- 1Service de Physiologie-Explorations Fonctionnelles, FHU APOLLO, Assistance Publique Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, Paris, France
- 2INSERM, UMR 1141 NeuroDiderot, Université de Paris, Paris, France
- 3INSERM, UMS 011, Population-based Epidemiological Cohorts Unit, Villejuif, France
- 4UMR 7057, CNRS, Laboratoire Matière et Système Complexes, Paris, France
Since the outbreak of the coronavirus (COVID-19) pandemic, most attention has focused on containing transmission and addressing the surge of critically ill patients in acute care settings. As we enter the second phase of the pandemic, emphasis must evolve to post-acute care of COVID-19 survivors. Persisting cardiorespiratory symptoms have been reported at several months after the onset of the infection. Information is lacking on the pathophysiology of exercise intolerance after COVID-19. Previous outbreaks of coronaviruses have been associated with persistent dyspnea, muscle weakness, fatigue and reduced quality of life. The extent of Covid-19 sequelae remains to be evaluated, but persisting cardiorespiratory symptoms in COVID-19 survivors can be described as two distinct entities. The first type of post-Covid symptoms are directly related to organ injury in the acute phase, or the complications of treatment. The second type of persisting symptoms can affect patients even with mild initial disease presentation without evidence of organ damage. The mechanisms are still poorly qualified to date. There is a lack of correlation between initial symptom severity and residual symptoms at exertion. We report exercise hyperventilation as a major limiting factor in COVID-19 survivors. The origin of this hyperventilation may be related to an abnormality of ventilatory control, by either hyperactivity of activator systems (automatic and cortical ventilatory control, peripheral afferents, and sensory cortex) or failure of inhibitory systems (endorphins) in the aftermath of pulmonary infection. Hyperventilation-induced hypocapnia can cause a multitude of extremely disabling symptoms such as dyspnea, tachycardia, chest pain, fatigue, dizziness and syncope at exertion.
Introduction
The infection with severe respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic on March 11, 2020. As of November 2020, the novel coronavirus, hereafter referred to as COVID-19, has affected more than 60 million people worldwide (Center, 2020; Simpson and Robinson, 2020). For the majority (81%), infection with COVID-19 manifests as a mild disease. Fever (88.7%), cough (57.6%), and dyspnea (45.6%) were the most commonly reported symptoms in a recent systematic review (Rodriguez-Morales et al., 2020). However, for a significant minority, and particularly those older than 65 years and with comorbidities, the infection requires management in an intensive care unit due to acute respiratory distress syndrome (ARDS) (Pascarella et al., 2020). Since the outbreak of the pandemic, most attention has focused on containing transmission and managing the wave of critically ill patients in acute care settings. As we enter the second phase of the pandemic, emphasis must evolve to post-acute care of COVID-19 survivors. For these patients, having defeated the virus is just the beginning of an unknown recovery path. There are increasing reports of persisting and recurrent symptoms at several months after the onset of the infection (Carfi et al., 2020; Goërtz et al., 2020), dyspnea at exertion being one of the most common complaints. The extent and severity of the lasting sequelae remain to be evaluated, but persisting cardiorespiratory symptoms in COVID-19 survivors can be described as two distinct entities. The first type of post-Covid consequences are directly related to organ damage in the acute phase, either due to the viral infection and the ensuing inflammatory response, or the complications of treatment in the intensive care setting (Li et al., 2006; Zhou et al., 2014; Mart and Ware, 2020). The persisting cardiorespiratory symptoms related to cardiac and lung injury in COVID-19 will be summarized below. The second type of persisting symptoms can affect patients even with mild initial disease presentation without evidence of severe organ damage. Sustained fatigue and exercise intolerance are the most frequent complaints in patients not requiring hospitalization (Goërtz et al., 2020; Tenforde et al., 2020). In these patients, there is a striking lack of correlation between initial symptom severity and residual symptoms at exertion. The mechanisms behind post-Covid exercise intolerance are most likely complex and still poorly qualified to date. In this article, we aimed to review the available evidence on persisting exercise intolerance experienced by COVID-19 survivors. We also report exercise hyperventilation as a major limiting factor that can cause a multitude of extremely disabling symptoms such as dyspnea, tachycardia, chest pain, fatigue, dizziness, and syncope at exertion. To our knowledge, this is the first report of a possible explanation for prolonged exercise intolerance in long-lasting COVID-19.
Persisting Cariorespiratory Symptoms in Coronavirus Survivors: Current State of Knowledge
Around 20% of patients with severe COVID-19 require in-hospital management (Simpson and Robinson, 2020). For the patients presenting with severe initial illness, the long-term consequences are yet to be elucidated. However, emerging studies show evidence of persistent cardiorespiratory symptoms months after hospital discharge. Weerahandi et al. (2020) studied severe COVID-19 patients who required at least 6 l/min of oxygen during hospital stay. At one-month follow-up, 74% of participants reported shortness of breath. Carfi et al. (2020) also assessed persistent symptoms in patients discharged from the hospital. At 2 months follow-up, half of the patients reported persistent fatigue, whereas dyspnea (43%) and chest pain (22%) were also highly prevalent. These findings are in line with the studies by Wong et al. (2020) and Garrigues et al. (2020), both of which found nearly half of the patients complaining of breathlessness at 3 months after hospital discharge.
