A case report of a patient with recurrent and severe infections highlighting the importance of considering inborn errors of immunity
- 1Pediatric Intensive Care Unit, Department of Pediatrics, New Jahra Hospital, Jahra Medical City, Kuwait
- 2Faculty of Pediatrics, Kuwait Institute for Medical Specialization, Kuwait City, Kuwait
- 3Department of Otorhinolaryngology, Zain Hospital, Sabah Health Region, Ministry of Health, Kuwait City, Kuwait
- 4Department of Otorhinolaryngology, Sabah Hospital, Sabah Health Region, Ministry of Health, Kuwait City, Kuwait
- 5Kuwait Medical Genetic Center, Sabah Health Region, Ministry of Health, Kuwait City, Kuwait
- 6Department of Pediatrics, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait
- 7Allergy & Clinical Immunology Unit, Department of Pediatrics, Sabah Hospital, Sabah Health Region, Ministry of Health, Kuwait City, Kuwait
Inborn errors of immunity (IEI) can often be misdiagnosed early in life due to their heterogenous clinical presentations. Interleukin-1 receptor-associated kinase 4 (IRAK-4) deficiency is one of the rare innate immunodeficiency disorders. We present the case of a patient who presented at the age of 15 days with meningitis and septic shock that responded to antibiotics. She was admitted again at the age of 45 days with pseudomonas aeruginosa bacteremia that was associated with increased inflammatory markers. Her third admission was at the age of 2.5 months due to left sided peri-orbital cellulitis that was again associated with elevated inflammatory markers. At 3.5 months, she experienced left orbital cellulitis, which was complicated by extensive sinus involvement, erosion, and abscess formation in the pterygopalatine fossa. Her condition progressed to septic shock and required multiple antibiotics and surgical interventions for drainage and control of the infection source. Both abscess and blood culture were positive for pseudomonas aeruginosa. An IEI was suspected but basic immunology testing was normal. Whole Exome Sequencing was performed and a novel mutation in IRAK4 was detected. In conclusion, we highlight the importance of raising awareness among pediatricians about the potentially lethal IEI and the need to consult specialists when these diseases are suspected. Among them is IRAK-4 deficiency which can be diagnosed by sophisticated functional assays and/or genetic testing.
Introduction
Inborn Errors of Immunity (IEI) are monogenic heterogeneous diseases that predispose patients to infections, immune dysregulation, and malignancy (1). Unfortunately, because the disease’s manifestations are not specific to IEI and many pediatricians are unaware of them, patients experience significant delay in diagnosis (2–5). Subsequently, these patients are at increased risk of morbidity, tissue damage, and death (6). The Jeffery Modell Foundation has developed 10 warning signs to improve awareness about IEI and to facilitate earlier diagnosis (7). Among these signs are recurrent and severe infections, especially if they are caused by unusual organisms.
Pseudomonas sepsis is rare in healthy, non-hospitalized infants and the empirical antibiotic treatment for sepsis does not include agents against this microorganism (8). This gram-negative bacillus is an opportunistic environmental microorganism that can cause severe infections in children with impaired defense mechanisms and chronic illnesses. In this report, we present the case of an infant who presented with recurrent life-threatening pyogenic Pseudomonas infection in the head and neck region. Although the initial immunology workup was unremarkable, eventually the infant was diagnosed with Interleukin -1 Receptor-Associated Kinase 4 (IRAK-4) deficiency, an inborn error of immunity. We aim to highlight the importance of considering IEI in the differential diagnosis of patients with recurrent infections.
Case report
The patient was born at full term with an average birth weight and no history of perinatal complications to consanguineous parents. She received Hepatitis B vaccine at birth. Family history was not suggestive of immunodeficiency.
She was admitted at the age of 15 days with fever, convulsions, septic shock and evidence of bacterial meningitis—as suggested by cerebrospinal fluid (CSF) studies [WBC 991 cells (22% polymorphs) and increased protein at 2.8 g/L (0.15–1.3)] but negative gram stain and bacterial culture as she had been pre-treated with a few doses of IV ampicillin and cefotaxime. Her complete blood count (CBC) showed white blood cells (WBC) 7.52 (109/L) (32% neutrophils) and C-reactive protein (CRP) was elevated at 15.7 mg/dl (normal <0.5) which normalized to 0.16 mg/dl after treatment with ampicillin and cefotaxime. Antibiotics were changed to intravenous vancomycin and meropenem. The blood culture was sterile.
