Near-Infrared Spectroscopy in Extremely Preterm Infants
- Division of Neonatology, Nationwide Children's Hospital, Columbus, OH, United States
With advances in neonatal care, survival of premature infants at the limits of viability has improved significantly. Despite these improvement in mortality, infants born at 22–24 weeks gestation are at a very high risk for short- and long-term morbidities associated with prematurity. Many of these diseases have been attributed to abnormalities of tissue oxygenation and perfusion. Near-infrared spectroscopy utilizes the unique absorption properties of oxyhemoglobin and deoxyhemoglobin to provide an assessment of regional tissue oxygen saturation, which can be used to calculate the fractional tissue oxygen extraction. This allows for a non-invasive way to monitor tissue oxygen consumption and enables targeted hemodynamic management. This mini-review provides a brief and complete overview of the background and physiology of near-infrared spectroscopy, practical use in extremely preterm infants, and potential applications in the neonatal intensive care unit. In this mini-review, we aim to summarize the three primary application sites for near-infrared spectroscopy, disease-specific indications, and available literature regarding use in extremely preterm infants.
Introduction
History, Physics, and Physiology of Near-Infrared Spectroscopy
Near-infrared energy exists in the 700–1,000 nm wavelength spectrum and was first discovered by the German astronomer (and composer) William Herschel in the 19th century (1). Jobsis et al. applied the principles of near-infrared spectroscopy (NIRS) to medical research in the 1970s and demonstrated that NIRS could monitor regional cerebral blood flow because light wavelengths in the near-infrared range are uniquely suitable to measuring conformational changes in hemoglobin (2, 3). Deoxyhemoglobin exists in a tense, low-oxygen affinity state. Upon binding of one oxygen molecule to a heme subunit, the hemoglobin molecule undergoes a conformational change that results in a more relaxed conformation that increases the affinity of the remaining subunits for oxygen, which forms the basis for the hemoglobin-oxygen dissociation curve (4, 5).
The conformations of deoxy- and oxyhemoglobin display distinct spectroscopic properties and absorption spectra (6). NIRS sensors measure the absorbance of light at wavelengths 700/850 nm, where the near-infrared absorption spectra are maximally separated between oxyhemoglobin and deoxyhemoglobin (7). The NIRS computer calculates the concentrations of deoxyhemoglobin and oxyhemoglobin using the modified Lambert-Beer law and presents the regional oxygen saturation (rSO2) as a ratio of oxyhemoglobin/(deoxyhemoglobin + oxyhemoglobin) (8, 9).
NIRS probes generally consist of a single light source (emitting light at two infrared wavelengths) and two down-stream photoreceptors, which measure light absorbance at different tissue depths (10). Absorption of near-infrared light is relatively low and has been detected at depths of several centimeters of biologic tissue (11). The light not detected upon return to the photodetector accounts for light absorbed by the deoxyhemoglobin and oxyhemoglobin. The NIRS computer calculates a tissue-specific rSO2 at a tissue depth of 1–2 cm for the entire microcirculation. rSO2 is reported as a weighted average of the ratio and therefore, approximately 75–85% of the rSO2 reflects the ratio of oxyhemoglobin to total hemoglobin in tissue-specific venules (12). When combined with arterial oxygen saturation (SaO2), rSO2 can be used to determine the local balance of oxygen delivery and consumption, similar to the mixed venous saturation (SvO2). rSO2 can be used to calculate the tissue fractional tissue oxygen extraction (FTOE), using the formula FTOE = (SaO2 – rSO2)/SaO2 (13, 14). rSO2 has been shown to correlate with SvO2 in pediatric cardiac surgery patients (13). Unlike SvO2, which requires central venous monitoring to measure, rSO2 can be measured non-invasively using NIRS probes.
