High-Flow Nasal Cannula Therapy in Children With Acute Respiratory Distress With Hypoxia in A Pediatric Intensive Care Unit–A Single Center Experience
- 1Department of Respiratory Therapy, Chang Gung Children's Hospital and Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan
- 2Chang Gung University School of Medicine, Taoyuan, Taiwan
- 3Division of Pediatric Critical Care Medicine and Pediatric Neurocritical Care Center, Chang Gung Children's Hospital and Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan
- 4Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan
Aim: High-flow nasal cannulas (HFNCs) show potential in the application of positive pressure, improving gas exchange, and decreasing work of breathing in patients with acute respiratory distress. The aims of this study were to elucidate the indications for HFNC therapy in children of all ages and diagnoses, and to evaluate the efficacy and risk factors for failure of HFNC therapy in children with acute respiratory distress with hypoxia in a pediatric intensive care unit.
Methods: We conducted this retrospective cohort study at a tertiary pediatric intensive care unit between January 1, 2018 and December 31, 2020. All children, from 1 month to 18 years of age, with acute respiratory distress with hypoxia and HFNC therapy were eligible. The clinical data were reviewed.
Results: One hundred and two children met the eligibility criteria for the study, of whom 57 (55.9%) were male, and the mean age was 7.00 ± 6.79 years. Seventy-eight (76.5%) of the children had underlying disorders. The most common indications for the use of HFNC therapy were pneumonia (40, 39.2%), sepsis-related respiratory distress (17, 16.7%), and bronchiolitis (16, 15.7%). The failure rate was 15.7% (16 of 102 children). Higher initial and maximum fraction of inspiration O2 levels and lower initial and lowest SpO2/FiO2 (S/F) ratio were early and possible signs of failure requiring escalation of respiratory support.
Conclusion: In our population, we found that HFNC therapy could be initiated as the first-line therapy for various etiologies of acute respiratory distress with hypoxia in a pediatric intensive care unit and for all age groups.
Introduction
Acute respiratory distress is the most common cause of pediatric intensive care unit admission. Invasive mechanical ventilation is an established effective supportive therapy for acute respiratory distress. However, it is associated with increased risks of nosocomial infections, lung and airway injuries, length of stay, and sedation-related complications (1–3).
High-flow nasal cannulas (HFNCs) are an increasingly used form of non-invasive respiratory support, and they have shown potential in reducing the need for intubation (4–7). HFNCs enable the administration of high concentrations of oxygen with adequate relative humidity and temperature, and they have been shown to improve airway resistance and lung compliance, achieve a certain level of continuous positive airway pressure (CPAP), eliminate dead space and decrease respiratory work (8–11). HFNC therapy has been used in infants with respiratory distress syndrome and infants with bronchiolitis, and it has been shown to decrease respiratory distress and intubation rates, increase patient comfort and ease of use compared with face masks or traditional cannulas, and shorten the length of stay in pediatric intensive care units (ICUs) (12–15).
Despite increasing evidence supporting the use of HFNCs as respiratory support for children with bronchiolitis, few studies have investigated the indications for HFNC therapy and the epidemiology of disease warranting HFNC therapy in older children in a pediatric ICU (16–25). Thus, the aims of this study were to elucidate the indications for HFNC therapy in children of all ages and diagnoses, and to evaluate the efficacy and risk factors for failure of HFNC therapy.
Methods
This was a retrospective cohort study using chart reviews of pediatric patients who received HFNC respiratory support at the pediatric ICU of Chang Gung Children's Hospital between January 1, 2018 and December 31, 2020. Acute respiratory distress was defined as hypoxemia (SpO2 < 94%) and signs of respiratory distress despite standard-flow oxygen therapy. All patients received standard-flow oxygen therapy via a traditional nasal cannula at 1–5 L/min, simple mask at 6–10 L/min or oxygen hood with 35–50% oxygen before they were switched to high flow (16, 17). The signs of respiratory distress included increased breathing rate and heart rate, color changes, grunting, nose flaring, retractions, wheezing, and sweating. The eligibility criteria for this study were: (1) age from 1 month to 18 years; and (2) patients with acute respiratory distress with hypoxia who used HFNC respiratory support for any period of time during their pediatric ICU admission. We excluded those who: (1) were older than 18 years and younger than 1 month; (2) had respiratory distress with low-flow oxygen therapy (such as a traditional nasal cannula at 1–5 L/min, simple mask at 6–10 L/min or oxygen hood with 35–50% oxygen) or respiratory failure with invasive mechanical ventilation; (3) required respiratory support post extubation and after weaning from continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BIPAP); and (4) had a history of long-term ventilator dependency. This study was approved by the Chang Gung Memorial Hospital Institutional Review Board (IRB number: 201801252B0C502 and 201901701B0).
