Abstract
Background:
Few studies have examined the reference value of the left ventricular structure and function in preterm infants. This study was designed to establish a point-of-care echocardiographic reference range of left ventricular structure and function based on different gestational age, weight, and body surface area (BSA) for preterm infants within 7 days after birth.
Methods:
We retrospectively studied 489 patients with traditional echocardiographic data of left ventricular (LV) M-mode: LV end diastolic dimensions (LVED), LV end systolic dimension (LVES), end-diastolic interventricular septal thickness (IVSd), end diastolic LV posterior wall thickness (LVPWd), left atrial (LA) and aortic root (AO) diameters, and index of LA/AO, LV ejection fraction (LVEF), LV fractional shortening (LVFS), and pulsed wave Doppler: aortic valve flow rate (AV), peak mitral valve flow rate E(MV-E), peak mitral valve flow rate A(MV-A), and MV-E/A. The LV dimensions and the maximum blood flow velocities of the aortic valves and mitral valves according to gestational age, birth weight, and body surface area (BSA) are presented in percentiles tables. Percentile curves of aforesaid four cardiac measurements (LVED, LA diameter (LAD), MV-E, MV-E/A) using the R language Generalized Additive Models for Location, Scale and Shape (GAMLSS) method were developed according to different gestational ages and weights.
Results:
Measurements of all cardiac dimensions and Doppler maximum velocities of AV, MV-E, and MV-E/A showed a correlation with gestational age, weight, and BSA. LVED, LAD, MV-E, and MV-E/A showed a trend of increasing values with gestational age and weight on the percentile curves.
Conclusion:
The percentile tables and graphs of these point-of-care echocardiographic data can provide reliable reference data for Chinese neonates. Normative values are recommended as a source of reference data for the identification of potentially abnormal echocardiography.
Introduction
Point-of-care echocardiography is an essential tool in critical care. Its role is emphasized in neonates, as other monitoring techniques may be unavailable (1). Point-of-care echocardiography—-performed by neonatologists in the neonatal intensive care unit (NICU)—-can quickly and dynamically evaluate patent ductus arteriosus, shock, heart failure, pericardial effusion, and persistent pulmonary hypertension in neonates (2–6). This enables neonatologists to make decisions based on the child’s clinical condition, medications, ventilation strategies, and fluid management and formulate a precise treatment plan. This critical heart condition is more likely to occur in premature infants within 7 days of the transition period (7–10). Therefore, point-of-care echocardiography is extremely important in guiding the clinical treatment of critically ill premature infants. In the past 20 years, several large sample studies have suggested normative data related to cardiac chambers and wall thickness in children; however, the age span of the targeted patient population is relatively large, ranging from to 0–18 years (11–14). In recent years, some large sample studies on the heart structure of preterm infants based on body weight and body surface area (BSA) have been conducted, but the weight distribution of preterm infants in these studies was less than 2,000 g (15, 16). One study focused on the normal value of the left ventricular(LV) mass (15), and the other focused on the normal value of the cardiac structure in the patent ductus arteriosus group (16). There are few studies on the reference value of the LV structure and function in premature infants within 7 days of birth (<37 weeks). Therefore, we aimed to establish reference values for LV inner diameter, ventricular wall thickness, and blood flow velocity using two-dimensional (2D), M-mode or Doppler echocardiographic measurements for Chinese neonates, to make a quick and accurate judgment in daily clinical practice.
Materials and Methods
This retrospective, observational, single-center study of the reports of point-of-care echocardiographic examinations was performed on a population of preterm Chinese infants in the first 7 days of life. Our study was conducted in the Department of Neonatal Intensive Care Unit, Peking University Third Hospital, from March 2017 to February 2020. The study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee (2020114-02), exempted informed consent.
Patient Data and Definitions
Inclusion criteria were: hemodynamically stable infants with at least one echocardiogram taken within 7 days after birth (including any patient with incomplete data). Exclusion criteria were: (1) Congenital heart disease and/or other congenital malformations; (2) hemodynamic significant patent ductus arteriosus (HSPDA); (3) sepsis; (4) persistent pulmonary hypertension; (5) renal failure; (6) necrotizing enterocolitis, gastrointestinal perforation or intestinal obstruction; (7) severe anemia or shock; (8) smaller or larger than gestational age; and (9) high-parameter invasive ventilation (mean average airway pressure > 8 cmH2O [1 cmH2O = 0.098 kPa]), and/or inhaled oxygen concentration degree > 0.40.
Hemodynamic significant patent ductus arteriosus was defined as a ductal diameter > 1.5 mm (left to right shunt) and left artrial (LA) diameter/aortic root (AO) > 1.5; or the requirement of positive inotropic drugs, or mechanical ventilation; or if pulmonary edema was present, or gastrointestinal intolerance (17–19).
Ultrasound Equipment
Echocardiographic examinations were performed with a Mindray M7 scanner produced by Shenzhen Mindray Bio-Medical Electronics Co., (Probe: P12-4S Frequency 4–12 MHZ).
Echocardiographic Measurements and Clinical Data
Echocardiograms were performed by a neonatologist, and examinations were completed by a neonatologist proficient in echocardiography techniques. Instant measurements were reviewed by a cardiologist. Measurements were made over three cardiac cycles, and the mean values were obtained in the standard precordial positions using the published American Child Echocardiography Guide and Standards and American NICU echocardiography Practice Guide (20, 21). The following cardiovascular measurements were obtained from parasternal long-axis views on cross-sectional LV M-mode (Figure 1): LV end-diastolic dimensions (LVED), LV end-systolic dimensions (LVES), end-diastolic interventricular septal thickness (IVSd), end diastolic LV posterior wall thickness (LVPWd), LA, and aortic root (AO) diameters, and index of LA/AO, left ventricular ejection fraction (LVEF), and left ventricular fractional shortening (LVFS). Measurements of LVED, LVES, IVSd, LVPWd, LVEF, and LVFS diameters were taken at the level of the posterior mitral valve leaflet, and measurements of the AO and LA diameters (AOD and LAD) were taken in end-systole at the level of the aortic valves. Maximum blood flow velocities were measured on the aortic and mitral valves and measured from an apical four-chamber and five chamber view using pulsed wave Doppler from a point at the center of the valves (Figure 1):aortic valve flow rate (AV), peak mitral valve flow rate E(MV-E), peak mitral valve flow rate A(MV-A), and MV-E/A. Among these traditional cardiac parameters LVED, LAD, and LA/AO represent the left heart volume load;LVFS and LVEF represent left ventricular systolic function; and MV-E and MV-E/A reflect left ventricular diastolic function. The aforementioned echocardiographic parameters, such as LAD, LA/AO, LVED, and MV-E/A have been examined as predictors of HSPDA (22, 23).
