Application of mixed reality navigation technology in primary brainstem hemorrhage puncture and drainage surgery: a case series and literature review
- 1Department of Neurosurgery, Chongqing Emergency Medical Center, Chongqing University Central Hospital, Chongqing, China
- 2Pre-hospital Emergency Department, Chongqing Emergency Medical Center, Chongqing University Central Hospital, Chongqing, China
- 3Qinying Technology Co., Ltd., Chongqing, China
Objective: The mortality rate of primary brainstem hemorrhage (PBH) is high, and the optimal treatment of PBH is controversial. We used mixed reality navigation technology (MRNT) to perform brainstem hematoma puncture and drainage surgery in seven patients with PBH. We shared practical experience to verify the feasibility and safety of the technology.
Method: We introduced the surgical procedure of brainstem hematoma puncture and drainage surgery with MRNT. From January 2021 to October 2022, we applied the technology to seven patients. We collected their clinical and radiographic indicators, including demographic indicators, preoperative and postoperative hematoma volume, hematoma evacuation rate, operation time, blood loss, deviation of the drainage tube target, depth of implantable drainage tube, postoperative complications, preoperative and 1-month postoperative GCS, etc.
Result: Among seven patients, with an average age of 56.71 ± 12.63 years, all had underlying diseases of hypertension and exhibited disturbances of consciousness. The average evacuation rate of hematoma was 50.39% ± 7.71%. The average operation time was 82.14 ± 15.74 min, the average deviation of the drainage tube target was 4.58 ± 0.72 mm, and the average depth of the implantable drainage tube was 62.73 ± 0.94 mm. Among all seven patients, four patients underwent external ventricular drainage first. There were no intraoperative deaths, and there was no complication after surgery in seven patients. The 1-month postoperative GCS was improved compared to the preoperative GCS.
Conclusion: It was feasible and safe to perform brainstem hematoma puncture and drainage surgery by MRNT. The technology could evacuate about half of the hematoma and prevent hematoma injury. The advantages included high precision in dual-plane navigation technology, low cost, an immersive operation experience, etc. Furthermore, improving the matching registration method and performing high-quality prospective clinical research was necessary.
Introduction
Primary brainstem hemorrhage (PBH) is spontaneous brainstem bleeding associated with hypertension unrelated to cavernous hemangioma, arteriovenous malformation, and other diseases. Hypertension is the leading risk factor for PBH, and other elements include anticoagulant therapy, cerebral amyloid angiopathy, et al. PBH is the deadliest subtype of intracerebral hemorrhage (ICH), accounting for 6%–10% of all ICH with an annual incidence of approximately 2–4/100,000 people [1–3]. The clinical characteristics of PBH are acute onset, rapid deterioration, poor prognosis, and high mortality (30%–90%) [1, 4, 5].
The inclusion criteria of previous ICH research all excluded PBH, such as STICH and MISTIE trials. There is no clear evidence for the optimal treatment of PBH, and the view of surgical treatment has noticeable regional differences. European and North American countries generally believe that severe disability or survival in a vegetative state is a high mental and economic burden for PBH patients and their families. These countries do not favor surgical treatment. However, many PBH surgical treatments have been carried out in China, Japan, and South Korea. Surgical treatment methods, surgical effects, monitoring methods, and complications have been investigated, and much experience has been accumulated.
In 1998, Korean scholars performed the first craniotomy to evacuate the brainstem hematoma [6]. However, in 1989, the Japanese scholar Takahama performed stereotactic brainstem hematoma aspiration surgery [7]. In our opinion, microsurgery craniotomy requires high electrophysiological monitoring and surgical skills, and these limitations are not conductive to popularization. Minimally invasive surgery has the characteristics of a simple operation, minimally invasive, and short operation time, and it is believed to reduce the damage to critical brainstem structures and protect brainstem function as much as possible. More and more minimally invasive treatments have been adopted to improve the precision of PBH puncture, including stereotactic frameworks, robotic-assisted navigation systems, 3D printing techniques, and even laser combined with CT navigation techniques.
Mixed reality navigation technology (MRNT) is based on virtual and augmented reality development. The technology uses CT images to construct a 3D head model and design an individual hematoma puncture trajectory. The actual environmental position is captured by a camera during surgery and was fused with 3D head model synchronously. MRNT not only display the model image combined with actual environment but also navigate the puncture trajectory in real time, allowing the surgeon to precisely control puncture angle and depth to achieve a perfect procedure. This technology makes the head utterly transparent during the surgery and brings an immersive experience to the surgeon.
MRNT has broad application prospects. However, it is still in its infancy, and its application in neurosurgery has rarely been reported. Furthermore, there is no report on application of MRNT in the surgical treatment of PBH. In this study, we used MRNT to perform brainstem hematoma puncture and drainage surgery in seven patients with PBH to share practical experience to verify the feasibility and safety of the technology.
