- 1Department of Physical Medicine and Rehabilitation, Sahloul Hospital, Sousse, Tunisia
- 2Department of Pneumology, Farhat HACHED Hospital, Sousse, Tunisia
- 3Research Laboratory “Heart Failure, LR12SP09”, Faculty of Medicine of Sousse, Hospital Farhat HACHED of Sousse, University of Sousse, Sousse, Tunisia
- 4Department of Training and Movement Science, Institute of Sport Science, Johannes Gutenberg-University Mainz, Mainz, Germany
- 5Interdisciplinary Laboratory in Neurosciences, Physiology and Psychology: Physical Activity, Health and Learning (LINP2), UPL, Paris Nanterre University, UFR STAPS, Nanterre, France
- 6High Institute of Sport and Physical Education of Sfax, University of Sfax, Sfax, Tunisia
Post-COVID19 patients suffer from persistent respiratory, cardiovascular, neurological, and musculoskeletal health complaints such as dyspnea, chest pain/discomfort, and fatigue. In Tunisia, the potential benefits of a cardiorespiratory rehabilitation program (CRRP) after COVID19 remain unclear. The main aim of this study was to evaluate the impact of a CRRP on submaximal exercise capacity, evaluated through the 6-min walk test (6MWT) data in post-COVID19 Tunisian patients. This was a cross-sectional study including 14 moderate to severe COVID19 patients aged from 50 to 70 years. CRRP was performed after the end of patients’ hospitalization in COVID19 units for extensive or severe extents of COVID19. Dyspnea (modified medical research council), spirometry data, handgrip strength values, 6MWT data, and 6-min walk work (i.e., 6-min walk distance x weight) were evaluated 1-week pre-CRRP, and 1-week post-CRRP. CRRP included 12 sessions [3 sessions (70 min each)/week for 4 weeks]. Exercise-training included aerobic cycle endurance, strength training, and educational sessions. Comparing pre- and post- CRRP results showed significant improvements in the means±standard deviations of dyspnea by 1.79 ± 0.80 points (p < 0.001), forced expiratory volume in one second by 110 ± 180 ml (p = 0.04), 6-min walk distance by 35 ± 42 m (p = 0.01), 6-min walk work by 2,448 ± 3,925 mkg (p = 0.048), resting heart-rate by 7 ± 9 bpm (p = 0.02) and resting diastolic blood pressure by 6 ± 10 mmHg (p = 0.045). In Tunisia, CRRP seems to improve the submaximal exercise capacity of post-COVID19 patients, mainly the 6-min walk distance and work.
1 Introduction
The coronavirus disease 2019 (COVID19) pandemic has overburdened healthcare systems and it poses a threat to the global economy and social disruption (Bessis, 2020). COVID19 is a respiratory infection with multisystem manifestations, affecting the respiratory, cardiovascular, neurological, and muscular systems (Xie et al., 2022). Several symptoms (e.g., dyspnea, dysrhythmias, stroke, headache, myalgia, and asthenia), and complications (e.g., respiratory failure, acute myocardial injury, thromboembolic events) have been reported for acute-COVID19 (Long et al., 2020; Murk et al., 2021). In post-acute COVID19, 40%–90% of patients continue to manifest symptoms for months, and the disease is named “long-COVID19” (Lopez-Leon et al., 2021). The “long-COVID19,” also called “post-acute-COVID19” or “persistent-COVID19 symptoms,” has various clinical manifestations affecting several systems, mainly the respiratory, cardiovascular, neurological, and muscular systems (e.g., dyspnea, post-activity polypnea, cough, chest pain/discomfort, resting tachycardia, fatigue), and alters the nutritional status (e.g., weight-loss) (Lopez-Leon et al., 2021; Skjorten et al., 2021; Ali et al., 2022; Ghram et al., 2022). Several studies have reported persistent physical impairments following hospital discharge (e.g., reduced forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) (Zhao Y. M. et al., 2020), decreased handgrip strength (HGS) (Cheval et al., 2021), and exertional dyspnea (modified medical research council (mMRC)) (Huang et al., 2021). In one study, the prevalence of musculoskeletal health complaints in “long-COVID19” patients was high at 38.7% (Ali et al., 2022). A reduced 6-min walk distance (6MWD) was reported by Huang et al. (2021), and it seems that 3 months after hospital discharge, one-third of long-COVID19 patients had a peak oxygen consumption <80% (Skjorten et al., 2021). Since survivors of moderate to severe COVID19 are significantly impaired in all activities of daily living (Skjorten et al., 2021), rehabilitation strategies are needed to improve post-COVID19 outcomes in this population. (Demeco et al., 2020; Hermann et al., 2020; Liu K. et al., 2020; Betschart et al., 2021; Bouteleux et al., 2021; Daynes et al., 2021; Gloeckl et al., 2021; Puchner et al., 2021; Spielmanns et al., 2021). The cardiorespiratory rehabilitation program (CRRP) is the cornerstone in the management of chronic cardiorespiratory diseases, and its benefits are well demonstrated (Ben Saad et al., 2008; Jenkins et al., 2018; Rezende Barbosa et al., 2018).
In COVID19, CRRP is a new management axis, and studies related to its impact on patients’ capacities are scarce (Demeco et al., 2020; Hermann et al., 2020; Liu K. et al., 2020; Betschart et al., 2021; Bouteleux et al., 2021; Daynes et al., 2021; Gloeckl et al., 2021; Puchner et al., 2021; Spielmanns et al., 2021). Two systematic reviews including almost 33 studies demonstrated the feasibility and efficiency of CRRP in the management of post-COVID19 patients (Demeco et al., 2020; Dixit et al., 2021). Almost all 33 retained studies were conducted in specialized rehabilitation units from industrialized countries. In low-income countries, such as Tunisia, the potential benefits of CRRP after COVID19 is unclear. Indeed, in these countries, CRRP centers are rare, no specialized rehabilitation equipment is available, and too few COVID19 patients have access to CRRP.
The objective of the present study, conducted in Tunisia, was to evaluate the impact of an ambulatory CRRP on perceived dyspnea (mMRC), spirometric, HGS, and 6-min walk test (6MWT) data. CRRP will be considered “efficient” if the delta CRRP changes (ΔCRRP = post-CRRP value minus pre-CRRP value) in the 6MWD and dyspnea mMRC scale exceed the recommended minimal clinically important differences (MCIDs) for respiratory chronic diseases [i.e., MCID = 30 m for 6MWD (Singh et al., 2014), MCID = one point for dyspnea (mMRC) (Crisafulli and Clini, 2010)].
