- 1Department of Pharmacognosy, School of Pharmacy, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
- 2Department of Pharmaceutical Chemistry and Pharmacognosy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
The genus Echinops belongs to the family of Asteraceae and comprises about 130 species. Many species belonging to the genus Echinops are traditionally used as medicinals mainly in Africa and Asia. The genus is reported to contain diverse secondary metabolites. The aim of this review is to critically evaluate the available research reports on the genus and systematically organize the findings. Information for this study was obtained using various search engines including PubMed and Google Scholar. This review revealed that the genus is used traditionally to treat pain, inflammation, respiratory diseases, diseases caused by different microorganisms, as an aphrodisiac, to fasten expulsion of placenta, and for removal of renal stones. More than 151 secondary metabolites have been reported from the genus in which thiophenic compounds held the biggest share. Various extracts, essential oils, and isolated compounds from members of this genus are shown to exhibit different biological effects mainly anti-microbial, anti-proliferative, and anti-inflammatory. However, there are a number of species in this genus that are claimed to have traditional medicinal uses but their biological effect not yet been evaluated.
Introduction
Echinops L., belongs to the family of Asteraceae, a family which is distributed all over the world except in Antarctica. Asteraceae is a monophyletic taxon distinguished by florets arranged on a receptacle in centripetal heads and bounded by bracts. It comprises 1,600−1,700 genera and 24,000−30,000 species (Funk et al., 2005). The genus Echinops belongs to the tribe Cardueae and is recognized by the presence of uniflowered capitula aggregated into second-order spherical or oval heads. This feature makes it unique within the tribe (Garnatje et al., 2005; Sánchez-Jiménez et al., 2010). It contains 120−130 species distributed across north and tropical Africa, the Mediterranean Basin, and central Asia. Members of this genus are mostly perennial with few annuals (Hedberg et al., 2004; Sánchez-Jiménez et al., 2010).
Many members of this genus are traditionally used to treat different diseases. Some are scientifically investigated for various biological activities and phytoconstituents. Previously, reviews that focus on single species, Echinops spinosus L. and E. echinatus Roxb. have been conducted (Bouzabata et al., 2018; Maurya et al., 2015). To the authors' knowledge, there is no study that reviewed the traditional use, phytochemistry, and biological activities of the whole genus. This review is aimed to critically evaluate available research reports on the genus and systematically organize and present the findings. It is attempted to include all articles published from 1990−2018 while some articles published before 1990 were included considering their significance. This review excluded unpublished findings and publications which were not available online and articles written in languages other than English. Chemical structures of only isolated and characterized compounds were provided while structures of compounds identified from essential oils and other chemical analysis were not. The main sources of the structures of isolated compounds were the research articles and these were confirmed using PubChem. Structures that were not available in the articles were obtained from theses, books, PubChem, and other reliable sources. Different search engines including PubMed and Google Scholar were employed to search literature using searching words such as Echinops, plant, phytochemical, phytochemistry, pharmacological activity, biological effect, and traditional use.
Traditional Uses
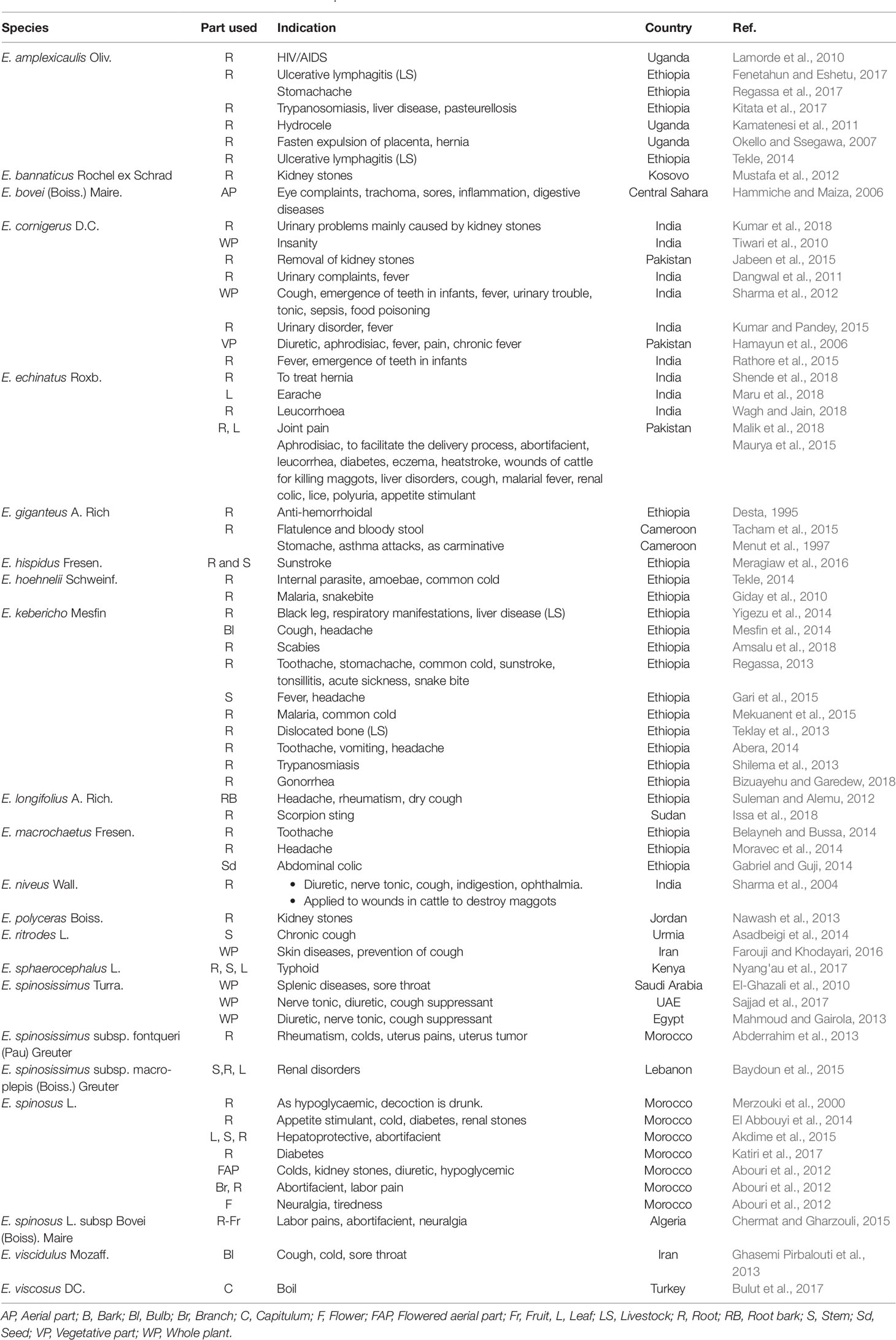
Ethnomedicinal claims on the genus Echinops to treat a number of ailments are depicted in Table 1. The common traditional uses can fall into three general groups. The frequently described application is to treat symptoms like inflammation, pain, and fever (Regassa, 2013; Rathore et al., 2015). The other common traditional use was to treat ailments related to respiratory tract including cough and sore throat (Ghasemi Pirbalouti et al., 2013; Sajjad et al., 2017). Members of the genus have been used as an aphrodisiac (Hamayun et al., 2006), facilitation of expulsion of retained placenta and delivery (Okello and Ssegawa, 2007; Qureshi and Bhatti, 2008), as an abortifacient (Abouri et al., 2012), treatment of uterus tumor (Abderrahim et al., 2013), and leucorrhoea (Wagh and Jain, 2018). Three species (E. bannaticus Rochel ex Schrad, E. cornigerus D.C., and E. polyceras Boiss.) reported to have been employed in the managment of kidney stones (Mustafa et al., 2012; Nawash et al., 2013; Kumar et al., 2018).
In addition to the traditional medicnal applications described in Table 1, the plants have nutritional value. In Iran, the bulb of E. viscidulus Mozaff is consumed as a vegetable (Ghasemi Pirbalouti et al., 2013). The roots of E. giganteus A. Rich. and E. spinosus are used as a spice in Morocco and Cameroon, respectively (Pavela et al., 2016; Tbatou et al., 2016). The use of E. giganteus might be attributed to the presence of nutrients including iron, phenols, carotenoids, and vitamins E and C in the plant (Abdou Bouba et al., 2012).
Phytochemicals
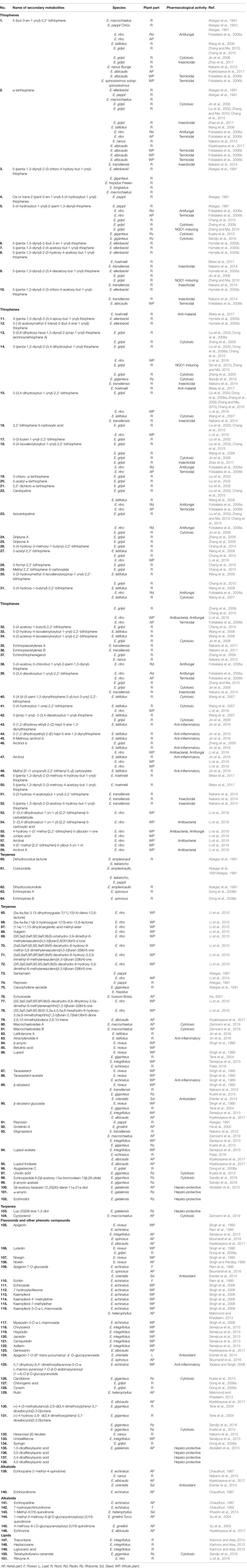
As presented in Table 2 and Figure 1, 151 compounds have been isolated and characterized using different spectroscopic/spectrometric techniques. Members of the genus Echinops contain primarily thiophenes and terpenes. Flavonoids and other phenolic compounds, alkaloids, lipids, and phenylpropanoids were also reported. The root of the plant is the main source of the thiophenes while most of the terpenes and flavonoids were isolated from the aerial part/the whole plant. The genus is also known for essential oil content and all morphological parts of the plants are reported to contain some of the essential oils. Around 53 of the isolated and characterized compounds are reported to have different biological activities. The structural formulae of isolated and characterized compounds are given in Figure 1.
Thiophenes
Thiophenes, the main bioactive constituents of the genus Echinops, are biosynthetically derived from fatty acids and reduced sulphur (Arroo et al., 1997). Majority of the thiophenic compounds comprise an acetylenic functional group and most of the thiophenes comprised two thiophene rings in their structure. The most abundant thiophenes which were reported from nine species were 5-(but-3-en-1-ynyl)-2,2'-bithiophene (1) and α-terthiophene (2). 5-(4-hydroxybut-1-ynyl)- 2-(pent-1,3-diynyl)-thiophene (5), 5-(penta-1,3-diynyl)-2-(3,4-dihydroxybut-1-ynyl)-thiophene (14), and 5-(4-hydroxy-1-butynyl)-2,2'-bithiophene (31) were isolated from five species. Thiophenes were detected in essential oils obtained from the different plants of this genus. 5-(3-buten-1-ynyl)-2,2'-bithienyl was detected in essential oils obtained from the roots of E. grijsii Hance, E. bannaticus, and E. sphaerocephalus L.
The biological activities of thiophenes were evaluated mainly in vitro and they have an insecticidal, anti-proliferative, and anti-fungal potential effects.
Terpenoids
Sesqui- and triterpenoids were reported mainly from the whole plant and aerial parts of the genus Echinops. Most of the sesquiterpenoids contain lactones. Sesquiterpene lactones are also the most prevalent secondary metabolites in the family of Asteraceae (Chadwick et al., 2013). Most triterpenoids exist in various forms including lactones, esters, and sterols along with their glycosides. The common sesquiterpenoid reported was costunolide (61), which was isolated from three species whereas lupeol (86) and lupeol acetate (94) were the common triterpenoids. Many sesquiterpenoids were also detected from the essential oils of the genus.
Flavonoids and Other Phenolic Compounds
Flavonoids from the genus Echinops were mainly flavones and mostly isolated from the whole plant and aerial parts of the members. Apigenin (105) is the most common flavonoidal aglycone and it was isolated from the flower and whole plant of E. niveus Wall., E. echinatus, E. integrifolius Kar. & Kir., and E. albicaulis Kar. & Kir. (Table 2). In addition to flavonoids, phenolic compounds including coumarins, phenylpropanoids, and lignans were reported (Tene et al., 2004; Dong et al., 2008a; Senejoux et al., 2013).
Alkaloids
The first alkaloids isolated from the genus Echinops were echinopsine (139), echinozolinone (140), and echinopsidine (141) from the aerial parts of E. echinatus (Chaudhuri, 1987). Later on, another alkaloid, 7-hydroxyechinozolinone (142), was isolated from the flowers of the same plant (Chaudhuri, 1992). Additional four alkaloids of which two were in glycosidic form were reported (Table 2). The alkaloids were mainly isolated from the aerial parts of the plants. The predominant alkaloid, which was isolated from four different species, was 1-methyl-4-quinolone (139).