Materials and methods
General information
With the approval of the Ethics Committee of the Chongqing Emergency Medical Center, we included seven patients diagnosed with PBH from January 2021 to October 2022. All underwent brainstem hematoma puncture and drainage surgery with MRNT under general anesthesia. Indications for surgery were patients who 1) were 18–80 years of age; 2) had hematoma volume greater than 5 mL and less than 15 mL; 3) had a diameter of the hematoma greater than 2 cm; 4) had hematoma deviating toward one side or the dorsal side; 5) had GCS less than 8; and 6) had surgery within 6–24 h after onset. Family members were informed and signed the consent form [8]. Exclusion criteria were patients who had 1) brainstem hemorrhage caused by cavernous hemangioma, arteriovenous malformation, and other diseases; 2) GCS >12; 3) bilateral pupil dilation; 4) unstable vital signs; 5) severe underlying disease; or 6) coagulation dysfunction.
Mixed reality navigation technology (MRNT)
All patients preparing for surgery were required to wear sticky analysis markers in the parieto-occipital region and undergo a CT scan before surgery. CT image scanning was performed with a 64-slice CT scanner (Lightspeed VCT 6, General Electric Company, United States of America). The image parameters included in the exposure were 3 mAS, the thickness was 5mm, and the image size was 512 × 512. The DICOM data were analyzed to construct the 3D model of the hematoma and head, and the volume of brainstem preoperative hematoma was calculated using software (Medical Modeling and Design System). In addition, the hematoma puncture trajectory was designed according to the constructed head model.
After general anesthesia, the sticky analysis markers were replaced with bone nail markers, keeping the same position [9]. Based on the principle of near-infrared optical navigation, the camera captured the actual space position in real-time, fused it with the markers of the 3D head model (HSCM3D DICOM), and transmitted the information to the wearable device (HoloLens). During surgery, the camera continuously tracked the position of the puncture needle to achieve navigation function. In short, the image processing software matched and fused information from camera systems and wearable device through multiple markers. When controlling the movement of surgical tools, the software also processed the dynamic tool position data and fused it with the virtual model through wireless transmission.
Surgical procedures
Hydrocephalus patients were first treated with external ventricular drainage (EVD), and the frontal Kocher point was selected as the cranial entry point. The procedures were cutting the skin, drilling the skull, cutting the dura mater, puncturing in the direction of the plane of binaural connection, fixing the drainage tube, and suturing it layer by layer.
The patient was placed in a prone position with the head frame fixed. The puncture point was 2 cm below the transverse sinus and 3 cm lateral to the midline of the hematoma side. After cutting the skin, the muscle was separated. The dura mater was cut through a drilled hole. Wearing HoloLens, the surgeon synchronously observed actual head structure and fused puncture trajectory from multiple angles and used dual-plane navigation technology [9] for hematoma puncture. After watching that the drainage tube was in place, the puncture needle was removed, and a 5 mL empty syringe was connected for suction. The drainage tube was fixed and sutured layer by layer. The head CT was reviewed immediately after the surgery, and the decision whether to inject urokinase according to the drainage tube’s position and the residual hematoma volume. Urokinase was injected from a drainage tube for 2-3 w units every 12 h, usually 4–6 times, and kept for 1.5 h before opening the tube. The retention time of the drainage tube was no more than 72 h after the surgery. The surgical procedure to apply MRNT is shown in Figure 1.
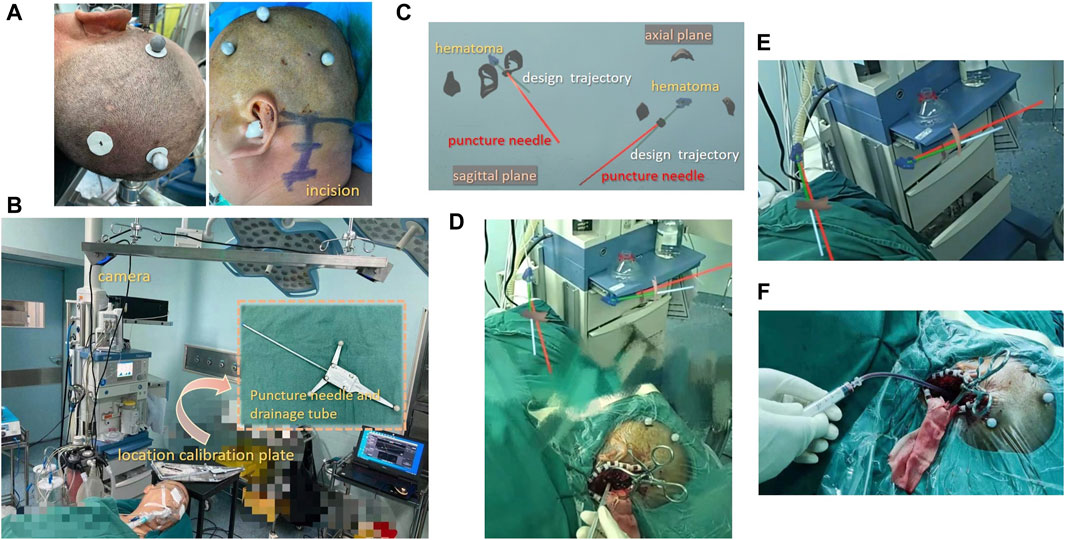
Figure 1. Surgical procedure for brainstem hematoma puncture and drainage surgery with MRNT (A) Patients were required to wear sticky analysis markers in the parieto-occipital region. (B) The camera captured the real space position of the calibration plate, puncture needle, and head. (C) Wearing HoloLens, the surgeon viewed the two planes of the image. (D) MRNT displays the model image and the actual environment synchronously, allowing the surgeon to perform precise surgery. (E) The real-time navigation of MRNT showed that the puncture needle was close to the hematoma target. (F) The surgeon was aspirating the hematoma.
