- 1Department of Clinical Pharmacy, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
- 2Department of Microbiology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
- 3Department of Clinical Pharmacy, Faculty of Clinical Pharmacy, Al Baha University, Al Baha, Saudi Arabia
- 4Department of Pharmaceutics, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
- 5Department of Infection Prevention and Control Program, Alnoor Specialist Hospital, Makkah, Saudi Arabia
- 6Infectious Diseases Department, Alnoor Specialist Hospital, Makkah, Saudi Arabia
- 7Plan and Research Department, General Directorate of Health Affairs of Makkah Region, Ministry of Health, Makkah, Saudi Arabia
- 8Medical Genetics Unit, Maternity and Children Hospital, Makkah Healthcare Cluster, Ministry of Health, Makkah, Saudi Arabia
- 9Department of Pharmacy Practice, Faculty of Pharmacy, Bahauddin Zakariya Univrsity, Multan, Pakistan
- 10Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 11Pharmaceutical Care Department, Alnoor Specialist Hospital, Ministry of Health, Makkah, Saudi Arabia
- 12General Department of Pharmaceutical Care, Ministry of Health, Riyadh, Saudi Arabia
- 13King Abdulaziz University, Jeddah, Saudi Arabia
- 14Usher Institute, The University of Edinburgh, Edinburgh, United Kingdom
Background: β-lactams remain the cornerstone of the empirical therapy to treat various bacterial infections. This systematic review aimed to analyze the data describing the dosing regimen of β-lactams.
Methods: Systematic scientific and grey literature was performed in accordance with Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The studies were retrieved and screened on the basis of pre-defined exclusion and inclusion criteria. The cohort studies, randomized controlled trials (RCT) and case reports that reported the dosing schedule of β-lactams are included in this study.
Results: A total of 52 studies met the inclusion criteria, of which 40 were cohort studies, 2 were case reports and 10 were RCTs. The majority of the studies (34/52) studied the pharmacokinetic (PK) parameters of a drug. A total of 20 studies proposed dosing schedule in pediatrics while 32 studies proposed dosing regimen among adults. Piperacillin (12/52) and Meropenem (11/52) were the most commonly used β-lactams used in hospitalized patients. As per available evidence, continuous infusion is considered as the most appropriate mode of administration to optimize the safety and efficacy of the treatment and improve the clinical outcomes.
Conclusion: Appropriate antibiotic therapy is challenging due to pathophysiological changes among different age groups. The optimization of pharmacokinetic/pharmacodynamic parameters is useful to support alternative dosing regimens such as an increase in dosing interval, continuous infusion, and increased bolus doses.
Introduction
In the past few years, increasing trend of antibiotic resistance challenges the efficacy of currently available antibiotics. It is because of the global dissemination of multi-drug resistant (MDR) microorganisms causing more than 23,000 death annually in the United States (Pacios et al., 2020). The higher mortality rates associated with methicillin-resistant Staphylococcus aureus (MRSA) were observed in East Africa (Wangai et al., 2019). A study reported that about 96,000 patients were died due to MDR infection in Southern Asia (Khan et al., 2016). Similarly, the morbidity rates associated with MDR are also high, particularly in low- and middle-income countries (LMICs) due to lack of resources, inadequate microbiological testing methods and treatment interventions (Atif et al., 2020). According to the Centers of Disease Control and Prevention (CDC), the economic burden associated with drug-resistant infections estimated US$3.5 billion annually (Caron et al., 2010). One of the major causes for the spread of these infections is the injudicious use of antibiotics. The injudicious use of antibiotics can contribute to increased mortality, morbidity, and overall healthcare costs (Lesprit and Brun-Buisson, 2008). The use of unnecessarily broad-spectrum antibiotics is common in empirical as well as targeted therapy (Mettler et al., 2007). Many healthcare professionals have limited knowledge regarding antibiotic use and resistance and do not follow guidelines. Expert-based strategies and policies regarding the initiation and implementation of an antibiotic stewardship program (ASP) are recommended by different organizations such as World Health Organization (WHO), CDC, and Infectious Diseases Society of America (IDSA) (van Limburg et al., 2014; Gross et al., 2019).
Antibiotic stewardship program (ASP) is one of the main effective approaches to promote the rational use of antibiotics and combat antibiotic resistance. ASP also helps to optimize the treatment of infectious diseases, improve prescribing behavior, ensure cost-effective therapy, minimize the side effects related to antibiotic use, including resistance (Pollack and Srinivasan, 2014). Lee and his colleagues implemented ASP in children’s hospital that results in the reduction of antibiotic acquisition costs of about US$200,000 (Lee et al., 2017). Data published on ASP in intensive care units have demonstrated significant improvement in antibiotic consumptions (Kaki et al., 2011; Haseeb et al., 2020; Haseeb et al., 2021a; Alghamdi et al., 2021). To optimize the antibiotic use, many strategies in ASP intervention including identification of patient with bacterial infection, appropriate selection of treatment using pharmacokinetics-pharmacodynamic (PK-PD) characteristic to optimize the antibiotic dosing and modalities, de-escalation of antibiotics and shortening of therapy duration were employed (Luyt et al., 2014).
Dose optimization includes optimization of antibiotic dosing based on patient characteristics (e.g., weight, age, renal/liver function), PK-PD parameters of the drug (e.g., concentration or time-dependent activity), and causative microorganisms (Haseeb et al., 2021b; Haseeb et al., 2022). An appropriate dosing is the mainstay of antibiotic therapy, which intensifies the PK and PD profiles of drugs and has a huge impact on therapeutic outcomes, dose-dependent toxicity as well as the emergence of antibiotic resistance (He et al., 2020). For instance, administering single dose of aminoglycosides instead of multiple doses not only improve bacterial eradication but also reduces the risk of ototoxicity and nephrotoxicity. The continuous infusion or prolonged/extended infusion of β-lactams instead of administering bolus is recommended as an advanced dose optimization strategy. This strategy not only improves therapeutic outcomes but also reduce the mortality rates for all patients infected with resistant pathogens. Multisite studies reported that for some particular antibiotics, PD profiles can be assessed to improve the efficacy by changing the mode of administration (Felton et al., 2012; Georges et al., 2012; Falagas et al., 2013). The awareness regarding how dosing strategies are employed is needed for the selection of appropriate antibiotics (Roberts et al., 2014).
The selection of dosing regimen for antibiotics are usually based on summary endpoints such as PK/PD indices and point estimates of effect in terms of MIC. Multisite site studies documented that antibiotics have been categorized in accordance with the relationship between effect and three PK/PD indices: 1) the ratio of the maximal unbound (free) drug concentration to MIC (fCmac/MIC), 2) the ratio of area under the drug-concentration-time curve to the MIC (fAUC/MIC), or 3) the percentage of a 24-h time interval that unbound drug concentration exceeds to MIC (ft>MIC) (Andes and Craig, 2002; MacGowan and Bowker, 2002; Nielsen et al., 2011). These indices are commonly utilized as targets in the dose selection process. In Monte Carlo simulations, between-patient variability in PK.PD parameters is considered and the probability of the target attainment (PTA) is estimated on the basis of stochastic simulations from the model (Mouton et al., 2004; Owens Jr et al., 2005). On the basis of existing literature, the activities of β-lactams antibiotics have been categorized as being dependent on the fT>MIC (Leggett et al., 1989; Gustafsson et al., 2001).
β-lactams (penicillins, cephalosporins and carbapenems) are broad-spectrum antibiotics that are used widely to treat various bacterial infections in healthcare settings particularly in intensive care units (ICUs) and may be targeted by antibiotic stewardship initiatives (Masich et al., 2018b). Optimal treatment needs appropriate dosage, modes of administration and dosing schedules (Delattre et al., 2017). The clinician’s knowledge concerning dose optimization of β-lactams is broadened but still faces some issues in implementing dosing-based approaches (Grupper et al., 2016). The initiation and implementation ASP and guidelines for β-lactams can improve the clinical outcomes and decrease the spread or emergence of antibiotic resistance (Hammond et al., 2019). Therefore, optimization of antibiotic therapy is an important consideration for clinician worldwide (Cotta et al., 2015). This systematic review was aimed to assess the data describing the dose optimization of β-lactams.
Materials and methods
Data sources and searches
We performed systematic scientific and grey literature search according to Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines from October 2021 to January 2022 (Moher et al., 2015). Two independent approaches were followed. Comprehensive grey literature and peer-reviewed literature were performed independently by two reviewers. The reference lists of the relevant articles and related reviews were also searched manually for additional studies. Complementary research was also performed to identify the most recent studies. The search items included “antibiotic” or “dose optimization” or “pharmacokinetic” or “pharmacodynamic” or “drug administration” or “β-lactams” or “penicillins” or “cephalosporins” or “carbapenems” or “ampicillin,” “amoxicillin,” or “piperacillin,” or “ceftriaxone” or “cefuroxime” or “cefixime” or “ceftaroline” or “ceftazidime” or “meropenem” or imipenem” or “doripenem” or “‘aztreonam.”
Inclusion and exclusion criteria
All the studies found were reviewed for eligibility. The studies retrieved from the aforementioned search strategies were combined and duplicates were removed. Full-text articles on dose optimization of antibiotics were included in this review. The inclusion criteria include articles written in English and published in peer-reviewed journals. Articles published after 2000 were included in this review to ensure the current dosing recommendation. However, the exclusion criteria were review articles, letters to editor, animal studies, no full-text availability, conference abstracts, and in vitro studies. Two reviewers screened titles and abstracts as per eligibility criteria to identify potential publications independently at first. Then full-text was assessed for final inclusion. The disagreements were resolved by discussion between 2 reviewers or by consulting third reviewers. The type of studies included were cohort study, case reports and randomized controlled trial (RCT).
