Serum vitamin D and obesity among US adolescents, NHANES 2011–2018
- 1Department of Pediatrics, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, China
- 2Department of Pediatrics, China-Japan Friendship Hospital, Beijing, China
- 3Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 4Center for Evidence-Based Medicine, Capital Institute of Pediatrics, Beijing, China
Background and objectives: Childhood obesity is highly prevalent worldwide. We aimed to assess whether serum 25-hydroxyvitamin D was associated with general/central obesity among US adolescents, and further to explore the mediatory impact of homeostasis model assessment of insulin resistance (HOMA-IR) on this association.
Methods: This study is cross-sectional in design. Study adolescents were enrolled from the National Health and Nutrition Examination Survey (NHANES), 2011–2018. Serum 25-hydroxyvitamin D categories associated with general (indexed by body mass index) and central (indexed by waist circumference to height ratio) obesity were regressed. The possible mediatory effect of HOMA-IR on this association was explored. The nonlinear and dose-response association was examined by restricted cubic spline (RCS) test.
Results: Total 2,696 adolescents were eligible for inclusion, and the mean age of all adolescents was 15.4 years. Overall, the percentage of general and central obesity was 38.0% and 38.6%, respectively. Compared with adolescents with sufficient vitamin D, adolescent with deficient and insufficient vitamin D intake were associated with general obesity and central obesity; fully-adjusted OR for general obesity was 1.602 (95% CI: 1.161–2.211) and 1.659 (1.385–1.986), and fully-adjusted OR for central obesity was 2.025 (1.445–2.837) and 1.557 (1.287–1.884), respectively, while there was no observable significance in adolescents with possibly harmful vitamin D. The proportion mediated by HOMA-IR was estimated to be 31.7% for global obesity and 50.3% for central obesity (both P < 0.05). More stratified analyses were presented, and identified that the association with general obesity was particularly present among Mexican American, while with central obesity among Non-Hispanic Black adolescents.
Conclusions: Our findings indicate that deficient or insufficient 25-hydroxyvitamin D concentrations were associated with the significant risk of general and central obesity among US adolescents, and approximately 30% and 50%, respectively, of these associations were mediated by HOMA-IR.
Introduction
Childhood obesity is a prevalent public health issue worldwide (1). Statistics from the World Health Organization show that the global prevalence of overweight or obesity in children and adolescents increased from 5% in 1975 to 18% in 2016. In the United States (US), 34.5% of adolescents 12–19 years of age were overweight or obese (2). Generally, obesity in adolescence persists into adulthood, and it can trigger the development of chronic diseases (such as diabetes and cardiovascular disease) and premature death, as well as psychological social and emotional well-being and self-esteem issues (3–5). Hence, identification of the risk factors of childhood obesity may enhance knowledge on underlying causes and inform therapeutic strategies toward a more effective prevention of obesity and resultant complications.
It is increasingly recognized that vitamin D is an essential nutrient responsible for health maintenance of bones and muscles (6). Vitamin D deficiency, as reflected by low 25-hydroxyvitamin D concentrations, is highly prevalent in children with obesity (7). Vitamin D is fat-soluble and can be affected by diet and sunlight, as well as obesity and sedentarism. Observational studies have shown that vitamin D deficiency was associated with general and central obesity in adults (7); however, evidence is sparse in children and adolescents. Biologically, vitamin D can regulate cell differentiation and growth via binding to vitamin D receptor in most body cells (8). Cell-signaling mechanisms linking vitamin D to obesity are multifaceted, possibly involving matrix metalloproteinases, mitogen-activated protein kinase pathways, reactive oxygen species, and nitric oxide synthase (8). In addition, vitamin D, from either exogenous or endogenous sources, becomes sequestered within adipose tissues (9), and excess adiposity may directly affect its bioavailability (10). Above lines of evidence collectively inspire us to speculate that vitamin D deficiency is a potential risk trigger for obesity in children and adolescents. However, the relationship between them can be bidirectional, since obesity can also lead to vitamin D deficiency (11).
Moreover, the relation between 25-hydroxyvitamin D and insulin resistance has been widely assessed (12–15). For example, in Turkey, serum 25-hydroxyvitamin D was found to be negatively correlated with insulin and homeostasis model assessment of insulin resistance (HOMA-IR) in children 5–17 years of age (16). In prepubertal Chilean children, there was an inverse association of 25-hydroxyvitamin D with adiposity and insulin resistance indicators (17). Given the close relation between insulin resistance and obesity, it is reasonable to speculate the association between 25-hydroxyvitamin D and obesity might be mediated through insulin resistance.
To address above two speculations, we aimed to assess whether serum 25-hydroxyvitamin D was associated with general/central obesity among US adolescents, and further to explore the mediatory impact of HOMA-IR on this association.
Methods
Data source and study subjects
This study used data from the National Health and Nutrition Examination Survey (NHANES), which is an ongoing two-year-cycle nationally representative survey in the US to monitor the health and nutritional status of adults and children. Detailed design and data collection of NHANES has been reported previously (18–20). All survey protocols were approved by the research ethics review board at the National Center for Health Statistics, and written informed consent was obtained from all respondents.