2 Patients and methods
This study is part of a project involving two parts. The first part constitutes the aim of this study. The second part will be the evaluation of the impact of CRRP on social disadvantage (i.e., physical activity, psychological data, health-related quality of life). Figure 1 details the present project flowchart.
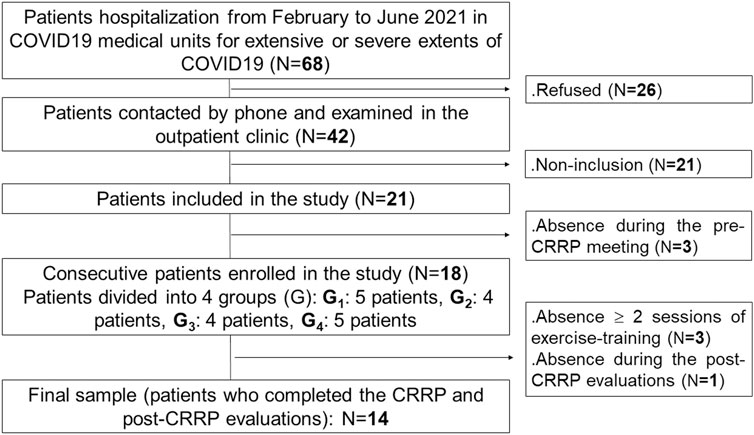
FIGURE 1. Study protocol. COVID19: coronavirus disease 2019. CRRP, cardio-respiratory rehabilitation program.
2.1 Study design
This was a cross-sectional study conducted by a multidisciplinary team, including the following three departments: the department of pulmonology, the department of physiology and functional explorations (Farhat HACHED hospital, Sousse, Tunisia), and the department of physical medicine and rehabilitation (Sahloul hospital, Sousse, Tunisia). This study was approved by the medical and research ethics committee of Farhat HACHED Hospital (Approval number FH2502/2021). Written informed consent was obtained from all patients after receiving an explanation of the study. This study was performed from February 2nd to September 26th 2021, including the Ramadan month (from April 13th to May 13th 2021). The period from February to June 2021 was reserved for the recruitment of COVID19 patients. The period from April to September 2021 was reserved for the practice of CRRP. The CRRP was performed at least 2 months after the end of the hospitalization in COVID19 units.
During the present study period (i.e., February 2nd to September 26th 2021), Tunisia decided to declare a 1-week nationwide lockdown starting from May 9th to May 16th 2021. During all the study steps, all recommended preventive measures to fight against COVID19 transmission (e.g., physical distancing of at least 1 m, wearing a fitted facemask properly, and cleaning hands frequently with alcohol-based hand rub or soap and water) were applied.
2.2 Study population
The source population was COVID19 patients living in Sousse (Tunisia) who needed hospitalization in a medical facility. The target population was patients hospitalized in COVID19 units of the aforementioned departments of pulmonology and physical medicine from February to June 2021 (Figure 1).
The following inclusion criteria were applied: confirmed diagnosis of COVID19, male patients, age >50 years, and chest computed tomography during the hospitalization period showing an extensive/severe extent of parenchymal lung injury (Revel et al., 2020). The applied exclusion criteria were: 1) COVID19 patients admitted in an intensive care unit; 2) contra-indications to 6MWT (Singh et al., 2014) [e.g., signs of unstable angina or myocardial infarction within the previous month, resting heart-rate ≥ 120 bpm, systolic blood pressure (SBP) ≥ 180 mmHg, diastolic blood pressure (DBP) ≥ 100 mmHg)]; 3) contra-indications to spirometry (Miller et al., 2005); and 4) orthopedic, rheumatologic, or muscular history, which may interfere with walking or HGS. Absence during two or more exercise-training sessions or the post-CRRP evaluation session was applied as an exclusion criterion.
2.3 Sample size
The sample size (N) was calculated according to the following predictive equation (Serhier et al., 2020): N = (Zα p (1-p))/i2, where “Zα” is the normal deviates for type I error (equal to 1.28 for 90% confidence level), “p” is the percentage of improvement of the main outcome (i.e., 6MWD) post-CRRP in COVID19 patients; and «i» is the precision (i = 0.15). According to a Chinese study (Liu K. et al., 2020), the 6MWD of trained COVID19 patients (n = 36, mean age: 69.4 years) was improved by 30.5% (p = 0.305) [went from 163 ± 72 (pre-CRRP) to 212 ± 82 m (post-CRRP)]. The application of the above-mentioned data in the predictive equation gave a sample size of 15 COVID19 patients. Assumption of 10% of absence during the exercise-training sessions or the post-CRRP evaluation session gave a revised sample of 17 COVID19 patients [17 = 15/(1–0.10)].
2.4 Coronavirus disease 2019 diagnosis and extent evaluation
COVID19 diagnosis was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) (Jamil et al., 2020). All patients underwent chest-computed tomography. The following two classifications were applied: 1) chest computed-tomography classification, including the following five levels based on the extent of parenchymal lung injury: absent or minimal (<10%), moderate (10%–25%), extensive (25%–50%), severe (50%–75%), and critical (>75%) (Revel et al., 2020), and 2) clinical classification (WHO, 2021), including the following four levels: mild, moderate, severe, and critical.
2.5 Applied protocol
The components of the CRRP were “derived” from previous international recommendations for COVID19 CRRPs (Barker-Davies et al., 2020; Carda et al., 2020; Spruit et al., 2020; Zhao H. M. et al., 2020), and from the American societies of cardiology and sports medicine recommendations for the practice of physical activity in chronically ill patients aged over 50 years (Nelson et al., 2007). Once four to five consecutive patients agreed to participate in the CRRP, they formed one group (Figure 1), perform the recommended tests, and begin the CRRP. Figure 2 summarizes the five steps of the study.
