- 1Laboratory of Pulmonary Investigation, Carlos Chagas Filho Biophysics Institute, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- 2Department of Physiology and Pharmacology, Biomedical Institute, Fluminense Federal University, Niterói, Brazil
- 3Laboratory of Cellular and Molecular Physiology, Carlos Chagas Filho Biophysics Institute, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- 4Department of Pathology, School of Medicine, University of São Paulo, São Paulo, Brazil
- 5Nuclear Medicine Service, Clementino Fraga Filho University Hospital, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- 6Department of Intensive Care, Laboratory of Experimental Intensive Care and Anesthesiology (L.E.I.C.A.), Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
- 7Department of Anesthesiology and Intensive Care Therapy, Pulmonary Engineering Group, University Hospital Carl Gustav Carus, Dresden University of Technology, Dresden, Germany
- 8Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy
- 9San Martino Policlinico Hospital, IRCCS for Oncology and Neurosciences, Genoa, Italy
Intraoperative positive end-expiratory pressure (PEEP) has been proposed to restore lung volumes and improve respiratory function in obesity. However, the biological impact of different PEEP levels on the lungs in obesity remains unknown. We aimed to compare the effects of PEEP = 2 cmH2O versus PEEP = 6 cmH2O during ventilation with low tidal volumes on lung function, histology, and biological markers in obese and non-obese rats undergoing open abdominal surgery. Forty-two Wistar rats (21 obese, 21 non-obese) were anesthetized and tracheotomized, and laparotomy was performed with standardized bowel manipulation. Rats were randomly ventilated with protective tidal volume (7 ml/kg) at PEEP = 2 cmH2O or PEEP = 6 cmH2O for 4 h, after which they were euthanized. Lung mechanics and histology, alveolar epithelial cell integrity, and biological markers associated with pulmonary inflammation, alveolar stretch, extracellular matrix, and epithelial and endothelial cell damage were analyzed. In obese rats, PEEP = 6 cmH2O compared with PEEP = 2 cmH2O was associated with less alveolar collapse (p = 0.02). E-cadherin expression was not different between the two PEEP groups. Gene expressions of interleukin (IL)-6 (p = 0.01) and type III procollagen (p = 0.004), as well as protein levels of tumor necrosis factor-alpha (p = 0.016), were lower at PEEP = 6 cmH2O than at PEEP = 2 cmH2O. In non-obese animals, PEEP = 6 cmH2O compared with PEEP = 2 cmH2O led to increased hyperinflation, reduced e-cadherin (p = 0.04), and increased gene expression of IL-6 (p = 0.004) and protein levels of tumor necrosis factor-alpha (p-0.029), but no changes in fibrogenesis. In conclusion, PEEP = 6 cmH2O reduced lung damage and inflammation in an experimental model of mechanical ventilation for open abdominal surgery, but only in obese animals.
Introduction
Several intraoperative ventilator strategies may prevent lung damage. Randomized clinical trials of intraoperative ventilation for abdominal surgery (Futier et al., 2013; Hemmes et al., 2014; Ferrando et al., 2018) have compared diverse ventilation strategies with respect to development of postoperative pulmonary complications (PPCs). In the IMPROVE trial (Futier et al., 2013), low tidal volume (VT) with moderate positive end-expiratory pressure (PEEP) and recruitment maneuvers (RMs) resulted in fewer PPCs compared with high-VT and no PEEP. In the PROVHILO trial (Hemmes et al., 2014), low-VT with high-PEEP levels and RMs, compared with low-VT and low-PEEP without RMs, did not protect against PPCs. In the iPROVE trial (Ferrando et al., 2018), higher PEEP or individualized PEEP setting compared with lower PEEP did not result in fewer PPCs. In an animal model of open abdominal surgery, both low-VT and moderate to high-PEEP and RMs resulted in lower driving pressure, mechanical power, and lung damage (Maia et al., 2017). The PROBESE trial (Bluth et al., 2019) compared high-PEEP and RM versus low-PEEP at low VT and found no significant differences in PPCs.
Obesity is a growing problem worldwide, which means that an increasing number of surgeries are being performed in obese patients (Schumann et al., 2015). Obese patients undergoing anesthesia have reduced lung volumes (Silva et al., 2012), which can be exacerbated by low VT, increasing atelectasis (Goldenberg et al., 2014). Intraoperative PEEP has been proposed to restore lung volumes and improve respiratory function (Reinius et al., 2009; Hedenstierna and Edmark, 2010; Imber et al., 2016). However, the biological impact of different levels of PEEP on the lungs in obesity remains unknown. We hypothesized that a protective tidal volume with a PEEP of 6 cmH2O (high PEEP for rats) might result in less mechanical and biological stress compared to a PEEP of 2 cmH2O (low PEEP for rats) during open abdominal surgery under general anesthesia in obese rats. The present study aimed to evaluate the impact of mechanical ventilation with high and low PEEP, both under low VT, on lung mechanics and histology, alveolar epithelial cell integrity, and biological markers associated with pulmonary inflammation, alveolar stretch, extracellular matrix, and epithelial and endothelial cell damage during open abdominal surgery in non-obese and obese rats.
Materials and Methods
Ethics Statement
This study was approved by the Research Ethics Committee of the Federal University of Rio de Janeiro Health Sciences Center (CEUA-117/16), Rio de Janeiro, Brazil (chair: Prof. Marcel Frajblat). All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the U.S. National Academy of Sciences. Experiments conformed with the “European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes” (Council of Europe No 123, Strasbourg 1985), and the present study followed the ARRIVE guidelines for reporting of animal research (Kilkenny et al., 2010).
Animal Preparation and Experimental Protocol
Forty-two Wistar rats were kept in a temperature-controlled room (23–24°C) with artificial dark–light cycles (lights on at 7 am. and off at 7 pm.). Virgin female rats (3 months old) were caged with male rats at a proportion of 3:1. During pregnancy and lactation, each female was housed in an individual cage with free access to water and food (commercial rat chow). To induce postnatal obesity (Ob group), 3 days after birth, litters were culled to three males per litter. In non-obese animals (nonOb group), the litter size was adjusted to 10 pups per litter. After weaning (day 21), both nonOb and Ob animals received commercial diet. From postnatal days 21 to 180, offspring body weight (g) was monitored every 7 days. At postnatal day 150, chest computed tomography was performed in nonOb (n = 14) and Ob animals (n = 14) to characterize obesity (see Supplementary Figure S1, Supplementary Digital Content S1, which describes additional methods).
