Practice patterns in pediatric infectious encephalopathy in four centers in Africa
- 1Department of Pediatrics and Child Health, St. Paul Hospital Millennium Medical College, Addis Ababa, Ethiopia
- 2Department of Pediatrics, University of Washington, Seattle, WA, United States
- 3Department of Molecular and Cell Biology, University of California, Berkeley, CA, United States
- 4Department of Critical Care Medicine, UPMC Children’s Hospital of Pittsburgh, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 5Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle Children’s, Seattle, WA, United States
- 6Department of Global Health, University of Washington, Seattle, WA, United States
- 7Division of Critical Care Medicine, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
- 8Division of Critical Care Medicine, Department of Anesthesiology and Critical Care, The Children’s Hospital of Philadelphia, Philadelphia, PA, United States
Introduction: Infectious encephalopathy (IE), including meningitis, infectious encephalitis, and cerebral abscess, remains prevalent and carries high mortality and morbidity in children, especially in low and middle income countries (LMIC). This study aims to describe the usual care and outcomes of pediatric IE in four LMIC hospitals in sub-Saharan Africa to support evidence-based care guideline development.
Methods: This is a secondary analysis of the Prevalence of Acute Critical Neurological disease in children: A Global Epidemiological Assessment—Developing Countries study, a 4-week, prospective, observational study in children (1 week to 17 years) with IE presenting to referral hospitals in Ethiopia, Kenya, Rwanda, and Ghana. Data collection included diagnostic testing, interventions, and patient outcomes [e.g., mortality, Pediatric Cerebral and Overall Performance Category Scores (PCPC and POPC)].
Results: Seventy-two children with IE were enrolled. Most patients were diagnosed with undifferentiated IE (78%, n = 56). Specific etiologies included cerebral malaria (10%, n = 7), viral encephalitis (4%, n = 3), tuberculosis (4%, n = 3), bacterial meningitis (3%, n = 2), and cerebral abscess (1%, n = 1). Fourteen patients (20%) had a head computed tomography performed. Thirty two (44%) children had a lumbar puncture but only 9 samples (28%) were sent for culture. Median time from diagnosis to antimicrobial therapy was 3 h (IQR 1–12 h). Half (51%, n = 33) of inpatients received intracranial pressure (ICP)-directed treatment but none underwent ICP monitoring. Mortality was 13% (n = 9). The percentage of children with a favorable cognitive score decreased from 95% (n = 62) prior to admission to 80% (n = 52) and 77% (n = 50) at discharge for PCPC and POPC respectively.
Discussion: IE led to considerable morbidity and mortality in this cohort, and evaluation and management varied across the care continuum. Resource limitations and diagnostic constraints may have affected diagnosis-directed therapy and other aspects of management. Further studies are needed to describe the epidemiology and management of IE in LMICs to inform future treatment protocols, the role of technological and human capacity building to support both basic monitoring and interventions, as well as creative new solutions to emergency and critical care in these settings.
Introduction
Infectious encephalopathy (IE), which includes meningitis, infectious encephalitis, and cerebral abscess, remains widely prevalent among children globally despite advancements in prevention. Meningitis and infectious encephalitis accounted for nearly 185,000 deaths and half a million DALYs in 2019 alone. The highest global burden of disease, morbidity, and mortality remains in low- and middle-income countries (LMIC) (1). Mortality due to infectious encephalopathy is twice as high in LMICs compared to high income countries (HICs) (2). With regard to morbidity, survivors of IE in childhood often experience incomplete recovery and many experience decreased quality of life (3–6).
Many factors likely contribute to the global disparities in outcomes. Bacterial meningitis is particularly morbid and has a high incidence in the “Meningitis Belt” in Sub-Saharan Africa stretching from Senegal to Ethiopia. LMICs have a higher prevalence of childhood HIV and malaria, and coinfection with IE confers worse outcomes in this patient population (5, 7–10). Poor healthcare infrastructure, sociopolitical instability, armed conflicts, as well as the challenge of accurately diagnosing neuro-infections, lead to delay in treatment and therefore poor outcomes in these settings (11, 12). International consensus statements and country-specific guidelines around meningitis and encephalitis (13–18) generally lack evidence specific to pediatric IE patients and typically do not account for resource constraints in LMICs. The World Health Organization does provide some high level guidance for meningitis care including antibiotic selection in endemic settings in Africa. However, the guidance does not provide specific management details (17). As a result, diagnosis and management of IE in these settings remain widely variable (19, 20).
