Endoscopic Treatment for Pediatric Esophageal Stenosis Induced by Chemical Burn, Congenitally, or After Surgical Repair of Esophageal Atresia
- 1Department of Gastroenterology, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Research Center of Digestive Disease, Central South University, Changsha, China
Objectives: To evaluate the safety and efficacy of endoscopic treatment for congenital pediatric esophageal stenosis or pediatric stenosis that develops after a chemical burn or surgical repair of esophageal atresia.
Methods: We retrospectively reviewed the medical records of 15 pediatric patients who underwent endoscopic treatments (dilation and/or stenting and/or incision) for congenital esophageal stenosis or esophageal stenosis that developed after a chemical burn or surgical repair of esophageal atresia, between January 2010 and January 2019. The patients were periodically followed-up to assess the safety and efficacy of treatment by comparing the diameter of stricture and dysphagia score before and after procedures, and complications or recurrence.
Results: All children successfully underwent the procedures. Fourteen of the 15 patients received endoscopic balloon dilation (EBD) as the first step of treatment, but EBD alone only resolved the symptoms in two patients. The remaining patients received other comprehensive treatments, such as EBD with endoscopic incision (EI), EBD with stent replacement, or a combination of EBD, stent replacement, and EI. Eleven (11/15, 73.3%) patients experienced symptomatic relief after endoscopic treatment, and recurrence was noted in four patients on 3–36 months after the final endoscopic treatment. All four patients underwent esophageal surgery to relieve their symptoms. Until October 2021, all patients experienced symptom relief, and their dysphagia scores decreased from 3–4 to 0–1 during the follow-up period of 8–121 months. The average diameter of stenosis was increased from 0.34 cm (range 0.2–0.7 cm) to 1.03 cm (range 0.8–1.2 cm). No severe complications occurred during endoscopic treatment and follow-up.
Conclusions: Endoscopic treatment is safe and effective for pediatric esophageal stenosis that is congenital or induced by chemical burns or surgical repair of esophageal atresia. Comparative large-scale studies are required to confirm our findings.
Introduction
There are many causes of esophageal stenosis in children, including esophageal atresia, inflammatory diseases, gastroesophageal reflux disease, eosinophilic esophagitis, congenital esophageal stenosis (CES), surgical complications of esophageal atresia, and chemical burns (1–3). The most common etiologies vary among countries. Burns are common in developing countries (4, 5). Dysphagia and vomiting are common clinical manifestations in children with esophageal stenosis. These patients usually have long-term morbidity, and multiple procedures may be required to relieve their symptoms. Esophageal dilatation usually serves as the first-line treatment because it effectively relieves symptoms. However, stenosis recurrence is common and research has shown that it can be difficult to maintain a satisfactory lumen diameter for over 4 weeks (1). Currently, there is no established treatment for patients with refractory stenosis. Some researchers have reported successful application of esophageal stents, endoscopic incision (EI), or mitomycin C following dilation (6). However, the efficacy of these newly proposed modalities remains unclear. Therefore, we reviewed the medical records of pediatric patients with congenital esophageal stenosis or esophageal stenosis that developed secondary to chemical burns or surgical repair of esophageal atresia in our hospital, who were treated with different endoscopic procedures, including endoscopic balloon dilation (EBD), endoscopic stent placement, and endoscopic incision (EI), to investigate the treatment outcomes of these interventions.
Patient Information
This retrospective study enrolled pediatric patients with congenital esophageal stenosis or stenosis that developed following a chemical burn or surgical procedure (surgical repair of esophageal atresia), who underwent endoscopic treatment in our hospital between January 2010 and January 2019. The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University.
Data, including those relating to demographic, clinical characteristics, outcomes of endoscopic treatment, and follow-up, were extracted from the patients' medical records. The patients' guardians were informed about the possible treatment options (including surgical and endoscopic methods) and potential adverse events associated with each option, and informed consent was obtained from them. Pediatric patients with blood coagulation disorders or severe cardiopulmonary diseases were excluded from the study. Usually, EBD was recommended as the primary treatment for stenosis shorter than 5 cm, and ESP was suggested for stricture longer than 5 cm, for recurrent stenosis, repeated EBD, EI, ESP, or combined therapy of them may be suggested. Surgery was also a choice for recurrent stenosis. The final treatment method each time was achieved after consideration of various factors (such as guardians' will, our condition, doctor's experience, etc.).