Essential Oils and Lipids
The genus Echinops is rich in bioactive essential oil constituents, which were mainly found in the roots. Various reports indicated the presence of terpenoids and thiophenes.
The root of E. grijisii was found to contain cis-β-farnesene and 5-(3-buten-1-ynyl)-bithiophene as main components (Guo et al., 1994). Essential oils from root, stem, leaf, and flowers of E. ellenbeckii comprised mainly β-maaliene, cyperene, caryophyllene oxide, and β-selinene from the respective plant parts (Hymete et al., 2004). The fresh inflorescences of E. graecus and E. ritro yielded methyl chavicol and (E)-2-hexenal, 1,8-cineole, and p-cymene as major constituents, respectively (Papadopoulou et al., 2006).
Essential oils from the root of E. bannaticus and E. sphaerocephalus were reported to contain 5-(3-buten-1-ynyl)-2,2'-bithienyl and α-terthienyl as major constituents, and also triquinane sesquiterpenoids (Radulović and Denić, 2013). The most abundant compounds from E. giganteus have been reported to be tricyclic sesquiterpenoids such as silphiperfol-6-ene and presilphiperfolan-8-ol followed by presilphiperfol-7-ene, cameroonan-7-α-ol, and (E)-caryophyllene (Pavela et al., 2016).
Ceramides, sulf-polyacetylene ester, and simple hydrocarbons were the nonpolar constituents from the genus (Figure 1). The ethyl acetate extract of E. integrifolius contained lupeolacetate, 1,3-butadiene-1-carboxylic acid, lupeol, (1R,3R,4R,5R)-(–)-quinic acid, palmitic acid, and D-threo-O-ethylthreonine as the main constituents (Karimov and Aisa, 2012). In a related study, GS-MS analysis of petroleum ether extract of the aerial part of E. integrifolius indicated the presence of methyl esters of fatty acids as well as saturated hydrocarbons such as octacosane, hentriacontane, hexacosane, tetratetraacontane, eicosane, and nonadecane. Trace amount of 2-octanone and 4,8,12,16-tetramethyl heptadecan-4-olide were also detected in E. integrifolius (Karimov and Aisa, 2013).
Biological Activities
Anti-Microbial Activity
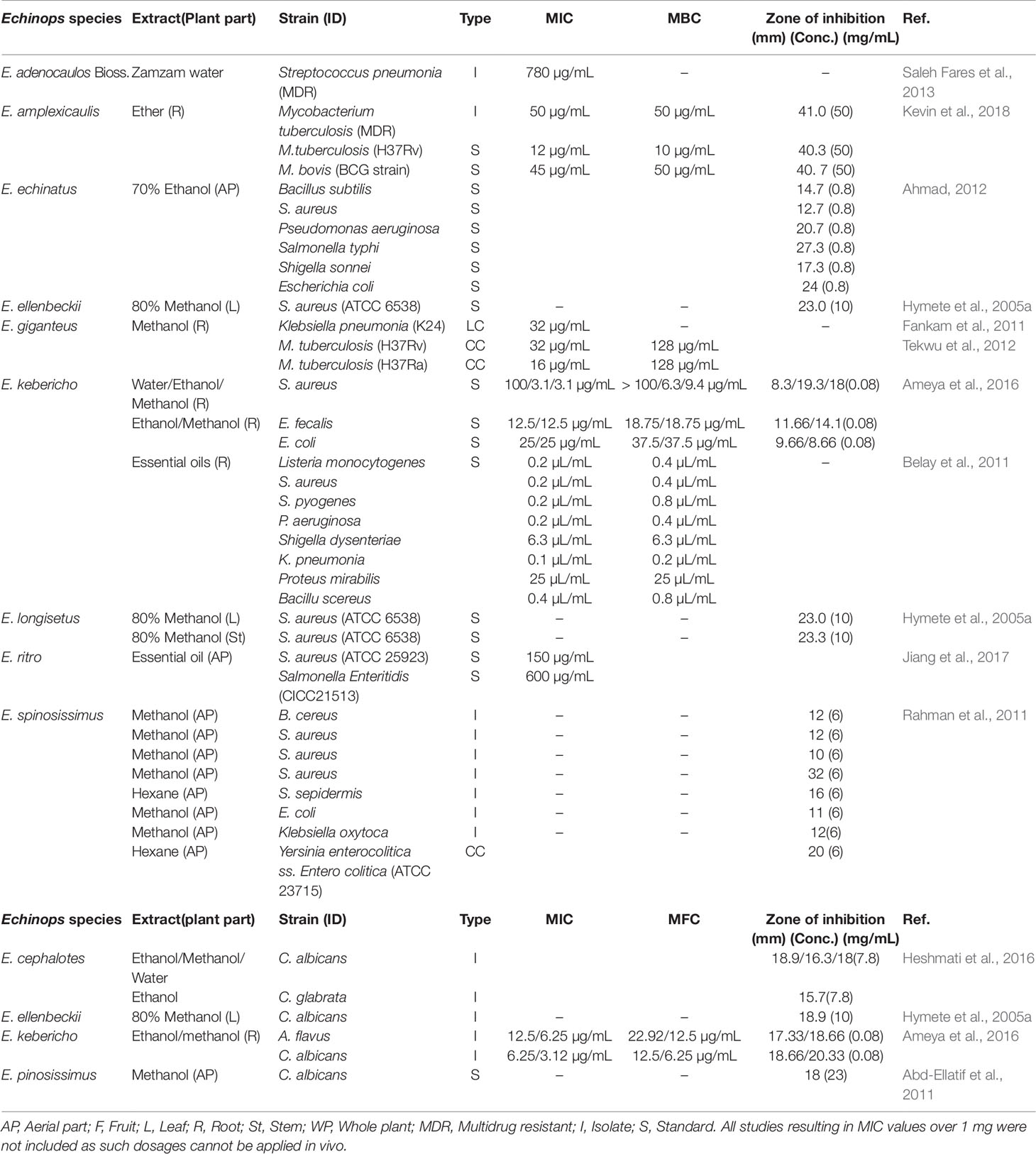
The genus Echinops is traditionally used to treat different infectious diseases including trachoma, sepsis, typhoid, gonorrhea, and ulcerative lymphangitis. It is also used to treat different ailments that might be caused by bacterial/fungal infections including fever, respiratory diseases, toothache, leucorrhoea, and earache. Thus, they have been investigated for their anti-microbial activities. Anti-bacterial and anti-fungal activities of extracts from the genus with their respective minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBI), minimum fungicidal concentration (MFC), and zone of inhibitions are presented in Table 3. These studies showed that both Gram-positive and Gram-negative bacteria were sensitive to the extracts/isolated compounds obtained from the genus.
Out of the tested strains, M. tuberculosis (H37Rv) showed higher sensitivity to the ether root extract of E. giganteus and methanolic extract of E. amplexicaulis Oliv. with MIC of 12 µg/mL and 32 µg/mL, respectively (Tekwu et al., 2012; Kevin et al., 2018). The methanolic root extract of E. amplexicaulis also showed a promising effect against a multidrug-resistant strain of M. tuberculosis with a MIC of 50 µg/mL (Kevin et al., 2018). The ethanolic root extract and essential oils obtained from E. kebericho Mesfin showed relatively strong effect against Staphylococcus aureus (Ameya et al., 2016) and Klebsiella pneumoniae (Belay et al., 2011). These results might justify the traditional application of E. kebericho in treating respiratory disease, toothache, and fever. The essential oil from E. ritro L. exhibited anti-bacterial effect and antibiofilm and disruption of the bacterial membrane were suggested as mechanisms of actions (Jiang et al., 2017).
Different extracts from members of the genus having anti-bacterial effect were analyzed for their chemical constituents. The unsaponifiable matter from the hexane extract of E. spinosissimus contained mainly taraxasterol, lupeol, pseudotaraxasterol, α-amyrin, β-amyrin, pseudotaraxasteryl acetate, lup-22(29)-en-3-yl acetate, β–sitosterol, and stigmasterol. The hexane extract showed anti-bacterial activity with MIC values of less than 125 µg/mL against different bacterial strains (Bacillus amyloliquefaciens, Micrococcus luteus, Bacillus subtilis, and Salmonella enteric) (Bouattour et al., 2016). Thiophens (31, 46, 54, and 59) isolated from the root of E. ritro possessed anti-bacterial effect against S. aureus with a MIC value of 8 µg/mL. This was similar to the effect observed for the positive control, levofloxacin. The anti-bacterial effects of thiophenes 31, 46, 55, 57, and 59 against Escherichia coli with a MIC of 64, 32, 64, 64, and 8 µg/mL, respectively, were also described (Li et al., 2019).
In addition to those described in Table 3, the root extract of Echinops spp from Ethiopia showed anti-bacterial activity through growth inhibition (Ashebir and Ashenafi, 1999). The study did not delineate the specific name of the plant, MIC/MBC, and zone of inhibitions which makes it challenging to compare with other study results. Methanolic extract of the whole plant of E. polyceras improved the effect of tetracycline on resistant strains of Pseudomonas aeruginosa (Aburjai et al., 2001). The effect of the plant without tetracycline however was not studied. The leaf and flower extracts of E. viscosus subsp. bithynicus were described to have anti-bacterial properties against E. coli, Micrococcus luteus, S. aureus, Mycobacterium smegmatis, P. aeruginosa, Enterobacter cloacae, and Bacillus megaterium. Even though the concentration of the extracts is not well defined in the study, the flower extract of E. microcephalus has been reported to have greater zone of inhibition than the standard drug, vancomycin (30 µg/disc) (Toroğlu et al., 2012).
Most of the anti-fungal studies on the genus revealed that the extracts/isolated compounds were effective mainly against Candida albicans with the most potent effect observed for the root methanolic extract of E. kebericho (MIC = 3.12 µg/mL) (Ameya et al., 2016).
Thiophenes (1, 2, 5, 18, 22, 23, 31, 38, and 39) from E. ritro have been described to possess significant anti-fungal activity against different fungal isolates. The most active thiophenes were 1 (IC50 = 4.2 µM) against Colletotrichum gloeosporioides, 2 (IC50 = 1.9 µM), and 5 (IC50 = 1.1 µM) against C. fragariae (Fokialakis et al., 2006a). A recent study also showed that thiophenes (31, 46, and 55) isolated from E. ritro exhibited anti-fungal effect against C. albicans with the MIC of 64, 32, and 64 µg/mL, respectively (Li et al., 2019). The anti-fungal activity of extracts obtained from E. viscosus subsp. bithynicus and E. microcephalus leaves and flowers were found to be active against Saccharomyces cerevisiae, Rhodotorula rubra, Mucor pusillus, and Kluyveromyces fragilis (Toroğlu et al., 2012).
