Burkitt's lymphoma in a young boy progressing to systemic lupus erythematosus during follow-up: a case report and literature review
- 1Department of Rheumatology and Immunology, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Hematology and Oncology, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Introduction: Patients with systemic lupus erythematosus (SLE) are at a higher risk of developing cancer, particularly hematological malignancies such as lymphoma and leukemia. However, existing studies on this topic that assess cancer incidence following SLE diagnosis are limited. In addition, SLE can be diagnosed after cancer, although such cases in children have been rarely reported.
Case report: We present the case of a 2.6-year-old boy who presented to our institute with fever and abdominal pain. His physical examination revealed a periumbilical mass, which was pathologically diagnosed as Burkitt's lymphoma. Autologous stem cell transplantation was performed to consolidate the effect of chemotherapy and reduce the risk of cancer relapse. He was diagnosed with SLE 5 years later, following the presentation of a fever with rash, positive autoantibodies, decreased complement, and kidney involvement. At the final follow-up, the patient was still alive and showed no recurrence of Burkitt's lymphoma or disease activity of SLE.
Conclusion: Despite the low frequency of SLE in children with lymphoma, cancer and SLE may be induced by a common mechanism involving B-cell cloning and proliferation. Therefore, hematologists and rheumatologists should be aware of the occurrence of these two conditions during patient follow-up.
Introduction
Over the past decade, several large-scale studies have elucidated the risk of systemic lupus erythematosus (SLE) in adults. A growing body of evidence has suggested that patients with SLE are at a higher risk of specific malignancies, such as non-Hodgkin's lymphoma (NHL) and leukemia, in comparison with the general population. This may be related to clonal proliferation and high B-cell activation. It has been reported that in SLE populations, the risk of hematological malignancy increased by approximately threefold, whereas endometrial, breast, ovarian, and prostate cancers showed a lower incidence (1, 2). However, it is inappropriate to extrapolate the characteristics of adult-onset SLE to pediatric populations due to the long disease course and the large clinical differences in disease activity, treatment, and organ involvement that exist between pediatric and adult-onset SLE. Moreover, the relationship between lymphoma and autoimmune diseases appears to be bidirectional, although most studies focused on the incidence of lymphoma following autoimmune disease diagnosis, especially in adult cohorts. To date, there is only one case reported on new-onset SLE in an adult woman with a history of lymphoma (3). Here, we report the first case report of a male child diagnosed with Burkitt's lymphoma that subsequently developed into SLE.
Case presentation
In April 2015, a 2.6-year-old boy was admitted to the Shanghai Children's Medical Center due to persistent fever and abdominal pain that lasted more than 2 months. His physical examination revealed hard lumps near the umbilicus in the left middle and right lower abdomen, with poor motion and no obvious tenderness. A contrast-enhanced abdominal computed tomography (CT) indicated a huge space occupation (6.5 cm × 8.8 cm × 12.4 cm) in the left abdomen and suspected a malignant abdominal tumor. Retroperitoneal partial tumor resection was performed, and the postoperative pathology was consistent with Burkitt’s lymphoma. The contrast-enhanced CT of other organs and bone scans did not show an abnormal space occupation, and bone marrow aspiration did not suggest bone marrow infiltration. Laboratory investigations showed normal white blood cell count, hemoglobin level, and platelet count and a slightly elevated C-reactive protein level (15 mg/L, normal <8 mg/L). Fluorescence in situ hybridization was negative for c-Myc, BCL2, and ALK rearrangements. Immune typing was negative for PAS, PASD, CDPP, CK, CGA, SYN, S100, CD3, CD4, ACK1, PGM1, MAC387, CD30, EMA, and PES and positive for CD20, CD79a, Ki67, PAX5, VIM, INI-1, and CD34. The etiological test was positive for Epstein–Barr virus (EBV) and immunoglobulin M (IgM), despite normal DNA quantification of EBV. No autoantibodies were detected at that time, and no significant travel, medical, or family ailment histories were noted. According to the World Health Organization 2016 classification criteria for hematopoietic and lymphoid tumors and St. Jude/Murphy staging criteria (4, 5), the patient was eventually diagnosed with stage 3 Burkitt's lymphoma due to a local but unresectable abdominal mass. Subsequently, he was enrolled in the R4 group of the CCCG-BNHL-2015 chemotherapy regimen since his lactate dehydrogenase level is >4 times the normal value (3532 U/L) (6). The patient underwent radical excision of the abdominal tumor after the fourth course of chemotherapy and completed six courses of chemotherapy. To consolidate the effects of chemotherapy and reduce cancer relapse, the patient underwent an autologous stem cell transplantation (ASCT) at the end of his chemotherapy. The patient was followed up regularly for nearly 4.5 years after ASCT, showing event-free survival.
