Impacts of an Interdisciplinary Developmental Follow-Up Program on Neurodevelopment in Congenital Heart Disease: The CINC Study
- 1Sainte-Justine University Hospital Research Center, Montreal, QC, Canada
- 2Department of Psychology, Université de Montréal, Montreal, QC, Canada
- 3School of Rehabilitation, Université de Montréal, Montreal, QC, Canada
- 4Clinique d'Investigation Neurocardiaque (CINC), Sainte-Justine University Hospital Center, Montreal, QC, Canada
- 5Division of Neurology, Department of Pediatrics, La Timone Hospital, Marseille, France
- 6Faculty of Medicine, Université de Montréal, Montreal, QC, Canada
Objectives: This study investigates the impact of an early systematic interdisciplinary developmental follow-up and individualized intervention program on the neurodevelopment of children with complex congenital heart disease (CHD) who required cardiac surgery.
Study Design: We prospectively enrolled 80 children with CHD: 41 were already followed at our neurocardiac developmental follow-up clinic from the age of 4 months, while 39 were born before the establishment of the program and therefore received standard health care. We conducted cognitive, motor, and behavioral assessments at 3 years of age. We used one-way multivariate analyses of variance to compare the neurodevelopmental outcome of both groups.
Results: Between-group analyses revealed a distinct neurodevelopmental profile with clinically significant effect size (P < 0.001, partial η2 = 0.366). Children followed at our clinic demonstrated better receptive language performances (P = 0.048) and tended to show higher scores on visuo-constructive tasks (P = 0.080). Children who received standard health care exhibited greater performances in working memory tasks (P = 0.032). We found no group differences on global intellectual functioning, gross and fine motor skills, and behaviors. Referral rates for specific remedial services were higher in patients followed at our neurocardiac clinic compared to the historical cohort (P < 0.005).
Conclusions: Overall, the impact of the developmental follow-up and individualized intervention program on neurodevelopmental outcomes remains subtle. Nevertheless, results, although limited by several factors, point toward an advantage for the children who took part in the program regarding receptive language skills over children who received standard health care. We hypothesize that group differences may be greater with growing age. Further research involving larger cohorts is needed to clearly assess the effectiveness of neurocardiac developmental follow-up programs at school age.
Introduction
Congenital heart disease (CHD) is the most common congenital anomaly, affecting up to 1% of live births (1–4), with the more severe cardiac malformations requiring surgery or catheter-based interventions to ensure survival (5, 6). Advances in prenatal diagnosis, surgical techniques, and medical therapies have led to a significant rise in the survival rates of children with CHD (5, 7). This, however, has been associated with an increase in long-term neurodevelopmental comorbidity (7), with up to 50% of children with CHD presenting impairment in motor, language, and/or cognitive functions (8–19), along with behavioral and psychosocial maladjustment (20). These comorbidities generally limit academic achievement, employability, earnings, and insurability, and ultimately reduce the quality of life of patients and their families (10, 20–23).
Given the aforementioned comorbidities, several studies have highlighted the need for an early and close developmental follow-up of children with CHD (24–29). In 2012, the American Heart Association and the American Academy of Pediatrics recommended systematic developmental surveillance, screening, and evaluation of patients with CHD throughout childhood to promote early diagnosis, implement relevant supportive strategies, and ultimately improve neurodevelopmental prognosis (30). Subsequently, many cardiac developmental follow-up programs have emerged in pediatric centers worldwide (26, 30, 31). At the Sainte-Justine University Hospital Center (Montreal, QC, Canada), we founded the Clinique d'Investigation Neurocardiaque (CINC) in 2013 to provide early systematic and interdisciplinary developmental follow-up for all children with critical CHD without genetic syndrome (e.g., trisomy 21). The establishment of the CINC program (i.e., identification of systematic time points for assessments, selection of assessment tools, recruitment of clinicians, etc.) is grounded on evidence from literature on the CHD population and similar clinical populations (e.g., preterm), and is gradually refined and adjusted according to probative research findings and hospital resources (19). The CINC interdisciplinary team is composed of a nurse practitioner coordinator, a pediatric cardiac surgeon, pediatric neurologists, cardiologists, developmental pediatricians, physical therapists, occupational therapists, a psychologist, a speech-language pathologist, and a nutritionist. Our program begins at the age of 4 months and consists of systematic and standardized developmental assessments at multiple pre-established ages. These include neurological and physical exams, motor and cognitive assessments, as well as socio-affective and behavioral screenings using parental questionnaires. While direct participation of professionals varies across assessments (e.g., 4-month neurologic exams are performed by developmental pediatricians or pediatric neurologists and 24-month cognitive and motor assessments are conducted by a neuropsychologist), all clinicians are invited to interdisciplinary clinical meetings, thus having a potential indirect involvement with every patient regardless of age. The timeline of the CINC developmental follow-up program is presented in Figure 1.
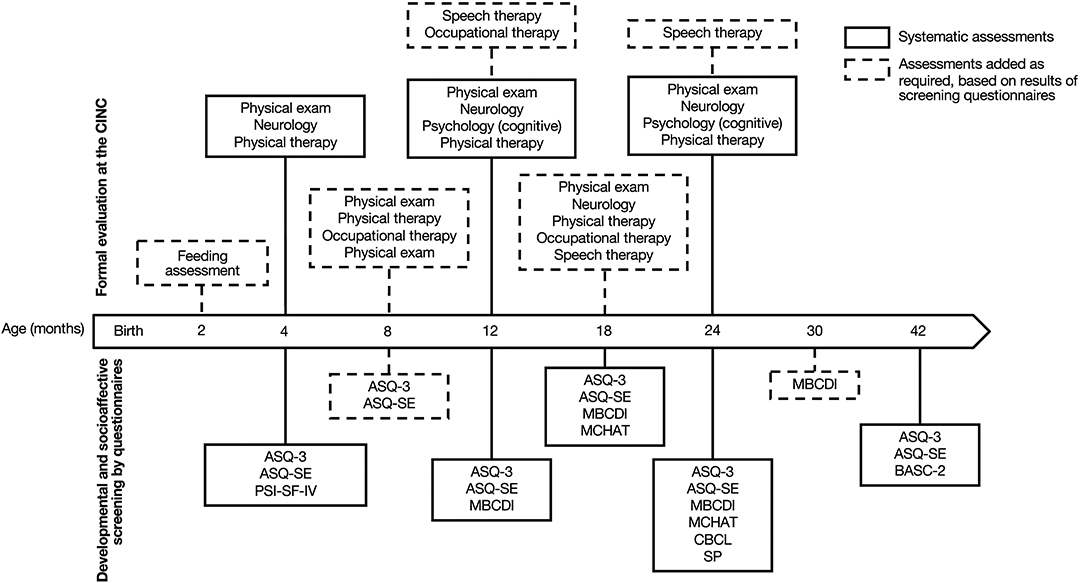
Figure 1. Timeline of developmental follow-up from 4 to 42 months of age Clinique d'Investigation Neurocardiaque (CINC). Systematic screening and assessments are represented in plain lines; additional examinations based on screening are represented in dotted lines. ASQ-3, Ages and Stages Questionnaire, Third edition; ASQ-SE, Ages and Stages Questionnaire: Social–emotional; MBCDI, MacArthur–Bates Communicative Development Inventories; MCHAT, Modified Checklist for Autism in Toddlers; BASC-2, Behavioral Assessment System for Children, Second Edition; SP, Sensory profile; CBCL, Child Behavior Checklist.
