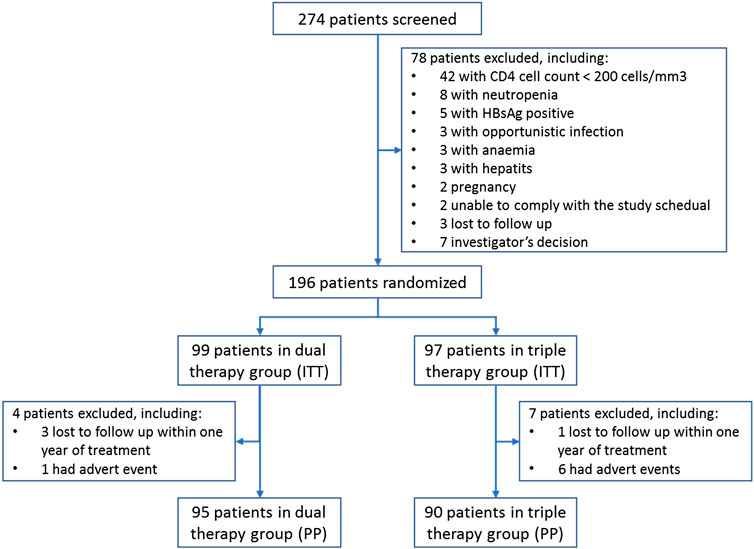
- Guangzhou Eighth People’s Hospital, Guangzhou Medical University, Guangzhou, China
Dual therapy with lopinavir/ritonavir (LPV/r) plus lamivudine (3TC) has been demonstrated to be non-inferior to the triple drug regimen including LPV/r plus two nucleoside reverse transcriptase inhibitors (NRTIs) in 48-week studies. However, little is known about the long-term efficacy and drug resistance of this simplified strategy. A randomized, controlled, open-label, non-inferiority trial (ALTERLL) was conducted to assess the efficacy, drug resistance, and safety of dual therapy with LPV/r plus 3TC (DT group), compared with the first-line triple-therapy regimen containing tenofovir (TDF), 3TC plus efavirenz (EFV) (TT group) in antiretroviral therapy (ART)-naïve HIV-1–infected adults in Guangdong, China. The primary endpoint was the proportion of patients with plasma HIV-1 RNA < 50 copies/ml at week 144. Between March 1 and December 31, 2015, a total of 196 patients (from 274 patients screened) were included and randomly assigned to either the DT group (n = 99) or the TT group (n = 97). In the primary intention-to-treat (ITT) analysis at week 144, 95 patients (96%) in the DT group and 93 patients (95.9%) in the TT group achieved virological inhibition with plasma HIV-1 RNA <50 copies/ml (difference: 0.1%; 95% CI, –4.6–4.7%), meeting the criteria for non-inferiority. The DT group did not show significant differences in the mean increase in CD4+ cell count (247.0 vs. 204.5 cells/mm3; p = 0.074) or the CD4/CD8 ratio (0.47 vs. 0.49; p = 0.947) from baseline, or the inflammatory biomarker levels through week 144 compared with the TT group. For the subgroup analysis, baseline high viremia (HIV-1 RNA > 100,000 copies/ml) and genotype BC did not affect the primary endpoint or the mean increase in CD4+ cell count or CD4/CD8 ratio from baseline at week 144. However, in patients with genotype AE, the DT group showed a higher mean increase in CD4+ cell count from baseline through 144 weeks than the TT group (308.7 vs. 209.4 cells/mm3; p = 0.038). No secondary HIV resistance was observed in either group. Moreover, no severe adverse event (SAE) or death was observed in any group. Nonetheless, more patients in the TT group (6.1%) discontinued the assigned regimen than those in the DT group (1%) due to adverse events. Dual therapy with LPV/r plus 3TC manifests long-term non-inferior therapeutic efficacy, low drug resistance, good safety, and tolerability compared with the first-line triple-therapy regimen in Guangdong, China, indicating dual therapy is a viable alternative in resource-limited areas.
Clinical Trial Registration: [http://www.chictr.org.cn], identifier [ChiCTR1900024611].
Introduction
Combined antiretroviral therapy (ART) for the treatment of HIV infection was established nearly 30 years ago, significantly decreasing AIDS-related morbidity and mortality globally (Gulick et al., 1997; Gunthard et al., 2016). In general, ART combination therapy comprises a three-drug regimen (Gunthard et al., 2016). In China, the government currently provides free ART for Chinese HIV-infected patients, with treatment comprising a first-line regimen of tenofovir disoproxil fumarate (TDF) plus lamivudine (3TC) plus efavirenz (EFV), and a second-line regimen of lopinavir/ritonavir (LPV/r) plus two nucleoside reverse transcriptase inhibitors (NRTIs) (Ning et al., 2017). However, in patients with a contraindication for TDF and could not obtain other NRTI alternatives, TDF-free simplified therapy, a potentially important alternative, holds several advantages, including low drug toxicity, lower pill burden, better adherence, and increased drug reserves in the future (Ahamed et al., 2016; Chwiki et al., 2017; De La Mata et al., 2017).
In recent years, simplified treatment strategies have garnered considerable interest. The first simplified strategy evaluated involved a boosted protease inhibitor (bPI) with a high genetic barrier to resistance. To date, dual therapy (DT) with bPI and 3TC has been found to be non-inferior to triple ART in multiple clinical trials (Calza et al., 2018; Perez-Molina et al., 2015), and is recognized as a confirmed switch therapy in current HIV treatment guidelines for use in special situations (European AIDS Clinical Society, 2017). In addition, in two separate studies, a simplified treatment regimen comprising LPV/r plus 3TC was demonstrated to be non-inferior to the standard treatment regimen in both antiretroviral therapy–naive and virologically suppressed HIV patients at week 48 (Cahn et al., 2014; Arribas et al., 2015).
However, a comprehensive comparison of EFV-based triple therapy (TT) vs. simplified strategies in HIV patients in China has not been published. To address this knowledge gap, we conducted a randomized, controlled, open-label, non-inferiority trial in antiretroviral-naïve HIV-1–infected patients comparing LPV/r plus 3TC DT with the Chinese first-line triple-therapy regimen (TDF, 3TC plus EFV). In China, where LPV/r is the only free protease inhibitor available to HIV patients, LPV/r is widely used (as it is in other countries with limited resources). We previously reported that DT with LPV/r plus 3TC was non-inferior to the first-line triple-therapy regimen at week 48 (Li et al., 2018). The study (ALTERLL) was continued using the same cohort, and we report here the antiretroviral long-term efficacy and safety of these regimens up to 144 weeks.