In August 2020, the patient was presented to our hospital again due to recurrent fever accompanied by a rash that lasted for 2 months. The rash was initially confined to the cheeks and appeared with erythematous scales. It was accompanied by pruritus with partial maculopapular scabs, which gradually spread to the extremities. Physical examination revealed oral ulcers and swollen cervical lymph nodes. The patient denied any other discomfort, such as joint pain, hair loss, or fatigue. etiological examination was negative for serologic EBV, cytomegalovirus, and tuberculosis. Routine blood examination suggested leukopenia (2.97 × 109/L), anemia (98 g/L) with a positive Coombs test, and thrombocytopenia (104 × 109/L). No naive lymphocytes were identified on bone marrow aspiration, and the bone marrow biopsy did not indicate lymphoma relapse. Auto-immunological examination indicated positive antinuclear antibody (ANA) with a titer of 1:1,000. Anti-ribosomal antibody (anti-rRNP), anti-Smith antibody (anti-Sm), anti-SSA/Ro52 antibody (anti-SSA/Ro52), anti-SSA/Ro60 antibody (anti-SSA/Ro60), anti-SSB antibody (anti-SSB), anti-double-stranded DNA antibody (anti-dsDNA), anti-nucleosome antibody, anti-histone antibody, and anti-ribosomal antibodies were all positive, whereas antiphospholipid antibodies were negative. Meanwhile, the level of complement 3 (C3 0.29 g/L, normal at 0.9–1.8 g/L), complement 4 (C4 0.04 g/L, normal at 0.1–0.4 g/L), and the total complement activities (CH50 U/ml, normal at 23–46 U/ml) were also low. Immunoglobulins G and globulins were elevated, with values of 26 g/L (normal at 6.3–15.0 g/L) and 40.8 g/L (normal at 18–32 g/L), respectively, while the rheumatoid factor was normal. Other biochemical tests indicated an elevated erythrocyte sedimentation rate (ESR) level (89 mm/h, normal at 0–20 mm/h) and normal liver and kidney functions. The maximum 24-h urinary protein level was 440 mg, with renal puncture indicating that the pathology coincided with lupus nephritis III + V. No pleural or pericardial effusion was identified on high-resolution CT of the chest or cardiac ultrasound. Ultrasonography of the thyroid and two salivary glands indicated an uneven echo of the thyroid and submandibular glands. The patient denied a family history of connective tissue disease, and whole-exome sequencing revealed no abnormalities. SLE was diagnosed according to the European League Against Rheumatism and the American College of Rheumatology (EULAR/ACR) 2019 diagnostic criteria (7), with a score of 20 (fever, subacute cutaneous lupus, anemia with positive Coombs test, low C3 and C4, and positive anti-Sm and anti-dsDNA). The patient may also have been diagnosed with SLE with features of Sjogren's syndrome since was positive for anti-SSA and anti-SSB and had a fever, high IgG level, leukopenia, elevated ESR level, and even abnormal submaxillary gland echo on ultrasonography.