Clinical and radiographic indicators
The indicators for analysis included: demographic indicators, preoperative and postoperative hematoma volume, hematoma evacuation rate, operation time, blood loss, deviation of the drainage tube target, depth of implantable drainage tube, postoperative complications, and preoperative and 1-month postoperative GCS, etc.
The deviation of the drainage tube target was defined as the distance between the tip of the drainage tube and the planned puncture hematoma target. The deviation calculation was done with the BLENDER 2.93.3 software, which used the 3D global coordinate system to visualize the distance.
The head CT examination was reviewed within 24 h after surgery, and the postoperative hematoma volume was measured by non-operators using previous software (Medical Modeling and Design System). Hematoma evacuation rate = (preoperative hematoma volume - postoperative hematoma volume)/preoperative hematoma volume.
Statistical analysis
All statistical analyses were performed with SPSS (version 21, IBM, Chicago, IL, United States). Quantitative variables are presented as means ± standard deviations. The normality of quantitative variables was assessed through the Kolmogorov-Smirnov test. If the distribution was found to be normal, paired t-test were performed. The categorical variables are presented as percentages and tested by χ2 or Fisher’s test. A p-value less than 0.05 was considered statistically significant.
Results
From January 2021 to October 2022, seven patients were diagnosed with PBH and underwent brainstem hematoma puncture and drainage surgery with MRNT. A summary of the demographic and clinical characteristics of the patients was provided in Table 1. Among the seven patients, five were men, with an average age of 56.71 ± 12.63 years (37–74 years). The seven cases had underlying hypertension, and four cases had diabetes. The average time from onset to admission was 4.2 ± 1.47 h. Seven patients had prominent disturbances of consciousness, four required ventilator assistance, and three had a high fever.
According to the brainstem hematoma classification advocated by Chung [10], 2 cases belonged to small unilateral tegmental type, 4 cases belonged to basal-tegmental type, and other 1 case belonged to bilateral tegmental type. The average volume of preoperative brainstem hematoma was 8.47 ± 2.22 mL (range, 5.45–12.2 mL), the average volume of postoperative brainstem hematoma was 4.16 ± 1.17 mL (range, 3.14–5.95 mL), and the differences were significant. The average hematoma evacuation rate was 50.39% ± 7.71% (range, 41.65%–63.23%). Four of the seven patients underwent EVD first (57.1%), and one underwent EVD 2 days after hematoma puncture and drainage surgery. The average operation time was 82.14 ± 15.74 min, the average blood loss was 32.2 ± 8.14 mL, the average deviation of the drainage tube target was 4.58 ± 0.72 mm (range, 3.36–5.32 mm), and the average depth of the implantable drainage tube was 62.73 ± 0.94 mm (range, 61.42–64.23 mm). Three patients were injected with urokinase after surgery, and the average retention time of the drainage tube was 53.56 ± 7.83 h.
There were no intraoperative deaths in seven patients. Two patients had slight intraoperative fluctuations in vital signs. The most common postoperative comorbidity was pneumonia (7/7, 100%), followed by gastrointestinal bleeding (5/7, 71.43%). There were no rebleeding incidents, ischemic stroke, intracranial infection, or epilepsy within 2 weeks after surgery. The preoperative high fever symptoms were relieved after surgery. Only one patient died due to pneumonia 12 days after surgery, one patient gave up 20 days after surgery. Two patients were conscious and three patients were still in a coma 1 month after surgery.
The average preoperative GCS was 6.57 ± 1.51, and the average postoperative GCS was 10.00 ± 2.83 1 month after surgery. The improvement was statistically significant. The representative cases are shown in Figure 2 and Figure 3.