At postnatal day 180, nonOb (n = 21) and Ob (n = 21) animals were sedated (diazepam 10 mg/kg intraperitoneally) and anesthetized (ketamine 75 mg/kg and midazolam 2 mg/kg intraperitoneally). The tail vein was cannulated (Jelco 24G, Becton, Dickinson and Company, New Jersey, United States) for continuous infusion of 50 mg.kg–1.h–1 ketamine, 2 mg.kg–1.h–1 midazolam, and 7 mL.kg–1.h–1 Ringer’s lactate (B. Braun, Rio de Janeiro, Brazil) during mechanical ventilation. Gelafundin® 4% (B. Braun, São Gonçalo, RJ, Brazil) was administered intravenously (in 0.5-mL increments) as needed to maintain mean arterial pressure (MAP) >60 mmHg. Depth of anesthesia was evaluated by the response to light touch with a fingertip on the rat’s whiskers (0 = awake, fully responsive to surroundings; 1 = not responsive to surroundings, rapid response to whisker stimulation; 2 = slow response; 3 = unresponsive to whisker stimulation), pupil diameter, position of the nictitating membrane, and movement in response to tail stimulation (Heil et al., 2016). Experiments were started when responses to a noise stimulus (handclap), whisker stimulation, and tail clamping were absent.
Local anesthetic (2% lidocaine, 0.4 mL) was infiltrated and a tracheostomy was performed via a midline neck incision for a 14-gauge cannula.
A catheter (18G, Arrow International, United States) was placed in the right internal carotid artery for blood sampling and gas analysis (Radiometer ABL80 FLEX, Copenhagen, NV, Denmark), as well as monitoring of MAP. Heart rate (HR), MAP, and rectal temperature were continuously monitored (Networked Multiparameter Veterinary Monitor LifeWindow 6000V, Digicare Animal Health, Boynton Beach, FL, United States). Body temperature was maintained at 37.5 ± 1°C using a heating bed.
Animals were then paralyzed (pancuronium 0.4 mg intramuscularly, followed by 1 mg/kg/h intravenously) (Spieth et al., 2015) and mechanically ventilated (Servo-I; MAQUET, Solna, Sweden) in volume-controlled mode with VT = 7 mL/kg, RR to maintain normocapnia (PaCO2 = 35–45 mmHg; around 45 bpm), inspiratory-to-expiratory ratio = 1:2, fraction of inspired oxygen = 0.4, and PEEP = 2 cmH2O for 5 min (Baseline). Arterial blood (300 μL) was drawn into a heparinized syringe to determine arterial oxygen partial pressure (PaO2), arterial carbon dioxide partial pressure (PaCO2), and arterial pH (pHa; ABL80 FLEX, Radiometer, Copenhagen, Denmark). Blood gas analysis was also performed 10 min after laparotomy and at the end of the experiments. FiO2 was maintained at 0.4 throughout the experiments. Obese and non-obese rats were then randomly assigned, using closed sealed envelopes, to receive the same protective VT (7 mL/kg) and two different PEEP levels: 2 cmH2O (PEEP2) and 6 cmH2O (PEEP6).
After group allocation (n = 7/group), laparotomy was performed, and animals were ventilated in volume-controlled mode for 4 h. The respiratory rate (RR) was adjusted to reach a minute ventilation of 160 mL/min. At the start of mechanical ventilation and every hour thereafter, a standardized bowel manipulation was performed as follows: under sterile conditions, lateral retractors were carefully placed, the bowel was gently taken out of the abdominal cavity and reintroduced within 20 s. The retractors were left in place, and the abdominal cavity was continuously humidified with warmed saline at 37°C. Lung mechanics, heart rate, MAP, and the amount of fluids infused were measured hourly. At the end of the experiment, animals were killed by injection of sodium thiopental (60 mg/kg), the lungs were extracted for postmortem analysis, and visceral fat mass was excised (mesenteric, epididymal and retroperitoneal white adipose tissue) and immediately weighed for evaluation of central adiposity. Seven nonOb and Ob rats were not tracheotomized, operated, or mechanically ventilated, and constituted the healthy, non-operated and non-ventilated control groups (NV-nonOb and NV-Ob, respectively).
Respiratory Data Acquisition and Processing
Airflow, as well as airway (Paw) and esophageal (Pes) pressures, were continuously measured. A pneumotachograph (internal diameter = 1.5 mm, length = 4.2 cm, distance between side ports = 2.1 cm) was connected to the tracheal cannula for airflow (V’) measurements (Mortola and Noworaj, 1983). The pressure gradient across the pneumotachograph was determined using a SCIREQ differential pressure transducer (UT-PDP-02, SCIREQ, Montreal, QC, Canada). VT was calculated by digital integration of the airflow signal obtained from the pneumotachograph, connected to the Y-piece of the ventilator tubing, while RR was calculated from the Pes swings as the frequency per minute of each type of breathing cycle. Airway pressure was measured with a SCIREQ differential pressure transducer (UT-PDP-75, SCIREQ, Montreal, QC, Canada). Esophageal pressure was measured using a 30-cm-long water-filled catheter (PE-205, Becton, Dickinson and Company) with side holes at the tip, connected to a differential pressure transducer (UT-PL-400, SCIREQ, Montreal, QC, Canada). The catheter was passed into the stomach and then slowly returned into the esophagus; its proper positioning was assessed with the “occlusion test”, as described elsewhere (Samary et al., 2015). Transpulmonary pressure (PL) was calculated during inspiration and expiration as the difference between Paw and Pes (Samary et al., 2015; Spieth et al., 2015; Felix et al., 2019). Values were recorded continuously using LabView-based software (National Instruments, Austin, TX). All signals were filtered (100 Hz), amplified in a 4-channel conditioner (SC-24; SCIREQ), and sampled at 200 Hz with a 12-bit analog-to-digital converter (National Instruments) (Felix et al., 2019).
Lung mechanics were assessed every hour by occluding the airway at end-inspiration for 5 s (Samary et al., 2015). Static lung elastance (Est,L = (P,Lend–insp-P,Lend–exp)/VT) and chest wall elastance (Est,w = (P,esend–insp-Pes,end–exp)/VT) were calculated [P,Lend–insp = P,rsend–insp (Paw at end inspiratory occlusion) – P,esend–insp (Pes at end-inspiratory occlusion) and P,Lend–exp = PEEP-Pes,end–exp (Pes at end-expiration)] offline using a routine written in MATLAB (version R2007a; The Mathworks Inc., Natick, MA, United States). All analyses were performed in a blinded manner, i.e., the observer was unaware of the experimental protocol.
