Modified ABCDEF-Bundles for Critically Ill Pediatric Patients - What Could They Look Like?
- 1Department of Pediatric Cardiology, Pulmonology and Pediatric Intensive Care Medicine, University Children's Hospital Tübingen, Tübingen, Germany
- 2Department of Pediatric Cardiology and Intensive Care Medicine, Hannover Medical School, Hannover, Germany
Background and Significance: Advances in pediatric intensive care have led to markedly improved survival rates in critically ill children. Approximately 70% of those children survive with varying forms of complex chronic diseases or impairment/disabilities. Length of stay, length of mechanical ventilation and number of interventions per patient are increasing with rising complexity of underlying diseases, leading to increasing pain, agitation, withdrawal symptoms, delirium, immobility, and sleep disruption. The ICU-Liberation Collaborative of the Society of Critical Care Medicine has developed a number of preventative measures for prevention, early detection, or treatment of physical and psychiatric/psychological sequelae of oftentimes traumatic intensive care medicine. These so called ABCDEF-Bundles consist of elements for (A) assessment, prevention and management of pain, (B) spontaneous awakening and breathing trials (SAT/SBT), (C) choice of analgesia and sedation, (D) assessment, prevention and management of delirium, (E) early mobility and exercise and (F) family engagement and empowerment. For adult patients in critical care medicine, research shows significant effects of bundle-implementation on survival, mechanical ventilation, coma, delirium and post-ICU discharge disposition. Research regarding PICS in children and possible preventative or therapeutic intervention is insufficient as yet. This narrative review provides available information for modification and further research on the ABCDEF-Bundles for use in critically ill children.
Material and Methods: A narrative review of existing literature was used.
Results: One obvious distinction to adult patients is the wide range of different developmental stages of children and the even closer relationship between patient and family. Evidence for pediatric ABCDEF-Bundles is insufficient and input can only be collected from literature regarding different subsections and topics.
Conclusion: In addition to efforts to improve analgesia, sedation and weaning protocols with the aim of prevention, early detection and effective treatment of withdrawal symptoms or delirium, efforts are focused on adjusting ABCDEF bundle for the entire pediatric age group and on strengthening families' decision-making power, understanding parents as a resource for their child and involving them early in the care of their children.
Introduction
Within the last decade, long term complications after intensive care therapy have moved further into focus for both adult and, in later years, pediatric patients (1).
Measurement of outcome parameters in pediatric intensive care patients has been performed for decades, including the development of different scales and questionnaires like the Pediatric Overall and Cerebral Performance Categories (POPC, PCPC) and Functional Status Scale (FSS) (2, 3).
Using these tools, assessment of outcome after pediatric intensive care has shown a decrease in mortality from 5.8% in 1989/1990 to 4.6% published in 2000 down to 2.4% in 2014 (1, 3, 4).
In that same study, Pollack et al. (1) found the rate of significant new morbidities to be 4.8%, double that of mortality, and concluded “that pediatric critical care may have exchanged mortality for morbidity over the last several decades”.
In light of these developments, long-term survival and health related quality of life have moved further into focus.
Both the event leading up to the ICU-stay (congenital or acquired, traumatic or medical) and the repeated trauma caused by necessary interventions and therapies have long lasting effects on patients and their families. In adult patients, long-term consequences of intensive care treatment have been recognized as a relevant problem with an increasing focus on its prevention during treatment (5, 6). Lately, research and knowledge regarding pediatric patients and their families is increasing in this regard, reliable methods for prevention and treatment however are still lacking (7, 8).
The associated combination of debilitating symptoms following long-term or deep sedation, mechanical ventilation and forced immobilization has been identified and described by Needham et al. (5) as post intensive care syndrome (PICS). It includes significant physical (pulmonary, neuromuscular, and physical function), cognitive (“critical illness–related brain injury,” memory loss, lack of concentration, learning impairment) and mental/emotional (PTSD, fear, or anxiety disorder) problems and disorders which last long after discharge. Up to 64% of surviving adult ICU-patients without preexisting impairment suffer from one or more of these aspects (9). Extremely relevant for long-term outcome for instance is ICU-acquired weakness, characterized by symmetric myo-and polyneuropathy. It affects up to 67% of patients on mechanical ventilation at time of awakening and oftentimes persists after discharge (10).
Critically ill patients can present with problems from all categories. Additionally, relatives and caretakers often suffer from mental or emotional long term impairment such as anxiety or post-traumatic stress disorder (11). This phenomenon is described as PICS-F for “family” and affects relatives of up to 75% of patients (5, 12).
PICS in adult patients has been studied in depth and several projects have made it their goal to improve treatment and avoid its development altogether. A collaboration of intensive care professionals has developed the so called ABCDEF-Bundles, a number of measures meant to prevent PICS in both patients and their families (6).
They consist of several evidence-based treatment options meant to prevent or, if necessary, treat symptoms of PICS.
The ABCDEF-Bundles include (A) assessment, prevention and management of pain, (B) spontaneous awakening and breathing trials (SAT/SBT), (C) choice of analgesia and sedation, (D) assessment, prevention, and management of delirium, (E) early mobility and exercise and (F) family engagement and empowerment.
Since their first description, the bundles have emerged as a well-founded system for liberating patients from mechanical ventilation and improving long-term outcome.
Their implementation has been shown to be very effective in caring for critically ill adult patients, showing, among others, improvement in survival and disposition at time of discharge, reduction in time on mechanical ventilation, use of physical restraints and occurrence of delirium (13, 14).
As all elements are overlapping or interconnecting at some point, they have shown to be most effective when implemented together (13).
The successful implementation of these bundles and their increasing incorporation in routine adult critical care leads to the assumption that a similar paradigm shift in pediatric critical care is urgently needed.
A well-known problem in pediatrics is a delay in the introduction of new therapies for children (15).
Studies on ABCDEF-Bundles in children are still rare. Reliable recommendations for prevention and treatment of PICS in children have not been developed.
Studies show the effect of one to three bundles for use in children, confirming that scoring and treatment for delirium and early mobility can be successfully implemented with positive results (16).
A survey conducted in 15 European countries shows a high variation by region concerning implementation of individual bundles in pediatric intensive care units (17).
In 2020 Walz et al. (18) published a review regarding ABCDEF-Bundles for use in children. They conclude that ABCDEF-Bundles are suspected to be of similar use in children as in adults, even though clinical studies to their effect still need to be conducted. For all aspects they recommend establishing protocols and multidisciplinary teams for implementation of bundles in pediatric critical care. There are no further recommendations on changes that might need to be made in order to adapt the bundles for use in children (18).
The prevalent use of deep sedation and prolonged immobilization in treating critically ill children contributes to physical impairment like ICU-acquired weakness, mental problems following delirium and poor neurocognitive outcome.
In addition, Manning et al. (8) described one difference in PICS between adult and pediatric patients concerning the involvement of families. Besides known emotional and mental disorders such as PTSD or anxiety, which occur in families of adult patients as well, families of critically ill children suffer severe challenges to social interactions which affect both parents and siblings in their daily life.
Following the well-known adage “children are not small adults,” ABCDEF-Bundles like any new therapies and methods, need to be adjusted for use in children, taking into account flexibility for a broad range of developmental stages.
There is scientific evidence for some aspects of the Bundles for use in children, for others further research is needed. In this publication, an overview of existing literature and methods is given as well as suggestions for further development.
