Safety and efficacy of biologic immunosuppressive treatment in juvenile idiopathic arthritis associated with inborn errors of immunity
- Rheumatology Unit, Meyer Children's Hospital IRCCS, Department of Health Sciences, University of Florence, Florence, Italy
Objectives: This study aims to describe clinical features, therapeutic outcomes, and safety profiles in patients affected by juvenile idiopathic arthritis (JIA) and inborn errors of immunity (IEI) treated with biological Disease-modifying antirheumatic drugs (DMARDs).
Methods: We enrolled three patients who were followed in the Pediatric Rheumatology Unit at Meyer Children's Hospital in Florence; these patients were affected by JIA, according to ILAR criteria, and IEI, according to the IUIS Phenotypical Classification for Human Inborn Errors of Immunity. Among them, two patients had 22q11.2 deletion syndrome (22q11.2DS) and one patient had X-linked agammaglobulinemia (XLA).
Results: Case 1: A 6-year and 2-month-old boy was affected by 22q11.2DS, associated with oligoarticular JIA, at the age of 2 years. He was treated with non-steroidal anti-inflammatory drugs (NSAIDs) and methotrexate, along with oral glucocorticoids but with no benefits. Treatment with etanercept allowed him to achieve remission after 10 months. Case 2: A 6-year and 2-month-old girl was affected by 22q11.2DS, associated with oligoarticular JIA, at the age of 3 years and 11 months. She was treated with NSAIDs, joint injections, and methotrexate but without clinical response. Treatment with Adalimumab allowed her to achieve remission after 6 months. Case 3: A 12-year and 2-month-old boy was affected by XLA, associated with polyarticular JIA, at the age of 9 years and 11 months. He was treated with NSAIDs, methotrexate, joint injections, and oral glucocorticoids with no benefits. He failed to respond to anti-TNF-alpha, tocilizumab, and abatacept. Currently, he is undergoing therapy with sirolimus plus abatacept, which allowed him to achieve remission after 4 months.
Conclusions: Results suggest that the use of immunosuppressive biological therapies can control disease activity in these patients. No adverse drug-related reactions were observed during the follow-up.
Introduction
Inborn errors of immunity (IEI) are a heterogeneous group of diseases affecting different components of the immune system. Overall, patients with IEI have an increased susceptibility to infectious diseases and autoimmune diseases, including juvenile idiopathic arthritis (JIA) (1). In 22q11.2 deletion syndrome (22q11.2DS), previously known as DiGeorge syndrome, a congenital chromosome deletion syndrome (22q11.2 del) characterized by decreased T-cell numbers secondary to thymic hypoplasia, JIA occurs 50 times more commonly than in the normal population (2). In X-linked agammaglobulinemia (XLA), a genetic disorder resulting in maturational disturbance of B-cell development due to a mutation in Bruton's tyrosine kinase (BTK), JIA has a prevalence of about 13% (3). We report a case series of three patients with IEI, receiving different immunosuppressive treatments due to the severe active disease of concomitant JIA.
Materials and methods
To be eligible for the case series description, patients should fulfill the diagnosis criteria of JIA, according to the ILAR criteria (4), and the diagnosis criteria of IEI, according to the IUIS (International Union of Immunological Societies) Phenotypical Classification for Human Inborn Errors of Immunity (5). Clinical charts of eligible patients were retrospectively reviewed at the disease onset and during the disease course. The disease onset of the first eligible patient was documented in August 2019. Over the period between January 2019 and December 2023, three patients fulfilled the inclusion criteria of the study. During the same time frame, 750 children with JIA were currently being followed at our unit.
