- 1Department of Vascular Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Cardiology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
About 230 million people worldwide suffer from peripheral arterial disease (PAD), and the prevalence is increasing year by year. Multiple risk factors, including smoking, dyslipidemia, diabetes, and hypertension, can contribute to the development of PAD. PAD is typically characterized by intermittent claudication and resting pain, and there is a risk of severe limb ischemia, leading to major adverse limb events, such as amputation. Currently, a major progress in the research field of the pathogenesis of vascular remodeling, including atherosclerosis and neointima hyperplasia has been made. For example, the molecular mechanisms of endothelial dysfunction and smooth muscle phenotype switching have been described. Interestingly, a series of focused studies on fibroblasts of the vessel wall has demonstrated their impact on smooth muscle proliferation and even endothelial function via cell-cell communications. In this review, we aim to focus on the functional changes of peripheral arterial cells and the mechanisms of the pathogenesis of PAD. At the same time, we summarize the progress of the current clinical treatment and potential therapeutic methods for PAD and shine a light on future perspectives.
Introduction
Peripheral arterial disease (PAD) is a kind of vascular disease in which the blood flow is restricted due to stenosis or obstruction of arteries, resulting in tissue ischemia. There are various disease included in PAD, such visceral artery aneurysm, arteritis, carotid artery stenosis and atherosclerosis obliterans (ASO). In this review, PAD mainly refers to ASO. With increasing incidence and mortality, PAD has affected more than 230 million people worldwide (Song et al., 2020). Traditional cardiovascular risk factors, including advanced age, hypertension, diabetes, and smoking, are strongly associated with the risk of PAD (Selvin and Erlinger, 2004; Allison et al., 2006; Fowkes et al., 2013; Whelton et al., 2018). Patients who suffer from PAD often have coronary or cerebrovascular disease as well. The typical symptom of PAD is intermittent claudication, mainly being manifested in the lameness of the lower limbs after walking for a certain distance. The above symptoms can be relieved by a short rest (Gerhard-Herman et al., 2017; Golledge, 2022). As the disease worsens, the patient develops resting pain (Jones and Farber, 2020). This is due to the aggravation of the lesion, resulting in occlusion of arteries, leading to ischemia and hypoxia of the skeletal muscle. After tissue ischemia, peripheral arteries display a positive enhancement of vascular remodeling, in which atherosclerosis is the main cause of peripheral vascular obstruction (Newman et al., 1993; Stock, 2022). On the other hand, ischemia can also trigger a series of cellular events that lead to peripheral vascular remodeling.
At the present, the exploration of the pathogenesis of peripheral artery disease is limited. We do not yet fully understand the complex cellular functional changes and the mechanisms underlying cell-to-cell interactions that drive PAD. Under physiological conditions, vascular cells, such as endothelial and smooth muscle cells (SMCs), and fibroblasts, are essential for maintaining stable vascular structure and function. When ischemia occurs, dysfunction of vascular cells leads to peripheral arterial remodeling. Currently, drug usage and revascularization approaches are the main treatments for PAD (Wiseman et al., 2017; Farb et al., 2021; Sogaard et al., 2021), although the effectiveness is limited. In this article, we aim to discuss the mechanism of peripheral vascular cell dysfunction under ischemic conditions, summarize the clinical treatment and potential therapeutic methods and highlight the potential future research focusing on PAD.
The role of endothelial cells in peripheral arterial disease
Vascular endothelium regulates the cellular and molecular transport between blood and tissues and plays an important role in maintaining the stability of vessel wall (Bazzoni and Dejana, 2004; Trimm and Red-Horse, 2022). Tissue ischemia leads to lower oxygen supply, free radical production, and other cellular response in most tissues. In response to tissue ischemia of the limb, the inflammatory response of endothelial cells (ECs) is closely related to the progression of vascular lesion development (Gimbrone and Garcia-Cardena, 2016; Duan et al., 2021). As a critical enhancer, tissue ischemia not only induced the expression of Vcam-1 and Icam-1 on ECs, but also upregulated the levels of inflammatory markers (IL-1β, IL-6 and TNF-α). Meanwhile, tissue ischemia also results in production of platelet-derived exosomes in serum that play an important role in a variety of pathological mechanisms (Li et al., 2017). Exosomes are mainly involved in immune response and cell-to-cell communication in the pathogenesis of atherosclerosis (Bu et al., 2021). As an intercellular communication carrier, exosomes can wrap miRNA for transfer. It has been found that miRNA regulate the expression of related target genes by matching the target sites of the 3′untranslated region (3′utr) of target genes (Ambros, 2004; Bartel, 2004). Evidence showed that ECs could endocytose exosomes to upregulate miR-25-3p, and suppress levels of α-smooth muscle actin (α-SMA), collagen I a1, Collagen III a1, IL-1β, IL-6, and TNF-α. miR-25-3p inhibits endothelial inflammation by inhibiting the expression of Adam10, and reducing the phosphorylation level of NF-kB signaling pathway (Yao et al., 2019). Exosomes containing miR-155 have been reported to transmigrate from smooth muscle cells (SMCs) to ECs, causing endothelial dysfunction and ultimately aggravating lesion formation of atherosclerosis (Zheng et al., 2017). Furthermore, miR-155 ameliorates ox-LDL-induced inflammation in human umbilical vein endothelial cells (HUVECs) by inhibiting SOCS1-dependent NF-kB signaling (Zhang et al., 2017), in which miR-19 attenuates endothelial dysfunction induced by ischemia by negatively regulating KLF10 and inhibiting TGF-β1/Smad signaling pathway (Xu et al., 2018). In addition, Jiang et al. (2019) found that miR-449a was significantly up-regulated in atherosclerosis lesions and also was able to promote proliferation and migration of ECs. Besides, it could also induce expression of α-SMA and Smad3, and inhibits E-cadherin expression in ECs. Eventually, it was found that miR-449a enhances the expression of VACM-1 and ICAM-1 induced by TNF-α in ECs (Jiang et al., 2019). These results indicate that miRNA is an important regulator of endothelial inflammation in response to ischemia, where exosomes are one of the important carriers for miRNA transport.
Ischemia-induced EC dysfunction can also lead to the overexpression of endothelin-1 (ET-1). As a potent vasoconstrictor, endothelin-1 (ET-1) is mainly secreted by ECs and interacts with two different G-protein-coupled receptors, ETA and ETB (Yanagisawa et al., 1988; Miyauchi and Masaki, 1999). SMCs express ETA and ETB receptors to mediate vasoconstriction, whereas ETB receptors on ECs mediate vasodilation through the release of nitric oxide (NO) and prostacyclin (Kirchengast and Munter, 1998; Luscher and Barton, 2000). ET-1 has been reported to play an important role in neointimal formation and atherosclerosis (Bacon et al., 1996; McKenna et al., 1998). ET-1 expression is upregulated in diseased vessels when stimulated by shear stress. It was found that loss of ET-1 was associated with decreased expression of VCAM-1 and MCP-1 in endothelial cells and decreased recruitment of inflammatory cells to the vessel wall (Anggrahini et al., 2009). In vitro studies also showed that ET-1 enhanced the expression of VCAM-1 in TNF-α-stimulated ECs (Ishizuka et al., 1999). ET-1 has been reported to induce proliferation of SMCs through ERK, p38-activated protein kinase and c-MyC-dependent signaling (Chen et al., 2006). ETA receptor signaling mediates SMC proliferation (Hafizi et al., 1999). Numerous studies revealed that ETA and ETB expression were decreased and SMC proliferation was attenuated in ET-1 null diseased vessels (Anggrahini et al., 2009). These results suggest that EC-derived endothelin-1 is involved in mediating SMCs proliferation and endothelial inflammation in a paracrine manner, thereby promoting vascular remodeling (Figure 1).
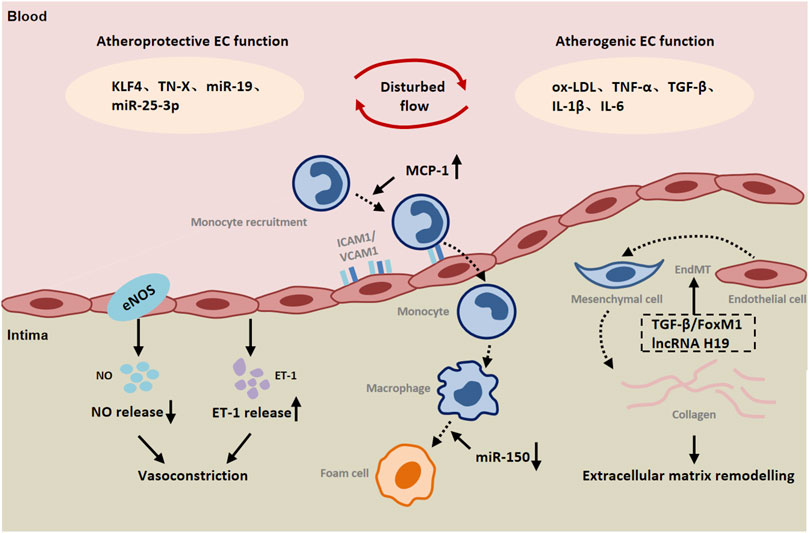
FIGURE 1. The role of endothelial cells (ECs) in peripheral arterial disease (PAD). Both atheroprotective and atherogenic mechanisms are operative in endothelial cells (ECs) exposed to disturbed flow. Endothelial cells are involved in communication with immune cells and smooth muscle cells by releasing a variety of inflammatory cytokines, chemokines and ET-1. Endothelial-to-mesenchymal transition (EndMT) contributes to vascular remodeling. Abbreviations: KLF4, Kruppel like factor 4; TN-X, Tenascin-X; oxLDL, oxidized low-density lipoprotein; IL-1β, interleukin 1 β; IL-6, interleukin 6; TGF-β, transforming growth factor-β; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; MCP-1, monocyte chemotactic protein-1; FoxM1, Forkhead Box M1; ET-1, endothelin-1.
