Pediatric paragonimiasis: a retrospective analysis of cases from a county in south-west China
- 1Department of Pediatrics, Yanjin Country People’s Hospital, Zhaotong, China
- 2Department of Pediatric Intensive Care Unit, Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
Introduction: The clinical manifestations of paragonimiasis are diverse and non-specific, and can easily lead to misdiagnosis. We aimed to analyze the clinical manifestations, laboratory features, treatment, and clinical outcome of children with paragonimiasis in order to improve recognition of this disease and avoid misdiagnosis.
Methods: Children diagnosed with paragonimiasis from August 2016 to July 2022 were included in the study. Information on population informatics, medical history, and laboratory features was extracted from case data. The clinical features of paragonimiasis were retrospectively analyzed.
Results: A total of 45 children were included in this study. All children had, at least, one risk factor. The clinical features mainly included fever, cough, pleural effusion, peritoneal effusion, and subcutaneous nodules. The main imaging findings were alveolar exudation, peritoneal effusion, pleural thickening, and local nodules. The “tunnel sign” finding on computed tomography (CT)/magnetic resonance imaging (MRI) was helpful in establishing the diagnosis of paragonimiasis. After praziquantel treatment, most of the children improved, and one child with cerebral paragonimiasis experienced sequelae.
Conclusion: Most children with paragonimiasis have a good prognosis, but few children can experience sequelae. Avoidance of untreated water and raw food is a simple, feasible, and effective preventive measure.
1. Introduction
Paragonimiasis is an important food-borne parasitic zoonosis caused by Paragonimus spp (1). It was estimated that there were about 23.2 million cases of paragonimiasis worldwide in 2005, including about 5 million heavily infected patients and cases that resulted in death (2, 3). In China, the national survey from 2001 to 2005 showed that the positive rate of serological tests for Paragonimus infection was 1.71%, and the positive rate of serological tests was higher in children under 9 years of age (4). Also, the prevalence of paragonimiasis was higher in Sichuan, Yunnan, and Chongqing due to their special dietary habits (5).
Human paragonimiasis is usually caused by drinking water containing metacercariae or consumption of raw or uncooked food contaminated with Paragonimus spp (1). There are more than 50 species of Paragonimus spp. In China, the main species are Paragonimus westermani and Paragonimus skrjabini (6). Paragonimus skrjabini and Paragonimus westermani have a similar life cycle.
Paragonimiasis is still one of the diseases that affect the health of children, especially in southwest China. The nonspecificity of clinical features can easily result in this disease condition being misdiagnosed as other diseases, such as tuberculosis and cancer (2, 3), especially in a situation in which the medical personnel do not have enough knowledge about the disease or do not pay enough attention to it. This can lead to delayed treatment and even a poor prognosis, as well as an increased medical burden. Thus, the aim of this retrospective study was to improve the recognition of paragonimiasis and reduce misdiagnosis, by analyzing the clinical manifestations, laboratory features, treatment, and prognosis of children with paragonimiasis during the past six years.
2. Materials and methods
2.1. Diagnosis of paragonimiasis
The diagnostic criteria were in accordance with the “Diagnosis of Paragonimiasis” (NO. WS 380-2012), which was drafted by the National Health Commission of the People's Republic of China in 2012 (6). In brief, the diagnosis of paragonimiasis was based on the risk factor, clinical manifestations, laboratory examination, and imaging examination. Suspected cases were defined as those having clinical features, risk factors and significantly elevated peripheral blood eosinophil ratios or absolute values.
Clinically diagnosed cases were defined as suspected cases that had one of following positive test results: (1) a positive intradermal test; (2) positive serum immunology test; (3) abnormal findings in imaging examination; and (4) biopsy showing characteristic pathological changes. Cases were defined as confirmed cases if Paragonimus eggs were found in the sputum or stool, or if Paragonimus worms or eggs were found in a subcutaneous mass or other living tissue and body fluids.
2.1.1. Clinical classification of paragonimiasis
The clinical classification was divided into thoracopulmonary paragonimiasis and extrapulmonary paragonimiasis. Patients with thoracopulmonary paragonimiasis may present with cough, chest pain, rusty or blood-stained sputum, pleural lesions, and other symptoms and signs. Patients with extrapulmonary paragonimiasis may have a subcutaneous mass, abdominal discomfort, and abnormal symptoms and signs of the nervous system. Some mildly infected patients may have no obvious clinical symptoms and signs.