Materials and Methods
Study Design
This study reports the results of a randomized, controlled, open-label, non-inferiority trial started in March 2015 comparing antiretroviral long-term efficacy and resistance in a DT regimen of LPV/r plus 3TC vs. a triple-therapy regimen of TDF plus 3TC plus EFV. Our research was conducted on the hypothesis of no difference between the two groups. Patients were eligible for enrollment if they were infected with HIV-1, older than 18 years of age, naive to ART, and had a CD4+ cell count over 200 cells/mm3 at the time of screening. The exclusion criteria included a) pregnancy or breastfeeding, b) coinfected with HBV or HCV, and c) complications from chronic liver disease or AIDS-associated opportunistic diseases within 30 days before screening.
Our research was divided into two phases, up to 48 weeks and 48–144 weeks. The results obtained at 48 weeks have previously been published (Li et al., 2018). Here, the time window for this study was extended to 144 weeks. The study was conducted at Guangzhou Eighth People’s Hospital, which is an infectious disease specialist hospital following up around 15,000 HIV-infected patients receiving long-term ART. The study was approved by the Ethics Committee of the Guangzhou Eighth People’s Hospital (Approval No. 20142154). In addition, written informed consent was obtained from all participants. This study was registered with the Chinese Clinical Trial Registry, number ChiCTR1900024611.
Randomization and Masking
A high viral load (usually considered HIV-1 RNA >100,000 copies/ml) may affect the efficacy of ART. To reduce the influence of this confounding factor, a stratified analysis was performed according to the screening level of plasma HIV-1 RNA (≤100,000 vs. >100,000 copies/ml). In each of the two stratifications, patients were randomly divided into two groups (at a ratio of 1:1) according to a computer-generated allocation schedule, and patients in each group received DT or TT. DT included lopinavir 400 mg and ritonavir 100 mg twice daily plus 3TC 300 mg once daily. TT consisted of TDF 300 mg plus 3TC 300 mg plus EFV 600 mg, all once daily. Since the study was open label, patients and investigators were unmasked to treatment allocation.
Procedures
Each patient attending the clinic was screened according to the inclusion and exclusion criteria. In total, 274 patients were screened, and 196 patients were eligible for inclusion and randomly assigned to either DT (n = 99) or TT (n = 97). Patients were assessed on the screening day, day 1 (baseline), and at weeks 12, 24, 48, 72, 96, 120, and 144, or at early termination. Plasma HIV-1 RNA was quantified, and blood specimens were preserved at baseline, weeks 12, 24, 48, 96, and 144. Clinical assessments were performed at every visit. HIV genotypic assays were performed at screening. Resistance testing was performed at screening and upon the development of confirmed protocol-defined virological failure. Virological failure was defined as two consecutive viral loads (≥7 days and ≤30 days apart) of more than 400 copies/ml at week 24 or later after ART.
All laboratory data were obtained from the central laboratory of the Guangzhou Eighth People’s Hospital. This laboratory met all the requirements of the Clinical Laboratory Improvement Amendments regulations and the National Guideline for Detection of HIV/AIDS (Society of Infectious Diseases, 2015). The estimated glomerular filtration rate (eGFR) was calculated using the 2009CKD-EPI equation, and reduced eGFR was defined as an eGFR <80 ml/min/1.73 m2 or a reduction in the GFR of 10% in the last year (Inker et al., 2012). The chronic immune activation indicators utilized in this study included CD4+CD38+, CD8+CD38+, CD4+HLA-DR+, CD8+HLA-DR+, CD4+CD69+, and CD8+CD69+ cells.
Adverse events, including discomfort symptoms, signs, and abnormal laboratory examinations, were compiled from baseline through 144 weeks.
Subgenotype Determination and Drug Resistance Gene Mutation Analysis
Plasma HIV-1 RNA load was detected using the Roche COBAS-TaqMan Assay (HIV-1 Test, version 2.0, Indianapolis, IN, United states). The target fragment for the subgenotyping includes 99 amino acid coding codons from the protease (PR) region, and resistance analysis consists of approximately 300 amino acid coding codons from the reverse transcriptase region (primer sequences listed in Supplementary Table S1). The subgenotype of HIV-1 was tested using Bioedit and MEGA5 software with the standard strains selected from the Los Alamos HIV database, and mutation sites were identified using the Stanford HIV-resistant database (Rhee et al., 2017; Zhang et al., 2017).
Flow Cytometric Analysis of T Cell Activation Indicators
Whole blood samples (5 ml) were collected from patients, and their peripheral blood mononuclear cells were isolated and preserved. The samples were cryopreserved at −80°C in 80% fetal calf serum, 10% RPMI–1640, and 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, LT: 67–68–5). The following human monoclonal antibodies were used for labeling: antihuman CD4-APC-H7 (Cat: 580158, LT: 7062573), antihuman CD8-FITC (Cat: 555634, LT: 6195899), antihuman HLA-DR-APC (Cat: 559866, LT: 6014674), antihuman CD69-BV421 (Cat: 562884, LT: 6154722), and antihuman CD38-PR-CyTM7 (Cat: 561646, LT: 7163804). These antibodies were purchased from BD Biosciences (San Jose, CA, United States).
Outcomes
The primary endpoint was the proportion of subjects with plasma HIV-1 RNA loads <50 copies/ml at week 144, referred to as virological response rate (Ning et al., 2017). Secondary endpoints included the change in CD4+ cell count and ratio of CD4+ and CD8+, resistance profile, adverse events, and changes in chronic immune activation indicators. If patients in the DT group developed virological failure, they were switched to receive TT on the basis of the national free ART directory (De La Mata et al., 2017; Ning et al., 2017).
Statistical Analyses
Estimation of optimal sample size was based on the assumption of no difference between the two groups and a predicted efficacy of 85% at week 144. In the ITT analysis with a 15% margin and a 10% withdrawal rate, we calculated that 99 participants per arm would be required.
Efficacy was analyzed in the ITT population. All randomized participants were included in the analysis based on the intentionality principle. Per-protocol (PP) population refers to all participants who completed the 144-week treatment and had no major protocol violations. Safety was analyzed in the safety population, defined as all subjects who received at least one dose of study medications. Subgroup analysis was carried out to analyze outcomes in patients with different baseline viral loads and genotypes.
All statistical analyses were performed using IBM SPSS Statistics 21 and MedCalc 16 software. Quantitative data are reported as mean ± standard deviation (SD), while qualitative data are reported as numbers and proportions. The Mann–Whitney U test was used to compare two groups of quantitative or ordinal data, and Fisher’s exact test was used to compare the nominal data. For all statistical data, a double-tailed test with a level of 0.05 was used, and differences with a p-value of less than 0.05 were considered significant.
Results
Basic Data of Patients
A summary of subject characteristics is presented in Figure 1. Seven patients in the TT group and four patients in the DT group were excluded from the PP population due to loss during follow-up (n = 4) or adverse events (n = 7). No significant differences in the demographics and baseline characteristics of the ITT population were observed between the treatment groups (Table 1).