Results
Case 1: A boy affected by 22q11.2DS, at the age of 2 years and 3 months, developed arthritis in the right ankle. MRI of the right ankle revealed synovitis and concomitant effusion in joint spaces. A diagnosis of oligoarticular JIA was made, and treatment with non-steroidal anti-inflammatory drug (NSAIDs) and methotrexate (MTX, dose 15 mg/mq s.c. once a week) was started. After 6 months with MTX, he complained of persistent arthritis in the right ankle, therefore leading to a steroid joint injection. One month later, the patient experienced another flare-up in the right ankle, and treatment with etanercept (0.8 mg/kg/week) was then started. Nonetheless his mono-articular course, the patient achieved a stable and persistent remission only after 10 months from starting Etanercept, while also maintaining MTX treatment. After at least 2 years of persistent remission, we attempted to wean and stop MTX over an additional 1 year. At the last follow-up, at the age of 6 years and 2 months, he is still in remission on etanercept and has started to wain this treatment. Over the course of JIA treatment with immunomodulatory drugs, the boy did not experience an increased rate of infections or serious adverse events.
Case 2: A girl affected by 22q11.2DS, at the age of 3 years and 6 months, developed left knee arthritis. MRI of the involved joint revealed signs of synovial thickening and joint effusion. She received NSAIDs for 16 weeks with no clinical response. Eight months later, a diagnosis of oligoarticular JIA was made, which was associated with severe uveitis, cataract, and iridolenticular synechia. She was treated with joint injections and MTX (dose 15 mg/mq s.c. once a week), but did not show improvement. After 8 months of treatment, MTX was stopped due to an increase in liver enzyme, and adalimumab (20 mg/2 weeks) was then started. After 6 months, the patient achieved clinical remission. At 5 years and 4 months, while undergoing therapy with adalimumab, she developed arthritis in the left knee, and by the age of 5 years and 11 months, she developed arthritis in both knees, leading to treatment with joint injections. At the last follow-up, at 6 years and 1 month, she is still being treated with adalimumab (increased to 40 mg/2 weeks for weight >30 kg), achieving clinical remission as regards arthritis and uveitis. The ocular condition remains stable, except for the ocular cataract, which was surgically treated. Over the course of Adalimumab treatment, no cases of infection or other adverse drug-related reactions were observed. Anti-adalimumab antibodies were periodically checked and yielded negative results (ELISA testing).
Case 3: A boy with XLA, in treatment with subcutaneous immunoglobulins since he was 2 years old, developed arthritis in the left knee at the age of 9 years and 11 months. After a few months, he developed arthritis in both ankles, knees, and elbows, as well as in I and V proximal interphalangeal (PIP) joints of the left hand, along with enthesitis of the right peroneal, tibial, and Achilles tendons. MRI scanning of both knees was performed, revealing signs of synovitis and modest joint effusion. Upon the diagnosis of polyarticular JIA, treatment with NSAIDs, MTX, and oral steroids was started. After 3 months, he developed new arthritis in both wrists and I and III PIP joints of the right hand. So, adalimumab (20 mg/2 weeks) was started, in addition to oral steroids for a month. Two months later, he developed arthritis in the knees and left ankle; thus, he received multiple joint injections. At the age of 10 years and 8 months, adalimumab was discontinued due to disease progression, and etanercept (0.8 mg/kg/weekly) was started, but without efficacy. Four months later, etanercept was switched to tocilizumab (8 mg/kg every 4 weeks), but arthritis persisted in both ankles, knees, and wrists. At the age of 11 years and 6 months, tocilizumab was switched to abatacept (10 mg/kg every 4 weeks) due to poor response. Despite additional joint injections for persistent arthritis in both knees, wrists, elbows, and PIP joints, his polyarthritis disease persisted. When he was 12 years old, sirolimus was added to the treatment regimen with abatacept and oral steroids, therefore discontinuing MTX, considering the possible role of mTOR protein in modulating immune response and influencing the production of inflammatory cytokines (6). Four months later, he achieved clinical remission, and steroids were then weaned. No cases of infection or serious adverse reactions were observed during treatment. In our cohort, no other autoimmune diseases, infections, or lymphoproliferative disorders were reported. In addition, to evaluate disease remission, the Juvenile Arthritis Disease Activity Score (JADAS) and Childhood Health Assessment Questionnaire (CHAQ) were scored. A summary of the demographic, clinical, laboratory, and therapeutic data of all three patients is presented in Table 1.