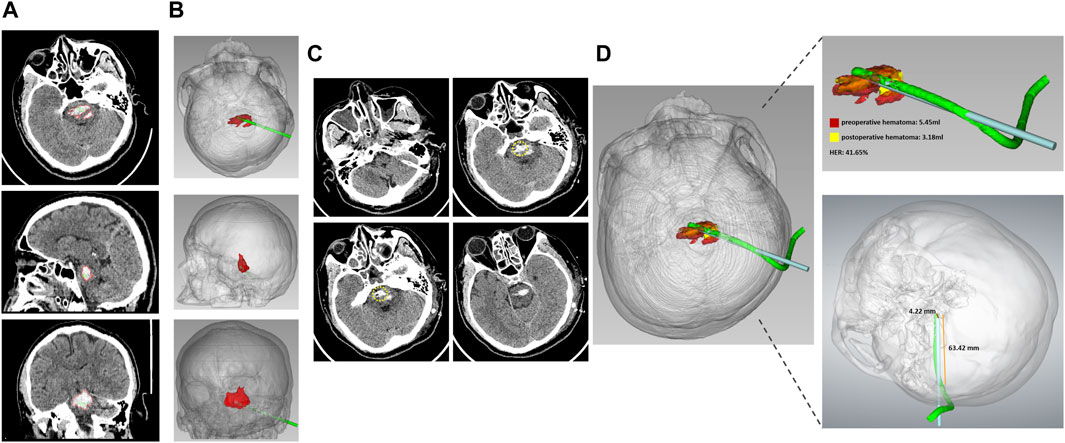
Figure 2. The representative case 2 (A) Preoperative CT showed PBH in the axial, sagittal, and coronal planes. (B) The 3D model constructed from CT images showed hematoma and designed the puncture trajectory from the axial, sagittal, and coronary positions. (C) Postoperative CT of the axial plane showed that the drainage tube location was precise. The yellow circle indicated the tip of the drainage tube. (D) Fusion of preoperative and postoperative 3D model showed that the preoperative hematoma volume was 5.45 mL, the postoperative hematoma volume was 3.18 mL, the hematoma evacuation rate was 41.65%, the deviation of the target drainage tube was 4.22 mm, and the depth of the implantable drainage tube was 63.42 mm.
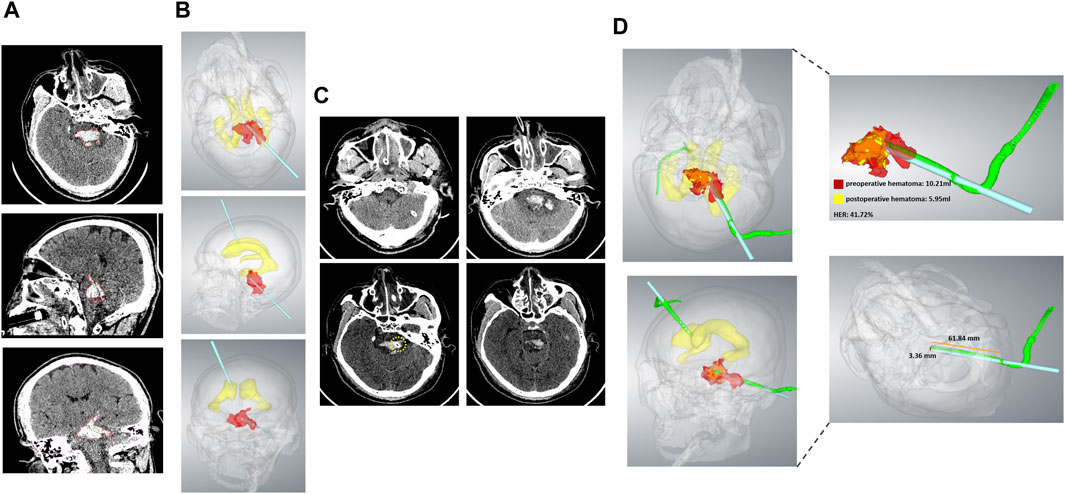
Figure 3. The representative case 5. (A) Preoperative CT showed PBH in the axial, sagittal, and coronal planes. (B) The 3D model constructed from CT images showed hematoma, lateral ventricular, and a designed puncture trajectory from axial, sagittal, and coronary positions. (C) Postoperative CT of the axial plane showed that the drainage tube location was precise. The yellow circle indicated the tip of the drainage tube. (D) Fusion of the preoperative and postoperative 3D model showed that the preoperative hematoma volume was 10.21 mL, the postoperative hematoma volume was 5.95 mL, the hematoma evacuation rate was 41.72%, the deviation of the drainage tube target was 3.36 mm. The depth of the implantable drainage tube was 61.84 mm.