Effect on Cancer Cell Lines
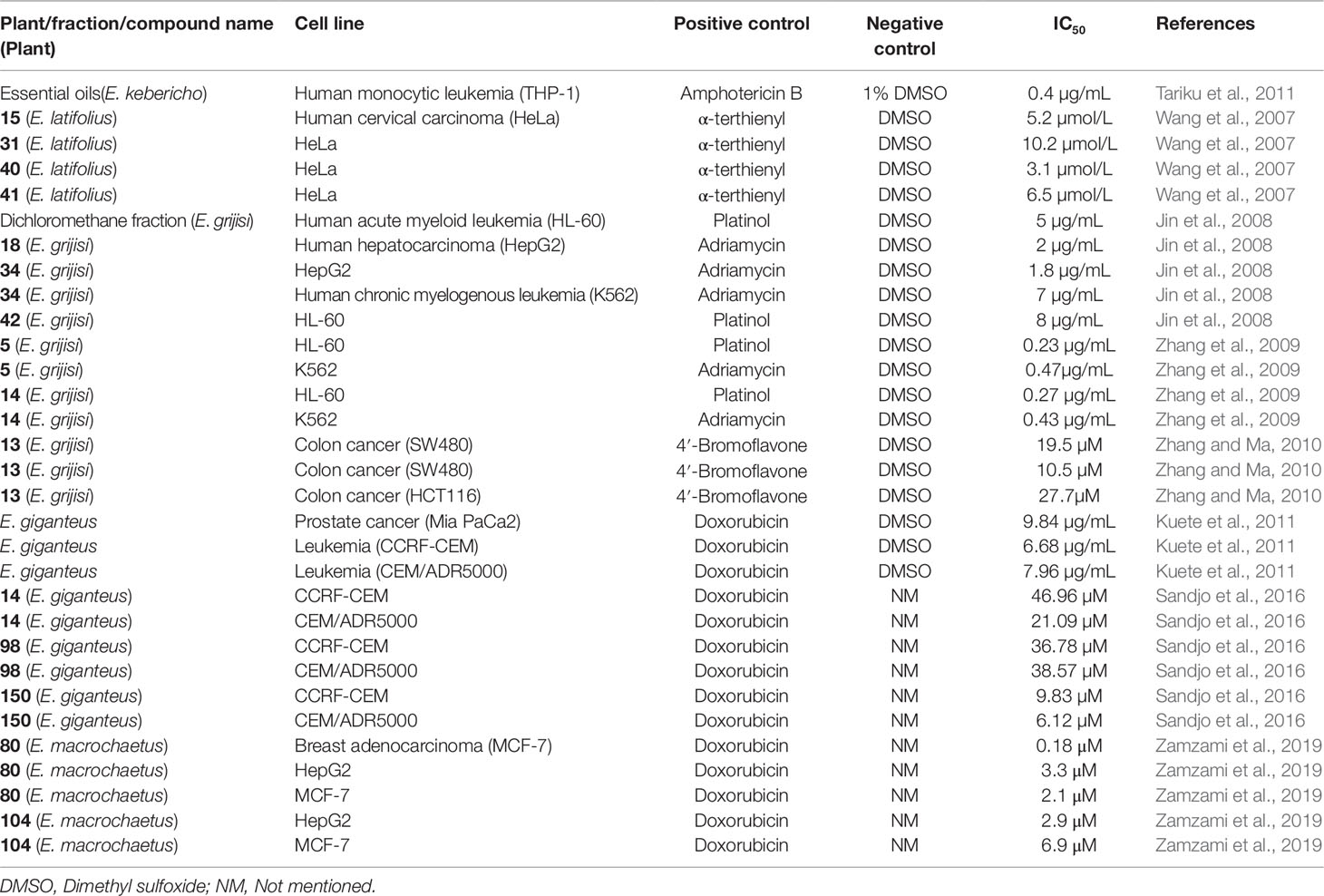
The traditional use of the genus Echinops in the treatment of cancer is not common nevertheless the species in this genus were explored for cytotoxic activity. The methanolic extract of E. kotschyi Boiss. against MOLT-4 and K562 cancer cell lines (Afshaki et al., 2012) and essential oils obtained from E. kebericho, which consist of 43 compounds predominantly dehydrocostus lactone, showed cytotoxic activity against human monocytic leukemia cell line (THP-1) with an IC50 value of 0.4 µg/L (Tariku et al., 2011).
Four thiophens isolated from E. latifolius Tausch., 5-(3,4-dihydroxybut-1-ynyl)-2,2'-bithiophene (15), 5-(4-hydroxy-1-butynyl)-2,2'-bithiophene (31), 5-{4-[4-(5-pent-1,3-diynylthiophene-2-yl)-but-3-yny}-2,2'-bithiophene (40), and 5-(4-hydroxybut-1-one)-2,2'-bithiophene (41) were tested against human malignant melanoma (A375-S2) and human cervical carcinoma (HeLa) cell lines. The four compounds displayed cytotoxic activity and the effect was more when the mixture of cell lines and compounds were exposed to ultraviolet A (UVA) light for 30 min. The effects of the four compounds were higher against HeLa cell line with IC50 values of 5.2, 10.2, 3.1, and 6.5 µmol/L, respectively (Wang et al., 2007).
Jin et al. (2008) illustrated the in vitro cytotoxic activity of the dichloromethane fraction of the crude ethanolic root extract of E. grijisi and thiophenes (1, 2, 9, 18, 23, 34, 39, and 42) isolated from this fraction. The fraction, as well as the isolated compounds showed different effects towards human hepatocarcinoma (HepG2 and MFC-7), human acute myeloid leukemia (HL-60), and human chronic myelogenous leukemia (K562) cell lines. The highest activities were observed for the dichloromethane fraction against HL-60 (IC50 = 5 µg/mL), 5-(4-isovaleroyloxybut-1-ynyl)-2,2'-bithiophene (18) against HepG2 (IC50 = 2 µg/mL), 5-(3-acetoxy-4-isovaleroyloxybut-1-ynyl)-2,2′-bithiophene (34) against HepG2 and K562 (IC50 = 1.8 and 7 µg/mL), and 5-(prop-1-ynyl)-2-(3,4-diacetoxybut-1-ynyl)-thiophene (42) against HL-60 (IC50 = 8 µg/mL). The dichloromethane fraction was tested in mice and did not show anti-tumor effect.
Similarly, Zhang et al. (2009) evaluated the cytotoxic effect of thiophenes isolated from E. grijisii on human cancer cell lines, HL60 and K562. Significantly potent effect was achieved with 5 (IC50 = 0.23 and 0.47 µg/mL) and 14 (IC50 = 0.27 and 0.43 µg/mL) against HL60 and K562, respectively. The thiophenes showed better activity against HL-60. A compound isolated from the root of E. grijsii, 5-(5,6-dihydroxy-hexa-1,3-diynyl)-2-(prop -1-ynyl)-thiophene (13), possessed anti-proliferative activity against human colon cancer cells, SW620, SW480, and HCT116 with IC50 values of 19.5 µM, 10.5 µM, and 27.7 µM, respectively, at 24 h. The proposed mechanism of action for the thiophene (13) was mitochondrial-mediated apoptosis (Zhang and Ma, 2010; Xu et al., 2015).
The methanolic extract from the underground part of E. giganteus also exhibited cytotoxic activity with an IC50 values of 9.84, 6.68, and 7.96 µg/mL against prostate cancer (Mia PaCa2) and two leukemia cells (CCRF-CEM and CEM/ADR5000), respectively (Kuete et al., 2011). In addition, the crude extract showed strong activity against breast cancer (MDA-MB-231-pcDNA3) with an IC50 value of 4.17 µg/mL. The secondary metabolites (5, 97, 126, and 131) from the methanolic extract of this plant were tested for their cytotoxic effect and showed lower effect than that of the crude extract (Kuete et al., 2013). In continuation of this study, 5-(3,4-dihydroxybut-1-ynyl)-2-(penta-1,3-diynyl)-thiophene (14), echinopsolide A (98), and tetrahydrofurano-ceramide (150) were isolated from E. giganteus. These three compounds tested against leukemia showed the highest activity on CCRF-CEM (IC50 values of 46.96, 36.78, and 9.83 µM, respectively) and CEM/ADR5000 (IC50 values of 21.09, 38.57, and 6.12 µM, respectively) cell lines (Sandjo et al., 2016).
Macrochaetosides A and B (80 and 81) and cyclostenol (104), isolated from aerial parts of E. macrochaetus Boiss., were tested for their cytotoxic activity. The activity was observed on cell lines of breast adenocarcinoma (MCF-7) (IC50 = 2.1 and 0.18 μM), human hepatocellular carcinoma (HepG2) (IC50 = 2.9 and 3.3 μM), and colorectal adenocarcinoma (HCT-116) (IC50 = 3.6 and 2.3 μM) for cyclostenol and macrochaetosides A, respectively. Macrochaetoside B only showed a cytotoxic activity against MCF-7 with an IC50 of 6.9 μM (Zamzami et al., 2019).
The vehicle used to dissolve the compounds for the cytotoxicity study is not mentioned in some of the reports (Sandjo et al., 2016; Zamzami et al., 2019). In one study, α-terthiophene (2) was used as a positive control against A375-S2 (IC50 = 10.6 µmol/L) and HeLa (IC50 = 6.3 µmol/L) cell lines (Wang et al., 2007). Similarly α-terthiophene showed cytotoxic effect towards K562 (IC50 = 50 µg/mL) and HepG2 (IC50 = 10µg/mL) (Jin et al., 2008).
The above-described effects on cancer cell lines could be mainly due to thiophenes. Terpenoids and ceramides were the other secondary metabolites having a cytotoxic effect. Among the cell lines tested, leukemia cell lines were comparatively more sensitive in which 5-(4-hydroxybut-1-ynyl)-2-(pent-1,3-diynyl)-thiophene (5) showed the most potent effect.
Even though the extracts and isolated compound from the genus showed promising effects against different cancer cell lines, the effects are ought to be further investigated using in vivo models.
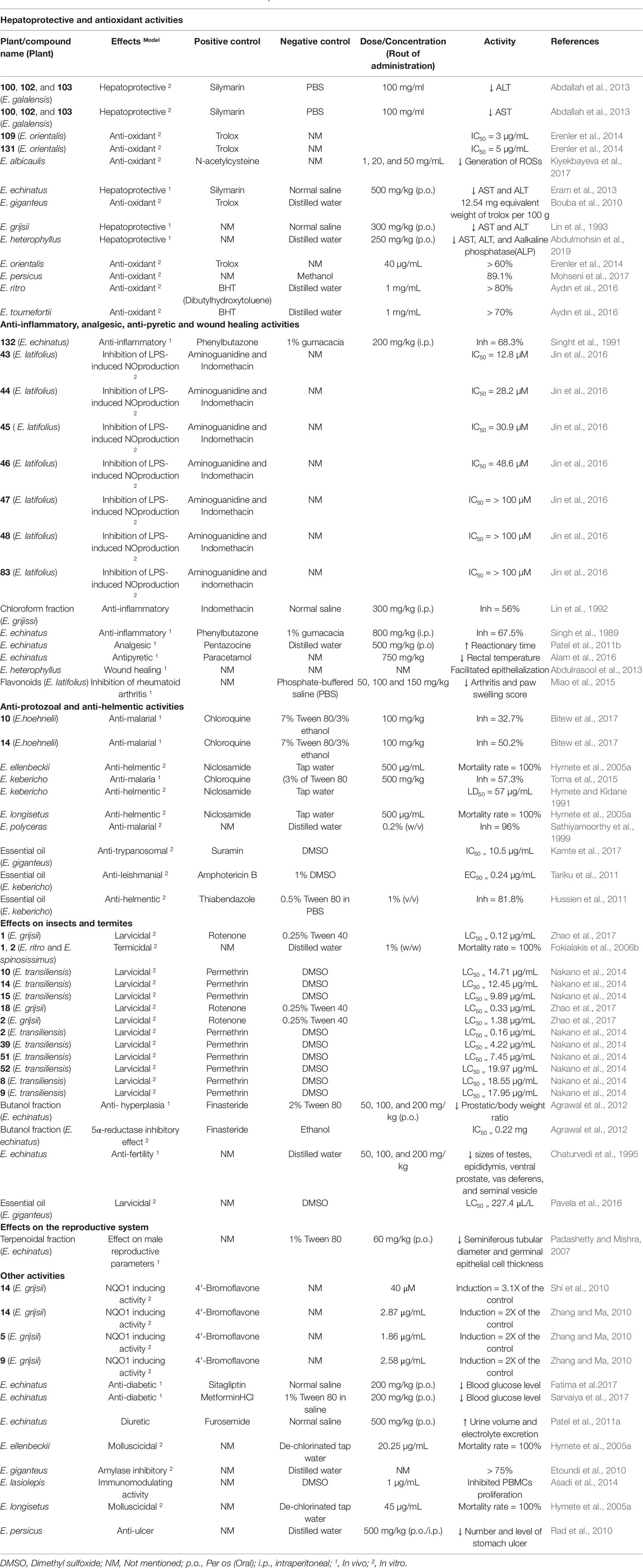
Hepato-Protective and Anti-Oxidant Activities
Members of the genus Echinops were also shown to have hepatoprotective and anti-oxidant activities. Most of the studies were conducted in carbon tetrachloride (CCl4)-induced liver damage, in which biomarkers of liver function like aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured.
The methanolic root extract, as well as n-butanol and aqueous fractions of E. grijsii, showed hepatoprotective activity in CCl4-induced liver damage in rats. The effect was prominent in the aqueous and butanol fractions, at a dose of 300 mg/kg, that markedly decreased AST and ALT levels (Lin et al., 1993). A study conducted by Eram et al. (2013) in CCl4-intoxicated rabbits justified the traditional claim of E. echinatus to treat jaundice (Gupta et al., 2010). The ethanolic aerial parts extract of E. echinatus at 500 and 750 mg/kg resulted in a significant decrease of ALT and AST, of which the lower dose (500 mg/kg) showed a higher effect (Eram et al., 2003). As presented in Table 1, flavonoids were isolated from the root of E. grijsii and the whole plant of E. echinatus. These might be responsible for the hepatoprotective effects of the extracts (Wang et al., 2015; Zang et al., 2017) and further investigations are required on phytoconstituents of the plants.
The hepatoprotective effect of compounds isolated from members of the genus Echinops was also investigated along with crude extracts. The protective effects of E. galalensis Schweinf. as well as isolated compounds β-sitosterol (89), apigenin-7-O-β-D-glucoside (109), 3β-acetoxy-taraxast-12,20(30)-diene-11α-21α–diol (100), α-amyrin (101), erythrodiol (102), lup-20(29)-ene-1,3-diol (103), and dicaffeoyl-quinic acid derivatives (135-138) on human hepatoma cell line (Huh7) have also been established. The highest protection was exhibited by 100, 102, and 103 and they significantly decreased the level of ALT. Except for the crude extract, all the tested samples decrease the level of AST and 89, 101, and 135 showed the highest effect (Abdallah et al., 2013). According to Abdallah et al. (2013), the protective effect of the extract and isolated compounds was suggested to be partly due to anti-oxidant effects of the samples.