Table 1. Demographic data, laboratory tests, clinical features, diagnostic data, therapeutic data, and outcomes at the last follow-up.
Discussion
The association between autoimmune diseases and inborn errors of immunity is well described in the literature, but poor evidence is available regarding the treatment outcomes. JIA is an antigen-driven autoimmune process, involving several mediators such as lymphocyte Th1, B cells, and macrophages (7). Autoimmune diseases have been associated with 22q11.2DS, probably linked to T-cell regulatory defects and impaired central tolerance. In XLA, low IgG levels, in addition to increasing susceptibility to infections, may be the basis of the inflammatory mechanism of JIA. Furthermore, BTK gene is associated with the Toll-like receptor (TLR) pathway, whose activation leads to the production of cytokines, notably TNF (1, 8).
Disorder of immune regulation can be the basis of autoimmunity and could be an initial or predominant manifestation of certain IEIs. Growing attention is being directed toward exploring potential predictors of immune dysregulation and autoimmunity in IEI. For example, in 22q11.2DS, low CD3+ T-cell counts, B-cell dysfunction, and consequently low serum levels of immunoglobulin can increase the rate of developing autoimmunity, manifesting as immune cytopenias as well as hypothyroidism, hyperthyroidism, diabetes mellitus, psoriasis, vitiligo, alopecia, and rheumatologic disease including juvenile idiopathic arthritis and systemic sclerosis (9). Although no studies specifically focus on JIA, this could represent an interesting perspective.
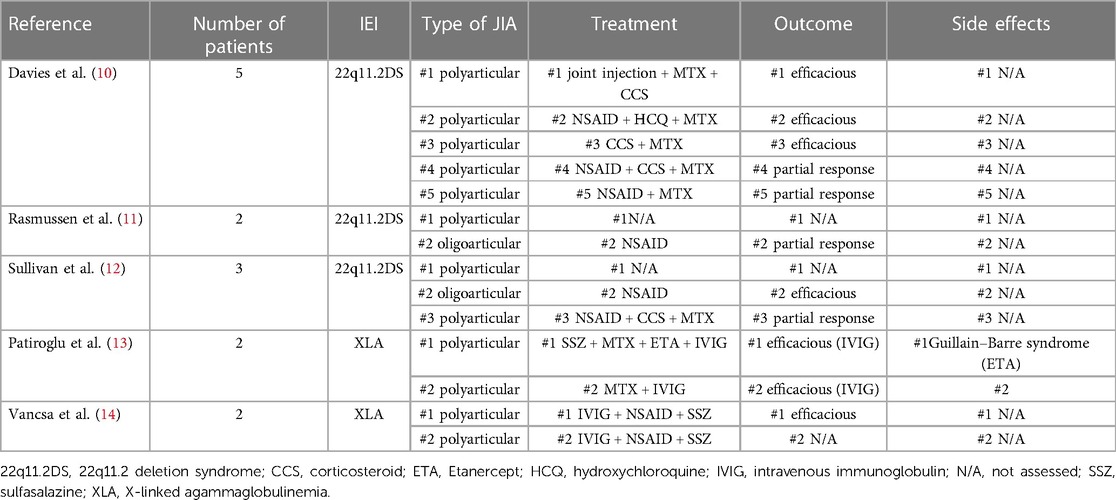
In our experience, JIA in IEI seems to exhibit a more severe disease course, with recurrent arthritis, the need for several joint injections and different corticosteroid courses, and, according to the literature, poor response to traditional immunosuppressive drugs (2, 10–14). Conversely to the literature, in patient #3, the immunoglobulin substitutive therapy did not change the course of arthritis (13). The use of biologic Disease-modifying antirheumatic drugs (bDMARDs) in patients with IEI remains controversial. Brief comparison of different treatments in patients with JIA and IEI in literature is presented in Table 2. Although mandatory due to the activity of the disease and its progression, their use is not free of side effects, including increased susceptibility to opportunistic infections.