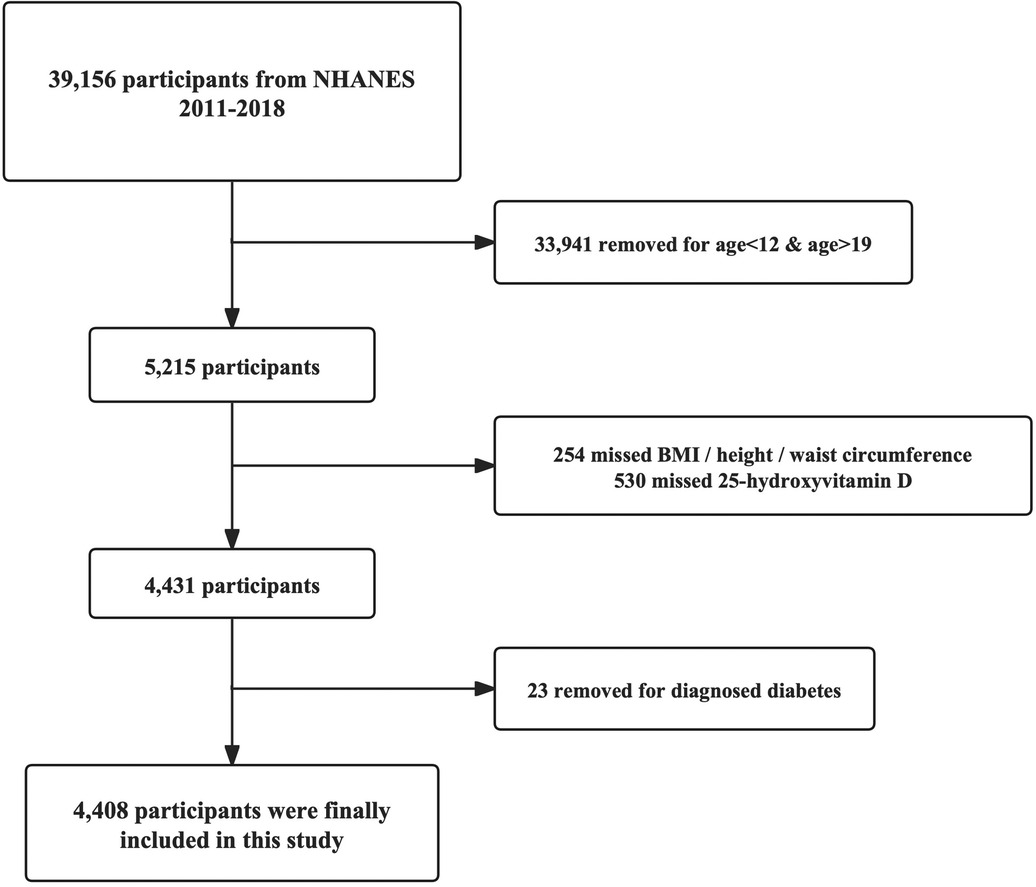
All subjects were selected from respondents attending 4 NHANES cycles, 2011–2018. Only adolescents 12–18 years of age were eligible for inclusion, with complete data on serum 25-hydroxyvitamin D concentrations and body mass index (BMI) or waist circumference. Adolescents were excluded if they had a diagnosis of diabetes mellitus. In total, 2,696 adolescents were finally analyzed in this study, and the selection process is illustrated in Figure 1.
General/central obesity
In this study, both general and central obesity were assessed. Specifically, body mass index (BMI) was chosen as an indicator of general obesity, and waist circumference to height ratio (WHtR) as an indicator of central obesity (21). Data on body measures were collected from the Mobile Examination Center (MEC) by trained health technicians.
BMI is calculated as weight in kilograms divided by height in meters squared. The classification are based on the Centers for Disease Control and Prevention's sex-specific 2,000 BMI-for-age growth charts for the United States. Overweight is BMI 85th percentile to <95th percentile. Obesity is BMI ≥ 95th percentile. Overweight and obesity was combined to define general obesity (18). WHtR is calculated as waist circumference in centimeters divided by height in centimeters. Central obesity is defined as WHtR of at least 0.5, as proposed previously (22).
Serum 25-hydroxyvitamin D
Serum 25-hydroxyvitamin D concentrations were assayed by the standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. According to the CDC (23), serum 25-hydroxyvitamin D concentrations less than 30 nmol/L were considered deficient, 30–50 nmol/L insufficient, 50–125 nmol/L sufficient, and exceeding 125 nmol/L possibly harmful.
HOMA-IR
Fasting glucose and insulin blood samples were collected by trained phlebotomists in the MEC. HOMA-IR were calculated by the formulas: fasting glucose (mmol/L)*fasting insulin (µU/ml)/22.5. HOMA-IR used for the mediation analysis were treated as categorical using the cut-offs for age and gender (24).
Other factors
During the in-home interview, demographic information was collected through the Computer-Assisted Personal Interview system, including age, sex, race/ethnicity, and poverty income ratio (PIR). PIR was calculated by dividing family income to the poverty guidelines specific for each survey year, and 2% was set as the threshold below which difficult financial conditions were assumed (25).
Dietary energy and vitamin D intakes were estimated using the mean of two 24-h dietary recall interviews from the Dietary Interview Questionnaire (19). Physical activity was obtained from the NHANES Physical Activity Questionnaire (PAQ). Different types of physical activities have different MET values and NHANES provides recommended MET values for sports of different types. Physical activity was calculated according to the following formula: physical activity (MET-h/week) = MET × weekly frequency × duration of each physical activity (20).
All variables under study can be found on the official NHANES website https://www.cdc.gov/nchs/nhanes/index.htm.
Statistical analyses
The Shapiro–Willk test was used to test the normality of distribution of continuous data, and according to its results, continuous variables were represented as median (interquartile range) because of skewed distribution. Categorical variables were represented as numbers and percentages. The Kruskal–Wallis test and χ2 test were used to compare the differences between groups according to vitamin D status. If significant, pairwise post-hoc Dunn's test. The association of serum 25-hydroxyvitamin D on a categorical scale with general and central obesity was assessed using the Logistic regression analyse. Three models were built. Model 1 did not adjust for any covariates. In model 2, we adjusted for age, sex, race/ethnicity, and PIR; In model 3, we further adjusted for energy intake and physical activity. We conducted stratified analyses according to age at baseline, sex, race, PIR and physical activity in the logistic regression models. Tests for interaction were performed by adding interaction terms in Model 3.
Besides overall association, subgroup analyses were also performed according to age, sex, race/ethnicity, PIR, and physical activity, respectively. The Sobel-Goodman mediation test was used to examine whether HOMA-IR can mediate the association of 25-hydroxyvitamin D with general and central obesity. Nonlinear and dose-response relation was examined by restricted cubic spline (RCS) curve. The possibility of unmeasured confounding factors was evaluated using the E-values (26).
Two-sided P below 0.05 was considered statistically significant. Data were analyzed using Stata software version 17 (StataCorp LP, TX, USA) and R programming environment version 4.2.3 available at website https://www.r-project.org/.