Methotrexate-induced hepatotoxicity was also used to evaluate the hepatoprotective effect of some of the plants. Using this model, the protective effect of ethanolic aerial part extract and flavonoid fraction of E. heterophyllus P.H. Davis was established in rabbits. The crude ethanolic extract (250 mg/kg) significantly decreased the serum proteins, liver enzymes, and oxidative stress markers than the flavonoid fraction (Abdulmohsin et al., 2019).
In liver diseases, excessive oxidative stress undoubtedly contributes to the progression and pathological expression of the disease and serves as a prognostic indicator (Zhu et al., 2012). The methanolic root extract of E. giganteus showed in vitro free radical scavenging effect with 12.54 mg equivalent weight of trolox per 100 g (Bouba et al., 2010). The aqueous extracts of E. ritro, E. tournefortii Ledeb. possessed 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging effect with inhibitions more than 80% and 70%, respectively, at 1 mg/mL (Aydın et al., 2016). A study that compared different types of extraction methods on antioxidant activity reported that hot extraction using methanolic-ethyl acetate of E. persicus showed higher in vitro free radical scavenging effect (89.14%) against DPPH (Mohseni et al., 2017). The free radical scavenging effect of crude seed and leaf extracts E. orientalis Trautv. as well as isolated compounds β-sitosterol (89) and 1-methylquinolin-4(1H)-one (139) from seeds and apigenin-7-O-β-D-glucoside (109) and apigenin-7-O-(6"-trans-p-coumaroyl-β-D-glucopyranoside (124) from leaf methanolic extract was demonstrated. The extracts showed a significant effect (> 60% at 40 µg/mL) while the effect of the isolated compounds was not significant against 2,2-diphenyl-1-picrylhydrazyl (DPPH). However, the two flavonoids (109 and 124) showed better scavenging effect towards 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical cation than the extracts and the other two compounds (89 and 139), with IC50 of 3 and 5 µg/mL (Erenler et al., 2014).
Active cell cultures of human peripheral blood mononuclear cells were also used to evaluate the anti-oxidant effect of aqueous methanolic extract of E. albicaulis aerial parts. The study showed that the active oxygen species (ROS) generation in the cells was significantly reduced at concentrations of 1, 20, and 50 mg/mL of the extract; however, the extract induced overproduction ROSs at higher concentrations (Kiyekbayeva et al., 2017).
Regardless of the effects described, the anti-oxidant activity evaluations are not still sufficient. In most of the reports the IC50 value for the in-vitro anti-oxidant effect are not mentioned. No single in vivo anti-oxidant model was employed. In some of the hepatoprotective effect studies standard drugs were not utilized and comparison was made only with the negative control (Table 5). The hepatoprotective effect of traditionally used plant, E. spinosus L. (Akdime et al., 2015), has not been scientifically investigated yet.
Anti-Inflammatory, Analgesic, Anti-Pyretic, and Wound Healing Activities
Traditionally, members of the genus Echinops are documented to have been used to treat inflammation, pain, and fever. Accordingly, several species have been explored for anti-inflammatory, analgesic, and anti-pyretic activities.
The whole plant ethanolic extract of E. echinatus showed anti-inflammatory activity against carrageenan and formaldehyde induced edema in rats with inhibitions of 67.5% and 51.8% at a dose of 800 mg/kg administered intraperitoneally and orally, respectively (Singh et al., 1989). A triterpenoid isolated from this plant, taraxasterol acetate (88), showed anti-inflammatory activity on carrageenan-induced pedal edema in rats with the highest inhibition of 68.3% and 63.2% at 200 mg/kg administered by the intraperitoneal and oral route, respectively (Sing et al., 1991). Flavanone glycoside, 5,7-dihydroxy-8,4'-dimethoxyflavanone-5-O-α-L-rhamno-pyranosyl-7-O-β-D-arabinopyranosyl (1→4)-O-β-D-glucopyranoside (125) isolated from E. echinatus, showed anti-inflammatory activity (Yadava and Singh, 2006). The methanolic root and aerial part extract of the plant showed analgesic properties in both hotplate and tail immersion models. The aerial part exhibited the highest activity by increasing the reaction time in both models to 7.99 and 7.77 sec, respectively, at 500 mg/kg, and it was comparable with the standard drug, pentazocine (Patel et al., 2011b). The ethanolic leaf and stem extract of E. echinatus showed antipyretic effect at a dose of 750 mg/kg in rabbits (Alam et al., 2016).
The methanolic root extracts of E. spinosus, E. grijissi, and E. latifolius exhibited significant anti-inflammatory activity (Lin et al., 1992; Rimbau et al., 1999). The ethyl acetate, chloroform, and n-hexane fractions obtained from the crude extract of E. grijissi showed significant anti-inflammatory activities in carrageenan-induced edema in rats, of which the chloroform fraction, at a dose of 300 mg/kg, exhibited inhibitory effect (56.7%) higher than that of indomethacin (Lin et al., 1992). Flavonoids, extracted from E. latifolius, were tested on rheumatoid arthritis using rats and inhibited the synovium proliferation through fibroblast-like synoviocytes apoptosis at 150 mg/kg (Miao et al., 2015).
A study was conducted to evaluate the anti-inflammatory activity of compounds isolated from E. latifolius, 5-(1,2-dihydroxy-ethyl)-2-(Z)-hept-5-ene-1,3-diynylthiophene (43), 5-(1,2-dihydroxyethyl)-2-(E)-hept-5-ene-1,3-diynylthiophene (44), 6-methoxy-arctinol-b (45), arctinol-b (46), latifolanone A (82), arctinol (47), methyl [5'-(1-propynyf)-2,2'-bithienyl-5-yl] carboxylate (48), and atractylenolide-II (83) on inhibition of lipopolysaccharide (LPS)-induced nitric oxide (NO) production. In the order of presented compound names, thiophenic compounds numbered 43-46 inhibited the NO production with IC50 ranging from 12.8–42.7 µM, whereas the IC50 of 47, 48, and 83 were reported to be more than 100 µM (Jin et al., 2016).
The whole plant extract of E. heterophyllus and the alkaloidal faction facilitated epithelialization and left no scars in rabbits (Abdulrasool et al., 2013). This is the only wound healing activity reported on members of this genus although the dose, vehicle, and the standard drug are not described.
The in vivo anti-inflammatory effects of the genus seemed to be not promising since the plants resulted in an inhibition of edema at higher doses. In spite of the studies stated above, scientific data justifying the traditional claim of E. bovei (Boiss.) Maire., E. cornigerus, E. kebericho, E. longifolius A. Rich., E. macrochaetus, and E. spinosissimus to treat rheumatism and pain are not provided yet.
Anti-Protozoal and Anti-Helmentic Activities
As presented in Table 3, E. hoehnelii Schweinf. and E. kebericho have been used in traditional treatment of malaria. These plants along with other species showed anti-malarial activity.
Aqueous extract of the aerial parts of E. polyceras exhibited strong (96%) in vitro growth inhibitory activity against Plasmodium falciparum. Nevertheless, the concentration of the extract used for the test and the standard drug used as positive control has not been reported (Sathiyamoorthy et al., 1999). A study on 70% ethanolic root extract E. kebericho resulted in an inhibition of parasitemia by 57.3% at a dose of 500 mg/kg in mice against Plasmodium berghei (Toma et al., 2015). A recent study conducted on the 70% methanolic extract from roots of E. kebericho exhibited 49.5% of inhibition at 1000 mg/kg in mice (Biruksew et al., 2018). This might suggest that the potency of E. kebericho extract could be dependent on the extraction solvent.
Dichloromethane faction of the 80% methanolic extract of E. hoehnelii, and thiophens (5-(penta-1,3-diynyl)-2-(3-chloro-4-acetoxy-but-1-ynyl)-thiophene (10), and 5-(penta-1,3-diynyl)-2-(3,4-dihydroxybut-1-ynyl)-thiophene (14)) possessed anti-malarial activity. The two compounds showed parasitemia inhibition of 32.7% and 50.2% at a dose of 100 mg/kg, respectively, against P. berghei in mice (Bitew et al., 2017).
Different studies showed that essential oils possess strong anti-protozoal effects. The essential oil isolated from E. kebericho displayed a strong activity against two Leishmania strains (L. aethiopica and L. donovani) with an EC50 values of 0.24 and 0.5 µg/mL (Tariku et al., 2011). Essential oil obtained from E. giganteus had anti-protozoal effect against Trypanosoma brucei with an IC50 of 10.5 µg/mL and GC-MS analysis of the oil revealed the presence of modheph-2-ene, presilphiperfolan-8-ol, presilphiperfol-7-ene, cameroonan-7-α-ol, and (E)-caryophyllene as the main constituents of the oil (Kamte et al., 2017).
The anti-helminthic effects of members of the genus were also described. The root 80% methanolic extract of E. kebericho showed higher anti-helmentic effect (LD50 = 57µg/mL) than niclosamide (LD50 = 84.5 µg/mL) against earthworms (Hymete and Kidane, 1991). The root 80% methanolic extracts of E. ellenbeckii as well as E. longisetus A. Rich. were active against earthworms with 100% mortality at 500 µg/mL (Hymete et al., 2005a). Essential oil from the root of E. kebericho showed lethal effect (81.8%) at a concentration of 1% (v/v) towards Haemonchus contortus (Hussien et al., 2011).
Effects on Insects and Termites
The leaves of Echinops spp, which are commonly known as “Kebericho” in Ethiopia, had a mosquito repellant effect against Anopheles arabiensis with the effectiveness of 92.47% as a smoke (Karunamoorthi et al., 2008).
The activity of thiophenes (2, 8, 9, 10, 14, 15, 39, 51, and 52) isolated from E. transiliensis Golosk. against Aedes aegypti was reported and the toxic effect increased with the number of thiophene moieties in the molecule. Strong activity was observed for 2''-terthiophene (2) with an LC50 value of 0.16 µg/mL (Nakano et al., 2014). Similarly, the root extract of E. grijsii possessed significant larvicidal activity against Aedes albopictus, Anopheles sinensis, and Culex pipienspallens with LC50 values of 2.65, 3.43, and 1.47 µg/mL, respectively.
Bioactivity-directed chromatographic separation of the essential oil obtained from E. grijsii led to the isolation of thiophenes. The larvicidal effects of the isolated compounds, 5-(3-buten-1-yn-1-yl)-2,2′-bithiophene (1) (LC50 0.34, 1.36, and 0.12 µg/mL), α-terthienyl (2) (LC50 1.41, 1.79, and 1.38 µg/mL), and 5-(4-isovaleroyloxybut-1-ynyl)-2,2'-bithiophene (18) (LC50 0.45, 5.36, and 0.33 µg/mL) against the three organisms mentioned above was described (Zhao et al., 2017). On the contrary, the larvicidal activity of essential oils from E. giganteus against Culex quinquefasciatus was relatively low (LC50 = 227.4 μL/L) (Pavela et al., 2016).
Fokialakis et al. (2006b) evaluated the termicidal effect of eight thiophenes (1, 2, 5, 10, 18, 23, 31, and 39) isolated from E. ritro, E. spinosissimus, E. albicaulis, and E. transiliensis on Coptotermes formosanus. The study revealed that all the thiophenes showed termicidal activity and 100% morality was observed after application of 5-(3-buten-1-ynyl)-2,2-bithiophene (1) and 2''-terthiophene (2) for 9 days at 2% and 1% (w/w), respectively. However, the exact concentrations of the compounds were not mentioned.
Effects on the Reproductive System
A number of species have been used for the management of various reproductive health problems (Table 1). In spite of the traditional claims, only E. echinatus has been evaluated for these biological activities.
Corresponding to its traditional use, the terpenoidal fraction from E. echinatus displayed anti-fertility properties at doses of 30 and 60 mg/kg in male rats (Padashetty and Mishra, 2007). Earlier studies also indicated that the root ethanolic extract of E. echinatus has anti-fertility properties through decrement in sizes of testes, epididymis, ventral prostate, vas deferens, and seminal vesicle at doses of 50, 100, and 200 mg/kg. In addition, the extract also decreased sperm motility and density with an inhibition of sepermatogenesis in rats (Chaturvedi et al., 1995). The butanol fraction of the root extract demonstrated a protective effect on testosterone-induced prostatic hyperplasia at a dose of 100 mg/kg in rats. The butanol fraction also showed better 5α-reductase inhibitory effect (IC50 = 0.22 mg/mL) than of the crude extract and other fractions followed by the water soluble fraction (IC50 = 0.43 mg/mL) (Agrawal et al., 2012). Similarly, the root petroleum ether extract of E. echinatus inhibited 5α-reductase. The enzyme plays an important role in the pathogenesis of benign prostatic hyperplasia (BPH), prostatic cancer, acne, alopecia, baldness in men, and hirsutism in women (Nahata and Dixit, 2014).