Early generalized and systematic intervention is also offered. It includes educational support to the child's family, such as information about infant growth and development, explanations of child behavior, and feedback from a professional on the parents' interaction with the child. Children may also obtain additional care according to their specific needs, therefore based on the results of the systematic assessments. This additional care consists of individualized recommendations to parents for educational activities, daily home exercises, or direct intervention with a therapist. Data from the scientific literature and the clinical experience of the CINC professionals have led to establish fixed criteria to determine the child's eligibility for further intervention (19, 32, 33). For instance, a child performing below the 10th percentile on the Alberta Infant Motor Scale (34) at 4 months will be considered at risk of gross motor delay (32, 33) and will receive individual sessions for motor intervention with the physical therapist (35). Similarly, a child with a score below the 2nd percentile in one scale of the MacArthur–Bates Communicative Development Inventories (MBCDI) (36), at the 10th percentile on the Communicative Gestures scale of the MBCDI, or below the 10th percentile in all of its scales will receive an extensive language assessment by a speech-language pathologist to determine the needs for speech therapy (19). A score in the monitoring zone or below the cutoff at the Ages and Stages Questionnaire, Third Edition (37), will also lead to a referral to a speech-language pathologist for evaluation. Finally, a child showing a failure to thrive, that is, an insufficient weight gain for age, an inappropriate weight loss, or a weight inferior to the 2nd percentile according to standards of growth, will be referred to a nutritionist.
In 2017, we published a case study (38) showing significant motor improvement in a 12-month-old girl with complex CHD who received early intervention with a physical therapist as part of the CINC program. Recently, Fourdain and colleagues described a substantial improvement of gross motor abilities in CINC patients aged up to 24 months, particularly in children at risk of motor delay who received physical therapy on a regular basis (35). Although these results suggest a positive impact of the CINC program, they could also be associated with spontaneous recovery after surgery (39, 40). Further research is therefore needed to quantify the benefits of neurocardiac follow-up programs on neurodevelopment. To this end, this study aimed to assess the impact of the CINC early systematic interdisciplinary developmental follow-up and individualized intervention program compared to standard health care on motor, cognitive, and behavioral development in 3-year-old children with critical CHD.
Materials and Methods
Patient Population
Since 2013, families of Sainte-Justine University Hospital Center's patients presenting with moderate to severe CHD requiring cardiac surgery have been offered a referral to the interdisciplinary neurocardiac clinic [Clinique d'Investigation Neurocardiaque (CINC)]. Children with genetic syndromes (e.g., trisomy 21) and children with severe or profound intellectual disability were not referred to the CINC as they already received specialized services in thematic clinics or rehabilitation centers. Since its opening, 16 (4%) families of the patients referred to the CINC declined the developmental interdisciplinary follow-up. Reasons for declining included being followed in another pediatric hospital in the Montreal area for 3 (19%), being followed in another specialized clinic in the Sainte-Justine University Hospital Center for 2 (13%), having been referred early to a pediatric rehabilitation center for 1 (6%), or being satisfied with the standard health care for 1 (6%). Nine (56%) families did not specify any reason for refusing the CINC follow-up. Currently, we provide early systematic and interdisciplinary developmental follow-ups to 338 children with CHD.
For this research study, we prospectively recruited a cohort of 43 children with complex CHD, followed longitudinally at the CINC. A total of 59 CINC patients were approached to participate in the present study: 14 (23.7%) did not respond to the invitation, 2 (3.4%) refused to participate due to lack of time, and 43 (72.9%) accepted the invitation and were scheduled for a research interdisciplinary standardized assessment at 3 years of age. Two (4.7%) children did not offer sufficient collaboration during the assessment to gather valid data. This resulted in 41 children whose data were included in this study and constituted the Surveillance Group. Among these, 5 (12.2%) did not attend the 4-month-old evaluation, 2 (4.9%) did not attend the 12-month assessment, and 2 (4.9%) did not attend the 24-month follow-up, with all children being present for at least two of these assessments.
To compare neurodevelopmental outcomes at the age of 3, we prospectively recruited a group of children with moderate to severe CHD who were born before the CINC was established and, as such, were not followed within the clinic's framework. These children received standard health care (pre-established follow-ups with the cardiologist and regular medical appointments with the pediatrician or family physician). As for the CINC referral criteria, we only included children who did not present genetic syndrome or severe or profound intellectual disability. Among the 63 eligible families contacted to participate in the study, 4 (6.3%) did not respond to the invitation, 19 (30.2%) refused to participate, and 40 (63.5%) accepted the invitation and were scheduled for the same research interdisciplinary standardized assessment at 3 years of age. The reasons provided when refusing to participate were lack of time for 6 (31.6%), long distance between home and the hospital for 6 (31.6%), stating that the child has no developmental delay for 4 (21%), parent or child having to undergo surgery for 2 (10.5%), and child being too afraid of hospitals for 1 (5.3%). One child did not offer sufficient collaboration during the assessment to gather valid data, resulting in 39 enrolled children who constituted the Historical Control Group.
A description of the timeline of participants' recruitment and assessment is presented in Figure 2. The study was approved by the institutional research ethics board of the Sainte-Justine University Hospital Center. All participants' parents gave written informed consent.
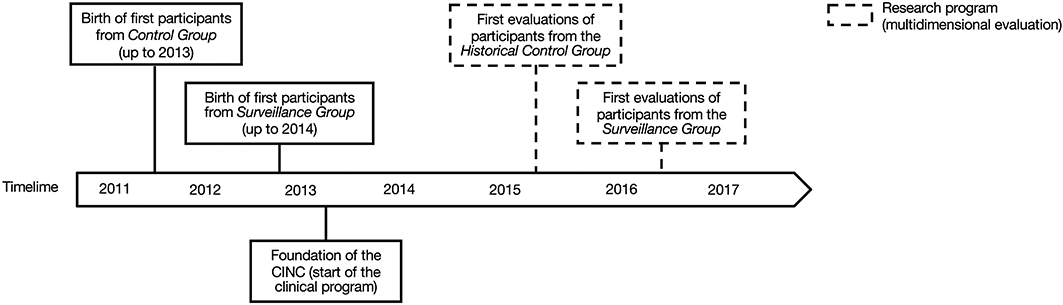
Figure 2. Timeline of participants' births (plain lines) and research assessments (dotted lines) for the Historical Control Group and the Surveillance Group.
Research Interdisciplinary Evaluations
Participants of the Surveillance Group and the Historical Control Group received an interdisciplinary neurodevelopmental evaluation as part of this research project. It included a cognitive assessment by neuropsychologists (SF and LCD), a gross motor assessment by a physical therapist (LD), a fine motor assessment by occupational therapists (KG and JP), and a behavioral assessment using a parental questionnaire. Interdisciplinary research assessments were conducted at the Sainte-Justine University Hospital Center during one full morning (90 min for the cognitive assessment, and 15 and 60 min for the fine and gross motor evaluations, respectively). Due to the young age of the participants, parents were present in the room with the child for the whole duration of the assessment. At the end of the meeting, professionals provided to parents a retroaction on the child's cognitive and motor performances, and behavioral skills during an interdisciplinary feedback session. Finally, the assessment findings and the resulting relevant recommendations were summarized in an interdisciplinary written report.
Cognitive assessment was performed using the Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition (WPPSI-IV) (41). For 3-year-olds, it includes three verbal subtests (Receptive Vocabulary, Information, and Picture Naming), two visuospatial subtests (Block Design and Object Assembly), and two working memory subtests (Picture Memory and Zoo Locations). It provides a global cognitive score (Full-Scale IQ) as well as three primary index scales (Verbal Comprehension, Visual Spatial, and Working Memory). Mean performances of 10 [standard deviation (SD) = 3] and 100 (SD = 15) are expected on the subtest and scale levels, respectively.
