Association of Mitochondrial DNA Polymorphisms With Pediatric-Onset Cyclic Vomiting Syndrome
- 1Division of Gastroenterology, Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 2Division of Medical Genetics, Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 3Department of Biochemistry, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
Background: Cyclic vomiting syndrome (CVS) is a functional gastrointestinal disorder characterized by recurrent stereotypic episodes of vomiting. The pathophysiology of CVS remains obscure. Previous studies have supported the hypotheses of mitochondrial dysfunction. However, data on association studies between mitochondrial DNA (mtDNA) polymorphisms and pediatric-onset CVS are limited and inconsistent. The aims of this study were to describe clinical characteristics, evaluate association of mtDNA polymorphisms 16519T and 3010A with pediatric-onset CVS and identify new mtDNA candidate variants.
Methods: This study involved Thai patients diagnosed with CVS according to the Rome III or IV criteria before the age of 15 years. Patients' demographic data, clinical characteristics, previous investigations and treatment outcomes were obtained. Blood samples were collected for next-generation (whole exome) sequencing, followed by analysis of chromosome M (mitochondrial. Variants were filtered according to clinical significance using ClinVar and MITOMAP. mtDNA polymorphisms in 148 normal Thai individuals were used as controls.
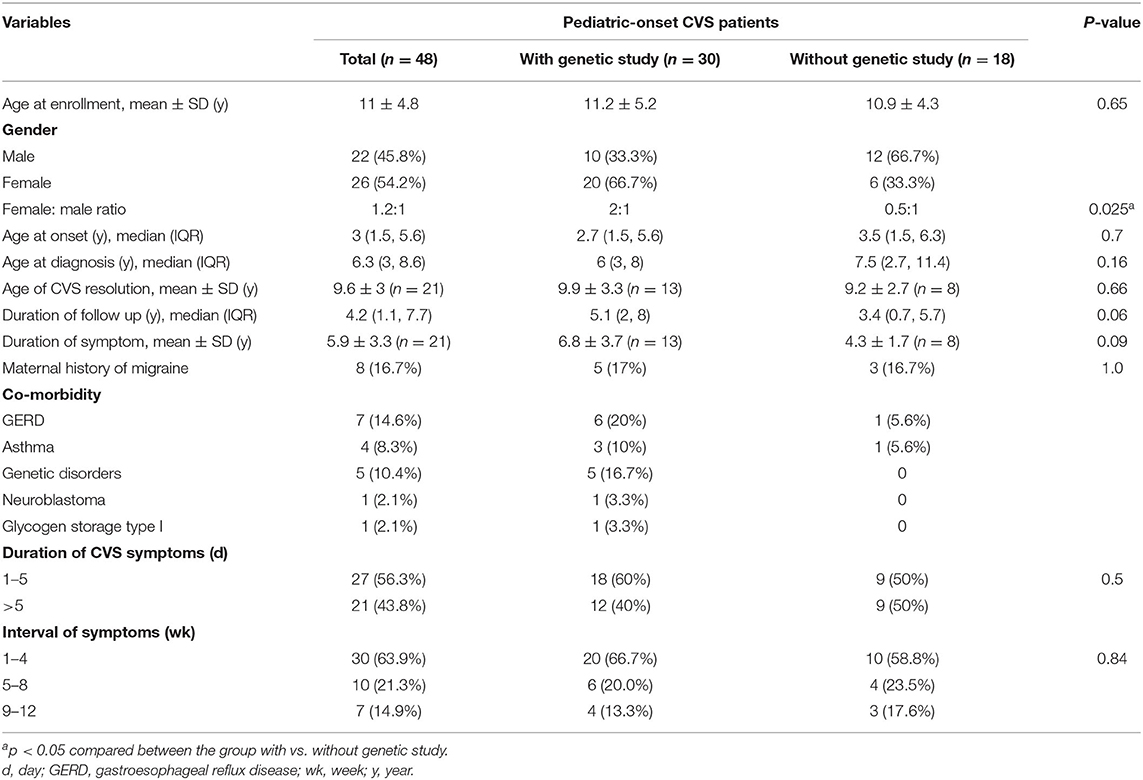
Results: Forty-eight children were enrolled in the clinical study, and 30 participated in the genetic analysis. The median age at onset and median age at diagnosis was 3.0 (1.5–5.6) and 6.3 (3.0–8.6) years, respectively. Maternal history of migraine was positive in 16.7%. About 45.7% (21 of 46) of the patients achieved complete clinical remission, with the mean symptom duration of 5.9 ± 3.3 years. The prevalence of mtDNA variants 16519T and 3010A among the patient group and Thai general population (control) were as follows: 40.0% (12/30) vs. 27.7% (P = 0.18) and 6.7% (2/30) vs. 0.7% (P = 0.07), respectively. Five known pathogenic variants were identified in 6 patients, including mtDNA 8528C in one patient who also had infantile hypertrophic cardiomyopathy. Six likely pathogenic variants were found but without statistical significance. We identified 11 variants with significant prevalence in the patient group. Though, these variants were classified as variants of unknown significance (VUS), several of them were located in mt functional regions and therefore they deserve further investigations as new candidates for association with pediatric CVS.