Quality assessment
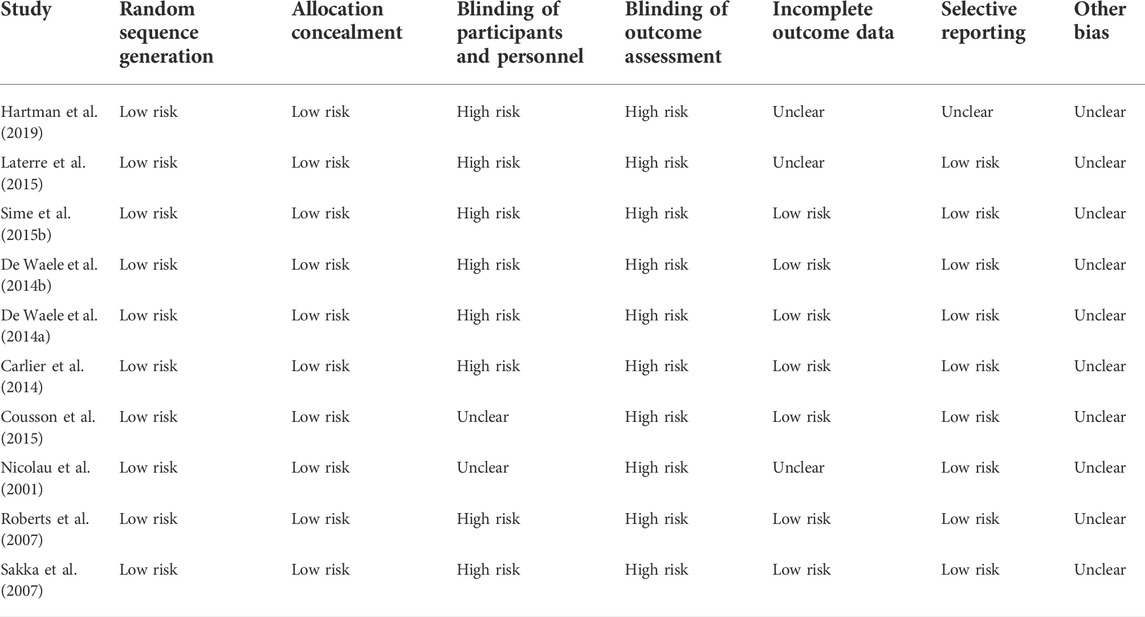
The quality assessment was carried out using New Castle-Ottawa Scale (NOS) scale for cohort studies and Cochrane bias tool for randomized controlled trials. The NOS scale categorizes the data into three subscales, i.e., selection, comparability and outcomes (Wells et al., 2014). However, the Cochrane assessment tool validates the randomized controlled studies (RCT) by assessing the risk of bias in each study (Higgins et al., 2019). This tool is structured into domains (random sequence generation, allocation concealment, blinding of patients and personnel, blinding of outcome assessment, incomplete outcome data and other bias) through which bias of each included study might be introduced in the results. The judgment is generally based on “high risk,” “low risk” and “unclear.” Each article was independently assessed by two experts. Reviewers compared their results and differences were then sorted by discussion.
Data extraction
The data was extracted from text, table and graph from each included study and was recorded in the pre-specified data collection form. This customized data form includes the following information; study characteristics (author’s name, year of publication, design, and sample size), patient characteristics (patient clinical condition, prescribed antibiotics, dosing regimen, outcomes of interests, and dosing recommendation). Data extraction was completed by one reviewer and it was then reviewed by another reviewer. Disagreements were addressed by discussion between two reviewers or consultation with the third reviewer if necessary.
Results
Characteristics of selected studies
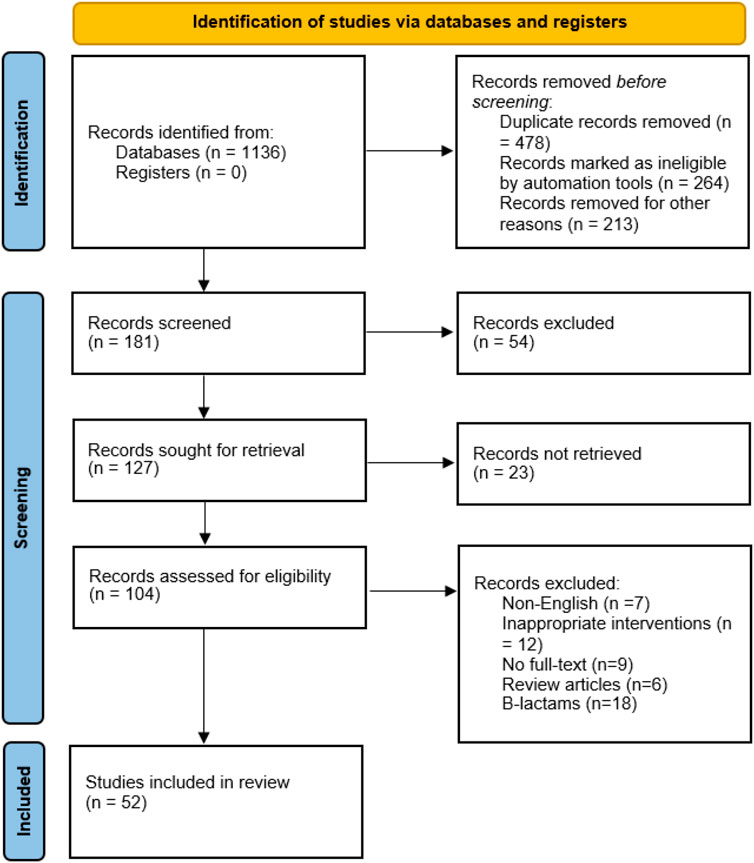
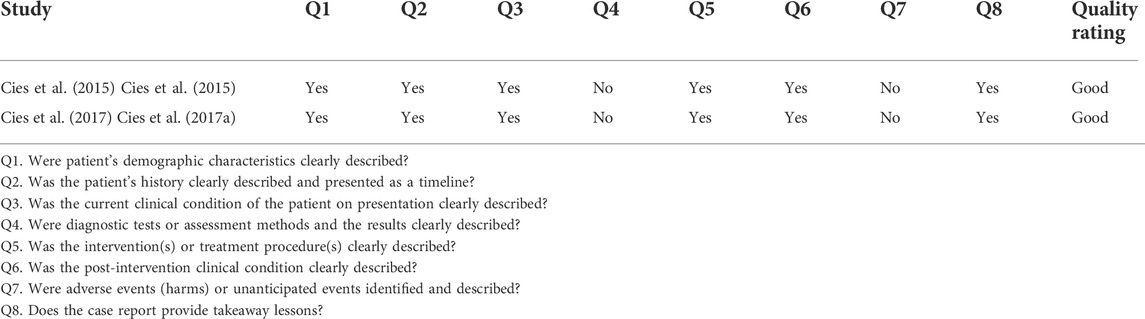
Of the 1,136 relevant published articles identified, 181 articles were initially proved eligible after duplicates were removed and abstracts screened. Various articles were retrieved from reference lists of the selected studies, other systematic reviews, and personal files. Majority of the studies were excluded some are Monte Carlo simulation studies where there were no patients involved. Of 127 articles, the data were not retrieved from 23 articles, therefore, excluded. After screening of articles, 104 articles met the eligibility criteria. A total of 52 studies were excluded due to following reasons: inappropriate intervention (N = 12), literature reviews (N = 6), non-English (N = 7), no full-text available (N = 9), and non-β-lactams (N = 18). The 52 articles met the inclusion criteria for this systematic review. The PRISMA flow diagram for studies selection is shown in the the Figure 1. Data extraction was performed for 47 full text articles with data on β-lactams. A complete list of all 47 articles and extracted Pk-data is presented in Tables 1, 2. All the 47 articles included were published in English of which 12 were RCT and 18 were cohort studies. The quality of case reports was not assessed because no validated tool is available. Therefore, we used Joanna Briggs institute (JBI) critical checklist for case reports (Ma et al., 2020).