Materials and Methods
Based on a narrative review of the existing literature on PICS and ABCDEF-Bundles in adult and pediatric patients, ABCDEF-Bundles are reevaluated and adapted for use in children by adjusting them as much as possible according to existing scientific evidence.
Taking into consideration current scientific evidence on analgesia and sedation, mechanical ventilation, management of delirium, mobilization and family involvement, pediatric ABCDEF-Bundles are being developed for implementation in the treatment of critically ill children.
Data sources: A systematic search of PubMed database was undertaken for full articles pertaining to ABCDEF Bundle and PICS, case series, observational and cohort studies and randomized controlled trials were included.
Study selection: No language or date barriers were set. Studies that met the following eligibility criteria were included: The study design aimed to describe the prevalence of PICS and the causes resulting from critical care treatment, as well as the description and effectiveness of ABCDEF Bundle on outcome.
Data extraction: Data were extracted by the primary researcher and accuracy checked by coauthors.
Data synthesis: A narrative synthesis was undertaken.
Results and Discussion
For adult patients in critical care medicine, research shows significant effects of ABCDEF- bundle-implementation on survival, mechanical ventilation, coma, delirium, and post-ICU discharge disposition.
For children, a recent survey showed an implementation of all aspects in only 9% of 161 PICUs in the US, Canada, Brazil and Europe (19). Although there are calls for implementation of these measures in pediatric intensive care, as with most medical developments, the use of adult therapies in children without crucial changes beyond adaptation for size and body weight has not been effective (18).
Therefore, adjustments are necessary where proven methods in adults do not show the same results in pediatrics.
Before addressing each of the elements, a framework for implementation needs to be established. In analyzing adherence to ABCDE-Bundles (not including “F” for family involvement and empowerment) the complexity of combined ABCDE-Bundles has been identified as one major obstacle to adherence to bundles. On first impression, bundles are associated with an increased workload within an already stressful work environment. Studies and reports to this effect are difficult to compare, as variables are not clearly defined and success or adherence is rated differently across publications (20).
Other aspects identified include concerns over patient stability or safety, providers lack of knowledge regarding reasons and goals behind bundles, unclear or difficult to follow protocols and lack of coordination within inter-professional teams (21).
In order to improve compliance with guidelines and facilitate implementation of bundles in daily critical care routines, structured and repeated training of all professionals involved is a necessity. Continuous reinforcement can be assured by establishing champions within the team, taking on responsibility for adherence to protocols and acting as intermediaries in case of doubt or questions as to the procedures. Protocols need to clearly define methods for assessment, prevention and treatment of symptoms, assign responsibility for different aspects to all professions involved and therefore dividing the burden of perceived increase in workload on many shoulders (20).
Clearly structured documentation within already established patient records without need for additional systems help monitor adherence as well as results and enable reevaluation and adjustment of bundles.
An analysis of ABCDEF-Bundle use in critically ill adult patients showed a dose and response effect, with an increase in effect dependent on the amount of bundle aspects implemented. While all aspects are at some point connected and have synergistic effects when used in combination, we therefore stipulate, that use of just some aspects should always be preferred over not using any at all because of limited resources (13, 16).
In 2016, Yaghmai et al. (22) demonstrated a deterioration in adherence to nurse-controlled sedation protocols after initial successful implementation, showing the need for continuous efforts in training and monitoring.
Considering the widely acknowledged problem of a “theory-to-practice gap” in all fields of academic study, including medical research, nursing science and others, the process of implementing some or all bundles should be guided by current recommendations from implementation science in order to reach permanent use and effectiveness (23, 24).
Assessment, Management, and Prevention of Pain and Choice of Sedation
Guidelines on analgesia and sedation differ according to regions and availability of substances and protocols should be adjusted accordingly.
Disoprivan, for instance, is recommended for use in children for up to 48 h within the United States but its use is not allowed for long-term sedation in Europe because of risk for propofol infusion syndrome in children under the age of 16 (25, 26).
We advocate for nurse-controlled protocols primarily using opioid infusion supplemented by alpha-2-agonists. Additionally, non-opioid drugs should be used for mild to moderate pain without the need for further sedative effect (25). Spinal anesthesia has been shown to effectively reduce opioid use in pediatric postoperative patients and has a significant benefit in providing hemodynamic stability in infants after surgery (27, 28).
In both adult and pediatric care, assessment of pain can best be accomplished by self-reporting using the numeric rating scale or visual analog scale. Unfortunately, in pediatric intensive care patients are oftentimes unable to participate due to either severity of illness or physiological developmental stages. There are several Scores available for use in such cases, i.e., the FLACC-Score or, in German speaking countries, the so called KUS-Skala (kindliche Unbehagens- und Schmerzskala) (29). They are validated for use in children <4 years and can also be used in older children with neurologic or developmental impairment (30, 31). All these scales are scored with points between 0 and 10 with any score ≥4 being seen as a reason for intervention. For postoperative assessment of sedated and even intubated children of all ages the Comfort-B-Scale is also available (32).
In general, the choice of scoring tool is not as important as the fact of scoring at all. It is recommended to evaluate pain regularly, we suggest every 8 h or more often in case of manifest pain and after intervention. Additionally, children under continuous analgesia and sedation can be scored using non-verbal scales in an attempt to differentiate between pain and undersedation for more appropriate intervention (33).
Prevention of pain should be achieved by using analgesia before any kind of potentially painful procedures, including endotracheal suction, blood draws, or other routine interventions.
Closely connected to bundle “A” is bundle “C,” Choice of sedation, which can also be achieved by implementing a protocol for analgesia and sedation.
In order to reduce stress and anxiety of patients as well as the safety risk to patients dependent on mechanical ventilation and catheters, undersedation needs to be avoided. On the other hand, oversedation carries the risk of prolonged mechanical ventilation, hemodynamic difficulties and an increase in withdrawal and delirium.
The goal should be sedation by continuous drug infusion which provides for patients in comfort, who are tolerating mechanical ventilation but are awake enough to perceive some of their environment and to communicate any discomfort which may be eliminated without the need for further sedation. In older children and adolescents, communication via drawing or writing should be made possible if tolerated by the patients.
The goal in both children and adults is the prevention of over- and undersedation with the long-term effect of reduction in withdrawal and delirium. For children, midazolam has been shown to increase delirium, decrease quality of sleep and prolong both length of mechanical ventilation and length of stay in the PICU. Most importantly, they have emerged as an independent risk factor for the development of pediatric delirium (34). While not all studies show a reduction in length of mechanical ventilation after implementation of sedation protocols, they do show a decrease in days with pain, withdrawal or delirium (35, 36). Use of sedation protocols has been shown to help in reducing use of benzodiazepines, support the interdisciplinary communication in order to set and manage goals of sedation and to lessen the presentation of iatrogenic withdrawal symptoms (37).
Several studies have shown alpha-2-agonists like dexmedetomidine and clonidine to have a sedative effect leading to a reduction in opioid- and benzodiazepine-requirement. At the same time, they prove to be less neurotoxic than other substances and lead to a lower occurrence in withdrawal and delirium (38, 39).
