- 1Galleon Pharmaceuticals Inc, Horsham, PA, United states
- 2Department of Pediatrics, Case Western Reserve University, Cleveland, OH, United states
- 3Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, United states
- 4Department of Biological Sciences, School of Biomedical Sciences, Kent State University, Kent, OH, United states
- 5Department of Pharmacology, Case Western Reserve University, Cleveland, OH, United states
We have reported that pretreatment with the clinically approved superoxide dismutase mimetic, Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), blunts the cardiorespiratory depressant responses elicited by a subsequent injection of fentanyl, in halothane-anesthetized rats. The objective of the present study was to determine whether Tempol is able to reverse the effects of morphine on arterial blood-gas (ABG) chemistry in freely-moving Sprague Dawley rats. The intravenous injection of morphine (10 mg/kg) elicited substantial decreases in pH, pO2 and sO2 that were accompanied by substantial increases in pCO2 and Alveolar-arterial gradient, which results in diminished gas-exchange within the lungs. Intravenous injection of a 60 mg/kg dose of Tempol 15 min after the injection of morphine caused minor improvements in pO2 and pCO2 but not in other ABG parameters. In contrast, the 100 mg/kg dose of Tempol caused an immediate and sustained reversal of the negative effects of morphine on arterial blood pH, pCO2, pO2, sO2 and Alveolar-arterial gradient. In other rats, we used pulse oximetry to determine that the 100 mg/kg dose of Tempol, but not the 60 mg/kg dose elicited a rapid and sustained reversal of the negative effects of morphine (10 mg/kg, IV) on tissue O2 saturation (SpO2). The injection of morphine caused a relatively minor fall in mean arterial blood pressure that was somewhat exacerbated by Tempol. These findings demonstrate that Tempol can reverse the negative effects of morphine on ABG chemistry in freely-moving rats paving the way of structure-activity and mechanisms of action studies with the host of Tempol analogues that are commercially available.
Introduction
Morphine is a widely administered and effective analgesic agent. However, along with this positive effect, morphine also depresses breathing and impairs gas-exchange in the lungs, which combine to produce negative effects on arterial blood-gas (ABG) chemistry (Dahan et al., 2010; Boom et al., 2012; Henderson et al., 2014; Baby et al., 2018). These effects of morphine–analgesia and the depression of breathing–can be prevented or reversed by administration of opioid receptor antagonists, such as naloxone (Dahan et al., 2010). Nevertheless, the development of drugs that overcome the negative effects of opioids on breathing without affecting analgesia is an important effort because of numerous scenarios when administration of opioid receptor antagonists are not tenable or optimal (van der Schier et al., 2014; Dahan et al., 2018). For instance, a patient struggling to breathe due to the effects of opioids given during surgery cannot receive naloxone because of the resulting loss of pain relief.
We recently reported that l-cysteine ethyl ester (Mendoza et al., 2013), glutathione ethyl ester (Jenkins et al., 2021), and d-cystine methyl and ethyl esters (Gaston et al., 2021) rapidly reverse the negative effects of opioids, such as morphine and fentanyl, on breathing, Alveolar-arterial gradient (i.e., the index of gas exchange within the lungs) and arterial blood-gas (ABG) chemistry while not reducing opioid analgesia. Since these reduced and oxidized thiol esters block the negative effects of opioids on breathing and because the potent reducing agent N-acetyl-l-cysteine methyl ester does not affect opioid-induced depression of breathing (Baby et al., 2021, it is unlikely that the thiol esters exert their effects by altering the redox state of cells involved in the effects of opioids on breathing. In support of this, there is no real consensus as to whether opioids induce oxidative stress. For example, morphine, buprenorphine and methadone can increase or decrease oxidative stress depending on the experimental conditions (Lee et al., 2004; Almeida et al., 2014; Motaghinejad et al., 2015; Skrabalova et al., 2018; Leventelis et al., 2019).
In another study that we expected to provide evidence that free radicals and superoxide anions play no roles in the ventilatory depressant effects of opioids, we pretreated isoflurane-anesthetized rats with the stable cell permeable free radical scavenger and superoxide dismutase-mimetic agent, Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) (Wilcox and Pearlman, 2008; Wilcox, 2010; Kim et al., 2016), and determined the cardiorespiratory and analgesic effects elicited by subsequent injection of fentanyl (Baby et al., 2021). To our surprise, Tempol markedly blunted the fentanyl-induced decreases in ventilation and arterial blood pressure whereas it did not blunt the antinociceptive actions of fentanyl. It seems somewhat unlikely that the effects of Tempol are only due to alterations in oxidative stress status since unlike Tempol, the powerful antioxidant and superoxide anion scavenger, N-acetyl-l-cysteine methyl ester, did not affect fentanyl-induced suppression of breathing (Baby et al., 2021). The clinical uses for fentanyl and morphine overlap, but there are many instances where one is preferred over the other (Dahan et al., 2010; 2018; Boom et al., 2012). Moreover, there are substantial similarities, but also equally substantial differences, in the abilities of fentanyl and morphine to affect cardiorespiratory parameters in humans and animals (Dahan et al., 2010; 2018; Boom et al., 2012; Henderson et al., 2014; Torralva and Janowsky, 2019) for reasons including, differential actions of the parent opioids and their metabolites on non-opioid receptors (Torralva et al., 2020). Questions arising from our first report on Tempol (Baby et al., 2021) ask 1) whether the presence of isoflurane anesthesia is a factor in the ability of Tempol to blunt the negative cardiorespiratory effects of fentanyl; 2) whether Tempol uniquely affects fentanyl or is active against other opioids; and 3) whether the efficacy of Tempol is related to it being a pretreatment. Obviously, development of therapeutic agents that effectively reverse the negative effects of opioids on cardiorespiratory function without directly interacting with opioid receptors would be of great value. Accordingly, we are actively exploring the ability of Tempol and its analogues (Baby et al., 2021) to reverse the negative effects of fentanyl and morphine on breathing and cardiovascular parameters in freely-moving rats. The fentanyl studies are on-going, but we have completed the morphine studies and present them here. More specifically, to address some of the above questions and to further explore the potential of Tempol as a therapeutic to effectively treat opioid-induced respiratory depression, we determined whether Tempol could reverse the negative effects of morphine on ABG chemistry and tissue O2 saturation (SpO2) in unanesthetized Sprague Dawley rats.
Materials and Methods
Ethics Statements
All animal studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80.23) revised in 1996. The protocols were approved by the Institutional Animal Care and Use Committee at Galleon Pharmaceuticals, Inc (Horsham, PA).
Animals and Preparations
Adult male Sprague Dawley rats (270–300 g, body weight) with femoral vein and femoral artery catheters in place were obtained from Harlan Laboratories, Inc (Indianapolis, IN). The rats were caged in a Innocage IVC rat caging system (InnoVive, San Diego, CA, United States) with standard housing conditions with free access to food and water. The vivarium temperature (22°C), humidity (35–40%) and light-dark cycle (12:12 h) were maintained consistently. On the day of the experiment, the rats were acclimatized to a 9″ x 5″ plastic container prior to the experiment. After the acclimation period, the venous catheter was extended 15″ with additional micro-renathane tubing (Braintree Scientific, Braintree, MA) with pin connectors attached to the catheter. The arterial catheter was extended 15” with additional micro-renathane tubing with a 3-way connector (Braintree Scientific, MA). One end of the three-way connector was connected to a heparinized saline filled pressure transducer (SP844-28; Memscap Inc. North Carolina, United States of America) to measure arterial blood pressures and heart rate. The other end of the 3-way connector was attached to heparinized saline filled syringe to collect arterial blood samples for arterial blood gases and pH measurement. In these studies described below, the doses of Tempol were chosen on the basis of other rat studies (Xu et al., 2004; Wilcox and Pearlman, 2008; Wilcox, 2010) and from preliminary studies in our laboratory.