Practical Considerations
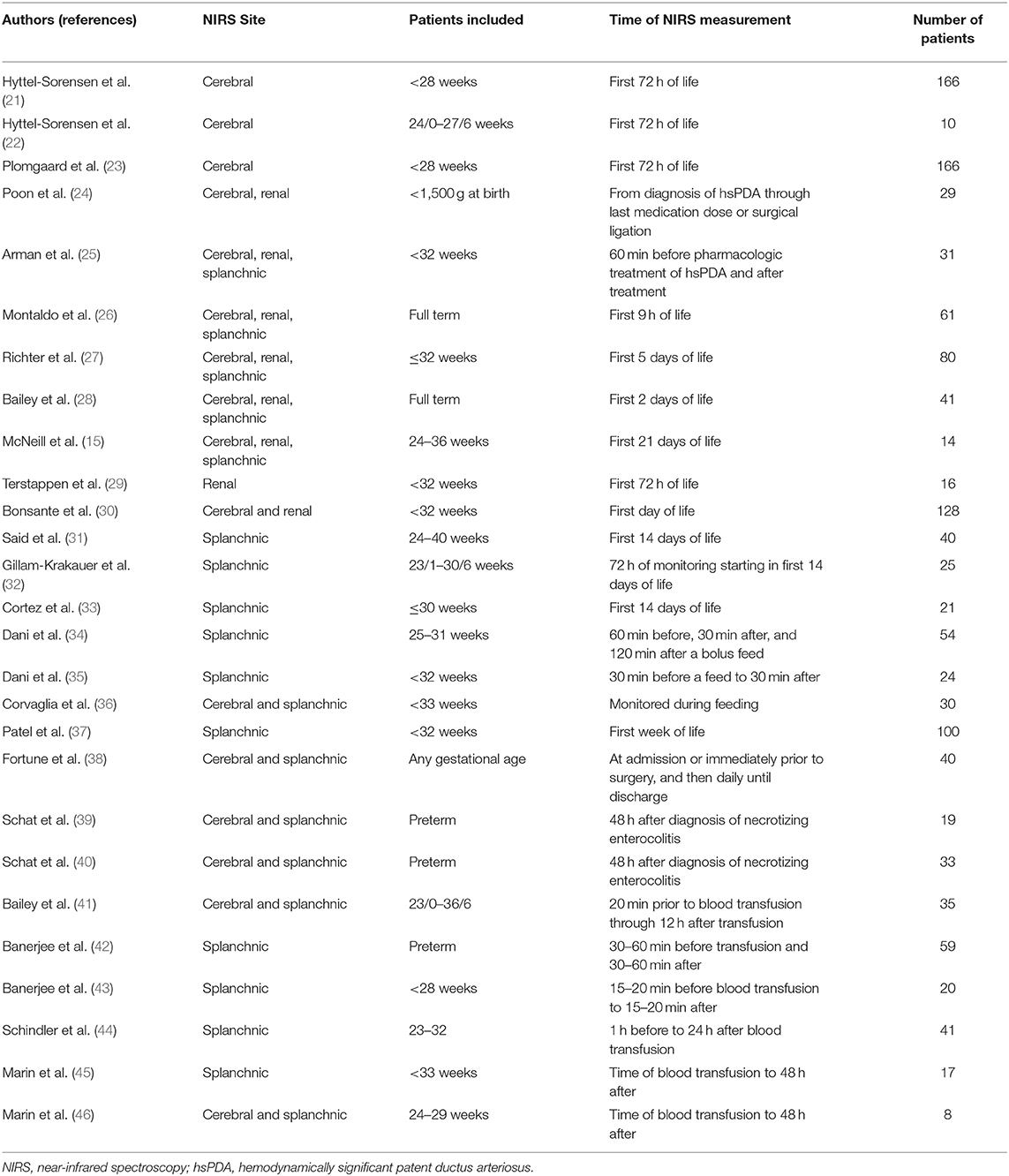
In neonates, NIRS probes are used to monitor cerebral rSO2 (crSO2), renal rSO2 (rrSO2), and splanchnic rSO2 (srSO2). NIRS probes for crSO2 monitoring are placed midline on the forehead to monitor the frontal lobe gray matter (15, 16). Renal NIRS probes are placed on the back, between the costal margin and iliac crest with the sensor lateral to the spine and reader tip wrapping around the flank (17). Splanchnic NIRS probes are placed over the liver or infra-umbilically (15). Though specific values vary across various NIRS devices (18–20), crSO2 is generally lower than rrSO2/srSO2 because the brain has greater metabolic activity and consumes more oxygen (15). Although adult reports suggest that applied light intensities are not harmful even with prolonged use (10), skin burns, pressure sores, and skin irritation resulting in persistent skin marks at term have been observed in studies of cerebral NIRS monitoring in very preterm infants (21). This review provides a summary (Table 1) of the potential applications of NIRS in the neonatal intensive care unit (NICU).
Cerebral NIRS
Background
The survival of extremely low birth weight infants has consistently improved over the past few decades. In addition to improving survival, optimizing neurodevelopmental outcomes is a key area of focus for all institutions that care for such vulnerable infants, especially since one review found that nearly half of surviving infants at <25 weeks had significant short- and long-term neurodevelopmental impairments (47).
Using NIRS in this patient population may help to detect or potentially ameliorate cerebral insults related to regional perfusion and ischemia, primarily intraventricular hemorrhage (IVH) and white matter injury (WMI) (48). Due to a poorly developed cerebral autoregulation system, premature infants are unable to adequately provide consistent cerebral blood flow in the face of stressors (49). NIRS can be helpful to continuously monitor regional brain tissue perfusion and provide objective data about the balance between oxygen delivery and demand. These values have previously been validated against jugular venous saturations in cohorts of neonates (50). While normative values continue to be an area of active research, one large multicenter study has published reference curves for the first 72 h of life in preterm infants (51).