Other Activities
A study showed that 5-(penta-1,3-diynyl)-2-(3,4-dihydroxybut-1-ynyl)-thiophene (14), isolated from the root of E. grijsii, has an induction effect on nicotinamide adenine dinucleotide phosphate (NAD(P)H): quinone oxidoreductase1 (NQO1), an enzyme that is involved in detoxification of toxic quinones. The induction effect was dose-dependent and the maximum effect was observed at a concentration of 40 μM and it was 3.1 folds of the control, 4'-bromoflavone (Shi et al., 2010). Similarly, compounds 5, 9, and 14, from the root of E. grijisii, had a strong NQO1-inducing effect and the concentrations that caused a twofold induction were 1.86, 2.58, and 2.87 μg/mL, respectively. Compounds 5 and 14 were found to have an alkylating effect on cysteine residues in NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) (Zhang and Ma, 2010).
The 70% hydro-alcoholic root extract of E. echinatus was reported to have significant anti-diabetic activity on alloxan-induced diabetic rats. The the extract treated animals (200 mg/kg) showed lower blood glucose level (164 mg/dL) compared to the negative control (277.6 mg/dL) after 21 days of treatment. In addition, the extract exhibited the ability to regenerate pancreatic islet cells and normal structure of glomeruli and proximal and distal convoluted tubules in kidneys (Fatima et al., 2017). Similarly, the methanolic root extract of E. echinatus exhibited a significant anti-diabetic effect at doses of 100 and 200 mg/kg on alloxan induced diabetic rats. The extract was also able to decrease serum cholesterol, serum triglyceride, serum low-density lipoprotein, serum very low-density lipoprotein, and serum alkaline phosphate significantly while it increased high-density lipoproteins (Sarvaiya et al., 2017).
The molluscicidal activities of 80% methanolic root extracts of E. ellenbeckii and E. longisetus with a 100% mortality rate at 20.25 and 45 µg/mL, respectively, was described (Hymete et al., 2005a). The pancreatic amylase inhibitory activity (> 75%) of aqueous root extract of E. giganteus was reported although the exact concentration of the extract was not mentioned (Etoundi et al., 2010). The latex of E. persicus at 500 mg/kg resulted in lower number and level of stomach ulcer compared to the negative control in rats (Rad et al., 2010). The methanolic extract of root and aerial parts of E. echinatus significantly increased urine volume and excretion at doses of 250 and 500 mg/kg (Patel et al., 2011a). The immunomodulating activity of aerial parts methanolic extract of E. lasiolepis Bunge has been reported. The extract at different concentrations (0.1, 1, 10, 100, and 200 µg/mL) inhibited peripheral blood mononuclear cells (PBMCs) proliferation of which 1 µg/mL showed optimum proliferation (30.66%) (Asadi et al., 2014).
Biological effects evaluated on genus Echinops and the doses with maximum effect are summarized in Tables 3–5.
Conclusion
The genus Echinops is well known for its use to treat pain and respiratory manifestations. The traditional claims were justified by different biological evaluations. Findings from in vitro studies indicated that members of the genus have a potential effect against different cancer lines, microbial strains, and insects. They also showed significant in vivo anti-inflammatory, analgesic, and hepatoprotective activities. Some of the extracts and isolated compounds showed promising effects. This includes the anticancer activity of compounds 5 and 14, antioxidant potential of 109, anti-leishmanial and anti-helmentic effects of E. kebericho, and the larvicidal effect of compound 1. The safety and efficacy of secondary metabolites responsible for the in vitro effects of extracts/fractions should further be investigated in in vivo models. The most abundant bioactive secondary metabolites in members of the genus are thiophenes and terpenoids which are also mentioned as responsible for the cytotoxic effect observed. In the current review, it has been observed that the potential uses of the species in the removal of kidney stones and use to solve nerve-related problems have not been scientifically addressed yet. Investigation of the anti-microbial activity of isolated compounds seems to be limited. We believe this review will provide summarized information to the scientific community working on the genus.
Author Contributions
HB developed concept of the review, conducted the literature review, extracted relevant information to the study, and drafted the manuscript. AH guided the literature search and edited the manuscript. Both authors have read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdallah, H. M., Ezzat, S. M., Dine, R. S., Abdel-Sattar, E., Abdel-Naim, A. B. (2013). Protective effect of echinops galalensis against CCl4-induced injury on the human hepatoma cell line (Huh7). Phytochem. Lett. 6, 73–78. doi: 10.1016/j.phytol.2012.10.01
Abd-Ellatif, S., Abdel Rahman, S. M., Deraz, S. F. (2011). Promising antifungal effect of some folkloric medicinal plants collected from El-Hammam habitat, Egypt against dangerous pathogenic and toxinogenic fungi. J. Agric. Biol. Sci. 6, 25–32.
Abderrahim, O., Martin, G. J., Abdelaziz, A. (2013). Botanical identification and ethno-medicinal uses of some underground part of medicinal plants collected and traded in Marrakech region. J. Med. Plants. Res. 7, 2165–2169. doi: 10.5897/JMPR11.1597
Abdulrasool, A. A., Fahmi, Z. M., Khadeem, E. J. (2013). Relative assess on wound healing and anti scar activity of crude Echinops heterophyllus extract and some of its bioactive fractions. Int. J. Pharm. Pharm. Sci. l5, 468–475.
Abdou Bouba, A., Njintang Yanou, N., Foyet, H., Scher, J., Montet, D., Mbofung, C. M. (2012). Proximate composition, mineral and vitamin content of some wild plants used as spices in Cameroon. Food Nutr. Sci. 3, 423–432. doi: 10.4236/fns.2012.34061
Abdulmohsin, H., Raghif, A. A., Manna, M. J. (2019). The protective effects of echinops heterophyllus extract against methotrexate-induced hepatotoxicity in rabbits. Asian. J. Pharm. Clin. Res. 12, 384–390. doi: 10.22159/ajpcr.2019.v12i1.30213
Abegaz, B. M. (1991). Polyacetylenic thiophenes and terpenoids from the roots of echinops pappii. Phytochemistry 30, 879–881. doi: 10.1016/0031-9422(91)85271-Z
Abegaz, B. M., Tadesse, M., Majinda, R. (1991). Distribution of sesquiterpene lactones and polyacetylenic thiophenes in Echinops. Biochem. Syst. Ecol. 19, 323–328. doi: 10.1016/0305-1978(91)90021-Q
Abera, B. (2014). Medicinal plants used in traditional medicine by oromo people, ghimbi district, southwest ethiopia. J. Ethnobiol. Ethnomed. 10, 40. doi: 10.1186/1746-4269-10-40
Abouri, M., Mousadik, A. E., Msanda, F., Boubaker, H., Saadi, B., Cherifi, K. (2012). An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int. J. Med. Plant. Res. 1, 99–123.
Aburjai, T., Darwish, R. M., Al-khalil, S., Mahafzah, A., Al-abbadi, A. (2001). Screening of antibiotic resistant inhibitors from local plant materials against two different strains of Pseudomonas aeruginosa. J. Ethnopharmacol. 76, 39–44.
Afshaki, S., Jafari, A., Javidnia, K., Firuzi, O. (2012). Antioxidant and cytotoxic activities of four plant extracts from dena region of Iran. Res. Pharm. Sci. 7, S853.
Agrawal, M., Nahata, A., Dixit, V. K. (2012). Protective effects of echinops echinatus on testosterone-induced prostatic hyperplasia in rats. Eur. J. Integr. Med. 4, 177–185. doi: 10.1016/j.eujim.2012.01.004
Ahmad, M., Ghafoor, N., Aamir, M. N. (2012). Antibacterial activity of mother tinctures of cholistan desert plants in Pakistan. Indian. J. Pharm. Sci. 74, 465–468. doi: 10.4103/0250-474X.108429
Akdime, H., Boukhira, S., Mansouri, L. E. L., El, A. H., Bousta, D. (2015). Ethnobotanical study and traditional knowledge of medicinal plants in ain leuh region (Middle-Atlas of Morocco). Am. J. Adv. Drug. Deliv. 3, 248–263.
Alam, M. K., Ahmed, S., Anjum, S., Akram, M., Shah, S. M., Wariss, H. M., et al. (2016). Evaluation of antipyretic activity of some medicinal plants from Cholistan desert Pakistan. Pak. J. Pharm. Sci. 29, 529–533.
Ameya, G., Gure, A., Dessalegn, E. (2016). Antimicrobial activity of echinops kebericho against human pathogenic bacteria and fungi. Afr. J. Tradit. Complement. Altern. Med. 13, 199–203.
Amsalu, N., Bezie, Y., Fentahun, M., Alemayehu, A., Amsalu, G. (2018). Use and conservation of medicinal plants by indigenous people of gozamin wereda, east gojjam zone of amhara region, ethiopia: an ethnobotanical approach. evidence-based. complement. altern. Med. 2018, 1–23. doi: 10.1155/2018/2973513
Arroo, R. R., Jacobs, J. J., Van Gestel, J. A., Kenkel, H., Jannink, W., Croes, A. F., et al.. (1997). Regulation of thiophene biosynthesis by sulphate in roots of marigolds. New. Phytol. 135, 175–181.
Asadbeigi, M., Mohammadi, T., Rafieian-Kopaei, M., Saki, K., Bahmani, M., Delfan, M. (2014). Traditional effects of medicinal plants in the treatment of respiratory diseases and disorders: an ethnobotanical study in the Urmia. Asian. Pac. J. Trop. Med. 7, S364–S368.
Asadi, M., Hadinedoushan, H., Mirghanizadeh, S. A., Karimollah, A., Dashti, F., Malek-hosseini, S. (2014). The effect of echinops lasiolepis extracts, native plant of yazd province, on peripheral blood mononuclear cellproliferation and IFN-γ Secretion. Int. J. Med. Lab. 1, 7—14.
Ashebir, M., Ashenafi, M. (1999). Evaluation of the anibactrtial activity of crude preparation of zingiber officinale (zinjibl) echinops spp. (kebericho), coriandrum sativum (dimbilal), and cymbopogan citraus (tej sar) on some food-borne pathogens. Ethiop. J. Hlth. Sci. 9, 33–39.
Aydın, Ç., Özcan, G. T., Turan, M., Mammadov, R. (2016). Phenolic contents and antioxidant properties of echinops ritro L. and E. tournefortii Jaup. Et. Spach extract. Int. J. Sec. Metabolite. 3, 74–81.
Baydoun, S., Chalak, L., Dalleh, H., Arnold, N. (2015). Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of mount hermon, Lebanon. J. Ethnopharmacol. 173, 139–156. doi: 10.1016/j.jep.2015.06.052
Belay, G., Tariku, Y., Kebede, T., Hymete, A., Mekonnen, Y. (2011). Ethnopharmacological investigations of essential oils isolated from five ethiopian medicinal plants against eleven pathogenic bacterial strains. Phytopharmacology 1, 133–143.
Belayneh, A., Bussa, N. F. (2014). Ethnomedicinal plants used to treat human ailments in the prehistoric place of harla and dengego valleys, eastern ethiopia. J. Ethnobiol. Ethnomed. 1, 18. doi: 10.1186/1746-4269-10-18
Biruksew, A., Zeynudin, A., Alemu, Y., Golassa, L., Yohannes, M., Debella, A., et al. (2018). Zingiber Officinale Roscoe and Echinops Kebericho Mesfin showed antiplasmodial activities against Plasmodium berghei in a dose-dependent manner in Ethiopia. Ethiop. J. Health. Sci. 28, 655. doi: 10.4314/ejhs.v28i5.17
Bitew, H., Mammo, W., Hymete, A., Yeshak, M. Y. (2017). Antimalarial activity of acetylenic thiophenes from echinops hoehnelii schweinf. Molecules 22, 1965. doi: 10.3390/molecules22111965
Bizuayehu, B., Garedew, B. (2018). A review on the ethnobotanical study of medicinal plants used for the treatment of gonorrhea disease in Ethiopia. Indian. J. Nat. Prod. Resour. 9, 183–193.
Bouattour, E., Fakhfakh, J., Dammak, F., Jabou, K., Damak, M. (2016). Hexane extract of echinops spinosissimus turra subsp. spinosus from tunisia: a potential source of acetylated sterols – investigation of its Biological Activities. Chem. Biodivers. 13, 1674–1684. doi: 10.1002/cbdv.2016001182016;1674–84
Bouba, A., Njintang, Y. N., Scher, J., Mbofung, C. M. F. (2010). Phenolic compounds and radical scavenging potential of twenty cameroonian spices. Agric. Biol. J. N. Am. 1, 213–224.
