Hyperglycemia in pregnancy did not worsen the short-term outcomes of very preterm infants: a propensity score matching study
- 1Department of Neonatology, Guangzhou Key Laboratory of Neonatal Intestinal Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
- 2Department of Neonatology, Women and Children’s Hospital, School of Medicine, Xiamen University, Xiamen, Fujian, China
- 3Xiamen Key Laboratory of Perinatal-Neonatal Infection, Xiamen, Fujian, China
- 4Department of Neonatology, Children’s Hospital of Fudan University, Shanghai, China
- 5Department of Pediatrics, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
- 6Department of Neonatology, Guiyang Maternal and Child Health Hospital·Guiyang Children’s Hospital, Guiyang, Guizhou, China
- 7Department of Pediatrics, Peking University Third Hospital, Beijing, China
- 8Department of Neonatology, Guangdong Province Maternal and Children’s Hospital, Guangzhou, Guangdong, China
- 9Department of Neonatology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
- 10Department of Neonatology, Children’s Hospital of Hebei Province, Shijiazhuang, Hebei, China
- 11Department of Neonatology, Children’ Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 12Department of Neonatology, The First Hospital of Jilin University, Changchun, Jilin, China
- 13Department of Neonatology, Quanzhou Maternity and Children’s Hospital, Quanzhou, Fujian, China
- 14Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 15Department of Neonatology, Liaocheng People’s Hospital, Liaocheng, Shandong, China
- 16Department of Neonatology, The Affiliate Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
- 17Department of Neonatology, Suzhou Municipal Hospital, Suzhou, Jiangsu, China
- 18Department of Neonatology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 19Department of Neonatology, Chengdu Women’ and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
- 20Guangdong Provincial Key Laboratory of Major Obstetric Diseases, Guangdong Provincial Clinical Research Center for Obstetrics and Gynecology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
Background: Hyperglycemia in pregnancy (HGP) has generally been considered a risk factor associated with adverse outcomes in offspring, but its impact on the short-term outcomes of very preterm infants remains unclear.
Methods: A secondary analysis was performed based on clinical data collected prospectively from 28 hospitals in seven regions of China from September 2019 to December 2020. According to maternal HGP, all infants were divided into the HGP group or the non-HGP group. A propensity score matching analysis was used to adjust for confounding factors, including gestational age, twin or multiple births, sex, antenatal steroid administration, delivery mode and hypertensive disorders of pregnancy. The main complications and the short-term growth status during hospitalization were evaluated in the HGP and non-HGP groups.
Results: A total of 2,514 infants were eligible for analysis. After matching, there were 437 infants in the HGP group and 874 infants in the non-HGP group. There was no significant difference between the two groups in main complications including respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, retinopathy of prematurity, patent ductus arteriosus, culture positive sepsis, intraventricular hemorrhage, periventricular leukomalacia, anemia, feeding intolerance, metabolic bone disease of prematurity, or parenteral nutrition-associated cholestasis. The incidences of extrauterine growth retardation and increased growth retardation for weight and head circumference in the non-HGP group were all higher than those in the HGP group after matching (P < 0.05).
Conclusions: HGP did not worsen the short-term outcomes of the surviving very preterm infants, as it did not lead to a higher risk of the main neonatal complications, and the infants’ growth improved during hospitalization.
1 Introduction
Hyperglycemia in pregnancy (HGP) is a maternal metabolic disorder, and its incidence is increasing worldwide. It includes two conditions known as gestational diabetes mellitus (GDM) and pregestational diabetes mellitus (PGDM), which are defined as abnormal glucose tolerance first found during pregnancy or diabetes before pregnancy, respectively. Some studies noted that HGP was associated with increased adverse effects on fetuses and infants, such as intrauterine hypoxia, preterm birth, birth injury, neonatal hypoglycemia, respiratory distress, and hyperbilirubinemia (1, 2). In addition, HGP can cause fetal overgrowth or backwardness in utero and increase the occurrence of macrosomia or small for gestational age (SGA) (3). These changes in the growth trajectory and metabolic level in infancy can even increase the risk of obesity and neurological damage in childhood or adulthood (4–7). Therefore, HGP has been regarded as a high-risk factor for short- and long-term adverse outcomes in offspring.
However, the impact of HGP on neonatal complications of very preterm infants (VPIs, gestational age <32 weeks) remains uncertain. There are some conflicting results in previous studies. Boghossian NS et al. (8) found that extremely preterm infants born to insulin-dependent diabetic mothers had higher risks of necrotizing enterocolitis (NEC), sepsis, and small head circumference but did not have an increased risk of patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), or bronchopulmonary dysplasia (BPD). Grandi C et al. (9) found that only NEC was significantly higher in very low birth weight infants born to mothers with HGP. Opara CN et al. (10) noted that HGP was associated with an increased incidence and severity of retinopathy of prematurity (ROP). In contrast, other reports indicated that HGP did not lead to an elevated risk of in-hospital mortality or severe morbidity in preterm infants, including respiratory distress syndrome (RDS), severe IVH (grade 3–4), PDA treatment, ROP treatment, BPD or NEC (11–13). These controversial results may be partly attributed to the differences in the characteristics of the study populations and diagnostic criteria for HGP.
Additionally, the influence of HGP on the growth of VPIs should be assessed in more detail. Previous studies have found maternal early-pregnancy blood glucose levels are associated with altered fetal growth patterns, characterized by decreased fetal growth rates in mid-pregnancy and increased fetal growth rates from late pregnancy onward, which indicate a limited effect of increased fetal growth rates in fetuses <32 weeks (14). In contrast, they are more prone to extrauterine growth retardation (EUGR) due to immaturity and various complications after birth (15). Severe EUGR will negatively impact the growth potential of infants (16).