The patient was administered glucocorticoids, hydroxychloroquine, and mycophenolate. Glucocorticoid administration was discontinued by August 2022 gradually. During the follow-up period, the anticardiolipin antibody began to be positive, and involvement of neuropsychiatric lupus and interstitial lung disease in other organs did not occur. During the most recent follow-up in May 2023, the patient is currently alive and has not experienced a relapse of Burkitt's lymphoma and disease activity of SLE. The latest ANA test was also positive, with a titer of 1:320, and only anti-SSA/Ro52, anti-SSA/Ro60, and anti-rRNP remained positive.
Discussion
At present, there is a growing body of research focusing on the relationship between autoimmune disease and cancer. Most related data have shown that malignancies are detected only after the diagnosis and treatment of autoimmune diseases, particularly SLE and Sjögren's syndrome. Neoplasia can occur either early or simultaneously. Studies have demonstrated an increased risk of malignancies in SLE patients, especially hematological malignancies and particularly NHL and acute lymphoblastic leukemia (ALL), which appear to be more common than other malignancies (1). The standardized incidence ratio (SIR) of NHL ranged from 4.39 to 7.4 in adult cohorts (2, 8, 9, 10, 11). One study based on the largest number of pediatric-onset SLE patients focused on cancer incidence and found that out of the 1,168 patients, 14 patients developed cancer, with an SIR of 4.13, and 3 patients developed NHL, with an SIR of 4.68 (12). In addition, only a small number of cases of cancer occurrence in children with SLE have been reported. However, it is somewhat reassuring that the incidence in absolute terms still translates into relatively few events. The characteristics of hematological malignancies in children with SLE are summarized in Tables 1 and 2 (12–20). The analysis of this data revealed no strong correlation between SLE severity and tumor development. Among these patients, only three developed into SLE following a diagnosis of ALL (17–19), and one patient underwent ASCT (18). Our case is the first report of a male patient who did not have a genetic predisposition or family history of connective tissue disease that developed into SLE, even secondary Sjögren's syndrome, following diagnosis of Burkitt's lymphoma and ASCT, suggesting a link between cancers and autoimmune disease.
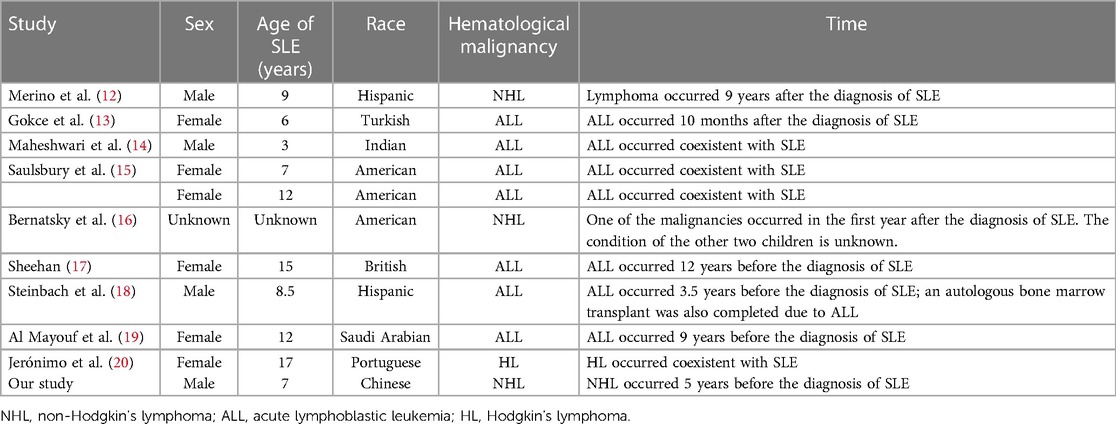
Table 1. General information for children with concurrent hematological malignancies and systemic lupus erythematosus (SLE).