FIGURE 1
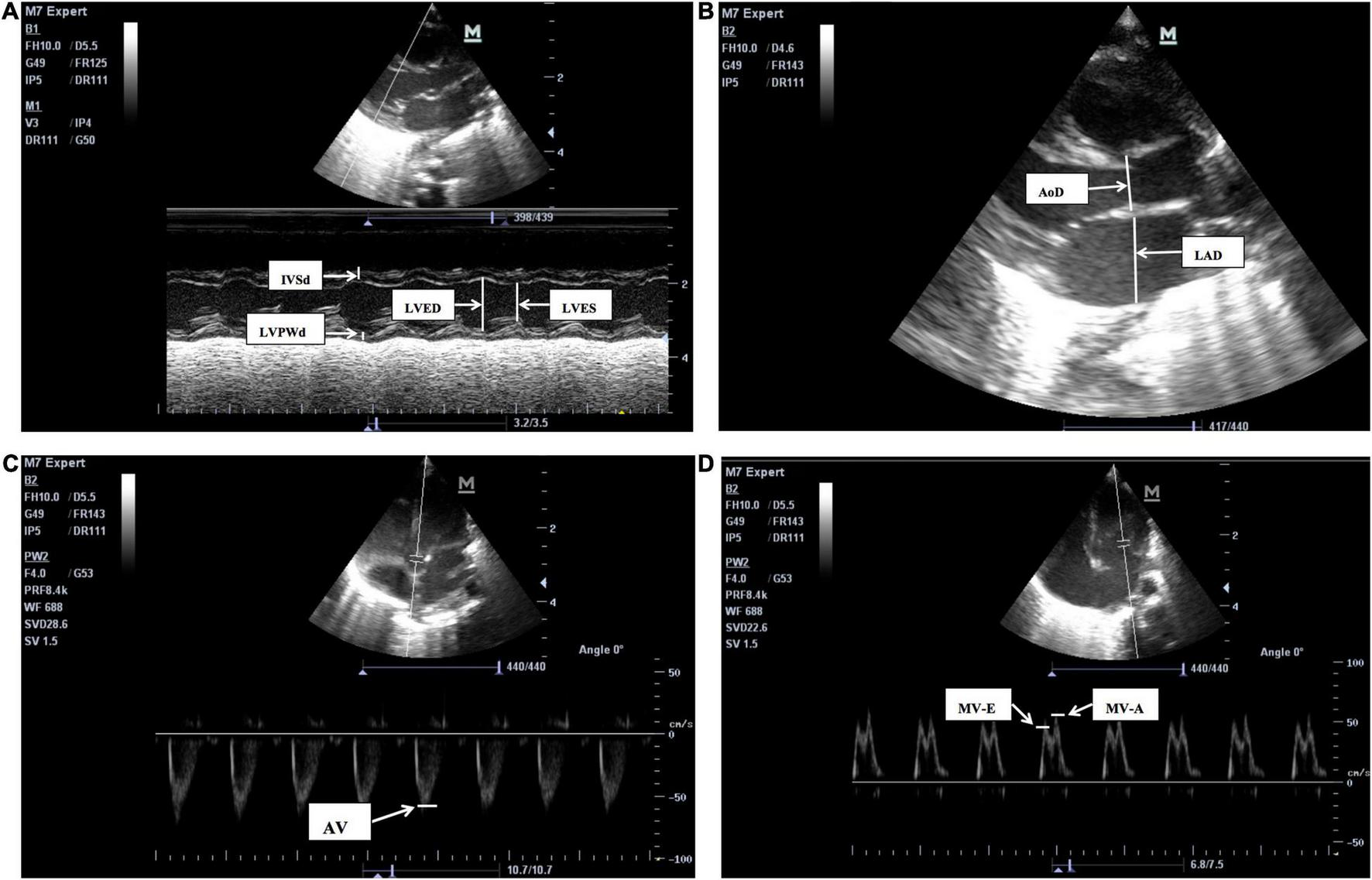
The two-dimensional (2D), M-mode or Doppler echocardiographic measurements pictures for LV structure and function. (A) The M-mode echocardiographic measurements for left ventricle and interventricular septum. IVSd, end-diastolic interventricular septum, LVPWd, left ventricular posterior wall end-diastolic thickness, LVED, Left ventricular end-diastolic, LVES, left ventricular end-systolic, LVFS, left ventricular fractional shortening, LVEF, left ventricular ejection fraction. LVFS (%), (LVED-LVES)/LVED × 100, LVEF is calculated by Teichholtz method. (B) The 2D measurements of left atrium diameters (LAD) and aortic root diameters (AoD), were taken in end-systole at the level of the aortic valves. LA/AO = LAD/AoD. (C) The pulsed wave Doppler measurements for aortic valve flow rate (AV). (D) The pulsed wave Doppler measurements for peak mitral valve flow rate E(MV-E), peak mitral valve flow rate A(MV-A), the peak mitral valve flow rate E to A (MV-E/A), MV-E/MV-A.
The following clinical variables were noted: antenatal steroids, birth weight, gestational age, body length, fetal birth mode, cause of premature birth, Apgar score, maternal pregnancy complications, and vital data parameters including ventilation mode, mean airway pressure and inhaled oxygen concentration, heart rate, respiratory rate, systolic pressure, diastolic pressure, and mean pressure during measurement.
Study Group
According to the gestational age (GA), the preterm infants were divided into four groups: <28 weeks, 28–31+6 weeks, 32–33+6 weeks, and 34–36+6 weeks, and also divided into five groups according to birth weight(BW): <1000, 1000–1499, 1500–1999, 2000–2499 and ≥2500 g. Based on BSA, infants were equally divided into seven groups, ranging from 0.07 to 0.20 m2.
Statistical Analysis
Data were analyzed using SPSS (Statistical Package for Social Sciences) version 24.0. BSA was determined using the DuBois and DuBois formula: BSA = 0.20247 × height(m)0.725 × weight(kg)0.425. Measurements of LVED, LVES, IVSd, LVPWd, LAD, AOD, AV, MV-E, and MV-E/A values were plotted against GA, BW, and BSA. Normality was then tested using the Kolmogorov–Smirnov test, and Spearman’s rank correlation was performed for each parameter. Descriptive statistics were presented as percentiles (3rd, 10th, 25th, 50th, 75th, 90th, and 97th) for the GA, BW, and BSA subgroups. The R language Generalized Additive Models for Location, Scale and Shape (GAMLSS) package was used to draw scatter plots of each measurement in relation to GA and BW, and box-cox transformation and curved lines of centiles (3rd, 10th, 25th, 50th, 75th, 90th, and 97th percentiles) show changes in the four important parameters (LVED, LAD, MV-E and MV-E/A) of GA and BW. A p-value less than 0.05 was considered significant.
Results
Our study included 489 preterm infants with a mean GA of 32 weeks (range: 24–36.7), mean BW of 1,700 g (range: 650–3,180), and mean BSA of 0.13 m2 (range: 0.07–0.20 m2). The general characteristics of the infants are presented in Table 1.
TABLE 1
| Characteristics | Result |
| Male: Female(N) | 264:225 |
| Length (cm) Median (IQR) | 41 (38–44) |
| SBP (mmHg) Median (IQR) | 69 (63–74) |
| DBP (mmHg) Median (IQR) | 37 (32–43) |
| MBP (mmHg) Median (IQR) | 48 (44–53) |
| Heart rate (times/minute) Median (IQR) | 143 (135–153) |
| Respiratory rate (times per minute) Median (IQR) | 40 (35–46) |
General characteristics of the preterm babies.
IQR, interquartile range.
In Table 2, the measurements of all cardiac dimensions and Doppler maximum velocities of AV, MV-E, and MV-E/A show a correlation with GA, BW, and BSA (p < 0.001). Echocardiographic parameters of LA/AO, LVEF, LVFS, and MV-A show no correlation with GA, BW, and BSA (p > 0.05).
TABLE 2
| Echocardiographic measurements | GA (wk) r (P) | BW (g) r (P) | BSA (m2) r (P) |
| LVED | 0.608 (<0.001) | 0.609 (<0.001) | 0.616 (<0.001) |
| LVES | 0.495 (<0.001) | 0.496 (<0.001) | 0.505 (<0.001) |
| IVSd | 0.209 (<0.001) | 0.275 (<0.001) | 0.254 (<0.001) |
| LVPWd | 0.216 (<0.001) | 0.288 (<0.001) | 0.269 (<0.001) |
| LAD | 0.430 (<0.001) | 0.445 (<0.001) | 0.437 (<0.001) |
| AoD | 0.481 (<0.001) | 0.508 (<0.001) | 0.503 (<0.001) |
| LA/AO | 0.07 (0.125) | 0.065 (0.154) | 0.062 (0.173) |
| LVEF | −0.021 (0.641) | −0.013(0.641) | −0.019 (0.668) |
| LVFS | 0.022 (0.623) | 0.033(0.470) | 0.027 (0.550) |
| AV | 0.263 (<0.001) | 0.292 (<0.001) | 0.278 (<0.001) |
| MV-A | −0.025 (0.578) | 0.049 (0.285) | 0.037 (0.418) |
| MV-E | 0.256 (<0.001) | 0.261 (<0.001) | 0.268 (<0.001) |
| MV-E/A | 0.344 (<0.001) | 0.301 (<0.001) | 0.312 (<0.001) |
Correlation of echocardiographic measurements with GA, BW, and BSA in preterm infants.