In our cohort, bDMARDs were added to the treatment regimen of all patients achieving clinical remission after several months and different attempts. Patient 1 received Etanercept plus MTX, while patient 2 received adalimumab and several joint injections, with good clinical control. Patient 3 had not responded to anti-TNF-alpha, tocilizumab, abatacept, and several joint injections, but showed good clinical response to sirolimus after 2 months. Apart from JIA associated with IEI, TNF-alpha inhibitors have been used with good clinical efficacy in patients with 22q11.2DS with associated IBD and in one patient with XLA with associated pyoderma gangrenosum (8–16).
In terms of safety, literature data suggest that biological therapies are generally well-tolerated, with common side effects that typically do not necessitate drug discontinuation. However, severe adverse events, including serious infections, have been observed, particularly when biological therapies are used in conjunction with other immunosuppressive drugs such as corticosteroids and methotrexate (17).
In our cohort, treatment with biological immunosuppressors over a follow-up of at least 16 months (range 16–38 months) did not show significant adverse events, including severe infections. None of the used bDMARDs were stopped due to adverse events, but rather due to no efficacy.
In our experience, biological DMARDs have been shown to be helpful and safe in controlling disease activity in these patients. Notwithstanding, the use of these kinds of therapies in this cohort of patients needs to be further studied, and regular follow-ups are necessary for monitoring the possible side effects of the treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
VA: Writing – original draft. IP: Writing – review & editing. IM: Writing – review & editing. EM: Writing – review & editing. MM: Writing – review & editing. GS: Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Amaya-Uribe L, Rojas M, Azizi G, Anaya J-M, Gershwin ME. Primary immunodeficiency and autoimmunity: a comprehensive review. J Autoimmun. (2019) 99:52–72. doi: 10.1016/j.jaut.2019.01.011
2. Verloes A, Curry C, Jamar M, Herens C, O'Lague P, Marks J, et al. Juvenile rheumatoid arthritis and del(22q11) syndrome: a non-random association. J Med Genet. (1998) 35(11):943–7. doi: 10.1136/jmg.35.11.943
3. El-Sayed ZA, Abramova I, Aldave JC, Al-Herz W, Bezrodnik L, Boukari R, et al. X-linked agammaglobulinemia (XLA): phenotype, diagnosis, and therapeutic challenges around the world. World Allergy Organ J. (2019) 12(3):100018. doi: 10.1016/j.waojou.2019.100018
4. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. (2011) 377(9783):2138–49. doi: 10.1016/S0140-6736(11)60244-4
5. Bousfiha A, Moundir A, Tangye SG, Picard C, Jeddane L, Al-Herz W, et al. The 2022 update of IUIS phenotypical classification for human inborn errors of immunity. J Clin Immunol. (2022) 42(7):1508–20. doi: 10.1007/s10875-022-01352-z
6. Venkatachari IV, Chougule A, Gowri V, Taur P, Bodhanwala M, Prabhu S, et al. Monogenic inborn errors of immunity in autoimmune disorders. Immunol Res. (2023) 71(5):771–80. doi: 10.1007/s12026-023-09391-3
7. Hahn Y-S, Kim J-G. Pathogenesis and clinical manifestations of juvenile rheumatoid arthritis. Korean J Pediatr. (2010) 53(11):921–30. doi: 10.3345/kjp.2010.53.11.921
8. Schwartzfarb EM, Weir D, Conlan WA, Romanelli P, Kirsner RS. Pyoderma gangrenosum in a patient with Bruton’s X-linked agammaglobulinemia: shared pathogenesis of altered tumor necrosis factor alpha? J Clin Aesthet Dermatol. (2008) 1(1):26–9. 21103306