Moreover, inflammation can induce endothelial-to-mesenchymal transition (EndMT), which leads to pathological vascular remodeling (Cho et al., 2018; Sanchez-Duffhues et al., 2019). EndMT is a phenomenon that ECs lose their endothelial markers and acquire mesenchymal characteristics. During EndMT, endothelial markers such as VE-cadherin and CD31 are lost, while the expression of mesenchymal markers such as fibroblast specific protein-1 (FSP-1), α-SMA, N-cadherin, and fibronectin is increased (Li et al., 2018; Kovacic et al., 2019). TGF-β is a key mediator of EndMT. Studies have shown that TGF-β promotes the phosphorylation of Smad2/3, and then regulates the expression of Snail, Twist, Slug and other transcription factors and promotes EndMT (Medici et al., 2011). Recently, increasing evidence highlights the potential role of Foxm1 in vascular fibrosis/remodeling (Balli et al., 2013). According to the report, TGF-β could induce the expression of Foxm1 in ECs. Foxm1 promoted the expression of TGF-β-induced vimentin, α-SMA and FSP1 in ECs through Smad2/3 signaling pathway, and inhibited the expression of VE-cadherin and CD31 (Song et al., 2019). FoxM1 has been reported to interact with Smad3 to maintain activation of the Smad2/Smad3/Smad4 complex in the nucleus. The complex translocated to the nucleus and activated the expression of ECM-related genes as well as Snail, and Twist genes (Xue et al., 2014; Sabbineni et al., 2018). It has been found that Snail inhibits VE-cadherin expression by directly binding to the E-box sequence within the VE-cadherin promoter (Cano et al., 2000). Activation of AMP-activated protein kinase (AMPK) upregulates the level of peroxidase proliferator-activated receptor-γcoactivator-1α (PGC1α), resulting in a decrease in phosphorylated Smad2 and the nuclear translocation of Snail, thereby inhibiting EndMT (Mao et al., 2022). The intracellular signaling pathway mediated by protein kinase Akt is closely related to the regulation of endothelial cell survival, proliferation, migration, glucose metabolism and gene expression (Shiojima and Walsh, 2002). Here, it was found that silencing Akt1 in endothelial cells resulted in increased expression of mesenchymal genes (e.g FN1 and KRT7) and decreased expression of eNOS, accompanied by activation of p38 MAP kinase. AKT1 can negatively regulate the expression of TGFβ2 and Smad2/3 phosphorylation, as well as the expression of Snail1 and FoxC2 (Sabbineni et al., 2019). In addition to the expression of mesenchymal markers, EndMT is also characterized by changes in EC morphology (Krenning et al., 2016). It was observed that normal ECs had a polygonal cobblestone shape, whereas AKT1-null ECs changed into a more spindle-like fibroblast shape. Inhibition of β-catenin reverses EndMT induced by loss of Akt1 (Sabbineni et al., 2019). Taken together, ischemia can directly and indirectly lead to endothelial-to-mesenchymal transition that plays a part in vascular remodeling in peripheral arteries.
It has been found that different forms of blood flow activate different signal transduction pathways in ECs, leading to vascular remodeling (Zhou et al., 2014; Nakajima and Mochizuki, 2017). In terms of peripheral arteries during ischemia, often there is atherosclerotic lesions resulting altered blood flow. Disturbed flow induces endothelium mesenchymal transition (EndMT). Unidirectional laminar flow inhibited TGF-β signaling as well as TGF-β-induced EndMT compared with disturbed flow (Moonen et al., 2015; Lee et al., 2017). The study found that laminar flow increased endothelial TN-X expression in a KLF4-dependent manner. TN-X expression in endothelial cells was negatively correlated with the expression of mesenchymal marker genes and inflammatory genes, such as FN1, CCL2, VACM-1, and the phosphorylation of Smad. TN-X directly interacts with TGF-β through its fibrin-like domain, thereby interfering with its binding to TGF-β receptors (Liang et al., 2022). Elevated TGF-β levels were also found in the plasma of TN-X deficient patients (Morissette et al., 2014). In addition, lncRNA H19 was significantly upregulated in TGF-β-induced EndMT. The expression of endothelial marker CD31 was up-regulated, and the inhibition of mesothelial cell marker FSP-1 was related to the inhibition of lncRNA H19. Moreover, the increased expression of lncRNA H19 could alleviate the inhibitory effect of miR-29a on EndMT-related gene expression (Shi et al., 2020). These results indicate that lncRNA H19 can compete with mir-29a and participate in the regulation of EndMT development through TGF-β/SMAD3 pathway (Figure 2).
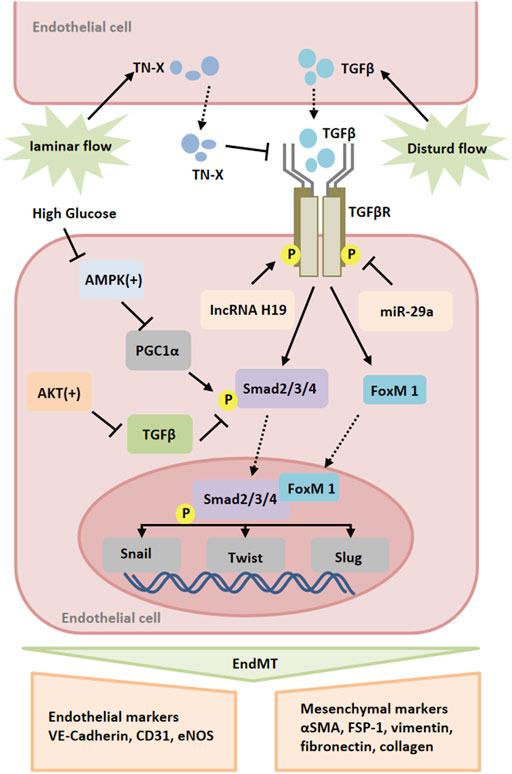
FIGURE 2. Signaling pathways of endothelial-to-mesenchymal transition (EndMT). Multiple stimuli, such as TGF and high glucose, induce the expression of transcription factors, such as Snail, Slug and Twist, which promote EndMT. These processes are regulated by various mediators, such as miR-25-3p, lncRNA H19, Smad2/3/4, FoxM1, AMPK and AKT. Abbreviations: AMPK, 5′-adenosine monophosphate (AMP)-activated protein kinase; FoxM1, Forkhead Box M1.
Tissue ischemia results in accumulation of free radicals locally, especially in peripheral arteries. In fact, oxidative stress is one of the important causes of vascular endothelial dysfunction (Cai et al., 2003). Elevated vascular reactive oxygen species (ROS) production is considered to be an early trigger mechanism for endothelial dysfunction, including oxidative stress, activation of proinflammatory signaling cascades, and reduction of endothelial nitric oxide (NO) bioavailability (Hsieh et al., 2014; Blaser et al., 2016; Wu et al., 2018). The NOX protein family contains several isoforms, including NOX1, NOX2, and NOX4, whose sole function is to produce ROS (Bedard and Krause, 2007). Under physiological conditions, NOX1 and NOX2 are expressed at low levels in the peripheral vascular, but up-regulated in pathological situations such as hypertension, diabetes and hyperlipidemia (Mollnau et al., 2002; Wendt et al., 2005). Increased NOX1 expression in ECs promotes the expression of MCP-1, VCAM-1, CTGF and collagen I/III/IV, which contributes to lesion formation (Gray et al., 2013). Moreover, there was a study demonstrates that NOX2 expression was upregulated in ECs of diseased vessels. Further studies revealed that decreased arterial superoxide production could significantly reduce lesion formation and increased NO bioavailability when NOX2 was deficient (Judkins et al., 2010). Endothelium-derived NO plays a protective role in vasculature by inhibiting the contraction and proliferation of vascular SMCs. NOX derived ROS can not only reduce the bioavailability of NO, but also directly react with NO to produce the oxidizing substance peroxynitrite (Judkins et al., 2010). Peroxynitrite has been reported to oxidize tetrahydrobiopterin (BH4), a cofactor of eNOS, leading to uncoupling of eNOS (Laursen et al., 2001). Compared with NOX1 and NOX2, NOX4 mainly plays a protective role in the cardiovascular system. NOX4 is predominantly expressed in ECs. Multiple recent reports indicate that Nox4 produces primarily H2O2 rather than superoxide (Schader et al., 2020). Low concentration of H2O2 contributes to vascular protection. H2O2 can not only maintain vasodilatation by activating protein kinase G, but also activate and induce eNOS expression (Indra et al., 1999; Garcia-Prieto et al., 2019). It has been found that H2O2 promoted vasodilation by enhancing Ca2+ release from endoplasmic reticulum stores, which in turn promoted the opening of Ca2+ -activated K+ channels (Hecquet et al., 2008). Katrin et al. proved that endogenous Nox4 accelerated the expression of eNOS, the formation of NO, and the expression of the antioxidant HO-1. HO-1 expression is controlled by the transcription factor Nrf-2. NOX4 reduces Nrf-2 degradation by producing H2O2, thereby promoting HO-1 expression. Loss of NOX4 not only exacerbates Ang II-induced vascular stenosis but also increases the rate of endothelial cell apoptosis, which is an important stimulator of vascular inflammation (Schroder et al., 2012). Moreover, NOX4 in endothelial cells can inhibit the expression of MCP-1, IL-1β, TGF-β and other proinflammatory factors, and limit vascular inflammation and macrophage infiltration. It has been reported that TGF-β/SMAD3 signaling pathway is involved in the regulation of CTGF expression and vascular fibrosis (Mao et al., 2020). The expression of EC-NOX4 was negatively correlated with the phosphorylation level of SMAD3 and the expression of CTGF (Gray et al., 2016). Thus, the balance between NOX-mediated free radical generation and eNOS pathway-generated NO production can be disturbed during ischemia, resulting endothelial dysfunction and eventually peripheral vascular remodeling.