LVED, left ventricular end-diastolic, LVES, left ventricular end-systolic, IVSd, end-diastolic interventricular septum, LVPWd, left ventricular posterior wall end-diastolic thickness, LAD, left atrium diameters, and AoD, aortic root diameters, LA to AO ratio (LA/AO), left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), aortic valve flow rate (AV), peak mitral valve flow rate E (MV-E), peak mitral valve flow rate A (MV-A), MV-E to MV-A ratio (MV-E/A).
Tables 3–11 show the nine cardiac measurement distributions as percentiles (3rd, 10th, 25th, 50th, 75th, 90th, and 97th) for each GA, BW, and BSA subgroup. Table 12 shows another four cardiac measurement distributions of 489 babies as percentiles (3rd, 10th, 25th, 50th, 75th, 90th, and 97th) within 7 days after birth.
TABLE 3
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 46 | 7.41 | 8.50 | 10.00 | 11.00 | 12.00 | 13.56 | 15.35 |
| 28–31+6 | 185 | 9.46 | 10.06 | 11.50 | 12.70 | 13.95 | 15.00 | 16.08 |
| 32–33+6 | 140 | 10.25 | 12.00 | 13.10 | 14.25 | 15.60 | 16.67 | 17.58 |
| 34–36+6 | 118 | 11.56 | 12.78 | 14.00 | 15.10 | 16.90 | 18.01 | 20.03 |
| BW(g) | ||||||||
| <1000 | 43 | 7.36 | 8.74 | 10.00 | 11.00 | 12.00 | 13.20 | 14.65 |
| 1000–1499 | 132 | 9.30 | 10.00 | 11.00 | 12.30 | 13.20 | 14.30 | 15.60 |
| 1500–1999 | 176 | 10.06 | 11.90 | 13.00 | 14.15 | 15.40 | 16.70 | 17.41 |
| 2000–2499 | 103 | 11.00 | 12.22 | 13.20 | 15.00 | 16.40 | 17.80 | 19.84 |
| ≥2500 | 35 | 12.82 | 13.62 | 14.00 | 15.20 | 16.20 | 17.52 | 19.00 |
| BSA(m2) | ||||||||
| –0.08 | 16 | 7.70 | 8.61 | 10.23 | 11.10 | 12.90 | 14.23 | – |
| –0.10 | 73 | 8.42 | 9.38 | 10.10 | 11.20 | 12.35 | 13.00 | 14.30 |
| –0.12 | 103 | 9.50 | 10.44 | 11.50 | 13.00 | 14.00 | 15.00 | 15.89 |
| –0.14 | 134 | 10.21 | 11.90 | 13.00 | 14.00 | 15.33 | 16.50 | 17.58 |
| –0.16 | 117 | 10.11 | 12.42 | 13.55 | 14.80 | 16.25 | 17.26 | 18.78 |
| –0.18 | 39 | 12.40 | 12.80 | 13.90 | 15.20 | 16.60 | 17.80 | 23.00 |
| –0.20 | 7 | 14.30 | 14.30 | 14.60 | 16.00 | 18.00 | – | – |
Left ventricular end-diastolic dimensions (mm) (from 3rd to 97th centiles) in relation to GA, BW, and BSA in preterm infants.
LVED, left ventricular end-diastolic.
TABLE 4
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 46 | 4.59 | 5.24 | 5.85 | 7.30 | 8.00 | 8.90 | 12.95 |
| 28–31+6 | 185 | 5.00 | 6.50 | 7.20 | 8.10 | 9.35 | 10.24 | 10.98 |
| 32–33+6 | 140 | 6.05 | 7.13 | 8.40 | 9.20 | 10.20 | 11.09 | 12.08 |
| 34–36+6 | 118 | 6.56 | 7.50 | 8.80 | 10.00 | 10.93 | 12.01 | 13.17 |
| BW(g) | ||||||||
| <1000 | 43 | 5.00 | 5.38 | 6.00 | 7.00 | 8.00 | 8.90 | 13.40 |
| 1000–1499 | 132 | 5.00 | 6.23 | 7.20 | 8.00 | 8.98 | 9.87 | 10.60 |
| 1500–1999 | 176 | 5.63 | 7.07 | 8.40 | 9.10 | 10.20 | 11.00 | 11.94 |
| 2000–2499 | 103 | 6.30 | 7.00 | 8.50 | 9.80 | 10.90 | 12.00 | 13.52 |
| ≥2500 | 35 | 4.26 | 7.62 | 8.80 | 10.00 | 10.70 | 11.98 | 13.00 |
| BSA(m2) | ||||||||
| –0.08 | 16 | 5.50 | 5.50 | 6.13 | 7.00 | 8.75 | 11.50 | – |
| –0.10 | 73 | 5.00 | 5.52 | 6.55 | 7.30 | 8.00 | 8.86 | 9.96 |
| –0.12 | 103 | 5.07 | 6.84 | 7.50 | 8.30 | 9.20 | 10.16 | 10.80 |
| –0.14 | 134 | 5.72 | 7.00 | 8.28 | 9.00 | 10.23 | 11.00 | 12.10 |
| –0.16 | 117 | 6.16 | 7.16 | 8.70 | 9.90 | 10.50 | 11.62 | 12.54 |
| –0.18 | 39 | 4.54 | 7.30 | 8.80 | 10.30 | 10.80 | 12.90 | 14.92 |
| –0.20 | 7 | 9.10 | 9.10 | 9.70 | 10.50 | 11.00 | – | – |
Left ventricular end-systolic dimensions (mm) (from 3rd to 97th centiles) in relation to GA, BW, and BSA in preterm infants.
LVES, left ventricular end-systolic.
TABLE 5
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 46 | 1.72 | 1.97 | 2.30 | 2.70 | 3.53 | 4.26 | 4.68 |
| 28–31+6 | 185 | 2.00 | 2.20 | 2.60 | 3.00 | 3.55 | 4.30 | 5.47 |
| 32–33+6 | 140 | 2.20 | 2.50 | 2.80 | 3.20 | 3.60 | 4.60 | 5.78 |
| 34–36+6 | 118 | 2.10 | 2.40 | 2.70 | 3.50 | 4.00 | 4.60 | 5.67 |
| BW(g) | ||||||||
| <1000 | 43 | 1.90 | 2.00 | 2.40 | 2.70 | 3.40 | 4.24 | 4.70 |
| 1000–1499 | 132 | 1.80 | 2.00 | 2.50 | 3.00 | 3.50 | 4.00 | 4.30 |
| 1500–1999 | 176 | 2.00 | 2.40 | 2.80 | 3.15 | 3.50 | 4.40 | 5.61 |
| 2000–2499 | 103 | 2.21 | 2.50 | 3.00 | 3.50 | 4.30 | 5.08 | 6.09 |
| ≥2 500 | 35 | 2.40 | 2.50 | 2.70 | 3.00 | 3.80 | 4.84 | 6.33 |
| BSA(m2) | ||||||||
| –0.08 | 16 | 2.00 | 2.00 | 2.28 | 3.05 | 3.60 | 4.15 | – |
| –0.10 | 73 | 1.62 | 2.00 | 2.35 | 2.70 | 3.40 | 4.20 | 4.78 |
| –0.12 | 103 | 1.91 | 2.10 | 2.50 | 3.00 | 3.50 | 4.00 | 4.30 |
| –0.14 | 134 | 2.01 | 2.50 | 2.80 | 3.20 | 3.73 | 4.55 | 5.68 |
| –0.16 | 117 | 2.30 | 2.50 | 3.00 | 3.40 | 4.00 | 4.70 | 6.13 |
| –0.18 | 39 | 2.10 | 2.40 | 2.70 | 3.20 | 4.00 | 5.30 | 6.08 |
| –0.20 | 7 | 2.50 | 2.50 | 2.70 | 3.00 | 3.50 | – | – |
End-diastolic interventricular septum dimensions (mm) (from 3rd to 97th centiles) in relation to GA, BW, and BSA in preterm infants.
IVSd, end-diastolic interventricular septum.