This study aims to build on prior knowledge gained from the Prevalence of Acute Critical Neurological disease in children: A Global Epidemiological Assessment—Developing Countries (PANGEA-DC) study and further evaluates usual care, diagnosis, management, and outcomes of pediatric IE in four public referral hospitals in Ethiopia, Kenya, Rwanda, and Ghana (2).
Materials and methods
This is a secondary analysis of PANGEA-DC, which was a 4-week, prospective, observational study in children with IE and traumatic brain injury conducted in 2017. Study sites included four public referral hospitals: Tikur Anbessa Specialized Hospital, in Addis Ababa, Ethiopia; Kenyatta National Hospital, in Nairobi, Kenya; University Teaching Hospital of Kigali, Rwanda; and the Wenchi Methodist Hospital, in Kumasi, Ghana. Study sites were selected as a convenience sample based on existing relationships with the PANGEA research group.
The observational study and data coordinating center (Pittsburgh, PA) were approved by the Institutional Review Board at the University of Pittsburgh, and local regulatory approval was obtained at each study site. Each site was a public referral center with a locally available emergency medical system. A limited number of narrow clinical protocols existed for pediatric patients with IE at the study sites (18). All centers were able to provide noninvasive and invasive ventilation, perform routine laboratory studies, and had head computed tomography (CT) scanners on site.
Children aged 1 week to 17 years presenting to the emergency department with either suspected or confirmed IE were included in this analysis. Participants meeting these criteria were identified by site investigators who provided clinical care or consultation at their respective centers. Data collection occurred using completion of a case report form (CRF) utilizing manual paper chart review and included patient and center demographics, diagnostic testing, medical interventions, and patient outcomes. Specifically, laboratory and brain imaging studies performed in the first 24 h of hospitalization, including cultures, organ supports, and monitoring, treatment for suspected intracranial hypertension (sedatives, analgesics, and hyperosmolar therapy) were specifically queried; not all therapies were available for each patient at each center on a given day. The data was de-identified and transcribed from the CRF to a central database at the University of Pittsburgh Data Coordination Center.
Patient outcomes included hospital mortality and change in Pediatric Cerebral Performance Category (PCPC) and Pediatrics Overall Performance Category (POPC) scores from prior to admission. The PCPC and POPC scores are standardized assessments commonly used to quantify new disability following illness. All scores were calculated by the center investigators; pre-illness scores were assigned based on caregiver interview, and discharge scores were assigned using the medical chart. A favorable score was considered a score of 1 or 2, consistent with no or very mild disability. A score of 6 is consistent with death (21). For the purposes of comparison testing, an unfavorable outcome was defined as a new discharge PCPC or POPC score >2, mortality, and/or a new morbidity (new feeding tube, new tracheostomy tube, hydrocephalus, dysautonomia, or new nosocomial infection).
The primary objective was to report monitoring, testing, therapeutics, and outcomes for children with IE presenting to one of the four hospitals in Sub-Saharan Africa as patients traveled through the care continuum.
Descriptive statistics were reported as median with interquartile range (IQR) as the data set was non-parametric. Less than 10% of the data was missing and therefore not imputed; thus, resulting in variable sample sizes for certain parameters. Data were compared using Chi-square testing, Mann–Whitney testing, Wilcoxon rank sum, and Spearman's correlation as appropriate. All p-values less than 0.05 were considered statistically significant. Stata software (College Station, TX) was used for the analysis.
Results
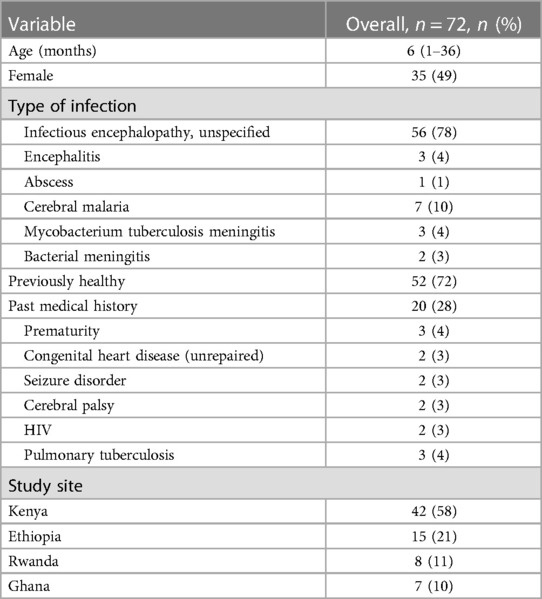
Seventy-two children with IE were enrolled on hospital presentation and followed until their discharge. The median age was 6 months and 49% (n = 35) of patients were female (Table 1). Of these patients, 42 (58%) were enrolled at the Kenyan site, 15 (21%) in Ethiopia, 8 (11%) in Rwanda, and 7 (10%) in Ghana. The majority of patients (72%, n = 52) had no reported past medical history.