Gross motor skills were assessed using the Peabody Developmental Motor Scale, Second Edition (PDMS-2) (42), which includes three gross motor subtests (Stationary, Locomotion, and Object Manipulation). It produces standard scores (Mean = 10; SD = 3) for each subtest as well as a Gross Motor Quotient (Mean = 100; SD = 15). Fine motor assessment was done using the Manual Dexterity scale of the Movement ABC, Second Edition (M-ABC-2) (43). It measures manipulative skills through three subtests (Posting Coins, Threading Beads, and Drawing Trail), each providing a standard score (Mean = 10; SD = 3). The M-ABC-2 also provides a Manual Dexterity composite score (Mean = 100; SD = 15).
Behavioral assessment was performed using the Parent Rating Scale of the Behavior Assessment System for Children, Second Edition (BASC-2), preschool version (44). It provides a T score (Mean = 50; SD = 10) for 12 syndrome scales and four composite scales (Externalizing Problems, Internalizing Problems, Behavioral Symptoms Index, and Adaptative Skills).
Perinatal, surgical, critical, and demographic variables were collected from medical records for participants of both groups. Anatomic CHD classification (45) and RACHS-1 scores (46) were extracted from the descriptions of heart defects and surgical procedures by a pediatric neurologist (BD). Information regarding the use of remedial services was collected through a parental report for both groups. For the Surveillance Group, additional information on remedial services was also retrieved from the CINC records.
Statistical Analyses
Descriptive statistics [means, medians, SDs, and 95% confidence intervals (95% CI)] were calculated for continuous variables, and number of participants and percentages were calculated for dichotomous and categorical variables. Unpaired t-tests, Mann–Whitney U-tests, and Chi-squared tests were used to compare both groups on continuous, categorical, and dichotomous variables, respectively.
One-way multivariate analyses of variance (MANOVA) were computed for intergroup comparisons on neurodevelopmental assessment scores. Post-hoc unpaired t-tests were carried out to identify significant differences between groups. Significance level was set to α = 0.05. Alpha adjustment for multiple comparisons was done according to false discovery rate (47).
Results
Patients' Characteristics
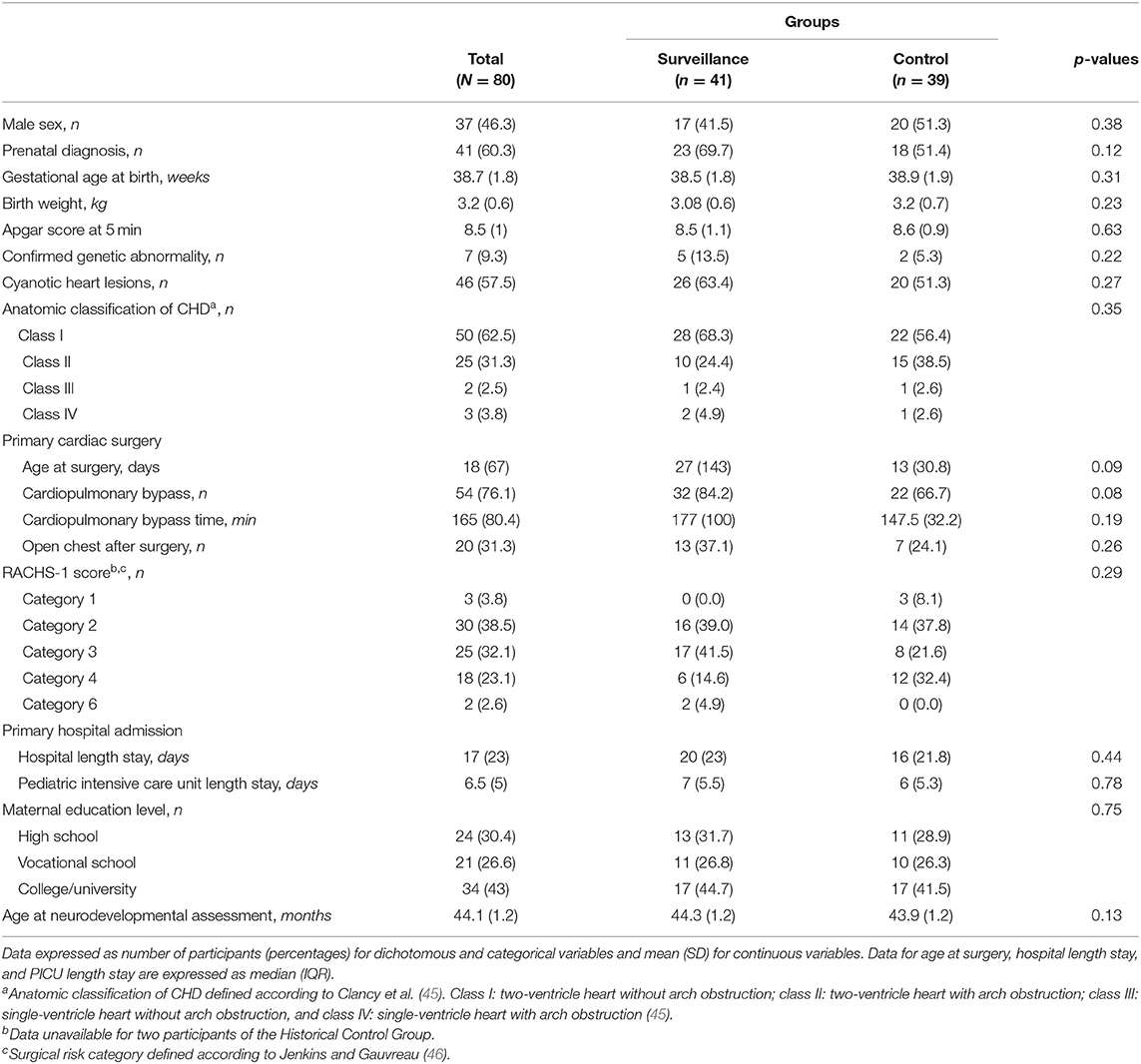
A total of 80 participants were included in this study: 41 children in the Surveillance Group and 39 in the Historical Control Group. Perinatal, surgical, critical, and demographic characteristics of the participants are presented in Table 1. The most common heart defects were transposition of the great arteries (39% in the Surveillance Group and 31% in the Historical Control Group), coarctation of the aorta (14% in the Surveillance Group and 28% in the Historical Control Group), and Tetralogy of Fallot (17% in the Surveillance Group and 18% in the Historical Control Group). We found a statistical tendency for a greater proportion of children of the Surveillance Group who required cardiopulmonary bypass during surgery compared to the Historical Control Group (P = 0.084). There were no other differences in perinatal, surgical, critical, and demographic characteristics between groups.
We found no differences between the 80 participants (41 in the Surveillance Group and 39 in the Historical Control Group) and children who declined participation in the study (16 CINC patients and 23 children born before the CINC was established; all P > 0.05) regarding sex or medical characteristics (i.e., gestational age at birth, prenatal diagnosis, anatomic CHD classification, birth weight, APGAR score at 5, age at surgery, CPB time, hospital length stay, PICU length stay, and open chest after surgery). No data were available regarding socio-economic status for those who declined participation.