All endoscopic treatments were performed under general anesthesia via tracheal intubation or under conscious sedation with the patient in the left lateral position. A single-channel endoscope (GIF-Q240/Q260J, Olympus) was used.
EBD Procedure
EBD was performed using a polyethylene balloon system (Rigiflex, Microvasive, Boston Scientific). The balloon diameter, usually 1.0 or 1.5 cm, was selected based on the age of the patient and the diameter of the stenosis. We usually set the balloon to an initial pressure of 3 atm, and this could be gradually increased to 5–8 atm after endoscopic evaluation. Expansions were maintained for 1–2 min, with a 2–5 min interval between expansions. Endoscopic examination was performed during the interval to detect any serious complications and confirm that the stenosis was sufficiently loose. Dilatations were continued until confirmation of either a loosened stricture or complications, such as mucosal laceration or hemorrhage.
Endoscopic Stent Placement Procedure
Esophageal stents were provided by Delman Technology Co. Ltd. (Jinan, Shandong, China). The length of the stent was selected based on the stricture length determined via endoscopy or imaging before the operation. Fully covered self-expandable metal stents are preferred because they can provide continuous dilation to the stenosis and are easy to remove after the relief of esophageal stenosis. Details of the steps involved in stent placement have previously been reported (7). After stent placement, endoscopy was repeated to confirm the appropriateness of the distance from the margin of the stent to the incisor.
EI Procedure
The steps used for EI were the same as those we previously reported (8). Under endoscopy, radial incisions were made along a virtual line connecting the oral end of the esophageal lumen and the anal end of the stenosis. Involvement of the muscularis propria and/or exposure of the bottom of the incision along the virtual line was regarded as a sufficient incision depth. After incision, the wound surface at the stenosis was carefully checked to confirm that there are no hemorrhages or perforations. Figure 1 depicts an example of EBD, endoscopic stent placement, and EI.
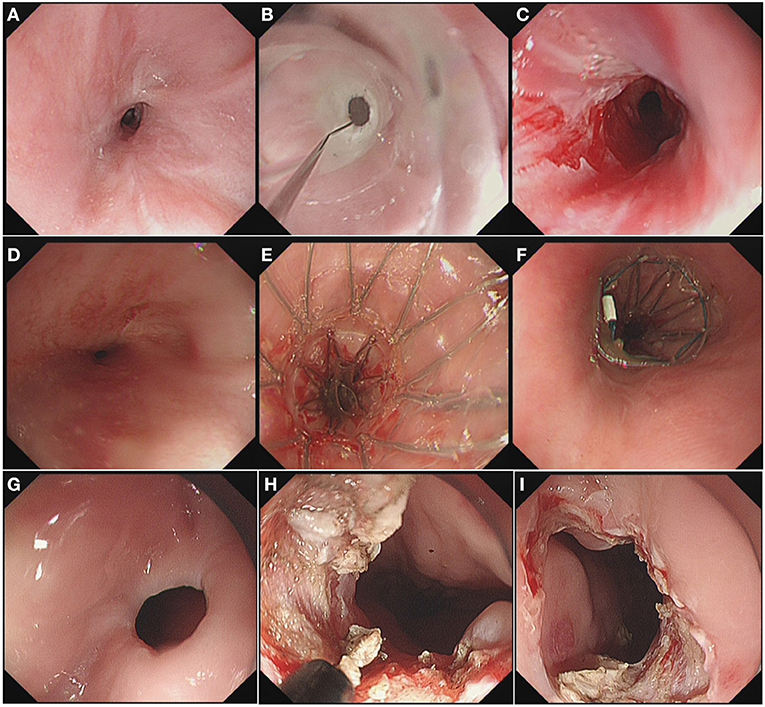
Figure 1. (A) Endoscopy Image of the stenosis before EBD. (B) EBD procedure. (C) Enlarged diameter of the stenosis after EBD. (D) Endoscopy Image of the stenosis before endoscopic stent placement. (E) Insertion of the stent. (F) Observation of the upper margin of the stent. (G) Endoscopy Image of the stenosis before EI. (H) Radial incisions under endoscopy. (I) Enlarged diameter of the stenosis after EI.