TABLE 6
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 46 | 1.42 | 1.87 | 2.10 | 2.40 | 2.75 | 3.12 | 4.12 |
| 28–31+6 | 185 | 1.60 | 2.00 | 2.10 | 2.50 | 3.00 | 3.40 | 4.00 |
| 32–33+6 | 140 | 2.00 | 2.10 | 2.40 | 2.70 | 3.00 | 3.50 | 4.00 |
| 34–36+6 | 118 | 2.00 | 2.20 | 2.40 | 2.85 | 3.20 | 3.61 | 4.13 |
| BW(g) | ||||||||
| <1000 | 43 | 1.40 | 1.68 | 2.00 | 2.40 | 2.90 | 3.00 | 4.14 |
| 1000–1499 | 132 | 1.60 | 2.00 | 2.10 | 2.40 | 2.90 | 3.17 | 3.90 |
| 1500–1999 | 176 | 2.00 | 2.00 | 2.40 | 2.70 | 3.00 | 3.50 | 4.14 |
| 2000–2499 | 103 | 2.00 | 2.20 | 2.50 | 2.90 | 3.40 | 3.80 | 4.26 |
| ≥2500 | 35 | 2.00 | 2.10 | 2.40 | 2.70 | 3.00 | 3.50 | 3.69 |
| BSA(m2) | ||||||||
| –0.08 | 16 | 1.60 | 1.60 | 1.85 | 2.40 | 2.88 | 3.30 | – |
| –0.10 | 73 | 1.60 | 1.94 | 2.05 | 2.40 | 2.90 | 3.20 | 4.00 |
| –0.12 | 103 | 1.81 | 2.00 | 2.10 | 2.50 | 3.00 | 3.20 | 3.88 |
| –0.14 | 134 | 2.00 | 2.00 | 2.40 | 2.70 | 3.10 | 3.50 | 4.29 |
| –0.16 | 117 | 2.00 | 2.20 | 2.40 | 2.90 | 3.20 | 3.72 | 4.00 |
| –0.18 | 39 | 1.76 | 2.10 | 2.40 | 2.90 | 3.20 | 3.50 | 4.18 |
| –0.20 | 7 | 2.10 | 2.10 | 2.50 | 2.60 | 3.20 | – | – |
Left ventricular posterior wall end-diastolic thickness dimensions (mm) (from 3rd to 97th centiles) in relation to GA, BW, and BSA in preterm infants.
LVPWd, left ventricular posterior wall end-diastolic thickness.
TABLE 7
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 46 | 4.78 | 5.00 | 5.98 | 7.05 | 8.80 | 10.29 | 11.04 |
| 28–31+6 | 185 | 5.06 | 5.96 | 6.65 | 7.80 | 8.95 | 10.00 | 11.17 |
| 32–33+6 | 140 | 5.92 | 6.70 | 7.80 | 8.60 | 9.70 | 10.80 | 11.55 |
| 34–36+6 | 118 | 5.87 | 7.09 | 8.10 | 9.20 | 10.05 | 11.42 | 12.74 |
| BW(g) | ||||||||
| <1000 | 43 | 3.82 | 4.90 | 5.90 | 7.00 | 8.10 | 10.30 | 11.07 |
| 1000–1499 | 132 | 5.00 | 5.90 | 6.40 | 7.50 | 8.40 | 9.50 | 10.50 |
| 1500–1999 | 176 | 6.00 | 6.64 | 7.80 | 8.70 | 9.70 | 10.80 | 11.60 |
| 2000–2499 | 103 | 5.72 | 7.00 | 8.00 | 9.20 | 10.00 | 11.22 | 12.49 |
| ≥2500 | 35 | 7.00 | 7.88 | 8.90 | 9.20 | 10.00 | 12.00 | 13.14 |
| BSA(m2) | ||||||||
| –0.08 | 16 | 4.70 | 4.91 | 5.58 | 7.25 | 9.73 | 10.92 | – |
| –0.10 | 73 | 4.82 | 5.04 | 6.00 | 7.00 | 8.05 | 8.92 | 10.43 |
| –0.12 | 103 | 5.40 | 6.00 | 6.70 | 8.00 | 9.00 | 10.00 | 10.93 |
| –0.14 | 134 | 6.00 | 6.45 | 7.70 | 8.55 | 9.70 | 10.80 | 11.60 |
| –0.16 | 117 | 5.90 | 7.00 | 8.00 | 9.00 | 10.00 | 11.32 | 12.28 |
| –0.18 | 39 | 5.94 | 7.80 | 8.90 | 9.20 | 10.40 | 11.90 | 14.16 |
| –0.20 | 7 | 7.00 | 7.00 | 7.70 | 10.00 | 12.00 | – | – |
Left atrium diameters (mm) (from 3rd to 97th centiles) in relation to GA, BW, and BSA in preterm infants.
LAD, left atrium diameters.
TABLE 8
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 46 | 4.08 | 4.40 | 4.90 | 5.15 | 5.83 | 6.50 | 7.79 |
| 28–31+6 | 185 | 4.37 | 5.00 | 5.40 | 6.00 | 6.70 | 7.00 | 7.97 |
| 32–33+6 | 140 | 5.00 | 5.10 | 6.00 | 6.50 | 7.10 | 7.80 | 8.28 |
| 34–36+6 | 118 | 5.10 | 5.69 | 6.20 | 7.00 | 7.50 | 8.32 | 9.20 |
| BW(g) | ||||||||
| <1000 | 43 | 4.00 | 4.08 | 4.60 | 5.10 | 5.80 | 6.50 | 6.84 |
| 1000–1499 | 132 | 4.50 | 5.00 | 5.30 | 5.95 | 6.50 | 7.00 | 7.80 |
| 1500–1999 | 176 | 5.00 | 5.10 | 5.90 | 6.50 | 7.00 | 7.63 | 8.24 |
| 2000–2499 | 103 | 5.00 | 5.48 | 6.20 | 7.00 | 7.30 | 8.26 | 9.16 |
| ≥2500 | 35 | 5.40 | 5.88 | 6.30 | 7.10 | 7.80 | 8.60 | 9.18 |
| BSA(m2) | ||||||||
| –0.08 | 16 | 4.00 | 4.14 | 4.93 | 5.45 | 5.98 | 6.29 | – |
| –0.10 | 73 | 4.00 | 4.50 | 4.95 | 5.60 | 6.00 | 6.60 | 7.62 |
| –0.12 | 103 | 4.82 | 5.00 | 5.40 | 6.00 | 6.70 | 7.00 | 7.50 |
| –0.14 | 134 | 5.00 | 5.10 | 6.00 | 6.50 | 7.03 | 7.70 | 8.40 |
| –0.16 | 117 | 5.00 | 5.40 | 6.00 | 6.70 | 7.35 | 8.02 | 8.85 |
| –0.18 | 39 | 5.40 | 5.90 | 6.60 | 7.00 | 8.00 | 8.60 | 9.92 |
| –0.20 | 7 | 6.00 | 6.00 | 6.00 | 6.70 | 7.50 | – | – |
Aortic root diameters (mm) (from 3rd to 97th centiles) in relation to GA, BW, and BSA in preterm infants.
AoD, aortic root diameters.