Protocols therefore should call for the sparing use of benzodiazepines in critically ill children, using opiates and alpha-2-agonist clonidine for firstline treatment (25, 33). Even with our knowledge of side effects and negative long-term effects, there are still patients who are sedated using benzodiazepines. In 2018 Shildt et al. could show that even with successful implementation of a benzodiazepine-sparing protocol, 30% of patients received midazolam infusion after sedation was found to be insufficient. The authors discuss whether some of those patients might have suffered from undetected delirium and question the influence of the practitioners' comfort with established routines using midazolam (40).
For any protocol based on titration of dosage to the effective level, there can be a reluctance in timely reduction. Therefore, regular scoring should involve active reevaluation of possible oversedation and protocols should call for attempted reduction in calm children.
The problem of iatrogenic withdrawal syndrome after long-term sedation is not included within the original ABCDEF-Bundles for adult patients. In 2019, Arroyo-Nonoa et al. (41) found only 8 works on IWS in adult critical care patients, with two published between 1998 and 2016 and 6 between 2017 and 2019. In contrast, this is one aspect where pediatric research is more advanced, having introduced and validated scoring tools for early detection (42, 43), after showing it to lead to relevant stress for both patients and parents (19). Additionally, IWS has been shown to be an independent risk factor for development of delirium, which in turn factors heavily within the long-term effects of critical care treatment (see bundle D below).
Nurse driven protocols for analgesia and sedation including tapering schedules contribute to the reduction in IWS (44). We therefore recommend using standardized IWS-scoring at least every 8 h, for instance using the Sophia observation of withdrawal score (Sophia observation and withdrawal score—pediatric delirium in conjunction with delirium screening) (43).
A possible strategy for the avoidance of oversedation might lie within increased family involvement in taking care of mechanically ventilated patients where nurse-to-patient ratios do not suffice for individualized care.
Both Spontaneous Awakening and Spontaneous Breathing Trials
A significant deviation occurs in adjusting bundle “B” for use in pediatric patients. In the original ABCDEF-Bundles for adult patients, “B” stands for “both spontaneous awakening and spontaneous breathing trials,” therefore tying it in closely between bundles A and C. It describes a standardized protocol for pauses in sedation and mechanical ventilation for assessing the patient while alert for any extubation readiness (6). In pediatric patients, a careful risk-benefit-analysis has to be performed. While mechanical ventilation is a vital part of critical care medicine, prolonged use brings with it risks such as need for deeper sedation, followed by hemodynamic instability, immobilization and infection, in turn leading back to a prolonged mechanical ventilation and length of PICU-stay (45–47).
Regular spontaneous awakening and breathing trials with pause in all sedation have been successful in reducing time on mechanical ventilation, length of PICU-stay and cumulative dosis of sedatives (45), but have not been proven effective in terms of short-term health related quality of life (48). Instead, compared to use of standardized sedation protocols with continuous reduction in sedation (40, 49), Vet et al. (50) showed daily sedation interruption in addition to protocolized sedation to increase mortality in critically ill children when compared to those under protocolized sedation only. There were no added benefits for clinical outcome in the combined group.
On the other hand, continuous titration of sedation might lead to a hesitancy in reducing sedatives after reaching a comfortable dosage and prolonging sedation, mechanical ventilation and length of stay (22). Likewise, it has been found that protocols for weaning from mechanical ventilation should include clear instructions for when to start reducing parameters in order to avoid unnecessary delay (51).
An early study on weaning and extubation readiness has shown a high percentage of children to be ready for extubation on their first extubation readiness test, suggesting a lack of extubation readiness tests in early stages of treatment and the danger of unnecessarily prolonged ventilation (52). Additionally, upper airway obstruction ranged as a main factor for extubation failure, which cannot be detected by spontaneous breathing trials (52).
However, for patients with congenital heart disease, spontaneous breathing trials and daily extubation readiness tests proved effective in reducing extubation failure and length of PICU-stay (53, 54).
We therefore propose focusing any aspects concerning analgesia and sedation within bundle A and renaming bundle B as “Breathing and mechanical ventilation” for use in pediatric critical care. We advocate for a proactive and continuous weaning protocol with standardized daily reevaluation of mechanical ventilation and assessment for weaning, regular reduction of ventilator parameters in conjunction with protocolized reduction in sedation once feasible and daily extubation readiness tests for identification for extubation as early as possible in hopes of further reducing time on the ventilator and maybe even length of stay on the PICU (52). In support of this goal, early use of non-invasive ventilation should be considered.
Assessment, Management and Prevention of Delirium
Delirium is a significant complication in critically ill children consisting of several symptoms of acute cerebral dysfunction. It has been found in up to 66% of patients in PICUs and is associated with prolonged time on mechanical ventilation, higher use of sedatives and physical restraints and leads to an increase in mortality as well as a reduction of health-related quality of life (55–57).
As with pain and sedation, regular assessment by using validated tools is the key for adequate management. Delirium remains underdiagnosed and misinterpreted in children and therefore undertreated, especially as children in hypoactive delirium are often seen as just especially calm and “easy” to comfort (58). All children admitted to a PICU should be subject to routine screening for withdrawal and early detection of symptoms and diagnosis of both hypo- and hyperactive as well as mixed forms of delirium in children
Although there are several possible scoring systems, such as the widely used tools of the Cornell Assessment of Pediatric Delirium (CAPD) or the Pediatric Confusion Assessment Method—Intensive Care Unit (pCAM-ICU) (59, 60), there is advantage in using the Sophia observation withdrawal—pediatric delirium assessment (SOS-PD). It has been validated for use in all pediatric age groups and, more importantly, differentiates between symptoms of withdrawal and delirium (61).
There are several modifiable risk factors for delirium in critically ill children, including mechanical ventilation, use of benzodiazepines as long-term sedatives, physical restraints, noise pollution and a lack of adequate nutrition which need to be considered in treatment (55, 57, 62, 63). On the other hand, we have no influence on independent factors such as age, sex, or severity and type of illness (34).
Delirium bundles have already been developed and described in detail.
Early use of non-pharmacological measures such as helping the children to reorient themselves after sedation, providing glasses and hearing aids and toys from home can prevent development of delirium or go a long way in treating symptoms that have already manifested (64). One most promising aspect in prevention of delirium presents standardized analgesia and sedation, which aims at a reduction in dosage (especially concerning benzodiazepines and anticholinergic substances) and a shortening in length of sedation (65).
The most important factors include treatment within a calm and comforting environment, including the presence of pictures or toys from home and the continuous care by a parent or other close caregiver. Orientation (or reorientation) in space and time should be encouraged by use of hearing aids and glasses, clocks and calendars and upright positioning in bed where tolerated (66, 67).
In severe cases, using low dose antipsychotic drugs as off-label medication (i.e., Quetiapine, Levomepromazine) might be feasible, but high quality studies to their affect are still lacking. Available studies show a high risk of side effects like extrapyramidal symptoms and changes in corrected QT-time (68–70). After close consideration in each case, benefits may outweigh the risks and should not be discounted completely. Nevertheless, these results emphasize the importance of non-pharmacological treatment of delirium and the necessity of a change of culture in pediatric intensive care toward prevention of delirium in critically ill children (67).
Child life specialists and other specialists should be present on the ward in order to treat patients, support and educate families and help train all other staff in dealing with delirium in critically ill children.
Early Mobility and Exercise
“E” stands for early mobilization in critically ill patients. It has been shown to have a positive effect on body function, reducing limitations on activity and improving muscle strength and ability to walk (71).