Discussion
The brainstem is small, deep in the skull, and includes the midbrain, pons, and medulla oblongata. The brainstem is the center of life, controlling respiration, heart rate, blood pressure, and body temperature. About 60%–80% of PBH occurs in the pons due to the rupture of the perforating vessels of the basilar artery [1, 2]. Hypertension is one of the most common causes of severe cerebrovascular disease. By causing mechanical and chemical damage to essential structures in the brainstem, such as the nucleus clusters and the reticular system, the hematoma quickly induces clinical symptoms such as coma, central hyperthermia, tachycardia, abnormal pupils, and hypotension. The prognosis is extremely poor, which presents a challenge to existing treatment methods.
The conservative treatment strategy for PBH is mainly related to the hypertensive treatment strategy for ICH [11]. Since the primary damage of PBH is irreversible, surgical treatment is believed to relieve mechanical compression of the hematoma and prevent secondary injury, improving prognosis [1, 12, 13]. However, there have been some controversies about surgical treatment. Due to the high mortality and disability rate of PBH, it is necessary to strictly evaluate the indications for surgery. Indications for surgery proposed by Shresha included a hematoma volume greater than 5 mL, a relatively concentrated hematoma, GCS less than 8, progressive neurological dysfunction, and uneventful vital signs, particularly requiring ventilatory assistance [14]. Huang established a brainstem hemorrhage scoring system and suggested patients with a score of 2–3 might benefit from surgical treatment. A score of 4 was a contraindication to surgical treatment [15]. A review of 10 cohort studies showed that the patients in the surgical group were 45–65 years old, unconscious, with a GCS of 3–8, and the hematoma volume was approximately 8 mL. The surgical group had a better prognosis and lower mortality than the conservative treatment group. The research also suggested that older age and coma were not contraindications for brainstem hemorrhage surgery [16]. According to the Chinese guidelines for brainstem hemorrhage, we specified the following surgical indications: age 18–80 years old, hematoma volume greater than 5 mL and less than 15 mL, hematoma diameter greater than 2 cm, hematoma deviated to one side or the dorsal side, GCS less than 8, surgery performed within 6–24 h after onset, and family consent [8].
The surgical treatments for PBH included microscopic craniotomy to evacuate the hematoma, which removed the hematoma as much as possible, performed hemostasis, and removed the fourth ventricular hematoma to smooth the circulation of cerebrospinal fluid. However, this technology required various intraoperative monitoring methods and proficient surgical skills. The most widely chosen method was stereotactic hematoma puncture and drainage surgery. To achieve precise puncture of the brainstem hematoma, surgeons had used invasive stereotaxic frames [17], robot-assisted navigation systems [18], the 3D printing technology navigation method [19], and laser combined with CT navigation technology [13]. The above techniques had shortcomings, including invasive placement positioning framework, the risk of skull bleeding and infection, expensive costs of robot-assisted and neuronavigation systems, the lengthy procedure of 3D printing technology, etc.
We innovatively used MRNT to perform brainstem hematoma puncture and drainage surgery. Our team used this technology to successfully perform intracranial foreign body removal [20] and minimally invasive puncture surgery for deep ICH, with a deviation of the drainage tube target of 5.76 ± 0.80 mm [9]. Based on previous experience and technical improvement, we applied technology to perform brainstem hematoma puncture and drainage surgery. The average volume of preoperative brainstem hematoma was 8.47 ± 2.22 mL, postoperative brainstem hematoma was 4.16 ± 1.17 mL, and the average hematoma evacuation rate was 50.39% ± 7.71%, which prevented hematoma primary compression and secondary injury. The surgical procedure under general anesthesia took an average of 82.14 ± 15.74 min, the average target deviation was 4.58 ± 0.72 mm, and the average depth of the implantable drainage tube was 62.73 ± 0.94 mm. The depth of the drainage tube was longer than that in the application of deep ICH, which required higher precision. Moreover, we found MRNT was safe in seven patients.