Results
Baseline characteristics
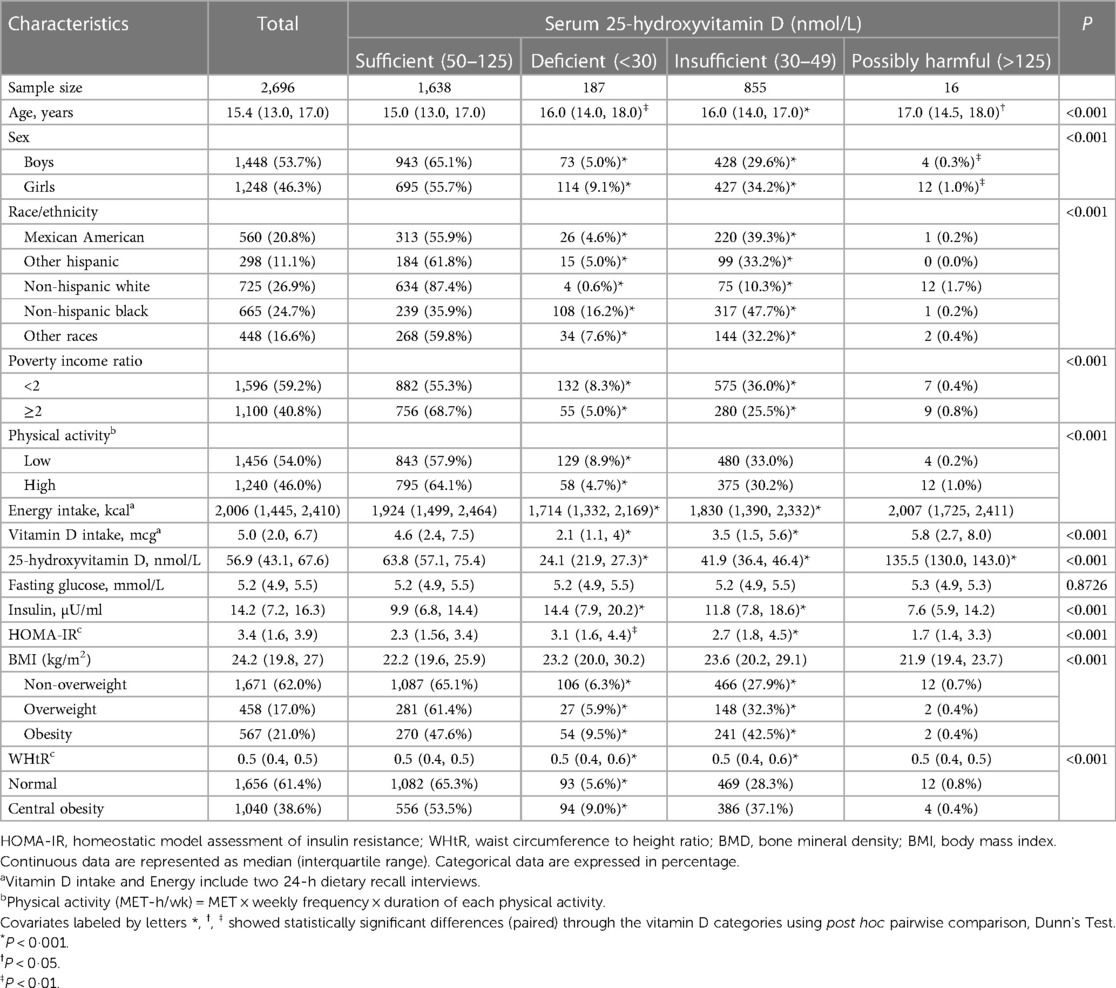
The baseline characteristics of 2,696 study adolescents by serum 25-hydroxyvitamin D categories are presented in Table 1. The mean age of all adolescents was 15.4 years, and boys accounted for 53.7%. The percentage of general and central obesity was 38.0% and 38.6%, respectively. Besides fasting glucose, all baseline characteristics differed significantly across 25-hydroxyvitamin D categories (P < 0.001).
Compared with adolescents with sufficient vitamin D, adolescents who had deficient and insufficient serum vitamin D were more likely to be older, girls, non-Hispanic Black (predominantly, both deficient and insufficient) and Mexican American (only insufficient), with low physical activity and PIR, with lower vitamin D and total energy intakes, with higher insulin levels and HOMA-IR, and with global and central obesity.
Overall analyses
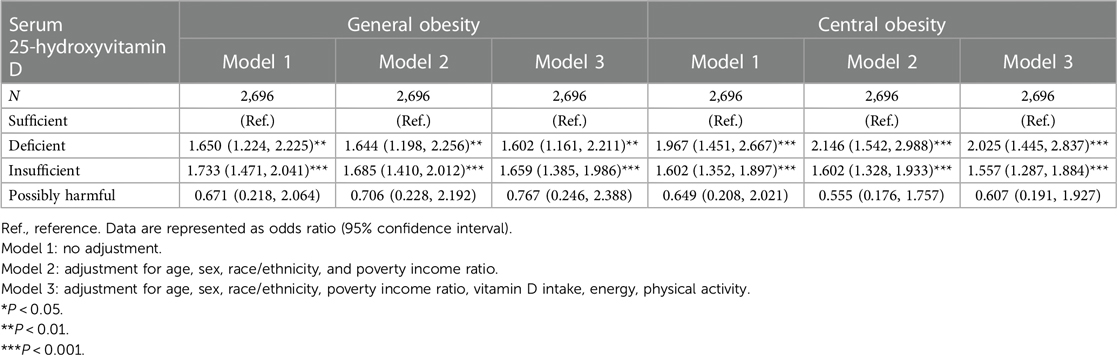
Table 2 shows the association of serum 25-hydroxyvitamin D with general and central obesity. Taking adolescents with sufficient 25-hydroxyvitamin D as a reference, the risk for both general and central obesity was significantly increased in adolescents with deficient and insufficient 25-hydroxyvitamin D before and after adjusting for confounding factors. In adolescents with deficient and insufficient vitamin D, fully-adjusted OR associated with general obesity was 1.602 (95% CI: 1.161–2.211) and 1.659 (1.385–1.986), with central obesity was 2.025 (1.445–2.837) and 1.557 (1.287–1.884), respectively. By contrast, no hint of statistical significance was seen in adolescent with possibly harmful 25-hydroxyvitamin D.
The dose-response relation of serum 25-hydroxyvitamin D with general and central obesity was also explored (Figure 2), and it was statistically significant at a level of 1‰. Based on the dose-response, vitamin D below about 55 nmol/L increases the risk of general and central obesity.