Fifty-six (78%) patients had no etiologic infectious organism or process identified as the cause of IE. Of those that did, the most common cause was cerebral malaria (n = 7), followed by unspecified viral encephalitis (4%, n = 3) and tuberculous meningitis (4%, n = 3).
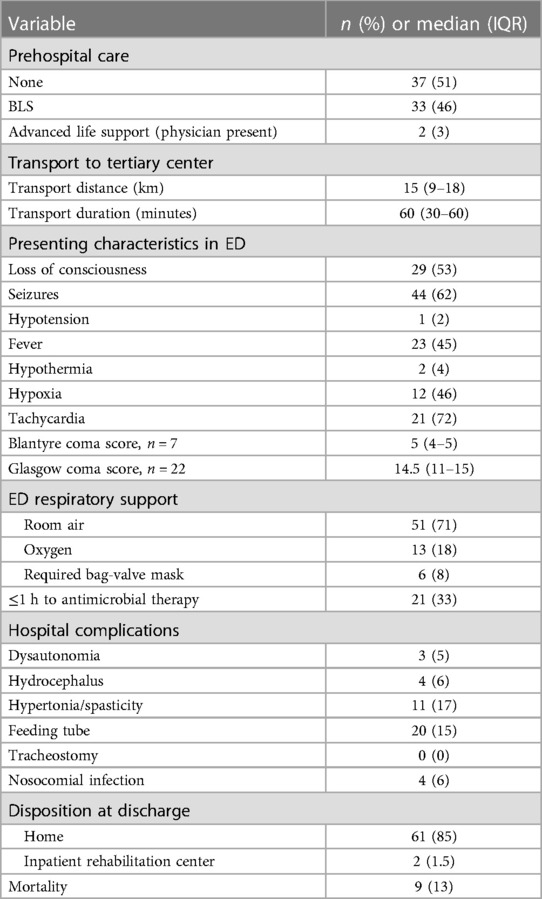
In the emergency department (ED), 36% (n = 26) of patients had a complete set of vital signs including temperature, heart rate, respiratory rate, blood pressure, and oxygen saturation recorded. The most commonly obtained vital sign was temperature (70%), and the least common was oxygen saturation (36%). For those who had any vital sign recorded (n = 51), 45% (23/51) were febrile, 4% (2/51) were hypothermic, 46% (12/26) were hypoxic, 72% (21/29) were tachycardic for age, and 2% (1/44) were hypotensive for age by World Health Organization (WHO) criteria. A Glasgow Coma Scale (GCS) score was completed on 22 patients in the ED with a median score of 14.5 (IQR 11–15). A Blantyre coma scale (BCS) score for preverbal children was completed on 7 patients with a median score of 5 (IQR 4–5). A lumbar puncture (LP) was performed in 44% of patients (n = 32), and 20% (n = 14) had a head computed tomography (CT) performed. Six of these patients (6/14, 43%) had evidence of abscess or infection on CT; the remainder were reported as normal. Laboratory work was performed on the majority of patients (90%, n = 65), with a full blood count as the most common investigation. Sixty-nine percent (n = 50) had a serum sodium value checked, with more than half (58%, n = 29/50) of these results revealing hyponatremia (sodium <135 mEq/L). Blood cultures were sent in 8% (n = 6) of patients and cerebrospinal fluid (CSF) cultures in 13% (n = 9) of patients.
No vasopressors or inotropes were initiated in the ED. Thirteen patients (18%) required oxygen delivered via simple face mask or nasal cannula. Six patients (13%) required bag-mask ventilation temporarily at some point during their ED evaluation. One patient was placed on non-invasive positive pressure ventilation in the ED. In total, 65 (90%) patients were admitted to the hospital; the majority (86%, n = 62) to the general pediatric ward, while 4% (n = 3) were admitted to the pediatric intensive care unit (PICU).
Time to antimicrobial therapy from diagnosis was documented for 92% of patients (n = 62) admitted to the hospital. Median time to receive an antimicrobial was 3 h (IQR 1–12 h, Range 0–80 h). A third of patients (33%, n = 21) received antimicrobial therapy within 1 h of diagnosis.