Arterial Blood Gases and pH Measurement
To collect arterial blood samples, any residual saline between the rat and the tubing connecting the syringe was removed and 250 µL of arterial blood was collected with a pre-heparinized 1 ml syringe. The syringe was capped and gently rotated up and down for 3 s to mix the heparin (Hann’s Pharma, Wilmington, DE) and blood to prevent any clotting in the blood gas machine. The sample was immediately injected into the blood gas analyzer, ABL 800 Flex (Radiometer, Westlake, OH) for the determination of pH, pCO2, pO2, sO2 and Alveolar-arterial (A-a) gradient. These samples were taken twice (at time-points 0 and 5 min) before the injection of morphine to ensure patency of the catheters and to obtain reliable baseline blood gases and pH values. Immediately following the second blood collection (at time-point 5 min), all rats received morphine (10 mg/kg, IV) given as a slow bolus in order to induce cardiorespiratory depression. Blood samples were taken 7 and 12 min later (12 and 17 min after initial blood samples were collected). Immediately after the second blood sample was taken (12 min post-morphine), the rats received a slow bolus injection of vehicle or Tempol at 60 or 100 mg/kg IV via the venous catheter. Arterial samples were taken 10 and 15 min afterwards (27 and 32 min post-morphine, after initial blood samples were collected, respectively).
Tissue O2 Saturation (SpO2) Analysis
SpO2 in conscious freely-moving rats was measured continuously using a MouseOx collar sensor (Starr Life Sciences Corp. Oakmont, PA, United States of America) placed over the carotid artery. On the day of the experiment, the rats were allowed to acclimatize to a 9″ x 5” plastic container. The MouseOx collar sensor fitted for the rat neck was connected to STARR-Link, an analog output module in conjunction with the MouseOx plus that converted the calculated parameters to an analog voltage output. The Starr-Link device placed outside the rat cage was interfaced with a PowerLab (ADInstruments, Inc. Colorado, CO, United States of America) and the data were digitized and continuously recorded using LabChart Pro7 software (ADInstruments, CO). SpO2 values were averaged in 30 s time bins to calculate the effects of Tempol on morphine-induced respiratory depression. More specifically, all of the rats received a slow bolus injection of morphine (10 mg/kg, IV) and after 15 min, the rats received a slow bolus injection of vehicle or Tempol at 60 or 100 mg/kg, IV. SpO2 was monitored for an additional 15 min.
Blood Pressure and Heart Rate Measurement
Blood pressure parameters and heart rate were continuously recorded in unrestrained freely-moving rats directly using femoral intra-arterial catheter as detailed previously (Baby et al., 2021). In brief, the arterial catheter was connected to a heparinized saline-filled pressure transducer (SP844-28; Memscap Inc. North Carolina, United States of America) and blood pressure signals were amplified using the Bridge Amp (FE221, AD Instruments, Inc.). The Bridge Amp was calibrated (2-point calibrations) before each experiment using sphygmomanometer. The arterial blood pressure wave-forms were sampled at 1-2k/sec and band-pass filtered between 0 and 1,000 Hz. Cyclic measurement algorithms in the LabChart 7 pro software (AD Instruments, Inc.) were used to calculate heart rate, diastolic blood pressure (DBP), systolic blood pressure (SBP) and mean arterial blood pressure (MAP) from the arterial blood pressure waveforms. The blood pressure waveforms were digitized (PowerLab, AD Instruments Inc.) and continuously recorded. The blood pressure and heart rate values were averaged every 30 s to calculate the effects of morphine and the Tempol effects on morphine-induced cardiovascular responses. The ratios of heart rate/MAP were determined throughout the experiments to provide an index of baroreceptor heart reflex activity as described previously (Lewis et al., 1999; Salman et al., 2020). With respect to the protocol, all rats received a slow bolus injection of morphine (10 mg/kg, IV) and after 15 min, the rats received a slow bolus injection of vehicle or Tempol at 60 or 100 mg/kg, IV. The cardiovascular parameters were all monitored for a further 15 min.
Drugs
Saline (vehicle) and morphine sulfate solution (50 mg/ml) were purchased from Hospira Inc (Lake Forest, IL, United States). Working dilutions of morphine (10 mg/ml) was prepared in sterile saline. Tempol was purchased from Tocris Bioscience (Minneapolis, MN, United States) and the stock solution (300 mg/ml) was prepared in sterile saline. Working dilutions (60 mg/ml and 100 mg/ml) were also prepared in sterile saline.
Data Analyses
All data are presented as mean ± SEM and were evaluated using one-way and two-way ANOVA followed by Bonferroni corrections for multiple comparisons between means using the error mean square term from the ANOVA (Wallenstein et al., 1980). Differences between means were taken to be significant at p < 0.05. Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc. La Jolla, CA).
Results
Effects of Tempol on Morphine-Induced Changes in ABG Chemistry and A-A Gradient
The changes in pH, pCO2, pO2 and sO2 elicited by the bolus injection of morphine (10 mg/kg, IV) and the subsequent injection of vehicle (saline) or a 60 mg/kg dose of Tempol (Tempol 60) in freely-moving rats are summarized in Figure 1. The injection of morphine elicited pronounced decreases in pH, pO2 and sO2 that were accompanied by equally pronounced increases in pCO2. These responses were similar in magnitude in the two groups. The injection of vehicle did not alter the morphine-induced responses whereas the 60 mg/kg dose of Tempol reversed the negative effects of morphine on pCO2 and pO2 with the effects on pH and sO2 not reaching significance. As seen in the top panel of Figure 2, the changes in pCO2 and pO2 elicited by morphine resulted in a rise in the A-a gradient (indicative of a mismatch of ventilation-perfusion) that was not affected by Tempol at doses 60 or 100 mg/kg. Changes in pH, pCO2, pO2 and sO2 elicited by the injection of morphine (10 mg/kg, IV) and subsequent injection of vehicle (saline) or 100 mg/kg dose of Tempol (Tempol 100) are summarized in Figure 3. The 100 mg/kg dose of Tempol elicited a sustained reversal of the negative effects of morphine on pH, pCO2, pO2 and sO2. Additionally it is important to note that in the bottom panel of Figure 2, the 100 mg/kg dose of Tempol elicited a significant decrease in the A-a gradient 5 min post-administration, whereas at 10 min post-administration there was no difference between the vehicle or Tempol-treated rats.
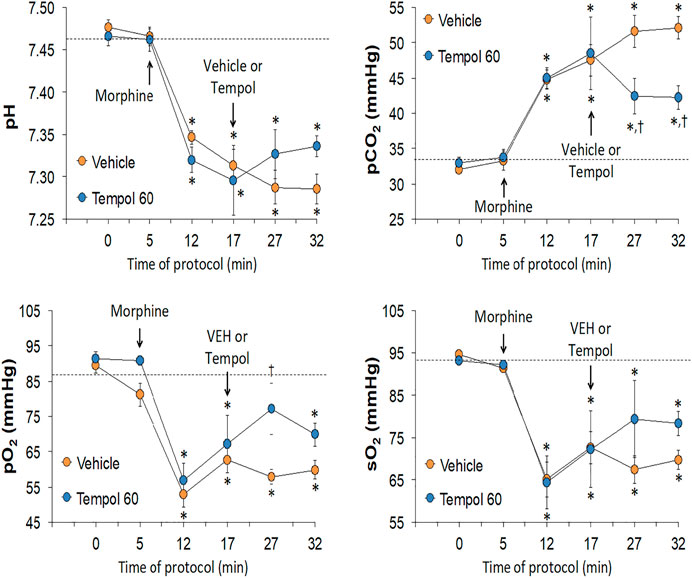
FIGURE 1. Changes in pH, pCO2, pO2 and sO2 elicited by a bolus injection of morphine (10 mg/kg, IV) and subsequent injection of vehicle (saline) or Tempol (, 60 mg/kg, IV; Tempol 60) in freely-moving rats. The data are presented as mean ± SEM. The numbers of rats in the vehicle and Tempol 60 groups were 7 and 3, respectively. *p < 0.05, significant change from baseline (time 0 value). †p < 0.05, Tempol-induced response versus vehicle-induced responses.