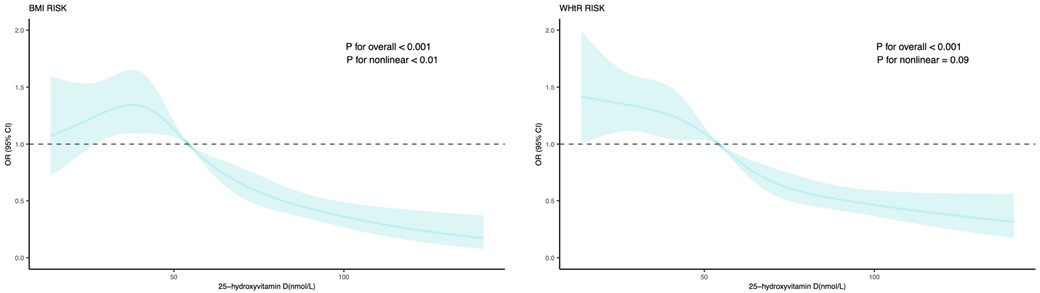
Figure 2. Dose-response relation of serum 25-hydroxyvitamin D with general and central obesity. Effect-size estimates were calculated after adjusting for age, sex, race/ethnicity, poverty income ratio, vitamin D intake, energy, physical activity. BMI, body mass index; WHtR, waist circumference to height ratio; OR, odds ratio; 95% CI, 95% confidence interval.
Mediatory effect
Table 3 shows the mediation effect of HOMA-IR on the association of serum 25-hydroxyvitamin D with general and central obesity. Total, natural direct, and natural indirect effects were explored, with statistical significance at a level of 5%. The proportion mediated by HOMA-IR reached as high as 31.7% for global obesity and 50.3% for central obesity (both P < 0.01).
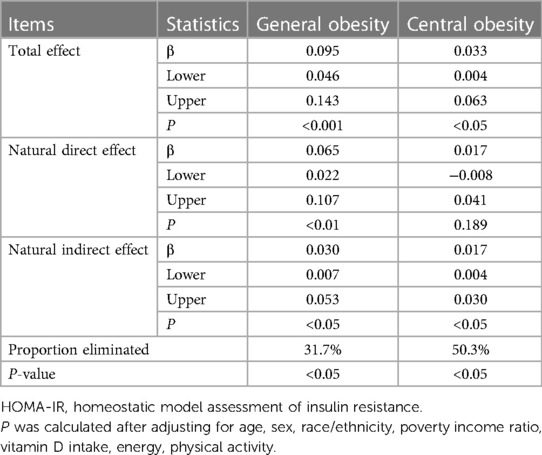
Table 3. Mediatory effect of HOMA-IR on the association of serum 25-hydroxyvitamin D with general and central obesity.
Subgroup analyses
To further account for possible confounding effects, subgroup analyses were conducted according to age, sex, race/ethnicity, PIR, and physical activity after adjusting for confounders and taking sufficient 25-hydroxyvitamin D as a reference (Table 4). The associations between vitamin D and obesity were not significantly modified by age, sex, race/ethnicity, PIR, and physical activity (all P-interaction >0.05).
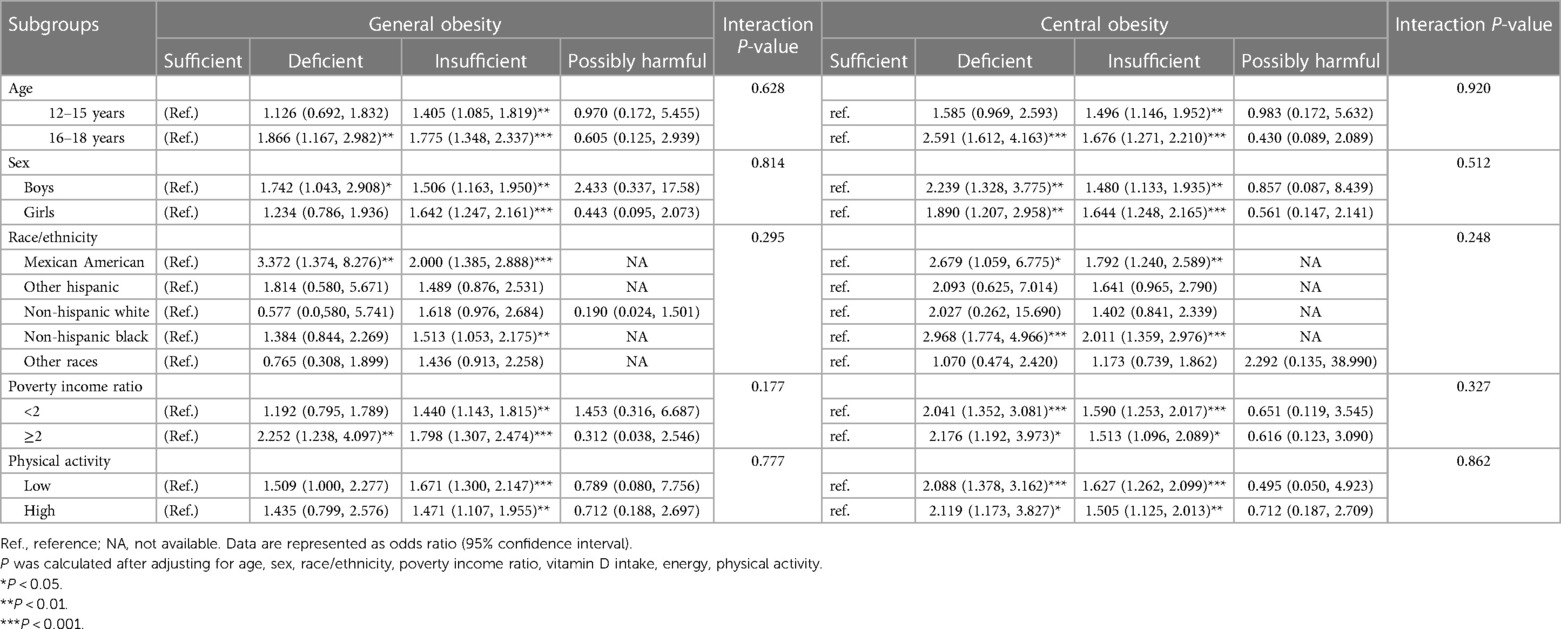
Table 4. Subgroup analyses on the association of serum 25-hydroxyvitamin D with general and central obesity.