The persistence of cardiorespiratory symptoms in survivors of severe COVID-19 can be partly explained by the pathophysiology of organ damage during the initial phase of the disease. The SARS-CoV-2 virus predominantly affects the respiratory system, although other organ systems can be compromised as well. The virus uses angiotensin-converting enzyme-2 (ACE2) receptors in pneumocytes of the epithelial alveolar lining to infect the host, thus causing lung injury (Varga et al., 2020). Diffuse alveolar damage was showed by several post-mortem studies (Carsana et al., 2020; Schaller et al., 2020), leading to hypotheses of residual pulmonary function impairment at long term. These hypotheses are supported by the available evidence on previous coronavirus outbreaks. A recent meta-analysis (Ahmed et al., 2020) of studies on SARS-CoV and MERS-CoV reported that approximately one third of hospitalized patients had persisting lung abnormalities after their acute illness. Prospective studies on SARS-CoV, MERS-CoV as well as short-term follow-up studies on the current SARS-CoV-2 epidemic are summarized in Table 1. These studies demonstrate mostly a mild pulmonary function impairment and the functional disability appears out of proportion to the degree of lung function impairment. For example, a 2005 study on SARS-CoV by Hui et al. (2005) found that the exercise capacity 6 months after disease onset was considerably lower than that of normal controls in the same age groups. However, significant impairment of surface area gas exchange was only found in 15% of the patients. Along with Hui’ study, Ong et al. (2004) performed cardiopulmonary exercises testing in 46 SARS-CoV patients at 3 months after hospital discharge. Half of the patients had abnormalities in the pulmonary function tests, but the impairment was mild in almost all cases. However, 41% of the patients had impairment of exercise capacity not due to ventilatory limitation. The discordance of the results of pulmonary function and exercise testing has been attributed to several factors, such as physical deconditioning, muscle weakness and poor motivation. The recent data in SARS-CoV-2 by Arnold et al. (2020) demonstrates that even 74% of survivors complained of persistent breathlessness and excessive fatigue at 3 months after hospital discharge. Yet abnormal pulmonary function was found in only 10% of the patients. Major limitations have to be taken into account when considering available evidence on long-term pulmonary function abnormalities. The majority of information is derived from single-center studies with a small sample of patients and a relatively short-term follow-up. However, most of the data support the idea that persistent cardiorespiratory symptoms in COVID-19 survivors cannot be accounted for by impairment of pulmonary function alone.
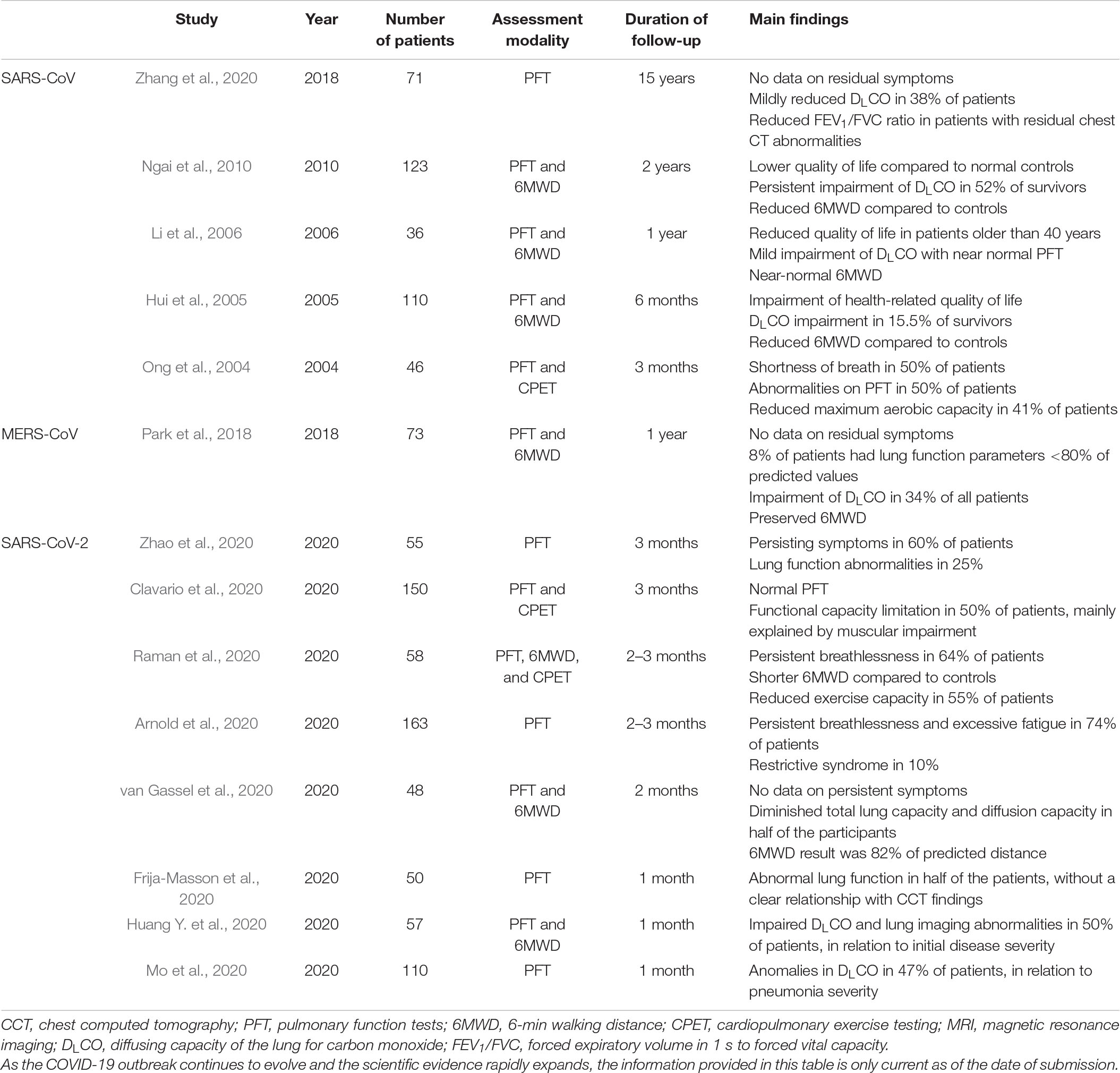
Table 1. prospective studies on residual symptoms, pulmonary function impairment and exercise intolerance following SARS-CoV, MERS-CoV and SARS-CoV-2 viral infections.