Continuous pulse oximetry and cardiovascular monitoring were not used on any ward or PICU patients. One PICU patient required vasoactive medications, and thus had a central venous catheter placed; no arterial catheters were placed. Two PICU patients required endotracheal intubation and invasive mechanical ventilation; median duration of mechanical ventilation was 2.5 days (IQR 1–4). A standard electroencephalogram (EEG) was performed for abnormal movements in one ward patient and was negative for seizures; another was performed in one PICU patient and was consistent with focal seizures. No patient had continuous EEG or brain magnetic resonance imaging performed.
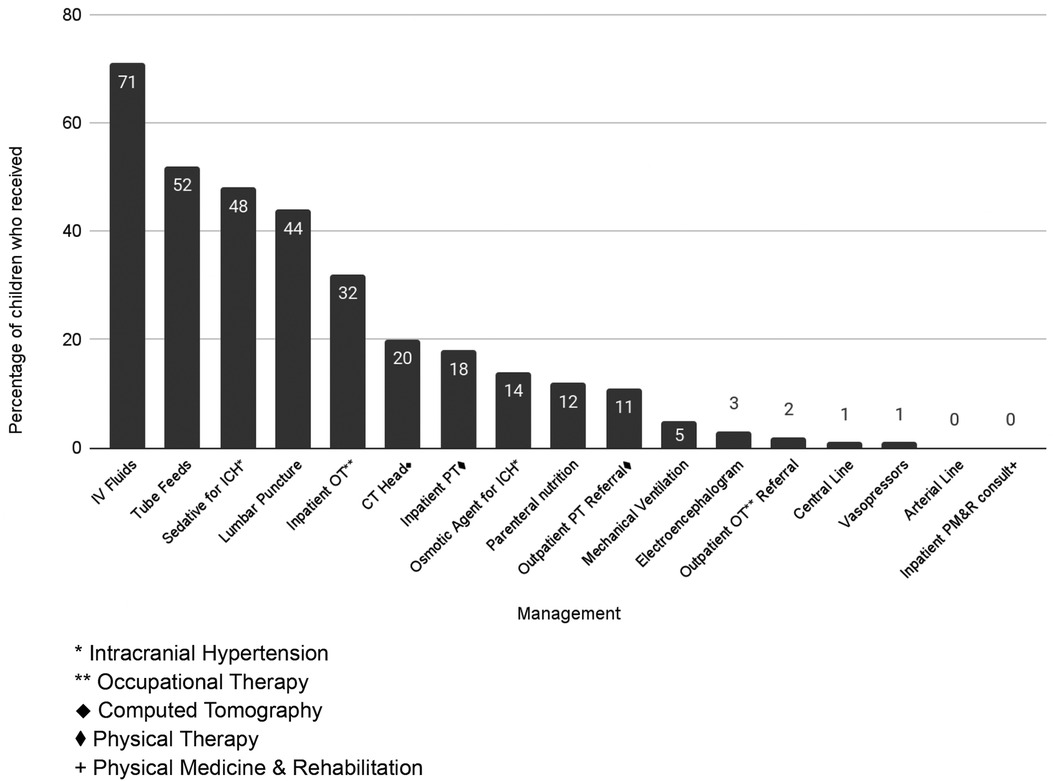
Around half (51%, n = 33) of inpatients received intracranial pressure (ICP)-directed treatment, however ICP monitoring was not available. The most common therapy was in the form of sedation/analgesia (49%, n = 32); seven patients received hyperosmolar therapy for presumed increased ICP (mannitol n = 2, hypertonic saline n = 5) (Figure 1). Nine patients (14%) received multiple medical therapies to decrease ICP. No patients received a decompressive craniectomy.
Median length of stay was 7 days (IQR 2–30) with median ICU length of stay 4.5 days (IQR 1–5 days). The majority of inpatients were discharged to home (83%, n = 54); nine children died. Two patients from Kenya were discharged elsewhere; one to an inpatient rehabilitation unit and another to a skilled nursing facility. Fifteen percent (n = 20) of children left the hospital with a new feeding tube, and 17% (n = 11) had new hypertonia/spasticity. Six percent of children (n = 4) had new hydrocephalus, two of whom required surgical ventriculoperitoneal shunt placement (Table 2).
Overall mortality was 13% (n = 9). Most children (95%, 62/65) had a favorable pre-illness PCPC score of 1 or 2. At hospital discharge, this proportion decreased to 80% (n = 52) with 44 children who scored 1, 8 scored 2, 2 scored 3, 1 scored 4, 1 scored 5, 9 scored 6 (Figure 2). The distribution and change in POPC score was similar; again, the majority (95%, n = 62) had a favorable pre-illness POPC score. At hospital discharge, the percentage of children with a favorable POPC score decreased to 77% (43 children scored 1, 7 scored 2; 4 children scored 3, 1 scored 4, 1 scored 5, and 9 scored 6 (Figure 2).