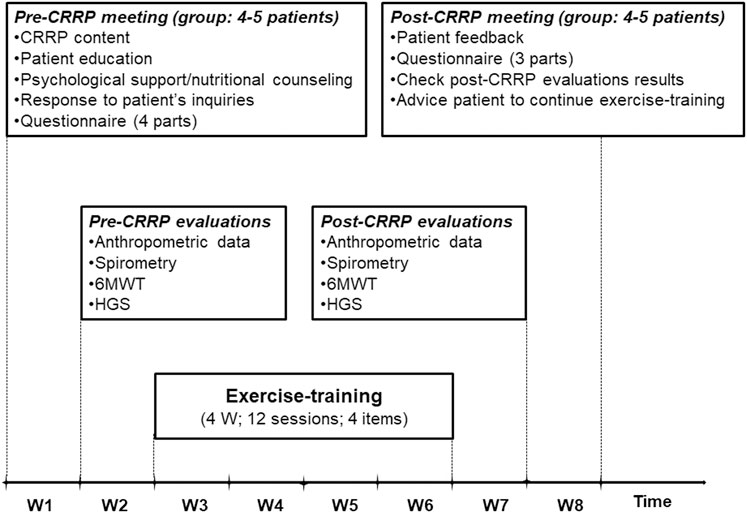
FIGURE 2. Cardiorespiratory rehabilitation program (CRRP). HGS, handgrip strength; W, week; 6MWT, 6-min walk test.
2.5.1 First step: Pre-CRRP meeting
The first step consists of a pre-CRRP meeting between two physicians (ET and WB in the authors’ list) and a group of four to five COVID19 patients. During this step, the following five actions were performed: 1) explanation of the CRRP content and its progress; 2) when applicable, education about how to manage comorbidities (e.g., diabetes-mellitus, arterial-hypertension), and encouraging smoking cessation; 3) psychological support (e.g., management of emotional distress, post-traumatic stress disorder, and strategies for coping with COVID19) (Simpson and Robinson, 2020), and nutritional counseling (Ghram et al., 2022); 4) response to patients’ inquiries; and 5) filling in the questionnaire.
The questionnaire was prepared in the local Arabic dialect by two trained physicians (ET and WB in the authors’ list). For each patient, the questionnaire was repeated by the same interviewer pre- and post- CRRP. The duration of the questionnaire was approximately 30 min for each patient. The questionnaire includes four parts. The first part (i.e., a general questionnaire), derived from the American thoracic society questionnaire (Ferris, 1978), was performed only pre-CRRP, and it involved clinical (e.g., lifestyle habits, medical history) and COVID19 (e.g., date of RT-PCR, hospitalization, number of days pre-CRRP, treatment, imaging) data. Cigarette smoking was evaluated in pack-years, and patients were classified into two groups [i.e., non-smoker (<5 pack-years), and smoker (≥5 pack-years)]. Hospital stay is the number of days of hospitalization for COVID19 management. The number of days pre-CRRP represents the number of days between COVID19 diagnosis (day of RT-PCR) and the first day of the onset of exercise-training. Dyspnea was assessed (pre- and post- CRRP) using the mMRC scale (Fletcher et al., 1959). The latter is a self-rating scale that measures the disability caused by breathlessness in daily activities (Mahler and Wells, 1988). This scale ranges from 0 to 4, where “0” is no breathlessness, except on strenuous exercise; and “4” is too breathless to leave the house, or breathless when dressing or undressing (Mahler and Wells, 1988). The remaining three parts of the questionnaire were reserved to explore the level of physical activity, current presence and tendency to anxiety or depression at the time of evaluation, and health-related quality of life. The data of the last three parts of the questionnaire will be explored in the second part of the project.
2.5.2 Second step: Pre-CRRP evaluations
During this step, the following four evaluations/tests were performed on the same day in the morning, and in the following order: anthropometric data, spirometry test, 6MWT, and HGS. The 6MWT and the HGS were performed on patients not wearing facemask.
Anthropometric data e.g., age, height (cm), weight (kg), and body mass index (BMI, kg/m2) were determined. The obesity status [underweight (BMI <18.5 kg/m2), normal weight (BMI: 18.5–24.9 kg/m2), overweight (BMI: 25.0–29.9 kg/m2), and obesity (BMI ≥30.0 kg/m2)] was noted (Tsai and Wadden, 2013).
The spirometry test was performed by an experiment technician using a portable spirometer (SpirobankG MIR, delMaggiolino 12500155 Roma, Italy), according to international guidelines (Miller et al., 2005). The collected spirometric data [i.e., (FVC, L), (FEV1, L), maximal mid-expiratory flow (L/s), and FEV1/FVC ratio (absolute value)] were expressed as absolute values and as percentages of predicted local values (Ben Saad et al., 2013).
The 6MWT was performed outdoors in the morning by one physician (HBS in the authors’ list), according to the international guidelines (Singh et al., 2014). The 6MWT was performed along a flat, straight corridor with a hard surface that is seldom traveled by others (40 m long, marked every 1 m with cones to indicate turnaround points). During the 6MWT, some data were measured at rest (Rest) and at the end (End) of the walk [e.g., dyspnea (visual analogue scale (VAS)), heart-rate, oxyhemoglobin saturation (SpO2, %); SBP and DBP (mmHg)], and the 6MWD (m, % of predicted value), and the number of stops were noted. For some 6MWT data, delta exercise changes (ΔExercise = 6MWTEnd value minus 6MWTrest value) were calculated [e.g., ΔSpO2, Δheart-rate, ΔDBP, ΔSBP, Δdyspnea (VAS)]. The test instructions given to the patients were those recommended by the international guidelines (Singh et al., 2014). Heart-rate was expressed as absolute value (bpm) and as percentage of the predicted maximal heart-rate [predicted maximal heart-rate (bpm) = 208—(0.7 x Age)] (Tanaka et al., 2001). Heart-rate and SpO2 were measured via a finger pulse oximeter (Nonin Medical, Minneapolis, MN). The heart-rateEnd (bpm) was considered as heart-rate target for lower limb exercise-training (Fabre et al., 2017). The predicted 6MWD and the lower limit of normal (LLN) were calculated according to local norms (Ben Saad et al., 2009). The 6-min walk work (i.e., the product of 6MWD and weight (Chuang et al., 2001; Carter et al., 2003)) was calculated. The VAS is an open line segment with the two extremities representing the absence of shortness of breath and the maximum shortness of breath (Sergysels and Hayot, 1997). Dyspnea (VAS) is evaluated by the physician from 0 (no shortness of breath) to 10 (maximum shortness of breath) (Sergysels and Hayot, 1997).
The HGS test measures the maximum-voluntary upper-limb muscle strength using an adjustable handgrip dynamometer (TKK5401®, Takei Scientific Instruments Co., Ltd., Niigata, Japan). The latter is a valid and reliable measure having a range of 5–100 kg of force, with increments of 1 kg (Cadenas-Sanchez et al., 2016). A brief demonstration and verbal instructions for the test were given to patients, and if necessary, the dynamometer was adjusted to the size of the hand. The measurements were taken in a standing position with the shoulder adducted and in neutral rotation, and the arms parallel but not in contact with the body. Participants were asked to tighten the dynamometer as hard as possible while exhaling. The test was repeated three times on each hand. The highest value of the three trials of the dominant hand was retained (Haidar et al., 2004), and it was expressed as absolute (kg) and relative (i.e., divided by weight) values.