She was admitted again at the age of 45 days with fever, rhinorrhea, and decreased oral intake. Cerebrospinal fluid (CSF) sample showed WBC of 9 (109/L) (11% polymorphs) and protein of 0.4 g/L. CRP was elevated at 26.6 mg/dl, procalcitonin was elevated at 5.4 ng/ml (normal <0.05 ng/ml). Blood culture grew pseudomonas aeruginosa. Her condition improved and CRP normalized after receiving antibiotics.
At 2 and a half months, she was admitted for the third time with low-grade fever, decreased activity, rhinorrhea, decreased oral intake, and left-sided peri-orbital cellulitis that was associated with elevated CRP at 17.8 mg/dl. Cerebrospinal fluid studies were unremarkable. The blood and CSF cultures were sterile.
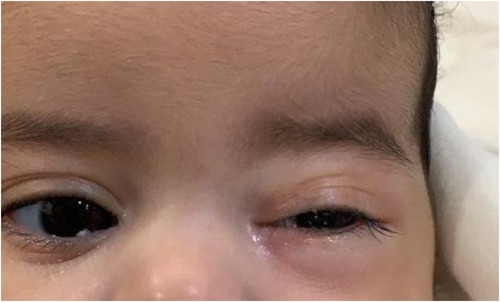
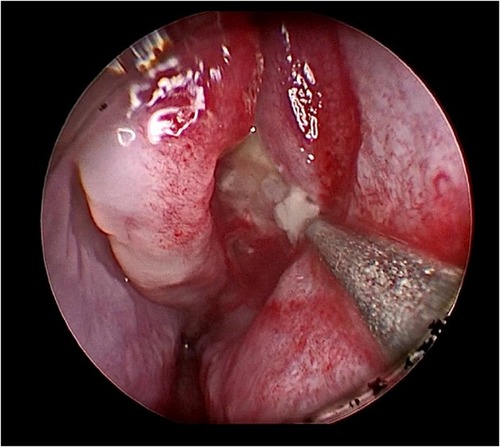
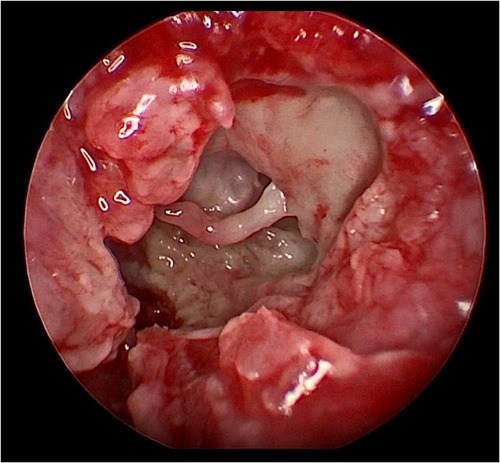
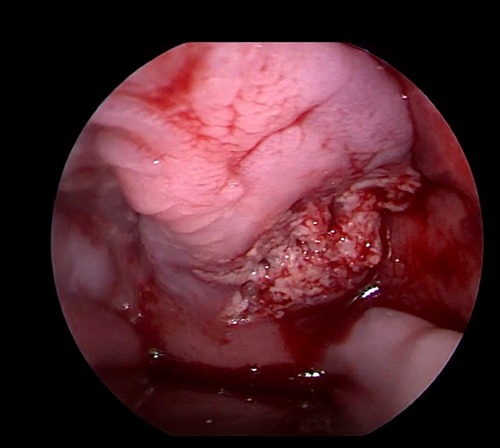
Her fourth admission was at the age of 3 and a half months with poor feeding, irritability, severe nasal congestion, left-sided facial swelling, redness with periorbital edema, and proptosis (Figure 1) consistent with a diagnosis of left orbital cellulitis. Computed tomography (CT) imaging of the head showed pterygopalatine fossa abscess with left cavernous sinus thrombosis. CBC showed a WBC count of 12.2 (109/L) (28% neutrophils) and CRP was elevated at 24 mg/dl. She was treated with IV Meropenem. After 2 days, her condition worsened with increased orbital swelling. She urgently underwent endoscopic sinus surgery to the left side for source control. Uncinectomy, anterior ethmoidectomy, and maxillary mega antrostomy with partial inferior turbinectomy were performed (Figure 2). There was purulent discharge involving the left maxillary sinus with bulging of the medial maxillary sinus into the septum. The posterior wall of the