Histology
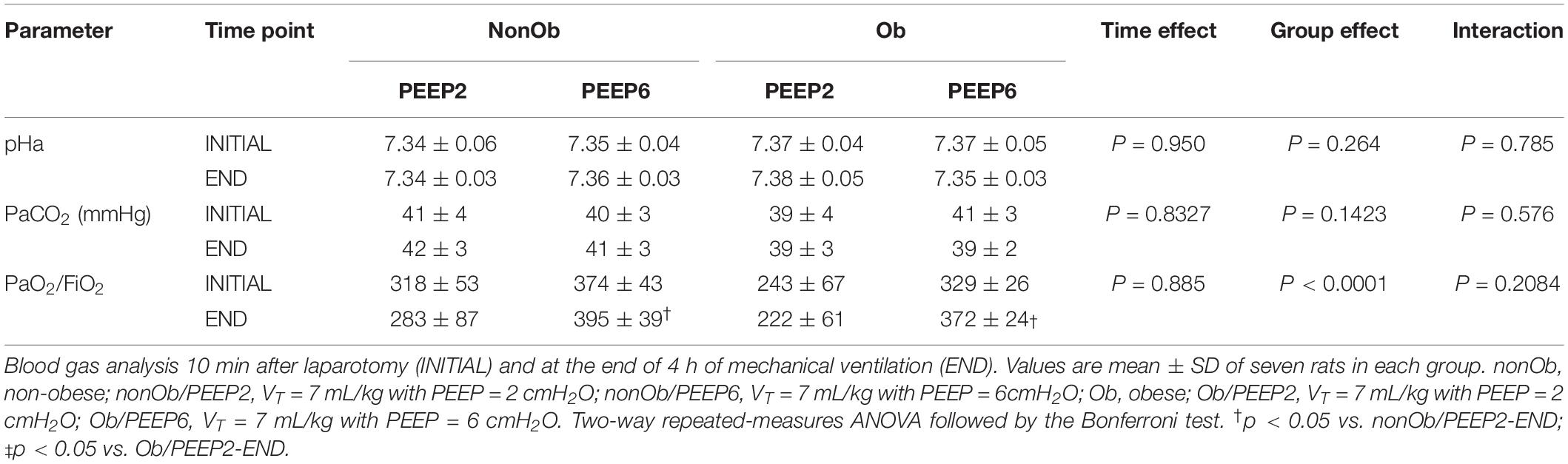
Immediately after euthanasia, heparin (1000 IU) was injected into the tail vein, the trachea was clamped at end-expiration, and the lungs were removed en bloc at PEEP = 3 cm H2O in all groups. The left lung was frozen in liquid nitrogen and submerged in Carnoy’s solution (Pássaro et al., 2009; Felix et al., 2019). Slices (4 μm thick) were stained with hematoxylin and eosin. Lung morphometric analysis was performed using an integrating eyepiece with a coherent system consisting of a grid with 100 points and 50 lines of known length coupled to a conventional light microscope (Olympus BX51, Olympus Latin America Inc., São Paulo, Brazil). The volume fractions of the lung occupied by collapsed alveoli (alveoli with rough or plicate walls) and hyperinflated structures (alveolar ducts, alveolar sacs, or alveoli wider than 120 μm) were determined by the point-counting technique at a magnification of × 200 across 10 random, non-coincident microscopic fields (Hsia et al., 2010; Wierzchon et al., 2017).
Immunohistochemistry
The right lower lung was immersed in immunohistochemistry solution. To evaluate the degree of epithelial cell damage, expression of E-cadherin (the major transmembrane protein of the adherens junction) was analyzed (Maia et al., 2017). Immunohistochemical procedures were performed on lung sections using a mouse polyclonal antibody against E-cadherin (cat. 610181, BD Transduction Laboratories, 1:300). Visualization and image capture were performed using a light microscope (Eclipse E800, Nikon, Japan) coupled to a digital camera (Evolution, Media Cybernetics Inc., Rockville, MD, United States) and Q-Capture 2.95.0 graphic interface software (version 2.0.5; Quantitative Imaging, Surrey, British Columbia, Canada). Expression of E-cadherin was analyzed using ImagePro Plus software (version 4.5.1, Media Cybernetics). The pathologist or technician working on lung morphometry and immunohistochemistry was blinded to the nature of the study.
Molecular Biology
The right middle lung was flash-frozen by immersion in liquid nitrogen and stored at −80°C for quantification of mRNA expression. Quantitative real-time reverse transcription polymerase chain reaction was performed to measure markers associated with inflammation [interleukin (IL)-6], mechanical pulmonary stretch (amphiregulin), epithelial cell injury [club cell secretory protein (CC-16)], endothelial cell damage [vascular cell adhesion molecule (VCAM)-1], and extracellular matrix damage [type III procollagen (PCIII), decorin, and metalloproteinase-9 (MMP-9)] (see Supplementary Table S1, Supplementary Digital Content S1, for primers). Central slices of the right lung were cut, collected in cryotubes, flash-frozen by immersion in liquid nitrogen, and stored at −80°C. Total RNA was extracted from frozen tissues using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s recommendations. RNA concentrations were measured by spectrophotometry in a Nanodrop ND-1000 system (Thermo Scientific, Wilmington, DE, United States). First-strand cDNA was synthesized from total RNA using a Quantitec reverse transcription kit (Qiagen, Hilden, Germany). Relative mRNA levels were measured by SYBR green detection in an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, United States). Samples were run in triplicate. For each sample, the expression of each gene was normalized to the acidic ribosomal phosphoprotein P0 (36B4) housekeeping gene and expressed as fold change relative to respective NV-nonOb (n = 7) and NV-Ob (n = 7) animals, using the 2–ΔΔCt method, where ΔCt = Ct (target gene)– Ct (reference gene) (Schmittgen and Livak, 2008).
Enzyme-Linked Immunosorbent Assay (ELISA)
The right upper lung was immediately frozen in liquid nitrogen and stored at −80°C for ELISA. Tumor necrosis factor (TNF)-α levels were quantified by ELISA in the lung tissue homogenate. All procedures were done according to the manufacturer’s protocol (PeproTech, London, United Kingdom) and normalized to total protein as assessed by Bradford’s reagent (Sigma-Aldrich, St Louis, MO, United States).
Statistical Analysis
The sample size calculation of each group was based on our experimental experience, which allowed detection of significant differences with the smallest possible number of animals, and on the respiratory effects observed in a previous study in rodents using comparable ventilator settings. A sample size of 7 animals per group would provide the appropriate power (1 − β = 0.8) to identify significant (α = 0.05) differences in IL-6 gene expression in lung tissue between ventilatory strategies based on PEEP2 and PEEP6 in obese animals (primary outcome), taking into account an effect size d = 1.76, a 2-sided test, and a sample size ratio = 1 (G∗Power 3.1.9.2, University of Düsseldorf, Düsseldorf, Germany). The secondary outcomes were lung mechanics and histology, alveolar epithelial integrity, and pulmonary inflammation.
Variables were tested for normality using the Kolmogorov–Smirnov test. Parametric data were expressed as means ±SD, and non-parametric data as median (interquartile range). To compare lung mechanics and blood gas analysis over time, two-way repeated-measures ANOVA followed by the Bonferroni test was used. Fraction area of alveolar collapse and hyperinflation, E-cadherin expression, and the protein levels of TNF-α in lung tissue between nonOb and Ob groups ventilated with PEEP6 or PEEP2 were compared using two-way ANOVA followed by Bonferroni’s test. Obese and non-obese non-ventilated animals presented different biological behavior; thus, the expression of biological markers in the Ob and nonOb groups ventilated with PEEP6 or PEEP2 was compared separately using the Mann–Whitney U test. All tests were performed in GraphPad Prism version 6.07 (GraphPad Software, San Diego, CA, United States). The significance level was set at 5%.