Postoperative Management
All patients were kept nil per os for 1 day, then on a liquid diet for 2–3days, and gradually allowed a soft diet within a month. Prophylactic intravenous proton pump inhibitors (PPIs) and antibiotics were occasionally used. Patients who underwent EBD and/or EI were scheduled for endoscopic examination every 2–3 months for the first 6 months after treatment. For patients who underwent stent placement, monthly chest X-ray examinations were scheduled to check for stent migration before final stent removal, and endoscopy was performed as necessary to adjust, reset, or remove the stent. Endoscopy was performed on all the patients for as long as recurrent dysphagia was experienced.
The time for stent removal was determined if any of the following criteria were met: (1) the stent had been in place for as long as 12 weeks, even if there was no migration, (2) the stent had fallen into the stomach, but dysphagia was relieved, or, (3) severe tissue overgrowth or ulceration on the upper and/or lower edges of the stent was observed. The stent was replaced if it shifted from the original position without improvement in the stenosis.
Evaluation of Dysphagia Before and After EI
The grade of dysphagia symptoms was defined by the following scoring system (9): 0, able to eat a normal diet; (1) unable to swallow certain solids, (2) able to swallow semisolid foods, (3) able to swallow liquids only, and 4, unable to swallow liquids.
Definition of Failure and Indication of Repeat Treatment
Treatment was considered unsuccessful when an endoscope with a diameter of 5.9 mm could not be passed through and the patient's dysphagia score exceeded 2. Additional endoscopic or surgical treatment was scheduled after such treatment failures.
Results
Patient Characteristics
The 15 patients were aged 1–14 years old, of these, 13 were boys and 2 were girls. There were 18 strictures in the 15 patients. All the patients received at least one type of treatment. All preoperative dysphagia scores were either 3 or 4. Detailed clinical data are presented in Table 1.
Treatment Outcome and Adverse Events
A total of 80 sessions of endoscopic treatment were administered to the 15 patients. This included 42 EBD sessions, 7 EI sessions, 1 stenting session, 19 EBD+Stenting sessions, 5 EBD+EI sessions, and 6 EI+Stenting sessions. All three types of endoscopic treatment were performed successfully. Fourteen of the 15 patients received EBD as the first step for treatment, but only two patients had their symptoms relieved by EBD alone. For patient 12, stent replacement alone was sufficient to relieve his symptoms. The remaining 12 patients received comprehensive treatment, such as EBD with EI, EBD with stent replacement, or a combination of EBD, stent replacement, and EI. Excluding patient 15, the overall median (IQR) treatment intervals of EBD, EI, EBD+Stenting, EBD+EI, and EI+Stenting were 2(1, 3), 4(2.5, 6), 10(3.5, 32.5), 3(3, 2), and 3(3, 5.35) months, respectively. Sustained symptom improvement was achieved in 73.3% (11/15) of the patients during a follow-up period of 8–121 months after treatment. The diameter of stricture was enlarged from 0.34 cm (range 0.2–0.7 cm) to 1.03 cm (range 0.8–1.2 cm) on average. Patient 1 developed subcutaneous emphysema, which resolved within a week without analgesic therapy. Patients 4, 8, 13, and 14 experienced recurrences at 31, 9, 3, and 36 months, respectively, after the final endoscopic treatment, and all of these patients underwent esophageal surgery to relieve their symptoms (Tables 1–3).
Discussion
The present study describes our experiences with endoscopic treatment of pediatric patients with esophageal stenosis that was congenital or induced by chemical burns or surgical repair of esophageal atresia. Unlike adult patients, in whom anastomotic stenosis after surgical resection of esophageal tumors is the leading source of stenosis, the etiological spectrum for children is comparatively broad. Nonetheless, the clinical presentations of children with esophageal stenosis tend to be very similar and are usually characterized by dysphagia, recurrent vomiting, and food impaction. Esophageal perforation may occur in some cases of corrosive ingestion (10). In addition to medical management, almost all patients with esophageal stenosis require either endoscopic dilatation or surgical intervention (11). To our knowledge, few articles discuss comprehensive endoscopic treatment of esophageal stenosis in children, as its incidence is relatively low worldwide.