While literature shows a solid scientific foundation for adult patients, implementation in PICUs is lacking (19). Interestingly, within this field of study there are more reviews available than clinical studies (72).
Additionally, in a recent review Nydahl et al. analyzed 33 reviews concerning early mobilization in critically ill patients. Out of these, only 3 were analyzing studies concerning pediatric patients (72).
Restrictions in time and space, lack of personnel and fear of adverse events such as dislodging of endotracheal tubes or central venous catheters all present (real and perceived) barriers to mobilization of critically ill children (17, 73, 74).
Throughout the available literature, a timeframe for early mobilization is not clearly defined (75). For children however, both Wieczorek and Choong have defined early mobilization as starting within 72 h of admission, starting with assessment for mobilization within 24 h (76, 77).
Depending on severity of illness, state of sedation and clearly indicated restrictions in movement or instabilities because of trauma or surgery, mobilization can be implemented as passive, assisted active or active mobilization (72, 76).
As with all other bundles, key is establishing a reliable protocol for daily review of the patients' goals, clear documentation of reached milestones for continuity of care and a multidisciplinary approach including rehabilitation specialists, nurses, doctors and parents, communicating different perspectives and defining common goals in daily rounds. Having a standardized protocol instead of individualized plans is associated with improved outcome and lessens the risk of implicit bias in planning the therapeutic approaches (76, 78).
Reinforcing the importance of the last of the bundles, F for “Family”, family presence has shown a marked influence on successful out-of-bed-mobilization for children (aOR 7.83). Unfortunately, the same study showed about one quarter of patients in pediatric intensive care to be completely immobilized (17).
Several international initiatives advocate for standardized early mobility in children, making a solid case for implementation in all PICUs, showing it to be safe and feasible, even in low resource regions (17, 77).
Family Engagement and Empowerment
Family education and empowerment are listed last within the concept of pediatric ABCDEF-Bundles but represent a key element. While PICS is relevant in critically ill children, trauma, or sudden illness of a child has a significant impact on all members of the household and other close relatives. Post-traumatic stress disorder, anxiety and sleep disorders in parents can disrupt daily life for the whole family for a long time after discharge, including for patients who leave the hospital without permanent impairment (8, 79).
In adult critical care, family engagement includes participation in rounds, ethics and palliative care consultations as well as the offer of being present during traumatizing situations such as CPR. All aspects have been shown to be beneficial for patients and family as well as staff (6).
A common reason for psychological long-term difficulties of family members is the parents' feeling of helplessness and lack of information experienced when coping with their child's illness (80).
In 2020 the EU PARK-PICU study evaluated family centered care by questioning if 24-h-presence by family members at bedside was possible (17). However, Meert et al. (81) described a much broader approach to family centered care in pediatric intensive care based on the recommendation by the American Academy of Pediatrics, calling for “an innovative approach to the planning, delivery, and evaluation of health care that is grounded in a mutually beneficial partnership among patients, families and providers that recognizes the importance of the family in the patient's life”.
Among the core aspects are open visitation hours for parents and individually prepared bedside visits by siblings. Even before the current pandemic led to restrictions in visitation all over the world, 24-h-attendence was made possible in about 88% of PICUs considering hospitals in the US, Canada, Brazil and Europe. Unfortunately, in Europe less than half of PICUs reported permitted 24 h presence by family members (19).
Apart from open doors and the theoretical possibility of being present 24 h a day, parents most benefited from having a place to sleep at the hospital and involvement in daily patient care as per their parental role as primary caregiver.
Another aspect are family centered rounds, including parents in case presentations and discussions at bedside after first informing parents of the purpose behind these rounds. Problems perceived by staff such as lack of teaching, inhibition regarding the discussion of difficult topics or prolonged time for rounds receded after adequate education among staff as to the benefits of family centered rounds.
Third, the offer of being present for traumatic events like CPR and for invasive procedures is included in family centered care. As with most other described aspects, education of all professionals involved and open communication with parents is key for success. Once those needs are met, family members overwhelmingly prefer being present and one study could show that parents present during CPR benefited by exhibiting less signs of intrusive thoughts, prolonged symptoms of grief and post traumatic avoidance behavior (82, 83).
One valuable tool is available in pediatric ICU-diaries (84). Adapted from adult intensive care as well, ICU-diaries enable parents to record impressions, information and feelings for later review and age-appropriate sharing with the patient after discharge. It has been shown to be beneficial to both parents and relatives for dealing with their own trauma as well as for patients in filling in gaps in memory (85).
Additionally, ICU-Diaries provided a helpful tool in not only enabling parents and patients to review and understand lived experiences, but also provided support in explaining difficult information to siblings and other relatives (86).
Unfortunately, current restrictions (in space and due to the pandemic) do not allow for 24-h presence of parents or other close relatives at the bedside. However, efforts should go toward lifting of any set visiting hours and allow extended presence of parents with children who are expected to benefit in terms of reduction of sedatives and improvement of psychological wellbeing. It must be taken into account that, for example, the presence of the family is most positively associated with mobilization out of bed, and probably with many other measures as well (17).
For long-term consideration, we propose further modifying and expanding the pediatric ABCDEF-Bundles.
A common denominator within all ABCDEF-Bundles is the topic of communication. Not exclusively regarding children but with a special emphasis within pediatric intensive care, parents and other caregivers are speaking for our patients and need to be included in all aspects of their care and relevant decision making. This necessitates a high level of information and discourse. Parallel to adult patients, communication with the patients themselves is another aspect needing consideration. And within any multiprofessional team such as those in critical care, communication between team members is of utmost importance. In a letter to the editor, Patak et al. (87) wrote “Perhaps communication should be a vital sign,” noting the lack of standardized assessment and documentation of patient communication. For further development of pediatric ABCDEF-Bundles we propose following their suggestion and renaming bundle “C” for Communication (87), including necessary education and training protocols for staff (37), standardized systems for information and discussion with parents including informational packets in different languages and reliable access to translator services where needed, implementation and further development of age appropriate communication strategies and tools for intubated or otherwise impaired critically ill children and providing support and infrastructure for calls and video communication with siblings and friends who are unable to visit. Considering the relevant psychological impact any trauma or severe illness has on parents, siblings and other relatives, psychologists should be an integral part of any PICU-team. Professional support for both patients and relatives might help early diagnosis and treatment of associated illness such as depression and anxiety and reduce long-term effects (12). Additionally, crisis-intervention training should be considered for any health care professional within the critical care setting.
The original “C” for choice of sedation will be incorporated into bundle A, being renamed “Analgesia and Sedation.”
Concerning this new bundle “C” as well as the established aspect “F,” several studies have looked at possibilities of health informational technologies in pediatric intensive care. Based on evidence, that most parents prefer receiving all information concerning their child's health as soon as it is available, rather than summarized at greater intervals, the effect of interactive monitors showing electronic health records for use by parents was evaluated and suggests an improvement in awareness for parents and support in informed decision making (88). In 2016, Brown et al. (89) also found the offer of electronic information tools within the ICU to be welcomed by both patients and relatives for receiving updated medical information for review.
Possibilities within this field seem endless, offering further options for improving individualized support for families, providing general as well as specific information and giving parents and caregivers the opportunity to review given information on their own terms and without the time constraints (real or perceived) often imposed on short updates by medical professionals.