Conclusion: There were no associations of mtDNA polymorphisms 16519T and 3010A with CVS in our pediatric cohort. Five pathogenic variants and 11 VUS were found associated with pediatric-onset CVS.
Introduction
Cyclic vomiting syndrome (CVS) is a functional gastrointestinal disorder that affects both adults and children of all ages and presents with characteristic manifestations of acute, stereotyped and recurrent episodes of intense nausea and vomiting lasting from hours to a few days (1, 2). The worldwide prevalence of pediatric CVS is 1.9–2.3% (2), with the incidence of 3.1 per 100,000 per year (3), and the female:male ratio is 1.5:1 (1). Coexisting neuromuscular disease and developmental delay are found in up to 25% of patients with CVS, known as CVS plus (4).
CVS is strongly associated with migraine as ~25–35% of children with CVS develop migraine headache later in adulthood and there is a high prevalence of migraine headache in their families (5–9). Currently, according to the International Classification of Headache Disorders, CVS is classified as an “episodic syndrome that may be associated with migraine” (10). The etiopathogenesis of CVS remains obscure. Many underlying potential mechanisms have been postulated including mitochondrial dysfunction causing cellular energy deficit, hyperactivity of hypothalamic-pituitary-adrenal axis in response to stimulation, autonomic abnormalities, and calcium channel abnormalities (2, 6, 11, 12). Many studies supporting the hypothesis of mitochondrial dysfunction include frequent presence of metabolic abnormalities patterns of mitochondrial disorders during the episodes (6, 8), a maternal inheritance pattern (6, 9, 13, 14), and response to mitochondrial targeted therapies such as coenzyme Q10, L-carnitine and riboflavin, as well as antimigraine medications (1, 12). Boles et al. (15) reported that most cases of CVS plus had body fluid metabolites and muscle biopsies consistent with mitochondrial disorders and were strongly associated with maternal inheritance pattern.
Previous studies have shown an association between mitochondrial DNA (mtDNA) polymorphisms and pediatric-onset CVS with some conflicting results (14, 16–19). A3243G mutation was reported in a family with CVS (17). Zaki et al. (19) described 16519T polymorphism in 70% (odds ratio, 6.2) and concomitant polymorphisms of 16519T and 3010A in 29% (odds ratio, 17) of children with CVS. Subsequently, Ye et al. (18) performed mtDNA sequencing in 13 Chinese children with CVS and showed no significant differences in the prevalence of 16519T and 3010A polymorphism in children with CVS and in the control group.
The objectives of this study were to describe clinical characteristics and evaluate association of mtDNA polymorphisms 16519T and 3010A with pediatric-onset CVS and identify novel mtDNA candidate variants associated with this condition.
Materials and Methods
This observational study was conducted at the pediatric gastroenterology clinic of the Faculty of Medicine Ramathibodi Hospital from April 2019 to April 2020. Inclusion criteria were the patients diagnosed with CVS under the age of 15 years between January 2000 and October 2019. Diagnosis of CVS was according to the Rome III (20, 21) during 2006–2015 and the Rome IV (22, 23) during 2016–2019. Patients diagnosed with CVS before 2006 were retrospectively classified according to the Rome IV. Blood samples were collected from the eligible patients for genetic analysis.
Clinical Data Collection
The medical records of the patients were reviewed retrospectively. Demographic data, comorbidities, clinical characteristics, family history of migraine headache, hospitalizations, precipitating factors, previous investigations, treatment received, and the course of CVS were obtained. To determine the CVS outcome, the patients were interviewed in the clinic, during the study period, using the structured questionnaire that included symptoms, episodes of attack and prophylactic medications in the previous year as well as an occurrence of migraine headache. Patients who had been missing for more than 6 months were contacted by phone to collect the data using the same questions. As for patients who were lost to follow-up and could not be reached by phone, their symptoms at the last clinic visit were employed as the outcome.
CVS outcomes were classified into three groups according to the frequency of episodes during the previous year of follow-up: complete, partial, and no remission. Complete remission was defined as no episodes, partial remission as ≥50% reduction in the frequency of episodes, and no remission as <50% reduction, when compared to the symptoms at presentation.
Diagnosis of CVS plus requires at least two hard manifestations of the following: skeletal myopathy, cranial nerve abnormality, ataxia, seizure disorder, cardiomyopathy, intellectual disability, attention-deficit/hyperactivity disorder, microcephaly, and autism (4).
Mitochondrial Genome Sequencing and Analysis
DNA was extracted from peripheral blood leukocytes using a DNA purification kit (Gentra Puregene Blood Kit; Qiagen, Hilden, Germany. Next-generation sequencing (NGS) was performed on a NovaSeq 6000 Sequencer (Illumina, San Diego, CA, USA) using SureSelect Human All Exon V5 kits (Agilent Technologies, Santa Clara, CA, USA) for target enrichment with 120-base-pair paired-end reads. The data were analyzed on Terra, a cloud-native platform for biomedical researchers. The processing steps were combined using the Workflow Description Language. Human genome reference version 38 (GRCh38) was used for alignment. The average depth of coverage was 42 with at least 30× coverage in 90% of the genome. The GATK Best Practice Workflow (version 4.1.3.0) for data processing and variant discovery was used to identify germline single-nucleotide polymorphisms (SNPs) and indels in mitochondria (chromosome M). To determine the effect of these variants, Variant Effect Predictor (ensembl-vep version 4.2) was used to annotate the variants discovered. Variants that did not pass quality control or had a heteroplasmy level of <10% (likely low clinical correlation) were eliminated from further analysis. Variants were filtered and prioritized for their clinical significance into pathogenic, likely pathogenic, variants of unknown significance, and benign. As for previously reported variants, we labeled its pathogenicity classification following its verdict as shown on the ClinVar/CLIN_SIG (https://www.ncbi.nlm.nih.gov/clinvar/docs/clinsig/) and the MITOMAP database (www.mitomap.org). We categorized the novel variants identified in the present study as VUS, and those with significant allele frequency were deposited into ClinVar database and subsequently assigned accession numbers (Supplementary Table S6). The known pathogenic variant was verified by Sanger sequencing. Primer sequences were provided in Supplementary Table S1.