Therefore, it is necessary to further identify the impact of HGP on neonatal complications and growth in VPIs. In this study, to minimize the differences in the study population and confounding factors, we analyzed the clinical data of VPIs from 28 hospitals in seven regions of China using propensity score matching (PSM) analysis.
2 Materials and methods
2.1 Study population
This was a secondary analysis of clinical data collected by the Nutritional Committee of Neonatology Branch of Chinese Medical Doctor Association, National Multicenter EUGR Collaborative Group. The collaborative group was founded in 2019 to investigate the incidence and risk factors for EUGR in VPIs during hospitalization in different regions of mainland China (17). The study protocol was approved by the Ethics Committee of the Women and Children's Hospital Affiliated to Xiamen University/Xiamen Maternity and Child Health Care Hospital (KY-2019-016). It was registered at http://www.chictr.org.cn, and the registration number was ChiCTR1900023418.
The inclusion criteria were as follows: (1) gestational age <32 weeks; (2) admission within 24 h after birth; and (3) a hospitalization stay ≥2 weeks. The exclusion criteria were as follows: (1) severe congenital malformations or inherited metabolic diseases; (2) in-hospital death, treatment interruption or automatic discharge; and (3) incomplete or missing data. The criteria for discharge were as follows: (1) a weight of 1 800–2,000 g or more; (2) a corrected age ≥36 weeks; (3) cured primary disease and stable vital signs (infants with BPD were allowed to be discharged with oxygen); and (4) oral feeding, with a milk volume reaching full enteral feeding. Finally, 2,514 infants were included in the analysis (17). In this study, they were divided into the HGP or non-HGP groups according to whether the mother had HGP or not.
2.2 Data collection
From September 2019 to December 2020, the clinical data of VPIs admitted to the neonatal intensive care unit (NICU) of the 28 included hospitals located in seven regions of mainland China were collected prospectively, including four maternal/child health centers, eleven children's hospitals and thirteen tertiary general hospitals. According to a unified questionnaire, the general clinical data of maternal and pregnancy disorders, perinatal conditions, neonatal growth, nutritional support during hospitalization, neonatal complications and main treatments were collected. To minimize bias among hospitals and investigators, comprehensive and systematic training was provided to all the staff involved in the survey. Data collected by the researcher at each collaborative NICU were supervised and checked by the NICU director, who was responsible for quality assurance.
2.3 Definitions and classifications
The diagnostic criteria of HGP referred to the guidelines issued by the WHO in 2013 (18). Intrauterine growth retardation (IUGR) referred to a birth weight, length or head circumference below the 10th percentile of the growth curve of infants with the same sex and gestational age (19). The diagnostic criteria of the main complications were the same as those previously described (17). Briefly, neonatal RDS was diagnosed in preterm infants with respiratory distress shortly after birth. The criterion for grading RDS was based on the chest x-ray, and grade 3–4 indicating severe RDS. BPD was defined as oxygen dependence for at least 28 days, and moderate to severe BPD was defined as the need for oxygen therapy, positive pressure ventilation or mechanical ventilation at the corrected age of 36 weeks or at discharge. The diagnosis and grading of NEC were defined according to the modified Bell criteria. The diagnosis of early-onset sepsis (EOS) or late-onset sepsis (LOS) was defined as clinical symptoms before or after 72 h of admission, with or without positive cultures from blood or cerebrospinal fluid samples. PDA was diagnosed by echocardiography after 72 h of admission, and hemodynamically significant PDA (hsPDA) was defined as an arterial duct diameter >1.5 mm, a left atrial diameter/aortic diameter ≥1.4 or a left ventricular end-diastolic diameter/aortic diameter ≥2.1 accompanied by one of the following clinical manifestations: heart murmur, tachycardia (sustained ≥160 beats/min), increased breathing, increased pulse pressure (>25 mm Hg), hypotension, water pulse, or cardiac enlargement. Treated PDA referred to hsPDA requiring fluid intake limitation, the administration of diuretics, ibuprofen, or acetaminophen, or surgical ligation. ROP and its grades were defined by the international classification of ROP. ROP treatment referred to intravitreal drug injection, laser therapy or surgery intervention. Both IVH and PVL were diagnosed by cranial ultrasonography or magnetic resonance imaging (MRI). Papile's criterion was used to grade IVH, and grades 3–4 was regarded as severe IVH. PVL was defined as the degeneration of white matter adjacent to the cerebral ventricles following cerebral hypoxia or brain ischemia. Anemia was defined as a hemoglobin (Hb) level ≤130 g/L in venous blood or ≤145 g/L in capillary blood within 2 weeks after birth and an Hb level ≤100 g/L in venous blood or ≤110 g/L in capillary blood two weeks later. EUGR referred to a weight, length or head circumference at 36 weeks of corrected age or at discharge that was below the 10th percentile according to the Fenton growth chart for preterm infants. Feeding intolerance (FI) was defined as gastric retention up to 25%−50% of the previous feeding amount, abdominal distension or bloody stool, vomiting or bile reflux after repeated feeding, or coffee-like substances in the stomach. Metabolic bone disease of prematurity (MBDP) was defined as a serum alkaline phosphatase level >900 IU/L, accompanied by a serum phosphorus level <1.8 mmol/L. Parenteral nutrition-associated cholestasis (PNAC) was defined as conjugated bilirubin levels >1.5 mg/dl (25 μmol/L) at 2 consecutive measurements by spectrophotometric quantitation, followed by parenteral nutrition for more than 14 days and the exclusion of other diseases. Total enteral feeding was defined as an enteral feeding amount reaching 150 ml/(kg·d) or a total calorie intake reaching 110 kcal/(kg·d). Growth velocity (GV) was calculated as follows: GV[g/(kg·d)] = [1,000×ln(Wn/W1)]/(Dn-D1), where Wn is the discharge weight (g), W1 is the birth weight (g), Dn is the length of hospital stay (d), and D1 is the time to return to birth weight (d).