Many reports have suggested that all endoscopic interventions should be performed under general anesthesia in pediatric patients (12). For children with esophageal stenosis, there have been many studies supporting EBD as a safe procedure with minimal morbidity and mortality, especially for anastomotic strictures, and those shorter than 5 cm (13, 14), making it a common first-line therapy for esophageal stenosis. Moreover, its safety and effectiveness have been emphasized, along with its long-term clinical success and nutritional promotion (15, 16). The size of the balloon catheter can vary from 0.4 to 2.2 cm, and the duration of balloon inflation can vary from 20 to 120 s (17). There is still no standard for the optimal timing of esophageal dilation. Two retrospective studies compared routine esophageal dilations (every 3 weeks, starting 3 weeks post-surgery) with dilations when symptoms developed in children post-esophageal atresia. They found no difference in outcomes or complications, but dilation times were significantly lower in the on-demand dilation group (18). Although more practical evidence is needed, the following rule of three has been widely accepted: dilate up to three times the diameter of stenosis, with an average of three dilations, and a minimum period of 3 weeks between dilation sessions (19).
Multiple EBDs are associated with a higher frequency of invasive operations and a greater risk of operation-related complications. Thus, endoscopic stent placement is prioritized. It has been widely accepted that temporary placement of self-expandable stents could be considered for refractory benign esophageal strictures in adult patients (20). For pediatric patients, Baghdadi et al. (21) performed a retrospective review of pediatric patients with esophageal atresia complicated by esophageal stenosis, in which patients who underwent endoscopic stent placement showed good outcomes after long-term follow-up. According to our prior experience, endoscopic stent placement is a good recommendation for pediatric patients with refractory esophageal stenosis, as it could provide continuous, radially oriented dilation pressures over a period of time. This is helpful for scar remodeling and decreasing the risk of perforation and restenosis (8). Moreover, for patients who undergo EBD and/or EI, damage to the esophageal wall is inevitable, and fully covered stents can protect the wound surface from gastric acid, thus potentially reducing the incidence of stenosis recurrence. Stents may remain for more than 4 weeks, which efficaciously lowers the frequency of invasive endoscopic operations and reduces the risk of complications during procedures. In this study, the median treatment interval was significantly longer in the EBD+stenting group than in the EBD group (10 vs. 4 months), which also confirmed the importance of stent placement after EBD. However, the migration rate after esophageal stent placement is high (22), and the possible complications including chest pain, reflux esophagitis, stent shedding, granulation tissue hyperplasia should not be ignored (23).
The efficacy and safety of EI have been proven in adults with anastomotic strictures and refractory benign strictures in recent years (24–31), but reports of its effectiveness in pediatric patients are rare. For adult patients with a Schatzki's ring, DiSario et al. (32) found that up to 64% of patients may relapse during follow-up, and the remission period was significantly longer with EI treatment than with EBD. The results of a prospective clinical trial involving 50 patients with Schatzki's rings showed that the asymptomatic survival of the EI group was higher than that of the EBD group (33). In our study, the median treatment interval of EI (4 months) was longer than that of EBD (2 months), which indicated that EI may result in longer asymptomatic survival than EBD. Additionally, EI could be an effective co-treatment for EBD and endoscopic stent placement. Our group (34) reported the case of a pediatric patient successfully treated with EI and esophageal stenting and performed an analysis of a case series (8) in which the symptoms of pediatric patients were successfully relieved after EI/EI+stent replacement when the previous attempts at dilation/stenting failed. It was found that EI efficacy was related to the stenosis length. Hordijk et al. (24) reported that patients with a stricture of <1.0 cm did not exhibit dysphagia recurrence during the 12 months of follow-up, whereas patients with a long-segment stricture size 1.5–5.0 cm required an average of three sessions to prevent a recurrence.