Multisystem involvement is the key pathophysiological feature of SARS-CoV-2 infection and can help explain the heterogeneous symptoms experienced by patients, as well as the complex mechanisms of long-lasting exercise intolerance. As described above, the virus binds to ACE2 receptors, which are present not only in the lungs, but are widely distributed in endothelial cells. Endothelial injury recruits inflammatory leukocytes, and contributes to tissue damage and cytokine release, as well as thrombosis and disseminated intravascular coagulation (Evans et al., 2020). Autopsy studies (Varga et al., 2020) show severe endothelial injury that is associated with the presence of intracellular virus. Widespread thrombosis with microangiopathy were also described (Ackermann et al., 2020). It has been postulated that endothelial injury and microangiopathy might be the reasons behind autonomic dysfunction, which is compatible with residual symptoms such as fatigue, palpitations and chest pain, among others. Cardiac injury has also been documented in patients hospitalized with moderate or severe COVID-19 (Clerkin et al., 2020; Driggin et al., 2020; Huang C. et al., 2020). Inflammatory myocarditis is frequently diagnosed in severe COVID-19 patients and may later lead to left ventricular failure. Inflammation induced pro-coagulatory state might lead to type 1 myocardial infarction (MI) due to plaque rupture or intracoronary thrombosis (Chieffo et al., 2020; Clerkin et al., 2020). Type 2 MI (myocardial injury in the absence of obstructive coronary artery disease) might be induced by prolonged hypoxia. Right ventricular failure secondary to acute pulmonary embolisms is also possible. Myocardial injury can persist after the acute phase and clinically manifest as dyspnea or chest pain at exertion. Therefore, appropriate follow-up to detect these complications is warranted. Two recent studies with cardiac magnetic resonance imaging (MRI) (Huang L. et al., 2020; Puntmann et al., 2020) showed ongoing cardiac involvement in a majority of patients months after a COVID-19 diagnosis. Another study by Raman et al. (2020) describes persistent MRI abnormalities seen in the lungs (60%) and the heart (26%) of COVID-19 patients at 2–3 months after hospital discharge, in relation to ongoing symptoms of breathlessness and excessive fatigue (in 64 and 55% of patients respectively). In the same study, cardiopulmonary exercise testing showed that 55% of patients had peak oxygen uptake (VO2 max) values lower than 80% of the predicted. In contrast, only 12% of patients had impaired pulmonary function tests.
Persisting cardiorespiratory symptoms may be in part related to initial disease severity and residual organ damage. In critically ill patients, acute respiratory distress (ARDS) – related consequences have been described previously (Desai et al., 1999; Mart and Ware, 2020) and are not specific to COVID-19. Other ICU-related complications such as ventilator-induced lung injury or critical illness-associated poly-neuropathy are well established (Zhou et al., 2014; Beitler et al., 2016) and are out of the scope of this article. The routine administration of high dose steroids to many patients with ARDS might lead to steroid myopathy and muscle wasting, resulting in residual exercise intolerance and excessive fatigue.
Most of the studies that have reported on sequelae of COVID-19 included participants whose illness was severe enough to require hospitalization. However, the majority of patients with COVID-19 are managed in outpatient settings. Long term outcomes might not be comparable due to multiple factors: varying degree of organ damage, different treatment and distinct patient demographics, as many patients admitted to hospital with COVID-19 are older, have more comorbidities and frailty. Despite mild initial presentation, emerging evidence indicates that it might take weeks for resolution of symptoms and return to usual health. Tenforde et al. (2020) reported that out of 274 patients tested for COVID-19 in an outpatient setting, one third complained of fatigue and dyspnea 2 weeks after symptom onset. Even among young adults aged 18–34 years with no chronic medical conditions, nearly one in five reported that they had not returned to their usual state of health. Thus it seems that the persistence of symptoms might not necessarily be related to patient age or initial clinical severity of the disease.