Results
A characterization of the rat model of obesity used in this study is presented in Supplementary Digital Content S2 (see Supplementary Figures S2–S4, which describe the comparisons between nonOb and Ob animals). The presentation of respiratory parameters and mean arterial pressure of nonOb and Ob animals at Baseline, are presented in Supplementary Digital Content S2 (Supplementary Table S2). Est,L and mean arterial pressure were higher and PaO2/FiO2 were lower in Ob than in nonOb animals, regardless of PEEP levels. No significant differences were observed in the amount of fluids between groups: mean ±SD, nonOb/PEEP2 = 19 ± 5 mL; nonOb/PEEP6 = 15 ± 2 mL; Ob/PEEP2 = 20 ± 4 mL; Ob/PEEP6 = 13 ± 7 mL.
Effects of PEEP in Obese and Non-obese Rats
Obese Rats
At END, PaO2/FiO2 was higher in Ob/PEEP6 than Ob/PEEP2, with no significant differences in pHa and PaCO2 (Table 1). Est,L and Est,w did not differ significantly between PEEP levels (Table 2). Alveolar collapse was lower in the Ob/PEEP6 group (Figure 1). No difference was observed in E-cadherin expression (Figure 2). Amphiregulin, CC-16, VCAM-1, decorin, and MMP-9 gene expressions in lung tissue did not differ between the Ob/PEEP6 and Ob/PEEP2 groups (Supplementary Digital Content S2, Supplementary Figure S5). On the other hand, IL-6 and PCIII gene expressions (Figure 3) and protein levels of TNF-α (Figure 4) were lower in Ob/PEEP6 compared with Ob/PEEP2 animals.
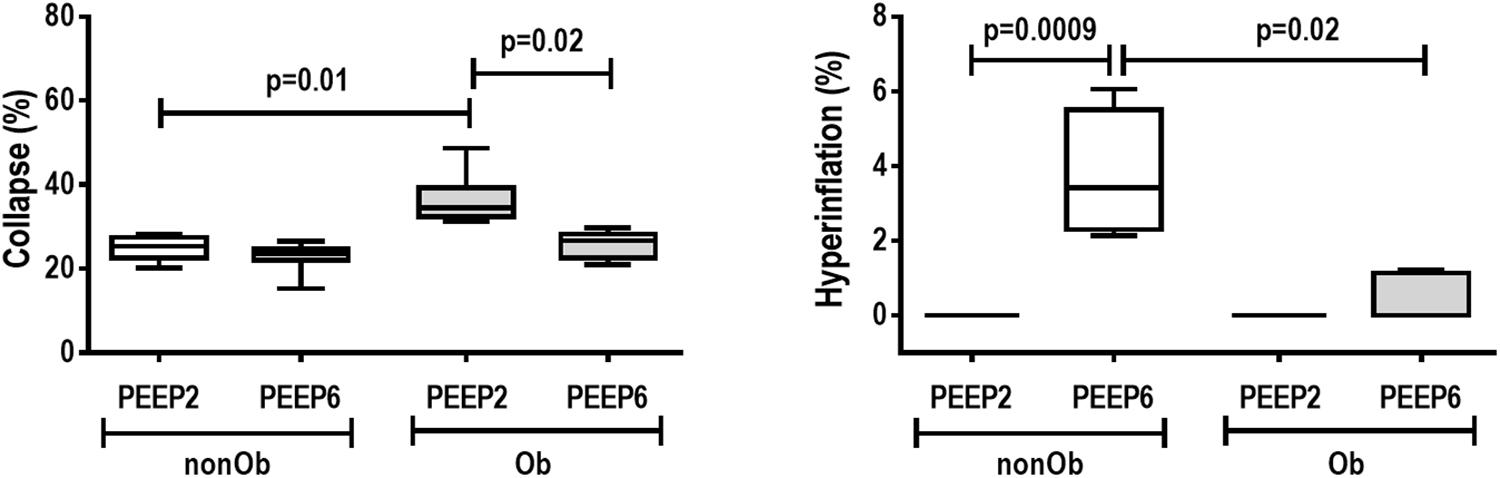
Figure 1. Lung morphometry in mechanically ventilated non-obese (nonOb) and obese (Ob) groups: Low-PEEP (2 cmH2O) and High-PEEP (6 cmH2O). Fraction area of alveolar collapse and lung hyperinflation. Boxes show the interquartile range (25th–75th percentile), while whiskers encompass the range (minimum-maximum) and horizontal lines represent the median in seven animals/group.
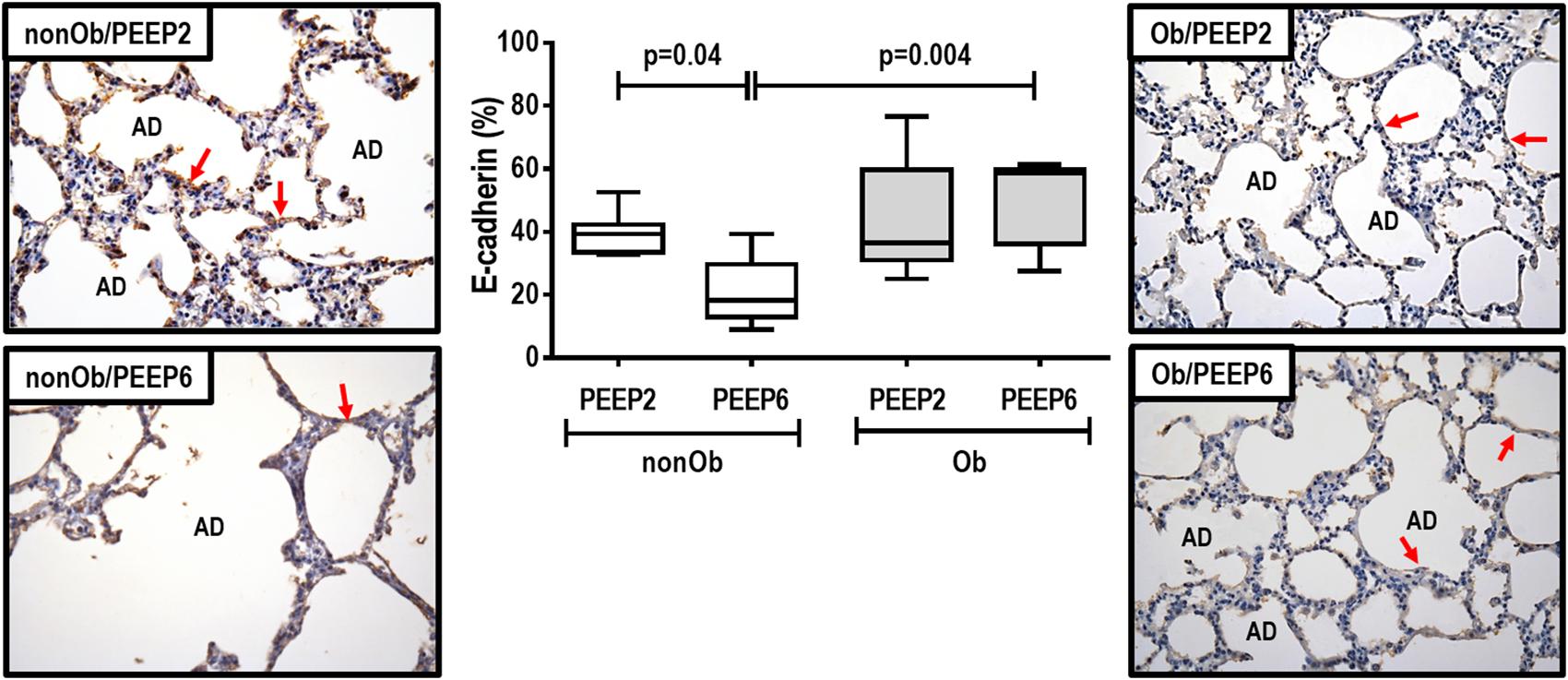
Figure 2. E-cadherin tissue expression and representative photomicrographs of immunohistochemical staining for E-cadherin in mechanically ventilated obese groups: Low-PEEP (2 cmH2O) and High-PEEP (6 cmH2O). Boxes show the interquartile range (25th–75th percentile), while whiskers encompass the range (minimum-maximum) and horizontal lines represent the median in seven animals/group. Red arrows represent E-cadherin immunohistochemical staining (brown). AD, alveolar ducts. Original magnification × 400.