maxillary sinus was eroded with exposure of the contents of the pterygopalatine fossa (Figure 3). In addition, there was erosion of the bone secondary to extensive inflammation in the hard palate (Figure 4). Multiple biopsies were taken for histopathology and cultures grew pseudomonas aeruginosa similar to her blood culture. Intravenous ciprofloxacin was added. After about 48 h of improvement, she deteriorated again with septic shock and progressive buccal and left facial cellulitis extending to the left lateral neck. Urgent head and neck CT with contrast revealed extensive progression of the pterygopalatine fossa abscess and thromboses of the left cavernous sinus, external and internal jugular veins (Figure 5). She was urgently taken to the operating room for source control including drainage of a hard palate collection that was bulging into the nasal floor (Figure 6). After about 2 weeks in the intensive care unit, she was successfully discharged to complete a prolonged IV antibiotic course.
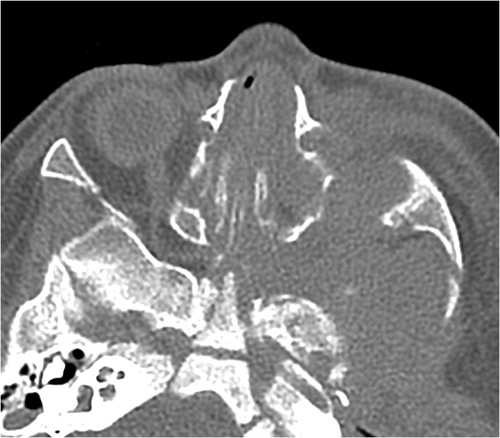
Figure 5. Axial CT scan of the paranasal sinuses shows a destructive inflammatory process of the left pterygopalatine fossa and widening of the foramen.

Figure 6. Coronal MRI of the paranasal sinuses shows an inflammatory process involving the left maxillary sinus, ethmoid sinus, floor of the orbit, and inferiorly into the sections of the hard palate.
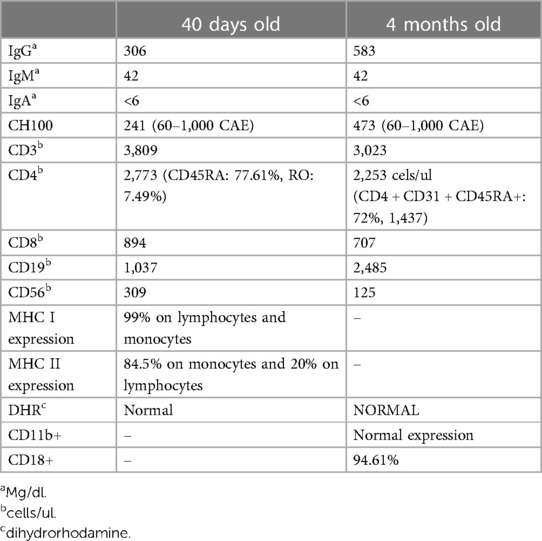
An IEI was suspected because of recurrent and severe infections. Her basic immunology test results done at the age of 40 days and at the age of 4 months were normal (Table 1). Due to the high suspicion of IEI despite normal results of basic immunology testing, Whole Exome Sequencing was performed using a next-generation sequencing platform through Kuwait Medical Genetic Center.