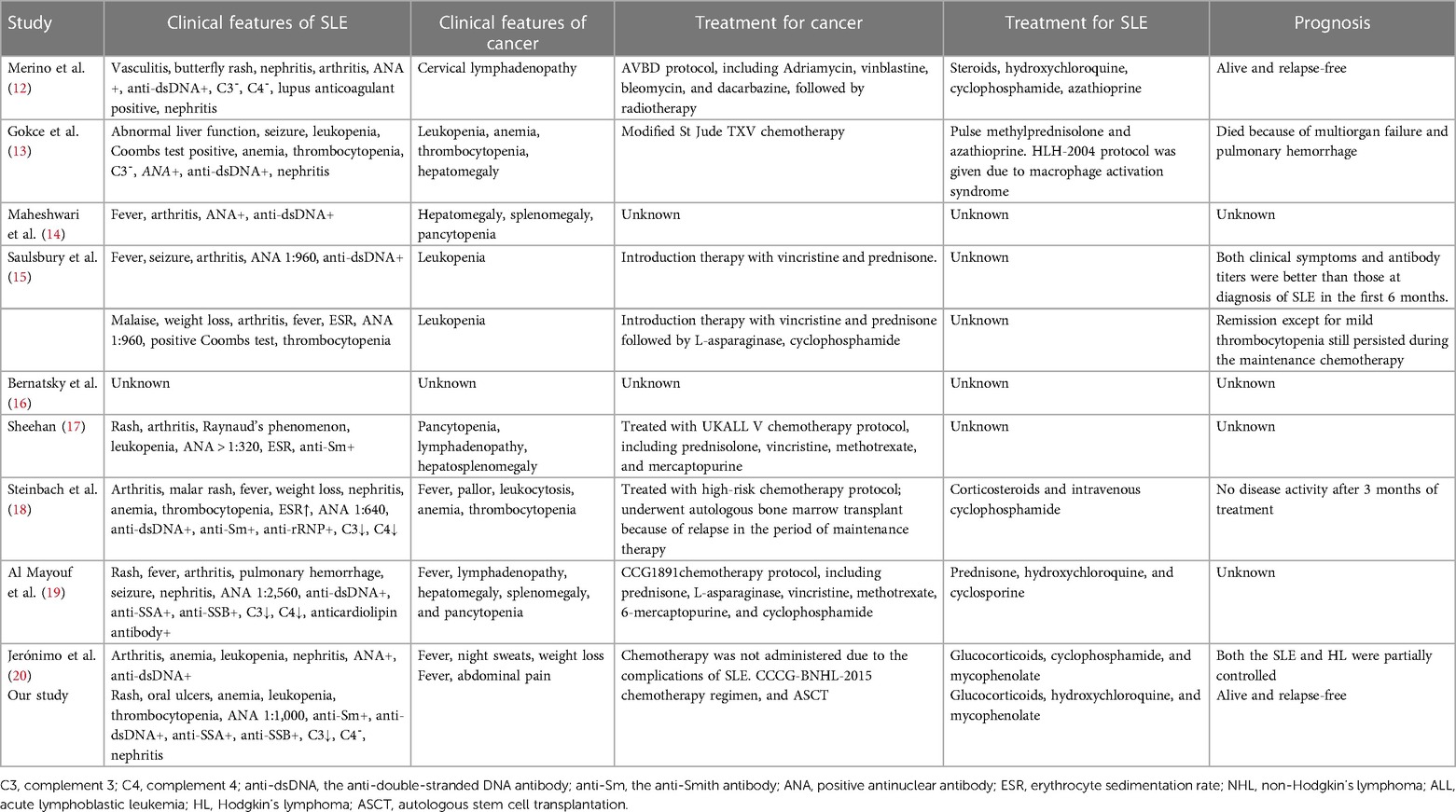
Table 2. Clinical characters for children with concurrent hematological malignancies and systemic lupus erythematosus (SLE).