Efficacy Outcomes
The proportion of patients in the DT group who reached the primary efficacy endpoint was non-inferior to that achieved in the TT group (Figure 2A). Thus, 95 patients (96.0%) in the DT group and 93 patients (95.9%) in the TT group demonstrated HIV-1 RNA loads of <50 copies/ml at week 144 (difference 0.1%: 95% CI, −4.6–4.7%) in the ITT analysis (Figure 2B). In the PP analysis, the proportions were 98.9% (94/95) in the DT group and 96.7% (87/90) in the TT group (difference 2.3%: 95% CI, −1.2–5.8%; Supplementary Figure S2B).
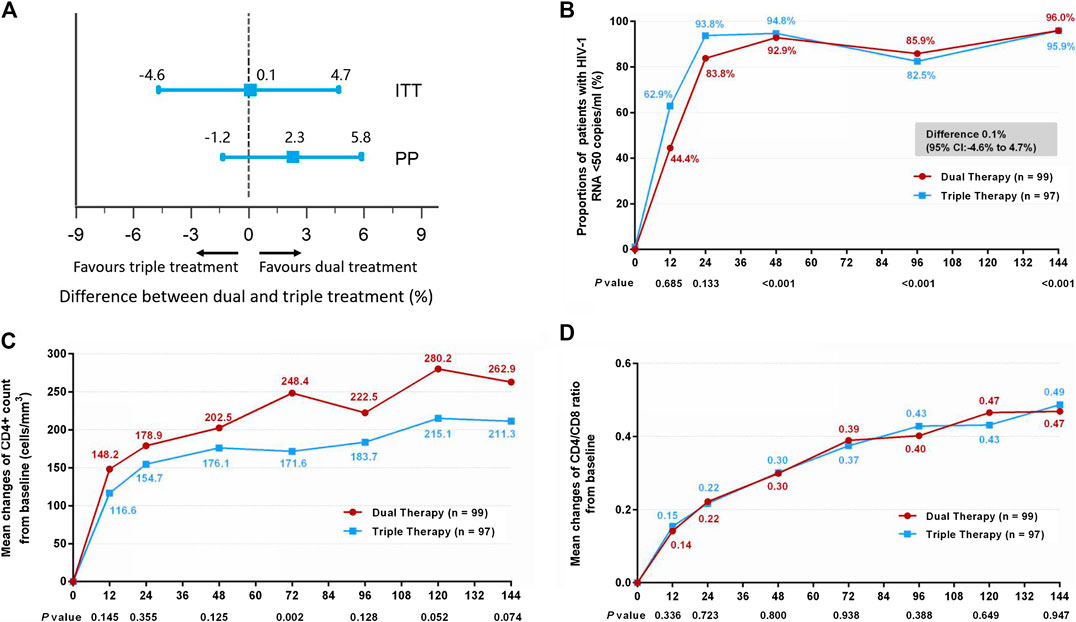
FIGURE 2. (A) Therapeutic response in the ITT and PP populations. The solid line represents no difference (dual treatment minus triple treatment) and the 15% non-inferiority margin. (B) Proportion of patients with HIV-1 RNA <50 copies/ml in ITT analysis. Proportions from two groups at different time points were tested for non-inferiority. p < 0.05 indicates that the dual treatment group is non-inferior to the triple treatment group. (C) Change in CD4+ count from baseline in ITT analysis. CD4+ counts from the two groups at different time points were tested with a Mann–Whitney U test. A p-value of less than 0.05 was considered significant. (D) Change in CD4/CD8 ratio from baseline in ITT analysis. CD4/CD8 ratios from the two groups at different time points were tested with a Mann–Whitney U test. A p-value less than 0.05 was considered significant.
Although the DT group showed higher increases in CD4+ cell count from baseline through 144 weeks than the TT group, both in the ITT (Figure 2C) (median, 247.0 cells/mm3 in DT group vs. 204.5 cells/mm3 in TT group) and PP (Supplementary Figure S2C) (median, 251.5 cells/mm3 in DT group vs. 204.5 cells/mm3 in TT group) analyses, the differences were not statistically significant (p = 0.074 and p = 0.075, respectively). A similar increase in the ratio of CD4+ to CD8+ was observed in the two groups, both in the ITT (Figure 2D) (median, 0.45 in DT group vs. 0.46 in TT group) and PP (Supplementary Figure S2D) (median, 0.45 in DT group vs. 0.45 in TT group) analyses.
Subgroup analysis was also conducted according to baseline HIV-1 RNA load. Within each subgroup (baseline HIV-1 RNA ≤ 100,000 copies/ml and baseline HIV-1 RNA > 100,000 copies/ml), no significant differences in the primary endpoint were observed between the DT and TT groups (Figures 3A–C) (difference −0.1%: 95% CI, −4.7–4.4% for the baseline HIV-1 RNA ≤100,000 copies/ml subgroup; difference 4.0%: 95% CI, −15.8–23.7% for the baseline HIV-1 RNA >100,000 copies/ml subgroup). Moreover, no significant difference was observed in the increase in CD4+ cell count from baseline through 144 weeks (Figures 3D,E) (median, 247 cells/mm3 vs. 190 cells/mm3 and 267.5 cells/mm3 vs. 264 cells/mm3 in the low load and high load subgroup, respectively), or in the ratio increase of CD4 to CD8 (Figures 3F,G) (median, 0.44 vs. 0.45 and 0.58 vs. 0.67 in the low load and high load subgroup, respectively). The results of PP analysis are consistent with those of the ITT analysis (Supplementary Figures S3B–G).