2.4 Statistical analysis
All data were analyzed using SPSS 26.0 software (IBM, Armonk, NY, USA). We conducted a 1:2 matched analysis by PSM with a nearest-neighbor matching algorithm to adjust for differences in baseline characteristics between the two groups, including gestational age, twin or multiple births, sex, antenatal steroid administration, delivery mode and hypertensive disorders in pregnancy. These covariates were selected based on reported studies (15, 20). Continuous variables are expressed as the mean ± standard deviation (SD) or median (IQR) according to whether the distribution was skewed and were analyzed using a t test or Mann–Whitney test. Categorical variables are expressed as frequencies (%) and were analyzed using Pearson's chi-square test. P < 0.05 was considered statistically significant.
3 Results
3.1 General characteristics
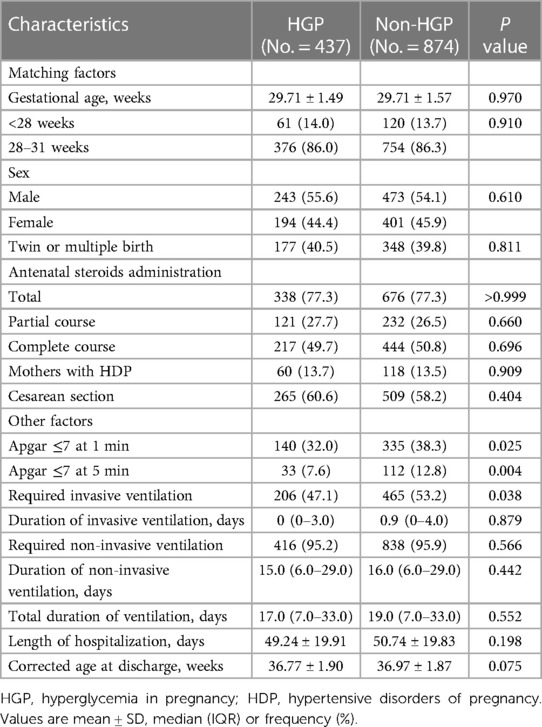
In total, 2,514 infants were eligible for statistical analysis (17). Before matching, there were 437 infants in the HGP group and 2,077 infants in the non-HGP group; after matching, the numbers of infants in the two groups were 437 and 874, respectively. The population characteristics of the two groups after matching are shown in Table 1. After matching, all six covariates were well balanced, with no significant differences between the two groups.
It was very interesting that fewer infants in the HGP group had low Apgar scores (≤7) both at 1 min and 5 min after matching (P < 0.05). Moreover, fewer infants in the HGP group required invasive ventilation after matching (47.1% vs. 53.2%, P = 0.038). No significant difference was found between the two groups in the median duration of invasive ventilation, the percentage of infants requiring noninvasive ventilation, the median duration of noninvasive ventilation, the median total duration of ventilation, or the mean corrected age at discharge after matching.
3.2 The impact of HGP on neonatal complications during hospitalization
To clarify the impact of HGP on the short-term outcomes of VPIs, we summarized the main complications of the hospitalized infants and made comparisons between the two groups. After matching, no difference was noted between the two groups in the total incidence of infants with RDS (total RDS and severe RDS), BPD (total BPD and moderate to severe BPD), NEC (NEC with Bell stage ≥Ⅱa and NEC surgery), ROP (total ROP and ROP treatment), PDA (total PDA diagnosed after 72 h and PDA treatment), IVH (total IVH and severe IVH), culture-positive sepsis (EOS and LOS), anemia (total anemia and anemia requiring red blood cell transfusion), PVL, FI, MBDP or PNAC. These are shown in Table 2.
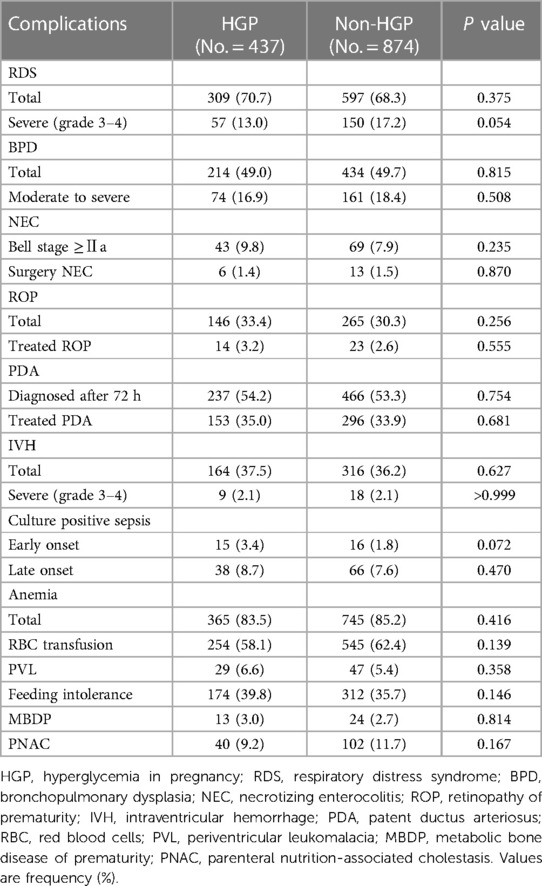
Table 2. The neonatal complications during hospitalization between the HGP group and the non-HGP group.