2.1.2. Risk factors
The risk factor extracted the following: (1) previous history of consumption of raw or semi-raw freshwater crabs and crayfish contaminated with Paragonimus, (2) history of consumption of raw or semi-raw mammalian meat (e.g., boar meat and spiny frog), and (3) history of drinking raw or untreated stream water.
2.2. Study population and data collection
From August 2016 to July 2022, 45 children with paragonimiasis infection were admitted to the department of pediatrics, Yanjin County People's Hospital, which is located in a mountainous county with multi-ethnic communities in southwest China. Firstly, patients diagnosed with paragonimiasis, pleural effusion, peritoneal effusion, and pericardial effusion were selected from the case system. Secondly, children with paragonimiasis whose etiological test results were positive for the antibody to Paragonimiasis (ELISA, Novo medical, Co. LTD) or in whom eggs/bodies were detected in the tissue were included in this analysis. Then, case data were collected, including blood routine tests (white blood cell count, eosinophilic percentage, hemoglobin concentration, and platelet count) and erythrocyte sedimentation rate (ESR), computed tomography (CT) or x-ray or magnetic resonance imaging (MRI) examination, and cardiac ultrasound and chest ultrasound. Finally, the following examination data were checked to ensure whether there is any misdiagnosis experience: all children with fever and cough were examined for tuberculous bacteria. Participants with pleural, pericardial and peritoneal effusion underwent puncture. Routine, biochemical, and etiological tests for puncture fluid were performed to exclude other pathogens and tumors.
2.3. Statistical methods
SPSS 22.0 was used for statistical analysis. The median was used for continuous variables because of the skewed distribution of data. Proportion (%) was selected for description of categorical variables.
2.4. Ethics approval and informed consent
Ethical approval for this study was obtained from the Ethics Committee of Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine (NO. XHEC-D-2022-247). As this was a retrospective study, the requirement of obtaining informed consent was waived.
3. Results
3.1. Patient demographics and clinical manifestations
A total of 45 children diagnosed with paragonimiasis infection were included in this study. The median age of these children was 96 months (minimum age of 18 months, and maximum age of 16 years). Among these 45 children, 31 children were male (68.9%) and 14 were female (31.1%); 44 children belonged to the Han race (97.8%) and 1 child belonged to the Miao race (2.2%). Majority of these children were from Yanjin county, while 1 child was from a neighboring county (Shuifu).
3.2. Clinical manifestations and laboratory features
All of these cases were clinically diagnosed. The clinical classification is shown in Table 1. Among these 45 cases, 25 were of the complex type, 14 were of the thoracopulmonary type, and 6 were of the extrapulmonary type. The clinical symptoms of different clinical types are shown in Supplementary Material S1. Clinical symptoms were closely related to the invaded systems. The most common symptoms were fever, cough, chest pain, and subcutaneous nodules.
For all children, results of routine blood tests and ESR were performed at the time of their first visit to the hospital (Supplementary Material S2). The median peripheral white blood cell count in venous blood samples was 11.9*109L, and the percentage of eosinophils was elevated in all but one child. ESR was higher than normal in all children. In all children, Paragonimus antibody test by the enzyme-linked immunosorbent assay (ELISA) was positive.
On pulmonary CT, the most common findings were pleural effusion (68.9%) and alveolar exudation (64.4%) (Supplementary Material S3, Figure 1). Brain edema, nodules, and cerebral hemorrhage were found in children with cerebral paragonimiasis (Supplementary Material S3, Figure 2). Pleural effusion was one of the most important findings of ultrasonography. In addition, ultrasound examination also showed abdominal effusion, pericardial effusion, and subcutaneous nodules.

Figure 1. A 5-year-old boy had habit of drinking untreated water. CT of the lungs showed cystic areas (A), pulmonary exudation (B) and pleural effusion (C).
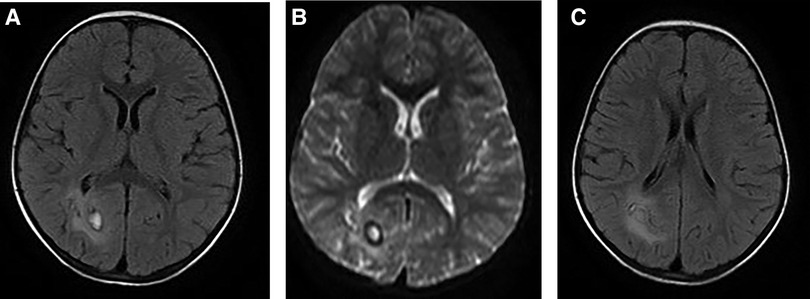
Figure 2. A 5-year-old girl visited our hospital due to limb movement disability. Brain MRI showed the lesion and its surrounding edema (A, B), and tunnel-like transitional signs (C).