A Cochrane review in 2017 found only one randomized clinical trial looking at the use of cerebral NIRS in preterm infants. While their conclusion did not demonstrate any positive effects of the use of NIRS in preventing brain injury, this study was only powered to detect differences in cerebral oxygenation with the use of NIRS (21). In fact, one of the earliest feasibility studies (SafeBoosC) demonstrated the ability to use NIRS in extremely premature infants and to maintain preterm infants within a predetermined crSO2 range (22). In the SafeBoosC-II study, subjects in the NIRS group were successfully maintained in the target crSO2 range as compared to a control group. While again not powered to detect clinical differences, there was an association between decreased levels of hypoxia and a lower incidence of severe IVH (23). The SafeBoosC investigators are currently conducting a phase-III follow-up study (SafeBoosC-III, www.safeboosc.eu) to detect differences in clinical outcomes with respect to continuous crSO2 monitoring in extremely preterm infants.
Intraventricular Hemorrhage
IVH remains an important cause of morbidity in mortality in extremely preterm infants (52). While multifactorial, much of the pathophysiology of the development of IVH is tied to the immature vasculature of the germinal matrix (53). It is thought that this impaired cerebral autoregulation is challenged by hypo- and hypercapnia, as well as episodic hypotension that is often compounded by the use of vasoactive agents; a common pathway here is the “pressure-passive” perfusion of the neonatal brain (54). As acute fluctuations in PaCO2 are associated with swings in cerebral blood flow, the use of NIRS in mechanically ventilated extremely preterm infants may help to detect and correct these changes before injury can occur (55).
Cerebral blood flow is intimately linked to blood pressure and crSO2 patterns passively change with the mean arterial pressure (56). As both extremes of blood pressure have important implications in the development of IVH, crSO2 monitoring may help identify such infants and facilitate judicious use of inotropes. One author has suggested using NIRS to minimize fluctuations in cerebral perfusion simply by monitoring those infants who cannot tolerate handling and may benefit from sedation or gentle containment maneuvers (55). Results of studies examining the significance of crSO2 and cerebral FTOE values with respect to the development of IVH have varied, as some have demonstrated that an increase in crSO2 and a decrease in cerebral FTOE precede severe IVH, while others have seen that these findings are a result of the severe IVH (48). While further research is required to elucidate the true prognostic value, trends in cerebral hemodynamics as measured in crSO2 and cerebral FTOE can potentially help detect regional perfusion changes that contribute to the pathophysiology of IVH.
White Matter Injury
Severe WMI is another significant contributor to poor long-term neurodevelopmental outcomes in extremely premature infants (57). NIRS is being studied and implemented to monitor for patterns of cerebral hypoxia and hypoperfusion that contribute to the development of WMI. The most recognized causes of hemodynamic and vascular disturbances to the fragile preterm brain include hypo- and hypercapnia, left-to-right shunting via a hemodynamically significant patent ductus arteriosus (hsPDA), hypoglycemia, anemia, and cortical thinning from post-hemorrhagic hydrocephalus (55). Changes in PaCO2 and hemodynamic fluctuations from a hsPDA are perhaps the two most studied etiologies. There are well-described associations of low PaCO2 and the development of WMI and cerebral palsy (58). Similar negative impacts are seen with anemia (59) and hypoglycemia (60).
Patent Ductus Arteriosus
Management of an hsPDA remains a challenging subject in neonatology. With a significant left-to-right shunt, impaired cardiac output can result in end-organ dysfunction. It is speculated that preterm infants with an hsPDA are exposed to decreased cerebral perfusion putting them at increased risk for brain injury (61, 62). While the exact crSO2 values during NIRS monitoring are a subject of ongoing research, it is postulated that values below 40–45% for as little as 30 min can have deleterious effects on cerebral blood flow and cerebellar growth (55). The application of NIRS to a preterm infant with an hsPDA may help guide management decisions about ductal closure. Recent prospective studies have shown that crSO2 and cerebral FTOE values can be trended during medical or surgical treatment of an hsPDA to help ascertain response (24, 25).