In a study by Muto et al. (25), a median of four sessions of preventive dilation were performed repeatedly to maintain patency. In our study, three patients underwent EI. Patient 13, who did not receive a stent replacement, suffered from recurrent dysphagia, supporting the notion that stent placement is beneficial for patients with a long-segment stricture. However, for patients who underwent EBD+EI or EI+Stenting, the treatment interval was 3(3, 2) months and 3(3, 5.35) months, respectively, which is shorter than that for EI alone 4(2.5, 6) months. However, for the EI+ stenting group, the P75 was significantly higher, suggesting that asymptomatic survival may be longer. However, as we only assessed five and six patients, respectively, in the EBD+EI and EI+Stenting groups, our findings need to be validated in a larger sample. Moreover, we suggest that EI should be performed by an experienced endoscopist, considering the risk of perforation due to the lack of epithelialization of the stricture.
In summary, EBD is suggested as the first and regular treatment step for children with esophageal stenosis shorter than 5 cm (22). Endoscopic stent placement is superior in maintaining continuous, radially oriented dilation pressures, and can serve as the first-line treatment for stenosis longer than 5 cm or recurrent stenosis. Furthermore, for patients who underwent EBD or EI, fully covered stents can protect the wound surface from gastric acid. EI could bring longer asymptomatic survival than EBD in our experience, and a combination therapy such as EBD+ESP, EI+ESP may result in a better efficacy for recurrent or refractory stenosis.
Moreover, there are other treatments for esophageal stenosis which we have not used. For example, an intralesional steroid injection may reduce the risk of recurrent stenosis by inhibiting collagen formation, accelerating collagen breakdown, and decreasing fibrotic healing (6). However, there is still a lack of consensus regarding the details of procedures involving optimal injection technique, frequency of injection, and dosage, especially for pediatric patients (35–37). Another example is mitomycin-C. Although studies have suggested that it may benefit patients with refractory strictures by decreasing collagen synthesis and scar formation (38–42), in a study conducted by Chapuy et al. (43), adding mitomycin-C treatment did not result in any benefit in strictures compared with repeated esophagea dilations alone. Thus, we did not choose to utilize these treatments for pediatric patients.
Our study has several limitations: first, this was a retrospective study with a relatively small sample size, therefore, selection and referral biases may exist. Second, this was a single-center study conducted in a tertiary hospital, and no patient received endoscopic drug therapy, such as intra-lesional injection of corticosteroids or mitomycin-C. Third, there was no comparison between endoscopic and surgical treatments, thus, it is difficult to confirm the advantages of endoscopic modalities. Furthermore, the comparison between different endoscopic treatments is limited because of the small number of patients, for example, only 3 patients received pure stent placement. Responses to different modalities of treatment for esophageal stenosis may also be different for esophageal atresia, chemical burns, and other causes of esophageal stenosis. Thus, larger comparative studies are required to confirm our findings and to suggest the best modality for pediatric esophageal stenosis.