Dose optimization of β-lactams in pediatrics
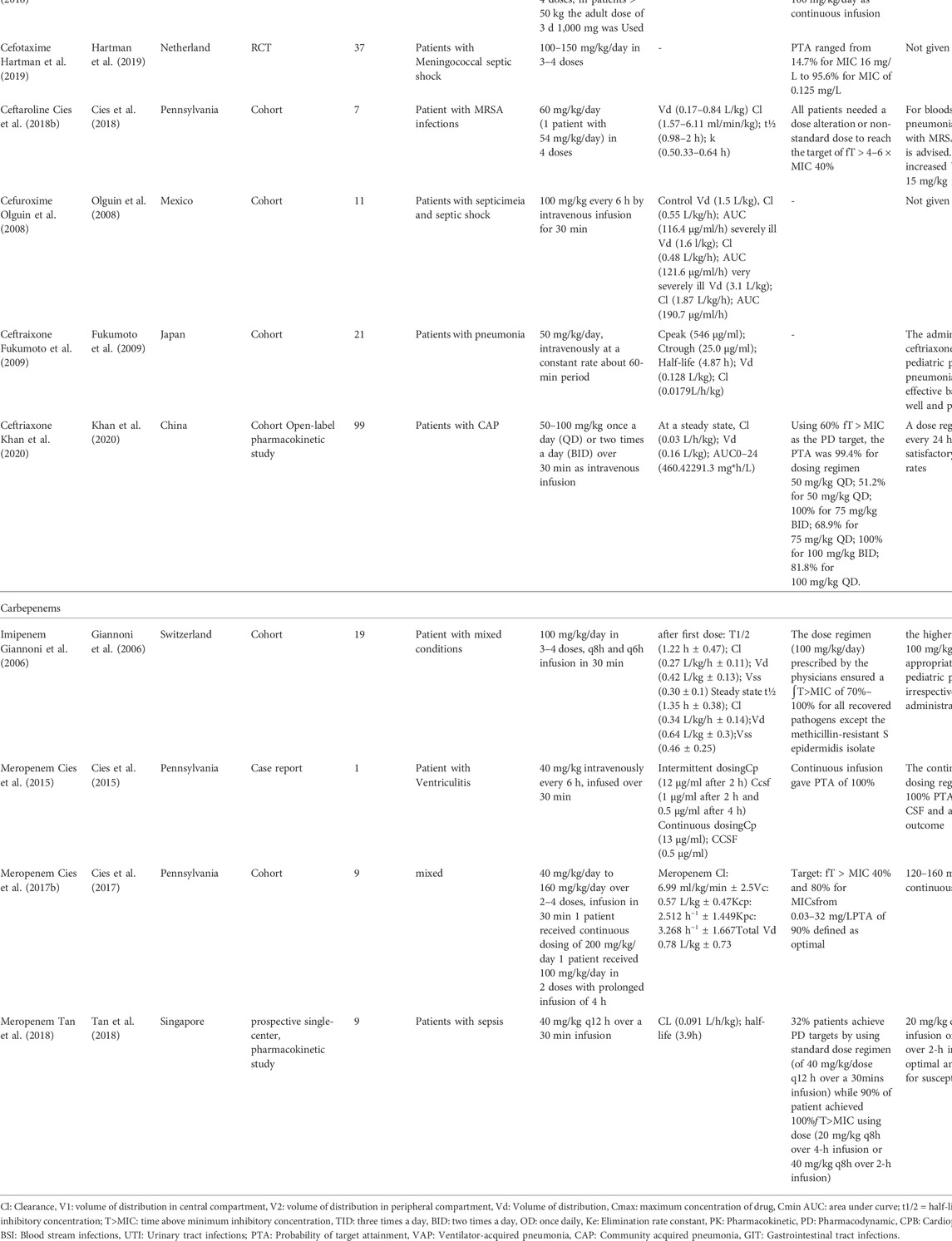
A total of twenty studies were reported among pediatrics (Table 1). Of 20 studies, eight studies were reported on penicillins (Cies et al., 2014; De Cock et al., 2015; Nichols et al., 2016; De Cock et al., 2017; Béranger et al., 2019; Tang et al., 2019; D’Agate et al., 2020; Wu et al., 2021), eight on cephalosporins (Olguin et al., 2008; Fukumoto et al., 2009; De Cock et al., 2016; Cies et al., 2018a; Béranger et al., 2018; Cies et al., 2019; Hartman et al., 2019) and 4 on carbapenems (Giannoni et al., 2006; Cies et al., 2015; Cies et al., 2017b; Tan et al., 2018) (Table 1). Four studies were reported on amoxicillin (De Cock et al., 2015; Tang et al., 2019; D’Agate et al., 2020; Wu et al., 2021). The normal dose for amoxicillin in selected studies ranged from 25 mg/kg to 125 mg/kg. Wu and his colleagues recommended to use other broad-spectrum antibiotic instead of amoxicillin for the treatment of E. coli infections. Another study reported that administration of dose (25 mg/kg every 6 h) of amoxicillin + clavulanic acid was stopped due to clinical failure in critically ill pediatrics with augmented renal functions (De Cock et al., 2015). The dose optimization and PK/PD parameters of piperacillin/tazobactam were discussed in four cohort studies (Cies et al., 2014; Nichols et al., 2016; De Cock et al., 2017; Béranger et al., 2019). The recommended dose of piperacillin range was from 150 mg/kg to 450 mg/kg. The continuous or extended infusion of piperacillin was shown to be effective in terms of safety and efficacy. De Cock et al. reported that loading dose followed by continuous infusion may improve the PD targets (De Cock et al., 2017).
Two cohort studies on cefazolin were reported in pediatrics (De Cock et al., 2016; Cies et al., 2019). The authors proposed a dose of 25 mg/kg by assessing the PK parameters using the Monta Simulation Model. The authors recommended that mixing cefazolin in the CPB circuit priming solution was effective in maintaining cefazolin serum concentration during surgery (De Cock et al., 2016). Two cefotaxime studies were included in this review (Béranger et al., 2018; Hartman et al., 2019). The recommended dose of cefotaxime ranges from 100 mg to 300 mg/kg as a continuous infusion that achieved 100% probability target attainment (PTA). Olguin et al. (2008) studied the PK parameters of cefuroxime on 11 patients with septicemia and septic shock. The authors recommended the dose of 100 mg/kg of body weight, administered every 6 h by intravenous infusion for 30 min. Cies et al. (2018a) discussed the PK-PD characteristics of Ceftaroline on 7 patients with MRSA infection. In this study, majority of the patients did not require additional alteration to achieve target attainment while a dose of 15 mg/kg was recommended for patients with increased volume of distribution.
One cohort study was reported on imipenem (Giannoni et al., 2006). All patients using dose regimen 100 mg/kg/day reached ∫T>MIC of 70%–100% for all isolated pathogens except methicillin-resistant staphylococcus epidermidis pathogen. Three studies were found reporting meropenem using the same dose (40 mg/kg) in pediatrics (Cies et al., 2015; Cies et al., 2017b). However, these studies recommended the continuous dosing regimen resulted in effective therapy.
Dose optimization of β-lactams in adults
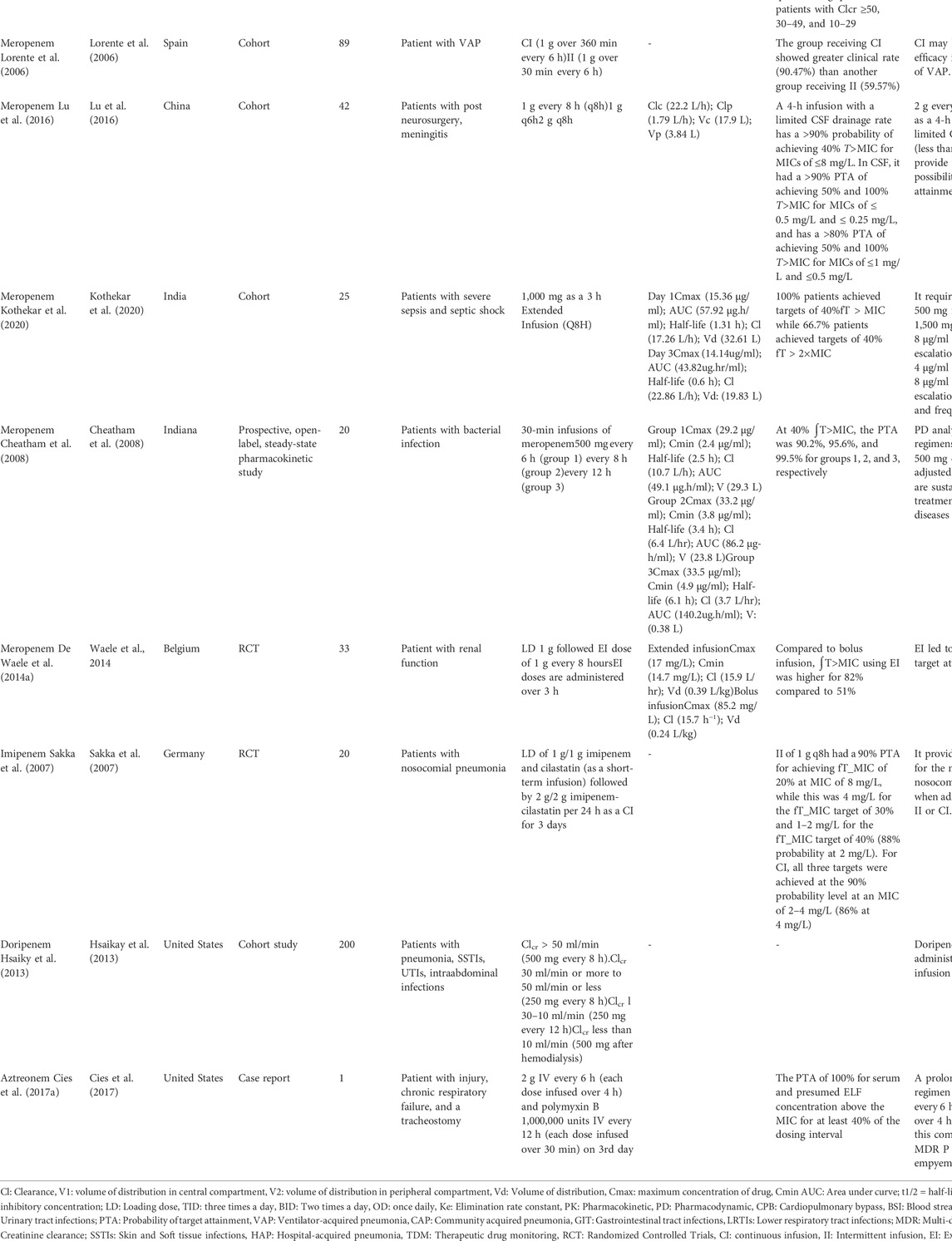
A total of 32 studies were reported in adults, of which 11 articles were on penicillins (Lodise et al., 2007a; Lorente et al., 2009; Roberts et al., 2010; Dow et al., 2011b; Yost and Cappelletty, 2011; De Waele J. et al., 2014; De Waele J. J. et al., 2014; Sime F. B. et al., 2015; Laterre et al., 2015; Yokoyama et al., 2015; Yokoyama et al., 2016), 11 on cephalosporin (Hanes et al., 2000; Joynt et al., 2001; Nicolau et al., 2001; Buijk et al., 2002; Lodise et al., 2007b; Lorente et al., 2007; Roberts et al., 2007; Chapuis et al., 2010; Carlier et al., 2014; Cousson et al., 2015; Kang et al., 2020), 10 on carbapenems (Lorente et al., 2006; Sakka et al., 2007; Cheatham et al., 2008; Dow et al., 2011a; Crandon et al., 2011; Hsaiky et al., 2013; De Waele J. J. et al., 2014; Lu et al., 2016; Yokoyama et al., 2018; Kothekar et al., 2020) and 1 on other β-lactams (aztreonam) (Cies et al., 2017a) (Table 2). One randomized controlled trial was conducted on temocillin in patients with intra-abdominal and lower respiratory tract infections (Laterre et al., 2015). A target of 80% ∫T>MIC was reached for the mean population for a MIC of 16 mg/L and a target of around 40 was reached for the mean population for a MIC of 32 mg/L. Two cohort studies were performed on patients receiving ampicillin + sulbactam (Yokoyama et al., 2015; Yokoyama et al., 2016). The standard dose of 1 g/0.5 g intravenously seemed to be adequate in terms of efficacy. However, dosing intervals can be increased to optimize the safety and efficacy of the treatment. Six studies were documented on piperacillin with or without combination with tazobactam, out of which two are randomized controlled trials (RCT) (Lodise et al., 2007a; Lorente et al., 2009; Roberts et al., 2010; Dow et al., 2011b; Yost and Cappelletty, 2011; Sime F. B. et al., 2015). Most of the studies recommended the dose of piperacillin of 4.5 g every 6 h or 8 h infused over 30 min. The administration of piperacillin + tazobactam using extended or continuous infusion achieve superior PK/PD targets.