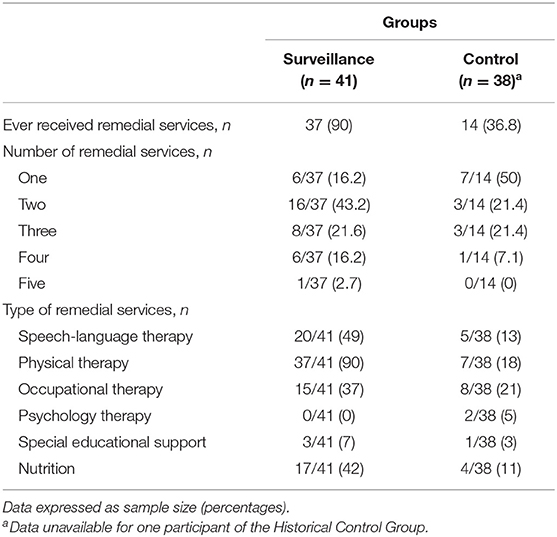
Based on parental reports, 14 (37%) children in the Historical Control Group received at least one remedial service before 3 years of age through references within standard health care. As expected, this rate was smaller compared to the Surveillance Group, in which 37 (90%) children received at least one remedial service before the age of 3, χ2 = 24.5, P < 0.001. Among the 14 children of the Historical Control Group who received services, only 7 (50%) had met more than one type of health professional, whereas 31 (84%) children in the Surveillance Group had been followed by at least two different health professionals, χ2 = 6.1, P = 0.013. Physical therapists, occupational therapists, speech-language pathologists, and nutritionists were the most consulted professionals in both groups. Among the 20 (49%) children in the Surveillance Group who received speech and language therapy, 30% received only indirect intervention, such as recommendations of daily activities to stimulate language development, whereas 70% received both indirect and direct speech therapy. In comparison, only 5 (13%) children in the Historical Control Group received services from a speech-language pathologist. A detailed description of remedial services received in both groups is presented in Table 2.
Cognitive, Motor, and Behavioral Results
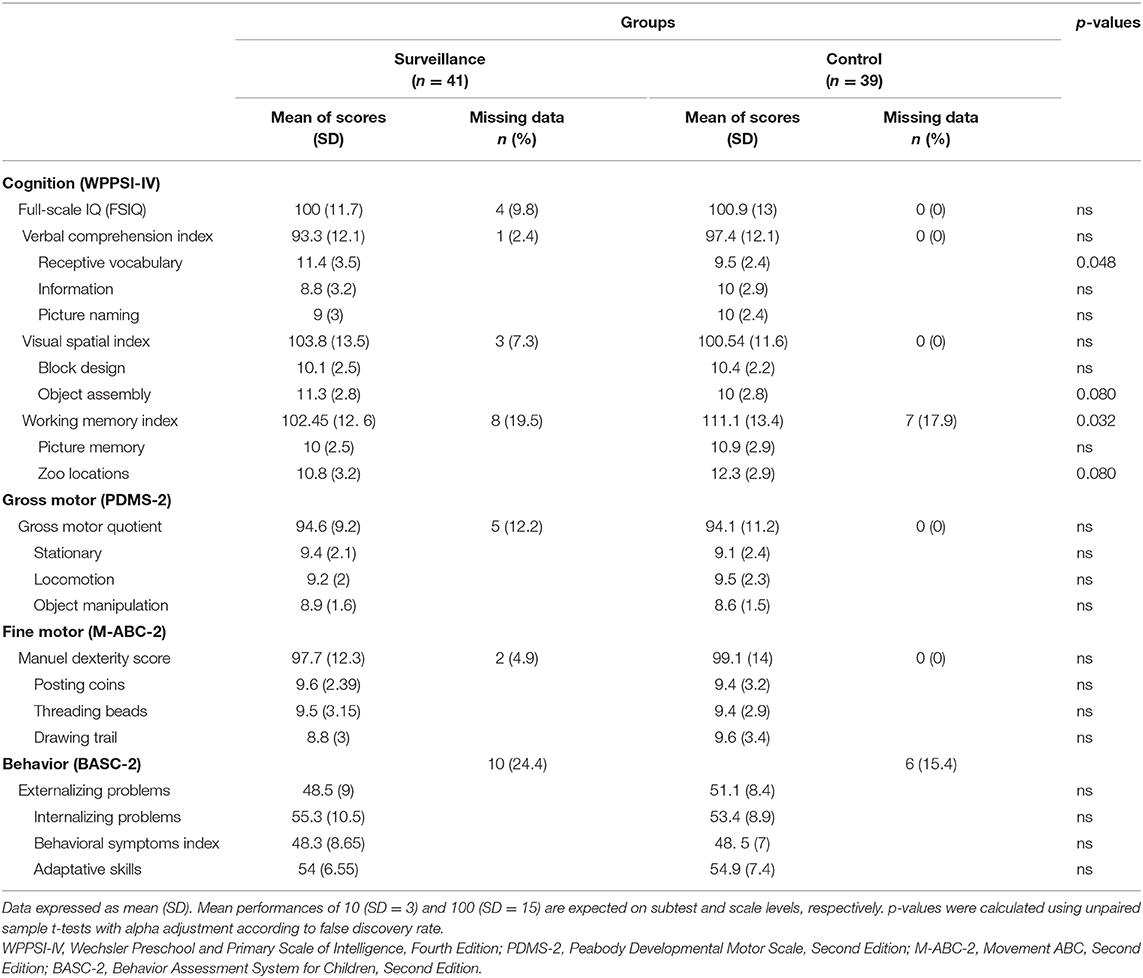
The mean age at testing was similar in both groups (P = 0.129) with assessments at 43.9 months (SD = 1.2) for the Historical Control Group and at 44.3 months (SD = 1.2) for the Surveillance Group. Table 3 shows groups' mean scores for cognitive, motor, and behavioral measures. Each mean score, including indices and subtests, was in the normal range, except for the Zoo Locations subtest and the Working Memory Index, which were both in the high average for the Historical Control Group. Although in the normal range, distributions of gross motor scores were slightly down shifted with an increased proportion of children performing under clinical cutoffs for both groups [12 (31%) from the Historical Control Group and 7 (19%) from the Surveillance Group performed equally to or below the 16th percentile (1 SD or more below norms)].
Differences Between Groups
The one-way MANOVA revealed significant differences between groups for performances on cognitive subtests, with a clinically significant effect size, F(6, 57) = 5.491, P < 0.001; Wilk's Λ = 0.634, partial η2 = 0.366. Post-hoc t-tests revealed that the Surveillance Group performed better than the Historical Control Group (P = 0.048) on the Receptive Vocabulary subtest. Individual results revealed that 3 (7%) children from the Surveillance Group performed equally to or below the 16th percentile (1 SD below mean), compared to 6 (16%) from the Historical Control Group on this subtest. t-tests also showed a statistical trend toward a greater score on the Object Assembly subtest and a lower score on the Zoo Locations subtest in the Surveillance Group compared to the Historical Control Group (both P = 0.08). We also found a significant group difference in cognitive primary indices, F(4, 49) = 3.449, P = 0.013; Wilk's Λ = 0.810, partial η2 = 0.190. Post-hoc comparisons indicated that the mean score for the Working Memory Index was significantly higher for the Historical Control Group compared to the Surveillance Group (P = 0.032), which is coherent with the results obtained on the subtest level. We found no significant effect of group on gross and fine motor scores as well as behavioral scales.
Discussion
Following the American Heart Association and the American Academy of Pediatrics recommendations of systematic developmental surveillance, screening, and evaluation of children with CHD (30), many neurocardiac developmental follow-up programs have emerged worldwide. The feasibility of implementing such programs has been demonstrated (26, 48) and factors improving follow-ups have been investigated (49, 50). The benefits of early intervention in supporting neurodevelopment have been shown in other clinical pediatric populations (e.g., preterms, autistic children) (51–55). Despite variability with regard to the type of interventions (provided at a clinic vs. home-based interventions) or professionals involved, studies have demonstrated a positive impact of early intervention programs compared to standard health care in enhancing cognitive and behavioral outcomes of children born preterm (55–58). In comparison, literature on interventional effect in CHD is only emerging but is gathering increasing attention. The aim of the present study was to assess the impact of the CINC early systematic interdisciplinary developmental follow-up program on neurodevelopmental outcomes of preschoolers with critical CHD compared to standard health care.