Sometimes, clinical manifestations, including arthritis, fever, lymphadenopathy, hepatosplenomegaly, leukopenia, anemia, and thrombocytopenia, are not specific to hematological malignancies or SLE. Therefore, for children with hematological malignancies, autoantibody tests must be completed in time if new symptoms cannot be explained entirely by the tumor or point to rheumatic diseases, and vice versa; bone marrow aspiration, or even bone or lymph node biopsies, should be performed in time if children with SLE have refractory leukopenia, anemia, thrombocytopenia, high lactate dehydrogenase (21), or persistent fever during treatment. Persistent dysplasia/cytopenia, a complicated disease course, multisystem involvement, drugs, and early age at SLE onset may all constitute the development of hematological malignancies (13). However, it is worth noting that ANA is not a specific screening indicator for rheumatic disease. Positive ANA may be found in patients with connective tissue diseases, infectious diseases, and nonspecific musculoskeletal pain (22, 23) and even in healthy populations (24, 25). Therefore, to make a comprehensive judgment, it is necessary to combine other antibodies, such as anti-dsDNA and anti-Smith, and to conduct a long-term follow-up of the immunological indicators of tumor patients with ANA positivity.
Moreover, when lymphoma and SLE are diagnosed in a young patient, differential diagnoses including monogenic lupus and birth immune error, especially autoimmune lymphoproliferative syndrome (ALPS), should be considered. ALPS is relegated to those disorders with variants in the activation-induced cell death pathway mediated by FAS, with somatic or germline variants in FAS, FASL, or CASP11 (26). However, genetic abnormalities alone cannot be used to diagnose ALPS. Usually, chronic (disease course of >6 months) lymphadenopathy and splenomegaly and elevated CD3 + TCRαβ+CD4−CD8− cells > 1% total lymphocytes or >2.5% CD3+ lymphocytes on flow cytometry are necessary for diagnosis. Other clinical criteria, such as autoimmune cytopenia and elevated vitamin B12, interleukin-10, and interleukin-18 levels may support the diagnosis. Meanwhile, patients with ALPS may also experience autoimmune cytopenia, including Coombs-positive autoimmune hemolytic anemia, immune thrombocytopenia, and autoimmune neutropenia (27). Our patient did not test positive for any gene variants related to ALPS or ALPS-like conditions after repeated analysis of the whole exon sequencing results. This negative result failed to indicate a common genetic background for the co-occurrence of lymphoma and SLE.
In addition, although a meta-analysis showed that mesenchymal stem cell transplantation has a favorable safety profile and good efficacy in improving the activity of lupus nephritis and renal function of SLE patients (28), earlier studies have found that hematopoietic stem cell transplantation (HSCT) or unexpected full hematopoietic engraftment following solid organ transplants may cause autoimmune disorders in both patients and mouse models (29, 30, 31, 32). SLE is a typical autoimmune disease characterized by the presence of many pathogenic autoantibodies (such as ANA, anti-dsDNA, anticardiolipin antibodies, or antibodies against various blood cell components). These autoantibodies may be the product of long-lived plasma cells in the bone marrow and may be present at low titers prior to HSCT without associated pathogenicity. If the regulatory cells that control these self-reactive plasma cells are inhibited by conditioning, the plasma cells can survive the intense lymphatic depletion during HSCT (33). In addition, the re-emergence of autoreactive B or T cells following HSCT may contribute to the autoimmunity in patients. In our case, SLE was diagnosed 4.5 years after ASCT; however, whether SLE-related antibodies were produced before the symptoms appeared, even at the time of development of Burkitt's lymphoma, or after ASCT remains unclear. This reminds us that it is necessary to pay attention to the screening and monitoring of autoantibodies during the process of diagnosis and follow-up.
Similar to other autoimmune diseases, the association between immune disorders, persistent chronic inflammation, and immunosuppressive therapy provides an appropriate background for the relationship between SLE and hematological malignancies. Several studies have suggested that SLE patients had increased viral load, EBV mRNA expression, EBV-directed antibodies, and EBV-directed cellular immunity compared to healthy controls (34, 35). This finding is consistent with the studies on the association between SLE and cancer, as EBV has been verified in the process of some malignancies, such as Burkitt's lymphoma, Hodgkin’s disease, and nasopharyngeal and gastric carcinomas (36). Our patient was positive for EBV-IgM at the time of the initial diagnosis of lymphoma. No agreement has yet been reached regarding the relationship between SLE disease activity, immunosuppressive intensity, and cancer incidence (1), although higher SLE activity and the use of cyclophosphamide may promote tumor progression (37, 38). Moreover, secondary Sjögren's syndrome may increase the risk of lymphoma because of B-cell clonal hyperplasia (37), which may also explain why lymphoma in patients may be easier to combine with or develop into Sjögren's syndrome during the process of treatment. Our patient was also considered to have developed secondary Sjögren's syndrome.