The role of smooth muscle cells in peripheral arterial disease
In normal arteries, SMCs remain quiescent and highly differentiated. However, they have dedifferentiation potential and plasticity compared with skeletal muscle and cardiomyocytes. Under pathological conditions, SMCs can switch from a contractile to a synthetic phenotype, which is also known as SMC phenotypic switching. During SMC phenotypic transition, the cell loses specific markers, such as smooth muscle actin and SMMHC (Hao et al., 2003; Gomez and Owens, 2012). This type of SMCs serves as main player for intima hyperplasia that is a key event for peripheral vascular remodeling (Owens et al., 2004). In response to ischemia and ischemia-mediated cytokines released from ECs, SMC undergo metabolic reprogramming upon activation to rapidly provide high energy and meet the biosynthetic requirements for cell proliferation (Kim et al., 2017). Increased glycolysis is essential for bioenergetic transfer during SMC proliferation and migration (Park et al., 2021). Pyruvate kinase (PKM2) is an important regulatory element in glycolysis and is mainly expressed in highly proliferating cells, such as stem cells and tumor cells (Xie et al., 2016; Liu et al., 2017). Emerging studies show that PKM2 is activated and nuclear translocated in neointimal SMC during neointimal formation. Nuclear PKM2 interacts with STAT3 and promotes STAT3 phosphorylation, thereby regulating MEK5 transcription in SMCs (Jain et al., 2021). At the same time, Liao et al. (Liao et al., 2015) found that STAT3 can regulate SMC phenotypic switching by interacting with myocardin. Furthermore, PKM2 was found to interact with β-catenin and enhance the expression of cyclin D1, GLUT1, and LDHA (Jain et al., 2021). Up-regulation of cyclin D1 and LDHA plays a key role in cell cycle progression and lactate production (Kim et al., 2017). Overexpression of glucose transporter GLUT1 in SMCs mediates their proliferation and phenotypic switching by regulating glucose transport/glucose metabolism (Pyla et al., 2013). It has been found that lactic acid, as a metabolite of glycolysis, can promote the synthetic phenotype of SMCs. Lactate-induced phenotypic modulation of SMCs is mediated by monocarboxylic acid transporters and N-myc downstream regulated gene (Yang et al., 2017). These results indicate that energy metabolism is an important factor influencing SMC functional transformation during tissue ischemia leading to vascular remodeling.
Concerning the molecular mechanisms of SMC phenotype changes, epigenetic inheritance also plays an important role in the process (Alexander and Owens, 2012). Epigenetic inheritance refers to heritable changes in the function of a gene without changes in its DNA sequence, which ultimately lead to changes in the phenotype. Epigenetic regulatory mechanisms include DNA methylation, histone acetylation, and noncoding RNA (Yao et al., 2016; Carter and Zhao, 2021). Increased histone acetyltransferase (HAT) activity stimulated SM22α expression, while increased histone deacetylase (HDAC) inhibited SM22α expression (Gomez et al., 2015). PDGF-BB and oxidized phospholipids induce KLF4 to recruit HDAC in the CArG box region. KLF4, ELK-1 and HDAC form a complex, which reduces the histone H3 acetylation level in the SM22α promoter by binding to G/C repressor elements, resulting in down-regulation of SM22α expression (Salmon et al., 2012). MicroRNA also play a key role in the regulation of SMC phenotype, especially the miR-143/145 cluster, which is a potent promoter for the contraction of SMC phenotype. miR-145 maintained the contractile phenotype of SMCs by inducing the expression of contractile genes, while down-regulating the expression of KLF4 and Elk-1 (Cordes et al., 2009; Lovren et al., 2012). Therefore, the mechanism of SMC phenotype switching involves not only different signal pathways but also epigenetic modification that leads to changes of gene expression profile.
There is an animal model of arterial ligation where artery ischemia occurs. The study has shown that increased expression of CyPA secreted by SMCs leads to intimal hyperplasia by promoting cell proliferation and migration. In ischemic arteries, CyPA can induce the increase of VCAM-1 expression, thereby recruiting inflammatory cells to the vessel wall. Additionally, ROS produced locally by inflammatory cells will further promote the release of CyPA from SMCs, which will form a proinflammatory cycle that promotes vascular remodeling (Satoh et al., 2008). Moreover, activated SMCs synthesize and secrete growth factors, proinflammatory factors and matrix proteins to form an extracellular environment, thereby triggering the infiltration and activation of surrounding proinflammatory cells and progenitor cells and further aggravating intimal hyperplasia (Geary et al., 2002). Concomitantly, it was found that neointimal hyperplasia occurred where there were breaches in the internal elastic lamina, and speculated that elastin disruption would promote neointimal hyperplasia (Lin et al., 2021). Elastin depletion in SMCs promotes their transition from a contractile to a synthetic phenotype (Rebello et al., 2021). What’s more, extracellular matrix glycoprotein biglycan enhances vascular smooth muscle cell proliferation through cdk2- and p27-dependent pathways (Shimizu-Hirota et al., 2004). This may be a reactive response to ischemia, which triggers transcriptional and phenotypic changes in SMCs, causing them to migrate into the intima and proliferate.
As mentioned above, ischemia alters the balance of redox process in the arterial wall. Redox signaling helps maintain the SMC contractile phenotype and proliferative response associated with vascular remodeling (Tong et al., 2010; Tong et al., 2015). As a member of the p38MAPK family, MAPK14 is a redox regulatory element. MAPK14 is considered to be a key regulator of SMC inflammation, proliferation and migration. Previous studies have found that SMC-MAPK14 can regulate MKL1 nuclear translocation and is a negative regulator of SMC contractile phenotype (Long et al., 2013). Some studies showed that in the neointima of mouse carotid arteries, SMCs in the neointima region showed high expression level of MAPK14 protein, accompanied by a decrease in the expression of MYH11. NOX4 is a key mediator of MAPK14 regulatory of SMC contractile gene expression (Wu et al., 2019). At the same time, knockdown of MAPK14 in SMC would lead to down-regulation of PDGFα and PDGFβ expression, thereby inhibiting the proliferation of SMCs. SMC-MAPK14 in neointima activates proinflammatory gene programs through a p65/NF-KB-dependent pathway. MAPK14 phosphorylates p65, which induces the expression of proinflammatory genes such as IL6, IL8, and CXCL1. Interestingly, in the neointimal region, high expression of MAPK14 was also observed in some CD45-positive inflammatory cells in addition to SMCs. The infiltrating inflammatory cells interact with inflammatory SMCs, leaving the vascular in a “hyperinflammatory” state (Wu et al., 2019). These results suggest that high expression of MAPK14 in SMCs in neointimal region regulates the pro-proliferative and pro-inflammatory properties of SMCs through two different pathways. Taken together, these studies suggest that SMCs play a central role in driving lesion development and vascular remodeling via “phenotypic switching” (Figure 3).
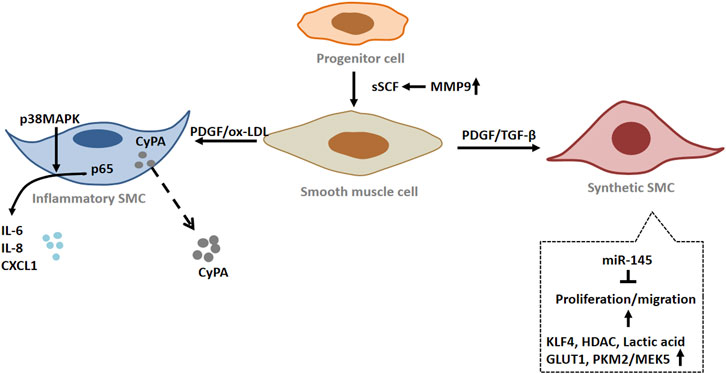
FIGURE 3. The role of smooth muscle cells (SMCs) in peripheral arterial disease (PAD). Under pathological conditions, smooth muscle cells (SMCs) undergo phenotypic switching. The proliferation and migration of SMC with synthetic phenotype were enhanced. Inflammatory SMCs produce and secrete a variety of inflammatory factors. When blood vessels are injured, vascular progenitor cells become activated and differentiate into SMCs. Abbreviations: SCF, stem cell factor; MMP9, matrix metalloproteinase 9; PDGF, platelet derived growth factor; IL-6, interleukin-6; IL-8, interleukin 8; CXCL1, C-X-C motif chemokine ligand 1; p38 MAPK, p38 mitogen activated protein kinase; HDAC, histone deacetylase; PKM2, pyruvate kinase M2; MEK5, mitogen-activated protein kinase 5; CyPA, cyclophilin A; p65, nuclear factor-kB p65; GLUT1, glucose transporter 1.
Mechanistically, there is a study found that stem cell factor (SCF)/c-Kit tyrosine kinase signaling pathway can regulate neointima formation. The investigators found that vascular injury not only resulted in local upregulation of membrane-bound SCF (mSCF) expression, but also increased circulating SCF (sSCF) levels. SCF not only promotes CD34 + cell mobilization, but also promotes progenitor adhesion to the exposed matrix on injured vessels, and mediates the differentiation of c-Kit + cells into SMCs (Wang et al., 2006). The researchers found that MMP-9 is activated and cleaves mSCF in response to vascular injury, increasing circulating SCF levels. Some studies have reported that activation of MMP-9 can also promote the release of soluble Kit ligand (sKitL), thus playing a role in progenitor mobilization (Heissig et al., 2002). By using genetic cell lineage tracing techniques, the direct evidence from our group demonstrated that c-Kit + cells can differentiate into SMCs in vitro and in vivo (Ni et al., 2019). In this aspect, detailed review has been published (Zhang et al., 2018). Thus, progenitor cells may be a source of SMCs during vascular remodelling.
The role of fibroblasts in peripheral arterial disease
Adventitia is the outermost connective tissue of blood vessels. In recent years, some reports have suggested that the adventitia is a key regulator that directly or indirectly modulates the structure and function of the vascular wall (Patel et al., 2000; Hu and Xu, 2011). Fibroblasts are the most important cell types in the adventitia of blood vessels, which may be the first cells of vascular to be activated in response to ischemia and hypoxia. When being activated, fibroblasts will produce more collagens, secret cytokines and be more actively proliferating (Anwar et al., 2012). Activation of fibroblasts by various stimuli can lead to differentiation of fibroblasts into a myofibroblast phenotype. The expression of α-SMA is an important signal for fibroblasts to differentiate into myofibroblasts. Myofibroblasts are key players in vascular remodeling due to their ability to respond promptly to changes in the local environment in vivo, including the production of extracellular matrix proteins, as well as various cytokines and ROS (Gabbiani, 2003). In addition, myofibroblasts can migrate from the adventitia to the media and even to the intima, thereby promoting vascular pathological remodeling (Li et al., 2000).