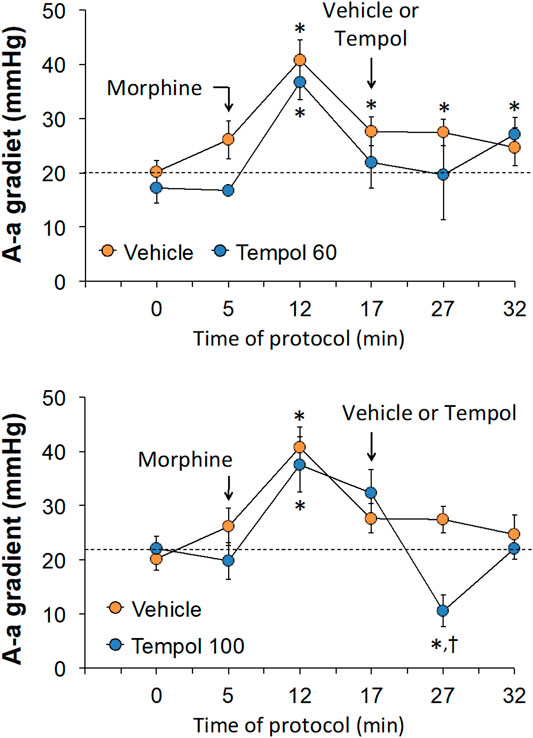
FIGURE 2. Changes in Alveolar-arterial (A-a) gradient elicited by the bolus injection of morphine (10 mg/kg, IV) and subsequent injection of vehicle (saline) or Tempol at doses of 60 mg/kg, IV (Tempol 60) (top panel) or 100 mg/kg, IV (Tempol 100) (bottom panel) in freely-moving rats. The data are presented as mean ± SEM. The numbers of rats in vehicle, Tempol 60 and Tempol 100 groups were 7, 3 and 7, respectively. *p < 0.05, significant change from baseline (time 0 value). †p < 0.05, Tempol-induced response versus vehicle-induced responses.
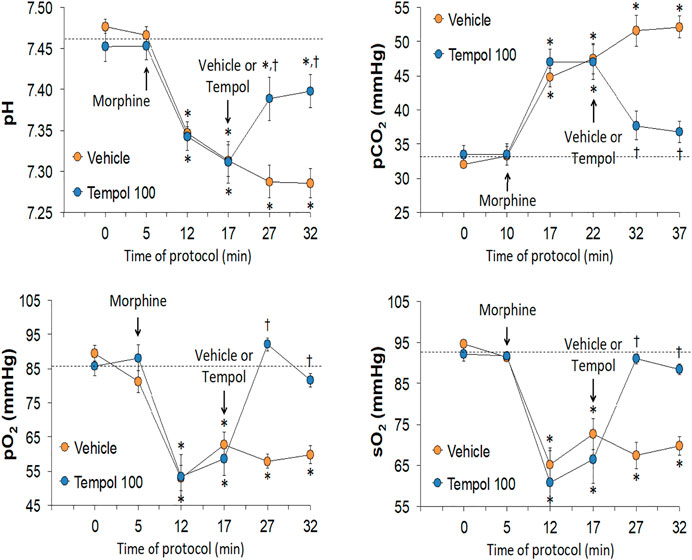
FIGURE 3. Changes in pH, pCO2, pO2 and sO2 elicited by the bolus injection of morphine (10 mg/kg, IV) and the subsequent injection of vehicle (saline) or Tempol (100 mg/kg, IV; Tempol 100) in freely-moving rats. The data are presented as mean ± SEM. The numbers of rats in the vehicle and Tempol 100 groups were 7 and 7, respectively. *p < 0.05, significant change from baseline (time 0 value). †p < 0.05, Tempol-induced response versus vehicle-induced responses.
Effects of Tempol on Morphine-Induced Changes in SpO2
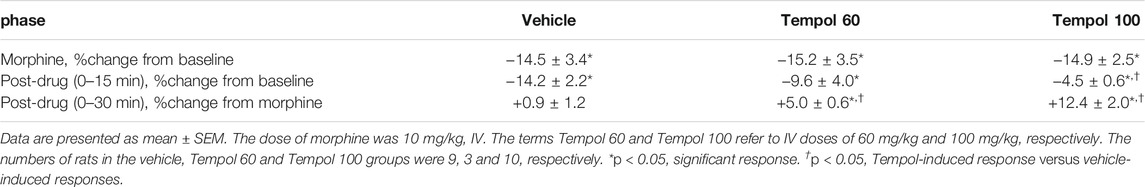
Baseline SpO2 values in the three groups are summarized in Supplemental Table S1. There were no between group differences in baseline SpO2 values. The changes in SpO2 elicited by an injection of morphine (10 mg/kg, IV) and subsequent injection of vehicle (saline), a 60 mg/kg dose of Tempol (Tempol 60) or a 100 mg/kg dose of Tempol (Tempol 100) in freely-moving rats are summarized in Figure 4. Morphine elicited a pronounced and long-lasting decrease in SpO2 that was not obviously affected by injection of vehicle. The injection of the 60 mg/kg dose of Tempol (Tempol 60) elicited a minor short-lived rise in SpO2, whereas the 100 mg/kg dose of Tempol (Tempol 100) elicited a prompt and sustained reversal of the negative effects of morphine on tissue oxygenation. As summarized in Table 1, the injection of morphine elicited similar cumulative decreases in SpO2 in the three groups (sum of responses that occurred from 0–15 min after injection). The injection of vehicle did not change the effects of morphine, such that the cumulative decrease in SpO2 over the 0–15 min post-vehicle injection phase (−14.2 ± 2.2) was similar to the 0–15 min post-morphine injection phase (−14.5 ± 3.4). In addition, the cumulative %change that occurred 0–30 min after injection of vehicle (using the 15 min post-morphine value as the pre-value) was not significant. In contrast, the cumulative responses after injection of the 60 mg/kg (Tempol 60) and 100 mg/kg (Tempol 100) doses of Tempol were significant with the 100 mg/kg dose being clearly stronger.
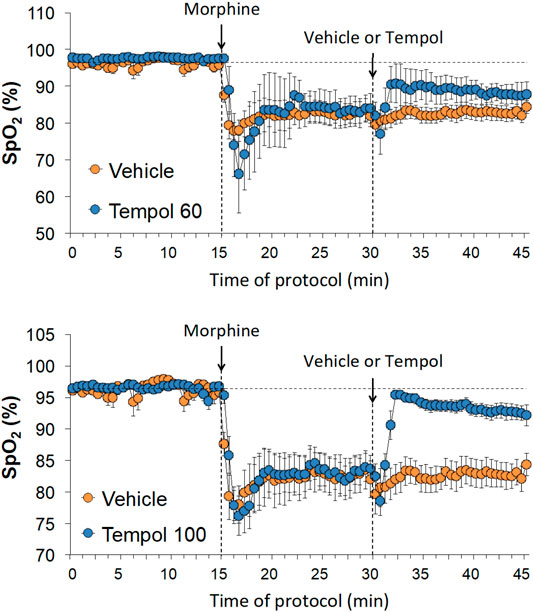
FIGURE 4. Changes in tissue oxygen saturation (SpO2) elicited by the bolus injection of morphine (10 mg/kg, IV) and the subsequent injection of vehicle (saline), Tempol at 60 mg/kg, IV (Tempol 60) or Tempol at 100 mg/kg, IV (Tempol 100) in freely-moving rats. The data are presented as mean ± SEM. The numbers of rats in the vehicle, Tempol 60 and Tempol 100 groups were 9, 3 and 10, respectively.