3.3 The effect of HGP on fetal and neonatal growth
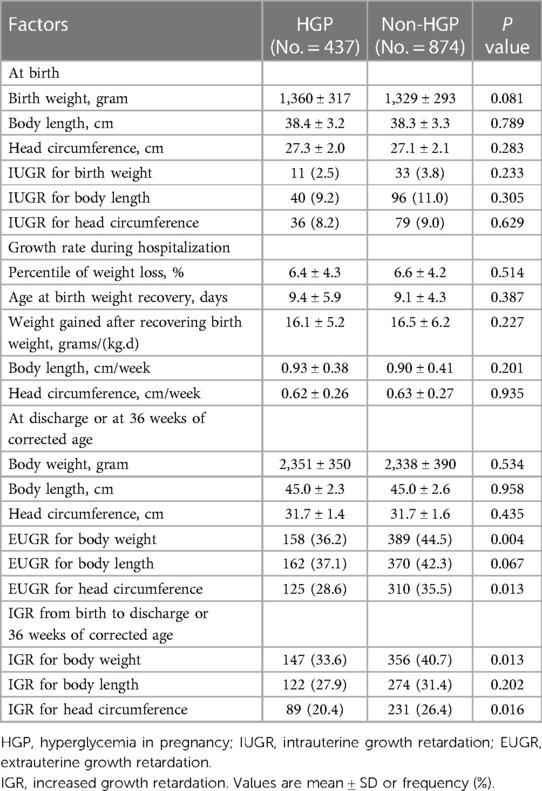
Birth weight, length, and head circumference can reflect fetal growth. After matching, the mean birth weight, length, and head circumference were similar between the two groups, as well as the incidence of IUGR respectively.
During hospitalization, there was no significant difference in the percentile of weight loss, age at birth weight recovery, weight gained per day after birth weight recovery, length or head circumference increased per week between the HGP group and the non-HGP group after matching.
At discharge or at 36 weeks of corrected age, a significant difference was noted only in EUGR for weight and head circumference (44.5% vs. 36.2%, P = 0.004; 35.5% vs. 28.6%, P = 0.013), not for length after matching.
In addition, we calculated the percentage of infants with increased growth retardation (IGR) from birth to discharge or at 36 weeks of corrected age. After matching, the percentages of IGR for weight and head circumference, but not for length, in the non-HGP group were higher than those in the HGP group (40.7% vs. 33.6%, P = 0.013; 26.4% vs. 20.4%, P = 0.016). All these are shown in Table 3.
3.4 Nutritional management during hospitalization
Nutrition intake is very important for infant growth. In this study, we also analyzed the nutritional management of the infants during hospitalization (Table 4). After matching, the initial time of enteral feeding in the non-HGP group was slightly later than that in the HGP group (24.0 (9.0–44.0) vs. 20.5 (5.0–30.0) h, P = 0.009). In addition, cumulative fasting time and age at reaching the oral calorie intake target were also longer in the non-HGP group than in the HGP group after matching (24.0 (17.0–34.0) vs. 23.0 (15.0–32.0) d, P < 0.05). After matching, age at achieving total enteral feeding, duration of parenteral nutrition, cumulative calories in the first week of hospitalization, age at reaching total calorie intake target, cumulative amino acid dose in the first week and during the whole hospitalization period, cumulative dose of fat emulsion in the first week and during the whole hospitalization period, were all similar between the two groups.
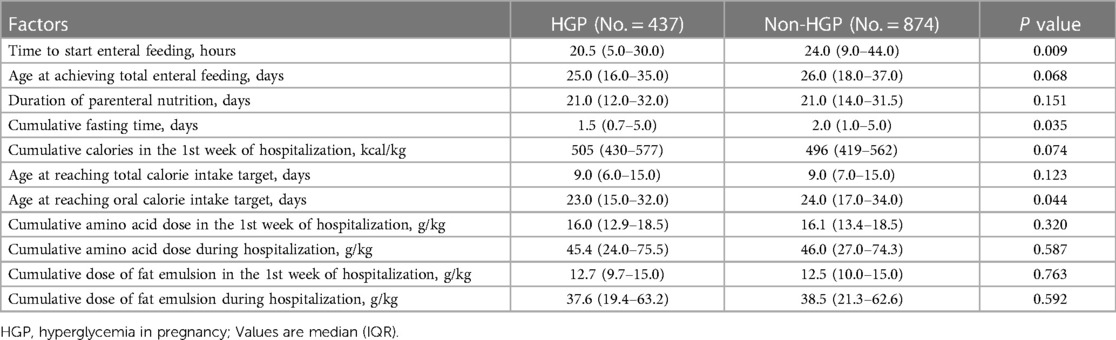
Table 4. Comparisons of nutritional intake during hospitalization between the HGP group and the non-HGP group.
4 Discussion
In this study, we identified that HGP did not worsen the short-term outcomes of the surviving VPIs. Specifically, the VPIs born to mothers with HGP did not have a higher risk of main complications, including RDS, BPD, NEC, ROP, PDA, IVH, PVL, culture-positive sepsis, anemia, FI, MBDP or PNAC. In addition, these infants had a lower incidence of EUGR for weight and head circumference at discharge or at 36 weeks of corrected age, as well as less IGR for weight and head circumference during hospitalization.
To date, only a few studies have explored the association between HGP and short-term outcomes in VPIs or very low birth weight infants (8–13, 21). The study results were consistent regarding the impact of HGP on the increased risk of RDS, BPD, severe IVH, PDA requiring treatment, PVL and birth asphyxia but were conflicting regarding NEC (8, 9) and ROP (10). These inconsistent results may be due to the poor control of confounding factors and the difference in the inclusion criteria and subjects. To minimize the confounding effect, we conducted a 1:2 matched analysis by PSM with a nearest-neighbor matching algorithm to adjust for the six covariates, including gestational age, sex, antenatal steroid administration, delivery mode, twin or multiple births, and maternal gestational hypertension. After matching, we did not find a significant difference in the occurrence of RDS, BPD, PDA requiring treatment, EOS, IVH or PVL between the HGP group and the non-HGP group, which is consistent with the studies mentioned above (11–13).