In another bid for expansion of ABCDEF-Bundles in pediatrics, Choong et al. (76) mention “G” for good nutrition and H for humanistic medicine.
Without question, physiological nutrition, and healthy sleep patterns are fundamental needs for children recuperating from severe illness and to prevent further deterioration (90). For a newly developed bundle G we therefore propose to include, again, standardized protocols for daily reevaluation of nutritional needs, determining severity of illness, weighing parenteral against early enteral nutrition, defining caloric needs and identifying patients in need of rehabilitational specialists for assessment and treatment of feeding and swallowing difficulties (91, 92). Next to “Good nutrition” we include “good sleep” and promote early support of a circadian rhythm, moving any possible intervention and diagnostics aside from emergencies into daylight hours and providing a calm and dark environment for uninterrupted sleep during nighttime for all children, irrelevant of their depth of sedation (25).
For a newly minted bundle “H”, however, we propose focusing on “home care,” using a humanistic approach throughout all bundles (Figure 1). For very few patients, their illness and treatment ends with discharge from PICU. Instead, more days on other wards within the hospital are often followed by ambulatory treatment and rehabilitation, including home care services, pediatricians and specialists, physiotherapy and many more. Other than during hospital stay, most of these different aspects often have to be coordinated by parents and caregivers themselves, which is made complicated by a scarcity of providers, especially in rural areas.
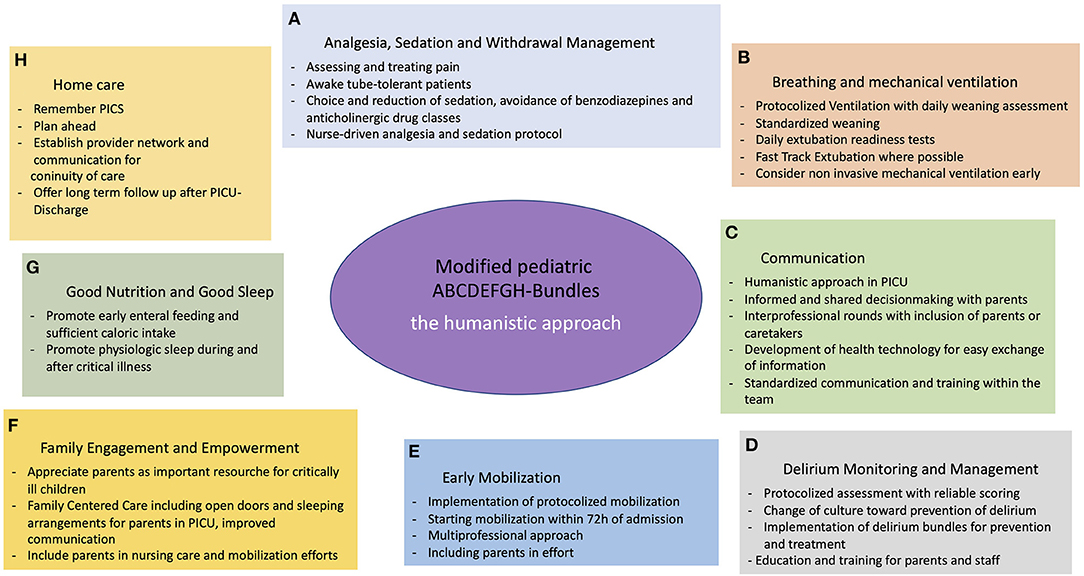
Figure 1. Modified pediatric ABCDEFGH-bundles—the humanistic approach. (A) Analgesia and Sedation; (B) Breathing and mechanical ventilation; (C) Communication; (D) Delirium Monitoring and Management; (E) Early Mobilization; (F) Familie Engagement and Empowerment; (G) Good Nutrition and Good Sleep; (H) Home care.
Long-term problems such as PICS or PTSD are often overlooked in these situations, leaving families without necessary support and treatment.
In order to ease this burden, we propose establishing follow-up services for parents and patients including screening for the development of PICS after discharge and coordinating services for rehabilitation specialists and other long-term health care providers. Comparable with ambitions in improving communication within the PICU-setting, this presents another aspect where health related technology should be developed, providing networking possibility and simplifying communication between providers for improved continuity of care after PICU discharge.
Conclusion
While further studies are needed and in progress for the evaluation of long-term benefits of ABCDEF-Bundles in pediatric critical care, there is sufficient evidence for modifying existing ABCDEF-Bundles from adult care for use in children.
For all entities it is paramount to use written protocols which include scoring and daily assessment for early detection of either symptoms of withdrawal, delirium or pain as well as readiness for extubation, early mobility or other opportunities for progress without delay. Standardized interdisciplinary rounds including parents or other caregivers shorten delays in communication and provide parents with valuable information and insight in their children's illness and therefore empower them to actively participate in their improvement.
Key aspect is continuous training of all professionals involved in order to shorten time to diagnosis for both patients and families at risk for PICS and other long-term difficulties. Evidence-based findings should also be established more quickly and more comprehensively in daily routine care, keeping in mind, that successful implementation of only parts of the complete set of bundles already shows benefit for the long-term outcome and expansion of measures can occur gradually and in accordance with individual resources and recommendations from implementation sciences. The complex interaction between the elements and the fast-developing scientific evidence within the separate entities requires any health care provider in pediatric intensive care medicine to stay up to date and adapt therapies and guidelines accordingly.