For cefuroxime, one RCT was reported (Carlier et al., 2014). The standard dose of cefuroxime prescribed by physicians was 1.5 g TID. Carrier et al. recommended that high-dose continuous infusion is more likely to reach PK/PD targets. The standard dose leads to 87% probability of target attainment (PTA) for patients with creatinine clearance (CLCr) of 50 ml/min and pathogen of MIC 8 mg/ml. Five studies were reported on ceftazidime (Hanes et al., 2000; Nicolau et al., 2001; Buijk et al., 2002; Lorente et al., 2007; Cousson et al., 2015). All these studies recommended the continuous infusion regimen that presents PK/PD advantages and predictable efficacy. Lorente et al. (2007) reported that the meantime that plasma ceftazidime concentration exceeded the MIC was higher for continuous infusion (100%) for susceptible, intermediate and resistant strains over intermittent infusion. Chapuis and his colleagues studied the PK/PD parameters of cefepime which identified a safety and efficacy window for a dose of 2 g every 12 h in patients with ClCr > 50 ml/min infected by pathogens with cefepime < 4 mg/ml. The dose of ceftriaxone included in three studies was 2 g once daily (Chapuis et al., 2010).
Eight studies were reported on dose optimization and PK/PD characteristics of meropenem (Lorente et al., 2006; Cheatham et al., 2008; Dow et al., 2011b; Crandon et al., 2011; De Waele J. J. et al., 2014; Lu et al., 2016; Yokoyama et al., 2018; Kothekar et al., 2020). In Cheathman et al. (2008) study, the PK/PD analysis recommended that dosing regimen of meropenem 500 mg every 6, 8, or 12 h, adjusted for the renal function is considered for treatment of various infection. Similarly, Kothekar et al. reported that dose optimization of meropenem is required in patients with severe sepsis and septic shock. The prescribed was 100 mg as 3 h extended infusion. The PTA was 100% at 40% ∫T>MIC and 66.7% at 40% ∫T > 2xMIC. Sakka et al. (2007) reported that imipenem-colistin provide robust coverage for most common nosocomial pathogens when administered either in intermittent or continuous infusion of 1 g q8h or in a continuous infusion of 2 g/day. Hsaikay et al. (2013) reported that doripenem should be administered via prolonged infusion regimen to optimize the efficacy of the treatment The dose of aztreonam 2 g every 6 h was effective in patients with pseudomonas aeruginosa empyema (Cies et al., 2017a).
Quality assessment
In NOS, a maximum of 13 stars assigned to each study. According to Agency for Healthcare Research and Quality (AHRQ) standards, a study who scored 3 or 5 stars in selection, 1 or 2 stars in comparability group and 2 or 3 stars in outcome groups is of good quality, study who scored 2 stars in selection domain, 1 or 2 stars in comparability domain and 2 or 3 stars in outcome domain is of fair quality, study who scored 0 or 1 start in selection group of 0 stars in comparability group or 0 or 1 star in outcome group is of poor quality. In this systematic review, out of 52 studies, 50 studies are of good quality and the remaining two studies are of fair quality (Table 3). The Cochrane bias tool assessed that all RCT studies are at lower risk of bias and all domains were discussed in Table 4. The two case reports included in the systematic review are of good quality (Table 5).
Discussion
Inappropriate antibiotic treatment is most often the result of inappropriate dose, delayed administration or more often an underestimation of current trends in resistance (Sulis et al., 2020). The bactericidal activity of antibiotics depends on the concentration of the drug with regards to the minimum inhibitory concentration (MIC) and the time that this exposure can be sustained (Kuti, 2016). The MIC represents the most fundamental PD measure for antibiotics against pathogens, presenting the potency of administered antibiotics (Onufrak et al., 2016). The dose optimization based on MIC would seem to provide rectification in the PD characteristics and target attainment (Hartman et al., 2020). However, the demerits using MIC values to optimize the dosing regimens were highlighted by Mouton et al. (2018). Therefore, MIC variation must be examined to avoid potential underdosing of the patient. Moreover, alteration in PK measure may affect the PD characteristics. In our systematic review, we have gathered information regarding the dosing pattern of β-lactams from 52 studies. The majority of the studies were carried out in intensive care units. Although antibiotic use is the cornerstone of intensive care treatment for critically ill patients with suspected infection (Pickens and Wunderink, 2019).
β-lactams include penicillins, cephalosporins, and carbapenems are widely used in the management and treatment of serious infection particularly in critically ill patients (Thakuria et al., 2013; Bozcal et al., 2017). All β-lactams showed time-dependent bactericidal activity, which is determined by the free antibiotic concentration-time above the MIC for microorganisms identified (%∫T>MIC) (Masich et al., 2018a; Pandey and Cascella, 2020). The optimal clinical outcomes may differ depending on the β-lactams, for example, the target attainment goals for piperacillin + tazobactam, cephalosporins and carbapenems were 50%∫T>MIC, 60%∫T>MIC and 40%∫T>, respectively (Masich et al., 2018a). Moreover, the maximal bactericidal activity can be achieved by increasing the drug levels i.e., four to five times above MIC, even so, the interaction to improved clinical outcomes is inconsistent. The specific percentage of dosing interval ∫T>MIC needed for optimal activity differs for different β-lactam classes. The variation in percentages have been associated with variation in the rate of killing and the post-antibiotic effect. Majority of the studies documented the clinical pharmacodynamic parameters of β-lactams against gram-negative bacteria (Manduru et al., 1997; Tam et al., 2002). Several studies suggested that the amount of time the plasma concentration of the drug remains 4-6 fold greater than MIC has to be maintained for 100% of the dosing time period, however other studies have reported a target of 60% ∫T>MIC depending on clinical outcome measures (clinical cure vs. reduced bacterial resistance) (Manduru et al., 1997; Tam et al., 2002; Crandon et al., 2010).
As per available evidence, the current knowledge of PK and target attainment is often suboptimal in patients following the standard dosing regimen of β-lactams. Most of the studies provide data on PK parameters (34/52). It is evident that changes in PK parameters occur in patients. Cies et al. (2018a) reported that volume of distribution was increased in 86% of patients and clearance was increased in 71% of patients receiving ceftaroline. Piperacillin (12/52) was the most commonly used β-lactams, followed by meropenem (11/52), and ceftazidime (5/52). Piperacillin and meropenem are widely used to treat various infections among the hospitalized patients because of their susceptibility against many gram-positive and gram-negative pathogens (Shah and Ryzner, 2013; Xu et al., 2019).
The common mode of administration of antibiotics recommended by many clinicians was intermittent intravenous administration (Kasiakou et al., 2005). However, optimal dosing strategies for the treatment of various infectious diseases remain controversial. Most of the β-lactams were administered as an intermittent bolus. However, on the basis of strong PK/PD data, the administration of antibiotics by continuous infusion is more effective than administration by intermittent infusion (Dulhunty et al., 2013). Many of included studies found that continuous or extended infusion increased the survival rates among hospitalized patients especially critically ill patients. The administration of β-lactams as continuous infusion increased blood and interstitial fluid concentration with greater time above the MIC as compared to intermittent dosing, especially for pathogens with MIC values, which are frequent in ICUs (Dulhunty et al., 2013). The potential benefits to patients as well as the healthcare system by implementing improved approaches of antibiotic delivery are substantial. In an era of increasingly expensive treatments, the administration of β-lactams are cost-effective in terms of drug costs and labor costs (Mouton and Vinks, 2007).