TABLE 9
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 46 | 32.00 | 37.90 | 46.50 | 52.80 | 58.25 | 68.90 | 174.89 |
| 28–31+6 | 185 | 30.58 | 34.60 | 41.50 | 52.00 | 63.50 | 78.80 | 92.68 |
| 32–33+6 | 140 | 36.00 | 42.10 | 50.50 | 59.50 | 71.00 | 78.90 | 88.00 |
| 34–36+6 | 118 | 36.57 | 41.00 | 51.00 | 61.50 | 71.25 | 82.10 | 93.72 |
| BW(g) | ||||||||
| <1000 | 43 | 26.56 | 32.40 | 39.00 | 50.00 | 60.00 | 69.80 | 192.20 |
| 1000–1499 | 132 | 30.00 | 36.30 | 42.00 | 50.00 | 59.75 | 71.00 | 80.03 |
| 1500–1999 | 176 | 34.62 | 41.00 | 50.00 | 59.50 | 72.00 | 82.00 | 94.38 |
| 2000–2499 | 103 | 36.12 | 40.00 | 52.00 | 63.00 | 71.00 | 83.60 | 96.88 |
| ≥2500 | 35 | 33.24 | 41.60 | 50.00 | 61.00 | 70.00 | 76.40 | 86.68 |
| BSA(m2) | ||||||||
| –0.08 | 16 | 24.00 | 29.60 | 34.75 | 52.80 | 59.00 | 123.20 | – |
| –0.10 | 73 | 30.22 | 34.00 | 42.50 | 50.00 | 59.50 | 67.00 | 75.56 |
| –0.12 | 103 | 32.00 | 36.40 | 41.00 | 50.00 | 61.00 | 72.60 | 82.64 |
| –0.14 | 134 | 34.10 | 42.50 | 52.00 | 61.00 | 76.25 | 84.50 | 100.75 |
| –0.16 | 117 | 36.00 | 40.00 | 50.00 | 60.00 | 71.00 | 82.00 | 96.00 |
| –0.18 | 39 | 34.40 | 46.00 | 55.00 | 63.00 | 70.00 | 78.00 | 87.80 |
| –0.20 | 7 | 36.00 | 36.00 | 41.00 | 66.00 | 74.00 | – | – |
Doppler maximum velocities of AV (cm/s) (from 3rd to 97th centiles) in relation to GA, BW, and BSA in preterm infants.
AV, aortic valve flow rate.
TABLE 10
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 46 | 20.00 | 23.00 | 30.00 | 37.00 | 47.25 | 68.50 | 94.36 |
| 28–31+6 | 185 | 20.00 | 22.00 | 26.50 | 33.00 | 40.00 | 50.00 | 58.84 |
| 32–33+6 | 140 | 22.00 | 28.10 | 35.00 | 40.00 | 45.75 | 54.90 | 63.00 |
| 34–36+6 | 118 | 23.00 | 27.00 | 34.00 | 41.00 | 48.25 | 59.10 | 70.44 |
| BW(g) | ||||||||
| <1000 | 43 | 17.96 | 20.00 | 23.00 | 32.00 | 42.00 | 59.20 | 94.72 |
| 1000–1499 | 132 | 20.00 | 22.00 | 25.00 | 32.00 | 41.00 | 50.00 | 57.01 |
| 1500–1999 | 176 | 24.00 | 27.00 | 33.00 | 38.50 | 45.00 | 55.00 | 63.69 |
| 2000–2499 | 103 | 20.12 | 27.00 | 36.00 | 40.00 | 46.00 | 58.60 | 67.00 |
| ≥2500 | 35 | 20.40 | 25.60 | 34.00 | 43.00 | 48.00 | 56.00 | 81.32 |
| BSA(m2) | ||||||||
| –0.08 | 16 | 20.00 | 20.00 | 23.50 | 34.00 | 46.50 | 93.20 | – |
| –0.10 | 73 | 20.00 | 22.00 | 24.50 | 32.00 | 41.00 | 51.20 | 57.00 |
| –0.12 | 103 | 20.12 | 22.40 | 27.00 | 33.00 | 41.00 | 51.80 | 62.88 |
| –0.14 | 134 | 23.05 | 26.50 | 32.00 | 38.00 | 45.00 | 55.00 | 65.90 |
| –0.16 | 117 | 20.00 | 27.00 | 33.50 | 40.00 | 45.00 | 56.20 | 62.00 |
| –0.18 | 39 | 21.40 | 37.00 | 41.00 | 45.00 | 54.00 | 63.00 | 81.40 |
| –0.20 | 7 | 25.00 | 25.00 | 26.00 | 33.00 | 45.00 | – | – |
Doppler maximum velocities of MV-E (cm/s) (from 3rd to 97th centiles) in relation to GA, BW and BSA in preterm infants.
MV-E, peak mitral valve flow rate E.
TABLE 11
| GA (wk) | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| <28 | 37 | 0.49 | 0.55 | 0.60 | 0.70 | 0.80 | 0.85 | 0.92 |
| 28–31+6 | 185 | 0.50 | 0.57 | 0.66 | 0.73 | 0.81 | 1.02 | 1.31 |
| 32–33+6 | 140 | 0.57 | 0.61 | 0.69 | 0.78 | 0.94 | 1.20 | 1.46 |
| 34–36+6 | 118 | 0.59 | 0.66 | 0.74 | 0.84 | 1.22 | 1.37 | 1.85 |
| BW(g) | ||||||||
| <1000 | 37 | 0.43 | 0.50 | 0.62 | 0.68 | 0.77 | 0.87 | 1.11 |
| 1000–1499 | 129 | 0.51 | 0.56 | 0.63 | 0.73 | 0.83 | 1.06 | 1.40 |
| 1500–1999 | 176 | 0.58 | 0.63 | 0.70 | 0.76 | 0.90 | 1.21 | 1.54 |
| 2000–2499 | 103 | 0.57 | 0.65 | 0.71 | 0.80 | 1.10 | 1.34 | 1.73 |
| ≥2500 | 35 | 0.48 | 0.58 | 0.73 | 0.87 | 1.14 | 1.31 | 2.36 |
| BSA(m2) | ||||||||
| –0.08 | 13 | 0.49 | 0.52 | 0.61 | 0.66 | 0.77 | 0.94 | – |
| –0.10 | 68 | 0.46 | 0.54 | 0.63 | 0.72 | 0.80 | 0.94 | 1.58 |
| –0.12 | 102 | 0.51 | 0.58 | 0.63 | 0.73 | 0.85 | 1.09 | 1.39 |
| –0.14 | 134 | 0.57 | 0.62 | 0.70 | 0.76 | 0.86 | 1.19 | 1.41 |
| –0.16 | 117 | 0.56 | 0.65 | 0.70 | 0.78 | 0.94 | 1.33 | 1.75 |
| –0.18 | 39 | 0.58 | 0.68 | 0.80 | 1.08 | 1.23 | 1.33 | 2.81 |
| –0.20 | 7 | 0.54 | 0.54 | 0.71 | 0.80 | 1.15 | – | – |
Doppler maximum velocities of MV-E/A (from 3rd to 97th centiles) in relation to GA, BW, and BSA in preterm infants.
MV-E/A, MV-E to MV-A ratio.
TABLE 12
| Cardiac parameters | n | 3rd | 10th | 25th | 50th | 75th | 90th | 97th |
| LA/AO | 489 | 0.89 | 1.00 | 1.14 | 1.33 | 1.52 | 1.67 | 1.87 |
| LVEF | 489 | 55.00 | 60.00 | 62.00 | 67.00 | 73.00 | 79.00 | 84.30 |
| LVFS | 489 | 26.00 | 29.00 | 31.00 | 34.00 | 38.00 | 44.00 | 50.00 |
| MV-A | 480 | 27.43 | 32.00 | 38.00 | 47.00 | 54.00 | 62.00 | 70.00 |
Percentiles of four cardiac parameters within seven days of 489 preterm infants.
Left atrium diameters to aortic root diameters ratio (LA/AO), left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), peak mitral valve flow rate A (MV-A).
Figures 2, 3 show four important cardiac measurements: percentile curves with data points for all 489 reference patients compared with their GA and BW, respectively. Percentile lines correspond to the 3rd, 10th, 25th, 50th, 75th, 90th, and 97th percentiles.