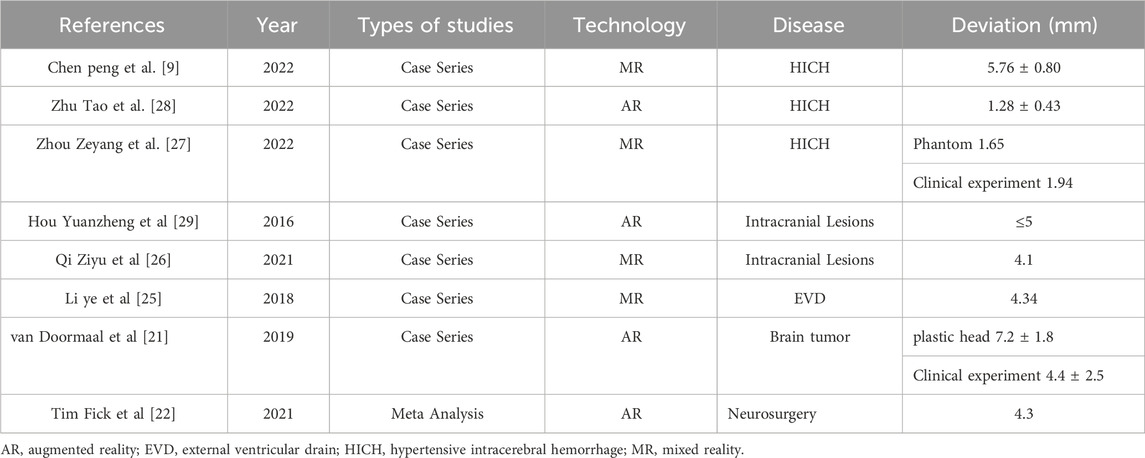
A comparison of the precision of augmented reality technology, mixed reality technology, and traditional stereotactic methods have been discussed in previous literature. Van Doormaal et al. conducted a holographic navigation study using augmented reality technology. They found that the fiducial registration error was 7.2 mm in a plastic head model, and the fiducial registration error was 4.4 mm in three patients [21]. A meta-analysis was conducted to systematically review the accuracy of augmented reality neuronavigation and compare it with conventional infrared neuronavigation. In 35 studies, the average target registration error of 2.5 mm in augmented reality technology was no different from that of 2.6 mm in traditional infrared navigation [22]. Moreover, In the study of neuronavigation using mixed reality technology, the researchers received a target deviation range of 4–6 mm [23–25].
The augmented reality technology application scenarios mainly involve intracranial tumors and rarely involve ICH. Qi et al. used mixed reality navigation technology to perform ICH surgery. They also used markers for point registration and image fusion. The results showed that the occipital hematoma puncture deviation was 5.3 mm due to the prone and supine position, and the deviation in the basal ganglia was 4.0 mm [26]. Zhou et al. also presented a novel multi-model mixed reality navigation system for hypertensive ICH surgery. The results of the phantom experiments revealed a mean registration error of 1.03 mm. The registration error was 1.94 mm in clinical use, which showed that the system was sufficiently accurate and effective for clinical application [27]. A summary of the deviations in the application of MR or AR was provided in Table 2.
In addition to precision puncture and hematoma drainage, surgical treatment of PBH also required further discussion on the timing of surgery, external ventricular drainage, and fibrinolytic drugs. Shrestha et al. found that surgical treatment within 6 h after onset was associated with a good prognosis [14]. The ultra-early operation alleviated the hematoma mass effect and reduced secondary injury. In particular, for patients with a severe condition, early hematoma aspiration could immediately eliminate harmful effects and prevent worse clinical outcomes [17] However, many primary hospitals are not equipped with PBH surgical treatment abilities. Patients have to waste a lot of time in the transfer process, which is a big challenge in clinical treatment. PBH can also cause cerebrospinal fluid circulation disorder that induces patients to become unconscious. External ventricular drainage is beneficial in improving cerebrospinal fluid circulation, managing intracranial pressure, and facilitating patient recovery [17]. In our study, external ventricular drainage was performed in five cases of seven patients. Previous research investigating the effects of rtPA on ICH and ventricular hemorrhage by MISTIE and CLAEA demonstrated that fibrinolytic drug administration did not increase the risk of hemorrhage [30–33]. Currently, there is no evidence and consensus to verify the effects of the thrombolytic drug used in PBH. We also found that urokinase did not increase the risk of bleeding and improve drainage efficiency, as reported in previous literature [13, 18].
Compared with the expensive neuronavigation system, mixed reality navigation technology was an independent research and development project, the equipment of the technology was simple, and the cost was low. The effect of the technology met the clinical application of intracerebral hemorrhage surgery, and was beneficial to popularization for primary hospital.
There were also some limitations in our technology. Firstly, in order to introduce our innovative mixed reality navigation technology earlier and faster, we reported few cases, so there are not enough data to verify the advancement of the technology. At present, it was difficult to perform a cohort study because of the small number of patients enrolled. We plan to carry out clinical study with other centers in the future. Secondly, navigation technology was mainly based on point-matching technology, which enabled the fusion of the image model with the actual space through markers. Implementing invasive markers in the skull might carry potential risks of bleeding or infection. Moreover, the procedure required CT examinations before surgery, which delayed surgery time, and increased costs. Some researchers proposed the face registration plan, but the target deviation of the face registration was higher than that of the point registration, and the clinical practicability was poor [34]. Clinical practice must explore a precise, simple, fast, and noninvasive matching and fusion innovative solution.