These findings were further confirmed by Goërtz et al. (2020), who performed a survey in ambulatory COVID-19 patients at 3 months after symptom onset. They showed that the majority of patients were still symptomatic, and reported a multitude of symptoms, ranging from cough, sore throat, muscle pain, dizziness, chest tightness, palpitations, weight loss, etc. Fatigue and dyspnea were the two most prevalent symptoms, described by 87 and 71% of patients respectively. These findings are especially alarming for such a young population (median age of 47 years) mostly without serious comorbidities and normal physical examination.
To date, the etiology of these heterogeneous long-Covid symptoms is poorly understood. It is possible that endothelial injury might play a role in the persistence of dysautonomic symptoms in COVID-19 survivors. Davido et al. (2020) described their experience working with outpatients who experienced mostly mild symptoms attributable to COVID-19. Subsequently they observed multiple persistent symptoms, especially intense fatigue, shortness of breath, chest tightness and tachycardia. Authors suggest that these symptoms are compatible with dysautonomia due to microangiopathy and endothelial injury. Miglis et al. (2020a, b) also described a subset of COVID-19 survivors presenting symptoms of autonomic dysfunction such as orthostatic intolerance and postural orthostatic tachycardia. Such symptoms are frequently reported after other viral infections and might be related to gastrointestinal fluid loss, prolonged bed rest and deconditioning of the cardiovascular system. However, further research is needed to further characterize the dysautonomic syndromes in COVID-19 survivors.
In contrast to hospitalized COVID-19 patients, conventional risk factors such as age and the presence of comorbidities do not seem to have an impact on the duration and severity of persistent symptoms. A study by O’Keefe et al. (2020) analyzed 273 non-hospitalized patients recovering from COVID-19. Interestingly, the authors found no correlation between symptom duration and patient factors such as age or comorbidities.
All in all, the pathophysiology of persistent cardiorespiratory symptoms in COVID-19 outpatients are not clearly understood to date. Even though these symptoms seem to be benign, their importance should not be underestimated. Persisting exercise intolerance can result in worsened quality of life, inability to return to work and increased use of health care systems, constituting a worldwide public health problem.
Hyperventilation as a Possible Cause of Persisting Exercise Intolerance
As a French Reference Center for Infectious Diseases, Bichat hospital has had an important number of COVID-19 patients, treated both in and out of hospital settings. All patients are systematically offered follow-up and undergo a thorough cardio-pulmonary exploration, including pulmonary function tests (PFT), a chest CT scan, a trans-thoracic echocardiogram, and cardiopulmonary exercise testing (CPET). Ethics committee approvals were obtained according to local requirements.
We report a case series of eight patients (seven women and one man) with important exertional dyspnea at 3 months after the onset of COVID-19 symptoms. All patients were aged 31–73 [median age 39 years (interquartile range 33–49)] and had no previous medical history (notably, no chronic cardiovascular or pulmonary diseases). All of the patients had a relatively mild course of COVID-19 and received ambulatory treatment without indications for hospitalization or oxygen therapy. Nijmegen test was available in five patients and median score was 35 (27–38). At 3 months after the symptom onset, all patients had near-normal pulmonary function tests and normal chest CT scans. Transthoracic echocardiogram showed preserved left ventricular ejection fraction as well as the absence of pulmonary hypertension (see Table 2). During CPET, dyspnea, palpitations, and dizziness were reproduced in all patients. For two patients CPET had to be interrupted due to syncope at exertion. All of the patients showed a significant impairment of exercise capacity: seven out of eight were incapable of reaching 100% of predicted workload and none of the patients reached their predicted VO2 max values. An elevated VE/VCO2 ratio was observed in five patients, suggesting exercise hyperventilation. All patients increased their ventilation and their tidal volume as soon as the effort started. However, we were not able to define the generic ventilatory profile since two patients increased their respiratory rate (RR) only after the aerobic threshold and one patient had no significant increase in RR. Unfortunately, we were not able to collect data on inspiratory capacity. Arterial blood gasses were also analyzed at rest as well as at maximum effort. The apparition of respiratory alkalosis with low arterial CO2 levels and significant base excess at exertion was observed in three patients. We hypothesized that hyperventilation-induced hypocapnia following COVID-19 infection and prolonged bed rest might be responsible for a multitude of extremely disabling symptoms such as dyspnea, tachycardia, chest pain, fatigue, dizziness and syncope at exertion. Indeed, clinical follow-up of patients showed progressive resolution of symptoms. Interestingly, most patients described paroxysmal dyspnea that could also occur at rest, which could indicate a wider breathing dysfunction. Normality of PFT is in accordance with our hypothesis that alteration of lung function does not solely account for residual symptoms.