HFNC Protocol
In January 1, 2018, we initiated an institutional protocol for the use of HFNCs, which was modified from a previous study conducted in a pediatric ICU (Figure 1) (3, 6). HFNC was delivered by an Optiflow System® (Fisher & Paykel, Auckland, New Zealand). The protocol includes guidelines for the indications, settings, monitoring and outcomes (success or failure) of HFNC therapy (3, 6). Fraction of inspiration O2 (FiO2) was adjusted to reach a pulse oximetry (SpO2) between 92 and 97%, and the flow setting was based on the patients' body weight: 0–15 kg: 2 L/kg/min; 16–30 kg: 35 L/min; 31–50 kg: 40 L/min; >50 kg: 50 L/min. We also monitored clinical parameters including heart rate, respiratory rate, and SpO2 as well as venous blood gas for pH and CO2. Disease severity and oxygenation were assessed according to the PRISM score, SpO2/FiO2 (S/F) ratio, and ROX index score [(SpO2/FiO2)/RR] (26, 27). The S/F ratio and ROX index score were calculated initially and every 4 h during the first 48 h after starting HFNC therapy or before stopping HFNC therapy. HFNC failure was defined as the need for escalation to non-invasive ventilation or invasive mechanical ventilation. The treating intensive care physician decided whether escalation of treatment was necessary, but it generally occurred if FiO2 > 0.6 or there was a worsening clinical state, and a similar protocol was followed in the PICU (17).
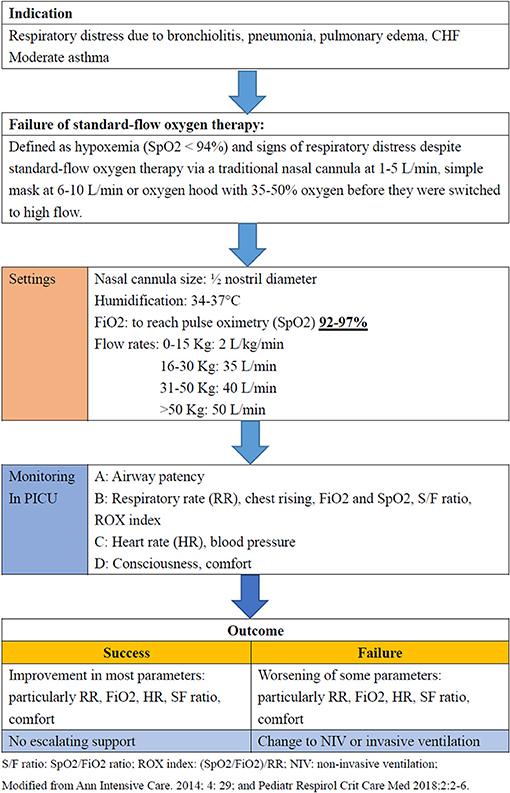
Figure 1. Protocol of high-flow nasal cannula therapy in the pediatric ICU at Chang Gung Children's Hospital, Taiwan.
Data Collection
The following information was collected for all patients: (1) demographics and underlying medical history; (2) primary indication and respiratory infection status; (3) clinical parameters of disease severity, including heart rate, breathing rate, SpO2, venous blood gas from a central venous catheter, including pH and PCO2, as well as Pediatric Risk of Mortality (PRISM) III score, the initial and lowest level S/F ratio and the ROX index score; (4) variables after HFNC respiratory support, including initial and maximum HFNC parameters (FiO2 and flow) and duration of HFNC use; and (5) outcomes. The primary indication was defined according to the discharge summary and treatment modalities used during the ICU stay. The primary outcome was defined as success or failure of HFNC respiratory support, and the second outcome was defined as 1-month mortality, and lengths of pediatric ICU and hospital stay.
Statistical Analysis
The patients' characteristics including demographic and HFNC utilization data are presented as percentage (%) or mean ± standard deviation (SD). We divided the patients into two groups: HFNC respiratory support success, and HFNC respiratory support failure. Between-group differences were analyzed using the chi-square test or Fisher's exact test for categorical variables, and the Student's t-test for normally distributed continuous variables. The Mann-Whitney test was used for non-normally distributed data. Associations with outcomes between the success and failure groups were determined using univariate analysis. Receiver operating characteristic (ROC) curves for the initial and lowest S/F ratio were plotted to predict the failure of HFNC respiratory support. The respective areas under the ROC curves and cut-off values were calculated. Statistical analysis was performed using SPSS software, version 23.0 (IBM, Inc., Chicago, IL). A two-sided p < 0.05 was considered to be statistically significant.