Effects of Tempol on Morphine-Induced Changes in Cardiovascular Parameters
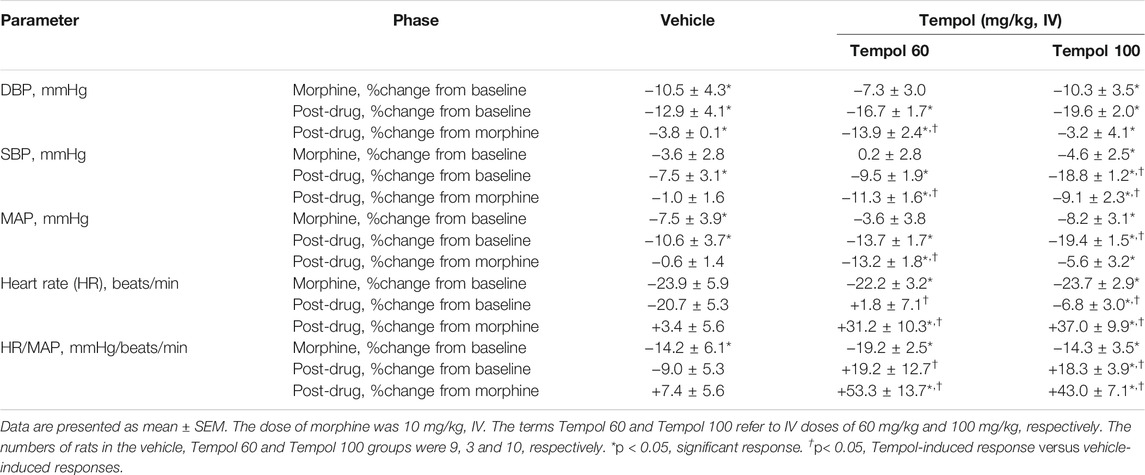
Baseline cardiovascular values in the three groups are summarized in Supplemental Table S1. There were no between group differences in any of the parameters. The changes in mean arterial blood pressure (MAP) and heart rate elicited by the injection of morphine (10 mg/kg, IV) and subsequent injection of vehicle (saline), a 60 mg/kg dose of Tempol (Tempol 60) or a 100 mg/kg dose of Tempol (Tempol 100) in freely-moving rats are summarized in Figure 5. Morphine elicited relatively minor initial decreases MAP but substantial decreases in heart rate. The injection of the 60 mg/kg dose of Tempol (Tempol 60) elicited a minor short-lived fall in MAP whereas the 100 mg/kg dose of Tempol (Tempol 100) elicited a prompt and sustained decrease in MAP. The 60 and 100 mg/kg doses of Tempol elicited a prompt and sustained reversal of the morphine-induced bradycardia. The changes in diastolic (DBP) and systolic (SBP) arterial blood pressures are summarized in Supplemental Figure S1. The data shows that changes in both DBP and SBP contribute equally to those described for MAP (see above). Moreover, as can be seen in Supplemental Figure S2, the changes in MAP and heart rate result in somewhat minor reductions in the ratio of heart rate/MAP (i.e., the index of baroreceptor heart rate reflex activity) following injection of morphine, suggesting a loss of baroreflex activity. The injection of Tempol, specifically at 100 mg/kg, elicited rapid and sustained increase in the heart rate/MAP ratio suggestive of pronounced increases in baroreceptor heart rate reflex activity. As summarized in Table 2, the injection of morphine elicited relatively minor cumulative decreases (about 10% or less) in DBP, SBP and MAP in the three groups of rats (sum of responses that occurred from 0–15 min post-injection). The cumulative decreases in DBP, SBP and MAP over the 0–15 min post-vehicle injection phase were similar to, or significantly greater than, those recorded during the 0–15 min post-morphine injection phase. In addition, the cumulative %change that occurred 0–15 min after injection of vehicle (using the final 15 min morphine value as the pre-value) were not significant for SBP or MAP. In contrast, the cumulative responses in DBP, SBP and MAP following the injection of the 60 and the 100 mg/kg doses of Tempol (as recorded between 15 and 30 min post-morphine) were all greater than those recorded between 0 and 15 min post-morphine injection. As also seen in Table 2, morphine elicited significant and similar cumulative decreases in heart rate and heart rate/MAP in the three groups of rats and that both doses of Tempol elicited sustained reversal of these effects of morphine.
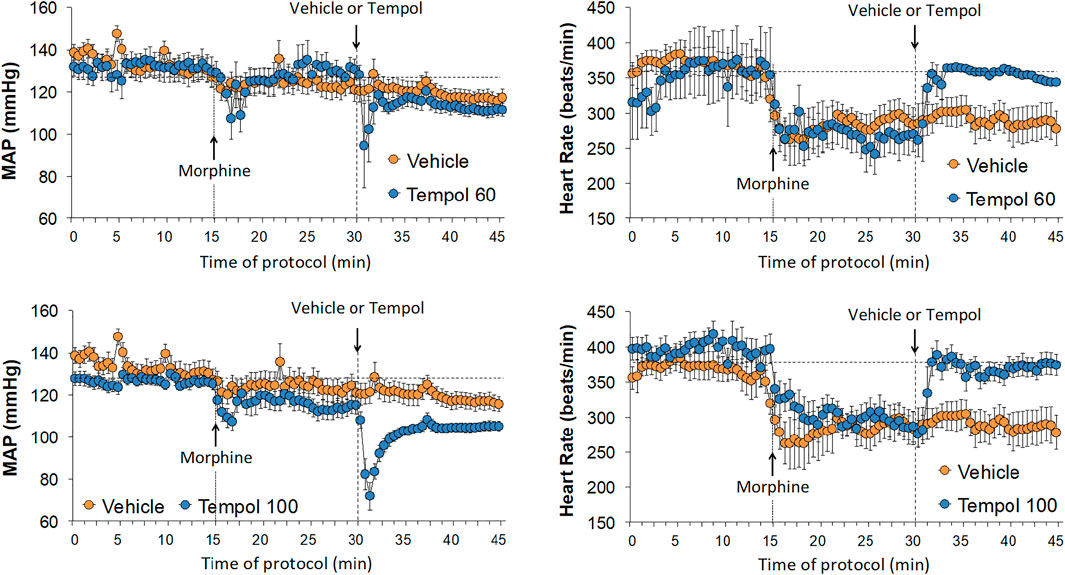
FIGURE 5. Changes in mean arterial blood pressure (MAP) and heart rate elicited by bolus injection of morphine (10 mg/kg, IV) and the subsequent injection of vehicle (saline), Tempol at 60 mg/kg, IV (Tempol 60) or Tempol at 100 mg/kg, IV (Tempol 100) in freely-moving rats. Data are shown as mean ± SEM. The numbers of rats in vehicle, Tempol 60 and Tempol 100 groups were 9, 3 and 10, respectively.
Discussion
The present study confirms that morphine (10 mg/kg, IV) has pronounced deleterious effects on ABG chemistry (decreases in pH, pO2 and sO2 accompanied by increases in pCO2) and noticeable decreases in gas-exchange in the lungs (i.e., elevated A-a gradient) in freely-moving male Sprague Dawley rats (May et al., 2013; Gaston et al., 2021). This study also confirms the findings of Grinnell et al. (2014) that morphine elicits a substantial decrease in tissue oxygenation saturation (SpO2) in rats. The administration of the 10 mg/kg dose of morphine elicited relatively minor reductions in DBP, SBP and MAP in our freely-moving rats with the available literature reporting minimal to substantial reductions in these parameters in non-anesthetized rats (Della Puppa et al., 1989; Thornhill et al., 1989; Thurston et al., 1993). Consistent with published literature, morphine also elicited robust and sustained decreases in heart rate, which have been reported to mainly involve an increase in vagal drive to the heart (Della Puppa et al., 1989; Thornhill et al., 1989; Thurston et al., 1993). The ratio of heart rate/MAP can be taken as an index of baroreceptor reflex activity (Lewis et al., 1999; Salman et al., 2020), and our data provide evidence that morphine decreased this ratio and therefore potentially decreased the activity of the baroreflex in our rats. There is remarkably little published data as to the exact effects of morphine or any other opioid on the baroreceptor reflex system in freely-moving (non-anaesthetized) rats although the reported evidence we could find demonstrated that morphine enhances baroreceptor reflex activity in anesthetized rats (Shanazari et al., 2011) whereas it inhibits baroreflex activity in humans (Kotrly et al., 1984).