In conclusion, our study showed that endoscopic treatment may serve as a safe and effective method for the treatment of pediatric esophageal stenosis—congenital or that caused by chemical burns or surgical repair of esophageal atresia. In most patients, endoscopic management (EBD, EI, stent placement, or a combination of these three methods) can relieve symptoms. Surgery is an alternative treatment option in the case of endoscopic failure.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
BZ contributed to drafting the manuscript. HP and LH contributed to data analysis and picture collecting. CL, LL, and XW contributed to the descriptions of detailed operation steps. YT and DL contributed to the conception of the study and revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thomson M, Tringali A, Dumonceau JM, Tavares M, Tabbers MM, Furlano R, et al. Paediatric gastrointestinal endoscopy: European Society for Paediatric Gastroenterology Hepatology and Nutrition and European Society of Gastrointestinal Endoscopy Guidelines. J Pediatr Gastroenterol Nutr. (2017) 64:133–53. doi: 10.1097/MPG.0000000000001408
2. Pearson EG, Downey EC, Barnhart DC, Scaife ER, Rollins MD, Black RE, et al. Reflux esophageal stricture–a review of 30 years' experience in children. J Pediatr Surg. (2010) 45:2356–60. doi: 10.1016/j.jpedsurg.2010.08.033
3. Romeo E, Foschia F, de Angelis P, Caldaro T, Federici di Abriola G, Gambitta R, et al. Endoscopic management of congenital esophageal stenosis. J Pediatr Surg. (2011) 46:838–41. doi: 10.1016/j.jpedsurg.2011.02.010
4. Ozdemir R, Bayrakci B, Teksam O, Yalcin B, Kale G. Thirty-three-year experience on childhood poisoning. Turk J Pediatr. (2012) 54:251–9. doi: 10.1016/j.cppeds.2012.01.004
5. Urganci N, Usta M, Kalyoncu D, Demirel E. Corrosive substance ingestion in children. Indian J Pediatr. (2014) 81:675–9. doi: 10.1007/s12098-013-1170-0
6. Vandenplas Y. Management of benign esophageal strictures in children. Pediatr Gastroenterol Hepatol Nutr. (2017) 20:211–5. doi: 10.5223/pghn.2017.20.4.211
7. Zhang J, Ren L, Huo J, Zhu Z, Liu D. The use of retrievable fully covered self-expanding metal stent in refractory postoperative restenosis of benign esophageal stricture in children. J Pediatr Surg. (2013) 48:2235–40. doi: 10.1016/j.jpedsurg.2013.06.005
8. Tan Y, Zhang J, Zhou J, Duan T, Liu D. Endoscopic incision for the treatment of refractory esophageal anastomotic strictures in children. J Pediatr Gastroenterol Nutr. (2015) 61:319–22. doi: 10.1097/MPG.0000000000000801
9. Atkinson M, Ferguson R, Ogilvie AL. Management of malignant dysphagia by intubation at endoscopy. J R Soc Med. (1979) 72:894–7. doi: 10.1177/014107687907201206
10. Jones DW, Kunisaki SM, Teitelbaum DH, Spigland NA, Coran AG. Congenital esophageal stenosis: the differential diagnosis and management. Pediatr Surg Int. (2010) 26:547–51. doi: 10.1007/s00383-010-2568-7
11. Terui K, Saito T, Mitsunaga T, Nakata M, Yoshida H. Endoscopic management for congenital esophageal stenosis: a systematic review. World J Gastrointestinal Endosc. (2015) 7:183–91. doi: 10.4253/wjge.v7.i3.183
12. Faccin G, Merlo F, Moretti T, Salano F. [Anesthesiologic problems in transluminal balloon dilatation of esophageal stenosis in children]. Minerva Anestesiol. (1990) 56:77–80.
13. Al Sarkhy A.A., Saeed A, Hamid YH, Al Asmi MM, Altokhais TI, Ullah AA, et al. Efficacy and safety of endoscopic dilatation in the management of esophageal strictures in children. Saudi Med J. (2018) 39:787–91. doi: 10.15537/smj.2018.8.22845
14. Fang SB Endoscopic balloon dilatation in pediatric patients with esophageal strictures: from the past to the future. Pediatr Neonatol. (2019) 60:119–20. doi: 10.1016/j.pedneo.2019.03.002