Allele Frequencies in Thai General Population
Allele frequencies of mtDNA polymorphisms were determined in 148 normal Thai controls as previously published and were used as ethnic-comparable frequencies (24). The samples were selected to represent the entire Thai population. Whole mitochondria were Sanger sequenced using 16 overlapping primer pairs.
Statistical Analysis
Statistical analysis was performed under closed supervision and reviewed by a biomedical statistician from Department of Clinical Epidemiology and Biostatistics Faculty of Medicine Ramathibodi Hospital, Mahidol University. Data from patients performed genetic test was compared with those without genetic test. Allele frequencies between patients with CVS and the general Thai population were compared and analyzed. SPSS version 21 for Windows (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Continuous variables were presented as mean ± standard deviation or median (interquartile range) and were analyzed using Mann–Whitney test. Categorical variables were presented as percentage and were analyzed by a Chi-square test. A P-value of <0.05 was considered statistically significant.
Results
Clinical Characteristics and Outcomes
Fifty-one patients were diagnosed with CVS; however, three had missing medical records. Therefore, 48 patients (54.2% female) were enrolled, and 30 participated in the genetic analysis. Exclusion from the genetic study was due to loss of contact (n = 14) and declining a genetic test (n = 4). The mean age ± SD at enrollment was 11.0 ± 4.8 (range 2.6–23) years, and the median (IQR) age at onset and at diagnosis was 3.0 (1.5–5.6) and 6.3 (3.0–8.6) years, respectively. A maternal family history of migraine headache was reported in 8 (16.7%) children. None had a family history of CVS or consanguinity (being descended from the same ancestor). There were no significant differences in age at onset, clinical remission of CVS, or maternal history of migraine between the patients who did and did not undergo genetic studies (Table 1; Supplementary Table S2).
Female sex was predominant among the patients who underwent genetic studies (66.7%), whereas male sex was predominant among the patients who did not (66.7%) (P = 0.025). Most (62.5%) patients had identifiable precipitating factors, among which psychological stress was the most common (50.0%). During vomiting episodes, the patients experienced lethargy (60.4%), abdominal pain (47.9%), hematemesis (37.5%), pallor (29.2%), hypertension (25%), headache (18.8%), and photosensitivity (4.2%). All patients diagnosed prior to establishing of the Rome IV exhibited a typical stereotyped pattern of episodes, which was included in the Rome IV but not in the Rome III diagnostic criteria.
Investigations to exclude possible organic causes were performed in all children with (1) age at onset <2 years; (2) clinical and blood chemistries suggestive of metabolic or other diseases; (3) treatment-refractory CVS. Upper GI studies were performed in 42 patients, with 4 (9.5%) reported non-specific findings (delay passage barium from 2nd to 3rd part of duodenum, pylorospasm, and gastroesophageal reflux). Abdominal ultrasonography was performed in 14 patients, with normal results. Esophagogastroduodenoscopy (EGD) was done in 33 patients with 16 (48.5%) reported abnormal but non-specific findings, including mild gastritis, mild esophagitis and prolapsed gastropathy. Twenty patients underwent brain CT scan which showed unremarkable results (Supplementary Table S2). Abnormal blood test results during the episodes included elevated blood lactate (6/29, 20.7%), hyperammonemia (4/35, 11.4%), abnormal plasma amino acid profiles (7/16, 43.8%), and abnormal urine organic acids (2/16, 12.5%) (Supplementary Table S2). Interestingly, one patient (Patient 7) developed a one-time episode of plasma citrullinemia without documented hyperammonemia but with a negative genetic analysis for urea cycle disorders and citrin deficiency, as performed by Sanger and whole-exome sequencing analysis. Treatment with a low-protein diet, ammonia-lowering agent, and CVS prophylaxis therapy alleviated this patient's symptoms. The patient was clinically well after discontinuation of treatment. Two patients had mild excretion of 3-methylglutaconic acid and lactic acid with abnormal urinary organic acid profile, and were treated as CVS with complete remission at 9 and 7 years of age, respectively. Genetic studies performed in all suspected cases did not reveal a specific metabolic disorder.
Prophylactic therapy included amitriptyline (22/45), cyproheptadine (14/45) and propranolol (12/45). At the median duration of follow-up of 4.2 years (IQR 1.1, 7.7 years), 21 out of 46 patients achieved complete clinical remission; the mean age at clinical remission was 9.6 ± 3.0 years, and the mean symptom duration was 5.9 ± 3.3 years. Migraine headache developed after CVS remission in 3/21 (14%) patients. Partial remission and no remission occurred in 21 and 4 patients, respectively. Among all patients, 13 still required hospitalization for episodic attacks.