β-l actams are frequently recommended by international and national treatment guidelines, have been prescribed for various infectious diseases. Therefore, ASPs should be implemented that helps the clinicians to use antibiotic appropriately from a pharmacological point of view that means excluding the pharmacological factors that potentially increase the risk of spread of resistance (Adembri et al., 2020). More accurately, antibiotics should be administered following PK/PD principles. When selecting the appropriate dosing regimen by keeping in view the PK/PD principles, the specified pathophysiological changes must be taken into consideration (Roberts et al., 2014). Moreover, multiple PK/PD software using a combination of TDM, Bayesian forecasting and PopPK can be utilized by pharmacists, clinical pharmacologists, and clinicians to maintain optimal target attainment (Abdulla et al., 2021). The guidelines on the use of TDM including an overview of suggested PD targets for several B-lactams antibiotics is also recommended by the French Society Anesthesia and Intensive Care Medicine (SFAR) (Guilhaumou et al., 2019). However, various softwares such as MIPD, NONMEM, MWPHARM++, ID-ODS, InsightRx Nova and AutoKinetics are available, close collaboration between pharmacists and clinicians are required to implement this feature to optimize the patient target attainment (Sime F. et al., 2015; Kantasiripitak et al., 2020). Model-informed precision dosing (MIPD) is an emerging approach that improves TDM process. This approach estimates the PK variability utilizing population PK model and predict the probability of target attainment for various dosing regimen (Gijsen et al., 2022). Despite of its advantages and availability of softwares, adoption of MIPD in clinical settings has been limited to date (Neely et al., 2018; Frymoyer et al., 2020).
The present study has some limitation that should be acknowledged when evaluating the data from included studies. Firstly, this study used limited databases with specific focus on titles describing the dose optimization of β-lactams antibiotics as no quantitative analysis was carried out. Moreover, limited grey literature search was conducted using additional search terms that identified relevant data. Secondly, some studies included the co-administration of two or more β-lactams antibiotics may alter the PK/PD parameters of both drugs. Thirdly, the difficulty in the assessment of efficacy concerning MIC was observed due to under-reporting.
Conclusion
This systematic review showed that appropriate antibiotic therapy is challenging due to a wide range of pathophysiological change among different age groups. This challenging perspective requires close collaboration between clinicians, pharmacists and clinical pharmacologists to optimize the effective treatment and improve the clinical outcome. The PK/PD analysis can be utilized to support alternative dosing regimens such as increase in dosing interval, continuous infusion, and increased bolus doses. The current study aimed to inspire both researchers and clinicians to identify and resolve these differences, not only by elucidating PK/PD parameters, but also providing guidelines for implementation in the healthcare settings, as this data is important to optimize antibiotic treatment in patient populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, AH, HF, SA, MA, and AS; methodology, AH, SA, ME, SAA, AFA, and ZS; review, AH, SA, and ZS; analysis, AH, SAA, and AZ; resources, AH, ME, SAA, and NO; writing—original draft preparation, AH, NO, SAA, AM, SSA, AK, and NO; writing—review and editing, AS, ZS, HF, and AK; supervision, HF, MA, and AS; funding acquisition, AH, AFA, NO, ME, and HF. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Deanship of Scientific research at the Umm Al-Qura University for supporting this work by grant code: 22UQU4290073DSR01.
Acknowledgments
We would like to thank the reviewers for their efforts to screen all studies in a timely manner and to resolve all conflicts while choosing the studies for the review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulla, A., Edwina, A. E., Flint, R. B., Allegaert, K., Wildschut, E. D., Koch, B. C., et al. (2021). Model-informed precision dosing of antibiotics in pediatric patients: A narrative review. Front. Pediatr. 9, 624639. doi:10.3389/fped.2021.624639
Adembri, C., Novelli, A., and Nobili, S. J. A. (2020). Some suggestions from PK/PD principles to contain resistance in the clinical setting—focus on ICU patients and gram-negative strains. Antibiotics 9 (10), 676. doi:10.3390/antibiotics9100676
Alghamdi, S., Berrou, I., Bajnaid, E., Aslanpour, Z., Haseeb, A., Hammad, M. A., et al. (2021). Antimicrobial stewardship program implementation in a Saudi medical city: An exploratory case study. Antibiotics 10 (3), 280. doi:10.3390/antibiotics10030280
Andes, D., and Craig, W. A. (2002). Animal model pharmacokinetics and pharmacodynamics: A critical review. Int. J. Antimicrob. Agents 19 (4), 261–268. doi:10.1016/s0924-8579(02)00022-5
Atif, M., Asghar, S., Mushtaq, I., Malik, I. J. J. o. i., and health, p. (2020). Community pharmacists as antibiotic stewards: A qualitative study exploring the current status of antibiotic stewardship program in bahawalpur, Pakistan. J. Infect. Public Health 13 (1), 118–124. doi:10.1016/j.jiph.2019.07.003
Béranger, A., Benaboud, S., Urien, S., Moulin, F., Bille, E., Lesage, F., et al. (2019). Piperacillin population pharmacokinetics and dosing regimen optimization in critically ill children with normal and augmented renal clearance. Clin. Pharmacokinet. 58 (2), 223–233. doi:10.1007/s40262-018-0682-1
Béranger, A., Oualha, M., Urien, S., Genuini, M., Renolleau, S., Aboura, R., et al. (2018). Population pharmacokinetic model to optimize cefotaxime dosing regimen in critically ill children. Clin. Pharmacokinet. 57 (7), 867–875. doi:10.1007/s40262-017-0602-9
Bozcal, E., Dagdeviren, M. J. P. F. S. T. A. t. N. R., and Rijeka, C. I. O. (2017). “Simplified techniques for analysis,” in Toxicity of β-lactam antibiotics: Pathophysiology, molecular biology and possible recovery strategies, 87–105.
Buijk, S. L., Gyssens, I. C., Mouton, J. W., Van Vliet, A., Verbrugh, H. A., and Bruining, H. A. (2002). Pharmacokinetics of ceftazidime in serum and peritoneal exudate during continuous versus intermittent administration to patients with severe intra-abdominal infections. J. Antimicrob. Chemother. 49 (1), 121–128. doi:10.1093/jac/49.1.121
Carlier, M., Noë, M., Roberts, J. A., Stove, V., Verstraete, A. G., Lipman, J., et al. (2014). Population pharmacokinetics and dosing simulations of cefuroxime in critically ill patients: Non-standard dosing approaches are required to achieve therapeutic exposures. J. Antimicrob. Chemother. 69 (10), 2797–2803. doi:10.1093/jac/dku195
Caron, W. P., Mousa, S. A. J. I., and resistance, d. (2010). Prevention strategies for antimicrobial resistance: A systematic review of the literature. Infect. Drug Resist. 3, 25–33. doi:10.2147/idr.s10018
Chapuis, T. M., Giannoni, E., Majcherczyk, P. A., Chioléro, R., Schaller, M. D., Berger, M. M., et al. (2010). Prospective monitoring of cefepime in intensive care unit adult patients. Crit. Care 14 (2), R51. doi:10.1186/cc8941
Cheatham, S. C., Kays, M. B., Smith, D. W., Wack, M. F., Sowinski, K. M. J. P. T. J. o. H. P., and Therapy, D. (2008). Steady‐state pharmacokinetics and pharmacodynamics of meropenem in hospitalized patients. Pharmacotherapy 28 (6), 691–698. doi:10.1592/phco.28.6.691
Cies, J. J., LaCoursiere, R. J., Moore, W. S., and Chopra, A. (2017a). Therapeutic drug monitoring of prolonged infusion aztreonam for multi-drug resistant Pseudomonas aeruginosa: A case report. J. Pediatr. Pharmacol. Ther. 22 (6), 467–470. doi:10.5863/1551-6776-22.6.467
Cies, J. J., Moore, W. S., Enache, A., and Chopra, A. (2018a). Ceftaroline for suspected or confirmed invasive methicillin-resistant Staphylococcus aureus: A pharmacokinetic case series. Pediatr. Crit. Care Med. 19 (6), e292–e299. doi:10.1097/pcc.0000000000001497
Cies, J. J., Moore, W. S., Enache, A., and Chopra, A. (2017b). Population pharmacokinetics and pharmacodynamic target attainment of meropenem in critically ill young children. J. Pediatr. Pharmacol. Ther. 22 (4), 276–285. doi:10.5863/1551-6776-22.4.276
Cies, J. J., Moore, W. S. I., Enache, A., and Chopra, A. (2018b). Ceftaroline for suspected or confirmed invasive methicillin-resistant Staphylococcus aureus: A pharmacokinetic case series. Pediatr. Crit. Care Med. 19 (6), e292–e299. doi:10.1097/pcc.0000000000001497
Cies, J. J., Moore, W. S., Calaman, S., Brown, M., Narayan, P., Parker, J., et al. (2015). Pharmacokinetics of continuous-infusion meropenem for the treatment of Serratia marcescens ventriculitis in a pediatric patient. Pharmacotherapy 35 (4), e32–e36. doi:10.1002/phar.1567
Cies, J. J., Moore, W. S., IIParker, J., Stevens, R., Al-Qaqaa, Y., Enache, A., et al. (2019). Pharmacokinetics of cefazolin delivery via the cardiopulmonary bypass circuit priming solution in infants and children. J. Antimicrob. Chemother. 74 (5), 1342–1347. doi:10.1093/jac/dky574
Cies, J. J., Shankar, V., Schlichting, C., and Kuti, J. L. (2014). Population pharmacokinetics of piperacillin/tazobactam in critically ill young children. Pediatr. Infect. Dis. J. 33 (2), 168–173. doi:10.1097/INF.0b013e3182a743c7
Cotta, M., Roberts, J., and Lipman, J. J. M. i. (2015). Antibiotic dose optimization in critically ill patients. Med. Intensiva 39 (9), 563–572. doi:10.1016/j.medin.2015.07.009
Cousson, J., Floch, T., Guillard, T., Vernet, V., Raclot, P., Wolak-Thierry, A., et al. (2015). Lung concentrations of ceftazidime administered by continuous versus intermittent infusion in patients with ventilator-associated pneumonia. Antimicrob. Agents Chemother. 59 (4), 1905–1909. doi:10.1128/AAC.04232-14
Crandon, J. L., Ariano, R. E., Zelenitsky, S. A., Nicasio, A. M., Kuti, J. L., and Nicolau, D. P. (2011). Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 37 (4), 632–638. doi:10.1007/s00134-010-2105-0
Crandon, J. L., Bulik, C. C., Kuti, J. L., and Nicolau, D. P. (2010). Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54 (3), 1111–1116. doi:10.1128/AAC.01183-09
D’Agate, S., Musuamba, F. T., and Della Pasqua, O. (2020). Dose rationale for amoxicillin in neonatal sepsis when referral is not possible. Front. Pharmacol. 11 (1434), 521933. doi:10.3389/fphar.2020.521933
De Cock, P. A. J. G., Mulla, H., Desmet, S., De Somer, F., McWhinney, B. C., Ungerer, J. P. J., et al. (2016). Population pharmacokinetics of cefazolin before, during and after cardiopulmonary bypass to optimize dosing regimens for children undergoing cardiac surgery. J. Antimicrob. Chemother. 72 (3), dkw496–800. doi:10.1093/jac/dkw496
De Cock, P. A. J. G., Standing, J. F., Barker, C. I. S., de Jaeger, A., Dhont, E., Carlier, M., et al. (2015). Augmented renal clearance implies a need for increased amoxicillin-clavulanic acid dosing in critically ill children. Antimicrob. Agents Chemother.Antimicrobial Agents Chemother. 59 (11), 7027–7035. doi:10.1128/aac.01368-15
De Cock, P. A. J. G., van Dijkman, S. C., de Jaeger, A., Willems, J., Carlier, M., Verstraete, A. G., et al. (2017). Dose optimization of piperacillin/tazobactam in critically ill children. J. Antimicrob. Chemother. 72 (7), 2002–2011. doi:10.1093/jac/dkx093
De Waele, J., Carlier, M., Hoste, E., Depuydt, P., Decruyenaere, J., Wallis, S. C., et al. (2014a). Extended versus bolus infusion of meropenem and piperacillin: A pharmacokinetic analysis. Minerva Anestesiol. 80 (12), 1302–1309.