Renal NIRS
Background
While use of renal NIRS in the NICU has primarily focused on full-term infants, there is emerging data and evidence for use in premature neonates, including the extremely preterm population. Estimations of normal values for rrSO2 in the first days of life in the term neonate have emerged from studies such as that done by Montaldo et al., which investigated renal NIRS values in term neonates starting in the first 15 min of life (26). Such values do not yet exist for premature infants, though some smaller studies examining this question have been published. In 2016 Richter et al. monitored a cohort of 80 infants ≤32 weeks gestation with renal NIRS for the first five postnatal days and found that exposure to maternal antihypertensive medications did not affect regional oxygenation and hemodynamics (27). rrSO2 in premature neonates has been observed to be lower than in term neonates within the first 48 h of life (26–28). In 2011 McNeil et al. sought to characterize normal neonatal rrSO2 using a cohort of 12 preterm infants 29–33 weeks gestation; the authors observed that rrSO2 starts in the mid-80s in the first week of life and decreases over time to mid-60s by the third week of life (15). Terstappen et al. demonstrated that rrSO2 was higher overall in growth restricted premature neonates in the first 72 h, compared with control neonates. During the first 72 h of life the rrSO2 of the growth restricted group decreased while those of the control group increased (29).
Acute Kidney Injury
One major potential future application of renal NIRS in the NICU is to monitor for and potentially prevent the development of acute kidney injury (AKI). In 2019 Bonsante et al. became the first to demonstrate the correlation between rrSO2 within the first 24 h of life and the future development of AKI in neonates <32 weeks gestation. Using renal NIRS in a cohort of 128 preterm infants, rrSO2 was monitored continuously for the first 24 h of life and serum creatinine was monitored daily for seven days. Low rrSO2 demonstrated a statistically significant association with the development of AKI (30). Until recently neonatal AKI has largely been an under-recognized disease with significant associated morbidity including long-term development of chronic kidney disease, and an incidence ranging 18–56% in the extremely low birth weight population (63–65). Elevated serum creatinine is the current gold standard for diagnosing AKI, but this takes at least 12–48 h to rise, at which point injury may be irreversible (66). Utilizing renal NIRS to monitor rrSO2 in neonates at-risk for AKI could potentially allow for earlier detection and intervention.
Splanchnic NIRS
Background
A third major area of potential use for NIRS in extremely premature infants is splanchnic NIRS to monitor gut oxygenation. There are limited studies validating the use of splanchnic NIRS in the NICU. In neonates undergoing surgical correction of congenital heart disease, srSO2 correlated with other established measures of oxygenation and perfusion, including lactate, gastric pH, and SvO2 (67). Said et al. compared srSO2 values with pulse oximetry and umbilical venous catheter oxygen saturations and showed good correlation between NIRS and reference value measurements (31). In stable preterm infants in the first 3 days of life, changes in srSO2 values in association with feeds were shown to correlate with changes in superior mesenteric artery blood flow velocity (32). Studies of healthy preterm neonates have shown a decrease in median srSO2 over the first week of life, followed by a subsequent increase (15, 33).
Monitoring of Feeds
NIRS have been utilized to study the response to various types feeding regimens in the preterm infant population, including breast milk compared to formula and bolus compared to continuous feeds (34–36). Dani et al. studied the impact of maternal breast milk, fortified human milk, and preterm infant formula on srSO2 at 30 and 120 min after initiation of a bolus feed in 25–31-week infants. srSO2 persistently decreased in the preterm formula group, unlike in the two human milk groups, suggesting that formula feeding leads to higher splanchnic FTOE than human milk (34).
A cross-over study in 2013 compared the effect of continuous vs. bolus feedings between appropriate for gestational age and small for gestational age infants at <32 weeks. srSO2 increased 30 min after a bolus feed in both groups, while splanchnic FTOE remained stable. On the other hand, srSO2 and splanchnic FTOE did not change during a continuous feed in either group of infants, suggesting that energy expenditure is lower during a continuous feed. During both bolus and continuous feeds, srSO2 is higher and FTOE is lower in the appropriate for gestational age cohort (35). Conversely, a cross-over study of preterm infants with a median gestational age of 30.1 weeks by Corvaglia et al. revealed increased srSO2 beginning at about 1 h after a bolus feed, whereas there was a decrease during continuous feedings (36). Unfortunately, none of these studies focus specifically on the most premature infants between 22 and 24 weeks at birth, and in fact, many limited enrollment to those infants born at >25–26 weeks.
Necrotizing Enterocolitis
Studies suggest that splanchnic NIRS may play a role in monitoring the hemodynamic changes associated with the development and progression of necrotizing enterocolitis (NEC). Using a cohort of 100 preterm infants <32 weeks gestation, Patel et al. showed that the mean srSO2 was lower in the first week of life in the neonates who later developed NEC, and these patients also had significantly more variation in srSO2 around feedings in the first 2 weeks (37). Additionally, clinical prediction models utilizing the cerebro-splanchnic oxygen ratio, which compares the srSO2 to crSO2 (38), and various biomarkers including plasmatic intestinal fatty acid-binding protein (39) have been investigated to improve the sensitivity of NEC prediction in preterm infants. In a prospective cohort study, Schat et al. investigated the utility of splanchnic NIRS to predict the course of NEC in preterm infants. The patients with complicated NEC, defined as bowel perforation requiring surgery or death, had significantly lower srSO2 with a higher splanchnic FTOE within 24 h after onset of symptoms when compared to the group that had uncomplicated NEC (40).