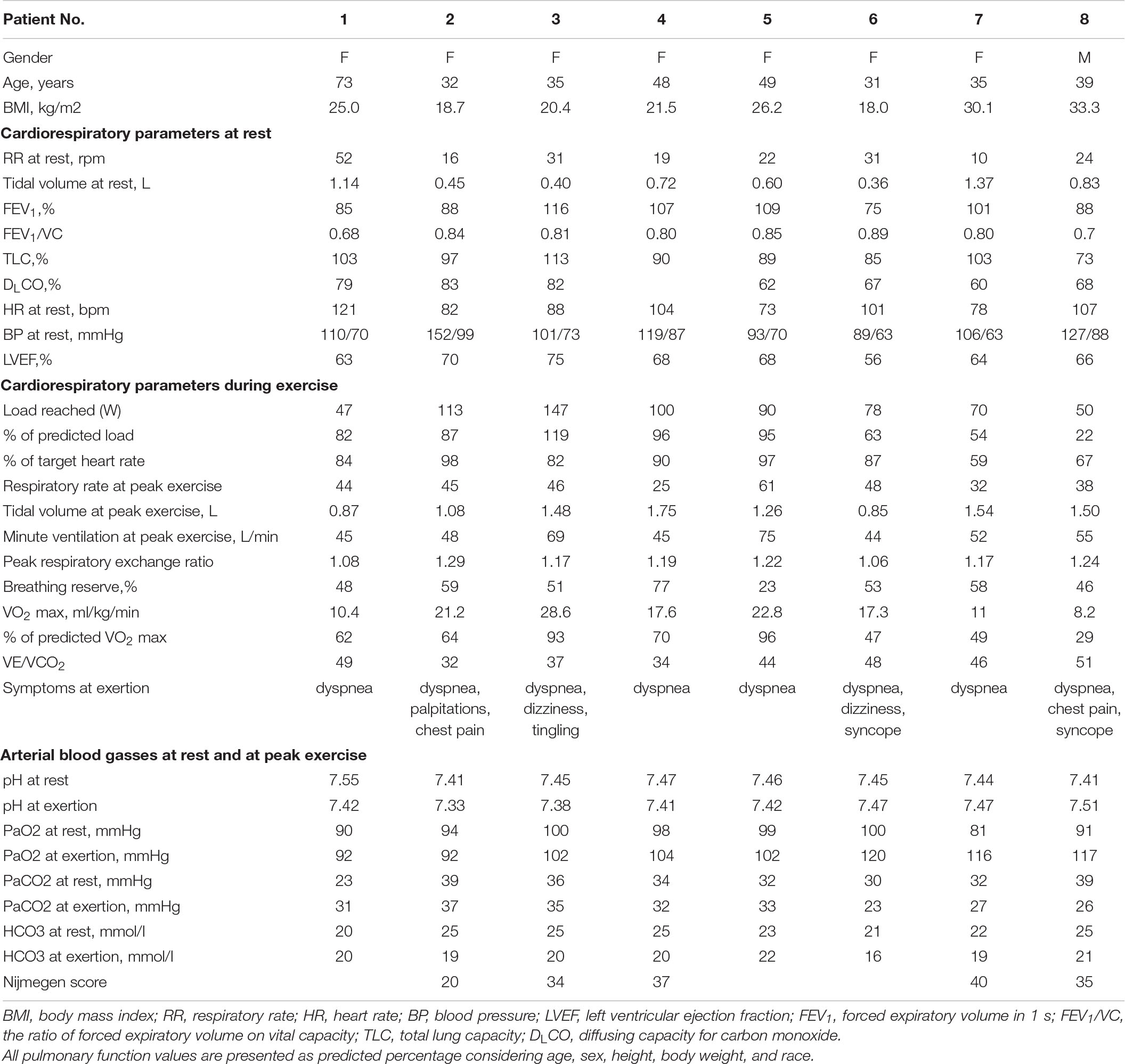
Table 2. Characteristics of patients presenting with hyperventilation syndrome in the aftermath of COVID-19, and findings of cardiopulmonary exercise testing.
Exercise hyperventilation is a condition defined by inappropriate alveolar hyperventilation, in regards to metabolic needs and mechanical stress in the body (Brat et al., 2019). It generally affects women more frequently than men, especially between the ages of 15 and 55 (Lum, 1975). The variety of symptoms experienced by the patient might take them to a wide range of specialist consultations, numerous investigations and inappropriate treatment.
The origin of this hyperventilation is unknown. The hypothesis of an abnormality of ventilatory control has been proposed, by either a stimulation of activator systems (automatic and cortical ventilatory control, peripheral afferents, and sensory cortex) or a suppression of inhibitory systems (endorphins) in the aftermath of a pulmonary infection (Gardner, 1994). Deciphering the mechanisms underlying this hyperventilation would require to examine chemo responsiveness by hypercapnic ventilatory challenge in patients recovering from COVID-19 and compare patients with and without hyperventilation.
Various forms of primary lung disease can influence the ventilatory control mechanisms and alter the respiratory center output. Hypoxemia-induced activation of peripheral chemoreceptors or stimulation of receptors by diseases affecting the airways or pulmonary interstitium can induce the respiratory center to increase its output, resulting in respiratory alkalosis. Similarly, patients recovering from acute pulmonary embolism, pneumonia, or chronic interstitial lung disease often hyperventilate, probably as a result of stimulation of one or more types of intrathoracic receptors, with or without the additional ventilatory stimulus induced by hypoxemia (Jack et al., 2003).