A major finding of the present study was that the intravenous injection of Tempol and especially at a 100 mg/kg dose was able to immediately reverse the pronounced negative effects of a 10 mg/kg intravenous dose of morphine on ABG chemistry, A-a gradient and SpO2 in the freely-moving rats. These findings complement our earlier report that pretreatment with a 100 mg/kg dose of Tempol markedly blunted the deleterious changes in ABG chemistry, A-a gradient and ventilatory parameters (e.g., frequency of breathing, tidal volume and minute ventilation) elicited by the subsequent injection of fentanyl in isoflurane-anesthetized rats (Baby et al., 2021). The present study did not directly address the mechanisms underlying the ability of Tempol to reverse the negative effects of morphine on ventilatory control processes pertinent to our study, however, the rapid and sustained effects of Tempol on ABG chemistry and SpO2 along with the relatively transient effects on A-a gradient (improved gas exchange in the lungs of morphine-treated rats) certainly suggests that the primary reason for the improved status of the ABG chemistry is due to the ability of Tempol to increase ventilatory performance (e.g., enhanced minute ventilation and inspiratory drive) in the morphine-treated rats.
The present study also found that the 60 and 100 mg/kg doses of Tempol elicited pronounced and sustained decreases in MAP in morphine-treated rats that were similar in magnitude to those that occur in naïve rats (Baby et al., 2021). The reductions in MAP in naïve rats is due to the direct activation of Ca2+-activated K+-channels (BKCa) channels in vascular smooth muscle rather than by scavenging free radicals and/or superoxide anion (Xu et al., 2004; 2005; 2006). The systemic administration of Tempol produces mild to profound dose-dependent bradycardia in freely-moving rats (Xu et al., 2004; 2005; 2006). Rather remarkably, the injection of Tempol (either 60 or 100 mg/kg) caused a pronounced and sustained increase in heart rate by mechanisms that we are currently exploring. We can only conjecture that Tempol somehow inhibits the signaling central signaling pathways by which morphine diminishes heart rate, including the activation of vagal drive (Della Puppa et al., 1989; Thornhill et al., 1989; Thurston et al., 1993). It is also important to consider the possibility that the increase in ventilation and improvements in gas-exchange within the lungs and therefore ABG chemistry following Tempol administration in morphine-treated rats, may be a result of the large reduction in blood pressure elicited by Tempol. For example, Viires et al. (1983) reported that the hypotension induced by pericardial temponade elicited an increase in ventilation in dogs by an increase in blood flow to the diaphragm (via a redistribution of systemic blood flow).
Currently, we do not have any knowledge about or any understanding of the molecular mechanisms by which Tempol reverses the negative effects of morphine on ABG chemistry and SpO2. However, we do believe that the ability of Tempol to activate BKCa channels (Xu et al., 2005; 2006) is most likely not a key contributor to the mechanisms by which Tempol reverses morphine-induced depression of ventilatory parameters responsible for the observed robust changes in ABG chemistry and SpO2 in the morphine-treated rats. Primary reasons for this belief are that 1) the activation of BKCa channels substantially diminishes carotid body glomus cell activity (Pichard et al., 2015; Wang et al., 2018) thereby dampening breathing, and 2) because pharmacological blockade of BKCa channels partially reverses opioid-induced respiratory depression in humans and animals (McLeod et al., 2014; Roozekrans et al., 2014; Dallas et al., 2015; Golder et al., 2015). Although the mechanisms responsible for the cardiovascular effects of morphine are multi-factorial, the direct and endothelium-dependent vasodilation of systemic and pulmonary arteries are both important mechanisms contributing to the underlying morphine-induced hypotension and the distribution of blood flow within the pulmonary circulation, respectively (Feuerstein, 1985; Johnson et al., 1985; Kaye et al., 2008; Toda et al., 2009; Behzadi et al., 2018). Nonetheless, though direct evidence is lacking, it would be reasonable to suggest that the ability of Tempol to reverse the negative effects of morphine on MAP involves effects on central (e.g., brainstem) circuitry and peripheral (e.g., sympathetic and parasympathetic ganglia and nerve terminals) that directly participate in the expression of the hypotension elicited by morphine or those that modulate this response (Feuerstein, 1985; Johnson et al., 1985; Toda et al., 2009; Behzadi et al., 2018). We have not determined whether the actions of Tempol seen in this study involve its proven capacity to scavenge free radicals and superoxide anion. However, our findings with Tempol against fentanyl (Baby et al., 2021) and morphine (present study) raise the possibility that Tempol, which is clinically approved to treat alopecia in humans (Wilcox and Pearlman, 2008; Wilcox, 2010), and many commercially-available Tempol analogues (Baby et al., 2021; Supplemental Figure S9) may be repurposed as an intravenous agent to both prevent and reverse the profound negative effects of fentanyl and morphine on breathing and arterial blood pressure while preserving analgesia in human subjects.
In summary, the present study extends our findings regarding the beneficial effects of Tempol against the negative effects of fentanyl on breathing, ABG chemistry and gas-exchange in the lungs, and the potential problems regarding the ability of Tempol to reduce arterial blood pressure (Baby et al., 2021). Previous studies have demonstrated the beneficial effects of Tempol in cell and animal models of neurodegenerative diseases, shock, hypertension, diabetes, ischemia-reperfusion injury, traumatic brain injury, tumororigenesis, chemotherapy-induced neuropathic pain and alopecia (Wilcox and Pearlman, 2008; Wilcox, 2010; Kim et al., 2016; Bernardy et al., 2017; Wang et al., 2018; Afjal et al., 2019; Chiarotto et al., 2019). Future studies with the array of commercially available Tempol analogues (Baby et al., 2021; Supplemental Figure S9) will help to define structure-activity relationships with respect to the wanted and unwanted effects of Tempol-like drugs. The findings may pave the way for the development of a novel series of drugs that can be used to overcome opioid-induced respiratory and hemodynamic depression. Of course, it is possible that designing Tempol analogues that do not have the hypotensive and vasodilator activities of Tempol may ultimately also eliminate efficacy against the negative effects of opioids on ventilatory function.
Study Limitations
The present study has several limitations that will be subject to future research studies in which we will 1) administer Tempol following the injection of fentanyl or even higher potency analogues (e.g., sufentanil) in freely-moving male and female rats, in order to determine the efficacy of Tempol as a reversal agent against the profound cardiorespiratory responses that are elicited by these high potency synthetic opioids, and 2) inject Tempol following injection of fentanyl (following bolus or infusion paradigms) in combination with diazepam and/or methamphetamine in freely-moving male and female rats to provide information as to the efficacy of Tempol against the cardiorespiratory effects of these combinations, which are ever increasing real-world scenarios (Warner et al., 2016; Macleod et al., 2019; Baker et al., 2021). Another important limitation of this study was that end-tidal PO2 and PCO2 were not continuously monitored. Such measurements would allow us to determine the temporal relationships between the changes in alveolar gases, ventilatory parameters, and arterial blood pressures because of morphine administration and subsequent administration of Tempol.