2.5.3 Third step: Exercise-training
Exercise-training consists of 12 sessions (i.e., three sessions/week for 4 weeks).
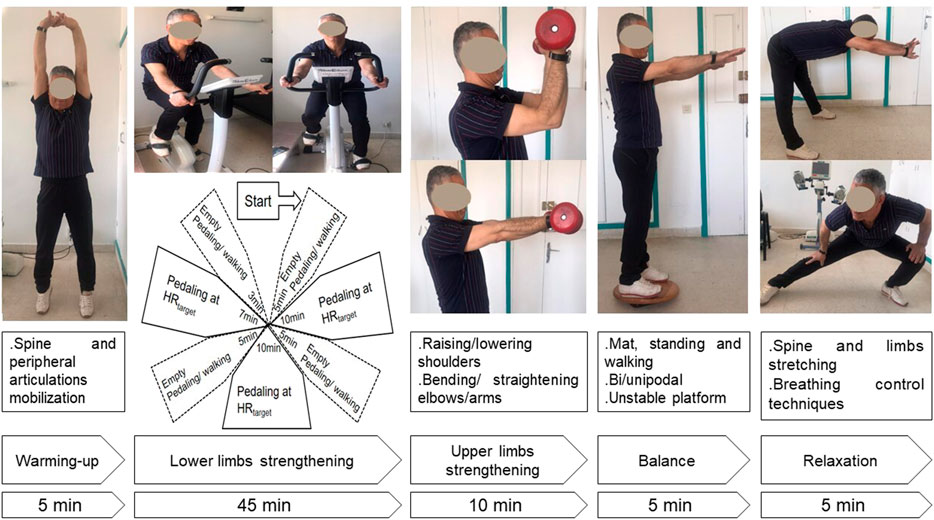
The duration of each session was 70 min. Exercise-training was performed in four groups of four or five patients. The typical exercise-training session included the following five items (Figure 3): warming-up for 5 minutes, lower limbs strengthening for 45 min, upper limbs strengthening for 10 min, balance posture and proprioception exercises for 5 minutes, and relaxation session for 5 minutes. During the first item (i.e., warming-up), light exercises were performed (i.e., walking slowly; mobilization of cervical, lumbar spine, and peripheral joints). During the second item (i.e., lower limbs strengthening), aerobic training on ergocycle was performed. The cycling intensity was standardized and personalized using a heart-rate monitor. As done in one previous similar study (Hermann et al., 2020), the heart-rate target was the heart-rateEnd ± 5 bpm determined during the 6MWT. In patients with chronic respiratory conditions, the heart-rate at the first ventilatory threshold measured during a cardiopulmonary exercise test was comparable and correlated to the heart-rate determined at the end of the 6MWT (Fabre et al., 2017). The latter heart-rate target (i.e., heart-rateEnd) allows individualizing the training intensity for each patient, and therefore optimizing the physical and physiological benefits of the CRRP (Fabre et al., 2017). The heart-rate monitor alarms were set around the heart-rate target. The patients were asked to gradually reach their heart-rate targets during the first 5 minutes and to maintain pedaling for 10 min at this intensity. Then, they were asked to return to empty pedaling or walking at their own pace for 5 minutes. They were again asked to complete one cycle of 10 min of target heart-rate training and 5 minutes of active recovery (e.g., empty pedaling or walking at their own pace), then to complete the last cycle of 7 minutes of target heart-rate training and 3 minutes of active recovery. During the third item (i.e., upper limbs strengthening), various muscle groups of the upper limbs were performed in sets of ten repetitions (e.g., raising and lowering shoulders, shoulder blade stabilization, bending and straightening elbows, raising arms). These exercises were performed without load during the first exercise-training sessions, then with dumbbells of increasing weights along exercise-training (Ben Saad and Ben Abdelkrim, 2005). During the fourth item, several exercises were performed to improve balance posture, proprioception, coordination, and stability. Positions exercises (i.e., floor exercises, seated, and standing exercises) were varied between sessions. Exercises of increasing difficulty on a mat, static and dynamic standing and walking, bipodal, and then unipodal exercises on an unstable platform were performed along the exercise-training (Ben Saad and Ben Abdelkrim, 2005). During the fifth item (i.e., relaxation), several exercises involving spine and limbs stretching (e.g., standing stretch, cat back exercises, sphinx position) and breathing exercises (e.g., controlled diaphragmatic breathing, coordination between inspiratory and expiratory times) were performed (Ben Saad and Ben Abdelkrim, 2005). During each exercise-training session, therapeutic education was carried out to strengthen the patients’ adherence to the lifestyle counseling provided during the pre-CRRP meeting (e.g., management of comorbidities and encouraging smoking cessation when applicable, psychological support, and nutritional counseling) (Ghram et al., 2022). All exercise-training items were performed on patients not wearing the facemask.
2.5.4 Fourth step: Post-CRRP evaluation
During this step, similar evaluations/tests to the second step were performed.
2.5.5 Fifth step: Post-CRRP meeting
During this step, the following issues were tackled: patients’ feedback, questionnaire (as conducted during the first step, except the general questionnaire), checking the results of post-CRRP evaluations, and advising patients to continue exercise-training.
2.6 Applied definitions for the submaximal exercise data
1) Abnormal 6MWD: 6MWD < LLN (Singh et al., 2014);
2) Clinically significant desaturation: ΔSpO2 > 5 point (Ben Saad et al., 2009; Ben Saad et al., 2014; Ben Saad et al., 2015; Ben Saad, 2020);
3) Walk intolerance signs: clinically significant dyspnea [i.e., dyspneaEnd (VAS) > 5/10] (Sergysels and Hayot, 1997; Ben Saad et al., 2014), and/or stopping during the 6MWT (Ben Saad et al., 2009; Ben Saad et al., 2014; Ben Saad et al., 2015; Ben Saad, 2020).