15-hydroxyeicosaenoic acid (15-HETE) is known as an important factor in vessel remodeling under ischemia/hypoxia, participating in the mediation of cell proliferation, migration and other biological processes (Ma et al., 2011). Zhang et al. (Zhang et al., 2016) found that 15-HETE induced fibroblasts to differentiate into myofibroblasts and promoted FGF-2 expression in adventitial fibroblasts by activating the p38MAPK/EGR-1 pathway. FGF2 not only increased the expression of α-SMA, but also promoted the proliferation of fibroblasts by inhibiting p27kip1 (Zhang et al., 2016). As a CDK inhibitor, p27kip1 negatively regulates the cell cycle by inhibiting cyclin activity and prevents the G1/S phase transition of cells, thereby inhibiting cell proliferation (Goncalves et al., 2015). Other studies have found that FGF-2 mediated cell phenotypic changes and increased nuclear FoxO3a through PI3K-Akt and ERK pathways during hypoxia-induced vascular remodeling (Khalil et al., 2005). At the same time, 15-HETE promotes the migration of adventitial fibroblasts and induces the up-regulation of STAT3 expression in fibroblasts. JNK1-mediated CREB activation was required for 15-HETE-induced STAT3 phosphorylation in VAFs. Phosphorylated STAT3 binds to the MMP2 promoter and induces the expression of MMP-2, thereby promoting the migration and phenotypic changes of fibroblasts (Zhang et al., 2015).
Fibroblast proliferation, migration, differentiation and collagen production are energy-dependent processes. Fatty acid oxidation is a process of energy metabolism that occurs in mitochondria by converting fatty acids into various products [e.g acetyl coenzyme A (acetyl-CoA)] to generate energy (Rodgers et al., 2020). Fatty acid binding protein 3 (FABP3) plays an important role in fatty acid oxidation, controlling fatty acid transport to maintain lipid metabolism and energy homeostasis (Storch and Corsico, 2008). An emerging study showed that FABP3 expression was increased in the adventitia of patients with arteritis and was positively correlated with adventitial fibroblast proliferation and ECM production such as FN1 and collagens (Wu et al., 2022). Elevated serum FABP3 levels have been reported to be associated with increased collagen1 expression and myocardial fibrosis (Maneikyte et al., 2020). Further studies revealed that FABP3 upregulation in adventitial fibroblast promoted the expression of carnitine palmitoyl transferase 1A (CPT1A) and carnitine/acylcarnitine carrier protein (CACT), two key enzymes in FAO, as well as the upregulation of adenosine triphosphate (ATP) (Wu et al., 2022). This suggests that FABP3-mediated progression of vascular fibrosis may be achieved by promoting fatty acid oxidation and ATP production in fibroblasts. Extracellular ATP plays an important role in vascular remodeling. It has been found that hypoxia stimulates the release of exogenous ATP, which can stimulate fibroblast proliferation by autocrine or paracrine pathways (Gerasimovskaya et al., 2002).
Adventitia plays a crucial role in the initiation and maintenance of vascular inflammation (Moreno et al., 2002). Vascular inflammation has traditionally been considered an inside-out response centered on the recruitment of leukocytes to the intima of vessels. However, a growing body of experimental evidence supports an outside-in hypothesis that vascular inflammation begins and persists in the adventitia and progresses toward the intima (Okamoto et al., 2001). After ischemia/hypoxia, activated adventitial fibroblasts promote the expression of chemokines and adhesion molecules and attract macrophages to the perivascular tissue and adventitia (Hu and Xu, 2011). There is a study revealed that JE/MCP-1 expression was up-regulated in fibroblasts at the early stage of atherosclerosis (Xu et al., 2007). Vascular endothelial growth factor (VEGF) is highly expressed in vascular endothelial cells and exerts its effects mainly through two tyrosine kinase receptors, Flt-1 and Flk-1 (Carmeliet, 2000). It has been found that Flt-1 is increased in adventitial fibroblasts of damaged arteries (Jin et al., 2007). Exogenous VEGF was found to regulate OPN protein expression by promoting ERK1/2 phosphorylation by acting on outer membrane fibroblast Flt-1. AF-derived OPN is a key mediator of VEGF/FLT-1-induced macrophage chemotaxis (Li et al., 2012). OPN can attract inflammatory cells to migrate and adhere to diseased blood vessels, and regulate cytokine production in macrophages, dendritic cells and T cells (Scatena et al., 2007). Further studies demonstrated that antagonistic Flt-1 could inhibit the proliferation of fibroblasts and neointima formation. It has been found that adventitia fibroblasts can migrate through the media to the neointima in damaged blood vessels. This implies that the contribution of Flt-1 to VEGF-mediated neointima formation may be due to inflammatory activation leading to adventitial cell proliferation and migration. In response to inflammatory stimulation, adventitial fibroblasts produce a large amount of NOX-derived ROS. Casino et al. found that adventitia-derived hydrogen peroxide enhanced vasoconstriction by activating p38 MAP kinase in medial SMC as well as inhibiting SHP-2 phosphorylation (Cascino et al., 2011). It was found that fibroblast-NOX2 modulates the growth of SMC in a GDF6-dependent manner. GDF6 is a member of TGF-β superfamily (Harrison et al., 2021) (Figure 4). We hypothesized that in vascular ischemia, leukocytes recruited by adventitial fibroblasts may infiltrate into the media through vasa vasorum, thereby promoting the development of inflammation. At the same time, fibroblasts are associated with peripheral vascular remodeling via secretion of cytokines that influence SMCs and even endothelium.
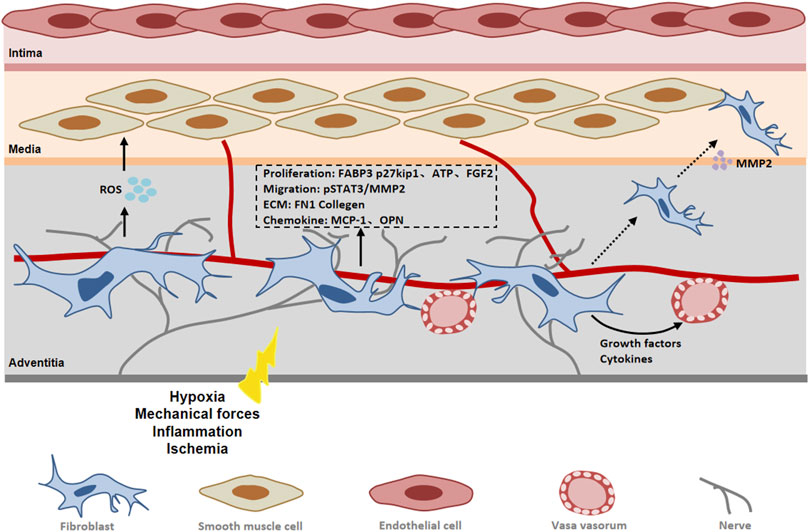
FIGURE 4. The role of fibroblast in peripheral arterial disease (PAD). In response to vascular injury, adventitial fibroblasts become activated and differentiate into myofibroblasts. Fibroblasts contribute to vascular remodeling through proliferation, migration and extracellular matrix synthesis. Abbreviations: FGF2, fibroblast growth factor 2; FABP3, fatty acid binding protein 3; MMP2, matrix metalloproteinase 2; pSTAT3, phosphorylated signal transducer and activator of transcription 3; ROS, reactive oxygen species; FN 1, fibronectin 1; OPN, osteopontin.
General treatment
Improved treatment for PAD must begin with lifestyle interventions, including smoking cessation, diet, and exercise. Smoking is an important risk factor for the development of PAD. It promotes thrombosis through effects on platelets, ECs, and the coagulation system by inducing oxidative stress and lowering nitric oxide (Celermajer et al., 1993; Anand, 2017). Therefore, quitting smoking can reduce the damage to vessels caused by nicotine in cigarettes and slow down the progression of PAD. Epidemiological studies connect healthy dietary patterns with lower incidence of clinical PAD. Improving the quality of diet is important for people with PAD, including fiber and fruit intake (Kulezic et al., 2019). Exercise therapy can maintain and improve the walking ability and quality of life of people with PAD through a series of supervised and systemic exercises (Hamburg and Balady, 2011). Supervised exercise training has been found to improve painless walking distance and maximum walking distance in patients with intermittent claudication (Parmenter et al., 2015). Another trial showed that weekly high-intensity home training, which stimulates moderate-to-severe ischemic leg pain, also significantly improved walking distance (McDermott et al., 2021). This suggests that PAD patients need to make exercise plans according to their own conditions and make timely adjustments to adapt to high-intensity exercise.
Therapy with drugs
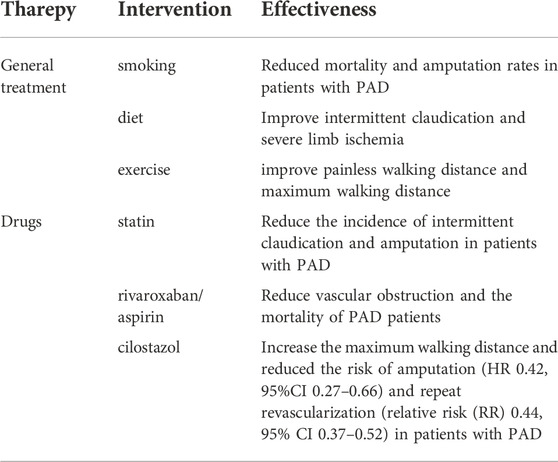
The American College of Cardiology (ACC)/American Heart Association (AHA) recommends high-strength statins for all patients with PAD (Gerhard-Herman et al., 2017). Statin therapy is the most recognized cholesterol-lowering drug in PAD drug therapy. Over the median 5.4-year follow-up period, the incidence of carotid murmurs and claudication was reduced in the simvastatin group (Pedersen et al., 1998). In a retrospective cohort study of diabetic patients with and without PAD, Sohn et al. (2013) compared outcomes in patients taking statins with those taking non-statin lipid-lowering drugs and found that statin therapy reduced amputation rates in patients with PAD. Anticoagulant therapy and antiplatelet therapy are also important to prevent vascular obstruction in patients with PAD. During a median of 23 months of follow-up, the combination of low-dose rivaroxaban and aspirin reduced mortality in patients with PAD as compared with aspirin alone (Eikelboom et al., 2021). In addition, Cilostazol has vasodilatation and antiplatelet function. By inhibiting phosphodiesterase activity in platelets and SMCs, cilostazol can increase the concentration of cAMP in platelets and SMCs and exert antiplatelet and vasodilatation effects (Hashimoto et al., 2006). There is a study showed that cilostazol not only significantly increased the maximum walking distance on the treadmill in PAD patients, but also reduced the risk of amputation (higher risk (HR) 0.42, 95% CI 0.27–0.66) and repeat revascularization (relative risk (RR) 0.44, 95% CI 0.37–0.52) in patients with PAD who underwent revascularization (Desai et al., 2021). Although these drug treatments can improve the symptoms of PAD, it is far from optimal. Understanding the molecular mechanism of the vascular disease development could offer some potential targets for the therapy (Table 1).