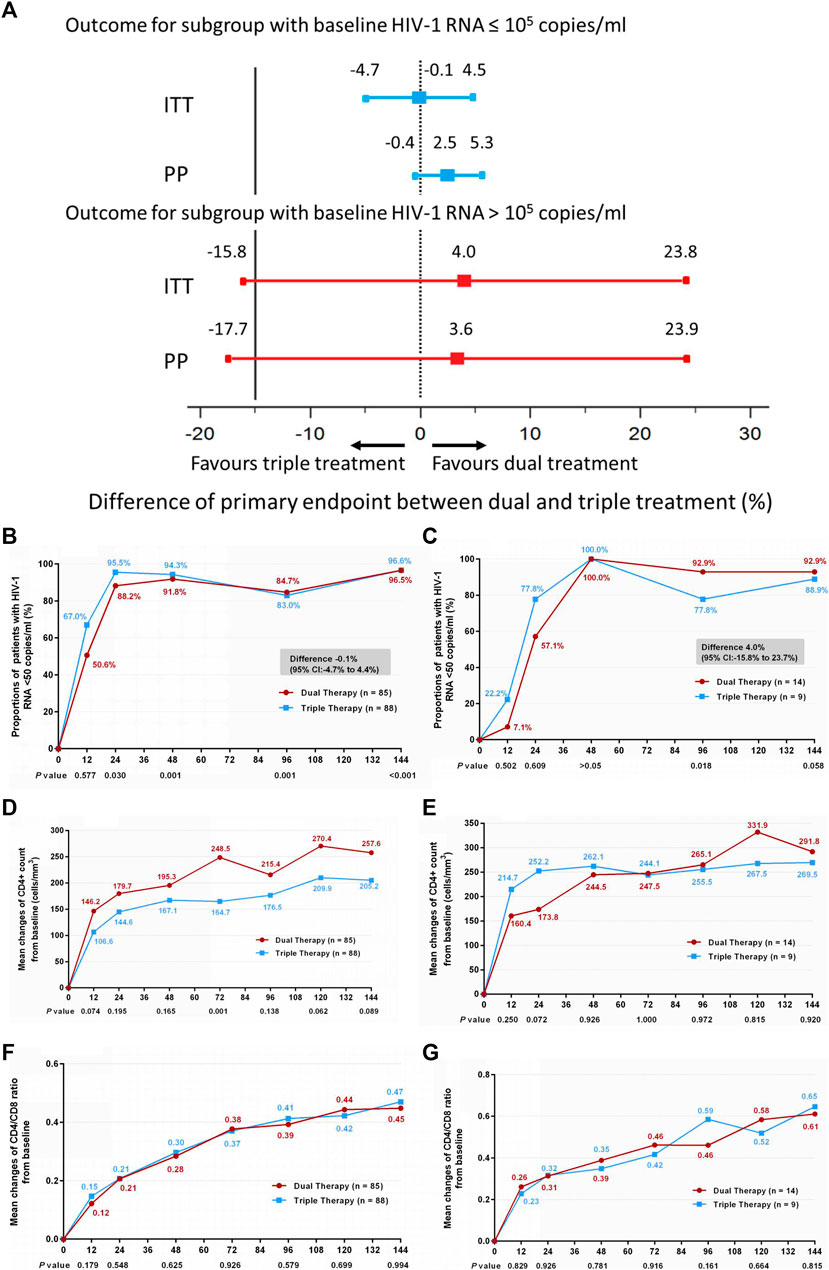
FIGURE 3. (A) Therapeutic response in the ITT and PP populations in subgroups stratified by baseline HIV-1 RNA. Solid lines represent no difference (dual treatment minus triple treatment) and the 15% non-inferiority margin (B,C). (B) Proportion of patients with HIV-1 RNA <50 copies/ml; proportion of patients with a baseline HIV-1 RNA ≤100,000 copies/ml in ITT analysis. (C) proportion of patients with a baseline HIV-1 RNA >100,000 copies/ml in ITT analysis. Proportions obtained from two groups at different time points were tested for non-inferiority. A p < 0.05 indicates that dual treatment group is non-inferior to the triple treatment group (D,E). (D) Change in CD4+ count from baseline in patients with a baseline HIV-1 RNA ≤100,000 copies/ml in ITT analysis. (E) Change in CD4+ count from baseline in patients with a baseline HIV-1 RNA >100,000 copies/ml in ITT analysis. CD4+ counts from the two groups at different time points were tested with a Mann–Whitney U test. A p-value of less than 0.05 was considered significant (F,G). (F) Change in CD4/CD8 ratio from baseline in patients with a baseline HIV-1 RNA ≤100,000 copies/mL in ITT analysis. (G) Change in CD4/CD8 ratio from baseline in patients with a baseline HIV-1 RNA >100,000 copies/mL in ITT analysis. CD4/CD8 ratio from the two groups at different time points were tested with a Mann–Whitney U test. A p-value of less than 0.05 was considered significant.
In addition, we compared treatment efficacy between the two treatment groups in patients with either genotype AE or genotype BC at week 144. No significant difference was observed in the proportions of patients with HIV-1 RNA loads <50 copies/ml (Figures 4A–C) (difference −3.6%: 95% CI, −13.4–6.3% in patients with genotype AE; difference 0.2%: 95% CI, −6.5–6.0% in patients with genotype BC), or in the increases in CD4/CD8 ratio (Figures 4D,E) (0.53 in both DT and TT groups, z = 0.134, p = 0.894). In patients with the AE genotype, the DT group showed a greater increase in CD4+ cell count from baseline through 144 weeks than the TT group (median, 289 cells/mm3 in the DT group vs. 173 cells/mm3 in the TT group, z = 2.076, p = 0.038). No significant difference in the elevation of CD4+ cell count was observed between the subgroups in patients with the BC genotype (median, 219.3 cells/mm3 in the DT group vs. 231.5 cells/mm3 in the TT group) (Figures 4F,G). The results of PP analysis were consistent with those of the ITT analysis (Supplementary Figures S4B–G).
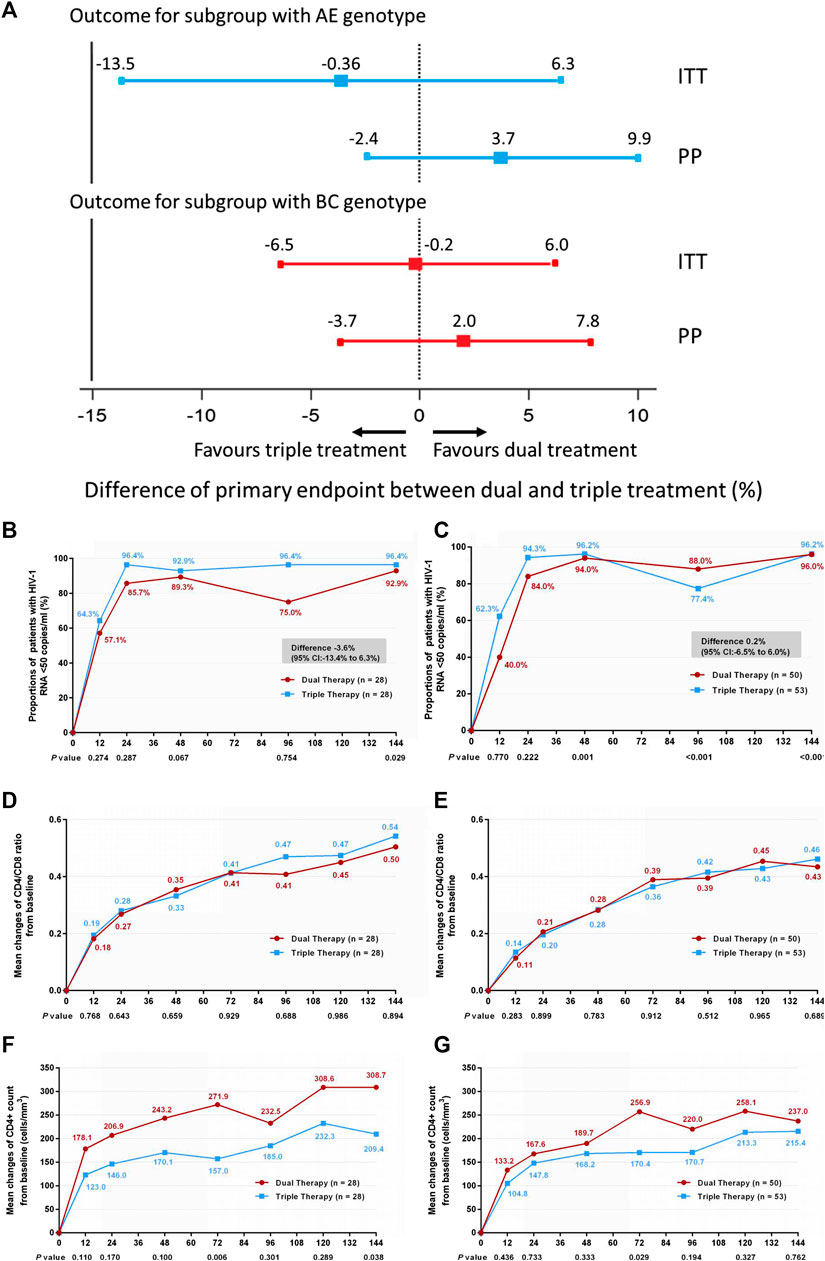
FIGURE 4. (A) Therapeutic response in the ITT and PP populations in subgroups stratified according to genotype. Solid lines represent no difference (dual treatment minus triple treatment) and the 15% non-inferiority margin (B,C). (B) Proportion of patients with HIV-1 RNA <50 copies/ml and with AE genotype in ITT analysis. (C) Proportion of patients with HIV-1 RNA <50 copies/ml and with BC genotype in ITT analysis. Proportions from two groups at different time points were tested for non-inferiority. A p < 0.05 indicates that dual treatment group is non-inferior to the triple treatment group (D,E). (D) Change in CD4/CD8 ratio from baseline in patients with AE genotype in ITT analysis. (E) Change in CD4/CD8 ratio from baseline in patients BC genotype in ITT analysis. CD4+ counts from the two groups at different time points were tested with a Mann–Whitney U test. A p-value of less than 0.05 was considered significant (F,G). (F) Change in CD4+ count from baseline in patients with AE genotype in ITT analysis. (G) Change in CD4+ count from baseline in patients with BC genotype in ITT analysis. CD4/CD8 ratios from the two groups at different time points were tested with a Mann–Whitney U test. A p-value of less than 0.05 was considered significant.
The chronic immune activation indicators, including CD4+CD38+, CD8+CD38+, CD4+HLA-DR+, CD8+HLA-DR+, CD4+CD69+, and CD8+CD69+ cells, were measured from baseline through 144 weeks. In both treatment groups, CD4+CD38+ and CD4+CD69+ increased in the first year after treatment, and then decreased over the following 2 years. CD4+HLA-DR+ showed a slight elevation trend during the 144 weeks, while CD8+CD38+, CD8+HLA-DR+, and CD8+CD69+ generally exhibited a downward trend from baseline through 144 weeks. However, no significant difference in the overall trend between the two groups over 144 weeks was observed (Supplementary Figure S5).