Even though the mechanisms of hyperventilation are not quite clear, the consequences of alveolar hyperventilation are well known (Laffey and Kavanagh, 2002). The most important physiological consequence of alveolar hyperventilation is the decrease in depolarization threshold of cell membranes. In case of respiratory alkalosis, H+ ions do not participate in membrane potential, and are transported out of the cell to decrease the blood pH, whereas K+ ions are transported into the cell. A relative excess of positive ions inside the membrane increases its potential, therefore decreasing the depolarization threshold. Neuronal hyperexcitability causes the activation of autonomous nervous system which in turn is responsible for neurovegetative symptoms described in hyperventilation syndrome. Muscular hyperexcitability results in increased muscular tone and arterial vasoconstriction due to smooth muscle cell contraction in the arterial wall. Resulting hypoperfusion of different organs might cause various symptoms related to ischemia. Hyperventilation related symptoms can range from dyspnea, palpitations, chest pain, muscle cramps, syncope to paresthesia, dizziness, headache, abdominal pain, nausea, fatigue, and anxiety.
There is no gold standard for the diagnosis of hyperventilation syndrome. Diagnostic tools include arterial blood gas measurement and various provocation tests in order to reproduce the symptoms. The Nijmegen Questionnaire (van Dixhoorn and Duivenvoorden, 1985) may be used as a screening instrument for early detection of hyperventilation syndrome, as well as an aid in diagnosis and therapy planning (with a sensibility of 91% and specificity 95%). Treatment options include respiratory physiotherapy with an experienced respiratory therapist, focusing on patient education and various techniques to control breathing. Fortunately, spontaneous resolution is common, especially with the gradual resumption of physical activity.
Conclusion
Persisting cardiorespiratory symptoms are rather common at several months after the onset of the COVID-19 infection. Currently, data is lacking on the pathophysiology of this post-COVID entity. In patients with severe initial disease presentation, persisting symptoms might be directly related to target organ damage as well as treatment complications. New studies have emerged describing the post-COVID syndrome even in patients with mild initial presentation, without a clear relationship with age and comorbidities. Persistent cardiorespiratory symptoms in these patients are most likely benign and unrelated to long-term organ damage. Muscle deconditioning, dysautonomia and exercise hyperventilation might partly explain the disabling symptoms in COVID-19 survivors. However, more studies are needed to further clarify the mechanisms behind this prolonged path to recovery.
Author Contributions
JM, PB, FA, LM, CB, and JF-M performed the cardiopulmonary function tests. JM and PB performed a literature review. JM and JF-M drafted the manuscript. PB, FA, LM, CB, and M-Pd’O contributed significantly to manuscript correction and finalization. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ackermann, M., Verleden, S. E., Kuehnel, M., Haverich, A., Welte, T., Laenger, F., et al. (2020). Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Eng. J. Med. 383, 120–128. doi: 10.1056/nejmoa2015432
Ahmed, H., Patel, K., Greenwood, D., Halpin, S., Lewthwaite, P., Salawu, A., et al. (2020). Long-term clinical outcomes in survivors of coronavirus outbreaks after hospitalization or icu admission: a systematic review and meta-analysis of follow-up studies. medRxiv [preprint] doi: 10.1101/2020.04.16.20067975
Arnold, D. T., Hamilton, F. W., Milne, A., Morley, A., Viner, J., Attwood, M., et al. (2020). Patient outcomes after hospitalisation with COVID-19 and implications for follow-up; results from a prospective UK cohort. medRxiv [preprint] doi: 10.1101/2020.08.12.20173526
Beitler, J. R., Malhotra, A., and Thompson, B. T. (2016). Ventilator-induced lung injury. Clin. Chest Med. 37, 633–646. doi: 10.1016/j.ccm.2016.07.004
Brat, K., Stastna, N., Merta, Z., Olson, L. J., Johnson, B. D., and Cundrle, I. Jr. (2019). Cardiopulmonary exercise testing for identification of patients with hyperventilation syndrome. PLoS One 14:e0215997. doi: 10.1371/journal.pone.0215997
Carfi, A., Bernabei, R., Landi, F., and Gemelli Against, C.-P.-A. C. S. G. (2020). Persistent symptoms in patients after acute COVID-19. JAMA 324, 603–605. doi: 10.1001/jama.2020.12603
Carsana, L., Sonzogni, A., Nasr, A., Rossi, R. S., Pellegrinelli, A., Zerbi, P., et al. (2020). Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 20, 1135–1140. doi: 10.1016/s1473-3099(20)30434-5
Center, J. H. C. R. (2020). COVID-19 Map - Johns Hopkins Coronavirus Resource Center. [online]. Available online at: https://coronavirus.jhu.edu/map.html (accessed November 26, 2020).