Summary and perspectives
In summary, the incidence of PAD is increasing year by year, which requires more clinical attention and early intervention. Current studies have shown that multiple mechanisms are involved in peripheral vascular remodeling in PAD, including EndMT, phenotypic transition of SMCs, and activation of fibroblasts. However, the current research is relatively limited, and we need to further explore the underlying mechanism. Firstly, endothelial functional changes in the development of PAD have not been fully understood, although some progress in the field was achieved, including NO/ROS balance, which was used as drugs for treatment of vascular disease. Current techniques of single cell RNA and protein sequencing combining with genetic approaches might be powerful tool for further understanding the mechanisms of cell dysfunctions. Secondly, for SMC biology, most studies focus on phenotype switching from contractile to secretary cells that proliferate and secrete matrix proteins. However, accumulating evidence indicates resident stem/progenitor cells may differentiate into SMCs where they participate in neointima hyperplasia (Wang et al., 2015). Finally, fibroblasts in the vessel wall have been paid attention recently. In this aspect, there is a large amount of work being to do. If researchers in these fields could make studies thoroughly, some major progress for the pathogenesis of PAD may be seen in near future.
At the present, the main treatment methods for PAD are drug therapy and surgery. However, patients with arterial occlusion who cannot undergo revascularization can only undergo amputation. In recent years, great progress has been made in the research of vascular stem cells. Animal studies have demonstrated that vascular stem cells can significantly improve blood supply to ischemic limbs. Novel therapies that promote tissue regeneration and stimulate vasculogenesis and angiogenesis are urgently needed for the treatment of the patients. Cell-based therapies have great potential for the treatment of ischemic diseases (Sprengers et al., 2008; Gupta and Losordo, 2011). Therefore, stem cell therapy could be a potential approach for the treatment of PAD in the future.
Author contributions
QX and YH conceived and designed the study. SL synthesized literature and drafted the review. HZ and RL participated in manuscript editing. All authors have read and approve the published version of the manuscript.
Funding
This study was supported by grants the National Natural Science Foundation of China (31830039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alexander, M. R., and Owens, G. K. (2012). Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 74, 13–40. doi:10.1146/annurev-physiol-012110-142315
Allison, M. A., Criqui, M. H., Mcclelland, R. L., Scott, J. M., Mcdermott, M. M., Liu, K., et al. (2006). The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 48, 1190–1197. doi:10.1016/j.jacc.2006.05.049
Anand, S. S. (2017). Smoking: A dual pathogen for arterial and venous thrombosis. Circulation 135, 17–20. doi:10.1161/CIRCULATIONAHA.116.025024
Anggrahini, D. W., Emoto, N., Nakayama, K., Widyantoro, B., Adiarto, S., Iwasa, N., et al. (2009). Vascular endothelial cell-derived endothelin-1 mediates vascular inflammation and neointima formation following blood flow cessation. Cardiovasc. Res. 82, 143–151. doi:10.1093/cvr/cvp026
Anwar, A., Li, M., Frid, M. G., Kumar, B., Gerasimovskaya, E. V., Riddle, S. R., et al. (2012). Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L1–L11. doi:10.1152/ajplung.00050.2012
Bacon, C. R., Cary, N. R., and Davenport, A. P. (1996). Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circ. Res. 79, 794–801. doi:10.1161/01.res.79.4.794
Balli, D., Ustiyan, V., Zhang, Y., Wang, I. C., Masino, A. J., Ren, X., et al. (2013). Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J. 32, 231–244. doi:10.1038/emboj.2012.336
Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 116, 281–297. doi:10.1016/s0092-8674(04)00045-5
Bazzoni, G., and Dejana, E. (2004). Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 84, 869–901. doi:10.1152/physrev.00035.2003
Bedard, K., and Krause, K. H. (2007). The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 87, 245–313. doi:10.1152/physrev.00044.2005
Blaser, H., Dostert, C., Mak, T. W., and Brenner, D. (2016). TNF and ROS crosstalk in inflammation. Trends Cell. Biol. 26, 249–261. doi:10.1016/j.tcb.2015.12.002
Bu, T., Li, Z., Hou, Y., Sun, W., Zhang, R., Zhao, L., et al. (2021). Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics 11, 9988–10000. doi:10.7150/thno.64229
Cai, H., Griendling, K. K., and Harrison, D. G. (2003). The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci. 24, 471–478. doi:10.1016/S0165-6147(03)00233-5
Cano, A., Perez-Moreno, M. A., Rodrigo, I., Locascio, A., Blanco, M. J., Del Barrio, M. G., et al. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell. Biol. 2, 76–83. doi:10.1038/35000025
Carmeliet, P. (2000). Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6, 389–395. doi:10.1038/74651
Carter, B., and Zhao, K. (2021). The epigenetic basis of cellular heterogeneity. Nat. Rev. Genet. 22, 235–250. doi:10.1038/s41576-020-00300-0
Cascino, T., Csanyi, G., Al Ghouleh, I., Montezano, A. C., Touyz, R. M., Haurani, M. J., et al. (2011). Adventitia-derived hydrogen peroxide impairs relaxation of the rat carotid artery via smooth muscle cell p38 mitogen-activated protein kinase. Antioxid. Redox Signal. 15, 1507–1515. doi:10.1089/ars.2010.3631
Celermajer, D. S., Sorensen, K. E., Georgakopoulos, D., Bull, C., Thomas, O., Robinson, J., et al. (1993). Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 88, 2149–2155. doi:10.1161/01.cir.88.5.2149
Chen, S., Qiong, Y., and Gardner, D. G. (2006). A role for p38 mitogen-activated protein kinase and c-myc in endothelin-dependent rat aortic smooth muscle cell proliferation. Hypertension 47, 252–258. doi:10.1161/01.HYP.0000198424.93598.6b
Cho, J. G., Lee, A., Chang, W., Lee, M. S., and Kim, J. (2018). Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front. Immunol. 9, 294. doi:10.3389/fimmu.2018.00294
Cordes, K. R., Sheehy, N. T., White, M. P., Berry, E. C., Morton, S. U., Muth, A. N., et al. (2009). miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460, 705–710. doi:10.1038/nature08195
Desai, K., Han, B., Kuziez, L., Yan, Y., and Zayed, M. A. (2021). Literature review and meta-analysis of the efficacy of cilostazol on limb salvage rates after infrainguinal endovascular and open revascularization. J. Vasc. Surg. 73, 711–721. e713. doi:10.1016/j.jvs.2020.08.125
Duan, H., Zhang, Q., Liu, J., Li, R., Wang, D., Peng, W., et al. (2021). Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol. Res. 168, 105599. doi:10.1016/j.phrs.2021.105599
Eikelboom, J. W., Bhatt, D. L., Fox, K. a. A., Bosch, J., Connolly, S. J., Anand, S. S., et al. (2021). Mortality benefit of rivaroxaban plus aspirin in patients with chronic coronary or peripheral artery disease. J. Am. Coll. Cardiol. 78, 14–23. doi:10.1016/j.jacc.2021.04.083
Farb, A., Malone, M., and Maisel, W. H. (2021). Drug-coated devices for peripheral arterial disease. N. Engl. J. Med. 384, 99–101. doi:10.1056/NEJMp2031360
Fowkes, F. G., Rudan, D., Rudan, I., Aboyans, V., Denenberg, J. O., Mcdermott, M. M., et al. (2013). Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 382, 1329–1340. doi:10.1016/S0140-6736(13)61249-0
Gabbiani, G. (2003). The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500–503. doi:10.1002/path.1427
Garcia-Prieto, C. F., Gil-Ortega, M., Plaza, A., Manzano-Lista, F. J., Gonzalez-Blazquez, R., Alcala, M., et al. (2019). Caloric restriction induces H2O2 formation as a trigger of AMPK-eNOS-NO pathway in obese rats: Role for CAMKII. Free Radic. Biol. Med. 139, 35–45. doi:10.1016/j.freeradbiomed.2019.05.016
Geary, R. L., Wong, J. M., Rossini, A., Schwartz, S. M., and Adams, L. D. (2002). Expression profiling identifies 147 genes contributing to a unique primate neointimal smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 22, 2010–2016. doi:10.1161/01.atv.0000038147.93527.35
Gerasimovskaya, E. V., Ahmad, S., White, C. W., Jones, P. L., Carpenter, T. C., and Stenmark, K. R. (2002). Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J. Biol. Chem. 277, 44638–44650. doi:10.1074/jbc.M203012200
Gerhard-Herman, M. D., Gornik, H. L., Barrett, C., Barshes, N. R., Corriere, M. A., Drachman, D. E., et al. (2017). 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: A report of the American College of Cardiology/American Heart association task force on clinical practice guidelines. Circulation 135, e726–e779. doi:10.1161/CIR.0000000000000471
Gimbrone, M. A., and Garcia-Cardena, G. (2016). Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636. doi:10.1161/CIRCRESAHA.115.306301
Golledge, J. (2022). Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 19, 456–474. doi:10.1038/s41569-021-00663-9