Boumaraf, M., Benyahia, S., Mekkiou, R., Benayache, S., Benayache, F. (2016). Flavonoids from ethyl acetate extract of echinops spinosus (Asteraceae). Der Pharma. Chemica. 8, 158–160.
Bouzabata, A., Mahomoodally, F., Tuberoso, C. (2018). Ethnopharmacognosy of Echinops spinosus L. in North Africa: a mini review. J. Complement. Med. Res. 8, 40–52. doi: 10.5455/jcmr.20180318051853
Bulut, G., Haznedaroğlu, M. Z., Doğan, A., Koyu, H., Tuzlacı, E. (2017). An ethnobotanical study of medicinal plants in acipayam (Denizli-Turkey). J. Herb. Med. 10, 64–81. doi: 10.1016/j.hermed.2017.08.001
Chadwick, M., Trewin, H., Gawthrop, F., Wagstaff, C. (2013). Sesquiterpenoids lactones: benefits to plants and people. Int. J. Mol. Sci. 14, 12780–12805. doi: 10.3390/ijms140612780
Chang, F. P., Chen, C. C., Huang, H. C., Wang, S. Y., Chen, J. J., Yang, C. S., et al. (2015). A new bithiophene from the root of echinops grijsii. Nat. Prod. Commun. 12, 2147–2149.
Chaturvedi, M., Mali, P. C., Dexit, V. P. (1995). Antifertility effects of the roots of echinops echinatus (Roxb.) in male rats. J. Phytol. Res. 8, 115–118.
Chaudhuri, P. K. (1987). Echinozolinone, an alkaloid from echinops echinatus. Phytochemistry 26, 587–589.
Chaudhuri, P. K. (1992). 7-hydroxyechinozolinone, a new alkaloid from the flowers of Echinops echinatus. J. Nat. Prod. 55, 249–250. doi: 10.1021/np50080a019
Chermat, S., Gharzouli, R. (2015). Ethnobotanical study of medicinal flora in the north east of algeria - an empirical knowledge in djebel zdimm (Setif). J. Mater. Sci. Eng. A 5, 50–59.
Dangwal, L. R., Rana, C. S., Sharma, A. (2011). Ethno-medicinal plants from transitional zone of nanda devi biosphere reserve, district chamoli, uttarakhand (India). Indian. J. Nat. Prod. Resour. 2, 116–120.
Desta, B. (1995). Ethiopian traditional herbal drugs. Part I: studies on the toxicity and therapeutic activity of local taenicidal medications. J. Ethnopharmacol. 45, 27–33.
Dong, L. I., Ning, L. I., Wan, X. I., Peng, Z. H., Zhong-jun, M. A., Xian, L. I. (2008a). Chemical constituents of the root of echinops grijisii Hance. Shenyang. Yao. Ke. Da. Xue. Xue. Bao. 8, 007.
Dong, M., Cong, B., Yu, S. H., Sauriol, F., Huo, C. H., Shi, Q. W., et al. (2008b). Echinopines A and B: sesquiterpenoids possessing an unprecedented skeleton from Echinops spinosus. Org. Lett. 10, 701–704. doi: 10.1021/ol702629z
El Abbouyi, P. A., Ansari, N. F., Khyari, P. S. E., Loukili, H. (2014). Inventory of medicinal plants prescribed by traditional healers in El Jadida city and suburbs (Morocco). Int. J. Green Pharm. 8, 242–251.
El-Ghazali, G. E., Al-Khalifa, K. S., Saleem, G. A., Abdallah, E. M. (2010). Traditional medicinal plants indigenous to Al-Rass province, Saudi Arabia. J. Med. Plants. Res. 4, 2680–2683. doi: 10.5897/JMPR09.556
Eram, S., Ahmad, M., Arshad, S. (2013). Experimental evaluation of echinops echinatus as an effective hepatoprotective. Sci. Res. Essays. 8, 1919–1923. doi: 10.5897/SRE2012.0766
Erenler, R., Yilmaz, S., Aksit, H., Sen, O., Genc, N., Elmastas, M., et al. (2014). antioxidant activities of chemical constituents isolated from echinops orientalis Trauv. Rec. Nat. Prod. 8, 32–36.
Etoundi, C. B., Kuaté, D., Ngondi, J. L., Oben, J. (2010). Anti-amylase, anti-lipase and antioxidant effects of aqueous extracts of some cameroonian spices. J. Nat. Prod. 3, 165–171.
Fankam, A. G., Kuete, V., Voukeng, I. K., Kuiate, J. R., Pages, J. (2011). Antibacterial activities of selected cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complement. Altern. Med. 11, 104. doi: 10.1186/1472-6882-11-104
Farouji, A. E., Khodayari, H. (2016). Ethnomedicinal plants of Farouj district, north khorasan province, Iran. J. Herbal. Drugs. 7, 21–36.
Fatima, S., Afroz, S., Qureshi, A. S. (2017). Anti-diabetic activity of hydro-alcoholic root extract of echinops echinatus and its beneficial effects on nephropathy in experimental rats. Indian. J. Res. Pharm. Biotechnol. 5, 19–27.
Fenetahun, Y., Eshetu, G. (2017). A review on ethnobotanical studies of medicinal plants use by agro-pastoral communities in, Ethiopia. J. Med. Plants. Stud. 5, 33–44.
Fokialakis, N., Cantrell, C., Duke, S. O., Skaltsounis, A. L., Wedge, D. E. (2006a). Antifungal activity of thiophenes from echinops ritro. J. Agric. Food Chem. 54, 1651–1655.
Fokialakis, N., Osbrink, W. L. A., Mamonov, L. K., Gemejieva, N. G., Mims, A. B., Skaltsounis, A. L., et al. (2006b). Antifeedant and toxicity effects of thiophenes from four echinops species against the formosan subterranean termite, coptotermes formosanus. Pest. Manag. Sci. 62, 832–838. doi: 10.1002/ps.1237
Funk, V. A., Bayer, R. J., Keeley, S., Chan, R., Watson, L., Gemeinholzer, B., et al.., (2005). Everywhere but antarctica: using a super tree to understand the diversity and distribution of the compositae. Biol. Skr. 55, 343–374.
Gabriel, T., Guji, T. (2014). Ethnopharmacological survey of medicinal plants in Agaro district, Jimma zone, South West Ethiopia. Int. J. Pharm. Sci. Res. 5, 3551. doi: 10.13040/IJPSR.0975-8232.5(8).3551-59
Gari, A., Yarlagadda, R., Wolde-Mariam, M. (2015). Knowledge, attitude, practice, and management of traditional medicine among people of Burka Jato Kebele, West Ethiopia. J. Pharm. Bioall. Sci. 7, 136–144. doi: 10.4103/0975-7406.148782
Garnatje, T., Susanna, A., Garcia-Jacas, N., Vilatersana, R., Vallès, J. (2005). A first approach to the molecular phylogeny of the genus Echinops (Asteraceae): Sectional delimitation and relationships with the genus Acantholepis. Folia. Geobot. 40, 407–419.
Ghasemi Pirbalouti, A., Momeni, M., Bahmani, M. (2013). Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan districts, Ilam province, Iran. Afr. J. Tradit. Complement. Altern. Med. 10, 368–385. doi: 10.4314/ajtcam.v10i2.24
Giday, M., Asfaw, Z., Woldu, Z. (2010). Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J. Ethnopharmacol. 132, 75–85. doi: 10.1016/j.jep.2010.07.046
Guo, D. A., Lou, Z. C., Liu, Z. A. (1994). Chemical components of volatile oil from Echinops grijisii Hance. Zhongguo. Zhong. Yao. Za. Zhi. 19, 100–101.
Gupta, R., Vairale, M. G., Deshmukh, R. R., Chaudhary, P. R., Wate, S. R. (2010). Ethnomedicinal uses of some plants used by Gond tribe of Bhandara district, Maharashtra. Indian J. Tradit. Knowl. 9, 713–717.
Hamayun, M., Khan, M. A., Chudhary, M. F., Ahmad, H. (2006). Studies on traditional knowledge of medicinal herbs of swat kohistan, District Swat, Pakistan. J. Herbs. Spices. Med. Plants. 12, 11–28. doi: 10.1300/J044v12n04_02
Hammiche, V., Maiza, K. (2006). Traditional medicine in central sahara: pharmacopoeia of tassili n'ajjer. J. Ethnopharmacol. 105, 358–367. doi: 10.1016/j.jep.2005.11.028
He, L., Chao, Q., Li, R., Lin, G., Huang, H. (2000). A new pentacyclic triterpene, gmeliniin A, from Echinops gmelinii Turcz. Chin. J. Chem. 18, 112–114.
Hedberg, I., Friis, I., Edwards, S. (2004). “Vol. 4, part 2',” in Flora of Ethiopia and Eritrea (Addis Ababa: Addis Ababa University), 15–23.
Heshmati, S., Madani, M., Amjad, L. (2016). Study of inhibitory effect of echinops cephalotes on candida spp isolated from vulvovaginal candidiasis Patients in Isfahan. J. Res. Med. Sci. 18, e7355. doi: 10.17795/zjrms-7355
Hussien, J., Urgessa, K., Regassa, F., Jemal, A., Abajebel, S., Hussien, N. (2011). Antihelmentic effects of the essential oil extracts of selected plants against Haemonchus contortus. Int. J. Agric. Res. 6, 290–298.
Hymete, A., Kidane, A. (1991). Screening for anthelmintic activity in two Echinops spp. Ethiop. Pharm. J. 9, 67–71.
Hymete, A., Iversen, T. H., Rohloff, J., Erko, B. (2005a). Screening of echinops ellenbeckii and echinops longisetus for biological activities and chemical constituents. Phytomedicine 12, 675–679. doi: 10.1016/j.phymed.2004.01.013
Hymete, A., Rohloff, J., Iversen, T. H. (2004). Chemical constituents of volatile fractions from echinops ellenbeckii O. Hoffm. J. Essent. Oil. Bear Pl. 7, 9–15. doi: 10.1080/0972-060X.2004.10643359
Hymete, A., Rohloff, J., Kjosen, H., Iversen, T. H. (2005b). Acetylenic thiophenes from the roots of echinops ellenbeckii from Ethiopia. Nat. Prod. Res. 19, 755–761. doi: 10.1080/1478641012000301711
Issa, T. O., Mohamed, Y. S., Yagi, S., Ahmed, R. H., Najeeb, T. M., Makhawi, A. M., et al. (2018). Ethnobotanical investigation on medicinal plants in Algoz area (South Kordofan), Sudan. J. Ethnobiol. Ethnomed. 14, 1–22. doi: 10.1186/s13002-018-0230-y
Jabeen, N., Ajaib, M., Siddiqui, M. F., Ulfat, M., Khan, B. (2015). A survey of ethnobotanically important plants of District Ghizer, Gilgit-Baltistan. FUUAST. J. Biol. 5, 153–160.
Jiang, B., Wang, F., Liu, L., Tian, S., Li, W., Yang, X., et al. (2017). Antibacterial activity and action mechanism of the echinops ritro L. essential oil against foodborne pathogenic bacteria. J. Essent. Oil Bear. Pl. 20, 1172–1183. doi: 10.1080/0972060X.2017.1399090
Jin, Q., Lee, J. W., Jang, H., Choi, J. E., Kim, H. S., Lee, D. (2016). Dimeric sesquiterpene and thiophenes from the roots of Echinops latifolius. Bioorg. Med. Chem. Lett. 26, 5995–5998. doi: 10.1016/j.bmcl.2016.10.017
Jin, W., Sh, i Q., Hong, C., Cheng, Y., Ma, Z., Qu, H. (2008). Cytotoxic properties of thiophenes from echinops grijissi Hance. Phytomedicine 15, 768–774. doi: 10.1016/j.phymed.2007.10.007
Ka, S. (2001). A pseudoguaiane sesquiterpene xylopyranoside from Echinops hussoni. Pharmazie 56, 415–417.