15. Chang CH, Chao HC, Kong MS, Chen SY, Chen CC, Lai MW. Clinical and nutritional outcome of pediatric esophageal stenosis with endoscopic balloon dilatation. Pediatr Neonatol. (2019) 60:141–8. doi: 10.1016/j.pedneo.2018.04.013
16. Cakmak M, Boybeyi O, Gollu G, Kucuk G, Bingol-Kologlu M, Yagmurlu A, et al. Endoscopic balloon dilatation of benign esophageal strictures in childhood: a 15-year experience. Dis Esophagus. (2016) 29:179–84. doi: 10.1111/dote.12305
17. Koivusalo A, Turunen P, Rintala RJ, van der Zee DC, Lindahl H, Bax NM. Is routine dilatation after repair of esophageal atresia with distal fistula better than dilatation when symptoms arise? Comparison of results of two European pediatric surgical centers. J Pediatr Surg. (2004) 39:1643–7. doi: 10.1016/j.jpedsurg.2004.07.011
18. Berger M, Ure B, Lacher M. Mitomycin C in the therapy of recurrent esophageal strictures: hype or hope? Eur J Pediatr Surg. (2012) 22:109–16. doi: 10.1055/s-0032-1311695
19. Bawazir O, Almaimani MO. Complications of esophageal strictures dilatation in children. A tertiary-center experience. Saudi Med J. (2020) 41:720–5. doi: 10.15537/smj.2020.7.25166
20. Ebigbo A, Karstensen JG, Aabakken L, Dinis-Ribeiro M, Spaander M, Le Moine O, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Cascade Guideline. Endosc Int Open. (2019) 7:E833–6. doi: 10.1055/a-0898-3523
21. Baghdadi O, Yasuda J, Staffa S, Ngo P, Zendejas B, Hamilton T, et al. Predictors and outcomes of fully covered stent treatment for anastomotic esophageal strictures in esophageal atresia. J Pediatr Gastroenterol Nutr. (2021) 74:221–6. doi: 10.1097/MPG.0000000000003330
22. Sarma MS, Tripathi PR, Arora S. Corrosive upper gastrointestinal strictures in children: difficulties and dilemmas. World J Clin Pediatr. (2021) 10:124–36. doi: 10.5409/wjcp.v10.i6.124
23. Fuccio L, Hassan C, Frazzoni L, Miglio R, Repici A. Clinical outcomes following stent placement in refractory benign esophageal stricture: a systematic review and meta-analysis. Endoscopy. (2016) 48:141–8. doi: 10.1055/s-0034-1393331
24. Hordijk ML, Siersema PD, Tilanus HW, Kuipers EJ. Electrocautery therapy for refractory anastomotic strictures of the esophagus. Gastrointestinal Endosc. (2006) 63:157–63. doi: 10.1016/j.gie.2005.06.016
25. Muto M, Ezoe Y, Yano T, Aoyama I, Yoda Y, Minashi K, et al. Usefulness of endoscopic radial incision and cutting method for refractory esophagogastric anastomotic stricture (with video). Gastrointestinal Endosc. (2012) 75:965–72. doi: 10.1016/j.gie.2012.01.012
26. Simmons DT, Baron TH. Electroincision of refractory esophagogastric anastomotic strictures. Dis Esophagus. (2006) 19:410–4. doi: 10.1111/j.1442-2050.2006.00605.x
27. Choi J, Choi SI. A new simple endoscopic incision therapy for refractory benign oesophageal anastomotic stricture. BMJ Case Rep. (2021) 14:e239798. doi: 10.1136/bcr-2020-239798
28. Mizusawa T, Kobayashi M, Terai S. Radial incision and cutting for refractory benign esophageal stricture. Digest Endosc. (2019) 31:e46–7. doi: 10.1111/den.13329
29. Liu D, Tan Y, Wang Y, Zhang J, Zhou J, Duan T, et al. Endoscopic incision with esophageal stent placement for the treatment of refractory benign esophageal strictures. Gastrointestinal Endosc. (2015) 81:1036–40. doi: 10.1016/j.gie.2014.10.037
30. Tan Y, Liu D. Endoscopic incision for the treatment of refractory esophageal anastomotic strictures: outcomes of 13 cases with a minimum follow-up of 12 months. Revista Espanola de Enfermedades Digestivas. (2016) 108:196–200. doi: 10.17235/reed.2016.4023/2015