Results
Demographics
During the study period, 102 children with acute respiratory distress were managed with HFNC therapy during their pediatric ICU stay (Figure 2). This represented 16.9% (102 of 603) of all pediatric ICU admissions due to acute respiratory distress over the same time period. Fifty-seven (55.9%) of the 102 children were male, and the mean age was 7.00 ± 6.79 years. There were no significant differences in sex and age between the two groups. Seventy-eight (76.5%) of the 102 children had an underlying medical history. The most common underlying medical history was a neurologic disorder (28, 27.5%), followed by hematologic disorder/malignancy (15, 14.7%), heart disorder (13, 12.7%) and asthma/history of wheezing (7, 6.9%). The most common indication for the use of HFNC therapy was pneumonia (40, 39.2%), followed by sepsis-related acute respiratory distress (17, 16.7%) and bronchiolitis (16, 15.7%). The initial S/F ratios were 211.87 ± 39.85 and 165.64 ± 46.49 in the success and failure groups, respectively. After disease progression, the lowest S/F ratios were 210.07 ± 41.72 and 147.43 ± 49.86, respectively. There were significant differences in the initial and lowest S/F ratios between the two groups (both p < 0.001). There were no other significant differences in underlying medical history, indication, PRISM III score and initial and lowest ROX index score between the two groups. The demographics of the 102 children are summarized in Table 1.
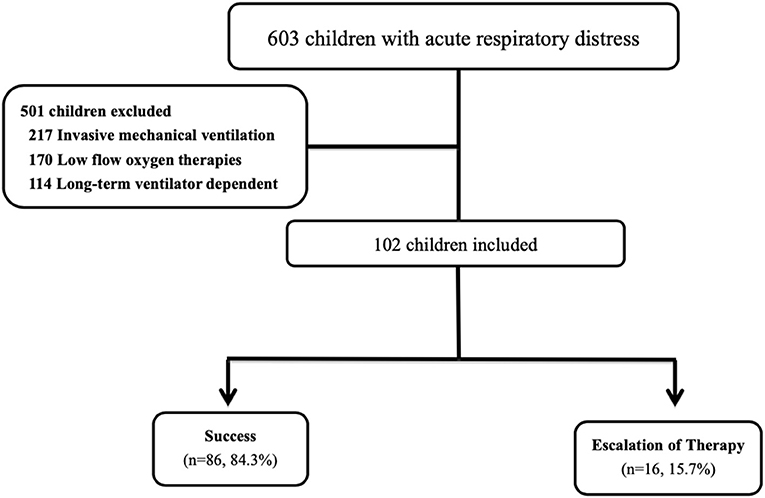
Figure 2. Flowchart of the included patients. During the study period, 102 children with acute respiratory distress were managed with HFNC therapy during their pediatric ICU stay. This represented 16.9% (102 of 603) of all pediatric ICU admissions due to acute respiratory distress over the same time period. The 16 (15.7%) children needed escalation of respiratory support, including five who received non-invasive ventilation and 11 who received intubation with mechanical ventilation.
