Effect of Different Milk Diet on the Level of Fecal Calprotectin in Very Preterm Infants
- Department of Woman and Child Health and Public Health, Child Health Area, Fondazione Policlinico Universitario A. Gemelli, IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy
Objective: To evaluate the course of fecal calprotectin (FC) in very preterm infants over the first 15 days of life in relation to the type of milk diet.
Methods: This study was part of a randomized controlled trial comparing two different ways of integrating the own mother's milk (OMM) for the evaluation of feeding tolerance in very preterm infants. In infants with gestational age of ≤ 32 weeks randomized to receive preterm formula (PF group) or pasteurized donor human milk (PDHM group) as a supplement to the OMM insufficient or unavailable, FC level was planned to be measured at the first meconium passage and at days 8 and 15 of life (T0, T1, and T2, respectively).
Results: FC data were available for all the 70 infants randomized, 35 in the PF group, and 35 in the PDHM group. The mean FC levels were similar in the two study groups at T0 and T1, whereas they were significantly higher in the PF group than the PDHM group at T2. FC values decreased over the first week of life in both groups and significantly increased over the second week of life only in the PF group.
Conclusions: Our study demonstrates a significant increase in FC levels when PF is used as a supplement to the OMM compared to the use of PDHM. Further studies are needed to establish if the higher FC levels in infants receiving PF are the expression of a normal immunological maturation rather than an initial inflammatory process.
Introduction
Calprotectin is a 36.4-KDa calcium and zinc-binding protein that constitutes the main component of cytosolic protein of neutrophils, monocytes, and macrophages. Calprotectin was shown to have bactericidal and fungicidal properties, and it may be involved in the regulation of inflammation (1, 2). Calprotectin is found in various body fluids in proportion to the degree of inflammation, but its concentration in the stool is ~6 times that of the plasma: this is the reason why the measurement of fecal calprotectin (FC) would likely be a sensitive and specific marker of gastrointestinal inflammation. The level of FC can be proportional to the quantity of neutrophils migrating through the gastrointestinal mucosa (3, 4).
In adults and children, FC is greatly used as a noninvasive marker for the diagnosis of inflammatory bowel disease and may serve as an important clinic test in order to evaluate the inflammatory state of the whole intestinal tract (5, 6). The reference value given for healthy adults and children older than 4 years of age is 50 μg/g of feces (7).
The current evidence suggests that FC is elevated in newborn infants suffering from necrotizing enterocolitis, but its significance as an early screening marker remains unknown. The reported sensitivity and specificity of the test remain unidentified, as there is a lack of consensus regarding the implementation of specific cutoff values (8).
Conflicting results have also been reported on the effect of the kind of feeding on the level of FC in infants. Some studies have shown higher FC in exclusively breastfed infants when compared to mixed- or formula-fed ones, and some have shown no difference (9–20).
The aim of this study was to evaluate the time course of FC in very preterm infants over the first 15 days of life in relation to the different kind of milk diet.
Materials and Methods
This study was part of a single-center, randomized, non-inferiority trial comparing two different ways of integrating the own mother's milk (OMM) over the first 2 weeks of life for the evaluation of feeding tolerance in very preterm infants (21). Briefly, the clinical trial planned that infants with gestational age (GA) of ≤ 32 weeks, without congenital malformations, connatal infections, or abnormal antenatal Doppler flow velocimetry, and for whom OMM was unavailable or insufficient to satisfy the planned enteral intakes, were randomized to receive pasteurized donor human milk (PDHM) or preterm formula (PF) as a supplement to the OMM. The PF diet consisted of 3.5 g of protein/100 Kcal formula (Plasmon PreZero, Plasmon, Italy). Donor human milk came from mothers who prematurely delivered and pasteurized within 24 h of collection by the Holder method (+ 62.5°C for 30 min). Minimal enteral feeding was initiated within the first 48 h of life and was continued at 20 ml/kg/day for up to 5 days. Subsequently, enteral nutrition volume was increased by 20 ml/kg/day. The parenteral nutrition (PN) was started soon after birth in all infants with a birth weight (BW) of <1,250 g and in infants with BW of <1,500 g requiring invasive ventilation. The PN was stopped when enteral intake was >125 ml/kg/day.