FIGURE 2
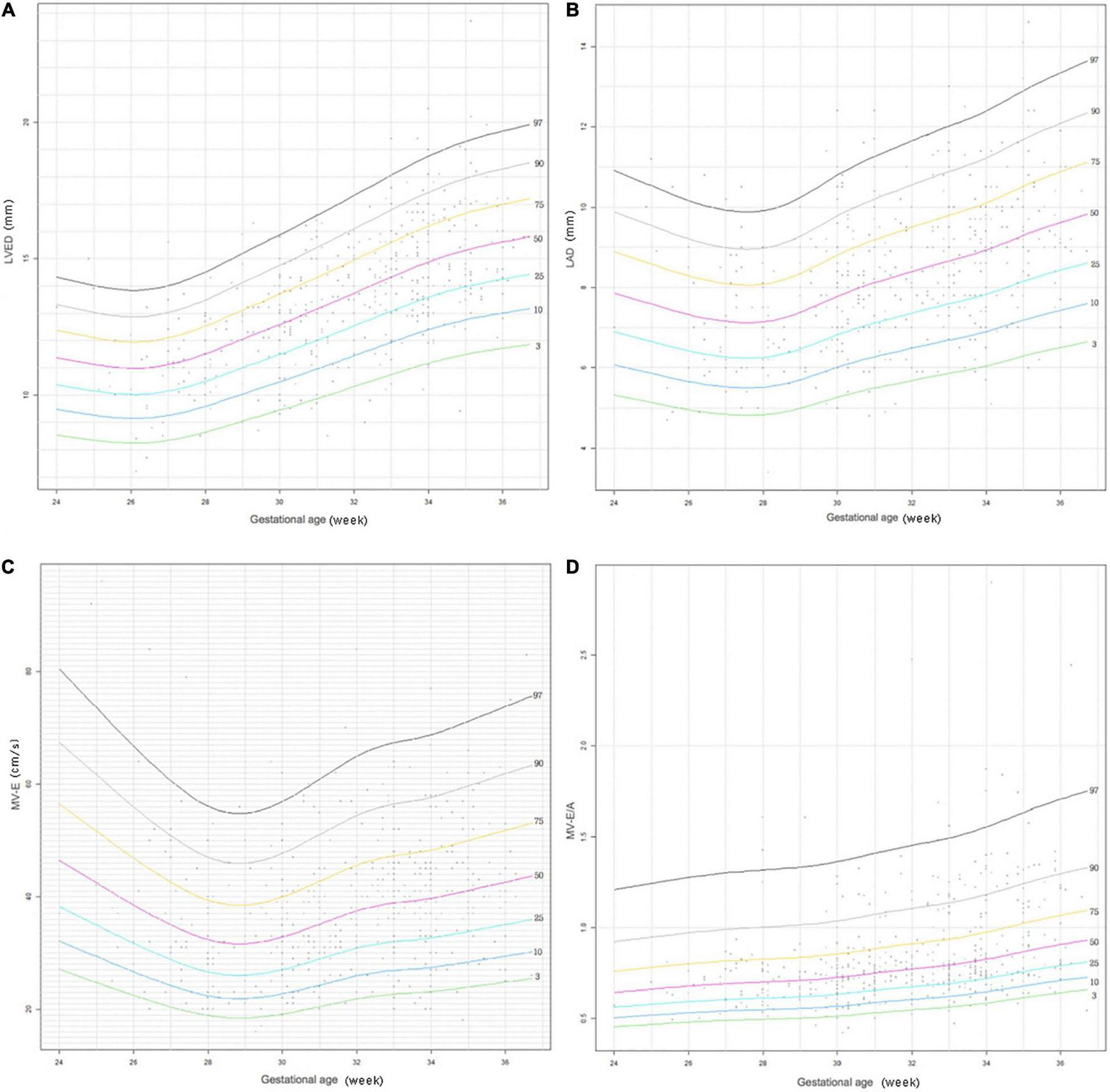
Reference centile curve for 489 preterm infants generated using R language GAMLSS method for (A) left ventricular end-diastolic (LVED), (B) left atrium diameter (LAD), (C) the peak mitral valve flow rate E (MV-E), and (D) the peak mitral valve flow rate E to A (MV-E/A) normalized by gestational age within 7 days of birth.
FIGURE 3
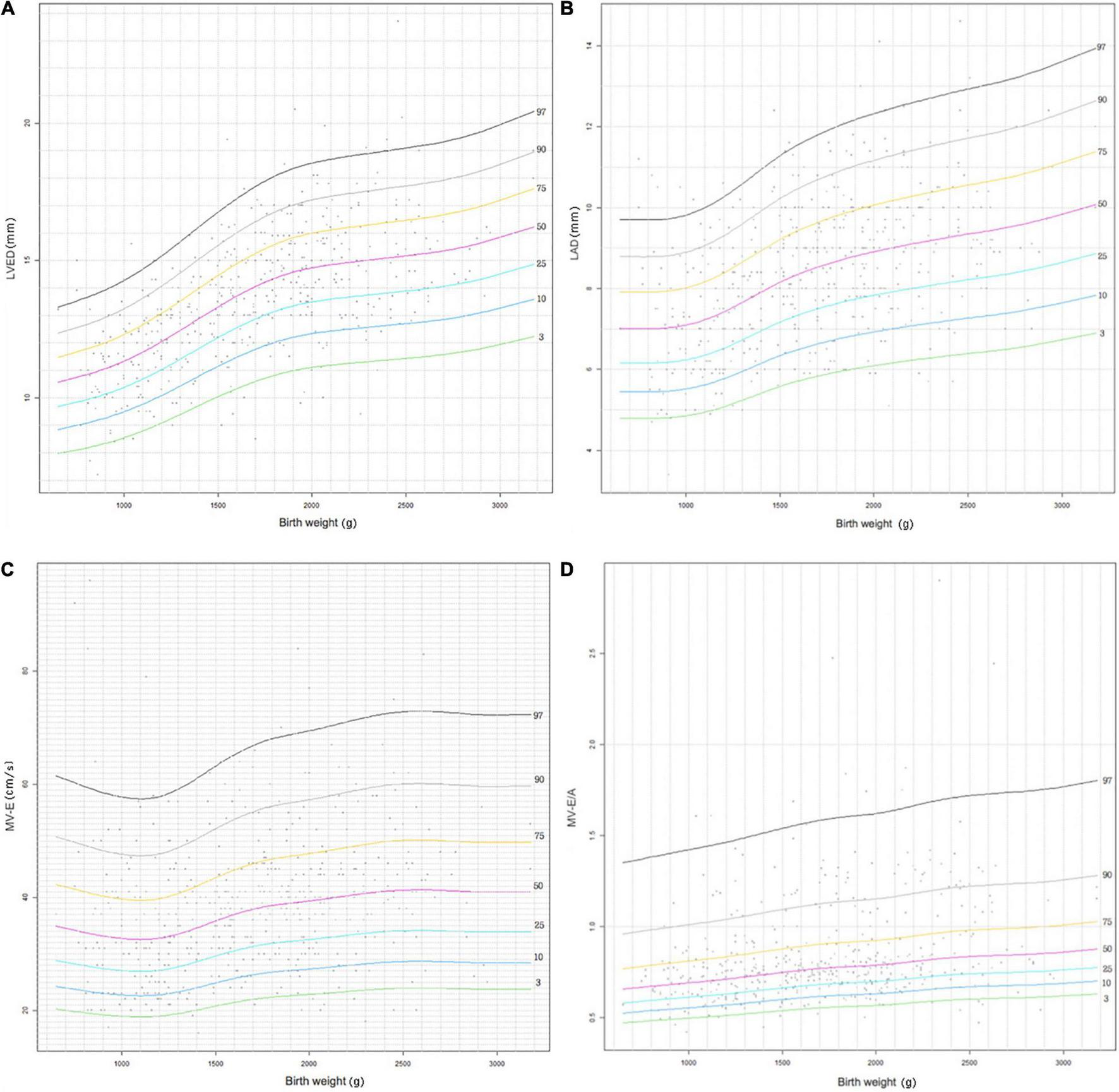
Reference centile curve for 489 preterm infants generated using R language GAMLSS method for (A) left ventricular end-diastolic (LVED), (B) left atrium diameter (LAD), (C) the peak mitral valve flow rate E (MV-E), and (D) the peak mitral valve flow rate E to A (MV-E/A) normalized by birth weight within 7 days of birth.
Discussion
We present reference ranges of the left cardiac dimensions, mitral and aortic valve maximum blood flow velocities for a complete population of preterm infants. The structure and function of the heart are considered to be related to race (24, 25). Lopez et al. (26) collected data from healthy non-obese children ≤ 18 years of age with a normal echocardiogram from 19 centers and studied the Z scores for common measurements adjusted for body surface area (BSA). They found that race had a small effect on the Z scores. Nevertheless, this study did not include preterm babies. Therefore, it is of great significance to establish a reference range for the heart size and peak valve flow rate of preterm infants during the perinatal period in our own country, which can help determine echocardiographic measurements of a particular patient as normal or abnormal.