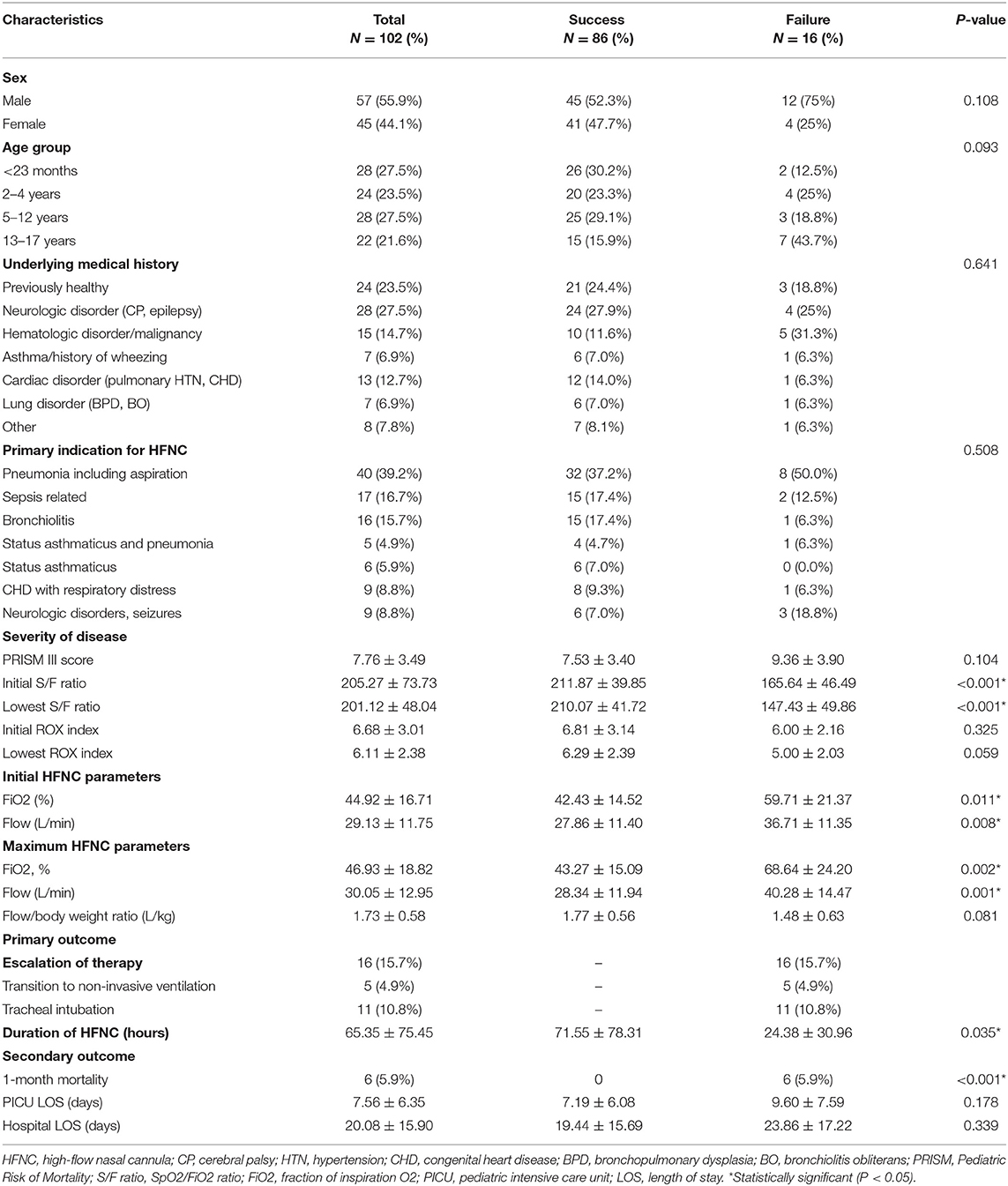
Table 1. Demographics of 102 children with acute respiratory distress requiring high-flow nasal cannula therapy during the study period.
Etiologies of Infection
Among the 102 patients, 33 had detectable pathogens (32.3%), including 13 bacterial infections from sputum cultures (7 Haemophilus influenzae, 4 Streptococcus pneumoniae, and 2 Staphylococcus aureus), 13 viruses [5 Adenovirus Ag from throat swabs or sputum specimens, 3 Respiratory syncytial virus (RSV) Ag from sputum specimens, 2 Human rhinovirus/Enterovirus PCR, 1 Influenza A PCR, 1 Influenza B PCR from throat swabs and 1 Parainfluenza A from a throat virus culture] and 5 Mycoplasma pneumonia PCR from throat swabs. In addition, two patients had combined bacterial and viral infections (Haemophilus influenzae and Respiratory syncytial virus).
Initial and Maximum HFNC Parameters and Clinical Parameters
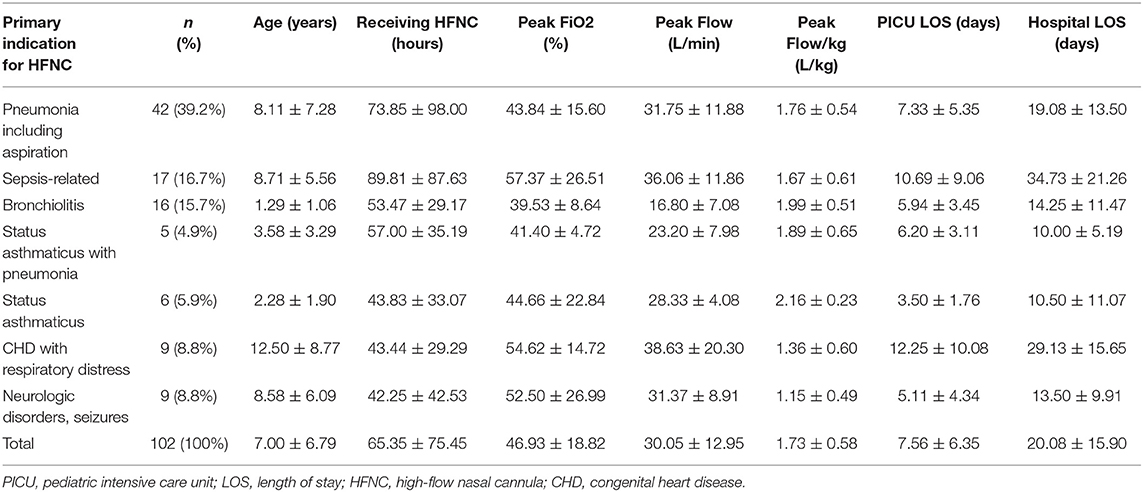
After starting HFNC therapy at the pediatric ICU, the initial FiO2 and flow rates were 44.92 ± 16.71% and 29.13 ± 11.75 L/min, respectively. After disease progression, the maximum FiO2 and flow rates were 46.93 ± 18.82% and 30.05 ± 12.95 L/min, respectively. The flow/body weight ratio was 1.73 ± 0.58 (L/kg). Table 2 summarizes the details of HFNC therapy by diagnostic indication. There were no significant differences in age, therapeutic interventions during hospitalization, and lengths of stay in the pediatric ICU and hospital between the different diagnostic indications.