De Waele, J. J., Carrette, S., Carlier, M., Stove, V., Boelens, J., Claeys, G., et al. (2014b). Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 40 (3), 380–387. doi:10.1007/s00134-013-3187-2
Delattre, I. K., Taccone, F. S., Jacobs, F., Hites, M., Dugernier, T., Spapen, H., et al. (2017). Optimizing β-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: Are first conventional doses effective? Expert Rev. anti. Infect. Ther. 15 (7), 677–688. doi:10.1080/14787210.2017.1338139
Dow, R. J., Rose, W. E., Fox, B. C., Thorpe, J. M., and Fish, J. T. J. I. D. i. C. P. (2011b). Retrospective study of prolonged versus intermittent infusion piperacillin-tazobactam and meropenem in intensive care unit patients at an academic medical center. Infect. Dis. Clin. Pract. Balt. Md. 19 (6), 413–417. doi:10.1097/ipc.0b013e31822e9bf5
Dow, R. J., Rose, W., Fox, B., Thorpe, J., and Fish, J. J. I. D. i. C. P. (2011a). Retrospective Study of Prolonged Versus Intermittent Infusion Piperacillin-Tazobactam and Meropenem in Intensive Care Unit Patients at an Academic Medical Center, 19, 413–417.
Dulhunty, J. M., Roberts, J. A., Davis, J. S., Webb, S. A., Bellomo, R., Gomersall, C., et al. (2013). Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin. Infect. Dis. 56 (2), 236–244. doi:10.1093/cid/cis856
Falagas, M. E., Tansarli, G. S., Ikawa, K., and Vardakas, K. Z. J. C. i. d. (2013). Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: A systematic review and meta-analysis. Clin. Infect. Dis. 56 (2), 272–282. doi:10.1093/cid/cis857
Felton, T., Hope, W., Lomaestro, B., Butterfield, J., Kwa, A., Drusano, G., et al. (2012). Population pharmacokinetics of extended-infusion piperacillin-tazobactam in hospitalized patients with nosocomial infections. Antimicrob. Agents Chemother. 56 (8), 4087–4094. doi:10.1128/AAC.00521-12
Frymoyer, A., Schwenk, H. T., Zorn, Y., Bio, L., Moss, J. D., Chasmawala, B., et al. (2020). Model-informed precision dosing of vancomycin in hospitalized children: Implementation and adoption at an academic children's hospital. Front. Pharmacol. 11, 551. doi:10.3389/fphar.2020.00551
Fukumoto, K., Aida, S., Oishi, T., Ueno, K. J. B., and Bulletin, P. (2009). Pharmacokinetics of ceftriaxione, a third-generation cephalosporin, in pediatric patients. Biol. Pharm. Bull. 32 (7), 1139–1141. doi:10.1248/bpb.32.1139
Georges, B., Conil, J. M., Ruiz, S., Seguin, T., Cougot, P., Fourcade, O., et al. (2012). Ceftazidime dosage regimen in intensive care unit patients: From a population pharmacokinetic approach to clinical practice via Monte Carlo simulations. Br. J. Clin. Pharmacol. 73 (4), 588–596. doi:10.1111/j.1365-2125.2011.04117.x
Giannoni, E., Moreillon, P., Cotting, J., Moessinger, A., Bille, J., Décosterd, L., et al. (2006). Prospective determination of plasma imipenem concentrations in critically ill children. Antimicrob. Agents Chemother.Antimicrobial Agents Chemother. 50 (7), 2563–2568. doi:10.1128/AAC.01149-05
Gijsen, M., Dreesen, E., Wauters, J., Debaveye, Y., and Spriet, I. (2022). The TARGET trial as a plea for model-informed precision dosing of piperacillin/tazobactam in patients with sepsis. Intensive Care Med. 48, 768–769. doi:10.1007/s00134-022-06679-0
Gross, A. E., Hanna, D., Rowan, S. A., Bleasdale, S. C., and Suda, K. J. (2019). “Successful implementation of an antibiotic stewardship program in an academic dental practice,” in Open forum infectious diseases (ofz067: Oxford University Press US).
Grupper, M., Kuti, J. L., and Nicolau, D. P. J. C. m. r. (2016). Continuous and prolonged intravenous β-lactam dosing: Implications for the clinical laboratory. Clin. Microbiol. Rev. 29 (4), 759–772. doi:10.1128/CMR.00022-16
Guilhaumou, R., Benaboud, S., Bennis, Y., Dahyot-Fizelier, C., Dailly, E., Gandia, P., et al. (2019). Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit. Care 23 (1), 104. doi:10.1186/s13054-019-2378-9
Gustafsson, I., Lowdin, E., Odenholt, I., and Cars, O. (2001). Pharmacokinetic and pharmacodynamic parameters for antimicrobial effects of cefotaxime and amoxicillin in an in vitro kinetic model. Antimicrob. Agents Chemother. 45 (9), 2436–2440. doi:10.1128/AAC.45.9.2436-2440.2001
Hammond, D. A., McCreary, E. K., Rech, M. A., Smith, M. N., Yeo, Q. M., Lusardi, K., et al. (2019). Perceptions and practices for beta‐lactam antibiotic dosing, administration, and monitoring in critically ill patients: Current views and use among critical care and infectious diseases pharmacists. J. Am. Coll. Clin. Pharm. 2 (5), 468–476. doi:10.1002/jac5.1084
Hanes, S. D., Wood, G. C., Herring, V., Croce, M. A., Fabian, T. C., Pritchard, E., et al. (2000). Intermittent and continuous ceftazidime infusion for critically ill trauma patients. Am. J. Surg. 179 (6), 436–440. doi:10.1016/s0002-9610(00)00388-3
Hartman, S. J. F., Boeddha, N. P., Ekinci, E., Koch, B. C. P., Donders, R., Hazelzet, J. A., et al. (2019). Target attainment of cefotaxime in critically ill children with meningococcal septic shock as a model for cefotaxime dosing in severe pediatric sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 38 (7), 1255–1260. doi:10.1007/s10096-019-03535-w
Hartman, S. J. F., Brüggemann, R. J., Orriëns, L., Dia, N., Schreuder, M. F., and de Wildt, S. N. (2020). Pharmacokinetics and target attainment of antibiotics in critically ill children: A systematic review of current literature. Clin. Pharmacokinet. 59 (2), 173–205. doi:10.1007/s40262-019-00813-w
Haseeb, A., Alqurashi, M. K., Althaqafi, A. S., Alsharif, J. M., Faidah, H. S., Bushyah, M., et al. (2022). A systematic review on clinical safety and efficacy of vancomycin loading dose in critically ill patients. Antibiotics 11 (3), 409. doi:10.3390/antibiotics11030409
Haseeb, A., Faidah, H. S., Al-Gethamy, M., Iqbal, M. S., Alhifany, A. A., Ali, M., et al. (2020). Evaluation of antimicrobial stewardship programs (ASPs) and their perceived level of success at makkah region hospitals, kingdom of Saudi arabia. Saudi Pharm. J. 28 (10), 1166–1171. doi:10.1016/j.jsps.2020.08.005
Haseeb, A., Faidah, H. S., Al-Gethamy, M., Iqbal, M. S., Barnawi, A. M., Elahe, S. S., et al. (2021a). Evaluation of a multidisciplinary antimicrobial stewardship program in a Saudi critical care unit: A quasi-experimental study. Front. Pharmacol. 11, 570238. doi:10.3389/fphar.2020.570238
Haseeb, A., Faidah, H. S., Alghamdi, S., Alotaibi, A. F., Elrggal, M. E., Mahrous, A. J., et al. (2021b). Dose optimization of colistin: A systematic review. Antibiotics 10 (12), 1454. doi:10.3390/antibiotics10121454
He, N., Dong, F., Liu, W., Zhai, S. J. I., and Resistance, D. (2020). A Systematic Review of Vancomycin Dosing in Patients with Hematologic Malignancies or Neutropenia, 13, 1807.