Conclusion
It was feasible and safe to perform brainstem hematoma puncture and drainage by MRNT. Early minimally invasive precise surgery could prevent hematoma primary and secondary injury, and improve the prognosis of patients with PBH. The advantages included high precision in dual-plane navigation technology, low cost, an immersive operation experience, etc. Furthermore, improving the matching registration method and performing high-quality prospective clinical research was necessary.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Chongqing Emergency Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XT: Writing–original draft, Data curation, Software. YaW: Writing–original draft. GT: Conceptualization, Project administration, Writing–original draft. YiW: Investigation, Resources, Software, Writing–original draft. WX: Resources, Formal Analysis, Writing–original draft, Writing–review and editing. YL: Methodology, Writing–original draft. YD: Writing–review and editing. PC: Writing–review and editing, Conceptualization, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Fundamental Research Funds for the Central Universities (2022CDJYGRH-015) and Medical Research Project of Science and Technology Bureau and Health Commission, Chongqing, China (2023MSXM076).
Conflict of interest
Author YiW was employed by Qinying Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen P, Yao H, Tang X, Wang Y, Zhang Q, Liu Y, et al. Management of primary brainstem hemorrhage: a review of outcome prediction, surgical treatment, and animal model. Dis Markers (2022) 2022:1–8. doi:10.1155/2022/4293590
2. Chen D, Tang Y, Nie H, Zhang P, Wang W, Dong Q, et al. Primary brainstem hemorrhage: a review of prognostic factors and surgical management. Front Neurol (2021) 12:727962. doi:10.3389/fneur.2021.727962
3. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol (2010) 9:167–76. doi:10.1016/s1474-4422(09)70340-0
4. Behrouz R. Prognostic factors in pontine haemorrhage: a systematic review. Eur Stroke J (2018) 3:101–9. doi:10.1177/2396987317752729
5. Balci K, Asil T, Kerimoglu M, Celik Y, Utku U. Clinical and neuroradiological predictors of mortality in patients with primary pontine hemorrhage. Clin Neurol Neurosurg (2005) 108:36–9. doi:10.1016/j.clineuro.2005.02.007
6. Hong JT, Choi SJ, Kye DK, Park CK, Lee SW, Kang JK. Surgical outcome of hypertensive pontine hemorrhages: experience of 13 cases. J Korean Neurosurg Soc (1998) 27:59–65.
7. Takahama H, Morii K, Sato M, Sekiguchi K, Sato S. Stereotactic aspiration in hypertensive pontine hemorrhage: comparative study with conservative therapy. No Shinkei Geka (1989) 17:733–9.
8. Chen L, Chen T, Mao G, Chen B, Li M, Zhang H, et al. Clinical neurorestorative therapeutic guideline for brainstem hemorrhage (2020 China version). J Neurorestoratology (2020) 8:232–40. doi:10.26599/jnr.2020.9040024
9. Peng C, Yang L, Yi W, Yidan L, Yanglingxi W, Qingtao Z, et al. Application of fused reality holographic image and navigation technology in the puncture treatment of hypertensive intracerebral hemorrhage. Front Neurosci (2022) 16:850179. doi:10.3389/fnins.2022.850179
10. Chung CS, Park CH. Primary pontine hemorrhage: a new CT classification. Neurology (1992) 42(4):830–4. doi:10.1212/wnl.42.4.830
11. Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American heart association/American stroke association. Stroke (2022) 53:e282–e361. doi:10.1161/str.0000000000000407
12. Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol (2012) 11:101–18. doi:10.1016/s1474-4422(11)70264-2
13. Wang Q, Guo W, Zhang T, Wang S, Li C, Yuan Z, et al. Laser navigation combined with XperCT technology assisted puncture of brainstem hemorrhage. Front Neurol (2022) 13:905477. doi:10.3389/fneur.2022.905477
14. Shrestha BK, Ma L, Lan Z, Li H, You C. Surgical management of spontaneous hypertensive brainstem hemorrhage. Interdiscip Neurosurg (2015) 2:145–8. doi:10.1016/j.inat.2015.06.005
15. Huang K, Ji Z, Sun L, Gao X, Lin S, Liu T, et al. Development and validation of a grading Scale for primary pontine hemorrhage. Stroke (2017) 48:63–9. doi:10.1161/strokeaha.116.015326
16. Zheng WJ, Shi SW, Gong J. The truths behind the statistics of surgical treatment for hypertensive brainstem hemorrhage in China: a review. Neurosurg Rev (2022) 45:1195–204. doi:10.1007/s10143-021-01683-2