The evolution of the clinical parameters and blood gas after the initiation of HFNC is shown in Table 3. There were significant improvements in heart rate, breathing rate, pulse oximetry (SpO2), S/F ratio, and ROX index score in the early HFNC period (0.5–8 h) and late HFNC period (8–24 h). No significant differences in pH and PCO2 were observed after the initiation of HFNC in the early HFNC period, however there were significant improvements in pH in the late HFNC period (8–24 h). No air leak syndrome or epistaxis were noted with the use of HFNCs.
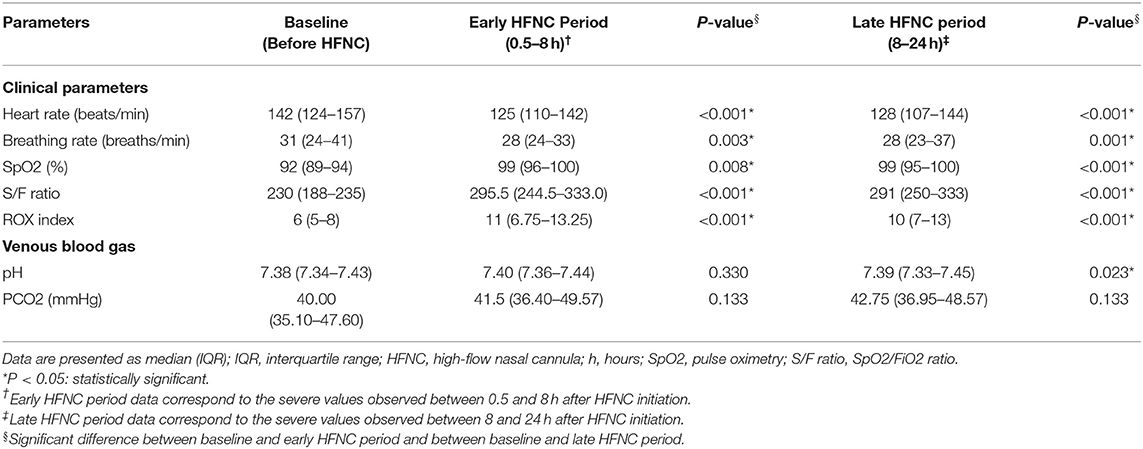
Table 3. Evolution of clinical parameters and blood gas after initiating high-flow nasal cannula therapy.
Outcomes
Most of the children (86 of 102, 84.3%) were successfully treated with HFNC during their pediatric ICU admission. The other 16 (15.7%) children needed escalation of respiratory support, including five who received non-invasive ventilation and 11 who received intubation with mechanical ventilation. The reasons for treatment failure were a rise in respiratory rate and desaturation in 13 (12.7%) children, and discontinuation of therapy due to discomfort in three (2.9%) children. Of the 16 cases who failed HFNC therapy, 11 (68.8%) failed during the first 24 h following the initiation of HFNC treatment. The mean time to failure was 24.38 ± 30.96 h. The overall 1-month mortality rate was 5.9% (6 of 102 children), and the lengths of stay in the pediatric ICU and hospital were 7.56 ± 6.35 and 20.08 ± 15.90 days, respectively.
Predictors of Failure
Among the data collected at baseline (Table 1), univariate analysis revealed that the failure group had significantly higher initial and maximum FiO2 levels than the success group (59.71 ± 21.37 vs. 42.43 ± 14.52%, p = 0.002; and 68.64 ± 24.20 vs. 43.27 ± 15.09%, p < 0.001, respectively). In addition, the initial and lowest Spo2/Fio2 ratio were also shown to be significant predictors of HFNC failure (both p < 0.001). The areas under the ROCs of initial and lowest S/F ratio for HFNC failure were 0.786 and 0.816, respectively, and both cut-off S/F ratio values were 212. Therefore, higher initial and maximum FiO2 levels and lower initial and lowest S/F ratio were early and possible signs of failure requiring escalation of respiratory support. However, there were no significant differences in other baseline data, including sex, age, underlying medical history, and primary indication for HFNC.