Anemia and Blood Transfusions
A final area of interest is the use of splanchnic NIRS in anemic preterm neonates receiving blood transfusions to further understand the association between NEC and blood transfusions. Blood transfusions have been shown to improve srSO2 and decrease splanchnic FTOE in extremely preterm infants (41–43). Changes in srSO2 when infants receive feeds during a transfusion have not been consistently shown, with some studies demonstrating no change (44) and others showing a decline (45). Very low birth weight infants who developed transfusion-associated NEC have greater variability in srSO2 compared to those who did not develop NEC (46). Additional research in this area is needed to further elucidate mechanisms of transfusion-associated NEC and methods of early prediction of high-risk infants and/or prevention.
Conclusions
The use of NIRS in preventing cerebral injury in extremely preterm infants remains an active area of challenging research, particularly as it pertains to establishing normative values and consensus within neonatology. The use of “pattern recognition” has often been suggested as a stop-gap measure, that is, recognizing trends in crSO2 and the underlying physiology that could be driving these changes (55). The many shared etiologies in cerebral injury in extremely preterm infants does allow for some simplification of clinical investigation when the crSO2 values begin to deviate from the “norm.”
While much of the research using NIRS in preterm infants has focused on cerebral NIRS, renal and splanchnic NIRS also have emerging data and potential for clinical and research opportunities. Further studies need to be performed to validate the use of NIRS and determine the appropriate use of this technology, but the possibility of having a non-invasive method of monitoring tissue-level hemodynamics and oxygen balance makes this research a worthwhile endeavor. One key consideration in research and clinical application is the specific NIRS device and sensor used, as there are multiple manufacturers and sizes of sensors. One study has shown consistently higher crSO2 using a pediatric or neonatal sensor when compared to an adult sensor, so close attention should be paid to the type of sensor used when interpreting absolute value rSO2 results provided in a study (68).
Many studies, even those focused on extremely preterm infants, do not include the most immature babies between 22 and 24 weeks gestation. As this is the patient population at the highest risk of morbidities and mortality associated with prematurity, they stand to benefit the most from precise management of hemodynamic status and monitoring for complications. Outcomes-driven research will ultimately determine if the use of NIRS will become mainstream in most institutions, but the use of regional perfusion monitoring to potentially prevent devastating morbidities is exciting and worth the effort.
Author Contributions
All authors contributed to the conception and design of this manuscript, drafting and critically revising the initial manuscript, and approval of the final version. All authors are accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. McClure WF. 204 years of near infrared technology: 1800–2003. J Near Infrared Spec. (2003) 11:487–518. doi: 10.1255/jnirs.399
2. Jobsis FF. Non-invasive, infra-red monitoring of cerebral O2 sufficiency, blood volume, HbO2-Hb shifts and bloodflow. Acta Neurol Scand Suppl. (1977) 64:452–3.
3. Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. (1977) 198:1264–7. doi: 10.1126/science.929199
4. Perutz MF, Wilkinson AJ, Paoli M, Dodson GG. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu Rev Biophys Biomol Struct. (1998) 27:1–34. doi: 10.1146/annurev.biophys.27.1.1
5. Hsia CC. Respiratory function of hemoglobin. N Engl J Med. (1998) 338:239–47. doi: 10.1056/NEJM199801223380407
6. Horecker BL. The absorption spectra of hemoglobin and its derivatives in the visible and near infra-red regions. J Biol Chem. (1943) 148:173–83.