Genetic methodology and result
Next-generation sequencing was carried out on an Ion Torrent S5 XL/Prime Machine, using Ion AmpliSeq Whole Exome sequencing Kit by Life Technologies to an average coverage depth of 70–100×. In-house bioinformatics analysis pipeline including signal processing, base calling, and variant annotation by alignment of reads to GRCh37/hg19 genome assembly. Primary filtering out of low-quality reads and probable artifacts were applied. Subsequent analysis and interpretation of the data were carried out for the sample. Identified variants and indels were filtered against external databases depending on their allele frequency focusing on rare variants with a minor allele frequency (MAF) of 1% or less. Evaluation was focused on coding exons along with flanking ±10 intronic bases. In Silico analysis of identified variants was performed using bioinformatics prediction programs. Classification of variants was performed based on ACMG guidelines (9).
Testing revealed that the proband was homozygous for a novel frameshift variant in IRAK4 ((NM_016123.4): c.274del; (p. Glu92AsnfsTer27). This variant was then confirmed by Sanger sequencing. The variant results in a 1-bp deletion in exon 3, generating a frameshift predicted to lead to a premature stop codon at position 27 downstream in the new reading frame. The variant has not been observed in gnomAD (Genome Aggregation Database) and was predicted to cause loss of normal protein function. Subsequent variant interpretation according to the ACMG guidelines (mutation type, predicted impact, absence in large control population, and the genotype–phenotype correlation), indicates that the variant can be classified as likely pathogenic with supporting evidence for pathogenicity criteria PVS1 (Very Strong strength level for pathogenicity in the ACMG) and PM2 (Moderate evidence of pathogenicity).
Discussion
We describe the case of a 4-month-old infant who presented with recurrent life-threatening infections including septicemia, meningitis, orbital cellulitis, and pyogenic head and neck infection caused by pseudomonas with an initial negative immunology workup. Typically, pseudomonas aeruginosa sepsis in children mainly occurs after prolonged hospitalization, and most commonly affects patients with chemotherapy-induced neutropenia or with IEI (10, 11).
IEI are genetic defects of the innate or adaptive immune system. The innate immune system is the first line of defense against pathogens and is critical to recognize microbes and start the inflammatory cascade (12). We described a novel frameshift variant in IRAK4 gene causing autosomal recessive immunodeficiency (OMIM # 607676). A review of the literature suggests that this variant creates a premature termination codon and is predicted to cause loss of normal protein function, resulting in severe IRAK4 deficiency (13). IRAK4 deficiency specifically abrogates Toll-like receptor TLR signaling. Defects in TLR signaling result in primary immune deficiency. Studies have described defects in components of the TLR signaling cascade, which includes MyD88, TIRAP, and the kinases IRAK1 and IRAK4 (14). Interleukin-1 receptor-associated kinase 4 (IRAK4) is the most upstream kinase in Toll/Interleukin-1 receptor (TIR) signaling (15) and is considered the master in the mammalian IRAK family. It plays a key role in the intracellular signal transduction from IL-1, IL-18, which are important mediators in the signal transduction of TLR and IL1R family members, collectively referred to as TIRs (16). The loss of IRAK4, or its intrinsic kinase activity, can entirely stop signaling through these pathways (17).
Due to defective activation of innate immune responses, IRAK-4-deficient patients are prone to recurrent pyogenic bacterial infections while preserving a normal resistance to common fungi, parasites, and viruses (11, 18, 19). Pseudomonas invasive infection has been reported in patients with such IEI but is less common than sepsis due to Gram-positive bacteria including Streptococcus pneumonia and Staphylococcus aureus (20–22). IRAK 4 deficient patients can present with meningitis, osteomyelitis, arthritis, abscesses, sepsis, and cellulitis. Immunological evaluation in IRAK4 deficiency usually reveals intact leukocyte populations, including T-, B-, NK-cell numbers, and T cells that exhibit normal proliferation. Serum immunoglobulin values and antibodies against specific vaccine antigens are usually within the normal range except for possible impaired responses to polysaccharide vaccines (23).