Discussion
In this retrospective study, we described the use of HFNC for children with acute respiratory distress at a tertiary pediatric ICU over a 3-year period. We focused on HFNC as the first-line therapy for various etiologies of acute respiratory distress with hypoxia and for all age groups. One hundred and two patients met the eligibility criteria for the study, and the failure rate was only 15.6% (16 of 102 children). In addition, there were no cases of air leak syndrome or epistaxis with HFNC therapy, Therefore, HFNC therapy appears to be a safe and effective method of non-invasive respiratory support.
The Indications for HFNC Therapy
HFNC therapy is most commonly used for infants with acute viral bronchiolitis. However, recent studies have suggested that HFNC therapy can also be effectively and safely used in patients with a wider age range and etiologies of respiratory distress (16–25). Coletti et al. investigated the use of HFNC in 620 children with a wide range of indications in their pediatric ICU, including a significant number of subjects with status asthmaticus (41%) and congenital heart disease with respiratory distress (10%), and they reported that 10.1% of the cases needed escalation of therapy to either non-invasive ventilation or intubation with mechanical ventilation (20). In addition, Baudin et al. described 177 subjects who received HFNC therapy in a similar pediatric ICU population, including 52% with congenital heart disease, 16% with bronchiolitis, and 7% with pneumonia. They reported that HFNC therapy failure occurred in 32 cases (22%), 28 of whom required transition to non-invasive ventilation, and five required endotracheal intubation (21). Kelly et al. also reported the use of HFNC therapy in 496 children with respiratory distress in the emergency department, including 46% with bronchiolitis, 28% with pneumonia and 8% with asthma. They reported that 8% of the cases failed therapy and required intubation with mechanical ventilation following HFNC therapy (22). In our study, we also used HFNC therapy for patients with a wide range of diagnoses, including a significant number with pneumonia (39.2%), sepsis-related respiratory distress (16.7%), and acute bronchiolitis (15.7%). Of our patients, 15.7% needed escalation of therapy to either non-invasive ventilation or intubation with mechanical ventilation.
The Risk Factors for Escalation of Therapy With the Use of HFNC Therapy
In clinical practice, it is important to have an objective method to determine if HFNC therapy is working or not. Roca et al. proposed an easy bedside tool using SaO2, FiO2 and respiratory rate to predict the success or failure of HFNC therapy, known as the ROX index (26). The authors found that a higher ROX index score was associated with HFNC success at all time points analyzed, and they concluded that a ROX index value of ≥ 4.88 at 12 h after the initiation of HFNC therapy was significantly associated with HFNC success. However, in children, predicting success using the ROX index can be much more difficult, because the respiratory rate can vary with age (27). In our study, there were no significant differences in initial and lowest ROX index scores between the two groups.
To date, few studies have assessed the risk factors for escalation of therapy to either non-invasive ventilation or intubation with mechanical ventilation, because most of the patients included in these studies have had a variety of indications and did not have severe forms of acute respiratory distress. Kelly et al. reported that failure occurred in the more critical children who presented to the pediatric emergency department with a triage respiratory rate greater than the 90th percentile for age, initial venous PCO2 >50 mm Hg and pH >7.30 (significant respiratory acidosis). A diagnosis of acute bronchiolitis seemed to be protective with respect to intubation following HFNC therapy (22). Kamit et al. reported that a lower SpO2/FiO2 (S/F) ratio at admission was a predictor of HFNC failure, and that achieving S/F > 200 at 60 min significantly predicted successful HFNC therapy (23). Betters et al. also reported that high FiO2 requirement, history of intubation, and cardiac co-morbidities were predictors of HFNC failure (24). Abboud et al. retrospectively analyzed children with viral bronchiolitis who failed HFNC (needing intubation) compared to children who were successfully treated with HFNCs, and found that improved respiratory rate and clearance of repeat pCO2 were predictors of success (25). In our study, higher initial and maximum FiO2 levels and lower initial and lowest S/F ratio were early and possible signs of failure requiring escalation of respiratory support. Therefore, these findings may help guide clinicians who would prefer to use HFNC therapy and avoid a delay in escalating therapy to either non-invasive ventilation or intubation with mechanical ventilation in children at a higher risk of failing HFNC therapy.