Cardiac point of care ultrasound (POCUS) is increasingly being utilized in neonatal intensive care units for providing information in real time to aid clinical decision making. Cardiac POCUS refers to a basic, time-sensitive, and focused diagnostic echocardiographic assessment, including Hemodynamic assessment, HSPDA, and PPHN. In this study, cardiac ultrasonography was performed by neonatologists with formal cardiac ultrasound training. Reference values of LVED, LAD, LVEF, LVFS, MV E/A, and LA/AO, measured in preterm infants within 7 days are helpful for judging left ventricular volume load, left ventricular systolic and diastolic function, and HSPDA, during cardiac POCUS.
The mean gestational age of the infants included in this study was 32 weeks. It is likely that extremely preterm infants under 32 weeks were more likely to develop systemic comorbidities such as HSPDA and PPHN within 7 days of birth, and were therefore excluded from the study.
Studies have found that heart size is positively correlated with GA, body weight or BSA of the newborn. The timings in previous studies were within 0, 7, and 28 days of the newborn’s birth and within 36 weeks of the postmenstrual age of preterm babies (13, 16, 27, 28) and are consistent with our findings. Therefore, for newborns, the heart size corresponding to each index can be used as a reference value. There are few research studies on the reference value of heart valve peak flow velocity. Twenty years ago, Skelton et al. (27) found that the peak flow velocities of the mitral valve, tricuspid valve, aorta, and pulmonary artery had no correlation with GA and BW; however, a recent study found that the MV-E/A value of full-term infants at the age of 4–7 days is higher than that at birth (29). Our study found that AV, MV-E, and MV-E/A were positively correlated with GA, BW, and BSA, which may be related to the small sample size of the previous study. This is consistent with recent research results and conforms to the law of continuous development and maturity of left myocardial function with age; based on the above rules, to establish a more comprehensive normal range of echocardiographic measurements in preterm infants, this study lists percentile tables based on GA, BW, and BSA. For a more intuitive and convenient reference, this study lists percentile curves of four important echocardiographic parameters based on GA and BW.
We found that the four indexes of echocardiography including LA/AO, LVEF, LVFS, and MV-A were not correlated with GA, BW, and BSA within 7 days of preterm infants. These four indexes are relatively independent and stable in the early postnatal period. LA/AO is currently the most commonly used method for predicting HSPDA. At present, many studies have suggested that HSPDA may occur when LA/AO ≥ 1.5 (17, 30). This study found that after excluding HSPDA, LA/AO in premature infants within 7 days of age, the median (interquartile range, IQR)is 1.33 (1.14–1.52). Therefore, when the LA/AO value is greater than the 75th percentile of the reference value, it is necessary to be alerted to the occurrence of HSPDA in premature infants. Regardless of adults or children, LVEF and LVFS are considered the most commonly used simple indicators to measure left ventricular systolic function (31, 32). This study found that neither of them changes with GA, BW, and BSA, which is consistent with the findings of a full-term neonatal heart reference value study (12). This shows that in the postnatal cycle transition period, the LV systolic function of premature infants maintained a relatively stable level, and the median (IQR) was 67 (62–73) and 34 (31–38), respectively. MV-A is the maximum peak flow velocity in the late diastole of the left ventricle, representing the contraction of the left atrium, and is one of the indicators reflecting the diastolic function of the left ventricle (32). This study found that the value of MV-A within 7 days of birth of preterm infants was not correlated with GA, BW, and BSA at birth, indicating that the left atrial contractility changes little in the early postnatal period, which may be related to the immature LV diastolic function of preterm infants.
The LVED and LAD indices are important indicators that reflect left ventricular volume load. It can be seen that LVED and LAD display a gradual increasing trend with increase in GA and BW in the centile curves (Figures 2, 3). The parameters have corresponding reference values for different gestational ages and birth weights. Therefore, the reference range of LVED and LAD in this study is helpful to judge whether left heart overload is present or not in preterm infants within 7 days of birth.
The indices of MV-E and MV-E/A are important indicators of LV diastolic function. A study on the LV function of the fetus at 20–36+6 weeks found that MV-E and MV-E/A of the fetus increased with GA (33). A meta-analysis of adults also found that MV-E/A increased with age (34). This study also found that MV-E and MV-E/A increased slightly with increase in GA and BW of preterm infants (Figures 2, 3). In addition, the median MV-E/A value of preterm infants with different gestational ages and birth weights within 7 days of birth was less than 1.00. Compared with full-term infants, MV-E/A decreased significantly at 28 days after birth in preterm infants (35). This shows that the LV diastolic function of premature infants in the early postnatal period is not yet mature. Therefore, the reference range of MV-E and MV-E/A in this study is helpful in determining whether the LV diastolic function of premature infants within 7 days of birth is abnormal.
The reference range of the percentile tables of other cardiac diameters, wall thicknesses, and Doppler blood flow parameters are helpful in judging normal or abnormal hearts.
Strengths and Limitations
We included a large sample of point-of-care echocardiographic data to establish the normal value of echocardiography of preterm infants from multiple angles. As a limitation, this was a retrospective, single-center study, and the time that each baby underwent echocardiography was not uniform. In this study, premature infants with stable circulatory conditions were selected as much as possible to reduce data deviation caused by time inconsistency. Finally, although 2D point-of-care echocardiography is important for cardiac structural and functional monitoring in preterm infants, we need to look at the overall clinical picture and also caveats about very limited data on three-dimensional functional assessments which are possible in older children and adults.
Conclusion
Data from this large study in preterm infants provide reference values of LV structure and function based on different GA, BW, and BSA for preterm infants within 7 days of birth. This data could prove to be invaluable in helping neonatologists to judge normal or abnormal hearts in time and make rapid and correct clinical decisions for critically ill premature infants in the cardiopulmonary transition period. We recommending the involvement of more centers and an intra-group difference analysis in prospective studies.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Peking University Third Hospital Medical Science Research Ethics Committee. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
D-FL: data collection, analysis, interpretation, literature search, and manuscript writing. X-MT and Y-FL: supervision, study design, and interpretation. HZ: data analysis and plotting. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all those neonatologist working at the Peking University Third Hospital in China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
SinghYTissotCFragaMVYousefNCortesRGLopezJet alInternational evidence-based guidelines on point of care ultrasound(POCUS)for critically ill neonates and children issued by the POCUS working group of the European society of paediatric and neonatal intensive care(ESPNIC).Crit Care. (2020) 24:65. 10.1186/s13054-020-2787-9
2.
de BoodeWPKluckowMMcNamaraPJGuptaS. Role of neonatologist-performed echocardiography in the assessment and management of patent ductus arteriosus physiology in the newborn.Semin Fetal Neonatal Med. (2018) 23:292–7. 10.1016/j.siny.2018.03.007
3.
de BoodeWPvan der LeeRHorsberg EriksenBNestaasEDempseyESinghYet alThe role of neonatologist performed echocardiography in the assessment and management of neonatal shock.Pediatr Res. (2018) 84(Suppl. 1):57–67. 10.1038/s41390-018-0081-1
4.
LevyPTTissotCHorsberg EriksenBNestaasERogersonSMcNamaraPJet alApplication of neonatologist performed echocardiography in the assessment and management of neonatal heart failure unrelated to congenital heart disease.Pediatr Res. (2018) 84(Suppl. 1):78–88. 10.1038/s41390-018-0075-z
5.
TissotCSinghY. Neonatal functional echocardiography.Curr Opin Pediatr. (2020) 32:235–44. 10.1097/MOP.0000000000000887
6.
de BoodeWPSinghYMolnarZSchubertUSavoiaMSehgalAet alApplication of neonatologist performed echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn.Pediatr Res. (2018) 84(Suppl. 1):68–77. 10.1038/s41390-018-0082-0
7.
El-KhuffashAMcNamaraPJ. Hemodynamic assessment and monitoring of premature infants.Clin Perinatol. (2017) 44:377–93. 10.1016/j.clp.2017.02.001
8.