CVS Plus
Six patients (83% female) were diagnosed with CVS plus, with a median age at onset of 4.3 (1.7–7.3) years; this age did not differ from that of the other patients. Two patients with CVS plus showed lactic acidosis. Three (50%) patients had a maternal history of migraine headache compared with 11.9% (5/42) of the non-CVS plus patients (P = 0.05; Supplementary Table S2). Complete remission rate was lower in CVS plus (1/6 or 16.7% vs. 20/40 or 50%, P = 0.19); and the frequency of no remission was higher in CVS plus (1/6 or 16.7% vs. 3/40 or 7.5%, P = 0.44).
Mitochondrial DNA Polymorphisms
The prevalence of mtDNA variants 16519T and 3010A among the patient group and Thai general population (control) were as follows: 40.0% (12/30) vs. 27.7% (P = 0.18) and 6.7% (2/30) vs. 0.7% (P = 0.07), respectively (Supplementary Table S3).
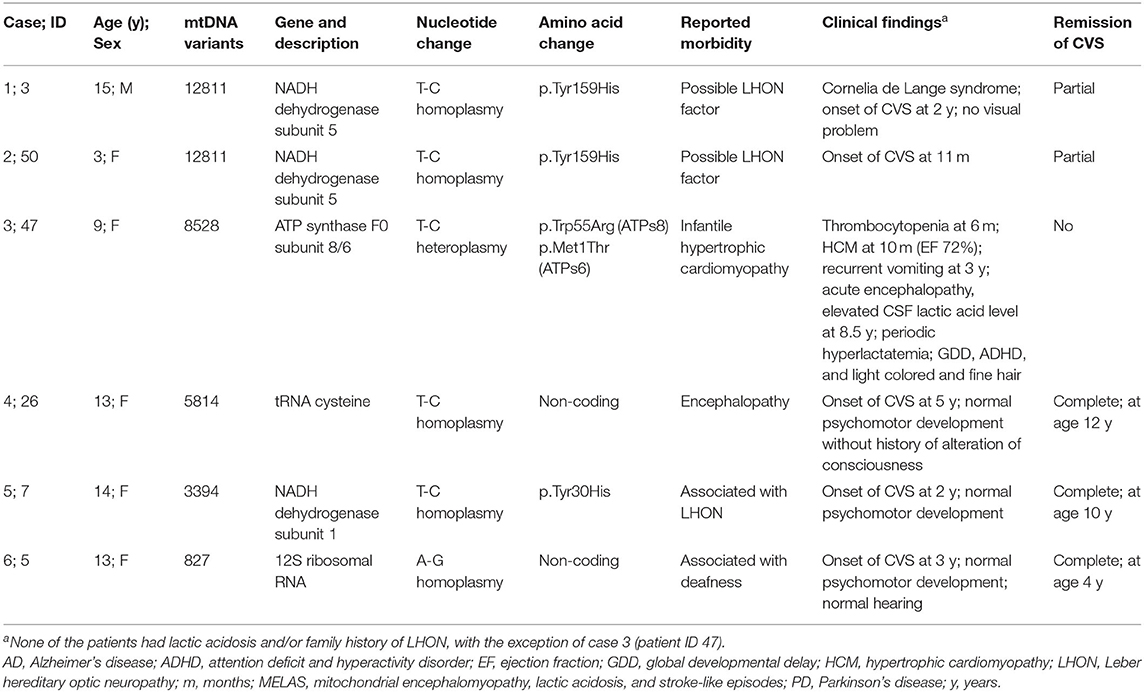
Mitochondrial genome analysis revealed 5 known pathogenic variants in 6 patients, 6 likely pathogenic variants in 30 patients (Tables 2, 3), and 312 VUS (with ≥10% level of heteroplasmy).
Among those with pathogenic variants, one patient (Patient 47) with T8528C (97% heteroplasmy) had clinical presentations that matched the reported morbidity. The patient presented with infantile-onset skin bruising owing to thrombocytopenia (20,000–60,000/mm3), hypertrophic cardiomyopathy (HCM) with outflow tract obstruction, and developmental delay. The HCM was gradually improved over time. At the age of 6 years, symptoms of cyclic vomiting began and platelet storage pool defect was suspected as evidenced by abnormal platelet aggregation and normal number of platelets. At the age of 8.5 years, the patient developed acute loss of consciousness with preceding 1-day history of agitation and confusion. Investigations revealed normal brain magnetic resonance imaging and magnetic resonance spectroscopy (MRI and MRS); elevated lactate level in cerebrospinal fluid (2.7 mmol/L) whilst normal blood lactate (1.2 mmol/L); elevated serum creatine kinase (1,079 U/L); normal profiles of blood ammonia, plasma amino acids, acylcarnitine profile and urinary organic acids; and mild HCM with ejection fraction of 76%. The patient recovered to her baseline within a couple days of general supportive care. Both parents had a normal echocardiogram. The mother harboring the T8528C variant at a low level of heteroplasmy (Supplementary Figure S1) reported no essential morbidities.