7. Ghosh A, Elwell C, Smith M. Review article: cerebral near-infrared spectroscopy in adults: a work in progress. Anesth Analg. (2012) 115:1373–83. doi: 10.1213/ANE.0b013e31826dd6a6
8. Sood BG, McLaughlin K, Cortez J. Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med. (2015) 20:164–72. doi: 10.1016/j.siny.2015.03.008
9. van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology. (2008) 94:237–44. doi: 10.1159/000151642
10. Scheeren TW, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput. (2012) 26:279–87. doi: 10.1007/s10877-012-9348-y
11. Vretzakis G, Georgopoulou S, Stamoulis K, Stamatiou G, Tsakiridis K, Zarogoulidis P, et al. Cerebral oximetry in cardiac anesthesia. J Thorac Dis. (2014) 6(Suppl. 1):S60–9. doi: 10.3978/j.issn.2072-1439.2013.10.22
12. Chakravarti S, Srivastava S, Mittnacht AJ. Near infrared spectroscopy (NIRS) in children. Semin Cardiothorac Vasc Anesth. (2008) 12:70–9. doi: 10.1177/1089253208316444
13. Tortoriello TA, Stayer SA, Mott AR, McKenzie ED, Fraser CD, Andropoulos DB, et al. A noninvasive estimation of mixed venous oxygen saturation using near-infrared spectroscopy by cerebral oximetry in pediatric cardiac surgery patients. Paediatr Anaesth. (2005) 15:495–503. doi: 10.1111/j.1460-9592.2005.01488.x
14. van Vonderen JJ, Roest AA, Siew ML, Walther FJ, Hooper SB, te Pas AB. Measuring physiological changes during the transition to life after birth. Neonatology. (2014) 105:230–42. doi: 10.1159/000356704
15. McNeill S, Gatenby JC, McElroy S, Engelhardt B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J Perinatol. (2011) 31:51–7. doi: 10.1038/jp.2010.71
16. Chock VY, Kwon SH, Ambalavanan N, Batton B, Nelin LD, Chalak LF, et al. Cerebral oxygenation and autoregulation in preterm infants (early NIRS study). J Pediatr. (2020) 227:94–100.e1. doi: 10.1016/j.jpeds.2020.08.036
17. Harer MW, Chock VY. Renal tissue oxygenation monitoring—an opportunity to improve kidney outcomes in the vulnerable neonatal population. Front Pediatr. (2020) 8:241. doi: 10.3389/fped.2020.00241
18. Hyttel-Sorensen S, Hessel TW, Greisen G. Peripheral tissue oximetry: comparing three commercial near-infrared spectroscopy oximeters on the forearm. J Clin Monit Comput. (2014) 28:149–55. doi: 10.1007/s10877-013-9507-9
19. Dullenkopf A, Frey B, Baenziger O, Gerber A, Weiss M. Measurement of cerebral oxygenation state in anaesthetized children using the INVOS 5100 cerebral oximeter. Paediatr Anaesth. (2003) 13:384–91. doi: 10.1046/j.1460-9592.2003.01111.x
20. Sorensen LC, Leung TS, Greisen G. Comparison of cerebral oxygen saturation in premature infants by near-infrared spatially resolved spectroscopy: observations on probe-dependent bias. J Biomed Opt. (2008) 13:064013. doi: 10.1117/1.3013454
21. Hyttel-Sorensen S, Greisen G, Als-Nielsen B, Gluud C. Cerebral near-infrared spectroscopy monitoring for prevention of brain injury in very preterm infants. Cochrane Database Syst Rev. (2017) 9:CD011506. doi: 10.1002/14651858.CD011506.pub2
22. Hyttel-Sorensen S, Austin T, van Bel F, Benders M, Claris O, Dempsey EM, et al. Clinical use of cerebral oximetry in extremely preterm infants is feasible. Dan Med J. (2013) 60:A4533. doi: 10.5167/uzh-79553
23. Plomgaard AM, Alderliesten T, Austin T, van Bel F, Benders M, Claris O, et al. Early biomarkers of brain injury and cerebral hypo- and hyperoxia in the SafeBoosC II trial. PLoS ONE. (2017) 12:e0173440. doi: 10.1371/journal.pone.0173440
24. Poon WB, Tagamolila V. Cerebral perfusion and assessing hemodynamic significance for patent ductus arteriosus using near infrared red spectroscopy in very low birth weight infants. J Matern Fetal Neonatal Med. (2019) 1–6. doi: 10.1080/14767058.2019.1644313
25. Arman D, Sancak S, Gursoy T, Topcuoglu S, Karatekin G, Ovali F. The association between NIRS and Doppler ultrasonography in preterm infants with patent ductus arteriosus. J Matern Fetal Neonatal Med. (2020) 33:1245–52. doi: 10.1080/14767058.2019.1639661
26. Montaldo P, De Leonibus C, Giordano L, De Vivo M, Giliberti P. Cerebral, renal and mesenteric regional oxygen saturation of term infants during transition. J Pediatr Surg. (2015) 50:1273–7. doi: 10.1016/j.jpedsurg.2015.04.004
27. Richter AE, Schat TE, Van Braeckel KN, Scherjon SA, Bos AF, Kooi EM. The effect of maternal antihypertensive drugs on the cerebral, renal and splanchnic tissue oxygen extraction of preterm neonates. Neonatology. (2016) 110:163–71. doi: 10.1159/000445283