Figure 3. Expression of biologic markers associated with lung damage in non-obese (nonOb) and obese (Ob) mechanically ventilated groups. Low-PEEP (2 cmH2O) and High-PEEP (6 cmH2O). Real-time polymerase chain reaction analysis of interleukin (IL)-6 and type III procollagen (PCIII). Relative gene expression was calculated as a ratio of average expression of each gene to the reference gene (36B4) and expressed as fold change relative to non-ventilated animals (NV). Boxes show the interquartile range (25th–75th percentile), while whiskers encompass the range (minimum-maximum) and horizontal lines represent the median in seven animals/group.
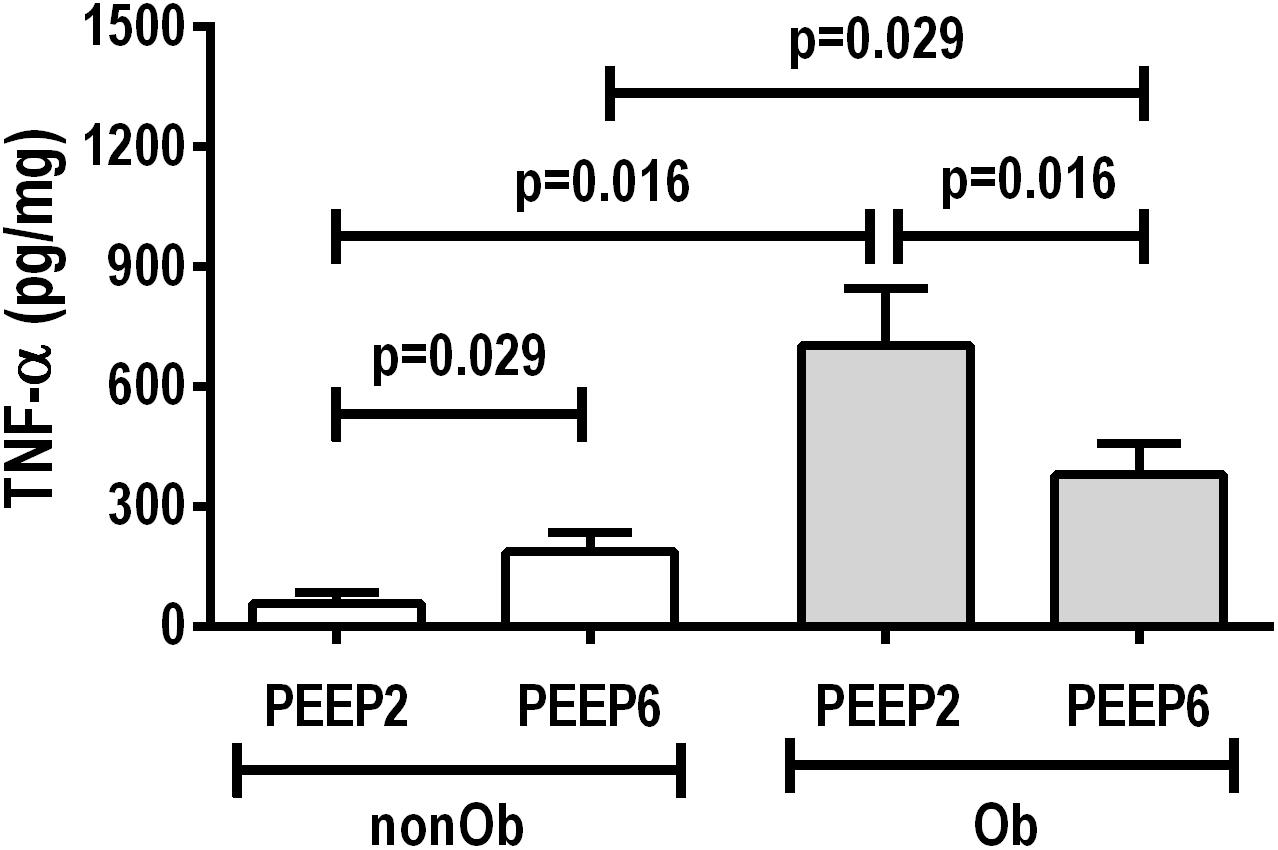
Figure 4. Protein levels of tumor necrosis factor (TNF)-α in lung tissue homogenate in non-obese (nonOb) and obese (Ob) mechanically ventilated groups: Low-PEEP (2 cmH2O) and High-PEEP (6 cmH2O). Bars represent means +SD of seven animals in each group.
Non-obese Rats
At END, PaO2/FiO2 was higher in nonOb/PEEP6 than in nonOb/PEEP2, with no significant differences in pHa and PaCO2 (Table 1). At INITIAL and END, Est,L and Est,w did not differ significantly between PEEP levels (Table 2), but was higher at END than INITIAL in the non-Ob/PEEP6 group (Table 2). The fraction area of alveolar collapse did not differ, and hyperinflation increased in the nonOb/PEEP6 group (Figure 1). E-cadherin expression was reduced in nonOb/PEEP6, suggesting alveolar epithelial cell damage (Figure 2). Gene expression of IL-6 was higher in nonOb/PEEP6 than nonOb/PEEP2 (Figure 3). No significant differences were observed in gene expressions of PCIII (Figure 3), amphiregulin, CC-16, VCAM-1, decorin, or MMP-9 (Supplementary Digital Content S2, Supplementary Figure S5). Protein levels of TNF-α were higher in nonOb/PEEP6 than nonOb/PEEP2 animals (Figure 4).