FC level was measured at the first meconium passage and at days 8 and 15 of life (T0, T1, and T2, respectively).
Stool samples were taken from the infants' diapers with sterile plastic spoons and put in sterile plastic screw-cap tubes. The samples were then frozen at −80°C until analysis. One hundred milligrams of stool were weighed, placed in a 15-ml conical tube, and agitated with a wooden stirrer. An extraction buffer was added, the sample vortexed to form a fine slurry, and then placed on a shaker for 25 min. One milliliter was removed and centrifuged at 10,000 g for 20 min. The supernatant was removed for analysis by ELISA (Eurospital, Trieste, Italy). The FC was expressed as μg/g of stool. Every assay included a standard curve and quality controls, all samples were done in duplicate, and the intra-assay coefficients of variation were <10%.
All parameters were checked for normal distribution using the Shapiro–Wilk test. Differences between the two groups were investigated using paired Student's t-test for normally distributed data or Wilcoxon signed-rank test otherwise. Chi-square test was used to compare categorical data. Data are presented as mean ± standard deviation (SD) or median–interquartile range. A p-value of <0.05 is considered to indicate statistical significance. Statistical analysis was performed using XLSTAT version 2014.5.03.
The institutional review boards approved the study, and written informed consent was obtained from the parents of all subjects before enrollment.
Results
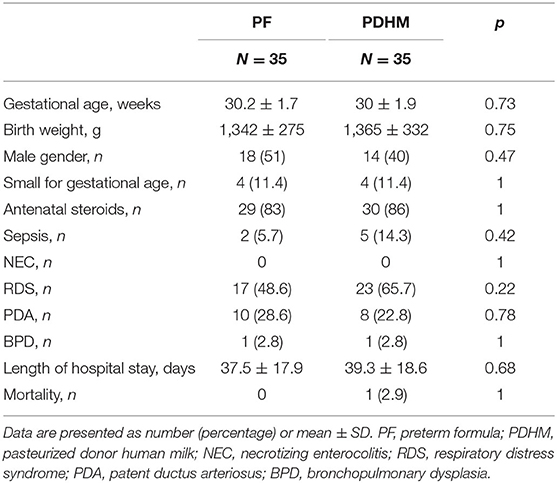
FC data were available for all the 70 preterm infants randomized in the original trial, 35 in the PF group and 35 in the PDHM group. Demographic and clinical data were well balanced between the two study groups, as detailed in Table 1.
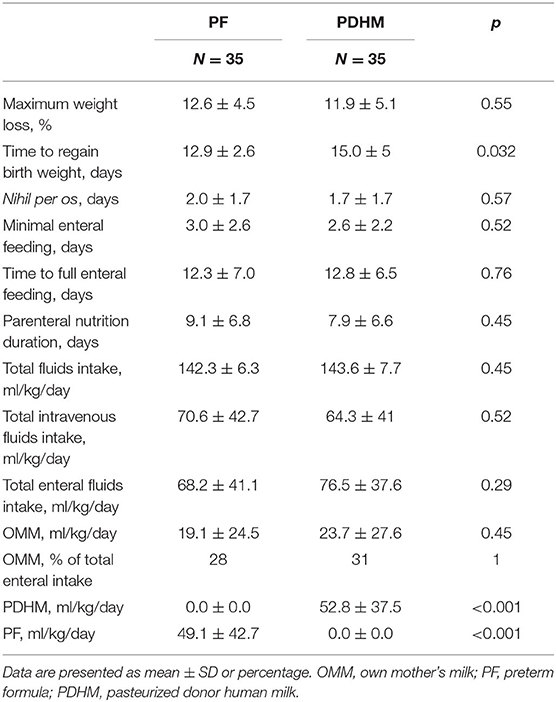
There was no difference in the maximum weight loss, but infants in the PF group regained birth weight 2 days earlier than infants in the PDHM group. No difference was observed in the duration of fasting, minimal enteral feeding, and parenteral nutrition. Time to reach full enteral feeding of 150 ml/kg/day of milk was similar in the two study groups. Infants in both groups received a similar total intravenous and enteral fluid intake, such as a similar amount of OMM, whereas the amounts of PF and PDHM were significantly different between groups, as expected (Table 2).