Physical therapy (PT) was available to 86% (n = 56) of inpatients and was utilized by 18 patients. Similarly, occupational therapy (OT) was available to 49% (n = 32) inpatients and utilized by 13 patients. Of the four survivors who had a worse PCPC score on discharge compared to pre-illness, two received PT; none received OT.
There was no association between hyponatremia (p = 0.694), anemia (p = 0.44), or acidosis (p = 0.08) and an unfavorable outcome. Additionally, there was no difference in outcome when comparing patients who received any ICP-directed therapy against those who did not (p = 0.113) and when comparing those who received lumbar punctures against those who did not (p = 0.061). There was a weak positive correlation (r = 0.222) between discharge PCPC score and length of stay.
Discussion
The PANGEA-DC program assessed access to care, treatments, and outcomes in neurocritical conditions with a focus on four countries in sub-Saharan Africa. This secondary analysis of PANGEA-DC data provided a deeper insight into management variability and system limitations in care of children with IE in these settings.
This analysis has demonstrated (1) inconsistent utilization of available diagnostic tools, including vital sign measurements, GCS, lumbar puncture for CSF sampling, laboratory workup including blood and CSF cultures, and brain imaging, (2) frequent treatment of intracranial hypertension (ICH) without the guidance of ICP monitoring, and (3) a substantial increase in neurological morbidity at discharge in the face of minimal use of physical and occupational therapy.
The majority of children in this cohort had unspecified encephalitis and no identified organism. In fact, most patients had no available culture data which limits diagnosis-directed therapy. However, empiric antimicrobial therapy was started in a timely fashion in most cases. Prompt administration of antibiotics when IE is suspected is well accepted as standard of care. However, there is a lack of consensus around a benchmark time frame for initiating antimicrobial therapy reflected in the variability between official recommendations and vague guidance in the WHO meningitis guidelines (14, 17, 22, 23). Several high quality studies have demonstrated negative impacts on mortality and outcomes when antibiotics are delayed beyond the first several hours of presentation (24–26). Furthermore, sepsis can be a comorbid process in IE patients and there are well established Surviving Sepsis guidelines with a goal of antimicrobial administration within 1 h (27). Future international guidelines for IE should likewise include evidence-based timeframes for delivery of antimicrobial therapy.
Interestingly, 90% of patients had some lab work completed and 44% of patients underwent lumbar puncture, indicating that there is capacity to obtain routine studies. This demonstrates the possibility of standardizing care to allow for a blood and CSF evaluation in cases of suspected IE which would include an infectious workup with cultures, while recognizing that good quality microbiology faces significant infrastructural, technical, and human resource challenges in many hospitals in LMICs. Consensus guidelines for management of IE patients generally do not recognize such limitations and therefore have less utility in these settings (13–17).
While brain imaging was less common (20%) for diagnosis, it can provide valuable information as it did in our patients when abscesses/other localized and loculated infections could be detected. In neonates and infants (with an open anterior fontanelle), cranial ultrasound can be a useful and inexpensive diagnostic method for suspected bacterial meningitis. Ultrasound machines are available at many centers in LMIC, and ultrasonography is a fast and safe procedure (28, 29). Sonographic abnormalities are observed in approximately 65% of pediatric patients with uncomplicated bacterial meningitis and up to 100% in children with bacterial meningitis and severe neurological symptoms (30). Ultrasound and doppler imaging may help provide a quick preliminary diagnosis for initiation of treatment, which can have a significant prognostic impact (31). Current guidelines recommend magnetic resonance imaging (MRI) and CT which are not always easily available in LMICs. Thus, we would advocate for ultrasound to be included in the list of brain imaging modalities as adjunct diagnostic support of IE if available.
We recognize however that clinical assessment and basic bedside monitoring can sometimes be the only available monitoring techniques in some resource-limited settings. The GCS represents a viable alternative to advanced imaging or invasive monitoring as it is easy to learn, reliable with training, and recognised internationally. In children under age 5 years, the modified Glasgow Coma Scale or the BCS can be used (32).