9. Deshpande DR, Demirdag YY, Marsh RA, Sullivan KE, Orange JS. Relationship between severity of T cell lymphopenia and immune dysregulation in patients with DiGeorge syndrome (22q11.2 deletions and/or related TBX1 mutations): a USIDNET study. J Clin Immunol. (2021) 41(1):29–37. doi: 10.1007/s10875-020-00854-y
10. Davies K, Stiehm ER, Woo P, Murray KJ. Juvenile idiopathic polyarticular arthritis and IgA deficiency in the 22q11 deletion syndrome. J Rheumatol. (2001) 28(10):2326–34. 11669177
11. Rasmussen SA, Williams CA, Ayoub EM, Sleasman JW, Gray BA, Bent-Williams A, et al. Juvenile rheumatoid arthritis in velo-cardio-facial syndrome: coincidence or unusual complication? Am J Med Genet. (1996) 64(4):546–50. doi: 10.1002/(SICI)1096-8628(19960906)64:4%3C546::AID-AJMG4%3E3.0.CO;2-N
12. Sullivan KE, McDonald-McGinn DM, Driscoll DA, Zmijewski CM, Ellabban AS, Reed L, et al. Juvenile rheumatoid arthritis-like polyarthritis in chromosome 22q11.2 deletion syndrome (DiGeorge anomalad/velocardiofacial syndrome/conotruncal anomaly face syndrome). Arthritis Rheum. (1997) 40(3):430–6. doi: 10.1002/art.1780400307
13. Patiroglu T, Akar HH, Gunduz Z, Sisko S, Ng YY. X-linked agammaglobulinemia in two siblings with a novel mutation in the BTK gene who presented with polyarticular juvenile idiopathic arthritis. Scand J Rheumatol. (2015) 44(2):168–70. doi: 10.3109/03009742.2014.995699
14. Váncsa A, Tóth B, Szekanecz Z. BTK gene mutation in two non-identical twins with X-linked agammaglobulinemia associated with polyarticular juvenile idiopathic arthritis. Isr Med Assoc J. (2011) 13(9):579–80. 21991724
15. Tam P-Y, Hanisch BR, Klammer K, DeVries AS. Measles vaccine strain from the skin rash of a DiGeorge patient receiving tumor necrosis factor inhibitor. Pediatr Infect Dis J. (2014) 33(1):117. doi: 10.1097/INF.0000000000000073
16. Uy R, Jacobs N, Mziray-Andrew C. Inflammatory bowel disease and diverticulosis in an adolescent with DiGeorge syndrome. J Pediatr Gastroenterol Nutr. (2016) 62(5):e43–5. doi: 10.1097/MPG.0000000000000497
17. Abubakar SD, Ihim SA, Farshchi A, Maleknia S, Abdullahi H, Sasaki T, et al. The role of TNF-α and anti-TNF-α agents in the immunopathogenesis and management of immune dysregulation in primary immunodeficiency diseases. Immunopharmacol Immunotoxicol. (2022) 44(2):147–56. doi: 10.1080/08923973.2021.2023173
Keywords: arthritis, juvenile, inborn errors of immunity, DiGeorge syndrome, Bruton-type agammaglobulinemia, adalimumab, etanercept, tocilizumab
Citation: Accardo V, Pagnini I, Maccora I, Marrani E, Mastrolia MV and Simonini G (2024) Safety and efficacy of biologic immunosuppressive treatment in juvenile idiopathic arthritis associated with inborn errors of immunity. Front. Pediatr. 12:1353825. doi: 10.3389/fped.2024.1353825
Received: 11 December 2023; Accepted: 9 February 2024;
Published: 26 February 2024.
Edited by:
Giorgia Martini, University Hospital of Padua, ItalyReviewed by:
Robert Naddei, Federico II University Hospital, ItalyGiorgio Costagliola, Azienda Ospedaliero Universitaria Pisana, Italy
© 2024 Accardo, Pagnini, Maccora, Marrani, Mastrolia and Simonini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: V. Accardo valerio.accardo@unifi.it
 V. Accardo
V. Accardo I. Pagnini
I. Pagnini I. Maccora
I. Maccora E. Marrani
E. Marrani M. V. Mastrolia
M. V. Mastrolia  G. Simonini
G. Simonini