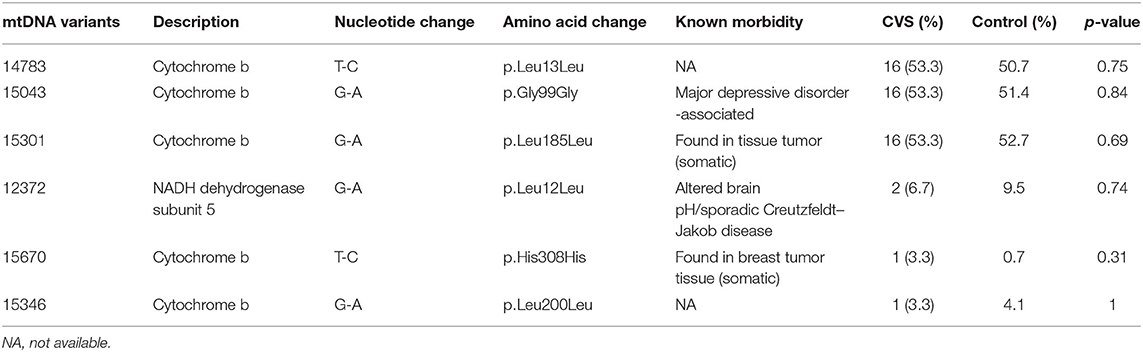
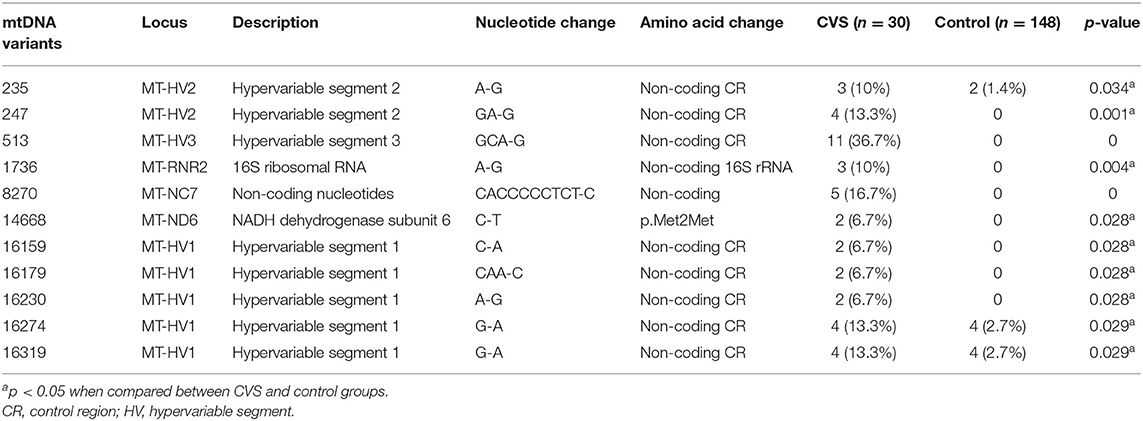
Among the 312 VUS, 11 were found to be significantly more prevalent among the CVS group than control group and had not been previously reported in patients with CVS (Table 4; Supplementary Tables S4, S5). These included 8 variants located at hypervariable (HV) segment of the control region and a variant of 12s ribosomal RNA gene, a non-coding nucleotide (Table 4; Supplementary Table S4).
Among the 6 CVS plus patients, 1 pathogenic (T8528C), as mentioned above), 3 likely pathogenic and 3 VUS were found but there was no statistically significant difference in term of prevalence when compared with non-CVS plus patients (Supplementary Table S5).
Discussion
Clinical and genetic studies on pediatric CVS among Asian affected population are limited and underrepresented in the literature. The existing genetic data are also conflicting which may reflect the scarcity of information. To our knowledge this is the largest cohort of Thai pediatric CVS and contribute as one of the milestone studies from Asian affected population.
In this cohort, we demonstrated similar clinical features to those in previous reports including vomiting characteristics and long duration of symptoms before diagnosis (5, 18, 25). However, the mean age of onset was slightly lower as compared to previous pediatric CVS studies (8, 18, 25). Maternal migraine headache was found in 16.7% in this study. Family history of migraine headache in pediatric CVS ranged from 11.9% in Chinese study (18) to 66–72% in western studies (8, 26). During the episodes, abnormal laboratory findings included elevated blood lactate, hyperammonemia, abnormal plasma amino acid profiles and urine organic acids.
Almost half of the patients achieved complete clinical remission at the mean age of 9.6 years, and among this group, the mean clinical course of CVS was 5.9 years. This outcome is consistent with our previous report (27) and other reports (25, 26). Three patients (14%) developed migraine after CVS remission while other studies reported around 25–35% (6, 7). Only one of the three patients underwent a mtDNA genetic test, which showed no 16519T or 3010A polymorphism.