Another notable finding of our study was the use of ICP-directed therapy in the absence of ICP monitoring. Practitioners reported using sedatives, analgesics, and hyperosmolar therapy (mannitol or hypertonic saline). It is not uncommon for practitioners in LMICs to utilize clinical judgment or non-invasive strategies to treat suspected intracranial hypertension, given lack of access to advanced technologies like continuous ICP monitoring. Investigators in South America report similar outcomes in using serial imaging as compared to ICP monitor use in severe traumatic brain injury (33). Ultrasound measuring optic nerve sheath diameter was used successfully in India for the identification of raised intracranial pressure in patients with tuberculous meningitis (28). Distinct changes in transcranial doppler (TCD) measurements were identified in African children with cerebral malaria that permitted phenotypic grouping associated with neurologic outcomes (34). However, robust evidence is required to demonstrate that techniques such as TCD and optic nerve sheath diameter identified with ultrasound detect raised intracranial pressure earlier, lead to appropriate interventions, and then improve outcomes.
Notably, both mortality and morbidity of our study patients was substantial with almost one third leaving the hospital with a new medical device (feeding tube or ventriculoperitoneal shunt). It has been suggested in HIC literature that earlier intervention with neurologically focused physical, occupational, and speech therapy can improve recovery and outcomes for patients following brain injury (35, 36). Access to and utilization of rehabilitative therapies was not consistent for our patient population which possibly contributed to the proportion of patients with an increase in their PCPC and POPC scores. Our study sites reflect an ongoing access issue to rehabilitative services in LMIC.
Limitations
Data collected for this study came from referral centers in four LMICs over a 1 month time frame. As a result, this data may not be generalizable to less-resourced community hospitals. In addition, the data does not capture patients who either had mild cases and did not present to care or those who died prior to transfer to tertiary care. Infectious diseases, especially vector-borne diseases are more common in Sub-Saharan Africa, and are often seasonal. The short data collection period may have missed common pathologies at different times of year. Unfortunately, a longer study duration was limited by the available funding. Limited diagnostic availability led to difficulty confirming the diagnosis of IE and identifying specific pathogens. Furthermore, some aspects of management reported in this study would be specific to certain pathologies and contraindicated in others, such as a lumbar puncture, making it difficult to compare practice patterns. The study did not allow for data collection on daily changes in physical exam or vital signs which limited full understanding of patient progression. Data on specific antimicrobial therapies was not available, which made it challenging to evaluate appropriateness of empiric or targeted therapy and limited generalizability on patient outcomes. Data on use of adjunctive therapy such as steroids was also not available. This study also had a small “n” with limited documentation and no long term follow up due to its point prevalence design. Longer term outcomes for patients such as changes to PCPC and POPC scores, evolving morbidity, readmission rates or post-discharge mortality are not represented here. The study did not include infants in the first week of life which may have excluded a large proportion of perinatally acquired IE.
Conclusion
Infectious encephalopathy is a common childhood disease in LMIC that can have devastating consequences. HICs have developed systems and clinical protocols around the management and treatment of IE, but in LMICs, evidence for such protocols and systems is more limited. The variability in care and the lack of resource use despite availability demonstrated in this study further underscore the need for such protocols. Resource limitations are a reality in LMIC care settings which was reflected in our data. Further studies are needed to describe the epidemiology and management of IE in LMICs to inform future treatment protocols, the role of technological and human capacity building to support both basic monitoring and interventions, as well as creative new solutions to emergency and critical care in these settings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board at the University of Pittsburgh. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
TB: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – review & editing, Writing – original draft. AO: Writing – original draft, Writing – review & editing. JB: Writing – original draft, Writing – review & editing. EF: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing, Formal Analysis, Funding acquisition. A-vSAvA: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing, Formal Analysis, Funding acquisition, Resources. MR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The parent study was funded by the Laerdal Foundation.