Chieffo, A., Stefanini, G. G., Price, S., Barbato, E., Tarantini, G., Karam, N., et al. (2020). EAPCI position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. Eur. Heart J. 41, 1839–1851. doi: 10.1093/eurheartj/ehaa381
Clavario, P., De Marzo, V., Lotti, R., Barbara, C., Porcile, A., Russo, C., et al. (2020). Assessment of functional capacity with cardiopulmonary exercise testing in non-severe COVID-19 patients at three months follow-up. medRxiv [preprint] doi: 10.1101/2020.11.15.20231985
Clerkin, K. J., Fried, J. A., Raikhelkar, J., Sayer, G., Griffin, J. M., Masoumi, A., et al. (2020). COVID-19 and cardiovascular disease. Circulation 141, 1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941
Davido, B., Seang, S., Tubiana, R., and de Truchis, P. (2020). Post-COVID-19 chronic symptoms: a postinfectious entity? Clin. Microbiol. Infect. 26, 1448–1449. doi: 10.1016/j.cmi.2020.07.028
Desai, S. R., Wells, A. U., Rubens, M. B., Evans, T. W., and Hansell, D. M. (1999). Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology 210, 29–35. doi: 10.1148/radiology.210.1.r99ja2629
Driggin, E., Madhavan, M. V., Bikdeli, B., Chuich, T., Laracy, J., Biondi-Zoccai, G., et al. (2020). Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 Pandemic. J. Am. Coll. Cardiol. 75, 2352–2371. doi: 10.1016/j.jacc.2020.03.031
Evans, P. C., Rainger, G., Mason, J. C., Guzik, T. J., Osto, E., and Stamataki, Z., et al. (eds) (2020). Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the esc council of basic cardiovascular science. Cardiovasc. Res. 116, 2177–2184. doi: 10.1093/cvr/cvaa230
Frija-Masson, J., Debray, M. P., Gilbert, M., Lescure, F. X., Travert, F., Borie, R., et al. (2020). Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur. Respir. J. 56:2001754. doi: 10.1183/13993003.01754-2020
Gardner, W. N. (1994). “Hyperventilation: diagnosis and therapy,” in Behavioral and Psychological Approaches to Breathing Disorders, eds H. Beverly and R. L. Timmons (New York: Springer Science + Business Media, LLC), 99–111. doi: 10.1007/978-1-4757-9383-3_7
Garrigues, E., Janvier, P., Kherabi, Y., Le Bot, A., Hamon, A., Gouze, H., et al. (2020). Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 81, e4–e6. doi: 10.1016/j.jinf.2020.08.029
Goërtz, Y. M. J., Van Herck, M., Delbressine, J. M., Vaes, A. W., Meys, R., and Machado, F. V. C. (2020). Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 6, 00542–2020. doi: 10.1183/23120541.00542-2020
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 395, 497–506. doi: 10.1016/S0140-6736(20)30183-5
Huang, L., Zhao, P., Tang, D., Zhu, T., Han, R., Zhan, C., et al. (2020). Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc. Imaging 13, 2330–2339. doi: 10.1016/j.jcmg.2020.05.004
Huang, Y., Tan, C., Wu, J., Chen, M., Wang, Z., Luo, L., et al. (2020). Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 21:163. doi: 10.1186/s12931-020-01429-6
Hui, D. S., Joynt, G. M., Wong, K. T., Gomersall, C. D., Li, T. S., Antonio, G., et al. (2005). Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 60, 401–409. doi: 10.1136/thx.2004.030205
Jack, S., Rossiter, H. B., Warburton, C. J., and Whipp, B. J. (2003). Behavioral influences and physiological indices of ventilatory control in subjects with idiopathic hyperventilation. Behav. Modif. 27, 637–652. doi: 10.1177/0145445503256318
Laffey, J. G., and Kavanagh, B. P. (2002). Hypocapnia. N. Engl. J. Med. 347, 43–53. doi: 10.1056/NEJMra012457
Li, T. S., Gomersall, C. D., Joynt, G. M., Chan, D. P., Leung, P., and Hui, D. S. (2006). Long-term outcome of acute respiratory distress syndrome caused by severe acute respiratory syndrome (SARS): an observational study. Crit. Care Med. 8, 302–308.
Lum, L. C. (1975). Hyperventilation: the tip and the iceberg. J. Psychosom. Res. 19, 375–383. doi: 10.1016/0022-3999(75)90017-3
Mart, M. F., and Ware, L. B. (2020). The long-lasting effects of the acute respiratory distress syndrome. Exp. Rev. Respir. Med. 14, 577–586. doi: 10.1080/17476348.2020.1743182
Miglis, M. G., Goodman, B. P., Chemali, K. R., and Stiles, L. (2020a). Re: ‘post-COVID-19 chronic symptoms’ by Davido et al. Clin. Microbiol. Infect. doi: 10.1016/j.cmi.2020.08.028 [Epub ahead of print].
Miglis, M. G., Prieto, T., Shaik, R., Muppidi, S., Sinn, D. I., and Jaradeh, S. (2020b). A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. doi: 10.1007/s10286-020-00727-9 [Epub ahead of print].