In conclusion, the present case further supports the existence of an inextricable link between autoimmune diseases and hematological malignancies as changes in the hematopoietic microenvironment may lead to immune system disorders. Despite the low frequency of SLE in children with lymphoma, this process may be induced through a common mechanism, including B-cell cloning and proliferation. Therefore, hematologists and rheumatologists should be aware of the occurrence of both cancer and SLE during patient follow-up.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Shanghai Children's Medical Center Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
CL: data curation, investigation, writing – original draft, writing – review and editing. CP: conceptualization, data curation, investigation, project administration, supervision, writing – review and editing. YiJ: data curation, investigation, resources, writing – review and editing. HH: data curation, investigation, resources, writing – review and editing. FD: formal analysis, investigation, writing – review and editing. XX: conceptualization, supervision, writing – review and editing. SB: data curation, supervision, writing – review and editing. XH: methodology, validation, writing – review and editing. YaJ: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, writing – review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Clinical Research Center For Systemic Lupus Erythematosus, Pediatric College, Shanghai Jiao Tong University School of Medicine (ELYZX202102).
Acknowledgments
The authors would like to thank the patient and his family for their consent to publish this report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ladouceur A, Tessier-Cloutier B, Clarke AE, Ramsey-Goldman R, Gordon C, Hansen JE, et al. Cancer and systemic lupus erythematosus. Rheum Dis Clin North Am. (2020) 46(3):533–50. doi: 10.1016/j.rdc.2020.05.005
2. Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: a systematic review and meta-analysis. Arthritis Res Ther. (2018) 20(1):270. doi: 10.1186/s13075-018-1760-3
3. Jiang Q, Qi C, Yang L. New-onset systemic lupus erythematosus in a woman with previous lymphoma during late pregnancy: a case report and literature review. Iran J Immunol. (2022) 19(2):213–7. doi: 10.22034/iji.2022.93584.2239
4. Jiang M, Bennani NN, Feldman AL. Lymphoma classification update: B-cell non-Hodgkin lymphomas. Expert Rev Hematol. (2017) 10(5):405–15. doi: 10.22034/iji.2022.93584.2239
5. McCarten KM, Nadel HR, Shulkin BL, Cho SY. Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr Radiol. (2019) 49(11):1545–64. doi: 10.1007/s00247-019-04529-8
6. Gao YJ, Fang YJ, Gao J, Yan J, Yang LC, Liu AG, et al. A prospective multicenter study investigating rituximab combined with intensive chemotherapy in newly diagnosed pediatric patients with aggressive mature B cell non-Hodgkin lymphoma (CCCG-BNHL-2015): a report from the Chinese Children's Cancer Group. Ann Hematol. (2022) 101(9):2035–43. doi: 10.1007/s00277-022-04904-w
7. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League against rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71(9):1400–12. doi: 10.1136/annrheumdis-2018-214819
8. Bernatsky S, Ramsey-Goldman R, Labrecque J, Joseph L, Boivin JF, Petri M, et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun. (2013) 42:130–5. doi: 10.1016/j.jaut.2012.12.009
9. Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. (2014) 25(10):2025–30. doi: 10.1093/annonc/mdu365