2.7 Statistical analysis
Quantitative and categorical data were presented as means ± standard deviation (95% confidence interval) and number (%), respectively. For each quantitative data (i.e., dyspnea (mMRC and VAS), weight, BMI, HGS, 6MWD, 6-min walk work, heart-rate, SpO2, SBP, DBP, and ΔExercise), a ΔCRRP was calculated. The Wilcoxon matched pairs test and the one-sided chi-2 test were used to compare the quantitative and categorical data pre- and post- CRRP, respectively. CRRP was considered “efficient” if the means of ΔCRRP for 6MWD and dyspnea (mMRC) exceeded the recommended MCIDs [i.e., 30 m for 6MWD (Singh et al., 2014)) and one point for dyspnea (Crisafulli and Clini, 2010)]. All statistical procedures were performed using statistical software (StatSoft, Inc. (2011). STATISTICA, version 12). The significance level was set at p < 0.05.
3 Results
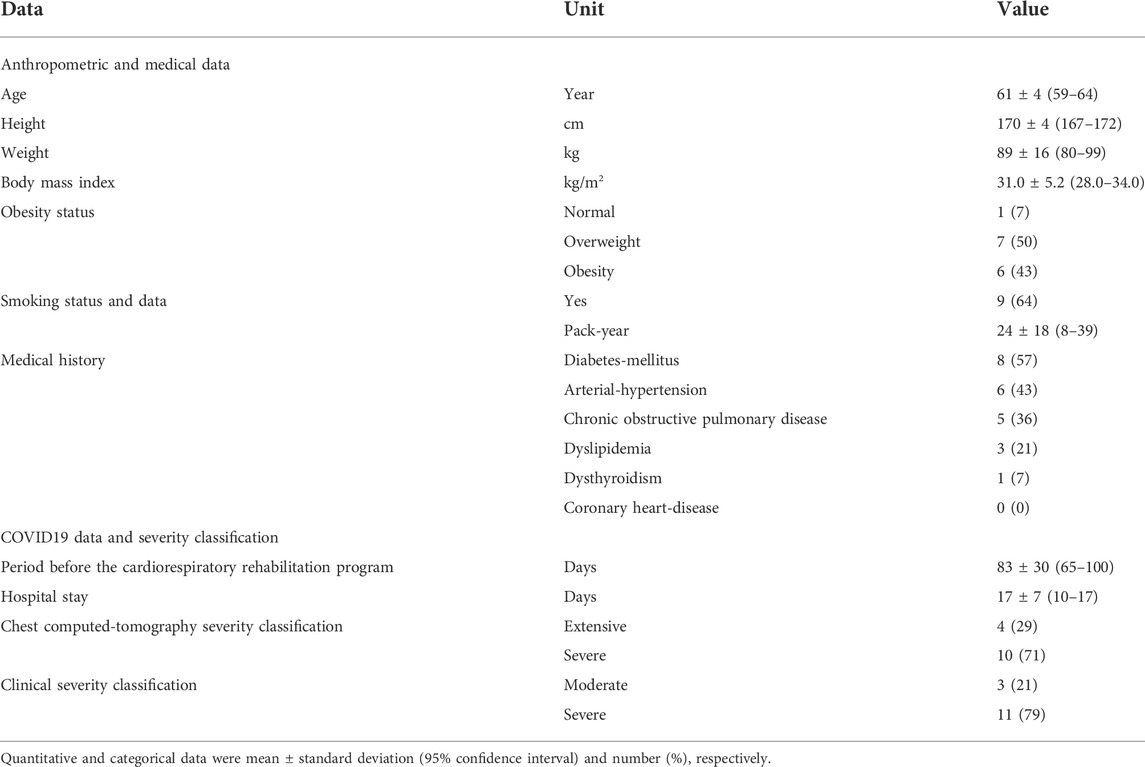
An initial sample of 68 patients was recruited. After the application of the inclusion/non-inclusion criteria, 18 patients were retained. Four patients withdrew during CRRP. Fourteen patients (age: 61 ± 4 years) completed the full CRRP and tests evaluations (Figure 1).
Table 1 details the patients’ characteristics. The profile of COVID19 patients was characterized by high frequencies of overweight and obesity (n = 13/14; 93%), level-2 chest computed-tomography at admission (n = 10/14; 71%), and smoking (n = 9/14; 64%). The two most frequent medical comorbidities were diabetes-mellitus and arterial-hypertension.
Table 2 illustrates the impact of CRRP on dyspnea, anthropometric, spirometric, and HGS data. Dyspnea (mMRC) was improved by 1.79 points, which exceeds the MCID of one point. FEV1 was improved by 110 ml (3.29%).
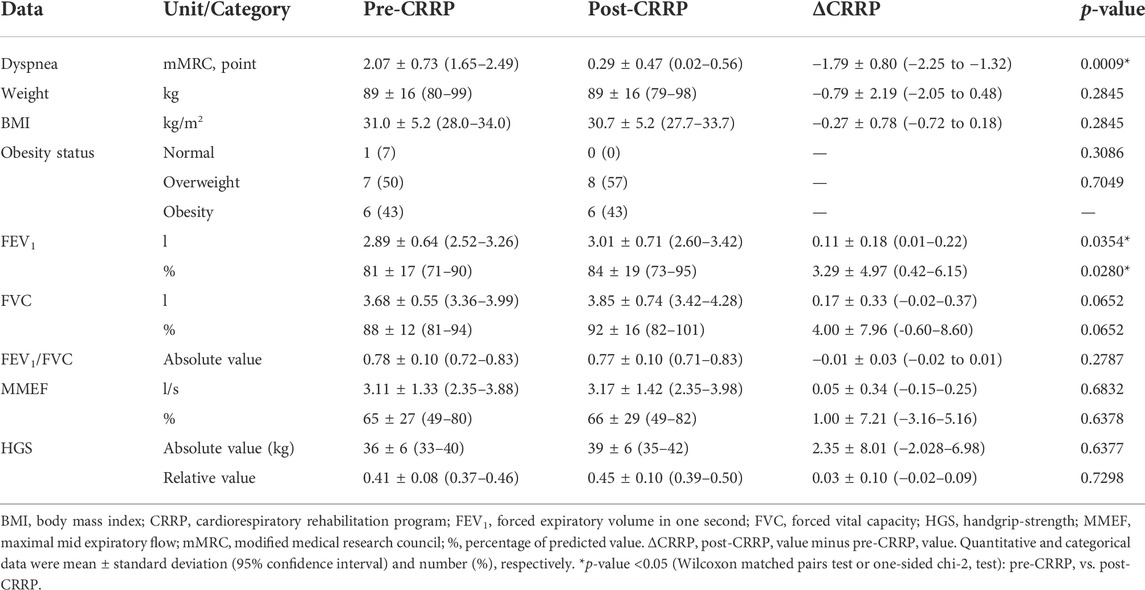
TABLE 2. Impact of CRRP on dyspnea, and anthropometric, spirometric and HGS data of patients with coronavirus disease 2019 (n = 14).