Mo, X., Jian, W., Su, Z., Chen, M., Peng, H., Peng, P., et al. (2020). Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 55:2001217. doi: 10.1183/13993003.01217-2020
Ngai, J. C., Ko, F. W., Ng, S. S., To, K. W., Tong, M., and Hui, D. S. (2010). The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 15, 543–550. doi: 10.1111/j.1440-1843.2010.01720.x
O’Keefe, J. B., Tong, E. J., Datoo, O., Keefe, G. A., and Tong, D. C. (2020). Predictors of disease duration and symptom course of outpatients with acute COVID-19: a retrospective cohort study. medRxiv [preprint] doi: 10.1101/2020.06.05.20123471
Ong, K. C., Ng, A. W., Lee, L. S., Kaw, G., Kwek, S. K., Leow, M. K., et al. (2004). Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur. Respir. J. 24, 436–442. doi: 10.1183/09031936.04.00007104
Park, W. B., Jun, K. I., Kim, G., Choi, J. P., Rhee, J. Y., Cheon, S., et al. (2018). Correlation between pneumonia severity and pulmonary complications in middle east respiratory syndrome. J. Korean Med. Sci. 33:e169. doi: 10.3346/jkms.2018.33.e169
Pascarella, G., Strumia, A., Piliego, C., Bruno, F., Del Buono, R., Costa, F., et al. (2020). COVID-19 diagnosis and management: a comprehensive review. J. Intern. Med. 288, 192–206. doi: 10.1111/joim.13091
Puntmann, V. O., Carerj, M. L., Wieters, I., Fahim, M., Arendt, C., Hoffmann, J., et al. (2020). Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5, 1265–1273. doi: 10.1001/jamacardio.2020.3557
Raman, B., Cassar, M. P., Tunnicliffe, E. M., Filippini, N., Griffanti, L., Alfaro-Almagro, F., et al. (2020). Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. medRxiv [preprint] doi: 10.1101/2020.10.15.20205054
Rodriguez-Morales, A. J., Cardona-Ospina, J. A., Gutierrez-Ocampo, E., Villamizar-Pena, R., Holguin-Rivera, Y., Escalera-Antezana, J. P., et al. (2020). Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med. Infect. Dis. 34:101623. doi: 10.1016/j.tmaid.2020.101623
Schaller, T., Hirschbuhl, K., Burkhardt, K., Braun, G., Trepel, M., Markl, B., et al. (2020). Postmortem examination of patients with COVID-19. JAMA 323, 2518–2520. doi: 10.1001/jama.2020.8907
Simpson, R., and Robinson, L. (2020). Rehabilitation after critical illness in people with COVID-19 infection. Am. J. Phys. Med. Rehabil. 99, 470–474. doi: 10.1097/PHM.0000000000001480
Tenforde, M. W., Kim, S. S., Lindsell, C. J., Billig Rose, E., Shapiro, N. I., Files, D. C., et al. (2020). Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - united states, march-june. MMWR Morb. Mortal. Wkly. Rep. 69, 993–998. doi: 10.15585/mmwr.mm6930e1
van Dixhoorn, J., and Duivenvoorden, H. J. (1985). Efficacy of nijmegen questionnaire in recognition of the hyperventilation syndrome. J. Psychosom. Res. 29, 199–206. doi: 10.1016/0022-3999(85)90042-x
van Gassel, R. J. J., Bels, J. L. M., Raafs, A., van Bussel, B. C. T., van de Poll, M. C. G., Simons, S. O., et al. (2020). High Prevalence of Pulmonary Sequelae at 3 Months After Hospital Discharge in Mechanically Ventilated COVID-19 Survivors. Am. J. Respir. Crit. Care Med. doi: 10.1164/rccm.202010-3823LE Online ahead of print.
Varga, Z., Flammer, A. J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A. S., et al. (2020). Endothelial cell infection and endotheliitis in COVID-19. Lancet 395, 1417–1418. doi: 10.1016/s0140-6736(20)30937-5
Weerahandi, H., Hochman, K. A., Simon, E., Blaum, C., Chodosh, J., Duan, E., et al. (2020). Post-discharge health status and symptoms in patients with severe COVID-19. medRxiv [preprint] doi: 10.1101/2020.08.11.20172742
Wong, A. W., Shah, A. S., Johnston, J. C., Carlsten, C., and Ryerson, C. J. (2020). Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur. Respir. J. 56:2003276. doi: 10.1183/13993003.03276-2020
Zhang, P., Li, J., Liu, H., Han, N., Ju, J., Kou, Y., et al. (2020). Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 8:8. doi: 10.1038/s41413-020-0084-5
Zhao, Y. M., Shang, Y. M., Song, W. B., Li, Q. Q., Xie, H., Xu, Q. F., et al. (2020). Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. E Clin. Med. 25:100463. doi: 10.1016/j.eclinm.2020.100463
Keywords: COVID-19, hyperventilation syndrome, dyspnea, cardiopulmonary exercise testing, SARS-CoV-2, exercise hyperventilation, persisting symptoms
Citation: Motiejunaite J, Balagny P, Arnoult F, Mangin L, Bancal C, d’Ortho M-P and Frija-Masson J (2021) Hyperventilation: A Possible Explanation for Long-Lasting Exercise Intolerance in Mild COVID-19 Survivors? Front. Physiol. 11:614590. doi: 10.3389/fphys.2020.614590
Received: 06 October 2020; Accepted: 24 December 2020;
Published: 18 January 2021.
Edited by:
Adriana Castello Costa Girardi, University of São Paulo, BrazilReviewed by:
Marli Maria Knorst, Federal University of Rio Grande do Sul, BrazilRen-Jay Shei, Mallinckrodt (United States), United States
Copyright © 2021 Motiejunaite, Balagny, Arnoult, Mangin, Bancal, d’Ortho and Frija-Masson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justina Motiejunaite, justina.motiejunaite@aphp.fr; orcid.org/0000-0001-8385-528X
 Justina Motiejunaite
Justina Motiejunaite Pauline Balagny1,3
Pauline Balagny1,3 Laurence Mangin
Laurence Mangin Justine Frija-Masson
Justine Frija-Masson