By age, the risk for general and central obesity conferred by deficient and insufficient serum 25-hydroxyvitamin D was more obvious in adolescents aged 16–18 years than 12–15 years. By sex and race/ethnicity, deficient serum 25-hydroxyvitamin D was associated with general obesity in boys but not in girls, and in adolescents of Mexican American descent but not of others. Deficient and insufficient serum 25-hydroxyvitamin D concentrations were associated with the significant risk of central obesity in both sexes and in adolescents of Mexican American and non-Hispanic Black descents.
PIR and physical activity did not significantly influence the risk for centripetal obesity among vitamin D both insufficient and deficient adolescents and the risk for global obesity among vitamin D insufficient adolescents, but among vitamin D deficient adolescents the risk for global obesity was statistically significant only among those with high PIR (note: for other vitamin D deficient PIR and physical activity subcategories, the risk was also high but did not rich significance).
Still, there was no observable significance across all subgroups in adolescents with probably harmful 25-hydroxyvitamin D.
The stratified estimated probabilities for general and central obesity with increasing serum 25-hydroxyvitamin D concentrations are illustrated in Supplementary Figure S1.
Unmeasured confounding evaluation
The possibility of unmeasured confounders was evaluated by using the E-values (Table 5). Relative to adolescents with sufficient 25-hydroxyvitamin D, E-values were estimated to be 1.846 and 1.897 in adolescent with deficient and insufficient 25-hydroxyvitamin D for general obesity, and 2.199 and 1.804 for central obesity. As these E-values were larger or almost equivalent to effect-size estimates of serum 25-hydroxyvitamin D associated with general and central obesity, the likelihood for the existence of unmeasured confounders was relatively low.
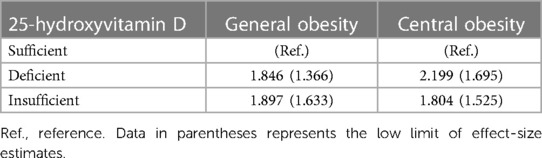
Table 5. E-values for the association of serum 25-hydroxyvitamin D with general and central obesity.
Discussion
This study aimed to assess whether serum 25-hydroxyvitamin D was associated with general/central obesity among US adolescents, and explore the mediatory impact of HOMA-IR on this association. The key findings of this study are that deficient and insufficient 25-hydroxyvitamin D concentrations were associated with general and central obesity among the USA adolescents, and approximately 30% and 50%, respectively, of these associations were mediated by HOMA-IR. To our knowledge, this is the thus far the first study that has explored the mediatory effect of HOMA-IR on the association between vitamin D and obesity in adolescents.
Some studies have reported that vitamin D was significantly associated with obesity in children and adolescents, whereas others failed to support this claim. For instance, in 494 children and adolescents from Colombia, insufficient 25-hydroxyvitamin D was associated with over 70% increased risk of overweight or obesity (27). In support of this association, a study of 2,680 children and adolescents from China demonstrated over 90% increased risk of overweight or obesity was attributable to deficient and insufficient vitamin D (28). By contrast, in 2,818 children and adolescents from China, vitamin D status was not associated with obesity (29). Another studies in 1,090 adolescents from Iran also failed to document any significant association between 25-hydroxyvitamin D and anthropometric measures (30). The reasons behind this controversy are manifold, likely relating to diverse origins of study populations, differing age groups, different eligibility criteria, low statistical power, or insufficient consideration of confounding factors. Bearing these reasons in mind, we employed the high-quality NHANES data, with continuous quality assurance and quality control, and among 2,696 adolescents 12–18 years of age assessed the association of 25-hydroxyvitamin D, the major circulating form of vitamin D, with both general and central obesity after considering a wide panel of confounders. It is worth noting that deficient and insufficient 25-hydroxyvitamin D concentrations were significantly and independently associated with the increased risk of both general and central obesity, consistent with the results of a recent meta-analysis by Fiamenghi and Mello (31). Nevertheless, we agree that much needs to be done before translating our findings into practice from both clinical and public health standpoints.
There are two possible mechanisms underlying the association between vitamin D and obesity. One is that vitamin D can directly influence fat accumulation, re-distribution and metabolism since it may regulates lipolysis and lipid synthesis and improve adipose tissue inflammation. Another possible mechanism is that vitamin D may indirectly contribute to weight gain and fat accumulation by regulating parathyroid hormone, calcium, and leptin (8, 32–34).
Based on above epidemiological and biological evidence, it is expected that proper supplementation of vitamin D, if involved, can form an effective preventive strategy for the onset and progression of obesity in adolescents. However, the evidence for the beneficial effect of vitamin D supplementation on BMI is conflicting, with no significant weight reduction observed in overweight and obese subjects after supplementation (35–37). On the other side, some authors observed a reduction in truncal subcutaneous fat and reversal to normoglycemia in the overweight/obese subjects after supplementation (35, 37–39). Therefore, this hypothesis requires more rigorous and evidence-based experiments to be designed for further research.
Since there is much controversy on the cause-effect direction of the association of general and central obesity with vitamin D (11), we performed supplementary analyses in the opposite way: the influence of general and central obesity on serum 25-hydroxy vitamin D levels and the mediatory impact of HOMA-IR on this association (Supplementary Material Tables S1,S2). The results were quite similar, both types of obesity were associated with low vitamin D levels, with HOMA-IR explaining significantly the association of central obesity with vitamin D levels, while less explaining the association of general obesity with vitamin D levels. Potential causative mechanisms for the low serum 25-hydroxyvitamin D concentrations in general/central obesity include volumetric dilution, sequestration into adipose tissue, limited sunlight exposure of overweight/obese people, and reduced vitamin D synthesis and activation in the skin, adipose tissue and the liver (35, 39, 40).