Acknowledgments
The authors thank the parents and children of our patients for their assistance. We also thank the remainder of the PANGEA-DC authors: Rashmi Kumar; Patrick T. Wilson; Abenezer Tirsit Aklilu; Tsegazeab Laeke Teklemariam; Shubhada Hooli; Lisine Tuyisenge; Easmon Otupiri; Anthony Fabio; John Gianakas; Patrick M. Kochanek; Derek C. Angus; Robert C. Tasker.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kassebaum NJ, Smith AGC, Bernabé E, Fleming TD, Reynolds AE, Vos T, et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res. (2017) 96(4):380–7. doi: 10.1177/0022034517693566
2. Fink EL, Kochanek PM, Tasker RC, Beca J, Bell MJ, Clark RSB, et al. International survey of critically ill children with acute neurologic insults: the prevalence of acute critical neurological disease in children: a global epidemiological assessment study*. Pediatr Crit Care Med. (2017) 18(4):330–42. doi: 10.1097/PCC.0000000000001093
3. Ramakrishnan M, Ulland AJ, Steinhardt LC, Moïsi JC, Were F, Levine OS. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med. (2009) 7(1):47. doi: 10.1186/1741-7015-7-47
4. Khandaker G, Jung J, Britton PN, King C, Yin JK, Jones CA. Long-term outcomes of infective encephalitis in children: a systematic review and meta-analysis. Dev Med Child Neurol. (2016) 58(11):1108–15. doi: 10.1111/dmcn.13197
5. McCormick DW, Wilson ML, Mankhambo L, Phiri A, Chimalizeni Y, Kawaza K, et al. Risk factors for death and severe sequelae in Malawian children with bacterial meningitis, 1997–2010. Pediatr Infect Dis J. (2013) 32(2):e54–61. doi: 10.1097/INF.0b013e31826faf5a
6. Ramanuj PP, Granerød J, Davies NWS, Conti S, Brown DWG, Crowcroft NS. Quality of life and associated socio-clinical factors after encephalitis in children and adults in England: a population-based, prospective cohort study. PLoS One. (2014) 9(7):e103496. (Bayer A, editor). doi: 10.1371/journal.pone.0103496
7. Arora A. UNICEF DATA. HIV estimates for children dashboard. (2019). Available at: https://data.unicef.org/resources/hiv-estimates-for-children-dashboard/ (accessed February 08, 2024).
8. Fact sheet about malaria [Internet]. Available at: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed February 08, 2024).
9. Cserti-Gazdewich CM, Dhabangi A, Musoke C, Ssewanyana I, Ddungu H, Nakiboneka-Ssenabulya D, et al. Inter-relationships of cardinal features and outcomes of symptomatic pediatric plasmodium falciparum malaria in 1,933 children in Kampala, Uganda. Am J Trop Med Hyg. (2013) 88(4):747–56. doi: 10.4269/ajtmh.12-0668
10. Mwangi I, Berkley J, Lowe B, Peshu N, Marsh K, Newton CRJC. Acute bacterial meningitis in children admitted to a rural Kenyan hospital: increasing antibiotic resistance and outcome. Pediatr Infect Dis J. (2002) 21(11):1042–8. doi: 10.1097/00006454-200211000-00013
11. De Jonge RC, Van Furth AM, Wassenaar M, Gemke RJ, Terwee CB. Predicting sequelae and death after bacterial meningitis in childhood: a systematic review of prognostic studies. BMC Infect Dis. (2010) 10(1):232. doi: 10.1186/1471-2334-10-232
12. Teixeira DC, Diniz LMO, Guimarães NS, Moreira HM de AS, Teixeira CC, Romanelli RM de C. Risk factors associated with the outcomes of pediatric bacterial meningitis: a systematic review. J Pediatr (Rio J). (2020) 96(2):159–67. doi: doi: 10.1016/j.jped.2019.07.003
13. Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. (2013) 57(8):1114–28. doi: 10.1093/cid/cit458
14. Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, et al. The management of encephalitis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis. (2008) 47(3):303–27. doi: 10.1086/589747
15. Pollard AJ, Nadel S, Ninis N, Faust SN, Levin M. Emergency management of meningococcal disease: eight years on. Arch Dis Child. (2007) 92(4):283–6. doi: 10.1136/adc.2006.102384
16. Lundbo LF, Benfield T. Risk factors for community-acquired bacterial meningitis. Infect Dis. (2017) 49(6):433–44. doi: 10.1080/23744235.2017.1285046
17. Meningitis. Available online at: Available at: https://www.who.int/health-topics/meningitis (accessed December 12, 2023).
18. Solomon T. A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bull World Health Organ. (2008) 86(3):178–86. doi: 10.2471/BLT.07.043307
19. Gudina EK, Tesfaye M, Adane A, Lemma K, Shibiru T, Pfister HW, et al. Challenges of bacterial meningitis case management in low income settings: an experience from Ethiopia Trop Med Int Health. (2016) 21(7):870–8. doi: 10.1111/tmi.12720
20. Ministry of Health. University of Nairobi Department of Paediatrics & Child Health. In: Basic Paediatric Protocols. 5th ed. Ministry of Health (2022). Available at: https://paediatrics.uonbi.ac.ke/basic-page/basic-paediatric-protocols (accessed February 08, 2024).
21. Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. (2000) 28(7):2616–20. doi: 10.1097/00003246-200007000-00072
22. Meyfroidt G, Kurtz P, Sonneville R. Critical care management of infectious meningitis and encephalitis. Intensive Care Med. (2020) 46(2):192–201. doi: 10.1007/s00134-019-05901-w
23. Ombelet S, Ronat JB, Walsh T, Yansouni CP, Cox J, Vlieghe E, et al. Clinical bacteriology in low-resource settings: today’s solutions. Lancet Infect Dis. (2018) 18(8):e248–58. doi: 10.1016/S1473-3099(18)30093-8
24. Eisen DP, Hamilton E, Bodilsen J, Køster-Rasmussen R, Stockdale AJ, Miner J, et al. Longer than 2 hours to antibiotics is associated with doubling of mortality in a multinational community-acquired bacterial meningitis cohort. Sci Rep. (2022) 12(1):672. doi: 10.1038/s41598-021-04349-7
25. Auburtin M, Wolff M, Charpentier J, Varon E, Le Tulzo Y, Girault C, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. (2006) 34(11):2758–65. doi: doi: 10.1097/01.CCM.0000239434.26669.65
26. Bodilsen J, Dalager-Pedersen M, Schønheyder HC, Nielsen H. Time to antibiotic therapy and outcome in bacterial meningitis: a Danish population-based cohort study. BMC Infect Dis. (2016) 16:392. doi: doi: 10.1186/s12879-016-1711-z
27. Society of Critical Care Medicine (SCCM). SCCM|surviving sepsis campaign guidelines 2021. Available at: https://sccm.org/Clinical-Resources/Guidelines/Guidelines/Surviving-Sepsis-Guidelines-2021 (accessed Serptember 14, 2023).
28. Sangani SV, Parikh S. Can sonographic measurement of optic nerve sheath diameter be used to detect raised intracranial pressure in patients with tuberculous meningitis? A prospective observational study. Indian J Radiol Imaging. (2015) 25(2):173–6. doi: 10.4103/0971-3026.155869
29. Sippel S, Muruganandan K, Levine A, Shah S. Review article: use of ultrasound in the developing world. Int J Emerg Med. (2011) 4(1):72. doi: 10.1186/1865-1380-4-72
30. Yikilmaz A, Taylor GA. Sonographic findings in bacterial meningitis in neonates and young infants. Pediatr Radiol. (2008) 38(2):129–37. doi: 10.1007/s00247-007-0538-6
31. Littwin B, Pomiećko A, Stępień-Roman M, Spârchez Z, Kosiak W. Bacterial meningitis in neonates and infants - the sonographic picture. J Ultrason. (2018) 18(72):63–70. doi: 10.15557/JoU.2018.0010
32. Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow coma scale at 40 years: standing the test of time. Lancet Neurol. (2014) 13(8):844–54. doi: 10.1016/S1474-4422(14)70120-6
33. Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. (2012) 367(26):2471–81. doi: 10.1056/NEJMoa1207363
34. O’Brien NF, Mutatshi Taty T, Moore-Clingenpeel M, Bodi Mabiala J, Mbaka Pongo J, Ambitapio Musungufu D, et al. Transcranial doppler ultrasonography provides insights into neurovascular changes in children with cerebral malaria. J Pediatr. (2018) 203:116–24.e3. doi: 10.1016/j.jpeds.2018.07.075
35. Dumas HM, Haley SM, Ludlow LH, Rabin JP. Functional recovery in pediatric traumatic brain injury during inpatient rehabilitation. Am J Phys Med Rehab. (2002) 81(9):661. doi: 10.1097/00002060-200209000-00005
Keywords: pediatric, encephalopathy, meningitis, low middle income countries (LMICs), infectious diseases, child, cerebral abscess
Citation: Bacha T, Obremskey A, Buxton J, Fink EL, von Saint Andre-von Arnim A and Raees M (2024) Practice patterns in pediatric infectious encephalopathy in four centers in Africa. Front. Pediatr. 12:1304245. doi: 10.3389/fped.2024.1304245
Received: 29 September 2023; Accepted: 2 February 2024;
Published: 23 February 2024.
Edited by:
Mohammod Jobayer Chisti, International Centre for Diarrhoeal Disease Research (ICDDR), BangladeshReviewed by:
Masashi Mizuguchi, The University of Tokyo, JapanCeren Günbey, Hacettepe University Hospital, Türkiye
© 2024 Bacha, Obremskey, Buxton, Fink, von Saint Andre-von Arnim and Raees. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tigist Bacha tigistbacha@yahoo.com
†These authors share first authorship
‡These authors have contributed equally to this work and share last authorship
 Tigist Bacha
Tigist Bacha Alexandra Obremskey
Alexandra Obremskey Jessica Buxton3
Jessica Buxton3  Ericka L. Fink
Ericka L. Fink Amelie von Saint Andre-von Arnim
Amelie von Saint Andre-von Arnim Madiha Raees
Madiha Raees