Obese Versus Non-obese Rats
At the same PEEP level (i.e., 2 or 6 cmH2O), pHa, PaCO2, and PaO2/FiO2 did not differ significantly between Ob and nonOb groups (Table 1). At INITIAL, Est,L was higher in Ob than nonOb animals regardless of PEEP level. At END, Est,L was higher in Ob than nonOb animals at PEEP2 (Table 2). At PEEP2, Ob animals exhibited greater alveolar collapse than their nonOb counterparts. At PEEP6, lung hyperinflation was lower (Figure 1) and E-cadherin expression higher (Figure 2) in Ob than in nonOb groups. TNF-α levels were also greater in Ob compared with nonOb animals, regardless of PEEP level (Figure 4).
Discussion
In an experimental model of ventilation for open abdominal surgery, PEEP = 6 cmH2O compared to PEEP = 2 cmH2O resulted in (1) less alveolar collapse and a lower pro-inflammatory and fibrogenic response in obese rats; and (2) increased hyperinflation, epithelial cell damage, and lung inflammation in non-obese rats. Our data suggest that PEEP6 reduced lung inflammation and fibrosis in obese rats undergoing open abdominal surgery, whereas the same PEEP6 resulted in lung damage in non-obese animals.
In the present study, we used a well-established metabolic programing model of obesity, which, compared with other models based on dietary interventions (Heil et al., 2016; Maia et al., 2019), better resembles the major hallmarks of clinical obesity (Plagemann et al., 2009). The difference in body weight between obese and non-obese animals, although modest, was significant (p = 0.0006). In the present study, CT showed increased visceral fat mass, which may be associated with reduced pulmonary density, as well as higher heterogeneity and an increase in hypoaerated areas in the lung. These morphological changes resulted in increased Est,L and reduced oxygenation in obese compared to non-obese animals. A low VT (6–8 mL/kg) was used since it has been associated with fewer PPCs (Severgnini et al., 2013). However, a recent study showed that obese patients are frequently ventilated with high-VT and low-PEEP (Ball et al., 2018), a combination that may cause lung damage. Beyond VT, there are controversies regarding the level of PEEP to be used during open abdominal surgery in non-obese patients (Futier et al., 2013; Hemmes et al., 2014; Güldner et al., 2015; Ferrando et al., 2018). Adequate PEEP levels may reduce atelectasis and improve lung mechanics and oxygenation, while high-PEEP may yield overdistension and impair ventilation-perfusion ratio and hemodynamics (Pelosi and Gregoretti, 2010). The PEEP levels tested in the present study (2 and 6 cmH2O) represented a two-fold increase compared with humans (4 and 12 cmH2O), due to differences in transpulmonary pressures between humans and rats (Caironi et al., 2011). These PEEP levels were previously tested in clinical studies (Nestler et al., 2017; Pereira et al., 2018; Bluth et al., 2019).
To the best of our knowledge, this is the first standardized, randomized preclinical translational study to compare the functional, morphological, and biological impacts of two levels of PEEP (low and high) during open abdominal surgery in non-obese and obese rats.
In obese animals, lung mechanics did not differ between PEEP = 2 cmH2O and PEEP = 6 cmH2O. Oxygenation improved with PEEP = 6 cmH2O, which may be associated with reduced alveolar collapse and heterogeneity score, yielding a less pro-inflammatory response (as measured by TNF-α protein levels and IL-6 gene expression) and less fibrogenesis (as measured by PCIII gene expression) in lung tissue. The absence of lung hyperinflation may be explained by the lower end-expiratory lung volumes before the application of PEEP, and, probably, by the presence of more compliant alveoli. At PEEP = 2 cmH2O, the continuous opening and closing of collapsed alveolar units during tidal breath may increase shear stress, thus promoting lung injury, inflammation (Bilek et al., 2003), and fibrogenesis (Farias et al., 2005). The absence of hyperinflation was likely associated with the absence of changes in gene expression of amphiregulin (Dolinay et al., 2004).
In non-obese animals, PEEP = 6 cmH2O, compared to PEEP = 2 cmH2O, did not modify lung mechanical parameters, but oxygenation was higher at PEEP = 6 cmH2O due to lung hyperinflation and increased ventilation-perfusion ratio. Alveolar collapse did not differ between PEEP = 2 cmH2O and PEEP = 6 cmH2O. PEEP = 6 cmH2O, compared to PEEP = 2 cmH2O, led to decreased E-cadherin as well as increased IL-6 gene expression and TNF-α protein content in lung tissue, suggesting damage to alveolar epithelial cells and greater lung inflammation, respectively. Based on these data, lung hyperinflation seemed to be associated with increased lung inflammatory response when compared with atelectasis per se. This reinforces the hypothesis that volutrauma is more injurious than atelectrauma in non-obese rats. The absence of changes in mediators other than those associated with inflammation is consistent with post hoc analyses of the PROVHILO trial, in which high-PEEP leads to minimal changes in biological markers (Serpa Neto et al., 2017).
The fact that lung mechanics were not affected by PEEP in non-obese or obese animals at END may be attributed to the following mechanisms: (1) different volume-pressure curve slope and position, resulting in diverse lung mechanical properties; and (2) different regional mechanical behavior of alveoli (less or more compliant), which included those opened before PEEP = 6 cmH2O and those reopened after PEEP = 6 cmH2O. In addition, chest wall mechanical properties did not differ among groups or over time. This may be explained by the increased abdominal compliance of small animals, which may balance the effects of the open-abdomen preparation.
In obese compared to non-obese animals, at PEEP = 6 cmH2O, lung hyperinflation was reduced, whereas E-cadherin expression increased. This increase in E-cadherin expression suggests that the integrity of alveolar epithelial cells was preserved in obese animals (Kasper et al., 1995; Goto et al., 2000). At PEEP = 2 cmH2O, alveolar collapse was increased and TNF-α levels in lung tissue were higher in obese animals, probably due to the enhanced lung inflammatory response associated with obesity (Mancuso, 2010).
A multicenter, international randomized controlled trial (PROBESE) compared the effects of two different levels of intraoperative PEEP (4 cmH2O versus 12 cmH2O) during protective low-VT ventilation in obese patients and observed no differences in PPCs (Bluth et al., 2019). Taking into account species differences, the PEEP levels used in our study and in PROBESE are comparable, but comparison between these two studies is unwarranted, since laparoscopy and recruitment maneuvers were performed in most patients.
Limitations
This study has some limitations that need to be addressed. First, a postnatal early overnutrition model was used; therefore, our results cannot be extrapolated to other models, including genetic variation (Kordonowy et al., 2012) and diet-induced obesity (Heil et al., 2016). Second, even considering the shorter lifespan of rats, the time of exposure of our animals to changes induced by obesity was relatively short. Nevertheless, since rats have a higher metabolic basal rate, a period of 150 days in rats would correspond to 14 years in humans (West and West, 2013). Third, our model of open abdominal surgery (laparotomy plus bowel manipulation), despite widespread use for experimental research (Maia et al., 2017), does not reproduce all aspects of the complex clinical scenario, where surgical trauma may be accompanied by bleeding and hemodynamic impairment. Fourth, in order to properly compare lung morphometry among groups, all animals had their PEEP level adjusted to 3 cmH2O for 2 min. Fifth, according to clinical trials and observational studies, ventilator strategies which feature high-PEEP are usually followed by RMs (Reinius et al., 2009; Pirrone et al., 2016). We chose to not include RMs or PEEP titration in our study, since this would have increased the number of groups and might have decreased the statistical power to find differences among groups. Sixth, the end-expiratory lung volume was not measured. Thus, further studies are required to better understand the changes in regional lung mechanical changes after each PEEP level.