Besides the convincing association between vitamin D and obesity, we explored the possible mediatory effect of insulin resistance on this association in this study. Growing data indicate the close relation between 25-hydroxyvitamin D and HOMA-IR, both of which were reported to be significantly associated with obesity in children and adolescents (41–43). Yet, a literature search has failed to reveal any evidence on the mediatory of HOMA-IR on the vitamin D-obesity association. To fill this gap in knowledge, we interestingly found that over 30% and 50% of these associations, respectively, for general and central obesity, can be mediated by HOMA-IR. The proposed mechanisms for the role of 25-hydroxyvitamin D in improving insulin resistance in obesity include reducing inflammation, enhancing peripheral and hepatic glucose uptake, and regulating insulin synthesis and secretion by pancreatic β cells through direct and indirect pathways (44). In addition, we also noticed that association with central obesity was lost when HOMA-IR was taken into account (which means that insulin resistance mediated completely this association), while with general obesity remained (insulin resistance did not mediate completely the association with general obesity). This is in accordance with the theory of dilution of vitamin D in fat tissue (11). However, association with insulin resistance has an additional, independent influence, apart from fat tissue dilution. This means that both mechanisms are generally included in general obesity, while in central obesity, the mechanism of insulin resistance predominates. Also in our supplementary study, approximately 28.6% and 36.6%, respectively, of these associations were mediated by HOMA-IR. The presence of vitamin D receptors and vitamin D metabolizing enzymes in insulin-sensitive organs suggests that vitamin D may be involved in glucose and lipid metabolism and may be associated with insulin sensitivity. Several studies support a role for vitamin D in regulating glucose and lipid metabolism in several insulin-sensitive tissues, including adipose tissue, skeletal muscle, liver, and pancreatic insulin secretion (14, 45). As well, a potential role for vitamin D in intestinal barrier function and metabolism has been proposed (46). However, the opposite direction can exist in the relation insulin resistance/diabetes and vitamin D metabolism. For example, Aatsinki et al. (47) showed that vitamin D metabolizing enzymes are altered in experimentally induced diabetic states (both type 1 and type 2 diabetes), leading to the repression of vitamin D bioactivation and induction of deficiency, by the mechanisms that include the peroxisome proliferator-activated receptor-gamma coactivator 1-α and estrogen-related receptor α (PGC-1α-ERRα), and the glucocorticoid receptor pathways (47).
To further investigate the association between 25-hydroxyvitamin D and obesity/central obesity, we performed subgroup analyses by age, sex, race, PIR, and PA. From the results, elder age, male, Mexican American and Non-Hispanic Black, poverty and low exercise were significantly associated with higher odds of obesity/central obesity. Age and ethnicity have been shown to affect serum vitamin D status (48, 49). Among them, in Mexican Americans and non-Hispanic blacks vitamin D levels were significantly associated with the prevalence of obesity. Moreover, there was an interesting finding in our study that the association with general obesity was particularly present among Mexican Americans, while with central obesity among Non-Hispanic Black adolescents. The explanations for such findings among these two populations could be the darker color of skin (50, 51), the increased prevalence of obesity and IR (52, 53), and specific lifestyle factors (exposure to sun, physical activity, low dietary vitamin D intake and rare use of supplements) (54). The possible reason that the associations were more significant with vitamin D insufficiency than with deficiency (Table 4) is that the number of subjects included in the deficient group was much lower than in the insufficiency group.
The E-value is the minimum strength of association (scaled by the risk ratio) between unmeasured confounders and treatments and outcomes in conditions where confounders have been measured to fully explain the association between a given treatment and outcome. Simply put, this means that, controlling for measured confounders, if the unmeasured confounding effect wants to completely erase the association effect between exposure and outcome in our study, then the unmeasured confounding effect should be minimized to this value in order to achieve this goal. So, even if there are covariates that are not considered for various reasons, the possibility of unaccounted confounding factors is low as reflected by the E-values (26).
There are several limitations in our study. Firstly, this was a cross-sectional study, which precludes comments on the causal impact of vitamin D on general and central obesity in adolescents. Secondly, some variables were not considered due to unavailability across surveys, such as intake of vitamin D supplement, sedentary behavior, sun exposure, parental genetics, and fat indicators in body composition. As reflected by the E-values, the possibility of unaccounted confounding factors was relatively low. Thirdly, vitamin D intake was collected by two 24-h dietary recalls, which may not reflect the habitual intake. Fourthly, only adolescents aged 12–18 years from US were included, and whether our findings can be extrapolated to the other age intervals and other races should be made with caution.
Despite these limitations, our findings indicate that deficient or insufficient 25-hydroxyvitamin D concentrations were associated with the significant risk of general and central obesity among US adolescents, and approximately 30% and 50% of these associations, respectively, were mediated by HOMA-IR. More stratified analyses were presented, and identified that the association with general obesity was particularly present among Mexican American, while with central obesity among Non-Hispanic Black adolescents.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Centers for disease control and prevention. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZC: Formal Analysis, Writing – original draft. XQ: Investigation, Writing – original draft. QW: Investigation, Methodology, Writing – original draft. JW: Conceptualization, Writing – review & editing. ML: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – review & editing. WN: Conceptualization, Methodology, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1334139/full#supplementary-material