Cognitive and Language Skills
Children of the Surveillance Group and the Historical Control Group demonstrated similar global intellectual functioning. We found a significant difference, with clinically significant effect size, in performances on the receptive vocabulary subtest and a statistical tendency on the visuo-constructive subtest between groups. These skills have been previously reported to be impaired in children with CHD (16, 19, 59–61). These results suggest an advantage of the children who took part in the CINC early systematic interdisciplinary developmental follow-up program regarding receptive language competency compared to children of the Historical Control Group who received standard health care.
As part of their developmental follow-up and based on the MBCDI screening results, 20 (49%) children of the Surveillance Group were referred to a speech-language pathologist for an extensive language assessment before the age of 3, with 70% who received both direct speech therapy and indirect intervention in the form of recommendations to stimulate language development. In comparison, only 5 (13%) parents of children from the Historical Control Group reported receiving services from a speech-language pathologist. These children were referred only when a language delay was already observed or if parental concerns were present, whereas all children of the Surveillance Group underwent early systematic language screening, which may result in earlier detection of language impairments and higher referral rates. The individualized intervention received by children from the Surveillance Group may have strengthened the development of their receptive language skills, as illustrated by a lower proportion of receptive difficulties at the age of 3 (7% in the Surveillance Group compared to 16% in the Historical Control Group being below 1 SD).
Additionally, we found that the children from the Surveillance Group tend to have better visuo-constructive skills compared to children from the Historical Control Group. Occupational therapists of the CINC sometimes recommended visuospatial and visuo-constructive activities for parents to perform with their child, including shape sorting games and stacking toys, to help stimulate perceptual–motor development and hand–eye coordination abilities. However, it is not possible at this point to state that we found an effect of the developmental follow-up program or the specific individualized intervention on these skills. This potential benefit in preserving the development of visuo-constructive skills remains to be demonstrated. Nonetheless, these results allow us to flag this set of competencies for further assessment at a growing age.
Beyond the benefits of direct intervention, literature has previously demonstrated the effects of a more global approach in improving neurocognitive functions in children at risk for developmental delay. For instance, several studies have demonstrated that intervention programs including parental support could benefit the neurodevelopment of preterm children (55, 58, 62, 63). In the CHD population, McCusker et al. (64) have documented better cognitive and communicative abilities in 8-month-old infants with CHD whose mother participated in the Congenital Heart Disease Program. Through psychoeducation, coaching, and therapy, this psychosocial program notably aimed at promoting child development by supporting maternal adjustment to the diagnosis and teaching effective coping strategies. The Congenital Heart Disease Program also demonstrated gains on measures of maternal mental health and family functioning, which may indirectly influence the child outcomes (65). In the CINC program, psychoeducational support is systematically offered to parents. Every visit is also an opportunity for parents to ask questions to pediatric specialists. If needed, the nurse practitioner coordinator also addresses parental concerns and offers additional support. In addition to the potential impact of the individualized direct intervention, the affective and psychoeducational support given to parents may have had a beneficial effect on the neurodevelopment of children with critical CHD. Additional research is needed to investigate the potential benefits of the CINC program in improving family well-being.
We found that children of the Surveillance Group showed a significantly lower mean score on the Working Memory Index compared to the children of the Historical Control Group. However, this result should be taken with caution as we cannot exclude that the high rate of missing data on this scale could have biased group comparisons (see Table 3). For a substantial number of children, we were not able to obtain adequate collaboration to acquire valid data for the two subtests composing this index (Zoo Locations and Picture Memory). As frequently observed by assessors in both groups, these two subtests specifically appeared to require substantial efforts from the participants. The high number of missing values could have resulted in an over-representation of the highest performances, potentially explaining the mean performance in the high average at the Working Memory Index for the Historical Control Group. This could also have reduced statistical power, thus not reflecting a true difference on memory span and working memory skills between groups.
Gross and Fine Motor Functions
Overall, gross motor assessment revealed mean scores in the normal range. However, this developmental domain still appears as a specific area of concern, with 31% of the Historical Control Group and 19% of the Surveillance Group performing equally to or below the 16th percentile (1 SD or more below norms). In both groups, gross motor disabilities were characterized by difficulties with standing balance, decreased lower limbs strength, proximal instability, and difficulty with hand–eye coordination. Although some studies document a gradual improvement in gross motor abilities up to the age of 3, difficulties in this domain persist at school entry where gross motor impairment rate generally exceeds normative expectations (9, 66).
We found no significant differences between groups for gross motor functions. Since gross motor impairments are known to be the first manifestations of altered neurodevelopment in infants with CHD, it is possible that family physicians and pediatricians, even outside specialized clinics, may be successful at detecting these difficulties and thus accurately referred patients to relevant rehabilitation services or offer parental recommendations on how to foster the child's motor development (67). Based on parental reports, 18% of children in the Historical Control Group had received treatments with a physical therapist in addition to the standard health care, physical therapy being one of the most frequently received interventional services. This could have preserved their gross motor skills and may explain the absence of significant differences between groups. As discussed by Kynø et al. (68) in the preterm population, the standard care offered to all patients with CHD at the pediatric intensive care unit (e.g., nesting, post-surgical positioning program, parents present as much as possible, etc.) may also have supported the child's development, therefore reducing outcome differences between groups. Nevertheless, the greater proportion of children in the Historical Control Group performing below the 1 SD cutoff suggests that children who did not undergo early systematic interdisciplinary developmental follow-ups are at higher risk for gross motor impairments compared to children who took part in the CINC program. We cannot exclude either that this absence of significant statistical differences in gross motor skills could be due to the small sample size.
Regarding fine motor functions, occupational therapists have observed limitations in the proximal stability of the upper limbs in a significant proportion of children from both groups. These limitations caused distal tremors and difficulties with motor coordination. Children seemed to succeed in compensating for these difficulties with both groups performing within the normal range in fine motor tasks. However, children frequently exhibited non-voluntary mouth movements, revealing that these tasks required substantial efforts. In future studies, systematic video recordings of children's evaluations could help analyze qualitative observations from specialists and thus allow the tracing of a more subtle neurodevelopmental profile. We hypothesize that these subtle limitations may progress into significant deficits with growing environmental demands. Conducting a follow-up at school age is thus crucial to document the developmental trajectory and to detect motor difficulties that could arise and significantly impact schooling and social life (9).
Behavioral Functioning
Children of the Surveillance Group and the Historical Control Group demonstrated similar results on the BASC-2, revealing behavioral functioning in the normative range for both groups. At that age, only 2 children (5%) of the Historical Control Group and no children from the Surveillance Group received services from a psychologist. However, as it is integrated within the CINC systematic program, psychologists conduct the 24-month-old developmental evaluation and thus have the possibility to provide parental counseling and psychological support without the need to refer the family to external psychological therapy. During the evaluation, assessors observed behavioral regulation issues (e.g., oppositional behaviors, hyperactivity, and difficulties to mobilize cognitive resources) that have not been reported in the parental questionnaire. Based on our experience and the literature, behavioral issues appear gradually with age in children with CHD and become more salient to parents when the child enters school. We are following these children and expect parents to report more behavioral issues at school age.