In this case report, we highlight the importance of raising awareness among pediatricians about the potentially lethal IEI and the need to consult specialists when these diseases are suspected. There are several inborn errors of immunity that can be severe but may not be detected in the initial immunologic workup like serum immunoglobulins and lymphocyte subsets testing. One example of such IEI is IRAK-4 deficiency which can be diagnosed by sophisticated functional assays then supported by genetic testing. It is recommended that IRAK-4 deficient patients receive prophylactic antibiotics including oral trimethoprim/sulfamethoxazole or penicillin. In addition, some patients may require monthly immunoglobulin infusions. It is imperative that such patients continue to receive regular vaccinations. Children who survive beyond childhood tend to develop less frequent invasive diseases (24). Inborn errors of immunity should be also be considered in populations with high incidence of consanguineous marriages which increases the risk of autosomal recessive disorders (25).
Conclusion
Pseudomonas aeruginosa sepsis and invasive pyogenic infections in early childhood in a previously healthy child signify the possible presence of an IEI. This case report highlights the importance of performing a thorough genetic investigation especially when the pre-test probability of an IEI is high. Making such a diagnosis is lifesaving. Moreover, in addition to targeted/definitive antimicrobial therapy, the role of surgical intervention for source control is crucial in alleviating the pyogenic burden of this disease.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical approval was not required for the study as this manuscript pertains only to a case report and specific ethics approval is not mandated in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian, for the publication of any potentially identifiable images or data included in this article.
Author contributions
FA: Writing – original draft, Writing – review & editing. MA: Writing – original draft. MA: Writing – original draft. AA: Writing – original draft, Writing – review & editing. RE: Software, Writing – original draft. WA: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. (2020) 40(1):24–64. doi: 10.1007/s10875-019-00737-x. Erratum in: J Clin Immunol. (2020).31953710
2. Gathmann B, Goldacker S, Klima M, Belohradsky BH, Notheis G, Ehl S, et al. The German national registry for primary immunodeficiencies (PID). Clin Exp Immunol. (2013) 173(2):372–80. doi: 10.1111/cei.12105
3. Mahlaoui N, Jais JP, Brosselin P, Mignot C, Beaurain B, Brito C, et al. Prevalence of primary immunodeficiencies in France is underestimated. J Allergy Clin Immunol. (2017) 40(6):1731–3. doi: 10.1016/j.jaci.2017.06.020
4. Al-Herz W, Al-Ahmad M, Al-Khabaz A, Husain A, Sadek A, Othman Y. The Kuwait national primary immunodeficiency registry 2004–2018. Front Immunol. (2019) 10:1754. doi: 10.3389/fimmu.2019.01754
5. Al-Herz W, Zainal ME, Salama M, Al-Ateeqi W, Husain K, Abdul-Rasoul M, et al. Primary immunodeficiency disorders: survey of pediatricians in Kuwait. J Clin Immunol. (2008) 28(4):379–83. doi: 10.1007/s10875-008-9191-6
6. Al-Herz W, Moussa MA. Survival and predictors of death among primary immunodeficient patients: a registry-based study. J Clin Immunol. (2012) 32(3):467–73. doi: 10.1007/s10875-011-9636-1
8. Yang MA, Lee J, Choi EH, Lee HJ. Pseudomonas aeruginosa bacteremia in children over ten consecutive years: analysis of clinical characteristics, risk factors of multi-drug resistance and clinical outcomes. J Korean Med Sci. (2011) 26:612–8. doi: 10.3346/jkms.2011.26.5.612
9. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
10. Huang YC, Lin TY, Wang CH. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty-three episodes. Pediatr Infect Dis J. (2002) 21:1049–52. doi: 10.1097/00006454-200211000-00015
11. Zhang Q, Smith JC, Zhu Q, Guo Z, MacDonald NE. A five-year review of Pseudomonas aeruginosa bacteremia in children hospitalized at a single center in southern China. Int J Infect Dis. (2012) 16:e628–32. doi: 10.1016/j.ijid.2012.03.014
12. Gobin K, Hintermeyer M, Boisson B, Chrabieh M, Ghandil P, Puel A, et al. IRAK4 deficiency in a patient with recurrent pneumococcal infections: case report and review of the literature. Front Pediatr. (2017) 5:83. doi: 10.3389/fped.2017.00083
13. Sullivan KE, Stiehm ER, editors. Stiehm’s immune deficiencies. Cambridge, MA, USA: Academic Press (2014). p. 687–710.