RDS has been reported to increase in infants born to mothers with GDM (2). In recent years, there were three meta-analysis studies referring to mothers with GDM and neonatal complications. One study was published in 2019 and noted that the odds ratio of RDS was higher in women with GDM and insulin use (22). The other two studies were published in 2022 and pointed out that regardless of the GDM screening criteria, the risk of RDS significantly increased in women with GDM compared with the non-GDM group (23, 24). The populations involved in all the above meta-analysis studies included term and preterm infants. In the survival VPIs, we did not find that HGP increased the risk of RDS. Prematurity is the chief cause of RDS, and the risk for developing RDS is negatively correlated with gestational age (25). Surfactant appears at approximately 24 weeks of gestation in the cytosol of type II pneumocytes and increases with gestational age. It is not measurable in the amniotic fluid until approximately 32 weeks (26). Insulin can inhibit the secretion of glucocorticoids, which can accelerate fetal lung maturation. Women with HGP always have hyperinsulinemia, which delays fetal lung maturation and partly contributes to RDS. In our study, because the VPIs had a high incidence of RDS, the statistical effect of GDM on lung maturation might be weakened.
Consistent with the studies of Persson M et al. and Razak A et al. (11, 13), our study found that the risk of NEC in the surviving VPIs born to mothers with HGP did not increase when compared with the non-HGP group. However, two other studies reported that maternal diabetes was a risk factor for NEC in preterm infants. One was from Latin America (9), and they examined the in-hospital mortality and morbidity in very low birth weight infants born to mothers with and without diabetes mellitus. After logistic regression analysis, they found that NEC (grades 2–3) was the only condition independently associated with diabetes mellitus. Another study was from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (8). They examined morbidity in infants born at 22–28 weeks of gestation to mothers with insulin-dependent diabetes. The results showed that the infants born to mothers with insulin use before pregnancy had a higher risk of NEC; however, in this study, the incidence of maternal hypertension was also higher in these infants, which was found to be associated with NEC among very low birth weight infants (27).
ROP remains one of the predominant causes of blindness in preterm infants. The incidence varies with gestational age and birth weight (28). Several studies have assessed the other risk factors for severe ROP, including sepsis, RDS, BPD, PDA, neonatal hyperglycemia, blood transfusion, supplemental oxygen administration, mechanical ventilation, and preeclampsia (29–31). Opara CN et al. (10) found that maternal diabetes was associated with ROP and that the strength of the association increased with increasing ROP severity in very low birth weight infants, but the baseline characteristics, including gestational age, birth weight, neonatal steroid use and sepsis, were significantly different. In our study, after matching the confounding covariates in the baseline characteristics between the two groups, we did not find a significant association between ROP and HGP in VPIs. This is similar to other studies (8–13).
In mothers with HGP, intrauterine hyperglycemia and hyperinsulinemia can affect fetal growth, and the impact can last for some time after birth. In our study, the incidence of IUGR at birth was low in VPIs and were comparable between the HGP group and the non-HGP group after matching. At discharge or at 36 weeks of corrected age, the incidence of EUGR became high. These results were similar to those of previous studies (32, 33). In addition, we found that the VPIs in the HGP group had a lower rate of EUGR for weight and head circumference, as well as less IGR during hospitalization. Although the clear mechanisms are still unknown, there are some possible explanations in previous reports. Animal experiments found that rats exposed to maternal diabetes during pregnancy exhibited hyperinsulinemia, which could accelerate physical growth by increasing the storage of fat and protein. Other studies reported that exposure to human milk from mothers with HGP could change hypothalamic function, which might affect the satiety center and the regulation of body weight and metabolism (34, 35). Interestingly, the metabolic state of germ-free mice receiving meconium from VPIs changed significantly with a reduction in the plasma levels of insulin and leptin and resulted in obvious growth restriction. This suggested that the unique microbiota in VPIs may be prone to growth failure (36). However, Ting Chen (37) et al. found that the richness and diversity of the gut microbiota in mothers with HGP decreased, and the pathways related to carbohydrate and nucleotide metabolism were enriched, suggesting that maternal HGP may promote the growth of high-energy-supplying microbiota by promoting succession and thereby alter the metabolism of offspring. On the other hand, postnatal nutrition intake is very important to the growth of VPIs. Delayed enteral feeding and increased fasting time are associated with EUGR (38, 39). In our study, although there was no significant difference between the two groups in the duration of parenteral nutrition or age at reaching the total calorie intake target, the initial time of enteral feeding in the non-HGP group was later than that in the HGP group. Moreover, the cumulative fasting time and age at reaching the oral calorie intake target were longer in the non-HGP group than in the HGP group.