It should be noted that CVS plus is not generally employed in daily clinical practice; however, in the present study of etiopathogenesis of CVS, it could raise concerns relating to differential diagnosis for the affected individuals, in particular pediatric population. Therefore, in this study, we decided to analyze this subgroup and search for potential distinctive etiology, if there was any. We found a higher prevalence of maternal migraine among the CVS plus group compared to non-CVS plus group (50 vs. 11.9%, P = 0.05), suggesting a higher rate of maternal inheritance in patients with CVS plus than in non-CVS plus. Patients with CVS plus had a lower complete remission rate (16.7 vs. 50%, P = 0.19); and a higher frequency of no remission (16.7 vs. 7.5%, P = 0.44). There were no statistical differences of the outcomes probably due to the small number of the patients in CVS plus group. These data suggest that pathogenesis of CVS plus may differ from non-CVS plus and may deserve more extensive investigations for etiologic diagnosis, including exome and mitochondrial genome sequencing for causal-disease relationship.
It is postulated that underlying mtDNA polymorphism might impact energy metabolism both in the resting state and a hyperexcitability state, including stress circumstance (6, 12). The mitochondrial genome encompasses 24 rRNA and tRNA genes and 13 genes encoding proteins involved in oxidative phosphorylation (complex I–V) (28). Some mitochondrial variants are judged as pathogenic or as having a “causality” effect, while others may contribute to the risk of developing multifactorial disorders or are considered to have an “association” effect.
Our study showed no significant association between mtDNA 16519T or 3010A SNPs and pediatric CVS. This result supports the finding in Chinese children with CVS (18) and contrasts the findings in Caucasian reports (16, 19). Explanations for these discrepancies include the small sample sizes, unintentional bias of subject recruitment, and differences in allele frequencies of the SNPs of interest among different ethnicities. More studies in Asian CVS children are required to confirm our findings.
The known pathogenic variants identified in this study (Table 2) have been reported in association with several conditions including Leber hereditary optic neuropathy; deafness; myopathy; cardiomyopathy; and encephalopathy, but not CVS. We have provided the first description of the association of these variants with pediatric-onset CVS. There were two (6.7%) occurrences of mtDNA T12811C variants in the CVS group compared with 4.73% in the control group, suggesting a strong correlation between this variant and CVS compared with other pathogenic SNPs.
Including our patient (Patient 47), seven patients with the mtT8528C allele have been identified (29, 30). All patients had infantile onset HCM, and about half (4/7 patients) had rapid progression leading to infantile death or requiring heart transplantation at a young age. To our knowledge, the present patient was the oldest living individual affected with mtT8528C-associated HCM. This study is the first to show an association between the mtT8528C allele and CVS, platelet dysfunction, abnormal hair, and behavioral disorders. Mitochondrial disorders caused by nuclear gene mutations are more severe than those caused by mtDNA mutations (31), with the exception of some rare variants including mtT8528C (29, 30). Given there has no previous association study of mtT8528C and CVS before, it suggests that mtT8528C and other mt pathogenic variant(s) can also has CVS as one of its related manifestations and that mt pathogenic variant(s) should be considered as possible SNPs associated with pediatric CVS regardless of the presence/absence of classical manifestation of mt disorders.
We found that patients with CVS tended to have at least one pathogenic variant when compared with the general population. Although the allele frequency of each pathogenic variant was not different from those in the control group, we cannot rule out a possible correlation between these variants and CVS/other manifestations. When the data of all SNPs were combined, the aggregate prevalence of pathogenic SNPs in the CVS group was 20% (6/30), which was higher than that in the reference population (9.5%) (P = 0.1). The absence of an association between the likely pathogenic variants and CVS is a straightforward finding because neither the individual nor aggregate prevalence of the variants differed between the two groups. Our data indicate that NGS with a mitochondrial analysis pipeline could be powerful for genomic research in CVS and related disorders.
Of the 11 VUS, one missense variant in complex I might affect mitochondrial function to some extent. Among the eight alleles found in HV segments of the control region, five were in the highly polymorphic HV1. However, part of the HV1, mt16040–16188 containing very low sequence variability, is important in mtDNA replication and possibly translation. A specific segment, mt16157–16172, is an important functional region: the termination-associated sequence (14). Therefore, the mtC16159A and mtCAA16179C variants (Table 4) might interfere with mitochondrial replication and translation. Among the variants in HV3, GCA513G of the D-loop (control region) appears to be a hotspot for somatic and germline mtDNA mutation and may reflect ancient mutation or ancestry (MITOMAP, https://www.mitomap.org/); therefore, it may or may not possess functional effects. We observed that C14668T variant was present in two patients with CVS plus and this variant was residing in the functional locus, MT-ND6 (Table 4; Supplementary Table S4). This may suggest that the variant deserves high priority for association study of mt polymorphisms with pediatric-onset CVS. Further studies are required to elucidate the functional consequences of this allele.
The strength of our study included whole mt genome being analyzed and having ethnic-comparable mt genome reference, which is important factor to avoid bias and increase reliability of the analysis for association study. However, we acknowledge some limitations. First, the clinical data were obtained by medical chart review and were incomplete because of the nature of this retrospective study. Second, although this is one of the few largest cohorts of pediatric CVS with whole mitochondrial genome analysis, the sample size was small. Third, statistical significance does not guarantee clinical relevance. Fourth, functional investigation was not performed to elaborate the pathological effects of the VUS identified. Fifthly, the presence of some individuals with CVS in the control group cannot be totally excluded because the individuals might not be aware of CVS during their childhood and the symptoms could be misinterpreted as infection related. Finally, the studied specimens were obtained from peripheral blood, which may not represent the mitochondrial genotypes in other tissues.