17. Du L, Wang JW, Li CH, Gao BL. Effects of stereotactic aspiration on brainstem hemorrhage in a case series. Front Surg (2022) 9:945905. doi:10.3389/fsurg.2022.945905
18. Zhang S, Chen T, Han B, Zhu W. A retrospective study of puncture and drainage for primary brainstem hemorrhage with the assistance of a surgical robot. Neurologist (2023) 28:73–9. doi:10.1097/nrl.0000000000000445
19. Wang Q, Guo W, Liu Y, Shao W, Li M, Li Z, et al. Application of a 3D-printed navigation mold in puncture drainage for brainstem hemorrhage. J Surg Res (2020) 245:99–106. doi:10.1016/j.jss.2019.07.026
20. Li Y, Huang J, Huang T, Tang J, Zhang W, Xu W, et al. Wearable mixed-reality holographic navigation guiding the management of penetrating intracranial injury caused by a nail. J Digit Imaging (2021) 34:362–6. doi:10.1007/s10278-021-00436-3
21. van Doormaal TPC, van Doormaal JAM, Mensink T. Clinical accuracy of holographic navigation using point-based registration on augmented-reality glasses. Oper Neurosurg (Hagerstown) (2019) 17:588–93. doi:10.1093/ons/opz094
22. Fick T, van Doormaal JAM, Hoving EW, Willems PWA, van Doormaal TPC. Current accuracy of augmented reality neuronavigation systems: systematic review and meta-analysis. World Neurosurg (2021) 146:179–88. doi:10.1016/j.wneu.2020.11.029
23. Incekara F, Smits M, Dirven C, Vincent A. Clinical feasibility of a wearable mixed-reality device in neurosurgery. World Neurosurg (2018) 118:e422–7. doi:10.1016/j.wneu.2018.06.208
24. McJunkin JL, Jiramongkolchai P, Chung W, Southworth M, Durakovic N, Buchman CA, et al. Development of a mixed reality platform for lateral skull base anatomy. Otol Neurotol (2018) 39:e1137–42. doi:10.1097/mao.0000000000001995
25. Li Y, Chen X, Wang N, Zhang W, Li D, Zhang L, et al. A wearable mixed-reality holographic computer for guiding external ventricular drain insertion at the bedside. J Neurosurg (2018) 1–8. doi:10.3171/2018.4.JNS18124
26. Qi Z, Li Y, Xu X, Zhang J, Li F, Gan Z, et al. Holographic mixed-reality neuronavigation with a head-mounted device: technical feasibility and clinical application. Neurosurg Focus (2021) 51:E22. doi:10.3171/2021.5.focus21175
27. Zhou Z, Yang Z, Jiang S, Zhuo J, Zhu T, Ma S. Surgical navigation system for hypertensive intracerebral hemorrhage based on mixed reality. J Digit Imaging (2022) 35:1530–43. doi:10.1007/s10278-022-00676-x
28. Zhu T, Jiang S, Yang Z, Zhou Z, Li Y, Ma S, et al. A neuroendoscopic navigation system based on dual-mode augmented reality for minimally invasive surgical treatment of hypertensive intracerebral hemorrhage. Comput Biol Med (2022) 140:105091. doi:10.1016/j.compbiomed.2021.105091
29. Hou Y, Ma L, Zhu R, Chen X, Zhang J. A low-cost iPhone-assisted augmented reality solution for the localization of intracranial lesions. PLoS One (2016) 11(7):e0159185. doi:10.1371/journal.pone.0159185
30. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet (2019) 393:1021–32. doi:10.1016/s0140-6736(19)30195-3
31. Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet (2017) 389:603–11. doi:10.1016/s0140-6736(16)32410-2
32. Montes JM, Wong JH, Fayad PB, Awad IA. Stereotactic computed tomographic-guided aspiration and thrombolysis of intracerebral hematoma: protocol and preliminary experience. Stroke (2000) 31:834–40. doi:10.1161/01.str.31.4.834
33. Vespa P, McArthur D, Miller C, O'Phelan K, Frazee J, Kidwell C, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care (2005) 2:274–81. doi:10.1385/ncc:2:3:274
Keywords: primary brainstem hemorrhage, mixed reality navigation technology, brainstem hematoma puncture and drainage surgery, neuronavigation, deviation
Citation: Tang X, Wang Y, Tang G, Wang Y, Xiong W, Liu Y, Deng Y and Chen P (2024) Application of mixed reality navigation technology in primary brainstem hemorrhage puncture and drainage surgery: a case series and literature review. Front. Phys. 12:1390236. doi: 10.3389/fphy.2024.1390236
Received: 23 February 2024; Accepted: 26 March 2024;
Published: 17 April 2024.
Edited by:
Guanqiu Qi, Buffalo State College, United StatesReviewed by:
Peng Zhang, First Affiliated Hospital of Wenzhou Medical University, ChinaGang Wu, Fudan University, China
Tao Li, Nanjing University, China
Copyright © 2024 Tang, Wang, Tang, Wang, Xiong, Liu, Deng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongbing Deng, 1422495819@qq.com; Peng Chen, chenpengpph@163.com
†These authors share first authorship
 Xiaoyong Tang1†
Xiaoyong Tang1†  Yongbing Deng
Yongbing Deng Peng Chen
Peng Chen