Moreover, this study only investigated male rats. Future studies must incorporate female animals to better understand the efficacy profile of Tempol. Additionally in our Tempol studies we have not established any of the molecular mechanisms by which readily cell-penetrant Tempol prevents and reverses the negative effects of fentanyl (Baby et al., 2021) and morphine (present study) on cardiorespiratory function, although published data suggests that it is unlikely to involve modulation of BKCa channels (Baby et al., 2021), or direct blockade of opioid receptors on the basis that Tempol spares morphine analgesia (Baby et al., 2021). With respect to potential plasma membrane and intracellular targets for Tempol, our collaborator Dr. Christopher Ellis (DEVCOM Chemical Biological Center, US Army) is employing phase (Public Health Assessment via Structural Evaluation) technology (Ellis et al., 2019) and molecular docking methods (Ellis et al., 2018) to identify protein sites (e.g., G-protein-coupled receptors, ion-channels, and membrane and intracellular enzymes) to which Tempol, morphine and fentanyl bind to, with emphasis on the signaling proteins/pathways most relevant to opioid-induced cardiorespiratory depression (Ellis et al., 2018). As mentioned previously (Baby et al., 2021), we will initially develop binding profiles for each drug of interest (e.g., Tempol, fentanyl) and then use phase to identify functionally relevant protein targets to develop potential insights into the mechanisms of action of Tempol. These studies will be augmented by molecular docking modeling approaches to predict binding affinities and displacement coefficients of the drugs at the sites identified in the first stage (phase computations) of these studies. This information from Dr. Ellis will play a vital role in our understanding of the functional interactions between Tempol and opioids with respect to protein targets and will directly inform future studies designed to optimize Tempol and derivatives to treat the negative effects of opioids while maintaining beneficial effects, such as analgesia.
Conclusion
The findings that Tempol reverses the negative effects of morphine on ABG chemistry and SpO2 in freely-moving Sprague Dawley rats is an important addition to the development of drugs to combat opioid-induced depression of respiratory and cardiovascular systems. From this and our earlier study (Baby et al., 2021) we now know that 1) Tempol is efficacious as a pretreatment against fentanyl (Baby et al., 2021), 2) Tempol is a reversal agent against morphine (present study), 3) Tempol is effective in isoflurane-anesthetized rats (Baby et al., 2021) and in freely-moving rats (present study), and 4) Tempol is efficacious against both fentanyl and morphine. Reversal strategies with a drug with the pharmacological profile of Tempol can be used in many clinical scenarios, such as in the operating room when the situation requires immediate and sustained reversal of opioid-induced respiratory depression without compromising analgesia, and in the increasingly greater set of scenarios when unintended/intended overdoses with an opioid occur. Future work involving elucidating the effects of Tempol on the intracellular signaling pathways activated by morphine and fentanyl, will entail examination of whether Tempol directly interacts with signaling pathways or modifies there activities indirectly by scavenging free radicals and/or superoxide anion. We will compare the efficacy of Tempol to free radical/superoxide anion scavengers with differing chemical structures using isothermal titration calorimetry on proteins within the morphine/fentanyl signaling pathway, high throughput screening methods including surface plasmon resonance and hydrogen deuterium exchange mass spectrometry (Marcsisin and Engen, 2010; Nguyen et al., 2015; Baranauskiene et al., 2019). These future studies are intended to define the precise signaling mechanisms by which Tempol prevents and reverses fentanyl- or morphine-induced depression of ventilatory function and arterial blood pressure without affecting analgesia/antinociception.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the IACUC Committee of Galleon Pharmaceuticals, Inc.
Author Contributions
The study was originated and constructed by SB, DD, and SL. Experimentation was done by SB, JD, and RG. The data were collated and statistically analyzed by SB, PG, DD, FC, and SL. The figures and tables were prepared by SB, PG, FC, and SL. All authors contributed to the writing of the original version of the manuscript and revision of the final document that was then submitted for publication.
Funding
These experiments were funded by grants from Galleon Pharmaceuticals, Inc. to SL and by a NIH/NIDA grant (0U1DA051373, Optimization of Novel Thiolesters as a Therapeutic Strategy for Combating Opioid Overdoses and Abuse) to SL. The leadership of Galleon Pharmaceuticals were not directly involved in this study as a commercial entity. Only the principal scientists of Galleon Pharmaceuticals, SB, JD, and RG, were involved in study design, collection, analysis, interpretation of data, writing of this article and the decision to submit it for publication.
Conflict of Interest
SB, JD, and RG were employed by Galleon Pharmaceuticals, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank the staff at the animal care facility at Galleon Pharmaceuticals, Inc., for their expert technical assistance. The authors also wish to thank Dr. James N. Bates (Department of Anesthesia, University of Iowa) for his assistance with clinical perspectives related to the findings and his helpful advice about the text provided in the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.749084/full#supplementary-material.
Abbreviations
A-a gradient, Alveolar-arterial gradient; ABG, arterial blood-gas chemistry; BKCa, Ca2+-activated K+-channels; DBP, diastolic arterial blood pressure; Tempol, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl; MAP, mean arterial blood pressure; SBP, systolic arterial blood pressure.
References
Afjal, M. A., Abdi, S. H., Sharma, S., Ahmad, S., Fatima, M., Dabeer, S., et al. (2019). Anti-inflammatory Role of Tempol (4-Hydroxy-2,2,6,6-Tetramethylpiperidin-1-Oxyl) in Nephroprotection. Hum. Exp. Toxicol. 38, 713–723. doi:10.1177/0960327119836203
Almeida, M. B., Costa-Malaquias, A., Nascimento, J. L., Oliveira, K. R., Herculano, A. M., and Crespo-Lopez, M. E. (2014). Therapeutic Concentration of Morphine Reduces Oxidative Stress in Glioma Cell Line. Braz. J. Med. Biol. Res. 47, 398–402. doi:10.1590/1414-431x20143697
Baby, S., Gruber, R., Discala, J., Puskovic, V., Jose, N., Cheng, F., et al. (2021). Systemic Administration of Tempol Attenuates the Cardiorespiratory Depressant Effects of Fentanyl. Front. Pharmacol. 12, 690407. doi:10.3389/fphar.2021.690407