Resistance Profile
Only two participants in the DT arm (2%) and two participants in the TT arm (2.06%) developed protocol-defined virological failure (difference, 0.04%: 95% CI −5.31–5.57%). Of these four patients, three decided to discontinue ART (two in the DT group and one in the TT group). The quantitative plasma viral load of the remaining patient was 401 copies/ml.
The primary drug resistance results of the 196 patients were as follows: 28 resistant mutations were detected; six cases showed altered sensitivities to the drugs (a drug resistance rate of 3.06%). Of the six cases of altered sensitivity, one case (in the DT group) was mildly resistant to PI, two cases (in the TT group) were resistant to NRTIs, and three cases (in the DT group) were resistant to non-NRTIs (NNRTIs); no cross-resistance was observed. None of the amplified samples at treatment failure in either group showed any secondary resistance mutations. No mutations associated with protease inhibitors were identified in either arm.
Safety Outcomes
A majority of the patients experienced at least one adverse event during the 144-week study period. In total, 195 patients reported adverse events, including 99 in the DT group and 96 in the TT group. More diarrhea cases were reported in the DT group (52.5% in DT group vs. 5.2% in TT group, p < 0.001), and more cases of rash, dizziness, insomnia, and “dreaminess” were reported in the TT group (8.1%, 3%, 3%, and 1% in the DT group vs. 23.7%, 53.6%, 15.5%, and 17.5% in TT group, respectively; p < 0.05). Regarding laboratory abnormalities, triglyceride and cholesterol elevations were higher in the DT group than in the TT group (18.2% and 8.1% vs. 5.2% and 2.1%, respectively).
More patients (n = 6, 6.1%) in the TT group discontinued the assigned treatment regimen due to adverse events than those in the DT group (n = 1, 1%). In this study, diarrhea caused by LPV/r was common (52.5%); all cases were classified as grade 1–2 diarrhea, and cases were mostly reported in the first month of treatment. The majority of these patients found relief from diarrhea without recourse to prescribed treatment; in a few cases, treatment with montmorillonite powder for a short period was prescribed. None of the patients changed their treatment schedule due to intolerable diarrhea.
Among level 3–4 abnormal laboratory indexes, one case of alanine transaminase (ALT) elevation and two cases of aspartate aminotransferase elevation were reported; all cases were classified as level 3, and a return to normal levels was observed after a 4-week treatment with oral hepato-protective agents. In addition, there were 10 cases with elevated total cholesterol (CHOL) in the DT group, 23 cases with elevated triglyceride, and 10 cases with elevated low-density lipoprotein; all were grade 3–4 abnormalities. However, all abnormal indexes in blood lipid levels improved after application of oral lipid-lowering agents. No hospitalization or life-threatening consequences were found in either group. No severe adverse event (SAE) or death was observed in either group (see data in Supplementary Table S2).
Discussion
Three-drug combination ART has become available and widely accepted since 1996, dramatically improving the prognosis of people living with HIV (Odone et al., 2014; Sezgin et al., 2018). However, ART is a lifelong commitment, and is associated with several significant problems, including long-term toxicities, potential drug–drug interactions, and a high pill burden, which ultimately lead to a reduction in drug adherence, resulting in frequent treatment discontinuations and the development of drug resistance (Blasco et al., 2013; Tourret et al., 2013; Moyle et al., 2015; Gatell Artigas et al., 2016). Due to the increased viral potency of modern antiretroviral drugs, repositioning to a two-drug combination therapy should be considered, potentially reducing adverse events, drug–drug interactions, and cost, while maintaining an excellent sustained antiviral effect. Various combinations of DT regimens have been studied. The combination of 3TC and a protease inhibitor with booster has shown non-inferiority in several randomized studies (Cahn et al., 2014; Perez-Molina et al., 2015).