Table 3 illustrates the impact of CRRP on submaximal exercise data. The 6MWD increased by 35 m, which is higher than the MCID of 30 m. Nine patients (64.3%) increased their 6MWD by more than 35 m, and the number (%) of COVID19 patients with abnormal 6MWD decreased from 3 (21%) to 0 (0%). The 6-min walk work increased by 2,448 mkg. The heart-rateRest (bpm, %) decreased by seven bpm (5%), and DBPRest decreased by 6 mmHg.
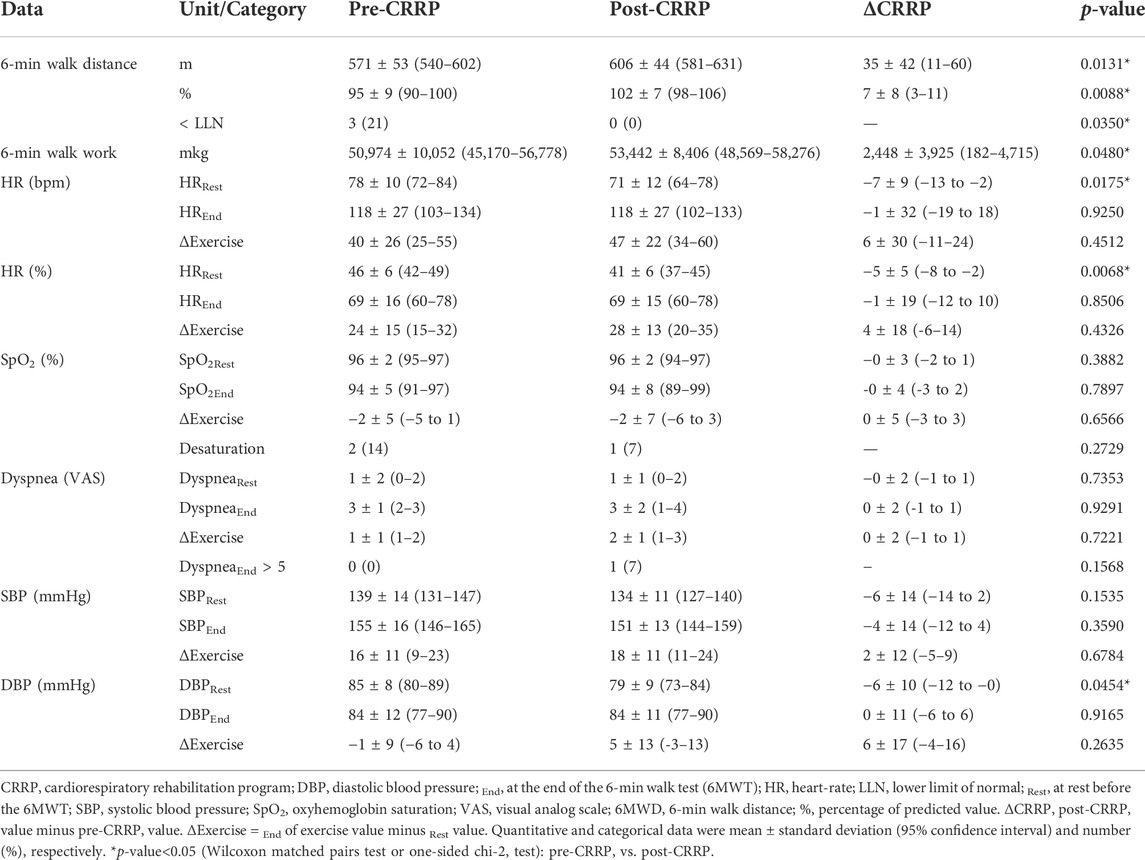
TABLE 3. Impact of CRRP on submaximal exercise data of patients with coronavirus disease 2019 (n = 14).
4 Discussion
The present Tunisian study demonstrated that the CRRP improves the submaximal exercise capacity of post-COVID19 patients. For instance, the 6MWD improved by 35 m, which exceeds the MCID of 30 m, and the dyspnea (mMRC) improved by 1.78 point, which exceeds the MCID of one point. To the best of the authors’ knowledge, this is the first North-African study investigating the impact of CRRP on post-COVID19 patients. The methodology and main outcomes of some similar studies including a single group of COVID19 patients (Hermann et al., 2020; Betschart et al., 2021; Bouteleux et al., 2021; Daynes et al., 2021; Gloeckl et al., 2021; Piquet et al., 2021; Puchner et al., 2021), and case-control studies (Liu K. et al., 2020; Spielmanns et al., 2021) are detailed in Tables 4, 5, respectively.