Baby, S. M., Gruber, R. B., Young, A. P., MacFarlane, P. M., Teppema, L. J., and Lewis, S. J. (2018). Bilateral Carotid Sinus Nerve Transection Exacerbates Morphine-Induced Respiratory Depression. Eur. J. Pharmacol. 834, 17–29. doi:10.1016/j.ejphar.2018.07.018
Baker, R., Leichtling, G., Hildebran, C., Pinela, C., Waddell, E. N., Sidlow, C., et al. (2021). "Like Yin and Yang": Perceptions of Methamphetamine Benefits and Consequences Among People Who Use Opioids in Rural Communities. J. Addict. Med. 15, 34–39. doi:10.1097/ADM.0000000000000669
Baranauskiene, L., Kuo, T. C., Chen, W. Y., and Matulis, D. (2019). Isothermal Titration Calorimetry for Characterization of Recombinant Proteins. Curr. Opin. Biotechnol. 55, 9–15. doi:10.1016/j.copbio.2018.06.003
Behzadi, M., Joukar, S., and Beik, A. (2018). Opioids and Cardiac Arrhythmia: A Literature Review. Med. Princ. Pract. 27, 401–414. doi:10.1159/000492616
Bernardy, C. C. F., Zarpelon, A. C., Pinho-Ribeiro, F. A., Calixto-Campos, C., Carvalho, T. T., Fattori, V., et al. (2017). Tempol, a Superoxide Dismutase Mimetic Agent, Inhibits Superoxide Anion-Induced Inflammatory Pain in Mice. Biomed. Res. Int. 2017, 9584819. doi:10.1155/2017/9584819
Boom, M., Niesters, M., Sarton, E., Aarts, L., Smith, T. W., and Dahan, A. (2012). Non-analgesic Effects of Opioids: Opioid-Induced Respiratory Depression. Curr. Pharm. Des. 18, 5994–6004. doi:10.2174/138161212803582469
Chiarotto, G. B., Cartarozzi, L. P., Perez, M., Biscola, N. P., Spejo, A. B., Gubert, F., et al. (2019). Tempol Improves Neuroinflammation and Delays Motor Dysfunction in a Mouse Model (SOD1G93A) of ALS. J. Neuroinflammation. 16, 218. doi:10.1186/s12974-019-1598-x
Dahan, A., Aarts, L., and Smith, T. W. (2010). Incidence, Reversal, and Prevention of Opioid-Induced Respiratory Depression. Anesthesiology 112, 226–238. doi:10.1097/ALN.0b013e3181c38c25
Dahan, A., van der Schrier, R., Smith, T., Aarts, L., van Velzen, M., and Niesters, M. (2018). Averting Opioid-Induced Respiratory Depression without Affecting Analgesia. Anesthesiology 128, 1027–1037. doi:10.1097/ALN.0000000000002184
Dallas, M. L., Peers, C., Golder, F. J., Baby, S., Gruber, R., and MacIntyre, D. E. (2015). GAL-021 and GAL-160 Are Efficacious in Rat Models of Obstructive and Central Sleep Apnea and Inhibit BKCa in Isolated Rat Carotid Body Glomus Cells. Adv. Exp. Med. Biol. 860, 361–370. doi:10.1007/978-3-319-18440-1_41
Della Puppa, A., Ford-Rice, F., Snyder, F. R., Cone, E., and London, E. D. (1989). Time Course of Verapamil Interaction with Morphine Effects on Physiological Parameters in Rats. J. Pharm. Pharmacol. 41, 617–623. doi:10.1111/j.2042-7158.1989.tb06542.x
Ellis, C. R., Kruhlak, N. L., Kim, M. T., Hawkins, E. G., and Stavitskaya, L. (2018). Predicting Opioid Receptor Binding Affinity of Pharmacologically Unclassified Designer Substances Using Molecular Docking. PLoS One 13, e0197734. doi:10.1371/journal.pone.0197734
Ellis, C. R., Racz, R., Kruhlak, N. L., Kim, M. T., Hawkins, E. G., Strauss, D. G., et al. (2019). Assessing the Structural and Pharmacological Similarity of Newly Identified Drugs of Abuse to Controlled Substances Using Public Health Assessment via Structural Evaluation. Clin. Pharmacol. Ther. 106, 116–122. doi:10.1002/cpt.1418
Feuerstein, G. (1985). The Opioid System and central Cardiovascular Control: Analysis of Controversies. Peptides 6 Suppl 2 (Suppl. 2), 51–56. doi:10.1016/0196-9781(85)90134-2
Gaston, B., Baby, S. M., May, W. J., Young, A. P., Grossfield, A., Bates, J. N., et al. (2021). D-Cystine di(m)ethyl ester reverses the deleterious effects of morphine on ventilation and arterial blood gas chemistry while promoting antinociception. Sci. Rep. 11, 10038. doi:10.1038/s41598-021-89455-2
Golder, F. J., Dax, S., Baby, S. M., Gruber, R., Hoshi, T., Ideo, C., et al. (2015). Identification and Characterization of GAL-021 as a Novel Breathing Control Modulator. Anesthesiology 123, 1093–1104. doi:10.1097/ALN.0000000000000844
Grinnell, S. G., Majumdar, S., Narayan, A., Le Rouzic, V., Ansonoff, M., Pintar, J. E., et al. (2014). Pharmacologic Characterization in the Rat of a Potent Analgesic Lacking Respiratory Depression, IBNtxA. J. Pharmacol. Exp. Ther. 350, 710–718. doi:10.1124/jpet.114.213199
Henderson, F., May, W. J., Gruber, R. B., Discala, J. F., Puskovic, V., Young, A. P., et al. (2014). Role of central and Peripheral Opiate Receptors in the Effects of Fentanyl on Analgesia, Ventilation and Arterial Blood-Gas Chemistry in Conscious Rats. Respir. Physiol. Neurobiol. 191, 95–105. doi:10.1016/j.resp.2013.11.005
Jenkins, M. W., Khalid, F., Baby, S. M., May, W. J., Young, A. P., Bates, J. N., et al. (2021). Glutathione Ethyl Ester Reverses the Deleterious Effects of Fentanyl on Ventilation and Arterial Blood-Gas Chemistry while Prolonging Fentanyl-Induced Analgesia. Sci. Rep. 11, 6985. doi:10.1038/s41598-021-86458-x
Johnson, M. W., Mitch, W. E., and Wilcox, C. S. (1985). The Cardiovascular Actions of Morphine and the Endogenous Opioid Peptides. Prog. Cardiovasc. Dis. 27, 435–450. doi:10.1016/0033-0620(85)90004-0
Kaye, A. D., Hoover, J. M., Hoover, A. J., Ibrahim, I. N., Fox, C., Bajwa, A., et al. (2008). Morphine, Opioids, and the Feline Pulmonary Vascular Bed. Acta Anaesthesiol. Scand. 52, 931–937. doi:10.1111/j.1399-6576.2008.01595.x
Kim, H. K., Hwang, S. H., and Abdi, S. (2016). Tempol Ameliorates and Prevents Mechanical Hyperalgesia in a Rat Model of Chemotherapy-Induced Neuropathic Pain. Front. Pharmacol. 7, 532. doi:10.3389/fphar.2016.00532
Kotrly, K. J., Ebert, T. J., Vucins, E. J., Roerig, D. L., and Kampine, J. P. (1984). Baroreceptor Reflex Control of Heart Rate during Morphine Sulfate, Diazepam, N2O/O2 Anesthesia in Humans. Anesthesiology 61, 558–563. doi:10.1097/00000542-198411000-00015
Lee, J., Kim, M. S., Park, C., Jung, E. B., Choi, D. H., Kim, T. Y., et al. (2004). Morphine Prevents Glutamate-Induced Death of Primary Rat Neonatal Astrocytes through Modulation of Intracellular Redox. Immunopharmacol. Immunotoxicol. 26, 17–28. doi:10.1081/iph-120029941
Leventelis, C., Goutzourelas, N., Kortsinidou, A., Spanidis, Y., Toulia, G., Kampitsi, A., et al. (2019). Buprenorphine and Methadone as Opioid Maintenance Treatments for Heroin-Addicted Patients Induce Oxidative Stress in Blood. Oxid. Med. Cel Longev. 2019, 9417048. doi:10.1155/2019/9417048
Lewis, S. J., Whalen, E. J., Beltz, T. G., and Johnson, A. K. (1999). Effects of Chronic Lesions of the Anteroventral Third Ventricle Region on Baroreceptor Reflex Function in Conscious Rats. Brain Res. 835, 330–333. doi:10.1016/s0006-8993(99)01561-9
Macleod, J., Steer, C., Tilling, K., Cornish, R., Marsden, J., Millar, T., et al. (2019). Prescription of Benzodiazepines, Z-Drugs, and Gabapentinoids and Mortality Risk in People Receiving Opioid Agonist Treatment: Observational Study Based on the UK Clinical Practice Research Datalink and Office for National Statistics Death Records. Plos Med. 16, e1002965. doi:10.1371/journal.pmed.1002965