Author Contributions
JE, FN, and FB wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Open Access Publishing Fund of University of Tübingen.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pollack MM, Holubkov R, Funai T, Clark A, Berger JT, Meert K, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. (2014) 15:821–7. doi: 10.1097/PCC.0000000000000250
2. Pollack MM, Holubkov R, Glass P, Dean JM, Meert KL, Zimmerman J, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. (2009) 124:e18–28. doi: 10.1542/peds.2008-1987
3. Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. (1992) 121:68–74. doi: 10.1016/S0022-3476(05)82544-2
4. Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Crit Care Med. (2000) 28:1173–9. doi: 10.1097/00003246-200004000-00043
5. Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. (2012) 40:502–9. doi: 10.1097/CCM.0b013e318232da75
6. Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF bundle in critical care. Crit Care Clin. (2017) 33:225–43. doi: 10.1016/j.ccc.2016.12.005
7. Herrup EA, Wieczorek B, Kudchadkar SR. Characteristics of postintensive care syndrome in survivors of pediatric critical illness: a systematic review. World J Crit Care Med. (2017) 6:124–34. doi: 10.5492/wjccm.v6.i2.124
8. Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ. Conceptualizing post intensive care syndrome in Children-The PICS-p Framework. Pediatr Crit Care Med. (2018) 19:298–300. doi: 10.1097/PCC.0000000000001476
9. Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. (2018) 46:1393–401. doi: 10.1097/CCM.0000000000003218
10. Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. (2015) 19:274. doi: 10.1186/s13054-015-0993-7
11. Colville G, Pierce C. Patterns of post-traumatic stress symptoms in families after paediatric intensive care. Intensive Care Med. (2012) 38:1523–31. doi: 10.1007/s00134-012-2612-2
12. Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. (2012) 40:618–24. doi: 10.1097/CCM.0b013e318236ebf9
13. Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. (2019) 47:3–14. doi: 10.1097/CCM.0000000000003482
14. Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California Community Hospitals: implementing PAD Guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. (2017) 45:171–8. doi: 10.1097/CCM.0000000000002149
15. Quintana Grijalba C, Rhee E, Zimmerman JJ. The ICU liberation ABCDEF bundle: utilization among critically ill little adults. Crit Care Med. (2022) 50:163–5. doi: 10.1097/CCM.0000000000005214
16. Di Nardo M, Boldrini F, Broccati F, Cancani F, Satta T, Stoppa F, et al. The LiberAction project: implementation of a pediatric liberation bundle to screen delirium, reduce benzodiazepine sedation, and provide early mobilization in a human resource-limited pediatric intensive care unit. Front Pediatr. (2021) 9:788997. doi: 10.3389/fped.2021.788997
17. Ista E, Scholefield BR, Manning JC, Harth I, Gawronski O, Bartkowska-Sniatkowska A, et al. Mobilization practices in critically ill children: a European point prevalence study (EU PARK-PICU). Crit Care. (2020) 24:368. doi: 10.1186/s13054-020-02988-2
18. Walz A, Canter MO, Betters K. The ICU liberation bundle and strategies for implementation in pediatrics. Curr Pediatr Rep. (2020) 8:69–78. doi: 10.1007/s40124-020-00216-7
19. Ista E, Redivo J, Kananur P, Choong K, Colleti J Jr, Needham DM, et al. ABCDEF bundle practices for critically ill children: an international survey of 161 PICUs in 18 countries. Crit Care Med. (2022) 50:114–25. doi: 10.1097/CCM.0000000000005168
20. Boehm LM, Dietrich MS, Vasilevskis EE, Wells N, Pandharipande P, Ely EW, et al. Perceptions of workload burden and adherence to ABCDE bundle among intensive care providers. Am J Crit Care. (2017) 26:e38–e47. doi: 10.4037/ajcc2017544
21. Costa DK, White MR, Ginier E, Manojlovich M, Govindan S, Iwashyna TJ, et al. Identifying barriers to delivering the awakening and breathing coordination, delirium, and early exercise/mobility bundle to minimize adverse outcomes for mechanically ventilated patients: a systematic review. Chest. (2017) 152:304–11. doi: 10.1016/j.chest.2017.03.054
22. Yaghmai BF, Di Gennaro JL, Irby GA, Deeter KH, Zimmerman JJ. A pediatric sedation protocol for mechanically ventilated patients requires sustenance beyond implementation. Pediatr Crit Care Med. (2016) 17:721–6. doi: 10.1097/PCC.0000000000000846
23. Brant JM. Bridging the research-to-practice gap: the role of the nurse scientist. Semin Oncol Nurs. (2015) 31:298–305. doi: 10.1016/j.soncn.2015.08.006
24. Bauer MS, Kirchner J. Implementation science: what is it and why should I care? Psychiatry Res. (2020) 283:112376. doi: 10.1016/j.psychres.2019.04.025
25. Smith HAB, Besunder JB, Betters KA, Johnson PN, Srinivasan V, Stormorken A, et al. 2022 Society of Critical Care Medicine Clinical Practice Guidelines on Prevention and Management of Pain, Agitation, Neuromuscular Blockade, and Delirium in Critically Ill Pediatric Patients With Consideration of the ICU Environment and Early Mobility. Pediatr Crit Care Med. (2022) 23:e74–110. doi: 10.1097/PCC.0000000000002873
26. Arzneimittelkommission. Mitteilungen: Schwere unerwünschte Arzneimittelwirkungen nach Propofol-Infusionen zur Sedierung. Dtsch Arztebl. (2004). 101:A-3447/B-2911/C-2759.
27. Tao B, Liu K, Wang D, Ding M, Yang N, Zhao P. Perioperative effects of caudal block on pediatric patients in laparoscopic upper urinary tract surgery: a randomized controlled trial. BMC Pediatr. (2019) 19:427. doi: 10.1186/s12887-019-1812-0
28. Oberlander TF, Berde CB, Lam KH, Rappaport LA, Saul JP. Infants tolerate spinal anesthesia with minimal overall autonomic changes: analysis of heart rate variability in former premature infants undergoing hernia repair. Anesth Analg. (1995) 80:20–7. doi: 10.1213/00000539-199501000-00005
29. Buttner W, Finke W, Hilleke M, Reckert S, Vsianska L, Brambrink A. [Development of an observational scale for assessment of postoperative pain in infants]. Anasthesiol Intensivmed Notfallmed Schmerzther. (1998) 33:353–61. doi: 10.1055/s-2007-994263
30. Giordano V, Edobor J, Deindl P, Wildner B, Goeral K, Steinbauer P, et al. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: a systematic review. JAMA Pediatr. (2019) 173:1186–97. doi: 10.1001/jamapediatrics.2019.3351
31. Manworren RC, Stinson J. Pediatric pain measurement, assessment, and evaluation. Semin Pediatr Neurol. (2016) 23:189–200. doi: 10.1016/j.spen.2016.10.001
32. Boerlage AA, Ista E, Duivenvoorden HJ, de Wildt SN, Tibboel D, van Dijk M. The COMFORT behaviour scale detects clinically meaningful effects of analgesic and sedative treatment. Eur J Pain. (2015) 19:473–9. doi: 10.1002/ejp.569
33. Amigoni A, Conti G, Conio A, Corno M, Fazio PC, Ferrero F, et al. Recommendations for analgesia and sedation in critically ill children admitted to intensive care unit. J Anesth Analg Crit Care. (2022) 2:9. doi: 10.1186/s44158-022-00036-9
34. Mody K, Kaur S, Mauer EA, Gerber LM, Greenwald BM, Silver G, et al. Benzodiazepines and development of delirium in critically ill children: estimating the causal effect. Crit Care Med. (2018) 46:1486–91. doi: 10.1097/CCM.0000000000003194
35. Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. (2015) 313:379–89. doi: 10.1001/jama.2014.18399