In this ALTERLL study, we demonstrate long-term sustained virologic efficacy of DT with lamivudine plus LPV/r over 144 weeks (3 years), with only rare cases developing virologic failure (2%, 2/99). DT with LPV/r plus 3TC is not inferior to the first-line regimen containing TDF, 3TC plus EFV, which is consistent with other findings (Cahn et al., 2014). The characteristics of LPV/r include a high gene barrier and high curative effect, which theoretically guarantees the efficacy of DT with lamivudine plus LPV/r. In practice, the GARDEL study (Cahn et al., 2014) and this study both confirm that DT with lamivudine plus LPV/r is not inferior to TT. In our study, DT was compared with 2NRTIS plus EFV, whereas in the GARDEL study, DT was compared with 2NRTIS plus LPV/r.
In comparison with the results obtained at 48 weeks (Li et al., 2018), the long-term effectiveness reported here at 144 weeks is more convincing. To confirm that dual ART is still effective in high HIV viral load populations and in different HIV genotypes, subgroup analyses according to baseline HIV-1 RNA and genotype were conducted. No significant differences were observed in the viral suppression rates at week 144 between the two groups among patients with high baseline viremia (HIV-1 RNA >100,000 copies/ml) or among patients with different genotypes. However, it should be noted that the number of cases with HIV-1 RNA >100,000 copies/ml was small, resulting in the 95% CI of the rate difference being too wide, and the lower limit exceeding −15%, which might affect the statistical results.
Regarding the chronic immune index, which reflects the restoration of immunity, no significant differences were observed in the increase in the ratio of CD4+ to CD8+ cells at week 144 between the two groups (or in the relevant subgroups with high/low baseline viremia or different genotypes). However, the DT group demonstrated a greater improvement in CD4+ cell count from baseline through 144 weeks than the TT group (although the differences were not statistically significant) and among patients with genotype AE (p < 0.05). Thus, it can be inferred from this study that simplified treatment of LPV/r plus 3TC is non-inferior to the standard triple regimen including TDF and 3TC plus EFV, and that DT may have advantages in some specific cases.
Another important concern is that of the improvement of chronic immune activation, for example, inflammatory cytokines (TNFα, IL-6), monocyte activation factor (soluble CD14), T-cell activation factors (HLA-DR, CD38), and exhaustion factor (PD-1) (Bastard et al., 2012; Hatano et al., 2013; Mendez-Lagares et al., 2013; Falasca et al., 2017). These immunity indicators have been reported to be associated with viral escape of HIV replication in sanctuaries, such as the brain and lymph nodes, where drugs may not reach the appropriate concentrations (Huang et al., 2016; Estes et al., 2017; Fletcher et al., 2018). In our study, there was a tendency for the T-cell activation markers to return to normal levels over 144 weeks in both groups, and no significant difference was observed between the two groups. This observation explains why DT is not inferior to TT in terms of virus suppression rate and immune reconstitution, and why DT can maintain a long-term effect over 144 weeks.
Virological failure was very rare in both arms of the study. Only four participants had viral rebounds >400 HIV-RNA copies/mL; treatment in three of these cases was subsequently discontinued due to low compliance. The quantitative plasma viral load of the remaining case was 401 copies/ml, which was lower than the viral load standard for drug resistance. In fact, in some similar simplified treatment studies, the presence of resistant sites, even for the NRTIs with low resistance barriers, cannot be induced. For example, in the OLE study, the observed drug resistance was due to previous irregular exposure to lamivudine (Arribas et al., 2015). In a simplified treatment study containing lamivudine and darunavir/ritonavir, virus rebound did not reveal a resistance site for lamivudine (Pulido et al., 2017). In the present study, no secondary resistance sites were observed, even if drug resistance was detected before treatment.
Thus far, current research has not reached a consensus on the adverse events associated with simplified treatment of LPV/r plus 3TC. In the SWORD one and two studies, adverse events were reported to be more frequent, and the incidence of discontinuation was increased in the arm receiving simplified treatment with LPV/r (Llibre et al., 2018). In the GEMINI study, significant differences in renal markers and bone markers were reported in the arms receiving DT or TT containing LPV/r at week 48 (Cahn et al., 2019). In our study, more patients in the TT group discontinued the assigned treatment regimen due to adverse events than those in the DT group, although the schedule containing lopinavir/ritonavir had higher incidences of diarrhea and metabolic disorders. Hyperlipidemia caused by LPV/r has been widely reported (Dai et al., 2019; Huang et al., 2019; Su et al., 2019). In agreement with these observations, our results confirm the occurrence of hyperlipidemia in the DT group, and show its side effects and its relation to other factors. For example, a rise in blood lipid levels in HIV-infected patients was more common than expected than in the general population (Grunfeld, 2010; Jain et al., 2018; Waters and Hsue, 2019). It should be noted that some patients had dyslipidemia before treatment, and that the extended follow-up time for this study increased the overall probability of dyslipidemia.
Our ALTERLL study is not without limitations. The limitations include the exclusion of CD4 < 200 patients, and the single-center, non–double-blind design of our study. However, we were still able to confirm that DT with LPV/r plus 3TC can maintain good long-term virologic efficacy. Furthermore, immune reconstitution in ART-naïve HIV-1–infected patients with genotype AE is greater following DT than immune reconstitution in patients following TT therapy with the first-line regimen TDF, 3TC plus EFV. In addition, drug resistance was relatively low in patients who had received DT (even after 144 weeks of treatment). Although LPV/r may lead to more metabolic disorders, these adverse events appear to be endurable and controllable. In conclusion, DT with LPV/r plus 3TC is a viable alternative in ART-naïve HIV-1–infected patients in resource-limited areas, including China.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Review Boards of Guangzhou Eighth People’s Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HH, XC, JC, JW, HZ, HL, WC, LL, and XT set up the study cohort and collected samples; PG, YL, and LL performed experiments; PD and CL followed up on patients and recorded data; XC and PG organized the data; PG and LL analyzed data and wrote the manuscript; WC, LL, and XT designed and directed the study. All authors contributed to revising the manuscript. All authors have read and approved the final version of the manuscript.
Funding
The study was supported by the Major National Science and Technology Projects during the 13th five-year plan period (2017ZX10202101-003, 2017ZX10202102-003-004), and the Guangzhou Science and Technology Innovation Committee project (new strategy for functional cure of AIDS-clinical and basic research, 201803040002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent to Publication
All patients agreed to the publication of anonymous data pertaining to them, including their demographic characteristics and experimental data. Written informed consent was obtained from all study participants.
Acknowledgments
We would like to thank the AbbVie company for technical support and language modification. We are also grateful for the statistical analyses performed by the statistician Hui Chen (Chinese Medical University).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.569766/full#supplementary-material.