14. Alsina L, Israelsson E, Altman MC, Dang KK, Ghandil P, Israel L, et al. A narrow repertoire of transcriptional modules responsive to pyogenic bacteria is impaired in patients carrying loss-of-function mutations in MYD88 or IRAK4. Nat. Immunol. (2014) 15:1134–42. doi: 10.1038/ni.3028
15. McElroy WT. Interleukin-1 receptor-associated kinase 4 (IRAK4) inhibitors: an updated patent review (2016–2018). Expert Opin Ther Pat. (2019) 29(4):243–59. doi: 10.1080/13543776.2019.1597850
16. Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Nat Acad Sci (2002) 99:5567–72. doi: 10.1073/pnas.082100399
17. Wang Z, Wesche H, Stevens T, Walker N, Yeh WC. IRAK-4 inhibitors for inflammation. Curr Top Med Chem. (2009) 9(8):724–37. doi: 10.2174/156802609789044407
18. Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. (2007) 204:24. doi: 10.1084/JEM20411OIA24
19. Bouma G, Doffinger R, Patel SY, Peskett E, Sinclair JC, Barcenas-Morales G, et al. Impaired neutrophil migration, and phagocytosis in IRAK-4 deficiency. Br J Haematol. (2009) 147:153–6. doi: 10.1111/j.1365-2141.2009.07838.x
20. Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, Arkwright PD, et al. Clinical features, and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore). (2010) 89:403–25. doi: 10.1097/MD.0b013e3181fd8ec3
21. Cardenes M, von Bernuth H, García-Saavedra A, Santiago E, Puel A, Ku CL, et al. Autosomal recessive interleukin-1 receptor-associated kinase 4 deficiency in fourth-degree relatives. J Pediatr. (2006) 148:549–51. doi: 10.1016/j.jpeds.2005.12.012
22. Nardo DD, Balka KR, Gloria YC. Interleukin-1 receptor–associated kinase 4 (IRAK4) plays a dual role in myddosome formation and Toll-like receptor signaling. (2018). Papers in Press.
23. Jia A, James E, Lu H Y. Clinical IRAK4 deficiency caused by homozygosity for the novel IRAK4 (c.1049delG, p.Gly350Glufs∗15) variant. Cold Spring Harb Mol Case Stud. (2020) 6:a005298. doi: 10.1101/mcs.a005298
24. Takada H, Ishimura M, Takimoto T, Kohagura T, Yoshikawa H, Imaizumi M, et al. Invasive bacterial infection in patients with interleukin-1 receptor-associated kinase 4 deficiency: case report. Medicine(Baltimore). (2016) 95(4):e2437. doi: 10.1097/MD.0000000000002437
Keywords: inborn errors of immunity, immunodeficiency, IRAK-4, pseudomonas aeruginosa, FESS [Functional endoscopic sinus surgery]
Citation: Altammar F, Alshamali M, Alqunaee M, Alali AJ, Elshafie RM and Al-Herz W (2024) A case report of a patient with recurrent and severe infections highlighting the importance of considering inborn errors of immunity. Front. Pediatr. 12:1340367. doi: 10.3389/fped.2024.1340367
Received: 17 November 2023; Accepted: 8 February 2024;
Published: 28 February 2024.
Edited by:
María Soledad Caldirola, Hospital General de Niños Ricardo Gutierrez, ArgentinaReviewed by:
Lina Maria Castano-Jaramillo, Fundación Hospital Pediátrico la Misericordia, ColombiaMichel J. Massaad, American University of Beirut, Lebanon
© 2024 Altammar, Alshamali, Alqunaee, Alali, Elshafie and Al-Herz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waleed Al-Herz waleed.alherz@ku.edu.kw; wemh@hotmail.com
 Fajer Altammar1,2
Fajer Altammar1,2  Ahmad J. Alali
Ahmad J. Alali Reem M. Elshafie
Reem M. Elshafie