Because pediatric CVS is a rare condition, we urge multicenter national and/or regional networks to perform a larger cohort study with an ethnic-comparable reference population, which may lead to a stronger association study. Additionally, development of a simple and less sophisticated method of mitochondrial functional analysis is needed for research in the fields of CVS and mitochondrial disorders.
Conclusion
In conclusion, the mtDNA polymorphisms 16519T and 3010A were not associated with pediatric-onset CVS in this pediatric cohort. However, 5 known pathogenic variants and 11 novel VUS were shown to be associated with pediatric-onset CVS. Our data suggest that NGS with a mitochondrial analysis pipeline is a powerful tool for mtDNA polymorphism study in CVS research.
Data Availability Statement
The data presented in the study are deposited in the ClinVar Database, accession numbers are shown in the Supplementary Table S6.
Ethics Statement
The studies involving human participants were reviewed and approved by Ramathibodi Hospital Human Research Ethics Committee (MURA2019/310). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
KV collected and analyzed the clinical data, performed the bioinformatics analysis of the patient group, and prepared the manuscript draft. BS supervised the bioinformatics analysis. PL supervised and SK performed the bioinformatics analysis of the control population. SN performed the bioinformatics analysis and sequencing. TT-A assisted with the data analysis. ST obtained a research grant. ST and DW designed the overall concept of the study, supervised the data analysis, and critically revised the manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was funded by the Faculty of Medicine Ramathibodi Hospital, Mahidol University (RF_62086).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the patients' families for their participation in this study; Ms. Umaporn Udomsubpayakul, M.sc (Biostat) for her assistance in statistical analysis; and Faculty of Medicine Ramathibodi Hospital for giving the Research Career Development Awards to DW and TT-A. Finally, we thank Angela Morben, DVM, ELS, from Edanz Group (https://en-author-services.edanz.com/ac), for editing a draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.876436/full#supplementary-material
References
1. Li BU, Lefevre F, Chelimsky GG, Boles RG, Nelson SP, Lewis DW, et al. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition consensus statement on the diagnosis and management of cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr. (2008) 47:379–93. doi: 10.1097/MPG.0b013e318173ed39
2. Romano C, Dipasquale V, Rybak A, Comito D, Borrelli O. An overview of the clinical management of cyclic vomiting syndrome in childhood. Curr Med Res Opin. (2018) 34:1785–91. doi: 10.1080/03007995.2018.1445983
3. Fitzpatrick E, Bourke B, Drumm B, Rowland M. The incidence of cyclic vomiting syndrome in children: population-based study. Am J Gastroenterol. (2008) 103:991–5; quiz 6. doi: 10.1111/j.1572-0241.2007.01668.x
4. Boles RG, Powers AL, Adams K. Cyclic vomiting syndrome plus. J Child Neurol. (2006) 21:182–8. doi: 10.2310/7010.2006.00040
5. Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol. (2012) 24:1001–6. doi: 10.1097/MEG.0b013e328355638f
6. Li BU, Misiewicz L. Cyclic vomiting syndrome: a brain-gut disorder. Gastroenterol Clin North Am. (2003) 32:997–1019. doi: 10.1016/S0889-8553(03)00045-1
7. Lin YP, Ni YH, Weng WC, Lee WT. Cyclic vomiting syndrome and migraine in children. J Formos Med Assoc. (2011) 110:382–7. doi: 10.1016/S0929-6646(11)60056-9
8. Moses J, Keilman A, Worley S, Radhakrishnan K, Rothner AD, Parikh S. Approach to the diagnosis and treatment of cyclic vomiting syndrome: a large single-center experience with 106 patients. Pediatr Neurol. (2014) 50:569–73. doi: 10.1016/j.pediatrneurol.2014.02.009
9. Stickler GB. Relationship between cyclic vomiting syndrome and migraine. Clin Pediatr (Phila). (2005) 44:505–8. doi: 10.1177/000992280504400606
10. (IHS) CotIHS Headache Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
11. Lee J, Wong SA, Li BU, Boles RG. NextGen nuclear DNA sequencing in cyclic vomiting syndrome reveals a significant association with the stress-induced calcium channel (RYR2). Neurogastroenterol Motil. (2015) 27:990–6. doi: 10.1111/nmo.12575
12. Raucci U, Borrelli O, Di Nardo G, Tambucci R, Pavone P, Salvatore S, et al. Cyclic vomiting syndrome in children. Front Neurol. (2020) 11:583425. doi: 10.3389/fneur.2020.583425
13. Boles RG, Adams K, Li BU. Maternal inheritance in cyclic vomiting syndrome. Am J Med Genet A. (2005) 133A:71–7. doi: 10.1002/ajmg.a.30524