5 Conclusions
In conclusion, our study found that HGP did not worsen the short-term outcomes of surviving VPIs, as it did not lead to a higher risk of the main neonatal complications and even led to improved growth during hospitalization. However, there are still some limitations in our study. First, the VPIs eligible for analyses were only survivors and were not based on the whole population. Second, more details, including the pattern of HGP (GDM or PGDM) and the treatment of HGP before delivery, could not be analyzed. Further studies in the future are needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Women and Children’s Hospital Affiliated to Xiamen University/Xiamen Maternity and Child Health Care Hospital (KY-2019-016). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YL: Writing – original draft, Data curation, Formal Analysis, Methodology, Software. WS: Data curation, Methodology, Software, Writing – review & editing. RZ: Data curation, Investigation, Methodology, Resources, Writing – review & editing. JM: Investigation, Resources, Supervision, Visualization, Writing – review & editing. LL: Investigation, Resources, Supervision, Visualization, Writing – review & editing. Y-MC: Data curation, Investigation, Methodology, Resources, Writing – review & editing. X-ZY: Investigation, Resources, Software, Writing – review & editing. Y-PQ: Investigation, Resources, Software, Writing – review & editing. LM: Investigation, Resources, Validation, Writing – review & editing. RC: Investigation, Resources, Validation, Writing – review & editing. HW: Investigation, Resources, Validation, Writing – review & editing. D-MC: Investigation, Resources, Validation, Writing – review & editing. LC: Investigation, Resources, Validation, Writing – review & editing. PX: Investigation, Resources, Validation, Writing – review & editing. HM: Investigation, Resources, Validation, Writing – review & editing. S-NW: Investigation, Resources, Validation, Writing – review & editing. F-LX: Investigation, Resources, Validation, Writing – review & editing. RJ: Investigation, Resources, Validation, Writing – review & editing. X-MT: Conceptualization, Project administration, Writing – review & editing. X-ZL: Conceptualization, Investigation, Project administration, Resources, Software, Writing – review & editing. FW: Conceptualization, Formal Analysis, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by Guidance Project of Xiamen Medical and Health (grant NO. 3502Z20199077 and 3502Z20214ZD1225), and Natural Science Foundation of Guangdong Province (grant NO. 2021A1515011225). The funding sources had no role in the study design and conduct, data analysis, or manuscript preparation.
Acknowledgments
We acknowledge and thank all the collaborative units in the following hospitals and centers for providing data for this study (Information of the Chinese Multi-center EUGR Collaborative Group). Department of Neonatology, Women's and Children's Hospital Affiliated to Xiamen University/Xiamen maternal and Child Health Hospital, Xiamen, Fujian, China (WS, ZZ, and X-ZL). Department of Neonatology, the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China (FW, Qianxin Tian, and Qiliang Cui). Department of Pediatrics, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China (JM, Yuan Yuan, and Ling Ren). Department of Neonatology, Guiyang maternal and Child Health Hospital Guiyang Children's Hospital, Guiyang, Guizhou, China (LL, Bizhen Shi, and Yumei Wang). Department of Pediatrics, Peking University Third Hospital, Beijing, China (Y-MC, Jinghui Zhang, and X-MT). Department of Neonatology, Pediatric Hospital of Fudan University, Shanghai, China (Yan Zhu, WS, RZ, and Chao Chen). Department of Neonatology, Guangdong Province Maternal and Children's Hospital, Guangzhou, Guangdong, China (Jingjing Zou and X-ZY). Department of Neonatology, General hospital of Ningxia Medical University, Yinchuan, Ningxia, China (Yuhuai Li, Baoyin Zhao, and Y-PQ). Department of Neonatology, Children's Hospital of Hebei Province, Shijiazhuang, Hebei, China (Shuhua Liu and LM). Department of Neonatology, Children’ hospital of Nanjing Medical University, Nanjing, Jiangsu, China (Ying Xu and RC). Department of Neonatology, The first hospital of Jilin University, Changchun, Jilin, China (Wenli Zhou and HW). Department of Neonatology, Quanzhou maternity and Children's Hospital, Quanzhou, Fujian, China (Zhiyong Liu and D-MC). Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China (Jinzhi Gao, Jing Liu, and LC). Department of Neonatology, Liaocheng People's Hospital, Liaocheng, Shandong, China (Cong Li, Chunyan Yang, and PX). Department of Neonatology, the Affiliate Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, China (Yayu Zhang, Sile Hu, and HM). Department of Neonatology, Suzhou Municipal Hospital, Suzhou, Jiangsu, China (Zuming Yang, Zongtai Feng, and Sannan Wang). Department of Neonatology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China (Eryan Meng, Lihong Shang, and Falin Xu). Department of Neonatology, Chengdu Women’ and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan, China (Shaoping Ou and RJ). Department of Neonatology, Hunan children's Hospital, Changsha, Hunan, China (Guinan Li) Department of Neonatology, People's Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, China (Long Li). Department of Neonatology, Guangzhou Women and Children's Medical Center, Guangzhou, Guangdong, China (Zhe Zhang). Department of Neonatology, Shanghai Children's Medical Center, Shanghai, China (Fei Bei). Department of Neonatology, Children's Hospital of Chongqing Medical University, Chongqing, China (Chun Deng). Department of Neonatology, the First People's Hospital of Yulin, Yulin, Guangxi, China (Ping Su). Department of Neonatology, the People's Hospital of Baoji, Baoji, Shanxi, China (Lingying Luo). Department of Pediatrics, Affiliated Hospital of Qingdao University, Qingdao, Shandong, China (Xiaohong Liu). Departments of Neonatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China (Lijun Wang). Departments of Neonatology, Xi’an Children's Hospital, Xi’an, Shanxi, China (Shuqun Yu).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am. (2019) 48(3):479–93. doi: 10.1016/j.ecl.2019.05.001