Abbreviations
LPV/r, Lopinavir/ritonavir; 3TC, lamivudine; NRTIs, Nucleoside reverse transcriptase inhibitors; TDF, Tenofovir disoproxil fumarate; EFV, Efavirenz; ART, Antiretroviral therapy; DT, Dual therapy; TT, Triple therapy; ITT, Intention-to-treat; AE, Adverse event; SAE, Severe adverse event; bPI, boosted protease inhibitor; eGFR, estimated glomerular filtration rate; DMSO, dimethyl sulfoxide; PP, per-protocol; SD, standard deviation.
References
Ahamed, J., Terry, H., and Choi, M. E. (2016). Transforming growth factor-beta1-mediated cardiac fibrosis: potential role in HIV and HIV/antiretroviral therapy-linked cardiovascular disease. AIDS 30, 535–542. doi:10.1097/qad.0000000000000982
Arribas, J. R., Girard, P. M., Landman, R., Pich, J., Mallolas, J., Martínez-Rebollar, M., et al. (2015). Dual treatment with lopinavir/ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect. Dis. 15 (7), 785–792. doi:10.1016/s1473-3099(15)00096-1
Bastard, J. P., Soulie, C., Fellahi, S., Haïm-Boukobza, S., Simon, A., Katlama, C., et al. (2012). Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir. Ther. 17 (5), 915–919. doi:10.3851/imp2093
Blasco, A. J., Llibre, J. M., Arribas, J. R., Boix, V., Clotet, B., Domingo, P., et al. (2013). Analysis of costs and cost-effectiveness of preferred GESIDA/National AIDS Plan regimens for initial antiretroviral therapy in human immunodeficiency virus infected adult patients in 2013. Enferm. Infecc. Microbiol. Clín. 31, 568–578. doi:10.1016/j.eimc.2013.06.002
Cahn, P., Andrade-Villanueva, J., Arribas, J. R., Gatell, J. M., Lama, J. R., Norton, M., et al. (2014). Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviraltherapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect. Dis. 14, 572–580. doi:10.1016/s1473-3099(14)70736-4
Cahn, P., Madero, J. S., Arribas, J. R., Antinori, A., Ortiz, R., Clarke, A. E., et al. (2019). Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 393 (10167), 143–155. doi:10.1016/S0140-6736(18)32462-0 Epub 2018 Nov 9. Erratum in: Lancet. 2018 Nov 28.
Calza, L., Cafaggi, M., Colangeli, V., Borderi, M., Barchi, E., Lanzafame, M., et al. (2018). Simplification to dual-therapy containing lamivudine and darunavir/ritonavir or atazanavir/ritonavir in HIV-infected patients on virologically suppressive antiretroviral therapy. Inf. Disp. 50 (5), 352–360. doi:10.1080/23744235.2017.1410285
Chwiki, S., Campos, M. M., Mclaughlin, M. E., Kleiner, D. E., Kovacs, J. A., Morse, C. G., et al. (2017). Adverse effects of antiretroviral therapy on liver hepatocytes and endothelium in HIV patients: an ultrastructural perspective. Ultrastruct. Pathol. 41 (2), 186–195. doi:10.1080/01913123.2017.1282066
Dai, L., Liu, A., Zhang, H., Wu, H., Zhang, T., Su, B., et al. (2019). Impact of lopinavir/ritonavir and efavirenz-based antiretroviral therapy on the lipid profile of Chinese HIV/AIDS treatment-naïve patients in beijing: a retrospective study. Curr. HIV Res. 17 (5), 324–334. doi:10.2174/1570162X17666191025115508
De La Mata, N. L., Kumarasamy, N., Ly, P. S., Ng, O. T., Nguyen, K. V., Merati, T. P., et al. (2017). Growing challenges for HIV programmes in Asia: clinic population trends, 2003-2013. AIDS Care 29, 1243–1254. doi:10.1080/09540121.2017.1282108
Estes, J. D., Kityo, C., Ssali, F., Swainson, L., Makamdop, K. N., Del Prete, G. Q., et al. (2017). Defifining total-body AIDS virus burden with implications for curative strategies. Nat. Med. 23 (11), 1271–1276. doi:10.1038/nm.4411
European AIDS Clinical Society (2017). EACS Guidelines for the clinical management and treatment of HIV-infected adults. Milan, Italy. Available at: https://www.eacsociety.org/files/guidelines_9.0-english.pdf.
Falasca, F., Di Carlo, D., De Vito, C., Bon, I., d’Ettorre, G., Fantauzzi, A., et al. (2017). Evaluation of HIV-DNA and inflflammatory markers in HIV-infected individuals with different viral load patterns. BMC Infect. Dis. 17 (1), 581. doi:10.1186/s12879-017-2676-2
Fletcher, C. V., Thorkelson, A., Winchester, L., et al. (2018). Comparative lymphoid tissue pharmacokinetics (PK) of integrase inhibitors (INSTI). Boston, MA, USA: Presented at CROI, March 4-7.
Gatell Artigas, J. M., Arribas Lopez, J. R., Lazaro, Y. D. M. P., and Blasco Bravo, A. J. (2016). Cost/efficacy analysis of preferred Spanish AIDS study group regimens and the dual therapy with lopinavir/ritonavir plus lamivudine for initial ART in HIV infected adults. Enferm. Infecc. Microbiol. Clín. 34, 427–430. doi:10.1016/j.eimc.2015.01.018
Grunfeld, C. (2010). Dyslipidemia and its treatment in HIV infection. Top. HIV Med. 18 (3), 112–118.