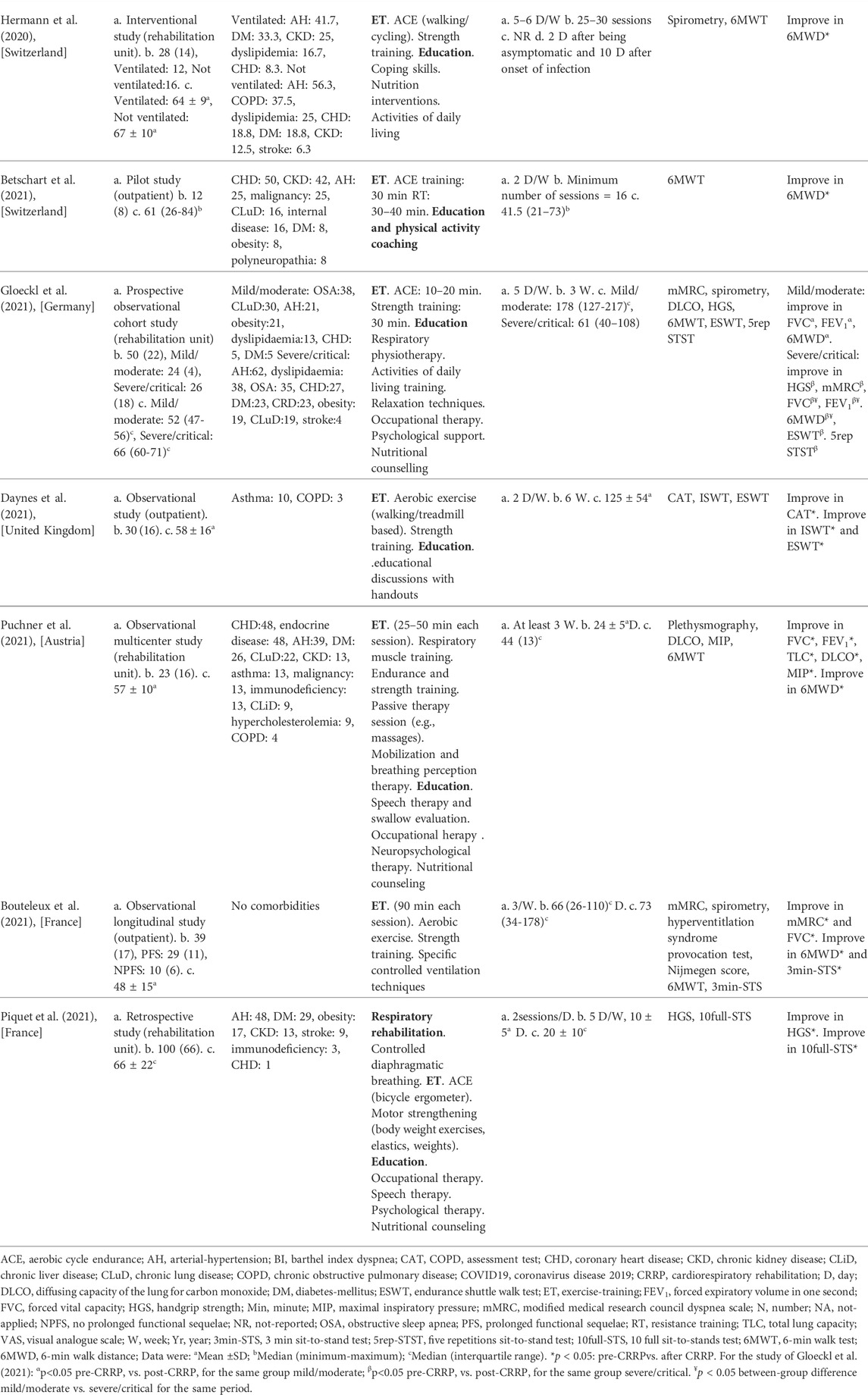
TABLE 4. Methodology and main outcomes of some studies including a single group of COVID19 patients, and aiming at evaluating the impacts of CRRP on COVID19 patients.
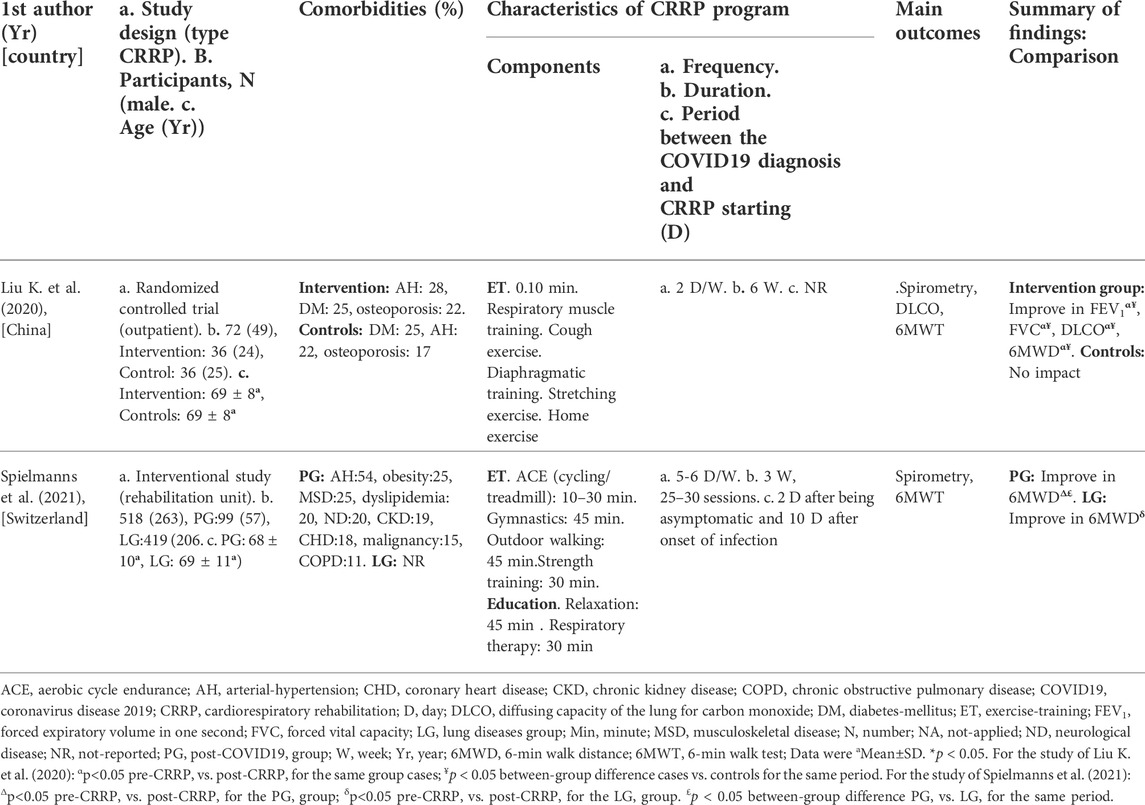
TABLE 5. Methodology and main outcomes of some case-control studies aiming at evaluating the impact of CRRP on COVID19 patients.
4.1 Discussion of results
In this study, the increase in the main outcome (i.e., 6MWD) was both “statistically” and “clinically” significant (mean of 35 m, which exceeds the MCID of 30 m (Singh et al., 2014)). At the end of CRRP, no COVID19 patients had an abnormal 6MWD, and the heart-rateRest and DBPRest decreased by seven bpm (5%) and 6 mmHg, respectively (Table 3).