3.3. Risk factors
These 45 children had multiple histories of drinking untreated water (n = 16, 35.56%), consuming semi-raw/raw freshwater crab meat (n = 25, 55.56%), or consuming semi-raw/raw meat (n = 10, 22.22%).
3.4. The experience of misdiagnosis
Before the diagnosis of paragonimiasis, 6 cases had been misdiagnosed (Table 2). Three patients presented with “fever, cough, and pleural effusion”, and were initially diagnosed as having pulmonary tuberculosis. A 5-year-old girl presented with “walking instability and vomiting”, and she was initially diagnosed with viral encephalitis. Two cases were misdiagnosed as tumors due to “fever, local mass, and multiple serous effusions”. The anti-tuberculosis treatment and anti-infective therapy were not effective, and the diagnosis was confirmed after detailed diet investigation and antibody detection of Paragonimus.
3.5. Treatment and follow-up outcomes
Praziquantel (Shenyang Hongqi Pharmaceutical Co. LTD) was the first drug of choice. It was given orally for three days at a dose of 75 mg/kg/day, three times a day, and then it was stopped for four days. The longest course of treatment was 12 weeks in a girl with cerebral paragonimiasis. For children with cerebral paragonimiasis, sedation, mannitol, furosemide and other intracranial pressure-lowering treatments were also given. In addition, adverse reactions to praziquantel, such as anaphylaxis, liver damage, and electrocardiographic abnormalities, were monitored. There was no adverse reaction to praziquantel during treatment.
In 43 cases, the original symptoms were markedly improved and serosal effusion disappeared. Eosinophils and ESR levels returned to normal within three months. The percentage of eosinophils returned to normal. CT or MRI showed narrowing and calcification of the lesion. Most children had a good prognosis, but one child with cerebral paragonimiasis experienced sequelae-limb motor disability. During the follow-up, two children, who were brothers, experienced a re-infection after symptom improvement due to consumption of raw meat again. They were first diagnosed with the thoracopulmonary type; and after treatment, cough symptoms disappeared and chest CT lesions calcified. After about five months, these two patients developed cough and subcutaneous nodules, and the antibody tests of paragonimiasis again turned positive; then, they were diagnosed as having the complex type. These two patients ate raw meat again.
4. Discussion
Despite significant improvement in economic and living conditions, paragonimiasis is still one of the diseases that affect the health of children, especially cerebral paragonimiasis. In this study, we retrospectively analyzed the clinical characteristics of 45 children with paragonimiasis in order to improve the recognition of this disease and reduce misdiagnosis.
The clinical manifestations of paragonimiasis in children are diverse and involve many systems, which is closely related to the life history of paragonimiasis. The 45 cases of paragonimiasis included in our study showed 4 clinical subtypes, and many of them were complex and involved multiple systems. The main clinical manifestations included fever, cough, pleural effusion, abdominal effusion, and subcutaneous nodules, which were consistent with previous studies (7). The results of laboratory and imaging examinations were basically consistent with the clinical symptoms.
Being a foodborne parasitic disease, risk factor is very important for establishing the diagnosis of paragonimiasis. The main ways of human infection with Paragonimus are drinking untreated water, consuming raw or semi-raw crayfish and freshwater crab, and consuming raw or semi-raw meat. All children included in this survey had a clear habit of drinking untreated water, eating raw crabs or crayfish, or eating uncooked meat, which is consistent with other studies (7–9). It is not easy for children to accurately recall their dietary history, and this can also lead to misdiagnosis or delayed diagnosis. We recommend careful and repeated questioning of the primary caregiver (sometimes not their parents). During follow-up, two boys (brothers) experienced re-infection because they did not change their habit of eating raw meat. Thus, changing inappropriate eating habits is a simple and effective way to prevent paragonimiasis.