Conclusion
In an experimental model of mechanical ventilation for open abdominal surgery, PEEP = 6 cmH2O reduced lung inflammation in obese rats. Conversely, in non-obese animals, PEEP = 6 cmH2O increased inflammation and alveolar epithelial cell damage.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
LM and MF participated in the design of the study, carried out the experiments, performed the data analyses, and drafted the manuscript. RS, LA, AC, NR, and MO contributed to the study design and carried out the experiments. MF and VC performed the analyses of lung morphology. SS and BG performed the CT analyses. LM, RS, and AC performed the analyses of lung mechanics. CS and MM carried out the molecular biology analyses. MS, MG, PP, PS, and PR contributed to the study design, supervised the experimental work and statistical analysis, wrote the manuscript, and supervised the entire project. All authors read and approved the final manuscript.
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico/Ministério da Saúde/DECIT (Grants 469716/2014-2, 465064/2014-0, and 400462/2014-1 to PR) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Grant E-26/103.118/2 to PR).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CB declared a shared affiliation, with no collaboration, with one of the authors, VC, to the handling Editor at the time of review.
Acknowledgments
The authors express their gratitude to Mr. Andre Benedito da Silva for animal care; Miss Maira Lima and Arlete Fernandes for their help during the experiments (Laboratory of Pulmonary Investigation, Carlos Chagas Filho Institute of Biophysics, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil); Prof. Ronir Raggio Luiz, Ph.D. (Institute of Public Health Studies, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil) for his help with statistics; Mrs. Moira Elizabeth Schottler (Rio de Janeiro, Brazil); and Mr. Filippe Vasconcellos (São Paulo, Brazil) for their assistance in editing the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.01513/full#supplementary-material
References
Ball, L., Hemmes, S. N. T., Neto, S. A., Bluth, T., Canet, J., Hiesmayr, M., et al. (2018). Intraoperative ventilation settings and their associations with postoperative pulmonary complications in obese patients. Br. J. Anaesth. 121, 899–908. doi: 10.1016/j.bja.2018.04.021
Bilek, A. M., Dee, K. C., and Gaver, D. P. (2003). Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J. Appl. Physiol. 94, 770–783. doi: 10.1152/japplphysiol.00764.2002
Bluth, T., Serpa Neto, A., Schultz, M. J., Pelosi, P., and Gama de Abreu, M. (2019). Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: a randomized clinical trial. JAMA 321, 2292–2305. doi: 10.1001/jama.2019.7505
Caironi, P., Langer, T., Carlesso, E., Protti, A., and Gattinoni, L. (2011). Time to generate ventilator-induced lung injury among mammals with healthy lungs: a unifying hypothesis. Intensive Care Med. 37, 1913–1920. doi: 10.1007/s00134-011-2388-9
Dolinay, T., Szilasi, M., Liu, M., and Choi, A. M. (2004). Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 170, 613–620. doi: 10.1164/rccm.200401-023OC
Farias, L. L., Faffe, D. S., Xisto, D. G., Santana, M. C., Lassance, R., Prota, L. F., et al. (2005). Positive end-expiratory pressure prevents lung mechanical stress caused by recruitment/derecruitment. J. Appl. Physiol. 98, 53–61. doi: 10.1152/japplphysiol.00118.2004
Felix, N. S., Samary, C. S., Cruz, F. F., Rocha, N. N., Fernandes, M. V. S., Machado, J. A., et al. (2019). Gradually increasing tidal volume may mitigate experimental lung injury in Rats. Anesthesiology 130, 767–777. doi: 10.1097/ALN.0000000000002630
Ferrando, C., Soro, M., Unzueta, C., Suarez-Sipmann, F., Canet, J., Librero, J., et al. (2018). Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir. Med. 6, 193–203. doi: 10.1016/S2213-2600(18)30024-9
Futier, E., Constantin, J.-M., Paugam-Burtz, C., Pascal, J., Eurin, M., Neuschwander, A., et al. (2013). A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. New Engl. J. Med. 369, 428–437. doi: 10.1056/NEJMoa1301082
Goldenberg, N. M., Steinberg, B. E., Lee, W. L., Wijeysundera, D. N., and Kavanagh, B. P. (2014). Lung-protective ventilation in the operating room: time to implement? Anesthesiology 121, 184–188. doi: 10.1097/ALN.0000000000000274
Goto, Y., Uchida, Y., Nomura, A., Sakamoto, T., Ishii, Y., Morishima, Y., et al. (2000). Dislocation of E-cadherin in the airway epithelium during an antigen-induced asthmatic response. Am. J. Respir. Cell Mol. Biol. 23, 712–718. doi: 10.1165/ajrcmb.23.6.4031
Güldner, A., Kiss, T., Neto, A., Hemmes, S. N., Canet, J., Spieth, P. M., et al. (2015). Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications. Anesthesiology 123, 692–713. doi: 10.1097/ALN.0000000000000754
Hedenstierna, G., and Edmark, L. (2010). Mechanisms of atelectasis in the perioperative period. Best Pract. Res. Clin. Anaesthesiol. 24, 157–169. doi: 10.1016/j.bpa.2009.12.002
Heil, L., Santos, C. L., Santos, R. S., Samary, C. S., Cavalcanti, V. C., Araújo, M. M., et al. (2016). The effects of short-term propofol and dexmedetomidine on lung mechanics, histology, and biological markers in experimental obesity. Anesth. Analg. 122, 1015–1023. doi: 10.1213/ANE.0000000000001114
Hemmes, S. N., Abreu, M., de Pelosi, P., and Schultz, M. J. (2014). High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 384, 495–503. doi: 10.1016/S0140-6736(14)60416-5
Hsia, C. C., Hyde, D. M., Ochs, M., and Weibel, E. R. (2010). An official research policy statement of the american thoracic society/european respiratory society: standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care 181, 394–418. doi: 10.1164/rccm.200809-1522ST
Imber, D., Pirrone, M., Zhang, C., Fisher, D., Kacmarek, R., and Berra, L. (2016). Respiratory management of perioperative obese patients. Respir. Care 61, 1681–1692. doi: 10.4187/respcare.04732
Kasper, M., Huber, O., Grossmann, H., Rudolph, B., Tränkner, C., and Müller, M. (1995). Immunocytochemical distribution of E-cadherin in normal and injured lung tissue of the rat. Histochem. Cell Biol. 104, 383–390. doi: 10.1007/bf01458132