28. Bailey SM, Hendricks-Munoz KD, Mally P. Cerebral, renal, and splanchnic tissue oxygen saturation values in healthy term newborns. Am J Perinatol. (2014) 31:339–44. doi: 10.1055/s-0033-1349894
29. Terstappen F, Paauw ND, Alderliesten T, Joles JA, Vijlbrief DC, Lely AT, et al. Elevated renal tissue oxygenation in premature fetal growth restricted neonates: An observational study. PLoS ONE. (2018) 13:e0204268. doi: 10.1371/journal.pone.0204268
30. Bonsante F, Ramful D, Binquet C, Samperiz S, Daniel S, Gouyon JB, et al. Low renal oxygen saturation at near-infrared spectroscopy on the first day of life is associated with developing acute kidney injury in very preterm infants. Neonatology. (2019) 115:198–204. doi: 10.1159/000494462
31. Said MM, Niforatos N, Rais-Bahrami K. Validation of near infrared spectroscopy to measure abdominal somatic tissue oxygen saturation in neonates. J Neonatal Perinatal Med. (2013) 6:23–30. doi: 10.3233/NPM-1365112
32. Gillam-Krakauer M, Cochran CM, Slaughter JC, Polavarapu S, McElroy SJ, Hernanz-Schulman M, et al. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. J Perinatol. (2013) 33:609–12. doi: 10.1038/jp.2013.3
33. Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J Matern Fetal Neonatal Med. (2011) 24:574–82. doi: 10.3109/14767058.2010.511335
34. Dani C, Coviello C, Montano S, Remaschi G, Petrolini C, Strozzi MC, et al. Effect on splanchnic oxygenation of breast milk, fortified breast milk, and formula milk in preterm infants. Pediatr Res. (2020) doi: 10.1038/s41390-020-0935-1
35. Dani C, Pratesi S, Barp J, Bertini G, Gozzini E, Mele L, et al. Near-infrared spectroscopy measurements of splanchnic tissue oxygenation during continuous versus intermittent feeding method in preterm infants. J Pediatr Gastroenterol Nutr. (2013) 56:652–6. doi: 10.1097/MPG.0b013e318287e9d7
36. Corvaglia L, Martini S, Battistini B, Rucci P, Aceti A, Faldella G. Bolus vs. continuous feeding: effects on splanchnic and cerebral tissue oxygenation in healthy preterm infants. Pediatr Res. (2014) 76:81–5. doi: 10.1038/pr.2014.52
37. Patel AK, Lazar DA, Burrin DG, Smith EO, Magliaro TJ, Stark AR, et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr Crit Care Med. (2014) 15:735–41. doi: 10.1097/PCC.0000000000000211
38. Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. (2001) 27:1401–7. doi: 10.1007/s001340100994
39. Schat TE, Heida FH, Schurink M, van der Laan ME, Hulzebos CV, Bos AF, et al. The relation between splanchnic ischaemia and intestinal damage in necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. (2016) 101:F533–9. doi: 10.1136/archdischild-2015-309838
40. Schat TE, Schurink M, van der Laan ME, Hulscher JB, Hulzebos CV, Bos AF, et al. Near-Infrared Spectroscopy to Predict the Course of Necrotizing Enterocolitis. PLoS ONE. (2016) 11:e0154710. doi: 10.1371/journal.pone.0154710
41. Bailey SM, Hendricks-Munoz KD, Wells JT, Mally P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am J Perinatol. (2010) 27:445–53. doi: 10.1055/s-0030-1247598
42. Banerjee J, Leung TS, Aladangady N. Blood transfusion in preterm infants improves intestinal tissue oxygenation without alteration in blood flow. Vox Sang. (2016) 111:399–408. doi: 10.1111/vox.12436
43. Banerjee J, Leung TS, Aladangady N. Effect of blood transfusion on intestinal blood flow and oxygenation in extremely preterm infants during first week of life. Transfusion. (2016) 56:808–15. doi: 10.1111/trf.13434
44. Schindler T, Yeo KT, Bolisetty S, Michalowski J, Tan AHK, Lui K. FEEding DURing red cell transfusion (FEEDUR RCT): a multi-arm randomised controlled trial. BMC Pediatr. (2020) 20:346. doi: 10.1186/s12887-020-02233-3
45. Marin T, Josephson CD, Kosmetatos N, Higgins M, Moore JE. Feeding preterm infants during red blood cell transfusion is associated with a decline in postprandial mesenteric oxygenation. J Pediatr. (2014) 165:464–71.e1. doi: 10.1016/j.jpeds.2014.05.009
46. Marin T, Moore J, Kosmetatos N, Roback JD, Weiss P, Higgins M, et al. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birthweight infants: a near-infrared spectroscopy investigation. Transfusion. (2013) 53:2650–8. doi: 10.1111/trf.12158
47. Jarjour IT. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr Neurol. (2015) 52:143–52. doi: 10.1016/j.pediatrneurol.2014.10.027