2. Preda A, Padureanu V, Mota M, Stefan AG, Comanescu AC, Radu L, et al. Analysis of maternal and neonatal complications in a group of patients with gestational diabetes mellitus. Medicina (Kaunas). (2021) 57(11):1170. doi: 10.3390/medicina57111170
3. Yang Y, Wang Z, Mo M, Muyiduli X, Wang S, Li M, et al. The association of gestational diabetes mellitus with fetal birth weight. J Diabetes Complications. (2018) 32(7):635–42. doi: 10.1016/j.jdiacomp.2018.04.008
4. Nouhjah S, Shahbazian H, Latifi SM, Malamiri RA, Ghodrati N. Body mass index growth trajectories from birth through 24 months in Iranian infants of mothers with gestational diabetes mellitus. Diabetes Metab Syndr. (2019) 13(1):408–12. doi: 10.1016/j.dsx.2018.10.002
5. Van Dam JM, Garrett AJ, Schneider LA, Hodyl NA, Goldsworthy MR, Coat S, et al. Reduced cortical excitability, neuroplasticity, and salivary cortisol in 11–13-year-old children born to women with gestational diabetes mellitus. EBioMedicine. (2018) 31:143–9. doi: 10.1016/j.ebiom.2018.04.011
6. Zhao YL, Ma RM, Lao TT, Chen Z, Du MY, Liang K, et al. Maternal gestational diabetes mellitus and overweight and obesity in offspring: a study in Chinese children. J Dev Orig Health Dis. (2015) 6(6):479–84. doi: 10.1017/S2040174415007205
7. Pathirana MM, Lassi ZS, Ali A, Arstall MA, Roberts CT, Andraweera PH. Association between metabolic syndrome and gestational diabetes mellitus in women and their children: a systematic review and meta-analysis. Endocrine. (2021) 71(2):310–20. doi: 10.1007/s12020-020-02492-1
8. Boghossian NS, Hansen NI, Bell EF, Brumbaugh JE, Stoll BJ, Laptook AR, et al. Outcomes of extremely preterm infants born to insulin-dependent diabetic mothers. Pediatrics. (2016) 137(6):e20153424. doi: 10.1542/peds.2015-3424
9. Grandi C, Tapia JL, Cardoso VC. Impact of maternal diabetes mellitus on mortality and morbidity of very low birth weight infants: a multicenter Latin America study. J Pediatr (Rio J). (2015) 91(3):234–41. doi: 10.1016/j.jped.2014.08.007
10. Opara CN, Akintorin M, Byrd A, Cirignani N, Akintorin S, Soyemi K. Maternal diabetes mellitus as an independent risk factor for clinically significant retinopathy of prematurity severity in neonates less than 1500 g. PLoS One. (2020) 15(8):e0236639. doi: 10.1371/journal.pone.0236639
11. Persson M, Shah PS, Rusconi F, Reichman B, Modi N, Kusuda S, et al. Association of maternal diabetes with neonatal outcomes of very preterm and very low-birth-weight infants: an international cohort study. JAMA Pediatr. (2018) 172(9):867–75. doi: 10.1001/jamapediatrics.2018.1811
12. Deryabina EG, Yakornova GV, Pestryaeva LA, Sandyreva ND. Perinatal outcome in pregnancies complicated with gestational diabetes mellitus and very preterm birth: case-control study. Gynecol Endocrinol. (2016) 32(sup2):52–5. doi: 10.1080/09513590.2016.1232215
13. Razak A, Faden M. Association of maternal diabetes mellitus with preterm infant outcomes: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2021) 106(3):271–7. doi: 10.1136/archdischild-2020-320054
14. Geurtsen ML, van Soest EEL, Voerman E, Steegers EAP, Jaddoe VWV, Gaillard R. High maternal early-pregnancy blood glucose levels are associated with altered fetal growth and increased risk of adverse birth outcome. Diabetologia. (2019) 62(10):1880–90. doi: 10.1007/s00125-019-4957-3
15. Zhao T, Feng HM, Caicike B, Zhu YP. Investigation into the current situation and analysis of the factors influencing extrauterine growth retardation in preterm infants. Front Pediatr. (2021) 9:643387. doi: 10.3389/fped.2021.643387
16. Pampanini V, Boiani A, De Marchis C, Giacomozzi C, Navas R, Agostino R, et al. Preterm infants with severe extrauterine growth retardation (EUGR) are at high risk of growth impairment during childhood. Eur J Pediatr. (2015) 174(1):33–41. doi: 10.1007/s00431-014-2361-z
17. Shen W, Zheng Z, Lin XZ, Wu F, Tian QX, Cui QL, et al. Incidence of extrauterine growth retardation and its risk factors in very preterm infants during hospitalization: a multicenter prospective study. Zhongguo Dang Dai Er Ke Za Zhi. (2022) 24(2):132–40. doi: 10.7499/j.issn.1008-8830.2111143
18. Organization WH. Diagnostic Criteria and Classification of Hyperglycemia First Detected in Pregnancy. (2013) Available online at: http://apps.who.int/iris/bitstream/10665/85975/1/who_nmh_mnd_13.2_eng.pdf
19. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
20. Zhao X, Ding L, Chen X, Zhu X, Wang J. Characteristics and risk factors for extrauterine growth retardation in very-low-birth-weight infants. Medicine (Baltimore). (2020) 99(47):e23104. doi: 10.1097/MD.0000000000023104
21. Hitaka D, Morisaki N, Miyazono Y, Piedvache A, Nagafuji M, Takeuchi S, et al. Neonatal outcomes of very low birthweight infants born to mothers with hyperglycaemia in pregnancy: a retrospective cohort study in Japan. BMJ Paediatr Open. (2019) 3(1):e000491. doi: 10.1136/bmjpo-2019-000491