Etiological diagnosis is the gold standard for the diagnosis of paragonimiasis. In this study, due to technical limitations, we only conducted ELISA. In this study, all children were positive for the Paragonimus antibody. In addition to ELISA detection of antibodies, other methods, such as detection of eggs in sputum or feces, and detection of worms or eggs in subcutaneous nodules, other tissues, or body fluids, are also used for etiological determination.
The diversity and lack of specificity of clinical symptoms, signs, and laboratory tests can easily lead to misdiagnosis. As shown in previous studies, paragonimiasis can be easily misdiagnosed as tuberculosis or cancer (10, 11); 6 cases had been misdiagnosed as tuberculosis, tumor, and viral encephalitis in our study. The radiographic findings, which presented in our results, are also found in other diseases, such as pneumonia, lung neoplasms, tuberculosis, central nervous system neoplasms, vascular diseases, and inflammatory disorders (10, 12, 13). In addition to paragonimiasis, a significant increase in the percentage of eosinophils is also seen in many diseases, such as allergic diseases, other parasitic infections, eosinophilic gastritis, hypereosinophilic syndrome, and mycotic infection (10).
The diagnosis of paragonimiasis requires a combination of symptoms, signs, laboratory tests, risk factor, and other factors. In areas with high incidence of pneumofluke disease, the possibility of paragonimiasis should be considered in patients with multi-system symptoms. It is recommended that patients should be carefully enquired about their dietary habits, and etiological detection of Paragonimus should be conducted as soon as possible. If necessary, a tissue biopsy can also be used for establishing the diagnosis (14). CT or MRI may reveal migratory symptoms or the tunnel sign, which are related to the migration of Paragonimus larvae, and these findings may be useful to establish the diagnosis (13, 14).
All children were treated with praziquantel and their symptoms improved after treatment. For cerebral paragonimiasis, surgical intervention is necessary if signs of cerebral herniation occur (14). In our study, four children with cerebral paragonimiasis were treated with praziquantel for up to 12 weeks. After treatment, headache, vomiting, and other symptoms were relieved, but a 5-year-old girl experienced sequelae (limb motor disability) due to persistence of cerebral tissue injury. In addition, patients with cerebral paragonimiasis may experience other sequelae, such as speech disorders and seizures (3, 8). Cerebral paragonimiasis is one of the most serious types of paragonimiasis, and it can cause headache, vomiting, movement disorders, seizures, and even death due to intracranial hypertension (3, 15, 16). All four children had symptoms, such as headache and intracranial hypertension, which were consistent with a previous study (14). The main pathogenic mechanism of cerebral paragonimiasis is direct and indirect damage to the brain tissue. Paragonimus larvae migrate to the brain tissue, directly destroying the local brain tissue. They can damage different parts of cerebral tissue, and the clinical symptoms also vary. Indirect injury is mainly caused by local brain edema, followed by intracranial hypertension. At this point, patients may present with symptoms of intracranial hypertension, such as headache, vomiting, papilledema, and hypertension (14).
However, this study has some limitations that should be acknowledged. It was a retrospective study, and a small number of children were included in the study.
5. Conclusion
An analysis of the clinical, laboratory, and imaging features of children diagnosed with paragonimiasis is helpful to improve the understanding and diagnosis of paragonimiasis. In children, the clinical manifestations of paragonimiasis are diverse and lack specificity, which can easily lead to misdiagnosis. The characteristic tunnel sign on CT or MRI is helpful for making the diagnosis. Children living in areas with high incidence of paragonimiasis and who have fever, cough, local nodules, and multiple serous effusions should be carefully enquired about their dietary habits. Praziquantel is the first drug of choice for the treatment of paragonimiasis. Most patients recover well after treatment, but severe cases may experience sequelae. People living in areas with high incidence of paragonimiasis should be encouraged to change their dietary patterns to prevent paragonimiasis. Avoiding drinking untreated water and eating cold and raw food is a simple but important measure to prevent paragonimiasis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
JY: contributed to program design, data analysis, writing manuscript; LG: contributed to data collection, case admission and analysis; PC, HZ, WC and MQ: contributed to case admission, data collection; CL: contributed to program design, proposal methodology, quality control, review and editing the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We sincerely thank the children and their parents who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1143262/full#supplementary-material.