Marcsisin, S. R., and Engen, J. R. (2010). Hydrogen Exchange Mass Spectrometry: What Is It and What Can It Tell Us?. Anal. Bioanal. Chem. 397, 967–972. doi:10.1007/s00216-010-3556-4
May, W. J., Gruber, R. B., Discala, J. F., Puskovic, V., Henderson, F., Palmer, L. A., et al. (2013). Morphine Has Latent Deleterious Effects on the Ventilatory Responses to a Hypoxic challenge. Open J. Mol. Integr. Physiol. 3, 166–180. doi:10.4236/ojmip.2013.34022
McLeod, J. F., Leempoels, J. M., Peng, S. X., Dax, S. L., Myers, L. J., and Golder, F. J. (2014). GAL-021, a New Intravenous BKCa-Channel Blocker, Is Well Tolerated and Stimulates Ventilation in Healthy Volunteers. Br. J. Anaesth. 113, 875–883. doi:10.1093/bja/aeu182
Mendoza, J., Passafaro, R., Baby, S., Young, A. P., Bates, J. N., Gaston, B., et al. (2013). L-cysteine Ethyl Ester Reverses the Deleterious Effects of Morphine on, Arterial Blood-Gas Chemistry in Tracheotomized Rats. Respir. Physiol. Neurobiol. 189, 136–143. doi:10.1016/j.resp.2013.07.007
Motaghinejad, M., Karimian, M., Motaghinejad, O., Shabab, B., Yazdani, I., and Fatima, S. (2015). Protective Effects of Various Dosage of Curcumin against Morphine Induced Apoptosis and Oxidative Stress in Rat Isolated hippocampus. Pharmacol. Rep. 67, 230–235. doi:10.1016/j.pharep.2014.09.006
Nguyen, H. H., Park, J., Kang, S., and Kim, M. (2015). Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors (Basel). 15, 10481–10510. doi:10.3390/s150510481
Pichard, L. E., Crainiceanu, C. M., Pashai, P., Kostuk, E. W., Fujioka, A., and Shirahata, M. (2015). Role of BK Channels in Murine Carotid Body Neural Responses In Vivo. Adv. Exp. Med. Biol. 860, 325–333. doi:10.1007/978-3-319-18440-1_37
Roozekrans, M., van der Schrier, R., Okkerse, P., Hay, J., McLeod, J. F., and Dahan, A. (2014). Two Studies on Reversal of Opioid-Induced Respiratory Depression by BK-Channel Blocker GAL021 in Human Volunteers. Anesthesiology 121, 459–468. doi:10.1097/ALN.0000000000000367
Salman, I. M., Ameer, O. Z., McMurray, S., Giarola, A. S., Sridhar, A., Lewis, S. J., et al. (2020). Laterality Influences Central Integration of Baroreceptor Afferent Input in Male and Female Sprague Dawley Rats. Front. Physiol. 11, 499. doi:10.3389/fphys.2020.00499
Shanazari, A. A., Aslani, Z., Ramshini, E., and Alaei, H. (2011). Acute and Chronic Effects of Morphine on Cardiovascular System and the Baroreflexes Sensitivity during Severe Increase in Blood Pressure in Rats. ARYA Atheroscler. 7, 111–117.
Skrabalova, J., Karlovska, I., Hejnova, L., and Novotny, J. (2018). Protective Effect of Morphine against the Oxidant-Induced Injury in H9c2 Cells. Cardiovasc. Toxicol. 18, 374–385. doi:10.1007/s12012-018-9448-0
Thornhill, J. A., Townsend, C., and Gregor, L. (1989). Intravenous Morphine Infusion (IMF) to Drug-Naive, Conscious Rats Evokes Bradycardic, Hypotensive Effects, but Pressor Actions Are Elicited after IMF to Rats Previously Given Morphine. Can. J. Physiol. Pharmacol. 67, 213–222. doi:10.1139/y89-036
Thurston, C. L., Starnes, A., and Randich, A. (1993). Changes in Nociception, Arterial Blood Pressure and Heart Rate Produced by Intravenous Morphine in the Conscious Rat. Brain Res. 612, 70–77. doi:10.1016/0006-8993(93)91645-9
Toda, N., Kishioka, S., Hatano, Y., and Toda, H. (2009). Interactions between Morphine and Nitric Oxide in Various Organs. J. Anesth. 23, 554–568. doi:10.1007/s00540-009-0793-9
Torralva, R., Eshleman, A. J., Swanson, T. L., Schmachtenberg, J. L., Schutzer, W. E., Bloom, S. H., et al. (2020). Fentanyl but Not Morphine Interacts with Nonopioid Recombinant Human Neurotransmitter Receptors and Transporters. J. Pharmacol. Exp. Ther. 374, 376–391. doi:10.1124/jpet.120.265561
Torralva, R., and Janowsky, A. (2019). Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J. Pharmacol. Exp. Ther. 371, 453–475. doi:10.1124/jpet.119.258566
van der Schier, R., Roozekrans, M., van Velzen, M., Dahan, A., and Niesters, M. (2014). Opioid-induced Respiratory Depression: Reversal by Non-opioid Drugs. F1000prime Rep. 6, 79. doi:10.12703/P6-79
Viires, N., Sillye, G., Aubier, M., Rassidakis, A., and Roussos, C. (1983). Regional Blood Flow Distribution in Dog during Induced Hypotension and Low Cardiac Output. Spontaneous Breathing versus Artificial Ventilation. J. Clin. Invest. 72, 935–947. doi:10.1172/JCI111065
Wallenstein, S., Zucker, C. L., and Fleiss, J. L. (1980). Some Statistical Methods Useful in Circulation Research. Circ. Res. 47, 1–9. doi:10.1161/01.res.47.1.1
Wang, Y., Hai, B., Ai, L., Cao, Y., Li, R., Li, H., et al. (2018). Tempol Relieves Lung Injury in a Rat Model of Chronic Intermittent Hypoxia via Suppression of Inflammation and Oxidative Stress. Iran J. Basic Med. Sci. 21, 1238–1244. doi:10.22038/ijbms.2018.31716.7714
Warner, M., Trinidad, J. P., Bastian, B. A., Minino, A. M., and Hedegaard, H. (2016). Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2010-2014. Natl. Vital Stat. Rep. 65, 1–15.
Wilcox, C. S. (2010). Effects of Tempol and Redox-Cycling Nitroxides in Models of Oxidative Stress. Pharmacol. Ther. 126, 119–145. doi:10.1016/j.pharmthera.2010.01.003
Wilcox, C. S., and Pearlman, A. (2008). Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides. Pharmacol. Rev. 60, 418–469. doi:10.1124/pr.108.000240
Xu, H., Bian, X., Watts, S. W., and Hlavacova, A. (2005). Activation of Vascular BK Channel by Tempol in DOCA-Salt Hypertensive Rats. Hypertension 46, 1154–1162. doi:10.1161/01.HYP.0000186278.50275.fa
Xu, H., Fink, G. D., and Galligan, J. J. (2004). Tempol Lowers Blood Pressure and Sympathetic Nerve Activity but Not Vascular O2- in DOCA-Salt Rats. Hypertension 43, 329–334. doi:10.1161/01.HYP.0000112304.26158.5c
Keywords: tempol, morphine, blood-gas chemistry, arterial blood pressure, tissue oxygen saturation, rats
Citation: Baby SM, Discala JF, Gruber R, Getsy PM, Cheng F, Damron DS and Lewis SJ (2021) Tempol Reverses the Negative Effects of Morphine on Arterial Blood-Gas Chemistry and Tissue Oxygen Saturation in Freely-Moving Rats. Front. Pharmacol. 12:749084. doi: 10.3389/fphar.2021.749084
Received: 29 July 2021; Accepted: 03 September 2021;
Published: 22 September 2021.
Edited by:
Nazareno Paolocci, Johns Hopkins University, United StatesReviewed by:
Chin Moi Chow, The University of Sydney, AustraliaLu Qin, Penn State Milton S. Hershey Medical Center, United States
Copyright © 2021 Baby, Discala, Gruber, Getsy, Cheng, Damron and Lewis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen J. Lewis, sjl78@case.edu
†Present address: Santhosh M. Baby, Translational Sciences Treatment Discovery, Galvani Bioelectronics, Inc., 1250 S Collegeville Rd., Collegeville, Pennsylvania 19,426.Email: santhosh.m.baby@galvani.bio
 Santhosh M. Baby1†
Santhosh M. Baby1† Joseph F. Discala
Joseph F. Discala Paulina M. Getsy
Paulina M. Getsy Derek S. Damron
Derek S. Damron Stephen J. Lewis
Stephen J. Lewis