Gulick, R. M., Mellors, J. W., Havlir, D., Eron, J. J., Gonzalez, C., McMahon, D., et al. (1997). Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337 (11), 734–739. doi:10.1056/nejm199709113371102
Gunthard, H. F., Saag, M. S., Benson, C. A., Hoy, J. F., Landovitz, R. J., Mugavero, M. J., et al. (2016). Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society-USA panel. J. Am. Med. Assoc. 316, 191–210. doi:10.3410/f.726500154.793532487
Hatano, H., Jain, V., Hunt, P. W., Lee, T. H., Sinclair, E., Do, T. D., et al. (2013). Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J. Infect. Dis. 208 (1), 50–56. doi:10.1093/infdis/jis630
Huang, X., Xu, L., Sun, L., Gao, G., Cai, W., Liu, Y., et al. (2019). Six-year immunologic recovery and virological suppression of HIV patients on LPV/r-based second-line antiretroviral treatment: a multi-center real-world cohort study in China. Front. Pharmacol. 10, 1455. doi:10.3389/fphar.2019.01455
Huang, Y., Hoque, M. T., Jenabian, M. A., Vyboh, K., Whyte, S. K., Sheehan, N. L., et al. (2016). Antiretroviral drug transporters and metabolic enzymes in human testicular tissue: potential contribution to HIV-1 sanctuary site. J. Antimicrob. Chemother. 71 (7), 1954–1965. doi:10.1093/jac/dkw046
Inker, L. A., Schmid, C. H., Tighiouart, H., Eckfeldt, J. H., Feldman, H. I., Greene, T., et al. (2012). Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 367, 681–681. doi:10.1056/nejmx120052
Jain, A., Kolvekar, T., and Nair, D. R. (2018). HIV infection and lipids. Curr. Opin. Cardiol. 33 (4), 429–435. doi:10.1097/HCO.0000000000000520
Li, L., He, H., Lan, Y., Chen, J., Zhong, H., Nie, J., et al. (2018). Dual therapy with lopinavir/ritonavir plus lamivudine could be a viable alternative for antiretroviral-therapy-naive adults with HIV-1 infection regardless of HIV viral load or subgenotype in resource-limited settings: a randomised, open-label and non-inferiority study from China. Indian J. Med. Microbiol. 36 (4), 513–516. doi:10.4103/ijmm.ijmm_18_172
Llibre, J. M., Hung, C-C., Brinson, C., Castelli, F., Girard, P. M., Kahl, L. P., et al. (2018). Effificacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomized, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 391 (10123), 839–849. doi:10.1016/s0140-6736(17)33095-7
Mendez-Lagares, G., Romero-Sanchez, M. C., Ruiz-Mateos, E., Genebat, M., Ferrando-Martínez, S., Muñoz-Fernández, M. Á., et al. (2013). Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble CD14. J. Infect. Dis. 207 (8), 1221–1225. doi:10.1093/infdis/jit025
Moyle, G. J., Orkin, C., Fisher, M., Dhar, J., Anderson, J., Wilkins, E., et al. (2015). A randomized comparative trial of continued abacavir/lamivudine plus efavirenz or replacement with efavirenz/emtricitabine/tenofovir DF in hypercholesterolemic HIV-1 infected individuals. PloS One 10, e0116297. doi:10.1371/journal.pone.0116297
Ning, C., Smith, K. M., Mccann, C. D., Hu, F., Lan, Y., Zhang, F., et al. (2017). Outcome of sentinel hospital-based and CDC-based ART service delivery: a prospective open cohort of people living with HIV in China. Sci. Rep. 7, 42637. doi:10.1038/srep42637
Odone, A., Amadasi, S., White, R. G., Cohen, T., Grant, A. D., and Houben, R. M. (2014). The impact of antiretroviral therapy on mortality in HIV positive people during tuberculosis treatment: a systematic review and metaanalysis. PloS One 9 (11), e112017. doi:10.1371/journal.pone.0112017
Perez-Molina, J. A., Rubio, R., Rivero, A., Pasquau, J., Suárez-Lozano, I., Riera, M., et al. (2015). Dual treatment with atazanavir/ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect. Dis. 15 (7), 775–784. doi:10.1016/s1473-3099(15)00097-3
Pulido, F., Ribera, E., Lagarde, M., Pérez-Valero, I., Palacios, R., Iribarren, J. A., et al. (2017). Dual therapy with darunavir and ritonavir plus lamivudine vs triple therapy with darunavir and ritonavir plus tenofovir disoproxil fumarate and emtricitabine or abacavir and lamivudine for maintenance of human immunodeficiency virus type 1 viral suppression: randomized, open-label, noninferiority DUAL-GESIDA 8014-RIS-EST45 trial. Clin. Infect. Dis. 65 (12), 2112–2118. doi:10.1093/cid/cix734
Rhee, S. Y., Varghese, V., Holmes, S. P., Van Zyl, G. U., Steegen, K., Boyd, M. A., et al. (2017). Mutational correlates of virological failure in individuals receiving a WHO-recommended tenofovir-containing first-line regimen: an international collaboration. EBioMedicine 18, 225–235. doi:10.1016/j.ebiom.2017.03.024
Sezgin, E., Van Natta, M. L., Thorne, J. E., Puhan, M. A., and Jabs, D. A. (2018). Secular trends in opportunistic infections, cancers and mortality in patients with AIDS during the era of modern combination antiretroviral therapy. HIV Med. 19 (6), 411–419. doi:10.1111/hiv.12609
Society of Infectious Diseases (2015). Chinese Medical Association Third edition of the guidelines for diagnosis and treatment of HIV/AIDS (2015). Chin. J. Clin. Infect. Dis. 57 (5), 385–401. doi:10.3760/cma.j.issn.1674-23972015.05.001
Su, B., Wang, Y., Zhou, R., Jiang, T., Zhang, H., Li, Z., et al. (2019). Efficacy and tolerability of lopinavir/ritonavir- and efavirenz-based initial antiretroviral therapy in HIV-1-Infected patients in a tertiary care hospital in beijing, China. Front. Pharmacol. 10, 1472. doi:10.3389/fphar.2019.01472
Tourret, J., Deray, G., and Isnard-Bagnis, C. (2013). Tenofovir effffect on the kidneys of HIV- infected patients: a double-edged sword? J. Am. Soc. Nephrol. 24, 1519–1527. doi:10.1681/asn.2012080857
Waters, D. D., and Hsue, P. Y. (2019). Lipid abnormalities in persons living with HIV infection. Can. J. Cardiol. 35 (3), 249–259. doi:10.1016/j.cjca.2018.11.005
Keywords: antiretroviral therapy, simplified regimen, randomized controlled study, lopinavir/ritonavir, inflammatory biomarker, efavirenz
Citation: Guo P-L, He H-L, Chen X-J, Chen J-F, Chen X-T, Lan Y, Wang J, Du P-S, Zhong H-L, Li H, Liu C, Li L-Y, Hu F-Y, Tang X-P, Cai W-P and Li L-H (2021) Antiretroviral Long-Term Efficacy and Resistance of Lopinavir/Ritonavir Plus Lamivudine in HIV-1-Infected Treatment-Naïve Patients (ALTERLL): 144-Week Results of a Randomized, Open-Label, Non-Inferiority Study From Guangdong, China. Front. Pharmacol. 11:569766. doi: 10.3389/fphar.2020.569766
Received: 05 June 2020; Accepted: 22 September 2020;
Published: 25 March 2021.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Yohana James Mashalla, University of Botswana, BotswanaAntonio Torsello, University of Milano-Bicocca, Italy
Copyright © 2021 Guo, He, Chen, Chen, Chen, Lan, Wang, Du, Zhong, Li, Liu, Li, Hu, Tang, Cai and LI. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-Hua Li, llheliza@126.com; Wei-Ping Cai, gz8hcwp@126.com
†These authors have contributed equally to this work.
 Peng-Le Guo
Peng-Le Guo Hao-Lan He†
Hao-Lan He† Ling-Hua Li
Ling-Hua Li