Higgins, J. P., Savović, J., Page, M. J., Elbers, R. G., and Sterne, J. A. J. C. h. f. s. r. o. i. (2019). Assessing risk of bias in a randomized trial, 205–228.
Hsaiky, L., Murray, K. P., Kokoska, L., Desai, N., and Cha, R. (2013). Standard versus prolonged doripenem infusion for treatment of gram-negative infections. Ann. Pharmacother. 47 (7-8), 999–1006. doi:10.1345/aph.1S032
Joynt, G. M., Lipman, J., Gomersall, C. D., Young, R. J., Wong, E. L. Y., and Gin, T. (2001). The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 47 (4), 421–429. doi:10.1093/jac/47.4.421
Kaki, R., Elligsen, M., Walker, S., Simor, A., Palmay, L., and Daneman, N. J. J. o. a. c. (2011). Impact of antimicrobial stewardship in critical care: A systematic review. J. Antimicrob. Chemother. 66 (6), 1223–1230. doi:10.1093/jac/dkr137
Kang, S., Jang, J. Y., Hahn, J., Kim, D., Lee, J. Y., Min, K. L., et al. (2020). Dose optimization of cefpirome based on population pharmacokinetics and target attainment during extracorporeal membrane oxygenation. Antimicrob. Agents Chemother. 64 (5), e00249-20. doi:10.1128/AAC.00249-20
Kantasiripitak, W., Van Daele, R., Gijsen, M., Ferrante, M., Spriet, I., and Dreesen, E. (2020). Software tools for model-informed precision dosing: How well do they satisfy the needs? Front. Pharmacol. 11, 620. doi:10.3389/fphar.2020.00620
Kasiakou, S. K., Lawrence, K. R., Choulis, N., and Falagas, M. E. J. D. (2005). Continuous versus intermittent intravenous administration of antibacterials with time-dependent action: A systematic review of pharmacokinetic and pharmacodynamic parameters. Drugs 65 (17), 2499–2511. doi:10.2165/00003495-200565170-00006
Khan, M. U., Hassali, M. A. A., Ahmad, A., Elkalmi, R. M., Zaidi, S. T. R., and Dhingra, S. J. P. o. (2016). Perceptions and practices of community pharmacists towards antimicrobial stewardship in the state of selangor, Malaysia. PLoS One 11 (2), e0149623. doi:10.1371/journal.pone.0149623
Khan, M. W., Wang, Y.-K., Wu, Y.-E., Tang, B.-H., Kan, M., Shi, H.-Y., et al. (2020). Population pharmacokinetics and dose optimization of ceftriaxone for children with community-acquired pneumonia. Eur. J. Clin. Pharmacol. 76 (11), 1547–1556. doi:10.1007/s00228-020-02939-4
Kothekar, A. T., Divatia, J. V., Myatra, S. N., Patil, A., Nookala Krishnamurthy, M., Maheshwarappa, H. M., et al. (2020). Clinical pharmacokinetics of 3-h extended infusion of meropenem in adult patients with severe sepsis and septic shock: Implications for empirical therapy against gram-negative bacteria. Ann. Intensive Care 10 (1), 4. doi:10.1186/s13613-019-0622-8
Kuti, J. L. J. R. M. C. L. C. (2016). Optimizing antimicrobial pharmacodynamics: A guide for your stewardship program. Rev. Medica Clin. Las Condes 27 (5), 615–624. doi:10.1016/j.rmclc.2016.08.001
Laterre, P. F., Wittebole, X., Van de Velde, S., Muller, A. E., Mouton, J. W., Carryn, S., et al. (2015). Temocillin (6 g daily) in critically ill patients: Continuous infusion versus three times daily administration. J. Antimicrob. Chemother. 70 (3), 891–898. doi:10.1093/jac/dku465
Lee, B. R., Goldman, J. L., Yu, D., Myers, A. L., Stach, L. M., Hedican, E., et al. (2017). Clinical impact of an antibiotic stewardship program at a children's hospital. Infect. Dis. Ther. 6 (1), 103–113. doi:10.1007/s40121-016-0139-5
Leggett, J. E., Fantin, B., Ebert, S., Totsuka, K., Vogelman, B., Calame, W., et al. (1989). Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159 (2), 281–292. doi:10.1093/infdis/159.2.281
Lesprit, P., and Brun-Buisson, C. J. C. o. i. i. d. (2008). Hosp. Antibiot. Steward. 21 (4), 344–349.
Lodise, T. P., Lomaestro, B., and Drusano, G. L. (2007a). Piperacillin-tazobactam for Pseudomonas aeruginosa infection: Clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. 44 (3), 357–363. doi:10.1086/510590
Lodise, T. P., Nau, R., Kinzig, M., Jones, R. N., Drusano, G. L., and Sörgel, F. (2007b). Comparison of the probability of target attainment between ceftriaxone and cefepime in the cerebrospinal fluid and serum against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 58 (4), 445–452. doi:10.1016/j.diagmicrobio.2007.03.015
Lorente, L., Jiménez, A., Martín, M. M., Iribarren, J. L., Jiménez, J. J., and Mora, M. L. (2009). Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int. J. Antimicrob. Agents 33 (5), 464–468. doi:10.1016/j.ijantimicag.2008.10.025
Lorente, L., Jiménez, A., Palmero, S., Jiménez, J. J., Iribarren, J. L., Santana, M., et al. (2007). Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: A retrospective, nonrandomized, open-label, historical chart review. Clin. Ther. 29 (11), 2433–2439. doi:10.1016/j.clinthera.2007.11.003
Lorente, L., Lorenzo, L., Martín, M. M., Jiménez, A., and Mora, M. L. (2006). Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann. Pharmacother. 40 (2), 219–223. doi:10.1345/aph.1G467
Lu, C., Zhang, Y., Chen, M., Zhong, P., Chen, Y., Yu, J., et al. (2016). Population pharmacokinetics and dosing regimen optimization of meropenem in cerebrospinal fluid and plasma in patients with meningitis after neurosurgery. Antimicrob. Agents Chemother. 60 (11), 6619–6625. doi:10.1128/AAC.00997-16
Luyt, C.-E., Bréchot, N., Trouillet, J.-L., and Chastre, J. J. C. c. (2014). Antibiotic stewardship in the intensive care unit. Crit. Care (Houten). 18 (5), 1–12. doi:10.1186/s13054-014-0480-6
Ma, L.-L., Wang, Y.-Y., Yang, Z.-H., Huang, D., Weng, H., and Zeng, X.-T. (2020). Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 7 (1), 7. doi:10.1186/s40779-020-00238-8
MacGowan, A., and Bowker, K. (2002). Developments in PK/PD: Optimising efficacy and prevention of resistance. A critical review of PK/PD in in vitro models. Int. J. Antimicrob. Agents 19 (4), 291–298. doi:10.1016/s0924-8579(02)00027-4
Manduru, M., Mihm, L. B., White, R. L., Friedrich, L. V., Flume, P. A., and Bosso, J. A. (1997). In vitro pharmacodynamics of ceftazidime against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 41 (9), 2053–2056. doi:10.1128/AAC.41.9.2053
Masich, A. M., Heavner, M. S., Gonzales, J. P., and Claeys, K. C. J. C. i. d. r. (2018b). Pharmacokinetic/pharmacodynamic considerations of beta-lactam antibiotics in adult critically ill patients. Curr. Infect. Dis. Rep. 20 (5), 9–8. doi:10.1007/s11908-018-0613-1
Masich, A. M., Heavner, M. S., Gonzales, J. P., and Claeys, K. C. (2018a). Pharmacokinetic/pharmacodynamic considerations of beta-lactam antibiotics in adult critically ill patients. Curr. Infect. Dis. Rep. 20 (5), 9. doi:10.1007/s11908-018-0613-1
Mettler, J., Simcock, M., Sendi, P., Widmer, A. F., Bingisser, R., Battegay, M., et al. (2007). Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: A prospective observational study. BMC Infect. Dis. 7 (1), 21–10. doi:10.1186/1471-2334-7-21
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1), 1–9. doi:10.1186/2046-4053-4-1
Mouton, J. W., Muller, A. E., Canton, R., Giske, C. G., Kahlmeter, G., and Turnidge, J. J. J. o. A. C. (2018). MIC-Based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 73 (3), 564–568. doi:10.1093/jac/dkx427
Mouton, J. W., Schmitt-Hoffmann, A., Shapiro, S., Nashed, N., and Punt, N. C. (2004). Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141. Antimicrob. Agents Chemother. 48 (5), 1713–1718. doi:10.1128/AAC.48.5.1713-1718.2004
Mouton, J. W., and Vinks, A. A. J. C. o. i. c. c. (2007). Continuous infusion of beta-lactams. Curr. Opin. Crit. Care 13 (5), 598–606. doi:10.1097/MCC.0b013e3282e2a98f
Neely, M. N., Kato, L., Youn, G., Kraler, L., Bayard, D., van Guilder, M., et al. (2018). Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob. Agents Chemother. 62 (2), e02042-17–02017. doi:10.1128/AAC.02042-17
Nichols, K., Chung, E. K., Knoderer, C. A., Buenger, L. E., Healy, D. P., Dees, J., et al. (2016). Population pharmacokinetics and pharmacodynamics of extended-infusion piperacillin and tazobactam in critically ill children. Antimicrob. Agents Chemother.Antimicrobial Agents Chemother. 60 (1), 522–531. doi:10.1128/AAC.02089-15