Gomez, D., and Owens, G. K. (2012). Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 95, 156–164. doi:10.1093/cvr/cvs115
Gomez, D., Swiatlowska, P., and Owens, G. K. (2015). Epigenetic control of smooth muscle cell identity and lineage memory. Arterioscler. Thromb. Vasc. Biol. 35, 2508–2516. doi:10.1161/ATVBAHA.115.305044
Goncalves, G. A., Camargo-Kosugi, C. M., Bonetti, T. C., Invitti, A. L., Girao, M. J., Silva, I. D., et al. (2015). p27kip1 overexpression regulates VEGF expression, cell proliferation and apoptosis in cell culture from eutopic endometrium of women with endometriosis. Apoptosis 20, 327–335. doi:10.1007/s10495-014-1079-8
Gray, S. P., Di Marco, E., Kennedy, K., Chew, P., Okabe, J., El-Osta, A., et al. (2016). Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 36, 295–307. doi:10.1161/ATVBAHA.115.307012
Gray, S. P., Di Marco, E., Okabe, J., Szyndralewiez, C., Heitz, F., Montezano, A. C., et al. (2013). NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127, 1888–1902. doi:10.1161/CIRCULATIONAHA.112.132159
Gupta, R., and Losordo, D. W. (2011). Cell therapy for critical limb ischemia: Moving forward one step at a time. Circ. Cardiovasc. Interv. 4, 2–5. doi:10.1161/CIRCINTERVENTIONS.110.960716
Hafizi, S., Allen, S. P., Goodwin, A. T., Chester, A. H., and Yacoub, M. H. (1999). Endothelin-1 stimulates proliferation of human coronary smooth muscle cells via the ET(A) receptor and is co-mitogenic with growth factors. Atherosclerosis 146, 351–359. doi:10.1016/s0021-9150(99)00178-1
Hamburg, N. M., and Balady, G. J. (2011). Exercise rehabilitation in peripheral artery disease: Functional impact and mechanisms of benefits. Circulation 123, 87–97. doi:10.1161/CIRCULATIONAHA.109.881888
Hao, H., Gabbiani, G., and Bochaton-Piallat, M. L. (2003). Arterial smooth muscle cell heterogeneity: Implications for atherosclerosis and restenosis development. Arterioscler. Thromb. Vasc. Biol. 23, 1510–1520. doi:10.1161/01.ATV.0000090130.85752.ED
Harrison, C. B., Trevelin, S. C., Richards, D. A., Santos, C. X. C., Sawyer, G., Markovinovic, A., et al. (2021). Fibroblast Nox2 (NADPH oxidase-2) regulates ANG II (angiotensin II)-Induced vascular remodeling and hypertension via paracrine signaling to vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 41, 698–710. doi:10.1161/ATVBAHA.120.315322
Hashimoto, A., Miyakoda, G., Hirose, Y., and Mori, T. (2006). Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis 189, 350–357. doi:10.1016/j.atherosclerosis.2006.01.022
Hecquet, C. M., Ahmmed, G. U., Vogel, S. M., and Malik, A. B. (2008). Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ. Res. 102, 347–355. doi:10.1161/CIRCRESAHA.107.160176
Heissig, B., Hattori, K., Dias, S., Friedrich, M., Ferris, B., Hackett, N. R., et al. (2002). Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 109, 625–637. doi:10.1016/s0092-8674(02)00754-7
Hsieh, H. J., Liu, C. A., Huang, B., Tseng, A. H., and Wang, D. L. (2014). Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 21, 3. doi:10.1186/1423-0127-21-3
Hu, Y., and Xu, Q. (2011). Adventitial biology: Differentiation and function. Arterioscler. Thromb. Vasc. Biol. 31, 1523–1529. doi:10.1161/ATVBAHA.110.221176
Indra, A. K., Warot, X., Brocard, J., Bornert, J. M., Xiao, J. H., Chambon, P., et al. (1999). Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible cre-ER(T) and cre-ER(T2) recombinases. Nucleic Acids Res. 27, 4324–4327. doi:10.1093/nar/27.22.4324
Ishizuka, T., Takamizawa-Matsumoto, M., Suzuki, K., and Kurita, A. (1999). Endothelin-1 enhances vascular cell adhesion molecule-1 expression in tumor necrosis factor alpha-stimulated vascular endothelial cells. Eur. J. Pharmacol. 369, 237–245. doi:10.1016/s0014-2999(99)00042-4
Jain, M., Dhanesha, N., Doddapattar, P., Nayak, M. K., Guo, L., Cornelissen, A., et al. (2021). Smooth muscle cell-specific PKM2 (pyruvate kinase muscle 2) promotes smooth muscle cell phenotypic switching and neointimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 41, 1724–1737. doi:10.1161/ATVBAHA.121.316021
Jiang, L., Hao, C., Li, Z., Zhang, P., Wang, S., Yang, S., et al. (2019). miR-449a induces EndMT, promotes the development of atherosclerosis by targeting the interaction between AdipoR2 and E-cadherin in Lipid Rafts. Biomed. Pharmacother. 109, 2293–2304. doi:10.1016/j.biopha.2018.11.114
Jin, X., Ge, X., Zhu, D. L., Yan, C., Chu, Y. F., Chen, W. D., et al. (2007). Expression and function of vascular endothelial growth factor receptors (Flt-1 and Flk-1) in vascular adventitial fibroblasts. J. Mol. Cell. Cardiol. 43, 292–300. doi:10.1016/j.yjmcc.2007.06.002
Jones, D. W., and Farber, A. (2020). Review of the global vascular guidelines on the management of chronic limb-threatening ischemia. JAMA Surg. 155, 161–162. doi:10.1001/jamasurg.2019.4928
Judkins, C. P., Diep, H., Broughton, B. R., Mast, A. E., Hooker, E. U., Miller, A. A., et al. (2010). Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am. J. Physiol. Heart Circ. Physiol. 298, H24–H32. doi:10.1152/ajpheart.00799.2009
Khalil, N., Xu, Y. D., O'connor, R., and Duronio, V. (2005). Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J. Biol. Chem. 280, 43000–43009. doi:10.1074/jbc.M510441200
Kim, J. H., Bae, K. H., Byun, J. K., Lee, S., Kim, J. G., Lee, I. K., et al. (2017). Lactate dehydrogenase-A is indispensable for vascular smooth muscle cell proliferation and migration. Biochem. Biophys. Res. Commun. 492, 41–47. doi:10.1016/j.bbrc.2017.08.041
Kirchengast, M., and Munter, K. (1998). Endothelin and restenosis. Cardiovasc. Res. 39, 550–555. doi:10.1016/s0008-6363(98)00143-6
Kovacic, J. C., Dimmeler, S., Harvey, R. P., Finkel, T., Aikawa, E., Krenning, G., et al. (2019). Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 190–209. doi:10.1016/j.jacc.2018.09.089
Krenning, G., Barauna, V. G., Krieger, J. E., Harmsen, M. C., and Moonen, J. R. (2016). Endothelial plasticity: Shifting phenotypes through force feedback. Stem Cells Int. 2016, 9762959. doi:10.1155/2016/9762959
Kulezic, A., Bergwall, S., Fatemi, S., Sonestedt, E., Zarrouk, M., Gottsater, A., et al. (2019). Healthy diet and fiber intake are associated with decreased risk of incident symptomatic peripheral artery disease - a prospective cohort study. Vasc. Med. 24, 511–518. doi:10.1177/1358863X19867393
Laursen, J. B., Somers, M., Kurz, S., Mccann, L., Warnholtz, A., Freeman, B. A., et al. (2001). Endothelial regulation of vasomotion in apoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103, 1282–1288. doi:10.1161/01.cir.103.9.1282
Lee, E. S., Boldo, L. S., Fernandez, B. O., Feelisch, M., and Harmsen, M. C. (2017). Suppression of TAK1 pathway by shear stress counteracts the inflammatory endothelial cell phenotype induced by oxidative stress and TGF-β1. Sci. Rep. 7, 42487. doi:10.1038/srep42487
Li, G., Chen, S. J., Oparil, S., Chen, Y. F., and Thompson, J. A. (2000). Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation 101, 1362–1365. doi:10.1161/01.cir.101.12.1362
Li, J., Tan, M., Xiang, Q., Zhou, Z., and Yan, H. (2017). Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb. Res. 154, 96–105. doi:10.1016/j.thromres.2017.04.016
Li, X. D., Chen, J., Ruan, C. C., Zhu, D. L., and Gao, P. J. (2012). Vascular endothelial growth factor-induced osteopontin expression mediates vascular inflammation and neointima formation via Flt-1 in adventitial fibroblasts. Arterioscler. Thromb. Vasc. Biol. 32, 2250–2258. doi:10.1161/ATVBAHA.112.255216
Li, Y., Lui, K. O., and Zhou, B. (2018). Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 15, 445–456. doi:10.1038/s41569-018-0023-y
Liang, G., Wang, S., Shao, J., Jin, Y. J., Xu, L., Yan, Y., et al. (2022). Tenascin-X mediates flow-induced suppression of EndMT and atherosclerosis. Circ. Res. 130, 1647–1659. doi:10.1161/CIRCRESAHA.121.320694
Liao, X. H., Wang, N., Zhao, D. W., Zheng, D. L., Zheng, L., Xing, W. J., et al. (2015). STAT3 protein regulates vascular smooth muscle cell phenotypic switch by interaction with myocardin. J. Biol. Chem. 290, 19641–19652. doi:10.1074/jbc.M114.630111
Lin, C. J., Hunkins, B. M., Roth, R. A., Lin, C. Y., Wagenseil, J. E., and Mecham, R. P. (2021). Vascular smooth muscle cell subpopulations and neointimal formation in mouse models of elastin insufficiency. Arterioscler. Thromb. Vasc. Biol. 41, 2890–2905. doi:10.1161/ATVBAHA.120.315681
Liu, F., Ma, F., Wang, Y., Hao, L., Zeng, H., Jia, C., et al. (2017). PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat. Cell. Biol. 19, 1358–1370. doi:10.1038/ncb3630
Long, X., Cowan, S. L., and Miano, J. M. (2013). Mitogen-activated protein kinase 14 is a novel negative regulatory switch for the vascular smooth muscle cell contractile gene program. Arterioscler. Thromb. Vasc. Biol. 33, 378–386. doi:10.1161/ATVBAHA.112.300645
Lovren, F., Pan, Y., Quan, A., Singh, K. K., Shukla, P. C., Gupta, N., et al. (2012). MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 126, S81–S90. doi:10.1161/CIRCULATIONAHA.111.084186