14. Wang Q, Ito M, Adams K, Li BU, Klopstock T, Maslim A, et al. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am J Med Genet A. (2004) 131:50–8. doi: 10.1002/ajmg.a.30323
15. Boles RG, Adams K, Ito M, Li BU. Maternal inheritance in cyclic vomiting syndrome with neuromuscular disease. Am J Med Genet A. (2003) 120A:474–82. doi: 10.1002/ajmg.a.20126
16. Boles RG, Zaki EA, Lavenbarg T, Hejazi R, Foran P, Freeborn J, et al. Are pediatric and adult-onset cyclic vomiting syndrome (CVS) biologically different conditions? Relationship of adult-onset CVS with the migraine and pediatric CVS-associated common mtDNA polymorphisms 16519T and 3010A. Neurogastroenterol Motil. (2009) 21:936–e72. doi: 10.1111/j.1365-2982.2009.01305.x
17. Salpietro CD, Briuglia S, Merlino MV, Di Bella C, Rigoli L. A mitochondrial DNA mutation (A3243G mtDNA) in a family with cyclic vomiting. Eur J Pediatr. (2003) 162:727–8. doi: 10.1007/s00431-003-1280-1
18. Ye Z, Xue A, Huang Y, Wu Q. Children with cyclic vomiting syndrome: phenotypes, disease burden and mitochondrial DNA analysis. BMC Gastroenterol. (2018) 18:104. doi: 10.1186/s12876-018-0836-5
19. Zaki EA, Freilinger T, Klopstock T, Baldwin EE, Heisner KR, Adams K, et al. Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia. (2009) 29:719–28. doi: 10.1111/j.1468-2982.2008.01793.x
20. Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. (2006) 130:1519–26. doi: 10.1053/j.gastro.2005.11.065
21. Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. (2006) 130:1527–37. doi: 10.1053/j.gastro.2005.08.063
22. Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. (2016) 150:1443–55. doi: 10.1053/j.gastro.2016.02.016
23. Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional disorders: children and adolescents. Gastroenterology. (2016) 150:1456–68. doi: 10.1053/j.gastro.2016.02.015
24. Kaewsutthi S, Phasukkijwatana N, Joyjinda Y, Chuenkongkaew W, Kunhapan B, Tun AW, et al. Mitochondrial haplogroup background may influence Southeast Asian G11778A Leber hereditary optic neuropathy. Invest Ophthalmol Vis Sci. (2011) 52:4742–8. doi: 10.1167/iovs.10-5816
25. Haghighat M, Rafie SM, Dehghani SM, Fallahi GH, Nejabat M. Cyclic vomiting syndrome in children: experience with 181 cases from southern Iran. World J Gastroenterol. (2007) 13:1833–6. doi: 10.3748/wjg.v13.i12.1833
26. Fitzpatrick E, Bourke B, Drumm B, Rowland M. Outcome for children with cyclical vomiting syndrome. Arch Dis Child. (2007) 92:1001–4. doi: 10.1136/adc.2007.116608
27. Treepongkaruna S, Jarasvaraparn C, Tanpowpong P, Lertudomphonwanit C. Short- and long-term outcomes of children with cyclic vomiting syndrome. J Med Assoc Thai. (2014) 97:1077–83.
28. Shoffner JM. Molecular analysis of oxidative phosphorylation diseases for detection of mitochondrial DNA mutations. Curr Protoc Hum Genet. (2001) Chapter 9:Unit 9. doi: 10.1002/0471142905.hg0909s13
29. Imai A, Fujita S, Kishita Y, Kohda M, Tokuzawa Y, Hirata T, et al. Rapidly progressive infantile cardiomyopathy with mitochondrial respiratory chain complex V deficiency due to loss of ATPase 6 and 8 protein. Int J Cardiol. (2016) 207:203–5. doi: 10.1016/j.ijcard.2016.01.026
30. Ware SM, El-Hassan N, Kahler SG, Zhang Q, Ma YW, Miller E, et al. Infantile cardiomyopathy caused by a mutation in the overlapping region of mitochondrial ATPase 6 and 8 genes. J Med Genet. (2009) 46:308–14. doi: 10.1136/jmg.2008.063149
Keywords: cyclic vomiting syndrome, DNA polymorphisms, Mt16519T, Mt3010A, mitochondrial next-generation sequencing, pediatric-onset cyclic vomiting syndrome
Citation: Veenin K, Wattanasirichaigoon D, Suktitipat B, Noojarern S, Lertrit P, Tim-Aroon T, Kaewsutthi S and Treepongkaruna S (2022) Association of Mitochondrial DNA Polymorphisms With Pediatric-Onset Cyclic Vomiting Syndrome. Front. Pediatr. 10:876436. doi: 10.3389/fped.2022.876436
Received: 15 February 2022; Accepted: 22 April 2022;
Published: 24 May 2022.
Edited by:
Francesco Valitutti, Ospedali Riuniti San Giovanni di Dio e Ruggi d'Aragona, ItalyReviewed by:
Silvia Salvatore, University of Insubria, ItalyPaolo Quitadamo, AORN Santobono-Pausilipon, Italy
Copyright © 2022 Veenin, Wattanasirichaigoon, Suktitipat, Noojarern, Lertrit, Tim-Aroon, Kaewsutthi and Treepongkaruna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suporn Treepongkaruna, suporn.tre@mahidol.ac.th; suporntr@gmail.com
 Kirana Veenin1
Kirana Veenin1  Duangrurdee Wattanasirichaigoon
Duangrurdee Wattanasirichaigoon Bhoom Suktitipat
Bhoom Suktitipat Thipwimol Tim-Aroon
Thipwimol Tim-Aroon Suporn Treepongkaruna
Suporn Treepongkaruna