Kamatenesi, M. M., Acipa, A., Oryem-Origa, H. (2011). Net medicinal plants of otwal and ngai sub counties in oyam district, northern uganda. J. Ethnobiol. Ethnomed. 7, 1–14. doi: 10.1186/1746-4269-7-7
Kamte, S. L. N., Ranjbarian, F., Campagnaro, G. D., Nya, P. C. B., Woguem, V., Womeni, H. M., et al. (2017). Trypanosoma brucei Iinhibition by essential oils from medicinal and aromatic plants traditionally used in cameroon (Azadirachta indica, Aframomum melegueta, Aframomum daniellii, Clausena anisata, Dichrostachys cinerea and Echinops giganteus). Int. J. Environ. Res. Public. Health. 14, 737. doi: 10.3390/ijerph14070737
Karimov, U. T., Aisa, H. A. (2012). Phytochemical study of the plant Echinops integrifolius growing in the Altai (XUAR PRC). Chem.Nat. Compd. 48, 903–905
Karimov, U. T., Aisa, H. A. (2013). Hydrocarbons and fatty acids from echinops integrifolius. Chem. Nat. Compd 49, 920–921. doi: 10.1007/s10600-013-0778-7
Karunamoorthi, K., Mulelam, A., Wassie, F. (2008). Laboratory evaluation of traditional insect/mosquito repellent plants against anopheles arabiensis, the predominant malaria vector in ethiopia. Parasitol. Res. 103, 529–534. doi: 10.1007/s00436-008-1001-9
Katiri, A., Barkaoui, M., Msanda, F., Boubaker, H. (2017). Ethnobotanical survey of medicinal plants used for the treatment of diabetes in the tizi n' test region (Taroudant Province, Morocco). J. Pharmacogn. Nat. Prod. 03, 1–10. doi: 10.4172/2472-0992.1000130
Kevin, K., John, K., Carolyn, N., Derrick, S., Lubega, A. (2018). In vitro anti-tuberculosis activity of total crude extract of echinops amplexicaulis against multi-drug resistant Mycobacterium tuberculosis. J. Health. Sci 6, 296–303. doi: 10.17265/2328-7136/2018.04.008
Khadim, E. J., Abdulrasool, A. A., Awad, Z. J. (2014). Phytochemical investigation of alkaloids in the iraqi echinops heterophyllus (Compositae). Iraqi J. Pharm. Sci. 23, 26–34.
Kitata, G., Abdeta, D., Amante, M. (2017). Ethnoknowledge of plants used in veterinary practices in midakegn district, west showa of oromia region, Ethiopia. J. Med. Plants. Stud. 5, 282–288.
Kiyekbayeva, L., Mohamed, N. M., Yerkebulan, O., Mohamed, E. I., Ubaidilla, D., Nursulu, A., et al. (2017). Phytochemical constituents and antioxidant activity of echinops albicaulis. Nat. Prod. Res. 32, 1203–1207. doi: 10.1080/14786419.2017.1323213
Kuete, V., Krusche, B., Youns, M., Voukeng, I., Fankam, A. G., Tankeo, S., et al. (2011). Cytotoxicity of some cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 134, 803–812. doi: 10.1016/j.jep.2011.01.035
Kuete, V., Sandjo, L. P., Wiench, B., Efferth, T. (2013). Cytotoxicity and modes of action of four cameroonian dietary spices ethno-medically used to treat cancers: echinops giganteus, xylopia aethiopica, imperata cylindrica and piper capense. J. Ethnopharmacol. 149, 245–253. doi: 10.1016/j.jep.2013.06.029
Kumar, H., Khajuria, A. K., Bisht, N. S. (2018). Traditional phytoremedies used to treatment urolithiasis in Pauri (Garhwal) Uttarakhand India. J. Pharmacogn. Phytochem. 7, 2941–2944.
Kumar, S., Pandey, S. (2015). An ethnobotanical study of local plants and their medicinal importance in tons river area, dehradun, uttarakhand. Indian. J. Trop. Biodivers. 23, 227–231.
Lamorde, M., Tabuti, J. R. S., Obua, C., Kukunda-Byobona, C., Lanyero, H., Byakika-Kibwika, P., et al. (2010). Medicinal plants used by traditional medicine practitioners for the treatment of HIV/AIDS and related conditions in Uganda. J. Ethnopharmacol. 130, 43–53. doi: 10.1016/j.jep.2010.04.004
Li, L., Ren, J., Cheng, Z., Zhu, H. (2010). Three new sesquiterpenoids from echinops ritro L. Helve. Chimica. Acta. 93, 1344–1349.
Li, L. B., Xiao, G. D., Xiang, W., Yang, X., Cao, K. X., Huang, R. S. (2019). Novel substituted thiophenes and sulf-polyacetylene ester from Echinops ritro L. Molecules 24, 805. doi: 10.3390/molecules24040805
Lin, C. C., Lin, C. H. (1993). Pharmacological and pathological studies on taiwan folk medicine (IX): the heptoprotective effect of the methanolic extract of Echinops grijissi. Am. J. Chin. Med. XXI, 21, 33–34.
Lin, C. C., Lin, C. H., Chiu, H. F., Hu, M. F. (1992). Pharmacological and pathological studies on taiwan folk medicine (VII): the anti-inflammatory effect of Echinops grijissi. Am. J. Chin. Med. XX, 20, 127–134.
Liu, Y., Ye, M., Guo, H. Z., Zhao, Y. Y., Guo, D. A. (2002). New thiophenes from echinops grijisii. J. Asian. Nat. Prod. Res. 4, 175–178. doi: 10.1080/1028602021000000071
Mahmood, A. A. R., Khadeem, E. J. (2013). Phytochemical investigation of flavonoids glycoside in the Iraqi Echinops heterophyllus (Compositae). Pharm. Glob. 4, 1.
Mahmoud, T., Gairola, S. (2013). Journal of medicinal plants studies traditional knowledge and use of medicinal plants in the eastern desert of egypt : a case study from Wadi El-Gemal National Park. J. Med. Plants. Stud. 1, 10–17.
Malik, K., Ahmad, M., Zhang, G., Rashid, N., Zafar, M., Sultana, S., et al. (2018). Traditional plant based medicines used to treat musculoskeletal disorders in Northern Pakistan. Eur. J. Integr. Med. 19, 17–64. doi: 10.1016/j.eujim.2018.02.003
Maru, R. N., Patel, H. R., Patel, R. S. (2018). Some plants used for the treatment of eye and earche from forest areas of Jhalod Taluka, Dahod District, Gujarat, India. Int. J. S. Res. Sci. Tech. 4, 578–581.
Maurya, S. K., Kushwaha, A. K., Seth, A. (2015). Ethnomedicinal review of Usnakantaka (Echinops echinatus Roxb.). Pharmacogn. Rev. 9, 149–154. doi: 10.4103/0973-7847.162138
Mekuanent, T., Zebene, A., Solomon, Z. (2015). Ethnobotanical study of medicinal plants in chilga district, northwestern ethiopia. J. Nat. Remedies. 15, 88–112. doi: 10.18311/jnr/2015/476
Menut, C., Lamaty, G., Weyerstahl, P., Marschall, H., Seelmann, I., Amvam Zollo, P. H. (1997). Aromatic plants of tropical Central Africa. Part XXXI. Tricyclic sesquiterpenes from the root essential oil of Echinops giganteus var. lelyi CD Adams. Flavour. Frag. J. 12, 415–421
Meragiaw, M., Asfaw, Z., Argaw, M. (2016). The status of ethnobotanical knowledge of medicinal plants and the impacts of resettlement in Delanta, northwestern Wello, northern Ethiopia. Evid. Based. Complement. Alternat. Med. 2016, 24. doi: 10.1155/2016/5060247
Merzouki, A., Ed-derfoufi, F., Mesa, J. M. (2000). Contribution to the knowledge of Rifian traditional medicine. II: folk medicine in Ksar Lakbir district (NW Morocco). Fitoterapia. 71, 278–307.
Mesfin, F., Seta, T., Assefa, A. (2014). An ethnobotanical study of medicinal plants in Amaro Woreda Ethiopia. Ethnobot. Res. Appl. 12, 341–354.
Miao, C. G., Zhou, G. L., Qin, M. S., Chen, J. Z., Li, C. F., Zhang, B. (2015). Treatment of rheumatoid arthritis with flavonoids of echinps latifolius tausch in rat model. Zhejiang. Da. Xue. Xue. Bao. Yi. Xue. Ban. 44, 43–48.
Mohseni, S., Sani, A. M., Tavakoli, M., Raoufi, A. M. (2017). Effect of extraction conditions on antioxidant activities of echinops persicus. J. Essent. Oil. Bear. Pl. 20, 1633–1644. doi: 10.1080/0972060X.2017.1399088
Moravec, I., Fernández, E., Vlkova, M., Milella, L. (2014). Ethnobotany of medicinal plants of northern ethiopia. Bol. Latinoam. Caribe. Plantas. Me. Aromát. 13, 126–134.
Mustafa, B., Hajdari, A., Krasniqi, F., Hoxha, E., Ademi, H., Quave, C. L., et al. (2012). Medical ethnobotany of the albanian alps in kosovo. J. Ethnobiol. Ethnomed. 8, 1–14. doi: 10.1186/1746-4269-8-6
Nahata, A., Dixit, V. K. (2014). Evaluation of 5α-reductase inhibitory activity of certain herbs useful as antiandrogens. Andrologia. 46, 592–601. doi: 10.1111/and.12115
Nakano, H., Ali, A., Rehman, J. U., Mamonov, L. K., Cantrell, C. L., Khan, I. A. (2014). Toxicity of thiophenes from echinops transiliensis (Asteraceae) against a aegypti (Diptera: Culicidae) larvae. Chem. Biodivers. 11, 1001–1009.
Nakano, H., Cantrell, C. L., Mamono, L. K., Osbrink, W. L. A., Ross, S. A. (2011). Echinopsacetylenes A and B, new thiophenes from Echinops transiliensis. Org. Lett. 13, 6228–6231. doi: 10.1021/ol202680a
Nakano, H., Cantrell, C. L., Mamonov, L. K., Kustova, T. S., Fronczek, F. R., Ross, S. A. (2012). Chemical constituents from Echinops nanus and Echinops transiliensis. Biochem. Syst. Ecol. 45, 127–129. doi: 10.1016/j.bse.2012.07.008
Nawash, O., Shudiefat, M., Al-Tabini, R., Al-Khalidi, K. (2013). Ethnobotanical study of medicinal plants commonly used by local bedouins in the badia region of Jordan. J. Ethnopharmacol. 148, 921–925. doi: 10.1016/j.jep.2013.05.044
Nyang'au, H. O., Maingi, J., Kebira, A., Muriithi, I. A., Muthoni, N. P., Ntarara, M. G., et al. (2017). The efficacy of some medicinal plants used locally within Transmara west, Narok County, Kenya against selected Enterobacteria and Candida. Infection 11, 13.
Okello, J., Ssegawa, P. (2007). Medicinal plants used by communities of Ngai Subcounty, Apac District, northern Ugand. Afr. J. Ecol. 45, 76–83.
Padashetty, S. A., Mishra, S. H. (2007). Effect of terpenoidal fraction of Echinops echinatus roots on reproductive parameters of male rats. J. Nat. Med. 61, 452–457. doi: 10.1007/s11418-007-0173-4
Papadopoulou, P., Couladis, M., Tzakou, O. (2006). Essential Oil Composition of two Greek Echinops species: E. graecus Miller and E. ritro L. J. Essent. Oil. Res. 18, 242–243. doi: 10.1080/10412905.2006.9699076
Patel, A. J. (2016). Isolation and characterization of lupeol from Echinops echinatus Roxb. Root. Eur. J. Pharm. Med. Res. 3, 385–387.
Patel, A. J., Patel, N. M., Patel, A. A., Patel, J., Patel, S. (2011b). Comparative analgesic activity of root and aerial part methanolic extracts of Echinops echinatus Roxb. Int. J. Pharm. Innov. 1, 23–29.
Patel, A. J., Patel, N. M., Patel, A. A., Patel, J., Patel, S. (2011a). Comparative diuretic activity of root and aerial part methanolic extracts of Echinops echinatus Roxb. Pharm. Lett. 3, 168–172.
Pavela, R., Maggi, F., Mbuntcha, H., Woguem, V., Fogang, H. P. D., Womeni, H. M., et al. (2016). Traditional herbal remedies and dietary spices from cameroon as novel sources of larvicides against filariasis mosquitoes? Parasitol. Res. 115, 4617–4626. doi: 10.1007/s00436-016-5254-4
Qureshi, R., Bhatti, G. R. (2008). Ethnobotany of plants used by the Thari people of Nara Desert, Pakistan. Fitoterapia. 79, 468–473. doi: 10.1016/j.fitote.2008.03.010
Rad, A. A., Najafzadeh-Varzi, H., Farajzadeh-Sheikh, A. (2010). Evaluation of anti-ulcer activity of echinops persicus on experimental gastric ulcer models in rats. Vet. Res. Forum. 1, 188–189.
Radulović, N. S., Denić, M. S. (2013). Essential oils from the roots of echinops bannaticus rochel ex schrad. and echinops sphaerocephalus L.(Asteraceae): chemotaxonomic and biosynthetic aspects. Chem. Biodivers. 10, 658–676. doi: 10.1002/cbdv.201200330
Rahman, S. M., Abd-Ellatif, S. A., Deraz, A. F., Khalil, A. A. (2011). Antibacterial activity of some wild medicinal plants collected from western mediterranean coast, Egypt: natural alternatives for infectious disease treatment. Afr. J. Biotechnol. 10, 10733–10743. doi: 10.5897.AJB11.007
Ram, S. N., Roy, R., Singh, B., Singh, R. P., Pandey, V. B. (1995). An acylfiavone glucoside of echinops echinatus flowers. Planta. Med. 62, 187.