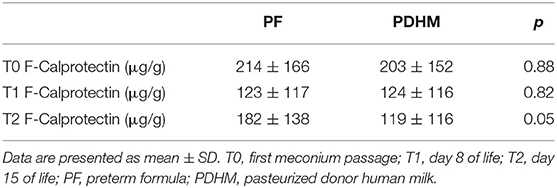
The mean FC levels were similar in the two study groups at T0 and T1, whereas they were significantly higher in the PF group than the PDHM at time 2 (Table 3).
FC values decreased over the first week of life in both groups and increased in a significant manner in the second week of life only in the PF group (Figure 1).
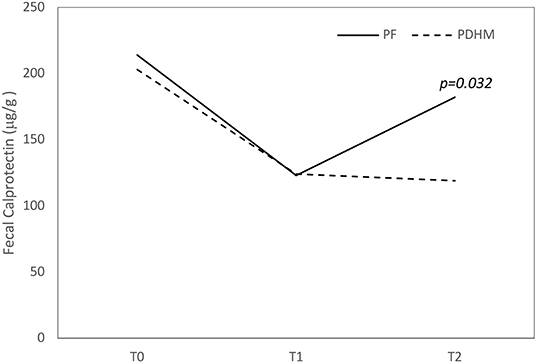
Figure 1. Trend of fecal calprotectin values over the first 15 days of life. The figure shows that fecal calprotectin concentration decreased over the first week of life in both groups and increased at 15 days only in the PF feed group. PF, preterm formula; PDHM, pasteurized donor human milk.
Discussion
In this study, we observed significantly higher FC levels at 15 days of life in very preterm infants who received PF as integration to OMM compared to those infants who received PDHM.
Previous studies reported conflicting results on the effect of feeding on the FC levels in infants since some authors observed higher FC levels in breastfed infants compared to mixed- or formula-fed ones (9–13), while others authors found no difference in the FC levels of infants fed breast milk vs. formula (14–20).
It is a challenge to explain why FC levels are higher in breastfed infants than formula-fed ones since FC is an index of bowel inflammation. On the contrary, it is evident that human milk contains several anti-inflammatory bioactive components that promote intestinal growth and maturation and reduce intestinal permeability more than formulas (22–25). It has been hypothesized that higher FC in breastfed neonates could be consistent with the promotion of maturation of the intestinal mucosa. High FC in the gut, induced by human milk, could provide a protective effect through its bactericidal and fungicidal properties and could contribute to modulation of the intestinal microflora (10, 11, 13).
This hypothesis seems to do not support our results as we found higher FC levels in the PF group compared to the PDHM group. We supposed that the significantly higher FC values in formula-fed infants at the 15th day of life could be explained with a subclinical inflammation of the bowel induced by PF.
It has been reported that preterm infants with feeding intolerance have statistically significant elevated levels of FC than those without feeding intolerance (26, 27) and that the FC levels decrease in a significant manner when PF-fed infants with feeding intolerance were shifted to receive amino acid-based formula (27). In our original trial, the infants who received PF as integration to OMM had a similar feeding tolerance than those who integrated their OMM with PDHM, since the time to achieve the full enteral feeding was the same for both groups (21), so we cannot attribute the higher FC levels in preterm infants receiving PF to the feeding intolerance.
A significant positive correlation has also been observed between the amount of volume of enteral feeding and FC levels (17, 28), suggesting that exposure to the increasing luminal dietary antigens might induce a state of physiological subclinical intestinal inflammation and then an increase in the calprotectin in the gut lumen. In our study, infants in both groups received the same mean enteral fluid intake, but FC increased significantly at 15 days of life only in the PF group.