Luscher, T. F., and Barton, M. (2000). Endothelins and endothelin receptor antagonists: Therapeutic considerations for a novel class of cardiovascular drugs. Circulation 102, 2434–2440. doi:10.1161/01.cir.102.19.2434
Ma, C., Li, Y., Ma, J., Liu, Y., Li, Q., Niu, S., et al. (2011). Key role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in pulmonary vascular remodeling and vascular angiogenesis associated with hypoxic pulmonary hypertension. Hypertension 58, 679–688. doi:10.1161/HYPERTENSIONAHA.111.171561
Maneikyte, J., Bausys, A., Leber, B., Feldbacher, N., Hoefler, G., Kolb-Lenz, D., et al. (2020). Dietary Glycine prevents folfox chemotherapy-induced Heart injury: A colorectal cancer liver metastasis treatment model in rats. Nutrients 12, E2634. doi:10.3390/nu12092634
Mao, L., Liu, L., Zhang, T., Wu, X., Zhang, T., and Xu, Y. (2020). MKL1 mediates TGF-beta-induced CTGF transcription to promote renal fibrosis. J. Cell. Physiol. 235, 4790–4803. doi:10.1002/jcp.29356
Mao, W., Fan, Y., Wang, X., Feng, G., You, Y., Li, H., et al. (2022). Phloretin ameliorates diabetes-induced endothelial injury through AMPK-dependent anti-EndMT pathway. Pharmacol. Res. 179, 106205. doi:10.1016/j.phrs.2022.106205
Mcdermott, M. M., Spring, B., Tian, L., Treat-Jacobson, D., Ferrucci, L., Lloyd-Jones, D., et al. (2021). Effect of low-intensity vs high-intensity home-based walking exercise on walk distance in patients with peripheral artery disease: The LITE randomized clinical trial. JAMA 325, 1266–1276. doi:10.1001/jama.2021.2536
Mckenna, C. J., Burke, S. E., Opgenorth, T. J., Padley, R. J., Camrud, L. J., Camrud, A. R., et al. (1998). Selective ET(A) receptor antagonism reduces neointimal hyperplasia in a porcine coronary stent model. Circulation 97, 2551–2556. doi:10.1161/01.cir.97.25.2551
Medici, D., Potenta, S., and Kalluri, R. (2011). Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem. J. 437, 515–520. doi:10.1042/BJ20101500
Miyauchi, T., and Masaki, T. (1999). Pathophysiology of endothelin in the cardiovascular system. Annu. Rev. Physiol. 61, 391–415. doi:10.1146/annurev.physiol.61.1.391
Mollnau, H., Wendt, M., Szocs, K., Lassegue, B., Schulz, E., Oelze, M., et al. (2002). Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 90, E58–E65. doi:10.1161/01.res.0000012569.55432.02
Moonen, J. R., Lee, E. S., Schmidt, M., Maleszewska, M., Koerts, J. A., Brouwer, L. A., et al. (2015). Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc. Res. 108, 377–386. doi:10.1093/cvr/cvv175
Moreno, P. R., Purushothaman, K. R., Fuster, V., and O'connor, W. N. (2002). Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: Implications for plaque vulnerability. Circulation 105, 2504–2511. doi:10.1161/01.cir.0000017265.52501.37
Morissette, R., Merke, D. P., and Mcdonnell, N. B. (2014). Transforming growth factor-beta (TGF-beta) pathway abnormalities in tenascin-X deficiency associated with CAH-X syndrome. Eur. J. Med. Genet. 57, 95–102. doi:10.1016/j.ejmg.2013.12.004
Nakajima, H., and Mochizuki, N. (2017). Flow pattern-dependent endothelial cell responses through transcriptional regulation. Cell. Cycle 16, 1893–1901. doi:10.1080/15384101.2017.1364324
Newman, A. B., Siscovick, D. S., Manolio, T. A., Polak, J., Fried, L. P., Borhani, N. O., et al. (1993). Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. Cardiovascular Heart study (CHS) collaborative research group. Circulation 88, 837–845. doi:10.1161/01.cir.88.3.837
Ni, Z., Deng, J., Potter, C. M. F., Nowak, W. N., Gu, W., Zhang, Z., et al. (2019). Recipient c-kit lineage cells repopulate smooth muscle cells of transplant arteriosclerosis in mouse models. Circ. Res. 125, 223–241. doi:10.1161/CIRCRESAHA.119.314855
Okamoto, E., Couse, T., De Leon, H., Vinten-Johansen, J., Goodman, R. B., Scott, N. A., et al. (2001). Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation 104, 2228–2235. doi:10.1161/hc4301.097195
Owens, G. K., Kumar, M. S., and Wamhoff, B. R. (2004). Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801. doi:10.1152/physrev.00041.2003
Park, H. Y., Kim, M. J., Lee, S., Jin, J., Lee, S., Kim, J. G., et al. (2021). Inhibitory effect of a glutamine antagonist on proliferation and migration of VSMCs via simultaneous attenuation of glycolysis and oxidative phosphorylation. Int. J. Mol. Sci. 22, 5602. doi:10.3390/ijms22115602
Parmenter, B. J., Dieberg, G., Phipps, G., and Smart, N. A. (2015). Exercise training for health-related quality of life in peripheral artery disease: A systematic review and meta-analysis. Vasc. Med. 20, 30–40. doi:10.1177/1358863X14559092
Patel, S., Shi, Y., Niculescu, R., Chung, E. H., Martin, J. L., and Zalewski, A. (2000). Characteristics of coronary smooth muscle cells and adventitial fibroblasts. Circulation 101, 524–532. doi:10.1161/01.cir.101.5.524
Pedersen, T. R., Kjekshus, J., Pyorala, K., Olsson, A. G., Cook, T. J., Musliner, T. A., et al. (1998). Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). Am. J. Cardiol. 81, 333–335. doi:10.1016/s0002-9149(97)00904-1
Pyla, R., Poulose, N., Jun, J. Y., and Segar, L. (2013). Expression of conventional and novel glucose transporters, GLUT1, -9, -10, and -12, in vascular smooth muscle cells. Am. J. Physiol. Cell. Physiol. 304, C574–C589. doi:10.1152/ajpcell.00275.2012
Rebello, K. R., Lemaire, S. A., and Shen, Y. H. (2021). Aortic neointimal formation: The role of elastin in conjunction with vascular smooth muscle cell origin. Arterioscler. Thromb. Vasc. Biol. 41, 2906–2908. doi:10.1161/ATVBAHA.121.317021
Rodgers, L. C., Cole, J., Rattigan, K. M., Barrett, M. P., Kurian, N., Mcinnes, I. B., et al. (2020). The rheumatoid synovial environment alters fatty acid metabolism in human monocytes and enhances CCL20 secretion. Rheumatol. Oxf. 59, 869–878. doi:10.1093/rheumatology/kez378
Sabbineni, H., Verma, A., Artham, S., Anderson, D., Amaka, O., Liu, F., et al. (2019). Pharmacological inhibition of beta-catenin prevents EndMT in vitro and vascular remodeling in vivo resulting from endothelial Akt1 suppression. Biochem. Pharmacol. 164, 205–215. doi:10.1016/j.bcp.2019.04.016
Sabbineni, H., Verma, A., and Somanath, P. R. (2018). Isoform-specific effects of transforming growth factor beta on endothelial-to-mesenchymal transition. J. Cell. Physiol. 233, 8418–8428. doi:10.1002/jcp.26801
Salmon, M., Gomez, D., Greene, E., Shankman, L., and Owens, G. K. (2012). Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22α promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ. Res. 111, 685–696. doi:10.1161/CIRCRESAHA.112.269811
Sanchez-Duffhues, G., Garcia De Vinuesa, A., Van De Pol, V., Geerts, M. E., De Vries, M. R., Janson, S. G., et al. (2019). Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J. Pathol. 247, 333–346. doi:10.1002/path.5193
Satoh, K., Matoba, T., Suzuki, J., O'dell, M. R., Nigro, P., Cui, Z., et al. (2008). Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation 117, 3088–3098. doi:10.1161/CIRCULATIONAHA.107.756106
Scatena, M., Liaw, L., and Giachelli, C. M. (2007). Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 27, 2302–2309. doi:10.1161/ATVBAHA.107.144824
Schader, T., Lowe, O., Reschke, C., Malacarne, P., Hahner, F., Muller, N., et al. (2020). Oxidation of HDAC4 by Nox4-derived H2O2 maintains tube formation by endothelial cells. Redox Biol. 36, 101669. doi:10.1016/j.redox.2020.101669
Schroder, K., Zhang, M., Benkhoff, S., Mieth, A., Pliquett, R., Kosowski, J., et al. (2012). Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 110, 1217–1225. doi:10.1161/CIRCRESAHA.112.267054
Selvin, E., and Erlinger, T. P. (2004). Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the national health and nutrition examination survey, 1999-2000. Circulation 110, 738–743. doi:10.1161/01.CIR.0000137913.26087.F0
Shi, S., Song, L., Yu, H., Feng, S., He, J., Liu, Y., et al. (2020). Knockdown of LncRNA-H19 ameliorates kidney fibrosis in diabetic mice by suppressing miR-29a-mediated EndMT. Front. Pharmacol. 11, 586895. doi:10.3389/fphar.2020.586895
Shimizu-Hirota, R., Sasamura, H., Kuroda, M., Kobayashi, E., Hayashi, M., and Saruta, T. (2004). Extracellular matrix glycoprotein biglycan enhances vascular smooth muscle cell proliferation and migration. Circ. Res. 94, 1067–1074. doi:10.1161/01.RES.0000126049.79800.CA
Shiojima, I., and Walsh, K. (2002). Role of Akt signaling in vascular homeostasis and angiogenesis. Circ. Res. 90, 1243–1250. doi:10.1161/01.res.0000022200.71892.9f
Sogaard, M., Nielsen, P. B., Skjoth, F., Eldrup, N., and Larsen, T. B. (2021). Temporal changes in secondary prevention and cardiovascular outcomes after revascularization for peripheral arterial disease in Denmark: A nationwide cohort study. Circulation 143, 907–920. doi:10.1161/CIRCULATIONAHA.120.047994
Sohn, M. W., Meadows, J. L., Oh, E. H., Budiman-Mak, E., Lee, T. A., Stone, N. J., et al. (2013). Statin use and lower extremity amputation risk in nonelderly diabetic patients. J. Vasc. Surg. 58, 1578–1585. e1571. doi:10.1016/j.jvs.2013.06.069