References
1. Lee EY, Yoon KH. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med. (2018) 12(6):658–66. doi: 10.1007/s11684-018-0640-1
2. Golden NH, Schneider M, Wood C, Committee on nutrition, Committee on adolescence, Section on obesity. Preventing obesity and eating disorders in adolescents. Pediatrics. (2016) 138(3):e20161649. doi: 10.1542/peds.2016-1649
3. Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S. Childhood and adolescent obesity in the United States: a public health concern. Glob Pediatr Health. (2019) 6:2333794X19891305. doi: 10.1177/2333794X19891305
4. Britz B, Siegfried W, Ziegler A, Lamertz C, Herpertz-Dahlmann BM, Remschmidt H, et al. Rates of psychiatric disorders in a clinical study group of adolescents with extreme obesity and in obese adolescents ascertained via a population based study. Int J Obes Relat Metab Disord. (2000) 24(12):1707–14. doi: 10.1038/sj.ijo.0801449
5. Bjerregaard LG, Jensen BW, Angquist L, Osler M, Sorensen TIA, Baker JL. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med. (2018) 378(14):1302–12. doi: 10.1056/NEJMoa1713231
6. Maeda SS, Borba VZ, Camargo MB, Silva DM, Borges JL, Bandeira F, et al. Recommendations of the Brazilian society of endocrinology and metabology (SBEM) for the diagnosis and treatment of hypovitaminosis D. Arq Bras Endocrinol Metabol. (2014) 58(5):411–33. English, Portuguese. doi: 10.1590/0004-2730000003388
7. Yao Y, Zhu L, He L, Duan Y, Liang W, Nie Z, et al. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int J Clin Exp Med. (2015) 8(9):14977–84. PMID: 2662898026628980
8. vinh quốc Lu’o’ng K, Nguyê˜n LT. The beneficial role of vitamin D in obesity: possible genetic and cell signaling mechanisms. Nutr J. (2013) 12:89. doi: 10.1186/1475-2891-12-89
9. Hofman-Hutna J, Hutny M, Matusik E, Olszanecka-Glinianowicz M, Matusik P. Vitamin D deficiency in obese children is associated with some metabolic syndrome components, but not with metabolic syndrome itself. Metabolites. (2023) 13(8):914. doi: 10.3390/metabo13080914
10. Calcaterra V, Cena H, Biino G, Grazi R, Bortoni G, Braschi V, et al. Screening questionnaire for vitamin D insufficiency in children with obesity. Children (Basel). (2022) 9(11):1685. doi: 10.3390/children9111685
11. Vranić L, Mikolašević I, Milić S. Vitamin D deficiency: consequence or cause of obesity? Medicina (B Aires). (2019) 55:541. doi: 10.3390/medicina55090541
12. Cătoi AF, Iancu M, Pârvu AE, Cecan AD, Bidian C, Chera EI, et al. Relationship between 25 hydroxyvitamin D, overweight/obesity status, pro-inflammatory and oxidative stress markers in patients with type 2 diabetes: a simplified empirical path model. Nutrients. (2021) 13(8):2889. doi: 10.3390/nu13082889
13. Contreras-Bolívar V, García-Fontana B, García-Fontana C, Muñoz-Torres M. Mechanisms involved in the relationship between vitamin D and insulin resistance: impact on clinical practice. Nutrients. (2021) 13(10):3491. doi: 10.3390/nu13103491
14. Pires LV, González-Gil EM, Anguita-Ruiz A, Bueno G, Gil-Campos M, Vázquez-Cobela R, et al. The vitamin D decrease in children with obesity is associated with the development of insulin resistance during puberty: the PUBMEP study. Nutrients. (2021) 13(12):4488. doi: 10.3390/nu13124488
15. Pramono A, Jocken JWE, Blaak EE. Vitamin D deficiency in the aetiology of obesity-related insulin resistance. Diabetes Metab Res Rev. (2019) 35(5):e3146. doi: 10.1002/dmrr.3146
16. Gun E, Uzun H, Bolu S, Arslanoglu I, Kocabay K. Serum 25-hydroxyvitamin D is associated with insulin resistance independently of obesity in children ages 5–17. Prim Care Diabetes. (2020) 14(6):741–6. doi: 10.1016/j.pcd.2020.06.006
17. Cediel G, Corvalan C, Aguirre C, de Romana DL, Uauy R. Serum 25-hydroxyvitamin D associated with indicators of body fat and insulin resistance in prepubertal Chilean children. Int J Obes (Lond. (2016) 40(1):147–52. doi: 10.1038/ijo.2015.148
18. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. (2018) 141(3):e20173459. Erratum in: Pediatrics. 2018 Sep;142(3): PMID: 29483202; PMCID: PMC6109602. doi: 10.1542/peds.2017-3459
19. Wang X, Zhang W, Huang J, Li H, Gao J. The relationship between vitamin K and metabolic dysfunction-associated fatty liver disease among the United States population: national health and nutrition examination survey 2017–2018. Front Nutr. (2023) 10:1086477. doi: 10.3389/fnut.2023.1086477
20. Tian X, Xue B, Wang B, Lei R, Shan X, Niu J, et al. Physical activity reduces the role of blood cadmium on depression: a cross-sectional analysis with NHANES data. Environ Pollut. (2022) 304:119211. doi: 10.1016/j.envpol.2022.119211
21. Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. (2017) 92(2):251–65. doi: 10.1016/j.mayocp.2016.09.017
22. Mokha JS, Srinivasan SR, Dasmahapatra P, Fernandez C, Chen W, Xu J, et al. Utility of waist-to-height ratio in assessing the status of central obesity and related cardiometabolic risk profile among normal weight and overweight/obese children: the bogalusa heart study. BMC Pediatr. (2010) 10:73. doi: 10.1186/1471-2431-10-73
23. IInstitute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press (2010).
24. Andrade MI, Oliveira JS, Leal VS, Lima NM, Costa EC, Aquino NB, et al. Identificação dos pontos de corte do índice homeostatic model assessment for insulin resistance em adolescentes: revisão sistemática (identification of cutoff points for homeostatic model assessment for insulin resistance index in adolescents: systematic review). Rev Paul Pediatr. (2016) 34(2):234–42. doi: 10.1016/j.rpped.2015.08.006
25. Gunanti IR, Marks GC, Al-Mamun A, Long KZ. Low serum concentrations of carotenoids and vitamin E are associated with high adiposity in Mexican-American children. J Nutr. (2014) 144(4):489–95. doi: 10.3945/jn.113.183137
26. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. (2017) 167(4):268–74. doi: 10.7326/M16-2607
27. Rojas LZ, Quintero-Lesmes DC, Gamboa-Delgado EM, Guio E, Serrano NC. Prevalence of vitamin D status and its association with overweight or obesity in a population of Colombian children and adolescents. J Nutr Sci. (2020) 9:e55. doi: 10.1017/jns.2020.47