It is almost evident that the type of milk diet influences the diversity of the gut microbiota in infants. The greatest microbial richness has been found in infants exclusively fed OMM (29). Cong et al. (30) found a more favorable microbial community in infants receiving exclusively OMM and a significantly lower diversity and a different microbial community in infants receiving PHDM and PF compared to infants receiving OMM. In particular, these authors found that infants receiving OMM had the highest abundance of Clostridiales, Lactobacillales, and Bacillales and the lowest abundance of Enterobacteriales. The groups of infants receiving OMM+PHDM as well as those infants receiving OMM+PF also had higher abundance of Clostridiales, Lactobacillales, Bacillales, and Bacteroidales compared to the groups of infants receiving only PHDM or PF or PHDM+PF (30).
The microbiota related to OMM stimulates the normal development of the gut-associated lymphoid tissue, representing the major external driving force in the maturation of the immune system after birth. When the gut microbiota becomes established, neutrophil infiltration into the intestinal mucosa occurs as an integral part of normal bowel development. This physiological inflammatory process would explain why infants who receive breast milk have higher levels of FC. In our study, infants in both groups received a similar amount of OMM, and the variable that can explain the difference in FC levels is attributable to the PF.
In our study, it is difficult to establish if the higher levels of FC found in PF group infants are the expression of a physiological process of intestinal maturation rather than the beginning of a pathological inflammatory process. However, the levels of FC we found were below the lowest reported cutoffs for bowel pathology in preterm infants (31, 32), as well as feeding tolerance was similar in both groups.
Regarding the course of FC over the first 15 days of life, we observed that the FC concentration decreased over the first week of life in both groups and increased at 15 days only in the PF-fed group. The course of FC in PF group infants is consistent with that reported by Zoppelli et al. (33), who found that in preterm infants, the FC levels, initially high in the meconium, dropped, reaching a nadir at approximately 8 days of life and then increased in a GA-dependent manner except in the most premature infants with GA <26 weeks. We could speculate that the failure to increase the FC levels in PDHM group infants might be interpreted as an inappropriate immune response of the bowel as what happens in extremely premature babies probably because the donor human milk, after the pasteurization process, is unable to induce the same maturation process induced by the raw OMM.
Our study has some strengths but also limitations. The strength of our study is that it is part of a randomized controlled trial and therefore there is a good control of all the variables that could influence the levels of FC. A limitation of the study is not having studied the intestinal microbiota which could have given more information about the significance of FC levels in relation to the integration of OMM with PF.
Our study demonstrates a significant increase in FC levels when PF is used as a supplement to the OMM when insufficient or unavailable compared to the use of PDHM.
Further studies are needed to establish whether this increased FC levels is the expression of a normal immunological maturation process rather than an initial inflammatory process related to the use of PF.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board, Department of Woman and Child Health and Public Health, Child Health Area, Fondazione Policlinico Universitario A. Gemelli, IRCCS; Università Cattolica del Sacro Cuore. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