48. El-Dib M, Soul JS. Monitoring and management of brain hemodynamics and oxygenation. Handb Clin Neurol. (2019) 162:295–314. doi: 10.1016/B978-0-444-64029-1.00014-X
49. Galinsky R, Lear CA, Dean JM, Wassink G, Dhillon SK, Fraser M, et al. Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Dev Med Child Neurol. (2018) 60:126–33. doi: 10.1111/dmcn.13629
50. Nagdyman N, Ewert P, Peters B, Miera O, Fleck T, Berger F. Comparison of different near-infrared spectroscopic cerebral oxygenation indices with central venous and jugular venous oxygenation saturation in children. Paediatr Anaesth. (2008) 18:160–6. doi: 10.1111/j.1460-9592.2007.02365.x
51. Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. (2016) 79:55–64. doi: 10.1038/pr.2015.186
52. Mukerji A, Shah V, Shah PS. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics. (2015) 136:1132–43. doi: 10.1542/peds.2015-0944
53. Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. (2014) 41:47–67. doi: 10.1016/j.clp.2013.09.007
54. Alderliesten T, Lemmers PM, Smarius JJ, van de Vosse RE, Baerts W, van Bel F. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr. (2013) 162:698–704.e2. doi: 10.1016/j.jpeds.2012.09.038
55. van Bel F, Mintzer JP. Monitoring cerebral oxygenation of the immature brain: a neuroprotective strategy? Pediatr Res. (2018) 84:159–64. doi: 10.1038/s41390-018-0026-8
56. Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. (2000) 106:625–32. doi: 10.1542/peds.106.4.625
57. Brown NC, Inder TE, Bear MJ, Hunt RW, Anderson PJ, Doyle LW. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J Pediatr. (2009) 155:32–8, 38.e1. doi: 10.1016/j.jpeds.2009.01.038
58. Greisen G, Vannucci RC. Is periventricular leucomalacia a result of hypoxic-ischaemic injury? Hypocapnia and the preterm brain. Biol Neonate. (2001) 79:194–200. doi: 10.1159/000047090
59. Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Monitoring regional tissue oxygen extraction in neonates <1250 g helps identify transfusion thresholds independent of hematocrit. J Neonatal Perinatal Med. (2014) 7:89–100. doi: 10.3233/NPM-1477213
60. Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. (2008) 122:65–74. doi: 10.1542/peds.2007-2822
61. Kluckow M, Lemmers P. Hemodynamic assessment of the patent ductus arteriosus: Beyond ultrasound. Semin Fetal Neonatal Med. (2018) 23:239–44. doi: 10.1016/j.siny.2018.04.002
62. Michel-Macias C, Morales-Barquet DA, Martinez-Garcia A, Ibarra-Rios D. Findings from somatic and cerebral near-infrared spectroscopy and echocardiographic monitoring during ductus arteriosus ligation: description of two cases and review of literature. Front Pediatr. (2020) 8:523. doi: 10.3389/fped.2020.00523
63. Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. (2011) 69:354–8. doi: 10.1203/PDR.0b013e31820b95ca
64. Carmody JB, Swanson JR, Rhone ET, Charlton JR. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. (2014) 9:2036–43. doi: 10.2215/CJN.05190514
65. Lee CC, Chan OW, Lai MY, Hsu KH, Wu TW, Lim WH, et al. Incidence and outcomes of acute kidney injury in extremely-low-birth-weight infants. PLoS ONE. (2017) 12:e0187764. doi: 10.1371/journal.pone.0187764
66. Kastl JT. Renal function in the fetus and neonate—the creatinine enigma. Semin Fetal Neonatal Med. (2017) 22:83–9. doi: 10.1016/j.siny.2016.12.002
67. Kaufman J, Almodovar MC, Zuk J, Friesen RH. Correlation of abdominal site near-infrared spectroscopy with gastric tonometry in infants following surgery for congenital heart disease. Pediatr Crit Care Med. (2008) 9:62–8. doi: 10.1097/01.PCC.0000298640.47574.DA
Keywords: neonate, ELGAN, ELBW, NIRS, hemodynamics, prematurity
Citation: Pavlek LR, Mueller C, Jebbia MR, Kielt MJ and Fathi O (2021) Near-Infrared Spectroscopy in Extremely Preterm Infants. Front. Pediatr. 8:624113. doi: 10.3389/fped.2020.624113
Received: 30 October 2020; Accepted: 23 December 2020;
Published: 21 January 2021.
Edited by:
Arjan Te Pas, Leiden University, NetherlandsReviewed by:
Stefano Nobile, A. Gemelli University Hospital Foundation (IRCCS), ItalyHercília Guimarães, University of Porto, Portugal
Copyright © 2021 Pavlek, Mueller, Jebbia, Kielt and Fathi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leeann R. Pavlek, leeann.pavlek@nationwidechildrens.org
 Leeann R. Pavlek
Leeann R. Pavlek Clifford Mueller
Clifford Mueller Maria R. Jebbia
Maria R. Jebbia Matthew J. Kielt
Matthew J. Kielt Omid Fathi
Omid Fathi