22. Li Y, Wang W, Zhang D. Maternal diabetes mellitus and risk of neonatal respiratory distress syndrome: a meta-analysis. Acta Diabetol. (2019) 56(7):729–40. doi: 10.1007/s00592-019-01327-4
23. Tehrani FR, Naz MSG, Bidhendi-Yarandi R, Behboudi-Gandevani S. Effect of different types of diagnostic criteria for gestational diabetes mellitus on adverse neonatal outcomes: a systematic review, meta-analysis, and meta-regression. Diabetes Metab J. (2022) 46(4):605–19. doi: 10.4093/dmj.2021.0178
24. Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. Br Med J. (2022) 377:e067946. doi: 10.1136/bmj-2021-067946
25. Dubin SB. Assessment of fetal lung maturity: in search of the holy grail. Clin Chem. (1990) 36(11):1867–9. doi: 10.1093/clinchem/36.11.1867
26. Leung-Pineda V, Gronowski AM. Biomarker tests for fetal lung maturity. Biomark Med. (2010) 4(6):849–57. doi: 10.2217/bmm.10.109
27. Bashiri A, Zmora E, Sheiner E, Hershkovitz R, Shoham-Vardi I, Mazor M. Maternal hypertensive disorders are an independent risk factor for the development of necrotizing enterocolitis in very low birth weight infants. Fetal Diagn Ther. (2003) 18(6):404–7. doi: 10.1159/000073132
28. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. (2013) 74(Suppl 1):35–49. doi: 10.1038/pr.2013.205
29. Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. (2018) 63(5):618–37. doi: 10.1016/j.survophthal.2018.04.002
30. Kumar P, Sankar MJ, Deorari A, Azad R, Chandra P, Agarwal R, et al. Risk factors for severe retinopathy of prematurity in preterm low birth weight neonates. Indian J Pediatr. (2011) 78(7):812–6. doi: 10.1007/s12098-011-0363-7
31. Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ, et al. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand neonatal network. Pediatrics. (2005) 115(4):990–6. doi: 10.1542/peds.2004-1309
32. Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. (2003) 111(5 Pt 1):986–90. doi: 10.1542/peds.111.5.986
33. Multicenter Study Collaborative Group for Evaluation of Outcomes in Very Low Birth Weight I. Risk factors for extrauterine growth retardation in very low birth weight infants: a multicenter study. Zhonghua Er Ke Za Zhi. (2020) 58(8):653–60. doi: 10.3760/cma.j.cn112140-20200326-00308
34. Plagemann A, Harder T, Janert U, Rake A, Rittel F, Rohde W, et al. Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Dev Neurosci. (1999) 21(1):58–67. doi: 10.1159/000017367
35. Fahrenkrog S, Harder T, Stolaczyk E, Melchior K, Franke K, Dudenhausen JW, et al. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr. (2004) 134(3):648–54. doi: 10.1093/jn/134.3.648
36. Hiltunen H, Hanani H, Luoto R, Turjeman S, Ziv O, Isolauri E, et al. Preterm infant meconium microbiota transplant induces growth failure, inflammatory activation, and metabolic disturbances in germ-free mice. Cell Rep Med. (2021) 2(11):100447. doi: 10.1016/j.xcrm.2021.100447
37. Chen T, Qin Y, Chen M, Zhang Y, Wang X, Dong T, et al. Gestational diabetes mellitus is associated with the neonatal gut microbiota and metabolome. BMC Med. (2021) 19(1):120. doi: 10.1186/s12916-021-01991-w
38. Kakatsaki I, Papanikolaou S, Roumeliotaki T, Anagnostatou NH, Lygerou I, Hatzidaki E. The prevalence of small for gestational age and extrauterine growth restriction among extremely and very preterm neonates, using different growth curves, and its association with clinical and nutritional factors. Nutrients. (2023) 15(15):3290. doi: 10.3390/nu15153290
Keywords: hyperglycemia in pregnancy, preterm infant, outcomes, complication, growth retardation
Citation: Li Y, Shen W, Zhang R, Mao J, Liu L, Chang Y-M, Ye X-Z, Qiu Y-P, Ma L, Cheng R, Wu H, Chen D-M, Chen L, Xu P, Mei H, Wang S-N, Xu F-L, Ju R, Tong X-M, Lin X-Z and Wu F (2024) Hyperglycemia in pregnancy did not worsen the short-term outcomes of very preterm infants: a propensity score matching study. Front. Pediatr. 12:1341221. doi: 10.3389/fped.2024.1341221
Received: 27 December 2023; Accepted: 27 February 2024;
Published: 6 March 2024.
Edited by:
A. Seval Ozgu-Erdinc, Ankara City Hospital, TürkiyeReviewed by:
Gabriela Corina Zaharie, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaRachana Singh, Tufts University, United States
© 2024 Li, Shen, Zhang, Mao, Liu, Chang, Ye, Qiu, Ma, Cheng, Wu, Chen, Chen, Xu, Mei, Wang, Xu, Ju, Tong, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Wu gdwufan@126.com Xiao-Mei Tong tongxm2007@126.com Xin-Zhu Lin xinzhufj@163.com
†These authors have contributed equally to this work
Abbreviations HGP, hyperglycemia in pregnancy; GDM, gestational diabetes mellitus; PGDM, pregestational diabetes mellitus; SGA, small for gestational age; VPIs, very preterm infants; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; RDS, respiratory distress syndrome; EUGR, extrauterine growth retardation; PSM, propensity score matching; NICU, neonatal intensive unit; GA, gestational age; IUGR, intrauterine growth retardation; EOS, early-onset sepsis; LOS, late-onset sepsis; hsPDA, hemodynamically significant PDA; MRI, magnetic resonance imaging; FI, feeding intolerance; MBDP, metabolic bone disease of prematurity; PNAC, parenteral nutrition-associated cholestasis; SD, standard deviation; IGR, increased growth retardation.
 Ying Li
Ying Li Wei Shen
Wei Shen Rong Zhang
Rong Zhang Jian Mao
Jian Mao Ling Liu6
Ling Liu6  Yan-Mei Chang
Yan-Mei Chang Xiu-Zhen Ye
Xiu-Zhen Ye Li Ma
Li Ma Rui Cheng
Rui Cheng Hui Wu
Hui Wu Dong-Mei Chen
Dong-Mei Chen Hua Mei
Hua Mei Rong Ju
Rong Ju Xiao-Mei Tong
Xiao-Mei Tong Fan Wu
Fan Wu