Use of Remedial Services
Referral rates for interventional services were higher in children who took part in our interdisciplinary developmental follow-up program (90%) compared to children who received standard health care (37%). These referral rates are in accordance with a previous study we conducted on a subsample of CINC patients where we reported that 79% were identified at the age of 4 months to be at high risk for gross motor delay and were referred to the CINC physical therapist for interventional treatment (35). In the current literature, apart from recognizing that the prevalence of children with CHD who need interventional services largely exceeds that of healthy children, there is no clear consensus regarding the referral rate for additional care, which depends on available public health resources as well as insurance coverage (50). For instance, Mussatto et al. (39) reported that 74% of 3-year-old children with CHD received remedial services from US regional early intervention programs or private therapy, and Calderon et al. (69) indicated that 53% of 5-year-old children with CHD from the Paris area received at least one rehabilitation service. Another study revealed that 40–95% of 8-year-old children with CHD who exhibited specific developmental delays did not receive the relevant services (28). In light of these findings, we think that an early systematic developmental follow-up program results in a potential earlier detection of neurodevelopmental impairments associated with CHD, thus generating higher referral rates and at an earlier age. However, this remains to be documented, and the relevance of higher referral rates to be demonstrated in future studies.
Clinical Characteristics
While no statistical differences were found for most perinatal, surgical, and critical characteristics between groups, a greater proportion of children of the Surveillance Group required cardiopulmonary bypass during surgery. This factor has been shown to be associated with worse neurodevelopmental outcomes (61, 70, 71). Children from the Surveillance Group may have been at higher risk of neurologic sequelae after surgery, thus affecting their neurodevelopmental outcome. The higher neurological burden of the CINC patients may have contributed to blurring group differences. More clinically equivalent groups may lead to greater effects of interventional treatment between groups.
Limitations
First, the small sample size may have prevented us from clearly demonstrating an advantage of the interdisciplinary developmental follow-up program over the existing standard health care, and limits the generalization of our findings to the whole complex CHD population. The sample size was restricted in accordance with the hospital's flow of patients with CHD. Specifically, the Historical Control Group was recruited and tested at a time period overlapping the beginning of the CINC program. Therefore, we reached a maximal sample size since all children subsequently born with a critical CHD were referred to the CINC. Second, the use of a historical control group may have introduced biases due to a different time of enrollment. Although no major changes in cardiac care (e.g., cardiac surgeon) occurred in the hospital over this timeframe, subtle changes may have influence group differences. We might also have conducted the evaluation too early in the developmental stage to observe significant differences in high-level cognitive functions, fine motor abilities, and behavior. We cannot exclude that the CINC program may have longer-term impacts that would be more precisely measured in an older cohort as opposed to preschoolers, thus stressing the importance of following these children up to school age and even later on. Finally, because evaluations were performed by the CINC professionals and served for the clinical follow-up of the CINC patients, it is to be noted that examiners were not blind to the children's group, which might have induced an assessment bias.
Conclusions
Results suggest that the CINC early systematic and interdisciplinary developmental follow-up program might prevent the emergence of receptive language difficulties in preschoolers. While these results are promising, they remain subtle at this age and several factors limit their generalization. It is therefore crucial to assess the impact of early systematic developmental follow-ups at school age when this impact might be greater and higher-order functions and learning abilities can be assessed. At the CINC, subsequent follow-ups occur at 5 and 8 years of age, with extensive motor, cognitive, and language assessments performed by occupational therapists, neuropsychologists, and speech-language pathologists, respectively. In addition, we are currently following the children from the Historical Control Group, who are now starting to turn 8 years, and we have been able to recruit more children in this group to increase statistical power. This second phase of the current study will allow us to more accurately assess the impact of our early systematic developmental program at school age.
While neurodevelopmental assessments show that cognitive, motor, and behavioral development of children with critical CHD appears globally within the normal range prior to entry at school, it is not to exclude that subtle limitations may progress into deficits at school age with growing environmental demands. Early systematic assessments with an interdisciplinary team therefore appear to be of great importance to document the developmental trajectory of these functions and to detect subtle impairments that may have a functional impact on behavior, learning, social functioning, and quality of life.
Data Availability Statement
The datasets generated for this study will not be made publicly available due to patient confidentiality.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Research Ethics Board of the Sainte-Justine University Hospital Center. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
SF participated in designing the study and data collection, conducted the analysis, drafted the initial manuscript, and reviewed and revised the manuscript. LC-D participated in data collection and analysis, drafted the initial manuscript, and reviewed and revised the manuscript. M-NS contributed to the analysis and reviewed and revised the manuscript. AD, KG, LD, ÉP, and JP contributed to the design of the study, collected the data, and reviewed and revised the manuscript. SP, AB-T, and IC contributed to the data collection and reviewed and revised the manuscript. NP contributed to the design of the study and reviewed and revised the manuscript. AG conceptualized and designed the study, supervised the collection of the data, contributed to the draft of the initial manuscript, and revised and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Heart and Stroke Foundation of Canada under grant numbers G-16-00012606 and G-17-0018253 as well as a Canada Research Chair held by AG and by Garnier Kids Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the families who participated in the CINC study. We also thank all the staff of the Clinique d'Investigation Neurocardiaque (CINC) of the Sainte-Justine University Hospital Center, Montreal, Quebec, Canada: Laurence Beaulieu-Genest, MD, Valérie Deslauriers, BSc, Erg, Amélie Fauvelle, MSc, Erg, Marie-Michèle Gagnon, MSc, PT, Julien Harvey, MSc, SLP, Julien Leroux, DPs, Psych, Magdalena Jaworski, MD, Geneviève Lupien, BSc, PT, Manuela Materassi, BSc, PT, Christine Montmigny, BSc, PT, Perrine Peckre, BSc, Erg, Elana Pinchefsky, MD, Anne-Marie-Vermette, BSc, RD, and Marie-Claude Vinay, PhD, Psych. We give particular credit to Lionel Carmant, MD, for his involvement in co-founding the CINC and his contribution throughout every step of this study. In memory of our friend and outstanding pediatric neurologist Ala Birca, MD.
References
1. van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BMJ. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. (2011) 8:50–60. doi: 10.1038/nrcardio.2010.166
2. Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. (2014) 130:749–56. doi: 10.1161/CIRCULATIONAHA.113.008396
3. Liu Y, Chen S, Choy M, Li N, Keavney BD. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48:455–63. doi: 10.1093/ije/dyz009
4. Loffredo CA. Epidemiology of cardiovascular malformations: prevalence and risk factors. Am J Med Genet. (2000) 97:319–25. doi: 10.1002/1096-8628(200024)97:4<319::AID-AJMG1283>3.0.CO;2-E
5. Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. (2013) 131:1–15. doi: 10.1542/peds.2012-3435
6. Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. (2010) 56:1149–57. doi: 10.1016/j.jacc.2010.03.085
7. Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW. Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation. (2016) 133:1951–62. doi: 10.1161/CIRCULATIONAHA.115.019881
8. Brown MD, Wernovsky G, Mussatto KA, Berger S. Long-term and developmental outcomes of children with complex congenital heart disease. Clin Perinatol. (2005) 32:1043–57. doi: 10.1016/j.clp.2005.09.008
9. Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. (2006) 148:72–7. doi: 10.1016/j.jpeds.2005.08.036
10. Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C, et al. Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics. (2001) 108:1325–31. doi: 10.1542/peds.108.6.1325
11. Castaneda AR, Jonas RA, Mayer JE Jr, Hanley F. D-transposition of the great arteries. In: Cardiac Surgery of the Neonate and Infant. Philadelphia, PA: WB Saunders Company (1994).