Song, P., Fang, Z., Wang, H., Cai, Y., Rahimi, K., Zhu, Y., et al. (2020). Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet. Glob. Health 8, e721–e729. doi:10.1016/S2214-109X(20)30117-0
Song, S., Zhang, R., Cao, W., Fang, G., Yu, Y., Wan, Y., et al. (2019). Foxm1 is a critical driver of TGF-beta-induced EndMT in endothelial cells through Smad2/3 and binds to the Snail promoter. J. Cell. Physiol. 234, 9052–9064. doi:10.1002/jcp.27583
Sprengers, R. W., Lips, D. J., Moll, F. L., and Verhaar, M. C. (2008). Progenitor cell therapy in patients with critical limb ischemia without surgical options. Ann. Surg. 247, 411–420. doi:10.1097/SLA.0b013e318153fdcb
Stock, J. K. (2022). Joint statement from the European Atherosclerosis Society and European Society of Vascular Medicine focuses on patients with peripheral arterial disease. Atherosclerosis 340, 48–50. doi:10.1016/j.atherosclerosis.2021.11.015
Storch, J., and Corsico, B. (2008). The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 28, 73–95. doi:10.1146/annurev.nutr.27.061406.093710
Tong, X., Hou, X., Jourd'heuil, D., Weisbrod, R. M., and Cohen, R. A. (2010). Upregulation of Nox4 by TGF{beta}1 oxidizes SERCA and inhibits NO in arterial smooth muscle of the prediabetic Zucker rat. Circ. Res. 107, 975–983. doi:10.1161/CIRCRESAHA.110.221242
Tong, X., Khandelwal, A. R., Qin, Z., Wu, X., Chen, L., Ago, T., et al. (2015). Role of smooth muscle Nox4-based NADPH oxidase in neointimal hyperplasia. J. Mol. Cell. Cardiol. 89, 185–194. doi:10.1016/j.yjmcc.2015.11.013
Trimm, E., and Red-Horse, K. (2022). Vascular endothelial cell development and diversity. Nat. Rev. Cardiol., 1–14. doi:10.1038/s41569-022-00770-1
Wang, C. H., Anderson, N., Li, S. H., Szmitko, P. E., Cherng, W. J., Fedak, P. W., et al. (2006). Stem cell factor deficiency is vasculoprotective: Unraveling a new therapeutic potential of imatinib mesylate. Circ. Res. 99, 617–625. doi:10.1161/01.RES.0000243210.79654.fd
Wang, G., Jacquet, L., Karamariti, E., and Xu, Q. (2015). Origin and differentiation of vascular smooth muscle cells. J. Physiol. 593, 3013–3030. doi:10.1113/JP270033
Wendt, M. C., Daiber, A., Kleschyov, A. L., Mulsch, A., Sydow, K., Schulz, E., et al. (2005). Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic. Biol. Med. 39, 381–391. doi:10.1016/j.freeradbiomed.2005.03.020
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Collins, K. J., Dennison Himmelfarb, C., et al. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart association task force on clinical practice guidelines. Circulation 138, e426–e483. doi:10.1161/CIR.0000000000000597
Wiseman, J. T., Fernandes-Taylor, S., Saha, S., Havlena, J., Rathouz, P. J., Smith, M. A., et al. (2017). Endovascular versus open revascularization for peripheral arterial disease. Ann. Surg. 265, 424–430. doi:10.1097/SLA.0000000000001676
Wu, S., Kong, X., Sun, Y., Dai, X., Yu, W., Chen, R., et al. (2022). FABP3 overexpression promotes vascular fibrosis in Takayasu's arteritis by enhancing fatty acid oxidation in aorta adventitial fibroblasts. Rheumatol. Oxf. 61, 3071–3081. doi:10.1093/rheumatology/keab788
Wu, W., Zhang, W., Choi, M., Zhao, J., Gao, P., Xue, M., et al. (2019). Vascular smooth muscle-MAPK14 is required for neointimal hyperplasia by suppressing VSMC differentiation and inducing proliferation and inflammation. Redox Biol. 22, 101137. doi:10.1016/j.redox.2019.101137
Wu, X., Zhang, H., Qi, W., Zhang, Y., Li, J., Li, Z., et al. (2018). Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell. Death Dis. 9, 171. doi:10.1038/s41419-017-0257-3
Xie, M., Yu, Y., Kang, R., Zhu, S., Yang, L., Zeng, L., et al. (2016). PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 7, 13280. doi:10.1038/ncomms13280
Xu, F., Ji, J., Li, L., Chen, R., and Hu, W. C. (2007). Adventitial fibroblasts are activated in the early stages of atherosclerosis in the apolipoprotein E knockout mouse. Biochem. Biophys. Res. Commun. 352, 681–688. doi:10.1016/j.bbrc.2006.11.073
Xu, Y. L., Zhang, M. H., Guo, W., Xue, Y., Du, X., Zhang, T., et al. (2018). MicroRNA-19 restores vascular endothelial cell function in lower limb ischemia-reperfusion injury through the KLF10-dependent TGF-β1/Smad signaling pathway in rats. J. Cell. Biochem. 119, 9303–9315. doi:10.1002/jcb.27207
Xue, J., Lin, X., Chiu, W. T., Chen, Y. H., Yu, G., Liu, M., et al. (2014). Sustained activation of SMAD3/SMAD4 by FOXM1 promotes TGF-beta-dependent cancer metastasis. J. Clin. Invest. 124, 564–579. doi:10.1172/JCI71104
Yanagisawa, M., Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., et al. (1988). A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332, 411–415. doi:10.1038/332411a0
Yang, L., Gao, L., Nickel, T., Yang, J., Zhou, J., Gilbertsen, A., et al. (2017). Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ. Res. 121, 1251–1262. doi:10.1161/CIRCRESAHA.117.311819
Yao, B., Christian, K. M., He, C., Jin, P., Ming, G. L., and Song, H. (2016). Epigenetic mechanisms in neurogenesis. Nat. Rev. Neurosci. 17, 537–549. doi:10.1038/nrn.2016.70
Yao, Y., Sun, W., Sun, Q., Jing, B., Liu, S., Liu, X., et al. (2019). Platelet-derived exosomal MicroRNA-25-3p inhibits coronary vascular endothelial cell inflammation through Adam10 via the NF-κB signaling pathway in ApoE-/- mice. Front. Immunol. 10, 2205. doi:10.3389/fimmu.2019.02205
Zhang, H., Zhao, Z., Pang, X., Yang, J., Yu, H., Zhang, Y., et al. (2017). Genistein protects against ox-LDL-induced inflammation through MicroRNA-155/SOCS1-mediated repression of NF-kB signaling pathway in HUVECs. Inflammation 40, 1450–1459. doi:10.1007/s10753-017-0588-3
Zhang, L., Chen, Y., Li, G., Chen, M., Huang, W., Liu, Y., et al. (2016). TGF-β1/FGF-2 signaling mediates the 15-HETE-induced differentiation of adventitial fibroblasts into myofibroblasts. Lipids Health Dis. 15, 2. doi:10.1186/s12944-015-0174-3
Zhang, L., Issa Bhaloo, S., Chen, T., Zhou, B., and Xu, Q. (2018). Role of resident stem cells in vessel formation and arteriosclerosis. Circ. Res. 122, 1608–1624. doi:10.1161/CIRCRESAHA.118.313058
Zhang, L., Li, Y., Liu, Y., Wang, X., Chen, M., Xing, Y., et al. (2015). STAT3-mediated MMP-2 expression is required for 15-HETE-induced vascular adventitial fibroblast migration. J. Steroid Biochem. Mol. Biol. 149, 106–117. doi:10.1016/j.jsbmb.2015.01.015
Zheng, B., Yin, W. N., Suzuki, T., Zhang, X. H., Zhang, Y., Song, L. L., et al. (2017). Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol. Ther. 25, 1279–1294. doi:10.1016/j.ymthe.2017.03.031
Zhou, J., Li, Y. S., and Chien, S. (2014). Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler. Thromb. Vasc. Biol. 34, 2191–2198. doi:10.1161/ATVBAHA.114.303422
Glossary
PAD peripheral arterial disease
ASO atherosclerosis obliterans
SMCs smooth muscle cells
ECs endothelial cells
α-SMA α-smooth muscle actin
HUVECs human umbilical vein endothelial cells
NO nitric oxide
EndMT endothelial-to-mesenchymal transition
AMPK AMP-activated protein kinase
eNOS endothelial nitric oxide synthase
ROS reactive oxygen species
HDAC histone deacetylase
15-HETE 15-hydroxyeicosaenoic acid
FABP3 fatty acid binding protein 3
CPT1A carnitine palmitoyl transferase 1A
CACT carnitine/acylcarnitine carrier protein
CXCL1 C-X-C motif chemokine ligand 1
PKM2 pyruvate kinase M2
CyPA cyclophilin A
GLUT1 glucose transporter 1
MMP2 matrix metalloproteinase 2
FN 1 fibronectin 1
ATP adenosine triphosphate
KLF4 Kruppel like factor 4
TN-X Tenascin-X
Ox-LDL oxidized low-density lipoprotein
IL-1β interleukin 1 β
IL-6 interleukin 6
IL-8 interleukin 8
ICAM-1 intercellular cell adhesionmolecule-1
VCAM-1 vascular cell adhesion molecule-1
MCP-1 monocyte chemotactic protein-1
FoxM1 Forkhead Box M1
ET-1 endothelin-1
SCF stem cell factor
MMP9 matrix metalloproteinase 9
PDGF platelet derived growth factor
TGF-β transforming growth factor-β
p38 MAPK p38 mitogen activated protein kinase
MEK5 mitogen-activated protein kinase 5
p65 nuclear factor-kB p65
FGF2 fibroblast growth factor 2
pSTAT3 phosphorylated signal transducer and activator of transcription 3
OPN osteopontin
Keywords: ischemia, endothelial cells, smooth muscle cells, fibroblasts, vascular disease
Citation: Lin S, Lin R, Zhang H, Xu Q and He Y (2022) Peripheral vascular remodeling during ischemia. Front. Pharmacol. 13:1078047. doi: 10.3389/fphar.2022.1078047
Received: 24 October 2022; Accepted: 21 November 2022;
Published: 01 December 2022.
Edited by:
Yan Yang, Southwestern Medical University, ChinaReviewed by:
Yao Lu, King’s College London, United KingdomMing Lei, University of Oxford, United Kingdom
Copyright © 2022 Lin, Lin, Zhang, Xu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingbo Xu, qingbo_xu@zju.edu.cn; Yangyan He, heyangyan19881022@163.com
 Shigang Lin
Shigang Lin Ruoran Lin
Ruoran Lin Hongkun Zhang
Hongkun Zhang Qingbo Xu
Qingbo Xu Yangyan He
Yangyan He