31. Liang C, Tan Y, Lu J, Le M, Liu D. Endoscopic incision for treatment of benign gastrointestinal strictures. Expert Rev Gastroenterol Hepatol. (2020) 14:445–52. doi: 10.1080/17474124.2020.1766966
32. DiSario JA, Pedersen PJ, Bichis-Canoutas C, Alder SC, Fang JC. Incision of recurrent distal esophageal (Schatzki) ring after dilation. Gastrointestinal Endosc. (2002) 56:244–8. doi: 10.1016/S0016-5107(02)70185-5
33. Wills JC, Hilden K, Disario JA, Fang JC. A randomized, prospective trial of electrosurgical incision followed by rabeprazole versus bougie dilation followed by rabeprazole of symptomatic esophageal (Schatzki's) rings. Gastrointestinal Endosc. (2008) 67:808–13. doi: 10.1016/j.gie.2007.10.062
34. Tan Y, Wang X, Liu D, Huo J. Endoscopic incision plus esophageal stenting for refractory esophageal stricture in children. Endoscopy. (2014) 46(Suppl 1):E111–2. doi: 10.1055/s-0034-1364880
35. Holder TM, Ashcraft KW, Leape L. The treatment of patients with esophageal strictures by local steroid injections. J Pediatr Surg. (1969) 4:646–53. doi: 10.1016/0022-3468(69)90492-8
36. Zein NN, Greseth JM, Perrault J. Endoscopic intralesional steroid injections in the management of refractory esophageal strictures. Gastrointestinal Endosc. (1995) 41:596–8. doi: 10.1016/S0016-5107(95)70198-2
37. Gandhi RP, Cooper A, Barlow BA. Successful management of esophageal strictures without resection or replacement. J Pediatr Surg. (1989) 24:745–9; discussion: 749–50. doi: 10.1016/S0022-3468(89)80529-9
38. Heran MK, Baird R, Blair GK, Skarsgard ED. Topical mitomycin-C for recalcitrant esophageal strictures: a novel endoscopic/fluoroscopic technique for safe endoluminal delivery. J Pediatr Surg. (2008) 43:815–8. doi: 10.1016/j.jpedsurg.2007.12.017
39. Uhlen S, Fayoux P, Vachin F, Guimber D, Gottrand F, Turck D, et al. Mitomycin C: an alternative conservative treatment for refractory esophageal stricture in children? Endoscopy. (2006) 38:404–7. doi: 10.1055/s-2006-925054
40. Rosseneu S, Afzal N, Yerushalmi B, Ibarguen-Secchia E, Lewindon P, Cameron D, et al. Topical application of mitomycin-C in oesophageal strictures. J Pediatr Gastroenterol Nutr. (2007) 44:336–41. doi: 10.1097/MPG.0b013e31802c6e45
41. Daher P, Riachy E, Georges B, Georges D, Adib M. Topical application of mitomycin C in the treatment of esophageal and tracheobronchial stricture: a report of 2 cases. J Pediatr Surg. (2007) 42:E9–11. doi: 10.1016/j.jpedsurg.2007.06.007
42. Chung J, Connolly B, Langer J, Marcon M, Temple M, Amaral JG. Fluoroscopy-guided topical application of mitomycin-C in a case of refractory esophageal stricture. J Vasc Intervent Radiol. (2010) 21:152–5. doi: 10.1016/j.jvir.2009.09.016
Keywords: esophageal stenosis, endoscopic treatment, esophageal atresia, endoscopic balloon dilation, endoscopic stent replacement, endoscopic incision
Citation: Zhou B, Peng H, Han L, Liang C, Lv L, Wang X, Liu D and Tan Y (2022) Endoscopic Treatment for Pediatric Esophageal Stenosis Induced by Chemical Burn, Congenitally, or After Surgical Repair of Esophageal Atresia. Front. Pediatr. 10:814901. doi: 10.3389/fped.2022.814901
Received: 14 November 2021; Accepted: 31 January 2022;
Published: 25 February 2022.
Edited by:
Steffan Loff, Klinikum Stuttgart, GermanyReviewed by:
José Estevão-Costa, Centro Hospitalar Universitário de São João (CHUSJ), PortugalBurak Tander, Acıbadem University, Turkey
Copyright © 2022 Zhou, Peng, Han, Liang, Lv, Wang, Liu and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deliang Liu, deliangliu@csu.edu.cn; Yuyong Tan, tanyuyong@csu.edu.cn
 Bingyi Zhou
Bingyi Zhou Hailing Peng
Hailing Peng Liu Han
Liu Han Chengbai Liang
Chengbai Liang Liang Lv
Liang Lv Xuehong Wang
Xuehong Wang Deliang Liu
Deliang Liu Yuyong Tan
Yuyong Tan