12. Hövels-Gürich HH, Seghaye M-C, Schnitker R, Wiesner M, Huber W, Minkenberg R, et al. Long-term neurodevelopmental outcomes in school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg. (2002) 124:448–58. doi: 10.1067/mtc.2002.122307
13. Laussen PC. Neonates with congenital heart disease. Curr Opin Pediatr. (2001) 13:220–6. doi: 10.1097/00008480-200106000-00002
14. Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. (2006) 16:92–104. doi: 10.1017/S1047951105002398
15. Wray J. Intellectual development of infants, children and adolescents with congenital heart disease. Dev Sci. (2006) 9:368–78. doi: 10.1111/j.1467-7687.2006.00502.x
16. Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the boston circulatory arrest trial. J Thorac Cardiovasc Surg. (2003) 126:1385–96. doi: 10.1016/S0022-5223(03)00711-6
17. Brosig CL, Bear L, Allen S, Hoffmann RG, Pan A, Frommelt M, et al. Preschool neurodevelopmental outcomes in children with congenital heart disease. J Pediatr. (2017) 183:80–6. doi: 10.1016/j.jpeds.2016.12.044
18. Butler SC, Sadhwani A, Stopp C, Singer J, Wypij D, Dunbar-Masterson C, et al. Neurodevelopmental assessment of infants with congenital heart disease in the early postoperative period. Congenit Heart Dis. (2018) 14:236–45. doi: 10.1111/chd.12686
19. Fourdain S, St-Denis A, Harvey J, Birca A, Carmant L, Gallagher A, et al. Language development in children with congenital heart disease aged 12–24 months. Eur J Paediatr Neurol. (2019) 23:491–9. doi: 10.1016/j.ejpn.2019.03.002
20. Hövels-Gürich HH. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. (2002) 87:506–10. doi: 10.1136/adc.87.6.506
21. Marino BS. New concepts in predicting, evaluating, and managing neurodevelopmental outcomes in children with congenital heart disease. Curr Opin Pediatr. (2013) 25:574–84. doi: 10.1097/MOP.0b013e328365342e
22. Massaro AN, El-dib M, Glass P, Aly H. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain Dev. (2008) 30:437–46. doi: 10.1016/j.braindev.2007.12.013
23. Mellion K, Uzark K, Cassedy A, Drotar D, Wernovsky G, Newburger JW, et al. Health-related quality of life outcomes in children and adolescents with congenital heart disease. J Pediatr. (2014) 164:781–8. doi: 10.1016/j.jpeds.2013.11.066
24. Chock VY, Chang IJ, Reddy VM. Short-term neurodevelopmental outcomes in neonates with congenital heart disease: the era of newer surgical strategies: postsurgical neurodevelopmental outcomes. Congenit Heart Dis. (2012) 7:544–50. doi: 10.1111/j.1747-0803.2012.00678.x
25. Dawson G, Bernier R. A quarter century of progress on the early detection and treatment of autism spectrum disorder. Dev Psychopathol. (2013) 25:1455–72. doi: 10.1017/S0954579413000710
26. Chorna O, Baldwin HS, Neumaier J, Gogliotti S, Powers D, Mouvery A, et al. Feasibility of a team approach to complex congenital heart defect neurodevelopmental follow-up: early experience of a combined cardiology/neonatal intensive care unit follow-up program. Circ Cardiovasc Qual Outcomes. (2016) 9:432–40. doi: 10.1161/CIRCOUTCOMES.116.002614
27. Brosig CL, Mussatto KA, Kuhn EM, Tweddell JS. Neurodevelopmental outcome in preschool survivors of complex congenital heart disease: implications for clinical practice. J Pediatr Health Care. (2007) 21:3–12. doi: 10.1016/j.pedhc.2006.03.008
28. Majnemer A, Mazer B, Lecker E, Leduc Carter A, Limperopoulos C, Shevell M, et al. Patterns of use of educational and rehabilitation services at school age for children with congenitally malformed hearts. Cardiol Young. (2008) 18:288–96. doi: 10.1017/S1047951108002114
29. Weinberg S, Kern J, Weiss K, Ross G. Developmental screening of children diagnosed with congenital heart defects. Clin Pediatr. (2001) 40:497–501. doi: 10.1177/000992280104000904
30. Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the american heart association. Circulation. (2012) 126:1143–72. doi: 10.1161/CIR.0b013e318265ee8a
31. Brosig C, Butcher J, Butler S, Ilardi DL, Sananes R, Sanz JH, et al. Monitoring developmental risk and promoting success for children with congenital heart disease: recommendations for cardiac neurodevelopmental follow-up programs. Clin Pract Pediatr Psychol. (2014) 2:153–65. doi: 10.1037/cpp0000058
32. Darrah J, Piper M, Watt M-J. Assessment of gross motor skills of at-risk infants: predictive validity of the Alberta Infant Motor Scale. Dev Med Child Neurol. (2008) 40:485–91. doi: 10.1111/j.1469-8749.1998.tb15399.x
33. Albuquerque PL de, Guerra MQ de F, Lima M, de C, Eickmann SH. Concurrent validity of the alberta infant motor scale to detect delayed gross motor development in preterm infants: a comparative study with the bayley III. Dev Neurorehabilitation. (2018) 21:408–14. doi: 10.1080/17518423.2017.1323974
34. Piper MC, Pinnell LE, Darrah J, Maguire T, Byrne PJ. Construction and validation of the Alberta Infant Motor Scale (AIMS). Can J Public Health. (1992) 83:46–50.
35. Fourdain S, Simard M-N, Dagenais L, Materassi M, Doussau A, Goulet J, et al. Gross motor development of children with congenital heart disease receiving early systematic surveillance and individualized intervention: brief report. Dev Neurorehabilitation. (2020) 1–7. doi: 10.1080/17518423.2020.1711541
36. Trudeau N. Les Inventaires MacArthur-Bates du Développement de la Communication (IMBCD), Montreal, QC (2008).
37. Squires J, Bricker D. Ages and Stages Questionnaires, Third Edition (ASQ-3): A Parent-Completed Child-Monitoring System. Baltimore, MD: Brookes Pu (2009).
38. Gallagher A, Dagenais L, Doussau A, Décarie J-C, Materassi M, Gagnon K, et al. Significant motor improvement in an infant with congenital heart disease and a rolandic stroke: the impact of early intervention. Dev Neurorehabilitation. (2017) 20:165–8. doi: 10.3109/17518423.2015.1132280
39. Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. (2014) 133:e570–7. doi: 10.1542/peds.2013-2309
40. Naef N, Wehrle F, Rousson V, Latal B. Cohort and individual neurodevelopmental stability between 1 and 6 years of age in children with congenital heart disease. J Pediatr. (2019) 215:83–9.e2. doi: 10.1016/j.jpeds.2019.08.036
41. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence – 4th Edition (WPPSI-IV). Bloomington, MN: Pearson (2012).
42. Folio M, Fewell R. Peabody Developmental Motor Scales, Second Edition (PDMS-2). Austin, TX: Pro-Ed (2000).
43. Henderson SE, Sugden DA, Barnett AL. Movement Assessment Battery for Children [Examiner's Manual]. 2nd ed. London: Pearson Assessments (2007).
44. Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. 2nd ed. Bloomington, MN: Pearson Assessments (2004).
45. Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. (2000) 119:347–57. doi: 10.1016/S0022-5223(00)70191-7
46. Jenkins KJ, Gauvreau K. Center-specific differences in mortality: preliminary analyses using the Risk Adjustment in Congenital Heart Surgery (RACHS-1) method. J Thorac Cardiovasc Surg. (2002) 124:97–104. doi: 10.1067/mtc.2002.122311
47. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
48. Soto CB, Olude O, Hoffmann RG, Bear L, Chin A, Dasgupta M, et al. Implementation of a routine developmental follow-up program for children with congenital heart disease: early results: developmental follow-up for congenital heart disease. Congenit Heart Dis. (2011) 6:451–60. doi: 10.1111/j.1747-0803.2011.00546.x
49. Michael M, Scharf R, Letzkus L, Vergales J. Improving neurodevelopmental surveillance and follow-up in infants with congenital heart disease: neurodevelopmental surveillance congenital heart disease. Congenit Heart Dis. (2016) 11:183–8. doi: 10.1111/chd.12333
50. Glotzbach KL, Ward JJ, Marietta J, Eckhauser AW, Winter S, Puchalski MD, et al. The benefits and bias in neurodevelopmental evaluation for children with congenital heart disease. Pediatr Cardiol. (2020) 41:327–33. doi: 10.1007/s00246-019-02260-7
51. Bonnier C. Evaluation of early stimulation programs for enhancing brain development. Acta Paediatr. (2008) 97:853–8. doi: 10.1111/j.1651-2227.2008.00834.x
52. Estes A, Vismara L, Mercado C, Fitzpatrick A, Elder L, Greenson J, et al. The impact of parent-delivered intervention on parents of very young children with autism. J Autism Dev Disord. (2014) 44:353–65. doi: 10.1007/s10803-013-1874-z
53. Rogers SJ, Estes A, Lord C, Vismara L, Winter J, Fitzpatrick A, et al. Effects of a brief Early Start Denver Model (ESDM)–based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. (2012) 51:1052–65. doi: 10.1016/j.jaac.2012.08.003
54. Spittle AJ, Barton S, Treyvaud K, Molloy CS, Doyle LW, Anderson PJ. School-age outcomes of early intervention for preterm infants and their parents: a randomized trial. Pediatrics. (2016) 138:e20161363. doi: 10.1542/peds.2016-1363
55. Spittle A, Treyvaud K. The role of early developmental intervention to influence neurobehavioral outcomes of children born preterm. Semin Perinatol. (2016) 40:542–8. doi: 10.1053/j.semperi.2016.09.006
56. Van Hus J, Jeukens-Visser M, Koldewijn K, Holman R, Kok J, Nollet F, et al. Early intervention leads to long-term developmental improvements in very preterm infants, especially infants with bronchopulmonary dysplasia. Acta Paediatr. (2016) 105:773–81. doi: 10.1111/apa.13387
57. Wu Y-C, Leng C-H, Hsieh W-S, Hsu C-H, Chen WJ, Gau SS-F, et al. A randomized controlled trial of clinic-based and home-based interventions in comparison with usual care for preterm infants: effects and mediators. Res Dev Disabil. (2014) 35:2384–93. doi: 10.1016/j.ridd.2014.06.009
58. Orton J, Spittle A, Doyle L, Anderson P, Boyd R. Do early intervention programmes improve cognitive and motor outcomes for preterm infants after discharge? A systematic review: Early Intervention for Preterm Infants. Dev Med Child Neurol. (2009) 51:851–9. doi: 10.1111/j.1469-8749.2009.03414.x
59. Bellinger DC, Bernstein JH, Kirkwood MW, Rappaport LA, Newburger JW. Visual-spatial skills in children after open-heart surgery. J Dev Behav Pediatr. (2003) 24:169–79. doi: 10.1097/00004703-200306000-00007
60. Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr. (2007) 151:73–8. doi: 10.1016/j.jpeds.2007.02.020
61. Hövels-Gürich HH, Bauer SB, Schnitker R, Willmes-von Hinckeldey K, Messmer BJ, Seghaye M-C, et al. Long-term outcome of speech and language in children after corrective surgery for cyanotic or acyanotic cardiac defects in infancy. Eur J Paediatr Neurol. (2008) 12:378–86. doi: 10.1016/j.ejpn.2007.10.004
62. Nordhov SM, Ronning JA, Dahl LB, Ulvund SE, Tunby J, Kaaresen PI. Early intervention improves cognitive outcomes for preterm infants: randomized controlled trial. Pediatrics. (2010) 126:e1088–94. doi: 10.1542/peds.2010-0778
63. Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. (2015) 24:CD005495. doi: 10.1002/14651858.CD005495.pub4
64. McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev. (2009) 36:110–7. doi: 10.1111/j.1365-2214.2009.01026.x
65. McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A randomized controlled trial of interventions to promote adjustment in children with congenital heart disease entering school and their families. J Pediatr Psychol. (2012) 37:1089–103. doi: 10.1093/jpepsy/jss092
66. Holm I, Fredriksen PM, Fosdahl MA, Olstad M, Vøllestad N. Impaired motor competence in school-aged children with complex congenital heart disease. Arch Pediatr Adolesc Med. (2007) 161:945–50. doi: 10.1001/archpedi.161.10.945
67. Dagenais L, Materassi M, Desnous B, Vinay M-C, Doussau A, Sabeh P, et al. Superior performance in prone in infants with congenital heart disease predicts an earlier onset of walking. J Child Neurol. (2018) 33:894–900. doi: 10.1177/0883073818798194
68. Kynø NM, Ravn IH, Lindemann R, Fagerland MW, Smeby NA, Torgersen AM. Effect of an early intervention programme on development of moderate and late preterm infants at 36 months: a randomized controlled study. Infant Behav Dev. (2012) 35:916–26. doi: 10.1016/j.infbeh.2012.09.004
69. Calderon J, Bonnet D, Pinabiaux C, Jambaqué I, Angeard N. Use of early remedial services in children with transposition of the great arteries. J Pediatr. (2013) 163:1105–10.e1. doi: 10.1016/j.jpeds.2013.04.065
70. Limperopoulos C, Majnemer A, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C, et al. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr. (2002) 141:51–8. doi: 10.1067/mpd.2002.125227
Keywords: congenital heart disease, neurodevelopment, neurocardiac program, early intervention, interdisciplinary, preschool age, developmental assessment
Citation: Fourdain S, Caron-Desrochers L, Simard M-N, Provost S, Doussau A, Gagnon K, Dagenais L, Presutto É, Prud'homme J, Boudreault-Trudeau A, Constantin IM, Desnous B, Poirier N and Gallagher A (2020) Impacts of an Interdisciplinary Developmental Follow-Up Program on Neurodevelopment in Congenital Heart Disease: The CINC Study. Front. Pediatr. 8:539451. doi: 10.3389/fped.2020.539451
Received: 01 March 2020; Accepted: 18 August 2020;
Published: 06 October 2020.
Edited by:
Oswin Grollmuss, Université Paris-Sud, FranceReviewed by:
Jo Wray, Great Ormond Street Hospital for Children National Health Service (NHS) Foundation Trust, United KingdomBenjamin Bierbach, University Hospital Bonn, Germany
Copyright © 2020 Fourdain, Caron-Desrochers, Simard, Provost, Doussau, Gagnon, Dagenais, Presutto, Prud'homme, Boudreault-Trudeau, Constantin, Desnous, Poirier and Gallagher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne Gallagher, anne.gallagher@umontreal.ca
 Solène Fourdain
Solène Fourdain Laura Caron-Desrochers
Laura Caron-Desrochers Marie-Noëlle Simard
Marie-Noëlle Simard Sarah Provost
Sarah Provost Amélie Doussau
Amélie Doussau Karine Gagnon
Karine Gagnon Lynn Dagenais
Lynn Dagenais Émilie Presutto4
Émilie Presutto4  Joëlle Prud'homme
Joëlle Prud'homme Annabelle Boudreault-Trudeau
Annabelle Boudreault-Trudeau Béatrice Desnous
Béatrice Desnous Anne Gallagher
Anne Gallagher