Nicolau, D. P., McNabb, J., Lacy, M. K., Quintiliani, R., and Nightingale, C. H. (2001). Continuous versus intermittent administration of ceftazidime in intensive care unit patients with nosocomial pneumonia. Int. J. Antimicrob. Agents 17 (6), 497–504. doi:10.1016/s0924-8579(01)00329-6
Nielsen, E. I., Cars, O., and Friberg, L. E. (2011). Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: A step toward model-based dose optimization. Antimicrob. Agents Chemother. 55 (10), 4619–4630. doi:10.1128/AAC.00182-11
Olguin, H. J., Asseff, I. L., Vieyra, A. C., Pérez, A. G., Saldaña, N. G., Quesada, A. C., et al. (2008). Effect of severity disease on the pharmacokinetics of cefuroxime in children with multiple organ system failure. Biol. Pharm. Bull. 31 (2), 316–320. doi:10.1248/bpb.31.316
Onufrak, N. J., Forrest, A., and Gonzalez, D. J. C. t. (2016). Pharmacokinetic and pharmacodynamic principles of anti-infective dosing. Clin. Ther. 38 (9), 1930–1947. doi:10.1016/j.clinthera.2016.06.015
Owens, R. C., Bhavnani, S. M., and Ambrose, P. G. (2005). Assessment of pharmacokinetic–pharmacodynamic target attainment of gemifloxacin against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 51 (1), 45–49. doi:10.1016/j.diagmicrobio.2004.08.019
Pacios, O., Blasco, L., Bleriot, I., Fernandez-Garcia, L., González Bardanca, M., Ambroa, A., et al. (2020). Strategies to combat multidrug-resistant and persistent infectious diseases. Antibiotics 9 (2), 65. doi:10.3390/antibiotics9020065
Pickens, C. I., and Wunderink, R. G. (2019). Principles and practice of antibiotic stewardship in the ICU. Chest 156 (1), 163–171. doi:10.1016/j.chest.2019.01.013
Pollack, L. A., and Srinivasan, A. J. C. I. D. (2014). Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 59 (3), S97–S100. doi:10.1093/cid/ciu542
Roberts, J. A., Abdul-Aziz, M. H., Lipman, J., Mouton, J. W., Vinks, A. A., Felton, T. W., et al. (2014). Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet. Infect. Dis. 14 (6), 498–509. doi:10.1016/S1473-3099(14)70036-2
Roberts, J. A., Boots, R., Rickard, C. M., Thomas, P., Quinn, J., Roberts, D. M., et al. (2007). Is continuous infusion ceftriaxone better than once-a-day dosing in intensive care? J. Antimicrob. Chemother. 59 (2), 285–291. doi:10.1093/jac/dkl478
Roberts, J. A., Kirkpatrick, C. M., Roberts, M. S., Dalley, A. J., and Lipman, J. (2010). First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int. J. Antimicrob. Agents 35 (2), 156–163. doi:10.1016/j.ijantimicag.2009.10.008
Sakka, S. G., Glauner, A. K., Bulitta, J. B., Kinzig-Schippers, M., Pfister, W., Drusano, G. L., et al. (2007). Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob. Agents Chemother. 51 (9), 3304–3310. doi:10.1128/aac.01318-06
Shah, P. J., and Ryzner, K. L. (2013). Evaluating the appropriate use of piperacillin/tazobactam in a community health system: A retrospective chart review. P Throughput 38 (8), 462–483.
Sime, F. B., Roberts, M. S., Tiong, I. S., Gardner, J. H., Lehman, S., Peake, S. L., et al. (2015b). Can therapeutic drug monitoring optimize exposure to piperacillin in febrile neutropenic patients with haematological malignancies? A randomized controlled trial. J. Antimicrob. Chemother. 70 (8), 2369–2375. doi:10.1093/jac/dkv123
Sime, F., Roberts, M., and Roberts, J. J. C. M. (2015a). Optimization of dosing regimens and dosing in special populations. Clin. Microbiol. Infect. 21 (10), 886–893. doi:10.1016/j.cmi.2015.05.002
Sulis, G., Daniels, B., Kwan, A., Gandra, S., Daftary, A., Das, J., et al. (2020). Antibiotic overuse in the primary health care setting: A secondary data analysis of standardised patient studies from India, China and Kenya. BMJ Glob. Health 5 (9), e003393. doi:10.1136/bmjgh-2020-003393
Tam, V. H., McKinnon, P. S., Akins, R. L., Rybak, M. J., and Drusano, G. L. (2002). Pharmacodynamics of cefepime in patients with Gram-negative infections. J. Antimicrob. Chemother. 50 (3), 425–428. doi:10.1093/jac/dkf130
Tan, W., Watt, K., Boakye-Agyeman, F., Cohen-Wolkowiez, M. M., Mok, Y., Yung, C., et al. (2018). Abstract PCCLB-32: Optimal dosing of meropenem in critically ill asian children receiving continuous renal replacement therapy using population pharmacokinetics. Pediatr. Crit. Care Med. 19 (6S), 253. doi:10.1097/01.pcc.0000538117.18545.8d
Tang, B.-H., Wu, Y.-E., Kou, C., Qi, Y.-J., Qi, H., Xu, H.-Y., et al. (2019). Population pharmacokinetics and dosing optimization of amoxicillin in neonates and young infants. Antimicrob. Agents Chemother. 63 (2), e02336-18–02318. doi:10.1128/AAC.02336-18
Thakuria, B., Lahon, K. J. J. o. c., and Jcdr, d. r. (2013). The beta lactam antibiotics as an empirical therapy in a developing country: An update on their current status and recommendations to counter the resistance against them. J. Clin. Diagn. Res. 7 (6), 1207–1214. doi:10.7860/JCDR/2013/5239.3052
van Limburg, M., Sinha, B., Lo-Ten-Foe, J. R., and van Gemert-Pijnen, J. E. W. C. (2014). Evaluation of early implementations of antibiotic stewardship program initiatives in nine Dutch hospitals. Antimicrob. Resist. Infect. Control 3 (1), 33. doi:10.1186/2047-2994-3-33
Wangai, F. K., Masika, M. M., Maritim, M. C., and Seaton, R. A. J. B. i. d. (2019). Methicillin-resistant Staphylococcus aureus (MRSA) in East Africa: Red alert or red herring? BMC Infect. Dis. 19 (1), 1–10. doi:10.1186/s12879-019-4245-3
Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., et al. (2014). Newcastle-Ottawa quality assessment scale cohort studies.
Wu, Y. E., Wang, Y. K., Tang, B. H., Dong, L., Li, X., Zhang, W., et al. (2021). Population pharmacokinetics and dosing optimization of amoxicillin in Chinese infants. J. Clin. Pharmacol. 61 (4), 538–546. doi:10.1002/jcph.1752
Xu, H., Kong, L., Wu, C., Xu, B., and Wu, X. (2019). Pharmacokinetics of meropenem in plasma and cerebrospinal fluid in patients with intraventricular hemorrhage after lateral ventricle drainage. Eur. J. Clin. Pharmacol. 75 (4), 595–597. doi:10.1007/s00228-018-02606-9
Yokoyama, Y., Matsumoto, K., Ikawa, K., Watanabe, E., Yamamoto, H., Imoto, Y., et al. (2015). Pharmacokinetics of prophylactic ampicillin-sulbactam and dosing optimization in patients undergoing cardiovascular surgery with cardiopulmonary bypass. Biol. Pharm. Bull. 38 (11), 1817–1821. doi:10.1248/bpb.b15-00334
Yokoyama, Y., Matsumoto, K., Ikawa, K., Watanabe, E., Yamamoto, H., Imoto, Y., et al. (2016). The pharmacokinetics of ampicillin–sulbactam in anuric patients: Dosing optimization for prophylaxis during cardiovascular surgery. Int. J. Clin. Pharm. 38 (4), 771–775. doi:10.1007/s11096-016-0286-5
Yokoyama, Y., Nishino, K., Matsumoto, K., Inomoto, Y., Matsuda, K., Nakamura, R. N., et al. (2018). Dosing optimization of meropenem based on a pharmacokinetic analysis in patients receiving hemodiafiltration and an in vitro model. J. Infect. Chemother. 24 (2), 92–98. doi:10.1016/j.jiac.2017.09.005
Keywords: dose optimization, beta-Lactams, carbapenems, penicillins, cephalosporins
Citation: Haseeb A, Faidah HS, Alghamdi S, Alotaibi AF, Elrggal ME, Mahrous AJ, Abuhussain SSA, Obaid NA, Algethamy M, AlQarni A, Khogeer AA, Saleem Z, Iqbal MS, Ashgar SS, Radwan RM, Mutlaq A, Fatani N and Sheikh A (2022) Dose optimization of β-lactams antibiotics in pediatrics and adults: A systematic review. Front. Pharmacol. 13:964005. doi: 10.3389/fphar.2022.964005
Received: 08 June 2022; Accepted: 09 August 2022;
Published: 21 September 2022.
Edited by:
Jessica K. Roberts, Cognigen, United StatesReviewed by:
Alan Abdulla, Erasmus Medical Center, NetherlandsMuhammad Usman, University of Veterinary and Animal Sciences, Pakistan
Copyright © 2022 Haseeb, Faidah, Alghamdi, Alotaibi, Elrggal, Mahrous, Abuhussain, Obaid, Algethamy, AlQarni, Khogeer, Saleem, Iqbal, Ashgar, Radwan, Mutlaq, Fatani and Sheikh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Haseeb, amhaseeb@uqu.edu.sa
 Abdul Haseeb
Abdul Haseeb Hani Saleh Faidah
Hani Saleh Faidah Saleh Alghamdi
Saleh Alghamdi Amal F. Alotaibi1
Amal F. Alotaibi1 Mahmoud Essam Elrggal
Mahmoud Essam Elrggal Ahmad J. Mahrous
Ahmad J. Mahrous Najla A. Obaid
Najla A. Obaid Asim A. Khogeer
Asim A. Khogeer Zikria Saleem
Zikria Saleem Muhammad Shahid Iqbal
Muhammad Shahid Iqbal Sami S. Ashgar
Sami S. Ashgar