10. Apor E, O'Brien J, Stephen M, Castillo JJ. Systemic lupus erythematosus is associated with increased incidence of hematologic malignancies: a meta-analysis of prospective cohort studies. Leuk Res. (2014) 38(9):1067–71. doi: 10.1016/j.leukres.2014.06.025
11. Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. (2005) 165(20):2337–44. doi: 10.1001/archinte.165.20.2337
12. Merino R, de Inocencio J, García-Miguel P, García-Consuegra J. Lymphoproliferative disorders in paediatric rheumatic diseases. A report of two cases. Clin Exp Rheumatol. (2004) 22(5):649–50.15485023
13. Gokce M, Bulus D, Bilginer Y, Gumruk F, Besbas N, Cetin M. Acute lymphoblastic leukaemia in a child with systemic lupus erythematosus. Lupus. (2012) 21(8):910–3. doi: 10.1177/0961203312436859
14. Maheshwari A, Pandey M, Rath B, Chandra J, Singh S, Sharma S. Clinical and laboratory observation systemic lupus erythematosus and acute lymphocytic leukemia: an unusual case. Indian J Med Paediatr Oncol. (2011) 32(3):154–6. doi: 10.4103/0971-5851.92816
15. Saulsbury FT, Sabio H, Conrad D, Kesler RW, Levien MG. Acute leukemia with features of systemic lupus erythematosus. J Pediatr. (1984) 105(1):57–9. doi: 10.1016/s0022-3476(84)80359-5
16. Bernatsky S, Clarke AE, Zahedi Niaki O, Labrecque J, Schanberg LE, Silverman ED, et al. Malignancy in pediatric-onset systemic lupus erythematosus. J Rheumatol. (2017) 44(10):1484–6. doi: 10.1186/ar4388
17. Sheehan NJ. Systemic lupus erythematosus after acute lymphoblastic leukaemia. Rheumatology (Oxford). (2002) 41(1):113–4. doi: 10.1093/rheumatology/41.1.113
18. Steinbach WJ, Sandborg CI. Development of systemic lupus erythematosus following autologous bone marrow transplant for acute lymphocytic leukemia. J Rheumatol. (2001) 28(6):1467–8.11409148
19. Al Mayouf SM, Seraihy A. Systemic lupus erythematosus following acute lymphocytic leukemia. Ann Saudi Med. (2006) 26(1):59–61. doi: 10.5144/0256-4947.2006.59
20. Jerónimo M, Silva S, Benedito M, Brito MJ. Linfoma de Hodgkin e autoimunidade: existirá uma relação? [Hodgkin's lymphoma and autoimmunity: is there a relationship?]. Acta Med Port. (2015) 28(6):749–53. doi: 10.20344/amp.6207
21. Cabral DA, Tucker LB. Malignancies in children who initially present with rheumatic complaints. J Pediatr. (1999) 134(1):53–7. doi: 10.1016/s0022-3476(99)70372-0
22. Litwin CM, Binder SR. ANA Testing in the presence of acute and chronic infections. J Immunoassay Immunochem. (2016) 37(5):439–52. doi: 10.1080/15321819.2016.1174136
23. Im JH, Chung MH, Park YK, Kwon HY, Baek JH, Lee SY, et al. Antinuclear antibodies in infectious diseases. Infect Dis (Lond. (2020) 52(3):177–85. doi: 10.1080/23744235.2019.1690676
24. Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. (1997) 40(9):1601–11. doi: 10.1002/art.1780400909
25. Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. (2012) 64(7):2319–27. doi: 10.1002/art.34380
26. Bride K, Teachey D. Autoimmune lymphoproliferative syndrome: more than a FAScinating disease. F1000Res. (2017) 6:1928. doi: 10.12688/f1000research.11545.1
27. Lambert MP. Presentation and diagnosis of autoimmune lymphoproliferative syndrome (ALPS). Expert Rev Clin Immunol. (2021) 17(11):1163–73. doi: 10.1080/1744666X.2021.1978842
28. Xia Y, Ye H, Li K, Shi B, Sun X, Wu J. Efficacy of mesenchymal stem cell therapy on lupus nephritis and renal function in systemic lupus erythematosus: a meta-analysis. Clin Invest Med. (2023) 46(1):E24–35. doi: 10.25011/cim.v46i1.39561