Limitations
There are some limitations to this study. First, this is a retrospective study with a limited cohort of children with acute respiratory distress receiving HFNC therapy at a single center. However, very few reports in the pediatric literature have reported HFNC therapy as initial respiratory support in children with acute respiratory distress, especially for pneumonia and sepsis-related respiratory distress. Experience with HFNC therapy for this indication is particularly lacking, and this is a strength of this study. Second, because few studies have assessed the use of HFNCs and the risk of intubation in children, there is low evidence or no guidelines for the escalation of treatment to CPAP or intubation. In our study, the criteria of escalating therapy from HFNC to either non-invasive ventilation or intubation with mechanical ventilation are different in different clinical scenarios. This may have influenced the failure rate, which may limit comparisons with other studies in this field. Third, broad age groups with a small number of cases may further limit the findings of this study. Fourth, in our study, most of the severe cases (217 of 501, 43.3%) of respiratory failure were not initially treated with HFNCs, but received invasive mechanical ventilation. Only 11 patients with borderline moderate to severe respiratory failure initially received HFNC therapy, and they were finally intubated. Because HFNC therapy is being increasingly used in our hospital, the overenthusiastic use leading to delayed intubation cannot be ruled out in this study. Fifth, HFNC has been reported to fail to offer adequate PEEP, even at higher flows, for patients with moderate to severe acute respiratory distress syndrome (28). In our study, FiO2 requirement (initial or maximum) > 60% was a predictor of HFNC failure. The safety and effectiveness of providing high FiO2 (>60%) with HFNCs without adequate PEEP, given the risk of oxygen-induced lung damage at high concentrations must be considered.
Conclusions
HFNC was used frequently over the 3-year study period for children with a wide range of ages and for a variety of indications. We found that HFNC could be initiated as the first-line therapy all age groups of children with various etiologies of acute respiratory distress in our pediatric ICU. Further prospective studies are needed to confirm the efficacy of HFNC therapy and to evaluate the risk factors of failure in different settings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Chang Gung Memorial Hospital Institutional Review Board (IRB number: 201801252B0C502 and 201901701B0). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
C-CC and J-JL: conceptualization and writing—original draft preparation. S-HH, Y-CL, and O-WC: methodology. O-WC: software. J-JL and O-WC: formal analysis. Y-CL, T-CC, O-WC, and E-PL: data curation. J-JL: writing—review and editing. J-JL and S-HH: supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported in part by grants from Chang Gung Memorial Hospital (CORPG3H0591).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was performed at the Pediatric Intensive Care Unit of Chang Gung Children's Hospital in Taoyuan, Taiwan. The authors would like to thank the physicians, patients and family members that provided clinical information.
References
1. Fedor KL. Noninvasive respiratory support in infants and children. Respir Care. (2017) 62:699–717. doi: 10.4187/respcare.05244
2. Lee M Jr, Nagler J. High-flow nasal cannula therapy beyond the perinatal period. Curr Opin Pediatr. (2017) 29:291–6. doi: 10.1097/MOP.0000000000000494
3. Franklin D, Schibler A. Nasal high-flow therapy in infants and children. Pediatr Respirol Crit Care Med. (2018) 2:2–6. doi: 10.4103/prcm.prcm_22_17
4. Zhao H, Wang H, Sun F, Lyu S, An Y. High-flow nasal cannula oxygen therapy is superior to conventional oxygen therapy but not to noninvasive mechanical ventilation on intubation rate: a systematic review and meta-analysis. Crit Care. (2017) 21:184. doi: 10.1186/s13054-017-1760-8
5. Mikalsen IB, Davis P, Øymar K. High flow nasal cannula in children: a literature review. Scand J Trauma Resusc Emerg Med. (2016) 24:93. doi: 10.1186/s13049-016-0278-4
6. Mile'si C, Boubal M, Jacquot A, Baleine J, Durand S, Odena MP, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. (2014) 4:29. doi: 10.1186/s13613-014-0029-5
7. Hutchings FA, Hilliard TN, Davis PJ. Heated humidified high flow nasal cannula therapy in children. Arch Dis Child. (2015) 100:571–5. doi: 10.1136/archdischild-2014-306590
8. Mile'si C, Baleine J, Matecki S, Durand S, Combes C, Novais ARB, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. (2013) 39:1088–94. doi: 10.1007/s00134-013-2879-y
9. Hough JL, Pham TMT, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. (2014) 15:e214–9. doi: 10.1097/PCC.0000000000000112
10. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. (2009) 103:1400–5. doi: 10.1016/j.rmed.2009.04.007
11. Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation: effects of oxygenation, comfort and clinical outcome. Am J Respir Crit Care Med. (2014) 190:282–8. doi: 10.1164/rccm.201402-0364OC
12. Pham TMT, O'Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. (2015) 50:713–20. doi: 10.1002/ppul.23060
13. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. (2014) 15:1–6. doi: 10.1097/PCC.0000000000000011
14. Kawaguchi A, Yasui Y, deCaen A, Garros D. The clinical impact of heated humidified high-flow nasal cannula on pediatric respiratory distress. Pediatr Crit Care Med. (2017) 18:112–9. doi: 10.1097/PCC.0000000000000985
15. Milési C, Essouri S, Pouyau R, Liet JM, Afanetti M, Portefaix A, et al. High flow nasal cannula (HFNC) versus nasal continuous positive airway pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: a multicenter randomized controlled trial (TRAMONTANE study). Intensive Care Med. (2017) 43:209–16. doi: 10.1007/s00134-016-4617-8
16. Spentzas T, Minarik M, Patters AB, Vinson B, Stidham G. Children with respiratory distress treated with high-flow nasal cannula. J Intensive Care Med. (2009) 24:323–8. doi: 10.1177/0885066609340622
17. Wraight TI, Ganu SS. High-flow nasal cannula use in a paediatric intensive care unit over 3 years. Crit Care Resusc. (2015) 17:197–201.
18. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. (2014) 3:CD009850. doi: 10.1002/14651858.CD009850.pub2
19. Riese J, Fierce J, Riese A, Alverson BK. Effect of a hospital-wide high-flow nasal cannula protocol on clinical outcomes and resource utilization of bronchiolitis patients admitted to the PICU. Hosp Pediatr. (2015) 5:613–8. doi: 10.1542/hpeds.2014-0220
20. Coletti KD, Bagdure DN, Walker LK, Remy KE, Custer JW. High-flow nasal cannula utilization in pediatric critical care. Respir Care. (2017) 62:1023–9. doi: 10.4187/respcare.05153
21. Baudin F, Gagnon S, Crulli B, Proulx F, Jouvet P, Emeriaud G. Modalities and complications associated with the use of high-flow nasal cannula: experience in a pediatric ICU. Respir Care. (2016) 61:1305–10. doi: 10.4187/respcare.04452
22. Kelly GS, Simon HK, Sturm JJ. High-flow nasal cannula use in children with respiratory distress in the emergency department: predicting the need for subsequent intubation. Pediatr Emerg Care. (2013) 29:888–92. doi: 10.1097/PEC.0b013e31829e7f2f
23. Kamit Can F, Anil AB, Anil M, Zengin N, Durak F, Alparslan C, et al. Predictive factors for the outcome of high flow nasal cannula therapy in a pediatric intensive care unit: Is the SpO2/FiO2 ratio useful? J Crit Care. (2018) 44:436–44. doi: 10.1016/j.jcrc.2017.09.003
24. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. (2017) 52:806–12. doi: 10.1002/ppul.23626
25. Abboud PA, Roth PJ, Skiles CL, Stolfi A, Rowin ME. Predictors of failure in infants with viral bronchiolitis treated with high-flow, high-humidity nasal cannula therapy*. Pediatr Crit Care Med. (2012) 13:e343–9. doi: 10.1097/PCC.0b013e31825b546f
26. Roca O, Messika J, Caralt B, García-de-Acilu M, Sztrymf B, Ricard JD, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. (2016) 35:200–5. doi: 10.1016/j.jcrc.2016.05.022
27. Yildizdas D, Yontem A, Iplik G, Horoz OO, Ekinci F. Predicting nasal high-flow therapy failure by pediatric respiratory rate-oxygenation index and pediatric respiratory rate-oxygenation index variation in children. Eur J Pediatr. (2021) 180:1099–106. doi: 10.1007/s00431-020-03847-6
Keywords: high-flow nasal cannula, child, acute respiratory distress, pediatric intensive care unit, hypoxic
Citation: Chang C-C, Lin Y-C, Chen T-C, Lin J-J, Hsia S-H, Chan O-W and Lee E-P (2021) High-Flow Nasal Cannula Therapy in Children With Acute Respiratory Distress With Hypoxia in A Pediatric Intensive Care Unit–A Single Center Experience. Front. Pediatr. 9:664180. doi: 10.3389/fped.2021.664180
Received: 04 February 2021; Accepted: 06 April 2021;
Published: 07 May 2021.
Edited by:
Phuc Huu Phan, Vietnam National Hospital of Pediatrics, VietnamReviewed by:
Dincer Riza Yildizdas, Çukurova University, TurkeyJhuma Sankar, All India Institute of Medical Sciences, India
Copyright © 2021 Chang, Lin, Chen, Lin, Hsia, Chan and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jainn-Jim Lin, lin0227@adm.cgmh.org.tw
†These authors have contributed equally to this work
 Chih-Ching Chang1†
Chih-Ching Chang1†  Jainn-Jim Lin
Jainn-Jim Lin Shao-Hsuan Hsia
Shao-Hsuan Hsia En-Pei Lee
En-Pei Lee