DeshpandePBaczynskiMMcNamaraPJJainA. Patent ductus arteriosus: the physiology of transition.Semin Fetal Neonatal Med. (2018) 23:225–31. 10.1016/j.siny.2018.05.001
9.
DaniCCorsiniICangemiJVangiVPratesiS. Nitric oxide for the treatment of preterm infants with severe RDS and pulmonary hypertension.Pediatr Pulmonol. (2017) 52:1461–8. 10.1002/ppul.23843
10.
ChioukhFZAmeurKBHmidaHBMonastiriK. Pericardial effusion with cardiac tamponade caused by a central venous catheter in a very low birth weight infant.Pan Afr Med J. (2016) 25:13. 10.11604/pamj.2016.25.13.8731
11.
KampmannCWiethoffCMWenzelAStolzGBetancorMWippermannCFet alNormal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe.Heart. (2000) 83:667–72. 10.1136/heart.83.6.667
12.
GüzeltaşAEroğluAG. Reference values for echocardiographic measurements of healthy newborns.Cardiol Young. (2012) 22:152–7. 10.1017/S1047951111001259
13.
OranBBodurASArslanDÇımenDGüvençO. Normal M mode values in healthy Turkish children.Turk J Med Sci. (2014) 44:756–63. 10.3906/sag-1208-13
14.
WangSSHongWJZhangYQChenSBHuangGYZhangHYet alRegression equations for calculation of z scores for echocardiographic measurements of left heart structures in healthy Han Chinese children.J Clin Ultrasound. (2018) 46:328–33. 10.1002/jcu.22579
15.
ChoudhrySSalterACunninghamTWLevyPTNguyenHHWallendorfMet alNormative left ventricular m-mode echocardiographic values in preterm infants up to 2 kg.J Am Soc Echocardiogr. (2017) 30:781–9.e4. 10.1016/j.echo.2017.04.010
16.
CaladoCCollinsCDrewSHolbertonJ. Reference echocardiographic measurements in very low birth weight preterm infants.Am J Perinatol. (2019) 36:303–10. 10.1055/s-0038-1667070
17.
KahveciHTaymanCLaloğluFKavasNCiftelMYılmazO. Relationship between hemodynamically significant ductus arteriosus and ischemia-modified albumin in premature infants.Indian J Clin Biochem. (2016) 31:231–6. 10.1007/s12291-015-0523-z
18.
ShepherdJLNooriS. What is a hemodynamically significant PDA in preterm infants?Congenit Heart Dis. (2019) 14:21–6. 10.1111/chd.12727
19.
HanssonLLindTWiklundUÖhlundIRydbergA. Fluid restriction negatively affects energy intake and growth in very low birthweight infants with haemodynamically significant patent ductus arteriosus.Acta Paediatr. (2019) 108:1985–92. 10.1111/apa.14815
20.
LaiWWGevaTShiraliGSFrommeltPCHumesRABrookMMet alGuidelines and standards for performance of a pediatric echocardiogram: a report from the task force of the pediatric council of the American society of echocardiography.J Am Soc Echocardiogr. (2006) 19:1413–30. 10.1016/j.echo.2006.09.001
21.
MertensLSeriIMarekJArlettazRBarkerPMcNamaraPet alTargeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training. Writing group of the American society of echocardiography (ASE) in collaboration with the European association of echocardiography (EAE) and the association for European pediatric cardiologists (AEPC).J Am Soc Echocardiogr. (2011) 24:1057–78. 10.1016/j.echo.2011.07.014
22.
SehgalAMcNamaraPJ. Does echocardiography facilitate determination of hemodynamic significance attributable to the ductus arteriosus?Eur J Pediatr. (2009) 168:907–14. 10.1007/s00431-009-0983-3
23.
KhositsethANuntnarumitPChongkongkiatP. Echocardiographic parameters of patent ductus arteriosus in preterm infants.Indian Pediatr. (2011) 48:773–8. 10.1007/s13312-011-0127-5
24.
LaBountyTMBachDSBossoneEKoliasTJ. Effect of race on echocardiographic measures of cardiac structure and function.Am J Cardiol. (2019) 124:812–8. 10.1016/j.amjcard.2019.05.049
25.
MajongaEDNorrishGRehmanAMKranzerKMujuruHANathooKet alRacial variation in echocardiographic reference ranges for left chamber dimensions in children and adolescents: a systematic review.Pediatr Cardiol. (2018) 39:859–68. 10.1007/s00246-018-1873-0
26.
LopezLColanSStylianouMGrangerSTrachtenbergFFrommeltPet alRelationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the pediatric heart network normal echocardiogram database.Circ Cardiovasc Imaging. (2017) 10:e006979. 10.1161/CIRCIMAGING.117.006979
27.
SkeltonRGillABParsonsJM. Reference ranges for cardiac dimensions and blood flow velocity in preterm infants.Heart. (1998) 80:281–5. 10.1136/hrt.80.3.281
28.
AbushabanLVelMTRathinasamyJSharmaPN. Normal reference ranges for pulmonary artery diameters in preterm infants.Pediatr Cardiol. (2017) 38:1377–84. 10.1007/s00246-017-1673-y
29.
Mrudhula TejaswiGSamanthJVasudevaALewisLKumarPNayakKet alFetal echocardiography at term in diabetic pregnancies helps predict the adverse neonatal outcome – results of a prospective observational study from South India.Indian Heart J. (2020) 72:576–81. 10.1016/j.ihj.2020.09.017
30.
YuLFXuCKZhaoMNiuLHuangXMZhangZQ. Bedside cardiopulmonary ultrasonography evaluates lung water content in very low-weight preterm neonates with patent ductus arteriosus.World J Clin Cases. (2021) 9:1827–34. 10.12998/wjcc.v9.i8.1827
31.
LopezLColanSDFrommeltPCEnsingGJKendallKYounoszaiAKet alRecommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American society of echocardiography pediatric and congenital heart disease Council.J Am Soc Echocardiogr. (2010) 23:465–95; quiz 576–7. 10.1016/j.echo.2010.03.019
32.
BokiniecRWłasienkoPSzymkiewicz-DangelJBorszewska-KornackaMK. Echocardiographic analysis of left ventricular function in term and preterm neonates at week 40 of postconceptional life.Kardiol Pol. (2019) 77:445–50. 10.5603/KP.a2019.0040
33.
PeixotoABBravo-ValenzuelaNJMMartinsWPMattarRMoronAFAraujo JúniorE. Reference ranges for the left ventricle modified myocardial performance index, respective time periods, and atrioventricular peak velocities between 20 and 36 + 6 weeks of gestation.J Matern Fetal Neonatal Med. (2021) 34:456–65. 10.1080/14767058.2019.1609933
34.
D’AscenziFPiuPCaponeVSciaccalugaCSolariMMondilloSet alReference values of left atrial size and function according to age: should we redefine the normal upper limits?Int J Cardiovasc Imaging. (2019) 35:41–8. 10.1007/s10554-018-1427-9
35.
HiroseAKhooNSAzizKAl-RajaaNvan den BoomJSavardWet alEvolution of left ventricular function in the preterm infant.J Am Soc Echocardiogr. (2015) 28:302–8. 10.1016/j.echo.2014.10.017
Summary
Keywords
reference values, echocardiography, point-of-care, preterm infant, cardiology
Citation
Lu D-F, Tong X-M, Liu Y-F and Zhang H (2022) Reference Values for Point-of-Care Echocardiographic Measurements of Preterm Infants in China. Front. Pediatr. 10:894152. doi: 10.3389/fped.2022.894152
Received
11 March 2022
Accepted
13 June 2022
Published
30 June 2022
Volume
10 - 2022
Edited by
Yogen Singh, Cambridge University Hospitals NHS Foundation Trust, United Kingdom
Reviewed by
Vikranth Bapu Anna Venugopalan, Sandwell and West Birmingham Hospitals NHS Trust, United Kingdom; Sajeev Job, Cambridge University Hospitals, United Kingdom
Updates
Copyright
© 2022 Lu, Tong, Liu and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Mei Tong, tongxm2007@126.com
This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.