36. Blackwood B, Tume LN, Morris KP, Clarke M, McDowell C, Hemming K, et al. Effect of a sedation and ventilator liberation protocol vs usual care on duration of invasive mechanical ventilation in pediatric intensive care units: a randomized clinical trial. JAMA. (2021) 326:401–10. doi: 10.1001/jama.2021.10296
37. Balit CR, LaRosa JM, Ong JSM, Kudchadkar SR. Sedation protocols in the pediatric intensive care unit: fact or fiction? Transl Pediatr. (2021) 10:2814–24. doi: 10.21037/tp-20-328
38. Hayden JC, Breatnach C, Doherty DR, Healy M, Howlett MM, Gallagher PJ, et al. Efficacy of alpha2-Agonists for sedation in pediatric critical care: a systematic review. Pediatr Crit Care Med. (2016) 17:e66–75. doi: 10.1097/PCC.0000000000000599
39. Andropoulos DB, Greene MF. Anesthesia and developing brains - Implications of the FDA warning. N Engl J Med. (2017) 376:905–7. doi: 10.1056/NEJMp1700196
40. Shildt N, Traube C, Dealmeida M, Dave I, Gillespie S, Moore W, et al. “Difficult to Sedate”: successful implementation of a benzodiazepine-sparing analgosedation-protocol in mechanically ventilated children. Children. (2021) 8:348. doi: 10.3390/children8050348
41. Arroyo-Novoa CM, Figueroa-Ramos MI, Puntillo KA. Opioid and benzodiazepine iatrogenic withdrawal syndrome in patients in the intensive care unit. AACN Adv Crit Care. (2019) 30:353–64. doi: 10.4037/aacnacc2019267
42. Franck LS, Scoppettuolo LA, Wypij D, Curley MAQ. Validity and generalizability of the Withdrawal Assessment Tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain. (2012) 153:142–8. doi: 10.1016/j.pain.2011.10.003
43. Ista E, de Hoog M, Tibboel D, Duivenvoorden HJ, van Dijk M. Psychometric evaluation of the Sophia Observation withdrawal symptoms scale in critically ill children. Pediatr Crit Care Med. (2013) 14:761–9. doi: 10.1097/PCC.0b013e31829f5be1
44. Neunhoeffer F, Kumpf M, Renk H, Hanelt M, Berneck N, Bosk A, et al. Nurse-driven pediatric analgesia and sedation protocol reduces withdrawal symptoms in critically ill medical pediatric patients. Paediatr Anaesth. (2015) 25:786–94. doi: 10.1111/pan.12649
45. Gupta K, Gupta VK, Jayashree M, Singhi S. Randomized controlled trial of interrupted versus continuous sedative infusions in ventilated children. Pediatr Crit Care Med. (2012) 13:131–5. doi: 10.1097/PCC.0b013e31820aba48
46. Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. (1998) 129:433–40. doi: 10.7326/0003-4819-129-6-199809150-00002
47. Zimmerman JJ, Harmon LA, Smithburger PL, Chaykosky D, Heffner AC, Hravnak M, et al. Choosing wisely for critical care: the next five. Crit Care Med. (2021) 49:472–81. doi: 10.1097/CCM.0000000000004876
48. Vet NJ, de Wildt SN, Verlaat CW, Mooij MG, Tibboel D, de Hoog M, et al. Short-term health-related quality of life of critically ill children following daily sedation interruption. Pediatr Crit Care Med. (2016) 17:e513–e20. doi: 10.1097/PCC.0000000000000956
49. Hanser A, Neunhoeffer F, Hayer T, Hofbeck M, Schlensak C, Mustafi M, et al. A nurse-driven analgesia and sedation protocol reduces length of PICU stay and cumulative dose of benzodiazepines after corrective surgery for tetralogy of Fallot. J Spec Pediatr Nurs. (2020) 25:e12291. doi: 10.1111/jspn.12291
50. Vet NJ, de Wildt SN, Verlaat CW, Knibbe CA, Mooij MG, van Woensel JB, et al. A randomized controlled trial of daily sedation interruption in critically ill children. Intensive Care Med. (2016) 42:233–44. doi: 10.1007/s00134-015-4136-z
51. Hartmann SM, Farris RW, Yanay O, DiBlasi RM, Kearney CN, Zimmerman JD, et al. Interaction of critical care practitioners with a decision support tool for weaning mechanical ventilation in children. Respir Care. (2020) 65:333–40. doi: 10.4187/respcare.06877
52. Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. (2009) 10:1–11. doi: 10.1097/PCC.0b013e318193724d
53. Abu-Sultaneh S, Hole AJ, Tori AJ, Benneyworth BD, Lutfi R, Mastropietro CW. An interprofessional quality improvement initiative to standardize pediatric extubation readiness assessment. Pediatr Crit Care Med. (2017) 18:e463–e71. doi: 10.1097/PCC.0000000000001285
54. Ferreira FV, Sugo EK, Aragon DC, Carmona F, Carlotti A. Spontaneous breathing trial for prediction of extubation success in pediatric patients following congenital heart surgery: a randomized controlled trial. Pediatr Crit Care Med. (2019) 20:940–6. doi: 10.1097/PCC.0000000000002006
55. Traube C, Silver G, Gerber LM, Kaur S, Mauer EA, Kerson A, et al. Delirium and Mortality in Critically Ill Children: Epidemiology and Outcomes of Pediatric Delirium. Crit Care Med. (2017) 45:891–8. doi: 10.1097/CCM.0000000000002324
56. Dervan LA, Killien EY, Smith MB, Watson RS. Health-related quality of life following delirium in the PICU. Pediatr Crit Care Med. (2021) 23:118–28. doi: 10.1097/PCC.0000000000002813
57. Silver G, Doyle H, Hegel E, Kaur S, Mauer EA, Gerber LM, et al. Association between pediatric delirium and quality of life after discharge. Crit Care Med. (2020) 48:1829–34. doi: 10.1097/CCM.0000000000004661
58. Siegel EJ, Traube C. Pediatric delirium: epidemiology and outcomes. Curr Opin Pediatr. (2020) 32:743–9. doi: 10.1097/MOP.0000000000000960
59. Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med. (2014) 42:656–63. doi: 10.1097/CCM.0b013e3182a66b76
60. Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, et al. Diagnosing delirium in critically ill children: validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med. (2011) 39:150–7. doi: 10.1097/CCM.0b013e3181feb489
61. Ista E, van Beusekom B, van Rosmalen J, Kneyber MCJ, Lemson J, Brouwers A, et al. Validation of the SOS-PD scale for assessment of pediatric delirium: a multicenter study. Crit Care. (2018) 22:309. doi: 10.1186/s13054-018-2238-z
62. Simone S, Edwards S, Lardieri A, Walker LK, Graciano AL, Kishk OA, et al. Implementation of an ICU Bundle: an interprofessional quality improvement project to enhance delirium management and monitor delirium prevalence in a single PICU. Pediatr Crit Care Med. (2017) 18:531–40. doi: 10.1097/PCC.0000000000001127
63. Weatherhead JR, Niedner M, Dahmer MK, Malas N, Owens T, Kawai Y. Patterns of delirium in a pediatric intensive care unit and associations with noise pollution. J Intensive Care Med. (2021) 2021:8850666211055649. doi: 10.1177/08850666211055649
64. Michel J. Implementation of a delirium bundle for pediatric intensive care patients. Front Pediatr. (2022) 10:826259. doi: 10.3389/fped.2022.826259
65. Madden K, Hussain K, Tasker RC. Anticholinergic medication burden in pediatric prolonged critical illness: a potentially modifiable risk factor for delirium. Pediatr Crit Care Med. (2018) 19:917–24. doi: 10.1097/PCC.0000000000001658
66. Rohlik G, Pfeiffer AJ, Collins CE, Parrett CR, Kawai Y. Improving pediatric delirium assessment documentation and implementation of a nonpharmacologic delirium management bundle in the cardiovascular intensive care unit. J Pediatr Nurs. (2021) 60:168–76. doi: 10.1016/j.pedn.2021.04.035
67. Michel J, Schepan E, Hofbeck M, Engel J, Simma A, Neunhoeffer F. Implementation of a delirium bundle for pediatric intensive care patients. Front Pediatr. (2022) 10:826259.