References
1. Zhou P, Chen N, Zhang RL, Lin RQ, Zhu XQ. Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol. (2008) 24(4):190–6. doi: 10.1016/j.pt.2008.01.001
2. Fürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. (2012) 12(3):210–21. doi: 10.1016/S1473-3099(11)70294-8
3. Chen J, Chen Z, Lin J, Zhu G, Meng H, Cui G, et al. Cerebral paragonimiasis: a retrospective analysis of 89 cases. Clin Neurol Neurosurg. (2013) 115(5):546–51. doi: 10.1016/j.clineuro.2012.06.025
4. Coordinatiing Office of the National Survey on the Important Human Parasitic Diseases. A national survey on current Status of the imporatant parasitic diseases in human population. Chin J Parasitol Parasit Dis. (2005) 23(z1):332–40. doi: 10.3969/j.issn.1000-7423.2005.z1.004
5. Liu Q, Wei F, Liu W, Yang S, Zhang X. Paragonimiasis: an important food-borne zoonosis in China. Trends Parasitol. (2008) 24(7):318–23. doi: 10.1016/j.pt.2008.03.014
6. National Health Commission of the People's Reople's Republic of China, Diagnosis of paragonimiasis (WS 380-2012). (2012).
7. Qian M, Li F, Zhang Y, Qiao Z, Shi Y, Shen J. A retrospective clinical analysis of pediatric paragonimiasis in a Chinese Children's Hospital from 2011 to 2019. Sci Rep. (2021) 11(1):2005. doi: 10.1038/s41598-021-81694-7
8. Xiao K, Zhao DX, Wang YW, Zou YS. Clinical analysis of 10 cases of cerebral paragonimiasis. Chin J Infect Chemother. (2021) 21(01):52–5. doi: 10.16718/j.1009-7708.2021.01.009
9. Laidemitt MR, Zawadzki ET, Brant SV, Mutuku MW, Mkoji GM, Loker ES. Loads of trematodes: discovering hidden diversity of paramphistomoids in Kenyan ruminants. Parasitology. (2017) 144(2):131–47. doi: 10.1017/S0031182016001827
10. Chen J, Qin L, Tang LF. Case report: intermittent chest pain with cough, fever, and pericardial effusion over the course of 1 year in an 11-year-old girl. Front Pediatr. (2022) 10:896824. doi: 10.3389/fped.2022.896824
11. Kong L, Hua L, Liu Q, Bao C, Hu J, Xu S. One delayed diagnosis of paragonimiasis case and literature review. Respirol Case Rep. (2021) 9(5):e00750. doi: 10.1002/rcr2.750
12. Zarrin-Khameh N, Citron DR, Stager CE, Laucirica R. Pulmonary paragonimiasis diagnosed by fine-needle aspiration biopsy. J Clin Microbiol. (2008) 46(6):2137–40. doi: 10.1128/JCM.02424-07
13. Cong CV, Anh TT, Ly TT, Duc NM. Paragonimiasis diagnosed by Ct-guided transthoracic lung biopsy: literature review and case report. Radiol Case Rep. (2022) 17(5):1591–7. doi: 10.1016/j.radcr.2022.02.046
14. Xia Y, Ju Y, Chen J, You C. Cerebral paragonimiasis: a retrospective analysis of 27 cases. J Neurosurg Pediatr. (2015) 15(1):101–6. doi: 10.3171/2014.10.PEDS14208
15. Chai JY. Paragonimiasis. Handb Clin Neurol. (2013) 114:283–96. doi: 10.1016/B978-0-444-53490-3.00023-6
Keywords: paragonimiasis, children, pleural effusion, peritoneal effusion, pericardial effusion
Citation: Jiang Y-x, Li G-q, Pan C-j, He Z-q, Wang C, Mu Q-r and Cao L-l (2023) Pediatric paragonimiasis: a retrospective analysis of cases from a county in south-west China. Front. Pediatr. 11:1143262. doi: 10.3389/fped.2023.1143262
Received: 12 January 2023; Accepted: 4 April 2023;
Published: 17 May 2023.
Edited by:
Azar Shokri, North Khorasan University of Medical Sciences, IranReviewed by:
Sachin Gajanan Damke, Shri Shankaracharya Institute of Medical Science, IndiaEmmanuel Ekanem, University of Calabar, Nigeria
Azadeh Mizani, Pasteur Institute of Iran (PII), Iran
© 2023 Jiang, Li, Pan, He, Wang, Mu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu-lu Cao caolulu0813@163.com; caolulu@xinhuamed.com.cn
 Yong-xin Jiang1
Yong-xin Jiang1  Lu-lu Cao
Lu-lu Cao