28. Tang Z, Huang S, Ma R, Zheng H, Zhu Y. Low vitamin D status is associated with obesity but no other cardiovascular risk factors in Chinese children and adolescents. Nutr Metab Cardiovasc Dis. (2020) 30(9):1573–81. doi: 10.1016/j.numecd.2020.05.019
29. Zou Y, Zhang R, Huang L, Zhao D, Su D, Meng J, et al. Serum levels of vitamin D, retinol, zinc, and CRP in relation to obesity among children and adolescents. Eur J Med Res. (2022) 27(1):51. doi: 10.1186/s40001-022-00670-7
30. Jari M, Qorbani M, Moafi M, Motlagh ME, Keikha M, Ardalan G, et al. Association of 25-hydroxy vitamin D levels with indexes of general and abdominal obesity in Iranian adolescents: the CASPIAN-III study. J Res Med Sci. (2015) 20(2):122–6. PMID: 2598376225983762
31. Fiamenghi VI, Mello ED. Vitamin D deficiency in children and adolescents with obesity: a meta-analysis. J Pediatr. (2021) 97(3):273–9. doi: 10.1016/j.jped.2020.08.006
32. Nimitphong H, Park E, Lee MJ. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr Res Pract. (2020) 14(6):553–67. doi: 10.4162/nrp.2020.14.6.553
33. Cipriani C, Pepe J, Piemonte S, Colangelo L, Cilli M, Minisola S. Vitamin D and its relationship with obesity and muscle. Int J Endocrinol. (2014) 2014:841248. doi: 10.1155/2014/841248
34. George JA, Norris SA, Toman M, Snyman T, Crowther NJ. Visceral adiposity is a predictor of parathyroid hormone levels in healthy adults. J Endocrinol Invest. (2016) 39:447–53. doi: 10.1007/s40618-015-0400-x
35. Karampela I, Sakelliou A, Vallianou N, Christodoulatos GS, Magkos F, Dalamaga M. Vitamin D and obesity: current evidence and controversies. Curr Obes Rep. (2021) 10(2):162–80. doi: 10.1007/s13679-021-00433-1
36. Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur J Endocrinol. (2008) 159:675–84. doi: 10.1530/EJE-08-0339
37. Corsello A, Macchi M, D'Oria V, Pigazzi C, Alberti I, Treglia G, et al. Effects of vitamin D supplementation in obese and overweight children and adolescents: a systematic review and meta-analysis. Pharmacol Res. (2023) 192:106793. doi: 10.1016/j.phrs.2023.106793
38. Bhatt SP, Misra A, Pandey RM, Upadhyay AD, Gulati S, Singh N. Vitamin D supplementation in overweight/obese Asian Indian women with prediabetes reduces glycemic measures and truncal subcutaneous fat: a 78 weeks randomized placebo-controlled trial (PREVENT-WIN trial). Sci Rep. (2020) 10:220. doi: 10.1038/s41598-019-56904-y
39. Zakharova I, Klimov L, Kuryaninova V, Nikitina I, Malyavskaya S, Dolbnya S, et al. Vitamin D insufficiency in overweight and obese children and adolescents. Front Endocrinol (Lausanne). (2019) 10:103. doi: 10.3389/fendo.2019.00103
40. Elkhwanky MS. Regulation of vitamin D metabolism by metabolic state in mice and humans. Discovery of molecular factors repressing vitamin D bioactivation and inducing deficiency in diabetes (Phd thesis). University of Oulu, Finland (2020). Acta Univ. Oul. D 1575. Available online at: https://oulurepo.oulu.fi/handle/10024/36528
41. Buchmann N, Eckstein N, Spira D, Demuth I, Steinhagen-Thiessen E, Norman K. Vitamin D insufficiency is associated with metabolic syndrome independent of insulin resistance and obesity in young adults—the Berlin aging study II. Diabetes Metab Res Rev. (2021) 37(8):e3457. doi: 10.1002/dmrr.3457
42. Pramono A, Jocken JWE, Adriaens ME, Hjorth MF, Astrup A, Saris WHM, et al. The association between vitamin D receptor polymorphisms and tissue-specific insulin resistance in human obesity. Int J Obes (Lond). (2021) 45(4):818–27. doi: 10.1038/s41366-021-00744-2
43. Li Z, Gueant-Rodriguez RM, Quilliot D, Sirveaux MA, Meyre D, Gueant JL, et al. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin Nutr. (2018) 37(5):1700–6. doi: 10.1016/j.clnu.2017.07.008
44. Ock SY, Ha KH, Kim BK, Kim HC, Shim JS, Lee MH, et al. Serum 25-hydroxyvitamin D concentration is independently inversely associated with insulin resistance in the healthy, non-obese Korean population. Diabetes Metab J. (2016) 40(5):367–75. doi: 10.4093/dmj.2016.40.5.367
45. Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesityrelated insulin resistance. Physiol Behav. (2008) 94(2):206–18. doi: 10.1016/j.physbeh.2007.10.010
46. Wasserman R. Vitamin D and the dual processes of intestinal calcium absorption. J Nutr. (2004) 134(11):3137–9. doi: 10.1093/jn/134.11.3137
47. Aatsinki SM, Elkhwanky MS, Kummu O, Karpale M, Buler M, Viitala P, et al. Fasting-induced transcription factors repress vitamin D bioactivation, a mechanism for vitamin D deficiency in diabetes. Diabetes. (2019) 68(5):918–31. doi: 10.2337/db18-1050
48. Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. (2011) 31:48–54. doi: 10.1016/j.nutres.2010.12.001
49. Parva NR, Tadepalli S, Singh P, Qian A, Joshi R, Kandala H, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011–2012). Cureus. (2018) 10:e2741. doi: 10.7759/cureus.2741
50. Ames BN, Grant WB, Willett WC. Does the high prevalence of vitamin D deficiency in African Americans contribute to health disparities? Nutrients. (2021) 13(2):499. doi: 10.3390/nu13020499
51. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. (1982) 1(8263):74–6. doi: 10.1016/s0140-6736(82)90214-8
52. Min J, Goodale H, Xue H, Brey R, Wang Y. Racial-ethnic disparities in obesity and biological, behavioral, and sociocultural influences in the United States: a systematic review. Adv Nutr. (2021) 12(4):1137–48. doi: 10.1093/advances/nmaa162
53. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. (2016) 118(11):1723–35. doi: 10.1161/CIRCRESAHA.115.306825
Keywords: 25-hydroxyvitamin D, obesity, HOMA-IR, adolescents, NHANES
Citation: Chen Z, Qiu X, Wang Q, Wu J, Li M and Niu W (2024) Serum vitamin D and obesity among US adolescents, NHANES 2011–2018. Front. Pediatr. 12:1334139. doi: 10.3389/fped.2024.1334139
Received: 6 November 2023; Accepted: 29 April 2024;
Published: 21 May 2024.
Edited by:
Melania Manco, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Lenycia De Cassya Lopes Neri, University of Pavia, ItalyIvana Šarac, University of Belgrade, Serbia
© 2024 Chen, Qiu, Wang, Wu, Li and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Wu, wujing20221120@163.com
Min Li, limin@bjzhongyi.com
Wenquan Niu, niuwenquan_shcn@163.com
†These authors share first authorship
 Zisu Chen1,†
Zisu Chen1,†  Jing Wu
Jing Wu Wenquan Niu
Wenquan Niu