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., and Altman, D. G. (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 1, 94–99. doi: 10.4103/0976-500X.72351
Kordonowy, L. L., Burg, E., Lenox, C. C., Gauthier, L. M., Petty, J. M., Antkowiak, M., et al. (2012). Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury. Am. J. Respir. Cell Mol. 47, 120–127. doi: 10.1165/rcmb.2011-0334OC
Maia, L. A., Cruz, F. F., de Oliveira, M. V., Samary, C. S., Fernandes, M. V. S., Trivelin, S. A. A., et al. (2019). Effects of obesity on pulmonary inflammation and remodeling in experimental moderate acute lung injury. Front. Immunol. 10:1215. doi: 10.3389/fimmu.2019.01215
Maia, L. A., Samary, C. S., Oliveira, M. V., Santos, C. L., Huhle, R., Capelozzi, V. L., et al. (2017). Impact of different ventilation strategies on driving pressure, mechanical power, and biological markers during open abdominal surgery in rats. Anesth. Analg. 125, 1364–1374. doi: 10.1213/ANE.0000000000002348
Mancuso, P. (2010). Obesity and lung inflammation. J. Appl. Physiol. 108, 722–728. doi: 10.1152/japplphysiol.00781.2009
Mortola, J., and Noworaj, A. (1983). Two-sidearm tracheal cannula for respiratory airflow measurements in small animals. J. Appl. Physiol. 55, 250–253. doi: 10.1152/jappl.1983.55.1.250
Nestler, C., Simon, P., Petroff, D., Hammermüller, S., Kamrath, D., Wolf, S., et al. (2017). Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br. J. Anaesth. 119, 1194–1205. doi: 10.1093/bja/aex192
Pássaro, C. P., Silva, P. L., Rzezinski, A. F., Abrantes, S., Santiago, V. R., Nardelli, L., et al. (2009). Pulmonary lesion induced by low and high positive end-expiratory pressure levels during protective ventilation in experimental acute lung injury. Crit. Care Med. 37, 1011–1017. doi: 10.1097/CCM.0b013e3181962d85
Pelosi, P., and Gregoretti, C. (2010). Perioperative management of obese patients. Best Pract. Res. Clin. Anaesthesiol. 24, 211–225. doi: 10.1016/j.bpa.2010.02.001
Pereira, S. M., Tucci, M. R., Morais, C. C., Simões, C. M., Tonelotto, B. F. F., Pompeo, M. S., et al. (2018). Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology 129, 1070–1081. doi: 10.1097/ALN.0000000000002435
Pirrone, M., Fisher, D., Chipman, D., Imber, D. A., Corona, J., Mietto, C., et al. (2016). Recruitment maneuvers and positive end-expiratory pressure titration in morbidly obese ICU patients. Crit. Care Med. 44, 300–307. doi: 10.1097/CCM.0000000000001387
Plagemann, A., Heidrich, I., Götz, F., Rohde, W., and Dörner, G. (2009). Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp. Clin. Endocrinol. 99, 154–158. doi: 10.1055/s-0029-1211159
Reinius, H., Jonsson, L., Gustafsson, S., Sundbom, M., Duvernoy, O., Pelosi, P., et al. (2009). Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis. Anesthesiology 111, 979–987. doi: 10.1097/ALN.0b013e3181b87edb
Samary, C. S., Santos, R. S., Santos, C. L., Felix, N. S., Bentes, M., Barboza, T., et al. (2015). Biological impact of transpulmonary driving pressure in experimental acute respiratory distress syndrome. Anesthesiology 123, 423–433. doi: 10.1097/ALN.0000000000000716
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Schumann, R., Shikora, S., Sigl, J., and Kelley, S. (2015). Association of metabolic syndrome and surgical factors with pulmonary adverse events, and longitudinal mortality in bariatric surgery. Br. J. Anaesth. 114, 83–90. doi: 10.1093/bja/aeu362
Serpa Neto, A., Campos, P. P., Hemmes, S. N., Bos, L. D., Bluth, T., Ferner, M., et al. (2017). Kinetics of plasma biomarkers of inflammation and lung injury in surgical patients with or without postoperative pulmonary complications. Eur. J. Anaesth. 34, 229–238. doi: 10.1097/EJA.0000000000000614
Severgnini, P., Selmo, G., Lanza, C., Chiesa, A., Frigerio, A., Bacuzzi, A., et al. (2013). Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 118, 1307–1321. doi: 10.1097/ALN.0b013e31829102de
Silva, L. P., Pelosi, P., and Rocco, P. (2012). Mechanical ventilation in obese patients. Minerva Anestesiol. 78, 1136–1145.
Spieth, P. M., Silva, P. L., Garcia, C. S., Ornellas, D. S., Samary, C. S., Moraes, L., et al. (2015). Modulation of stress versus time product during mechanical ventilation influences inflammation as well as alveolar epithelial and endothelial response in rats. Anesthesiology 122, 106–116. doi: 10.1097/ALN.0000000000000415
West, D., and West, B. J. (2013). Physiologic time: a hypothesis. Phys. Life Rev. 10, 210–224. doi: 10.1016/j.plrev.2013.04.006
Keywords: inflammation, epithelial cell damage, mechanical ventilation, positive-end expiratory pressure, obesity
Citation: Maia LA, Fernandes MVS, Santos RS, Agra LC, Carvalho AC, Rocha NN, Oliveira MV, Santos CL, Morales MM, Capelozzi VL, Souza SAL, Gutfilen B, Schultz MJ, Gama de Abreu M, Pelosi P, Silva PL and Rocco PRM (2019) Effects of Protective Mechanical Ventilation With Different PEEP Levels on Alveolar Damage and Inflammation in a Model of Open Abdominal Surgery: A Randomized Study in Obese Versus Non-obese Rats. Front. Physiol. 10:1513. doi: 10.3389/fphys.2019.01513
Received: 22 September 2019; Accepted: 02 December 2019;
Published: 17 December 2019.
Edited by:
Jeremy Andrew Simpson, University of Guelph, CanadaReviewed by:
Marina Soro, Hospital Clínic Universitari de València, SpainCarmen Silvia Valente Barbas, University of São Paulo, Brazil
Copyright © 2019 Maia, Fernandes, Santos, Agra, Carvalho, Rocha, Oliveira, Santos, Morales, Capelozzi, Souza, Gutfilen, Schultz, Gama de Abreu, Pelosi, Silva and Rocco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricia R. M. Rocco, prmrocco@biof.ufrj.br; prmrocco@gmail.com
 Lígia de A. Maia
Lígia de A. Maia Marcos V. S. Fernandes
Marcos V. S. Fernandes Raquel S. Santos
Raquel S. Santos Laís C. Agra
Laís C. Agra Anna Carolinna Carvalho
Anna Carolinna Carvalho Nazareth de N. Rocha1,2
Nazareth de N. Rocha1,2 Milena V. Oliveira
Milena V. Oliveira Marcelo M. Morales
Marcelo M. Morales Vera L. Capelozzi
Vera L. Capelozzi Sergio A. L. Souza
Sergio A. L. Souza Bianca Gutfilen
Bianca Gutfilen Paolo Pelosi
Paolo Pelosi Pedro L. Silva
Pedro L. Silva Patricia R. M. Rocco
Patricia R. M. Rocco