Rathore, S., Tiwari, J. K., Malik, Z. A. (2015). Ethnomedicinal survey of herbaceous flora traditionally used in health care practices by inhabitants of dhundsir gad watershed of garhwal himalaya, India. Global. J. Res. Med. Plants. Indigen. Med. 4, 65–78.
Regassa, R. (2013). Assessment of indigenous knowledge of medicinal plant practice and mode of service delivery in Hawassa city, southern Ethiopia. J. Med. Plants. 7, 517–535.
Regassa, R., Bekele, T., Megersa, M. (2017). Ethnobotonical study of traditional medicinal plants used to treat human ailments by halaba people, southern Ethiopia. J. Med. Plants. Stud. 36, 36–47.
Rimbau, V., Cerdan, C., Vila, R., Iglesias, J. (1999). Antiinflammatory activity of some extracts from plants used in the traditional medicine of North-African countries (II). Phytother. Res. 13, 128–132.
Sajjad, A., Adnan, S., Hasnain Alam, H., Mohamed, E. A. R. (2017). Ethno botanical study of traditional native plants in Al Ain UAE. Int. J. Adv. Res. Biol. Sci. 4, 165–174. doi: 10.22192/ijarbs.2017.04.02.020
Saleh Fares, G. O., Abdallah, L., Almasri, M., Slaileh, A., Zurba, Z. (2013). Antibacterial activity of selected palestinian wild plant extracts against multidrug-resistant clinical isolate of streptococcus pneumoniae. JPR : BioMedRx: Int J. 1, 963–969.
Sánchez-Jiménez, I., Lazkov, G. A., Hidalgo, O., Garnatje, T. (2010). Molecular systematics of Echinops L. (Asteraceae, Cynareae): A phylogeny based on ITS and trnL-trnF sequences with emphasis on sectional delimitation. Taxon. 59, 698–708.
Sandjo, L. P., Kuete, V., Siwe, X. N., Poumale, H. M. P. (2016). Cytotoxicity of an unprecedented brominated oleanolide and a new furoceramide from the Cameroonian spice, Echinops giganteus. Nat. Prod. Res. 30, 2529–2537. doi: 10.1080/14786419.2015.1120724
Sarvaiya, D. D., Sheth, N. R., Dudhrejiya, A. V. (2017). Antidiabetic and antioxidant activity of roots of Echinops echinatus Roxb. Pharmacologyonline. 2, 10–39.
Sathiyamoorthy, P., Lugasi-Evgi, H., Schlesinger, P., Kedar, I., Gopas, J., Pollack, Y., et al. (1999). Screening for cytotoxic and antimalarial activities in desert plants of the negev and bedouin market plant products. Pharm. Biol. 37, 188–195.
Senejoux, F., Demougeot, C., Karimov, U., Muyard, F., Kerram, P., Aisa, H. A., et al. (2013). Chemical constituents from echinops integrifolius. Biochem. Syst. Ecol. 47, 42–44. doi: 10.1016/j.bse.2012.10.013
Sharma, J., Gairola, S., Gaur, R. D., Painuli, R. M. (2012). The treatment of jaundice with medicinal plants in indigenous communities of the sub-himalayan region of uttarakhand, India. J. Ethnopharmacol. 143, 262–291. doi: 10.1016/j.jep.2012.06.034
Sharma, P. K., Chauhan, N. S., Lal, B. (2004). Observations on the traditional phytotherapy among the inhabitants of Parvati valley in western Himalaya, India. J. Ethnopharmacol. 92, 167–176. doi: 10.1016/j.jep.2003.12.018
Shende, A. N., Mohture, V. M., Korpenwar, A. N. (2018). Traditional medicinal plants used against various diseases in Nagbhid tahsil, Chandrapur (MS) India. Int. J. Life. Sci. A 12, 135–142.
Shi, J., Zhang, X., Jiang, H. (2010). 2-(Penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl)thiophene, a novel NQO1 inducing agent from Echinops grijsii Hance. Molecules 15, 5273–5281. doi: 10.3390/molecules15085273
Shilema, A., Zerom, K., Mussa, A. (2013). Ethnoveterinary practices against animal trypanosomosis in Amaro district, Southern Ethiopia. Int. J. Med. Plants Res. 2, 238–241.
Sing, B., Ram, S. N., Pandeys, V. B., Joshis, V. K., Gambhir, S. S. (1991). Studies on antiinflammatory activity of taraxasterol acetate from echinops echinatus in rats and mice. Phytother. Res. 5, 103–106.
Singh, B., Gambhir, S. S., Pandjwb, V. B., Joshf, V. K. (1989). Anti-inflammatory activity of Echinops echinatus. J. Ethnopharmacol. 25, 189–199.
Singh, R. P., Pandey, V. B. (1990). Nivetin, a neoflavonoid from Echinops niveus. Phytochemistry. Phytochemistry 29, 680–681.
Singh, R. P., Singh, K. N., Pandey, V. B. (1990). Constituents of echinops niveus. Fitoterapia. 61, 279.
Singh, S., Upadhyay, R. K., Pandey, M. B., Singh, J. P., Pandey, V. B. (2006). Flavonoids from Echinops echinatus. J Asian Nat Prod Res. 8, 197–200.
Su, Y. F., Luo, Y., Guo, C. Y., Guo, D. A. (2004). Two new quinoline glycoalkaloids from Echinops gmelinii. J. Asian. Nat. Prod. Res. 6, 223–227. doi: 10.1080/10286020310001653327
Suleman, S., Alemu, T. (2012). A survey on utilization of ethnomedicinal plants in Nekemte town, East Wellega (Oromia), Ethiopia. J. Herbs. Spices. Med. Plants. 18, 34–57. doi: 10.1080/10496475.2011.645188
Tacham, W. N., Fonge, B. A., Fonkou, T. (2015). Traditional medicine and ethnobotanical use of wild plants by the Mundani people of Wabane, South West Region, Cameroon. J. Ethnobiol. Tradit. Med. Photon. 125, 1060–1080.
Tariku, Y., Hymete, A., Hailu, A., Rohloff, J. (2011). In vitro evaluation of antileishmanial activity and toxicity of essential oils of artemisia absinthium and echinops kebericho. Chem. Biodivers. 8, 614–623.
Tbatou, M. A., Belahyan, A. B., Belahsen, R. E. (2016). Wild edible plants traditionally used in the countryside of El Jadida, coastal area in the Center of Morocco. Life. Sci. Leafl. 75, 28–48.
Teklay, A., Abera, B., Giday, M. (2013). An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J. Ethnobiol. Ethnomed. 9, 65. doi: 10.1186/1746-4269-9-65
Tekle, Y. (2014). An ethno-veterinary botanical survey of medicinal plants in Kochore district of Gedeo Zone, Southern Nations Nationalities and Peoples Regional State (SNNPRs), Ethiopia. J. Sci. Innov. Res. 3, 433–445.
Tekwu, E. M., Askun, T., Kuete, V., Nkengfack, A. F., Nyasse, B., Etoa, F. X., et al (2012). Antibacterial activity of selected cameroonian dietary spices ethno-medically used against strains of Mycobacterium tuberculosis. J. ethnopharmacol. 142, 374–382. doi: 10.1016/j.jep.2012.05.003
Tene, M., Tane, P., Sondengam, B. L., Connolly, J. D. (2004). Lignans from the roots of echinops giganteus. phytochemistry 65, 2101–2105. doi: 10.1016/j.phytochem.2004.05.014
Tiwari, J. K., Ballabha, R., Tiwari, P. (2010). Ethnopaediatrics in garhwal Himalaya, Uttarakhand, India (Psychomedicine and Medicine). N. Y. Sci. J. 3, 123–126.
Toma, A., Deyno, S., Fikru, A., Eyado, A., Beale, A. (2015). In vivo antiplasmodial and toxicological effect of crude ethanol extract of Echinops kebericho traditionally used in treatment of malaria in Ethiopia. Malar. J. 14, 196. doi: 10.1186/s12936-015-0716-1
Toroğlu, S., Keskin, D., Vural, C., Kertmen, M., Cenet, M. (2012). Comparison of antimicrobial activity of echinops viscosus subsp. bithynicus and E. microcephalus leaves and flowers extracts from turkey. Int. J. Agric. Biol. 14, 637–640.
Wagh, V. V., Jain, A. K. (2018). Status of ethnobotanical invasive plants in western Madhya Pradesh, India. S. Afr. J. Bot. 114, 171–180. doi: 10.1016/j.sajb.2017.11.008
Wang, Y., Li, X., Meng, D., Li, N., Zhang, Y. (2008). Chemical constituents of thiophenes from Echinops latifolius Tausch. Shenyang. Yao. Ke. Da. Xue. Xue. Bao. 8.
Wang, Y., Li, X., Li, L., Meng, D., Li, Z., Li, N. (2007). Two new thiophenes from Echinops latifolius and their phototoxic activities. Planta. Med. 73, 696–698. doi: 10.1055/s-2007-981541
Wang, Y., Li, X., Meng, D.-L., Li, Z.-L., Zhang, P., Xu, J. (2006). Thiophenes from Echinops latifolius. Nat. Prod. Res. 8, 585–588. doi: 10.1080/10286020500176724
Wang, M., Sun, J., Jiang, Z., Xie, W., Zhang, X. (2015). Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am. J. Chin. Med. 43, 241–254.
Xu, D. G., Lv, W., Dai, C. Y., Zhu, F. F., Xu, G. H., Ma, Z. J., et al. (2015). 2 2-(Pro-1-ynyl)-5-(5,6-dihydroxypenta-1,3- diynyl) thiophene induces apoptosis through reactive oxygen species-mediated JNK activation in human colon Cancer SW620 Cells. Anat. Rec. 298, 376–385. doi: 10.1002/ar.23045
Yadava, R. N., Singh, S. K. (2006). New anti-inflammatory active flavanone glycoside from the Echinops echiantus Roxb. Indian. J. Chem. 45, 1004–1008.
Yigezu, Y., Haile, D. B., Ayen, W. Y. (2014). Ethnoveterinary medicines in four districts of jimma zone, Ethiopia: cross sectional survey for plant species and mode of use. BMC. Vet. Res. 10, 1–12. doi: 10.1186/1746-6148-10-76
Zamzami, T. A., Abdallah, H. M., Shehata, I. A., Mohamed, G. A., Alfaifi, M. Y., Elbehairi, S. E. I., et al. (2019). Macrochaetosides A and B, new rare sesquiterpene glycosides from Echinops macrochaetus and their cytotoxic activity. Phytochem. Lett. 30, 88–92. doi: 10.1016/j.phytol.2019.01.025
Zhang, P., Jin, W. R., Shi, Q., He, H., Ma, Z. J., et al. (2008). Two novel thiophenes from Echinops grijissi Hance. J. Asian. Nat. Prod. Res. 10, 977–981. doi: 10.1080/10286020802240467
Zhang, P., Liang, D., Jin, W., Qu, H., Cheng, Y., L, i, X., et al. (2009). Cytotoxic thiophenes from the root of Echinops grijisii Hance. Zeitschrift für. Naturforschung C. 1, 193–196.
Zhang, X., Ma, Z. (2010). Characterization of bioactive thiophenes from the dichloromethane extract of Echinops grijisii as Michael addition acceptors. Anal. Bioanal. Chem. 39, 1975–1984. doi: 10.1007/s00216-010-3729-1
Zhang, H., Tan, X., Yang, D., Lu, J., Liu, B., Baiyun, et al. (2017). Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget. 8, 40982. doi: 10.18632/oncotarget.17334
Zhao, M. P., Liu, Q. Z., Liu, Q., Liu, Z. L. (2017). Identification of Larvicidal Constituents of the Essential Oil of Echinops grijsii Roots against the three species of mosquitoe. Molecules 22, 205. doi: 10.3390/molecules2202020
Keywords: Echinops, thiophene, phytochemistry, Asteraceae, pharmacological activity, traditional use
Citation: Bitew H and Hymete A (2019) The Genus Echinops: Phytochemistry and Biological Activities: A Review . Front. Pharmacol. 10:1234. doi: 10.3389/fphar.2019.01234
Received: 17 November 2018; Accepted: 27 September 2019;
Published: 01 November 2019.
Edited by:
Alexander N. Shikov, Saint-Petersburg State Chemical Pharmaceutical Academy, RussiaCopyright © 2019 Bitew and Hymete. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helen Bitew, helenbitew@gmail.com; helen.bitew@mu.edu.et
 Helen Bitew
Helen Bitew Ariaya Hymete
Ariaya Hymete