29. Lambertenghi-Deliliers GL, Annaloro C, Della Volpe A, Oriani A, Pozzoli E, Soligo D. Multiple autoimmune events after autologous bone marrow transplantation. Bone Marrow Transplant. (1997) 19(7):745–7. doi: 10.1038/sj.bmt.1700711
30. Goeser LE, Chiu YE, Lerret SM, Nocton JJ. Systemic lupus erythematosus in a 15-year-old with graft-versus-host disease following liver transplant and unexpected full hematopoietic engraftment. JAAD Case Rep. (2022) 24:4–7. doi: 10.1016/j.jdcr.2022.03.015. eCollection 2022 Jun.35518274
31. Levite M, Zinger H, Mozes E, Reisner Y. Systemic lupus erythematosus-related autoantibody production in mice is determined by bone marrow-derived cells. Bone Marrow Transplant. (1993) 12(3):179–83.8241973
32. Daikeler T, Labopin M, Di Gioia M, Abinun M, Alexander T, Miniati I, et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT autoimmune disease working party. Blood. (2011) 118(6):1693–8. doi: 10.1182/blood-2011-02-336156
33. Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. (2004) 199(11):1577–84. doi: 10.1084/jem.20040168
34. Houen G, Trier NH. Epstein-Barr virus and systemic autoimmune diseases. Front Immunol. (2021) 11:587380. doi: 10.3389/fimmu.2020.587380
35. Jog NR, James JA. Epstein Barr virus and autoimmune responses in systemic lupus erythematosus. Front Immunol. (2021) 11:623944. doi: 10.3389/fimmu.2020.623944
36. Pattle SB, Farrell PJ. The role of Epstein-Barr virus in cancer. Expert Opin Biol Ther. (2006) 6(11):1193–205. doi: 10.1517/14712598.6.11.1193
37. Bernatsky S, Ramsey-Goldman R, Joseph L, Boivin JF, Costenbader KH, Urowitz MB, et al. Lymphoma risk in systemic lupus: effects of disease activity versus treatment. Ann Rheum Dis. (2014) 73(1):138–42. doi: 10.1136/annrheumdis-2012-202099
Keywords: Burkitt’s lymphoma, systemic lupus erythematosus, autologous stem cell transplantation, children, case report
Citation: Liu C, Pan C, Jin Y, Huang H, Ding F, Xu X, Bao S, Han X and Jin Y (2024) Burkitt's lymphoma in a young boy progressing to systemic lupus erythematosus during follow-up: a case report and literature review. Front. Pediatr. 12:1348342. doi: 10.3389/fped.2024.1348342
Received: 2 December 2023; Accepted: 18 February 2024;
Published: 1 March 2024.
Edited by:
Rabia Miray Kisla Ekinci, Ministry of Health, TürkiyeReviewed by:
Deniz Gezgin Yıldırım, Gazi University, TürkiyeHakan Kisaoglu, Republic of Turkey Ministry of Health Sciences, Türkiye
© 2024 Liu, Pan, Jin, Huang, Ding, Xu, Bao, Han and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanliang Jin jinyanliang@scmc.com.cn
Abbreviations SLE, systemic lupus erythematosus; CT, computed tomography; EBV, Epstein–Barr virus; IgM, immunoglobulin M; ASCT, autologous stem cell transplantation; ANA, antinuclear antibody; anti-rRNP, anti-ribosome antibody; anti-Sm, anti-Smith antibody; anti-SSA/Ro52, anti-SSA/Ro52 antibody; anti-SSA/Ro60, anti-SSA/Ro60 antibody; anti-SSB, anti-SSB antibody; anti-dsDNA, anti-double-stranded DNA antibody; C3, complement 3; C4, complement 4; CH50, total complement activities; ESR, erythrocyte sedimentation rate; EULAR/ACR, European League Against Rheumatism and the American College of Rheumatology; NHL, non-Hodgkin’s lymphoma; ALL, acute lymphoblastic leukemia; SIR, standardized incidence ration; HSCT, hematopoietic stem cell transplantation; ALPS, autoimmune lymphoproliferative syndrome.
 Chenxi Liu
Chenxi Liu Ci Pan2
Ci Pan2  Yingying Jin
Yingying Jin Hua Huang
Hua Huang