All authors have read and approved the manuscript for submission have made a substantial contribution to the conception, design, gathering of data and a contribution to the writing and intellectual content of the article and acknowledge that they have exercised due care in ensuring the integrity of the work. SC conceived the design of the study, interpreted the data, and revised it critically for intellectual content. MP drafted the article and interpreted the data. AP analyzed the data for the work. CC, GP, CT, MT, and AL acquired the data and researched the scientific literature. All authors revised the article and have given final approval of the version to be published. GV final approved the work to be published.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. (1990) 336:763–65. doi: 10.1016/0140-6736(90)93237-j
2. Baldassarre ME, Altomare MA, Fanelli M, Carbone D, Di Bitonto G, Mautone A, et al. Does calprotectin represent a regulatory factor in host defense or a drug target in inflammatory disease? Endocr Metab Immune Disord Drug Targets. (2007) 7:1–5. doi: 10.2174/187153007780059441
3. Dale I, Brandtzaeg P, Fagerhol MK, Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. (1985) 84:24–34. doi: 10.1093/ajcp/84.1.24
4. Ayling RM, Kok K. Fecal calprotectin. Adv Clin Chem. (2018) 87:161–90. doi: 10.1016/bs.acc.2018.07.005
5. Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen CF, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. (1997) 50:113–23. doi: 10.1136/mp.50.3.113
6. Logan R. Faecal calprotectin for the diagnosis of inflammatory bowel disease. BMJ. (2010) 341:c3636. doi: 10.1136/bmj.c3636
7. Fagerberg UL, Lööf L, Merzoug RD, Hansson LO, Finkel Y. Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr. (2003) 37:468–72. doi: 10.1097/00005176-200310000-00013
8. Pergialiotis V, Konstantopoulos P, Karampetsou N, Koutaki D, Gkioka E, Perrea DN, et al. Calprotectin levels in necrotizing enterocolitis: a systematic review of the literature. Inflamm Res. (2016) 65:847–52. doi: 10.1007/s00011-016-0963-9
9. Dorosko SM, Mackenzie T, Connor RI. Fecal calprotectin concentrations are higher in exclusively breastfed infants compared to those who are mixed-fed. Breastfeed Med. (2008) 3:117–9. doi: 10.1089/bfm.2007.0036
10. Savino F, Castagno E, Calabrese R, Viola S, Oggero R, Miniero R. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology. (2010) 97:299–304. doi: 10.1159/000255161
11. Groer M, Ashmeade T, Louis-Jacques A, Beckstead J, Ji M. Relationships of feeding and mother's own milk with fecal calprotectin levels in preterm infants. Breastfeed Med. (2016) 11:207–12. doi: 10.1089/bfm.2015.0115
12. Asgarshirazi M, Shariat M, Nayeri F, Dalili H, Abdollahi A. Comparison of fecal calprotectin in exclusively breastfed and formula or mixed fed infants in the first six months of life. Acta Med Iran. (2017) 55:53–8.
13. Lee YM, Min CY, Choi YJ, Jeong SJ. Delivery and feeding mode affects fecal calprotectin levels in infants <7months old. Early Hum Dev. (2017) 108:45–8. doi: 10.1016/j.earlhumdev.2017.03.014
14. F, Campeotto M J, Butel N, Kalach S, Derrieux C, Aubert-Jacquin L, Barbot, et al. High faecal calprotectin concentrations in newborn infants. Arch Dis Child Fetal Neonatal Ed. (2004) 89:F353–5. doi: 10.1136/adc.2002.022368
15. Campeotto F, Kalach N, Lapillonne A, Butel MJ, Dupont C, Kapel N. Time course of faecal calprotectin in preterm newborns during the first month of life. Acta Paediatr. (2007) 96:1531–3. doi: 10.1111/j.1651-2227.2007.00457.x
16. Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. (2008) 94:267–71. doi: 10.1159/000151645
17. Rougé C, Butel M J, Piloquet H, Ferraris L, Legrand A, Vodovar M, et al. Fecal calprotectin excretion in preterm infants during the neonatal period. PLoS ONE. (2010) 5:e11083. doi: 10.1371/journal.pone.0011083
18. Li F, Ma J, Geng S, Wang J, Ren F, Sheng X. Comparison of the different kinds of feeding on the level of fecal calprotectin. Early Hum Dev. (2014) 90:471–5. doi: 10.1016/j.earlhumdev.2014.06.005
19. Rosti L, Braga M, Fulcieri C, Sammarco G, Manenti B, Costa E. Formula milk feeding does not increase the release of the inflammatory marker calprotectin, compared to human milk. Pediatr Med Chir. (2011) 33:178–81.
