abbreviations
Anti-IL1, anti-Interleukin 1; Anti-IL6, anti-Interleukin 6; Anti-TNF, anti-Tumor Necrosis Factor; APTT, Activated Partial Thromboplastin Time; ECG, Electrocardiogram; ESR, Erythrocyte Sedimentation Rate; PCR, Polymerase Chain Reaction; PT, Prothrombin Time; IL-6, Interleukin-6; PIMS-TS, Pediatric Inflammatory Multisystem Syndrome-temporally associated with SARS-CoV-2; pro-BNP, pro-Brain Natriuretic Peptide; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
References
1. Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Heal. (2020). doi: 10.1016/S2352-4642(20)30177-2. [Epub ahead of print].
2. Munro APS, Faust SN. Children are not COVID-19 super spreaders: time to go back to school. Arch Dis Child. (2020) 105:618–9. Available online at: http://adc.bmj.com/content/early/2020/05/05/archdischild-2020-319474.abstract
3. Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. (2020) 39:469–77. doi: 10.1097/INF.0000000000002700
4. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) 395:1607–8. doi: 10.1016/S0140-6736(20)31094-1
5. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020) 395:1771–8. doi: 10.1016/S0140-6736(20)31103-X
6. Zahra B, Mathilde M, Fanny B, Diala K, Antoine L, Samya A, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. (2020) 142:429–36. doi: 10.1161/CIRCULATIONAHA.120.048360
7. Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the Coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. (2020) 9:393–8. doi: 10.1093/jpids/piaa069
8. Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. (2020) 10:69. doi: 10.1186/s13613-020-00690-8
9. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. (2020) 369:m2094. doi: 10.1136/bmj.m2094
10. Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis K. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. (2020). doi: 10.1053/j.gastro.2020.05.079. [Epub ahead of print].
11. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. J Am Med Assoc. (2020) 324:259–69. doi: 10.1001/jama.2020.10369
12. Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. J Am Med Assoc. (2020). doi: 10.1001/jama.2020.10374. [Epub ahead of print].
13. Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. (2020). doi: 10.1016/j.jpeds.2020.06.044. [Epub ahead of print].
14. Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. (2020) 79:999–1006. doi: 10.1136/annrheumdis-2020-218614
15. Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol. (2020) 1–11. doi: 10.1007/s00246-020-02391-2. [Epub ahead of print].
16. Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, et al. Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York City. J Pediatr. (2020). doi: 10.1016/j.jpeds.2020.06.045. [Epub ahead of print].
17. Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Posada R, Sordillo EM, et al. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience. J Med Virol. (2020). doi: 10.1002/jmv.26224. [Epub ahead of print].
18. Hameed S, Elbaaly H, Reid CEL, Santos RMF, Shivamurthy V, Wong J, et al. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. (2020) 202543. doi: 10.1148/radiol.2020202543. [Epub ahead of print].
19. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. Children and Adolescents. N Engl J Med. (2020) 383:334–46. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/32598831
20. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. (2020) 383:347–58. doi: 10.1056/NEJMoa2021756
21. Waltuch T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. (2020) S0735-6757(20)30403-4. doi: 10.1016/j.ajem.2020.05.058
22. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
23. Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human Coronavirus and Kawasaki disease. J Infect Dis. (2005) 191:499–502. doi: 10.1086/428291
24. Menikou S, Langford PR, Levin M. Kawasaki disease: the role of immune complexes revisited. Front Immunol. (2019) 10:1–11. doi: 10.3389/fimmu.2019.01156
25. Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. (2015) 100:1084–8. doi: 10.1136/archdischild-2014-307536
26. Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Bradley Segal J, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. (2020) 10:537–40. Available online at: http://hosppeds.aappublications.org/content/early/2020/04/06/hpeds.2020-0123.abstract
27. European Cenre fo Disease Prevention and Control. Paediatric inflammatory Multisystem Syndrome and Sars-Cov-2 Rapid Risk Assessment. (2020). Available online at: https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment
28. Royal College of Paediatrics and Child Health. Paediatric Multisystem Inflammatory Syndrome Temporally Associated With COVID-19. (2020) (accessed May 6, 2020)p. 1–6. Available online at: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19
29. Center for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019. (COVID-19) (2020). Available online at: https://emergency.cdc.gov/han/2020/han00432.asp
30. World Health Organisation. Multisystem Inflammatory Syndrome in Children and Adolescents With COVID-19. (2020). p. 1–3. Available online at: https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
31. Tullie L, Ford K, Bisharat M, Watson T, Thakkar H, Mullassery D, et al. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Heal. (2020) 4:e19–20. doi: 10.1016/S2352-4642(20)30165-6
32. Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. (2020) 395:1741–3. doi: 10.1016/S0140-6736(20)31129-6
33. Isba R, Edge R, Jenner R, Broughton E, Francis N, Butler J. Where have all the children gone? Decreases in paediatric emergency department attendances at the start of the COVID-19 pandemic of 2020. Arch Dis Child. (2020) 105:704. Available online at: http://adc.bmj.com/content/early/2020/05/05/archdischild-2020-319385.abstract
34. Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Heal. (2020) 4:e10–1. doi: 10.1016/S2352-4642(20)30108-5
35. Lynn RM, Avis JL, Lenton S, Amin-Chowdhury Z, Ladhani SN. Delayed access to care and late presentations in children during the COVID-19 pandemic: a snapshot survey of 4075 paediatricians in the UK and Ireland. Arch Dis Child. (2020). doi: 10.1136/archdischild-2020-319848. [Epub ahead of print].
36. Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Eurosurveillance. (2020) 25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010
37. Maconochie IK, Bingham R, Eich C, López-Herce J, Rodríguez-Núñez A, Rajka T, et al. European resuscitation council guidelines for resuscitation 2015. Section 6. Paediatric life support. Resuscitation. (2015) 95:223–48. doi: 10.1016/j.resuscitation.2015.07.028
38. Wilkinson E. RECOVERY trial: The UK covid-19 study resetting expectations for clinical trials. BMJ. (2020) 369:m1626. doi: 10.1136/bmj.m1626
39. National Institute of Health and Care Excellence (NICE). NICE Clinical Guideline 160: Fever in Under 5s: Assessment and Initial Management Clinical Guideline. (2013). Available online at: http://www.nice.org.uk/guidance/cg160
40. Ladhani SN, Amin-Chowdhury Z, Amirthalingam G, Demirjian A, Ramsay ME. Prioritising paediatric surveillance during the COVID-19 pandemic. Arch Dis Child. (2020) 105:613–5. Available online at: http://adc.bmj.com/content/early/2020/05/19/archdischild-2020-319363.abstract
Keywords: children, COVID-19, SARS-CoV-2, PIMS-TS, MIS-C, Kawasaki-like disease, fever
Citation: Nijman RG, De Guchtenaere A, Koletzko B, Ross Russell R, Copley S, Titomanlio L, del Torso S and Hadjipanayis A (2020) Pediatric Inflammatory Multisystem Syndrome: Statement by the Pediatric Section of the European Society for Emergency Medicine and European Academy of Pediatrics. Front. Pediatr. 8:490. doi: 10.3389/fped.2020.00490
Received: 26 May 2020; Accepted: 13 July 2020;
Published: 28 August 2020.
Edited by:
Christèle Gras-Le Guen, Centre Hospitalier Universitaire (CHU) de Nantes, FranceReviewed by:
Eitan Naaman Berezin, Santa Casa of Sao Paulo, BrazilJulia Clark, Children's Health Queensland, Australia
Copyright © 2020 Nijman, De Guchtenaere, Koletzko, Ross Russell, Copley, Titomanlio, del Torso and Hadjipanayis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruud G. Nijman, r.nijman@imperial.ac.uk
 Ruud G. Nijman
Ruud G. Nijman Ann De Guchtenaere2
Ann De Guchtenaere2  Berthold Koletzko
Berthold Koletzko Rob Ross Russell
Rob Ross Russell Adamos Hadjipanayis
Adamos Hadjipanayis