68. Sassano-Higgins S, Freudenberg N, Jacobson J, Turkel S. Olanzapine reduces delirium symptoms in the critically ill pediatric patient. J Pediatr Intensive Care. (2013) 2:49–54. doi: 10.3233/PIC-13049
69. Slooff VD, van den Dungen DK, van Beusekom BS, Jessurun N, Ista E, Tibboel D, et al. Monitoring haloperidol plasma concentration and associated adverse events in critically ill children with delirium: first results of a clinical protocol aimed to monitor efficacy and safety. Pediatr Crit Care Med. (2018) 19:e112–e9. doi: 10.1097/01.pcc.0000537953.48378.d2
70. Joyce C, Witcher R, Herrup E, Kaur S, Mendez-Rico E, Silver G, et al. Evaluation of the safety of quetiapine in treating delirium in critically ill children: a retrospective review. J Child Adolesc Psychopharmacol. (2015) 25:666–70. doi: 10.1089/cap.2015.0093
71. Tipping CJ, Bailey MJ, Bellomo R, Berney S, Buhr H, Denehy L, et al. The ICU mobility scale has construct and predictive validity and is responsive. A multicenter observational study. Ann Am Thorac Soc. (2016) 13:887–93. doi: 10.1513/AnnalsATS.201510-717OC
72. Nydahl P DR, Hermes C, Nessizius S, Moritz R. Evidenz der Frühmobilisierung. DIVI. (2021) 12:76–82. doi: 10.3238/DIVI.2012.0076-0082
73. Walker TC, Kudchadkar SR. Early mobilization in the pediatric intensive care unit. Transl Pediatr. (2018) 7:308–13. doi: 10.21037/tp.2018.09.02
74. Patel RV, Redivo J, Nelliot A, Eakin MN, Wieczorek B, Quinn J, et al. Early mobilization in a PICU: a qualitative sustainability analysis of PICU up! Pediatr Crit Care Med. (2021). 22:e233–42. doi: 10.1097/PCC.0000000000002619
75. Clarissa C, Salisbury L, Rodgers S, Kean S. Early mobilisation in mechanically ventilated patients: a systematic integrative review of definitions and activities. J Intensive Care. (2019) 7:3. doi: 10.1186/s40560-018-0355-z
76. Choong K, Canci F, Clark H, Hopkins RO, Kudchadkar SR, Lati J, et al. Practice recommendations for early mobilization in critically ill children. J Pediatr Intensive Care. (2018) 7:14–26. doi: 10.1055/s-0037-1601424
77. Wieczorek B, Ascenzi J, Kim Y, Lenker H, Potter C, Shata NJ, et al. PICU Up!: Impact of a quality improvement intervention to promote early mobilization in critically ill children. Pediatr Crit Care Med. (2016) 17:e559–e66. doi: 10.1097/PCC.0000000000000983
78. Morrow BM. Building a culture of early mobilization in the pediatric intensive care unit-a nuts and bolts approach. Transl Pediatr. (2021) 10:2845–57. doi: 10.21037/tp-20-324
79. Stremler R, Haddad S, Pullenayegum E, Parshuram C. Psychological outcomes in parents of critically ill hospitalized children. J Pediatr Nurs. (2017) 34:36–43. doi: 10.1016/j.pedn.2017.01.012
80. Abela KM, Wardell D, Rozmus C, LoBiondo-Wood G. Impact of pediatric critical illness and injury on families: an updated systematic review. J Pediatr Nurs. (2020) 51:21–31. doi: 10.1016/j.pedn.2019.10.013
81. Committee on Hospital Care and Institute for Patient-and Family-Centered Care. Patient- and family-centered care and the pediatrician's role. Pediatrics. (2012) 129:394–404. doi: 10.1542/peds.2011-3084
82. Meert KL, Clark J, Eggly S. Family-centered care in the pediatric intensive care unit. Pediatr Clin North Am. (2013) 60:761–72. doi: 10.1016/j.pcl.2013.02.011
83. Robinson SM, Mackenzie-Ross S, Campbell Hewson GL, Egleston CV, Prevost AT. Psychological effect of witnessed resuscitation on bereaved relatives. Lancet. (1998) 352:614–7. doi: 10.1016/S0140-6736(97)12179-1
84. Dryden-Palmer KD. PICU diaries: a simple and promising family-centered intervention. Pediatr Crit Care Med. (2019) 20:208–9. doi: 10.1097/PCC.0000000000001838
85. McIlroy PA, King RS, Garrouste-Orgeas M, Tabah A, Ramanan M. The effect of ICU diaries on psychological outcomes and quality of life of survivors of critical illness and their relatives: a systematic review and meta-analysis. Crit Care Med. (2019) 47:273–9. doi: 10.1097/CCM.0000000000003547
86. Herrup EA, Wieczorek B, Kudchadkar SR. Feasibility and perceptions of PICU diaries. Pediatr Crit Care Med. (2019) 20:e83–e90. doi: 10.1097/PCC.0000000000001814
87. Patak L, Tate JA, Happ MB. C-The missing tenet within the ABCDEF bundle. Crit Care Med. (2020) 48:e629–30. doi: 10.1097/CCM.0000000000004311
88. Asan O, Scanlon MC, Crotty B, Holden RJ, Flynn KE. Parental perceptions of displayed patient data in a PICU: an example of unintentional empowerment. Pediatr Crit Care Med. (2019) 20:435–41. doi: 10.1097/PCC.0000000000001895
89. Brown SM, Bell SK, Roche SD, Dente E, Mueller A, Kim TE, et al. Preferences of current and potential patients and family members regarding implementation of electronic communication portals in intensive care units. Ann Am Thorac Soc. (2016) 13:391–400. doi: 10.1513/AnnalsATS.201509-638OC
90. Bechard LJ, Duggan C, Touger-Decker R, Parrott JS, Rothpletz-Puglia P, Byham-Gray L, et al. Nutritional status based on body mass index is associated with morbidity and mortality in mechanically ventilated critically ill children in the PICU. Crit Care Med. (2016) 44:1530–7. doi: 10.1097/CCM.0000000000001713
91. Sirianansopa K, Rassameehirun C, Chomtho S, Suteerojntrakool O, Kongkiattikul L. Optimal enteral nutrition support preserved muscle mass in critically ill children. J Nutr Metab. (2022) 2022:7004543. doi: 10.1155/2022/7004543
92. Tume LN, Valla FV, Joosten K, Jotterand Chaparro C, Latten L, Marino LV, et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med. (2020) 46:411–25. doi: 10.1007/s00134-019-05922-5
Keywords: pediatric critical care, post intensive care syndrome, PICS, ABCDEF-bundles, family centered care, PICUs (pediatric intensive care unit)
Citation: Engel J, von Borell F, Baumgartner I, Kumpf M, Hofbeck M, Michel J and Neunhoeffer F (2022) Modified ABCDEF-Bundles for Critically Ill Pediatric Patients - What Could They Look Like? Front. Pediatr. 10:886334. doi: 10.3389/fped.2022.886334
Received: 28 February 2022; Accepted: 11 April 2022;
Published: 02 May 2022.
Edited by:
Sinno Simons, Erasmus Medical Center, NetherlandsReviewed by:
Jerry John Zimmerman, Seattle Children's Hospital, United StatesWarwick Wolf Butt, Royal Children's Hospital, Australia
Copyright © 2022 Engel, von Borell, Baumgartner, Kumpf, Hofbeck, Michel and Neunhoeffer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juliane Engel, juliane.engel@med.uni-tuebingen.de
 Juliane Engel
Juliane Engel Florian von Borell
Florian von Borell Isabella Baumgartner
Isabella Baumgartner Matthias Kumpf1
Matthias Kumpf1  Michael Hofbeck
Michael Hofbeck Jörg Michel
Jörg Michel Felix Neunhoeffer
Felix Neunhoeffer