20. Oswari H, Prayitno L, Dwipoerwantoro P G, Firmansyah A, Makrides M, Lawley B, et al. Comparison of stool microbiota compositions, stool alpha1-antitrypsin and calprotectin concentrations, and diarrhoeal morbidity of Indonesian infants fed breast milk or probiotic/prebiotic-supplemented formula. J Paediatr Child Health. (2013) 49:1032–9. doi: 10.1111/jpc.12307
21. Costa S, Maggio L, Alighieri G, Barone G, Cota F, Vento G. Tolerance of preterm formula versus pasteurized donor human milk in very preterm infants: a randomized non-inferiority trial. Ital J Pediatr. (2018) 44:96. doi: 10.1186/s13052-018-0532-7
22. Wagner CL, Taylor SN, Johnson D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin Rev Allergy Immunol. (2008) 34:191–204. doi: 10.1007/s12016-007-8032-3
23. Turfkruyer M, Verhasselt V. Breast milk and its impact on maturation of the neonatal immune system. Curr Opin Infect Dis. (2015) 28:199–206. doi: 10.1097/QCO.0000000000000165
24. Cacho NT, Lawrence RM. Innate immunity and breast milk. Front Immunol. (2017) 8:584. doi: 10.3389/fimmu.2017.00584
25. Nolan LS, Parks OB, Good M. A review of the immunomodulating components of maternal breast milk and protection against necrotizing enterocolitis. Nutrients. (2019) 12:14. doi: 10.3390/nu12010014
26. Moussa R, Khashana A, Kamel N, Elsharqawy SE. Fecal calprotectin levels in preterm infants with and without feeding intolerance. J Pediatr. (2016) 92:486–92. doi: 10.1016/j.jped.2015.11.007
27. Jang HJ, Park JH, Kim CS, Lee SL, Lee WM. Amino acid-based formula in premature infants with feeding intolerance: comparison of fecal calprotectin level. Pediatr Gastroenterol Hepatol Nutr. (2018) 21:189–95. doi: 10.5223/pghn.2018.21.3.189
28. Josefsson S, Bunn SK, Domellöf M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr. (2007) 44:407–13. doi: 10.1097/MPG.0b013e3180320643
29. Zanella A, Silveira R C, Roesch L F W, Corso A L, Dobbler P T, Mai V, et al. Influence of own mother's milk and different proportions of formula on intestinal microbiota of very preterm newborns. PLoS ONE. (2019) 14:e0217296. doi: 10.1371/journal.pone.0217296
30. Cong X, Judge M, Xu W, Diallo A, Janton S, Brownell EA, et al. Influence of feeding type on gut microbiome development in hospitalized preterm infants. Nurs Res. (2017) 66:123–33. doi: 10.1097/NNR.0000000000000208
31. Kapel N, Campeotto F, Kalach N, Baldassare M, Butel MJ, Dupont C. Faecal calprotectin in term and preterm neonates. J Pediatr Gastroenterol Nutr. (2010) 51:542–7. doi: 10.1097/MPG.0b013e3181e2ad72
32. MacQueen BC, Christensen RD, Yost CC, Gordon PV, Baer VL, Schlaberg R, et al. Reference intervals for stool calprotectin in preterm neonates and their utility for the diagnosis of necrotizing enterocolitis. J Perinatol. (2018) 38:1379–85. doi: 10.1038/s41372-018-0108-9
Keywords: fecal calprotectin, inflammation, immunomodulation, milk diet, preterm infants
Citation: Costa S, Patti ML, Perri A, Cocca C, Pinna G, Tirone C, Tana M, Lio A and Vento G (2020) Effect of Different Milk Diet on the Level of Fecal Calprotectin in Very Preterm Infants. Front. Pediatr. 8:552. doi: 10.3389/fped.2020.00552
Received: 01 June 2020; Accepted: 30 July 2020;
Published: 16 September 2020.
Edited by:
Iliana Bersani, Bambino Gesù Children Hospital (IRCCS), ItalyReviewed by:
Guglielmo Salvatori, Bambino Gesù Children Hospital (IRCCS), ItalyMaría Gormaz, Agencia Valenciana de Salud, Spain
Copyright © 2020 Costa, Patti, Perri, Cocca, Pinna, Tirone, Tana, Lio and Vento. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simonetta Costa, simonetta.costa@policlinicogemelli.it
 Simonetta Costa
Simonetta Costa Maria Letizia Patti
Maria Letizia Patti Alessandro Perri
Alessandro Perri Carmen Cocca
Carmen Cocca Giovanni Pinna
Giovanni Pinna Chiara Tirone
Chiara Tirone Milena Tana
Milena Tana  Alessandra Lio
Alessandra Lio Giovanni Vento
Giovanni Vento