The mean increase in 6MWD reported in this study was intermediate with the values reported in the literature (Liu K. et al., 2020; Betschart et al., 2021; Gloeckl et al., 2021) (Tables 4, 5). The 35-m 6MWD mean was closer to these reported in some studies [e.g., 48 m for mild/moderate patients (Gloeckl et al., 2021), 50 m (Liu K. et al., 2020)], but it was lower than the values reported in some other studies [e.g., 88 m (Betschart et al., 2021), 124 m for severe/critical patients (Gloeckl et al., 2021), 130 m (Hermann et al., 2020), 176 m mean (Puchner et al., 2021)]. Similar to some studies (Hermann et al., 2020; Betschart et al., 2021; Gloeckl et al., 2021), the improvement in 6MWD noted in this study was “clinically significant.” Indeed, before CRRP, three patients had an abnormal 6MWD; and after CRRP, all patients had normal 6MWD (p = 0.03) (Table 3). This finding is inconsistent with the one reported in a German study (Gloeckl et al., 2021), where 79% of mild/moderate patients had an abnormal 6MWD after 3 weeks of inpatient rehabilitation. Because weight directly affects the work/energy required to perform the 6MWT (Holland et al., 2014; Singh et al., 2014), the 6-min walk work was calculated. The latter, which is the product of 6MWD and weight, provides a better estimate of the work required to perform the 6MWD than distance alone (Holland et al., 2014; Singh et al., 2014). In this study, since the 6-min walk work increased significantly (Table 3), and since there were no statistically significant changes in patients’ weight or BMI (Table 2), these confirm that the 6MWD improve is independent of changes in weight or BMI. To the best of the authors’ knowledge, no previous study investigated the change of the 6-min walk work before/after a CRRP in COVID19 patients. Additional studies are needed to better characterize the utility of 6-min walk work in rehabilitation programs of COVID19 patients.
The decrease in heart-rateRest and DBPRest noted in this study (Table 3) could have some clinical importance. It appears that increased heart-rateRest (after adjustment for fitness) is an independent risk factor for all-cause mortality in males (Aladin et al., 2014), and a previous report indicated that a 10-bpm increase in heart-rateRest may increase all-cause mortality by 17% (Aune et al., 2017). High blood pressure is among the most important modifiable risk factors for cardiovascular disease and death (Williams et al., 2018). After the CRRP, the mean DBPRest decreased from 85 to 79 mmHg, which is an interesting outcome since the 2018 European society of cardiology recommends an optimal DBPRest target between 70 and 80 mmHg for patients with all risk levels (Williams et al., 2018). The present decrease in heart-rateRest is comparable to previously reported findings in older adults indicating a beneficial effect for endurance-based exercise-training (Schmidt et al., 2014; Akwa et al., 2017) as well as combined exercise-training (Delecluse et al., 2004; Ammar et al., 2021) with a significant reduction of heart-rateRest ranging from 4.5 to eight bpm. Presumably, the present beneficial cardiac effects of twelve-CRRP sessions could be the result of an enhancement of the cardiovascular autonomic control, with possible modification in the sympathovagal balance (Gamelin et al., 2007; Ammar et al., 2021). However, the exact mechanisms require further investigation. Additionally, the reduced values of both heart-rateRest and DBPRest at post-CRRP could be explained by the improvement in fitness (Greenland et al., 1999), and/or sleep quality (Soler et al., 2013; Yuksel et al., 2014), and/or nutritional status (Singh et al., 2000). The aforementioned results have not been discussed in previous studies evaluating the effects of CRRP on 6MWT data in COVID19 patients (Table 4, 5).
In our study, while dyspnea (mMRC) and FEV1 were improved by 1.79 points and 110 ml, respectively, HGS remained unchanged (Table 2). The finding related to dyspnea is in line with previous reports (Table 4) indicating that CRRP improves perceived dyspnea [whatever its mode of evaluation; e.g., mMRC (Bouteleux et al., 2021; Gloeckl et al., 2021), chronic obstructive pulmonary disease assessment test (Daynes et al., 2021)], even in severe/critical COVID19 patients (Gloeckl et al., 2021). In our study, mMRC improvement was higher than the one point MCID (Crisafulli and Clini, 2010). To the best of the authors’ knowledge, this is the first study investigating MCID dyspnea after a CRRP in post-COVID19 patients. In these patients, improvement in perceived dyspnea is capital since dyspnea is significantly associated with higher mortality (Shi et al., 2020), and it is a predictive factor of reduced functional capacity (Wong et al., 2021).
Our results concerning spirometry data are comparable with those investigating the impact of CRRP on lung function data (Liu K. et al., 2020; Bouteleux et al., 2021; Gloeckl et al., 2021; Puchner et al., 2021) (Tables 4, 5). In the latter studies, at least one lung function parameter was improved [FEV1 (Liu K. et al., 2020; Gloeckl et al., 2021; Puchner et al., 2021), FVC (Liu K. et al., 2020; Bouteleux et al., 2021; Gloeckl et al., 2021; Puchner et al., 2021), total lung capacity (Puchner et al., 2021), diffusing lung capacity for carbon monoxide (DLCO) (Liu K. et al., 2020; Puchner et al., 2021)]. Concerning the improvement in FEV1, the 110-ml increase observed in our study was lower than the values reported in the literature [e.g., 200 ml (Puchner et al., 2021), 340 ml (Liu K. et al., 2020)]. The improvement in lung function data could be explained by the breathing exercises and the respiratory muscle training applied during CRRP (Liu K. et al., 2020; Gloeckl et al., 2021; Puchner et al., 2021). Improvement in lung function data, such as FVC and FEV1, is useful to improve risk stratification in patients with intermediate coronary heart disease (Lee et al., 2010). FVC is implicated in predicting cardiovascular events and thus mortality (Lee et al., 2010). In addition, even in healthy people, there is a positive correlation between spirometric data (e.g., FEV1 and FVC) and 6MWD (Ben Saad et al., 2009).
In our study, the HGS remained unchanged (Table 2). Our findings were inconsistent with those reported by two previous studies, where HGS improved by 3 kg (i.e., from 18 to 21 kg) (Piquet et al., 2021) or 5 kg (from 25 to 30 kg) (Gloeckl et al., 2021) (Table 4). The absence of improvement in HGS could be explained by the fact that the pre-CRRP HGS value (i.e., 37 kg) was in the norms [i.e., >27 kg (Cruz-Jentoft et al., 2019)], and by the inclusion of moderate to severe COVID19 patients (Table 1). In the two above-cited studies reporting improvement in HGS, patients were classified as severe or critical (Gloeckl et al., 2021), and both the pre- and the post- CRRP HGS values were below the norms (Piquet et al., 2021). HGS measurement is important since it is associated with frailty and with an increased risk of mortality (Cheval et al., 2021). Indeed, COVID19 survivors have an increased risk of acute sarcopenia (Welch et al., 2020) due to the loss of muscle mass, fiber denervation, neuromuscular junction damage, and upregulation of protein breakdown (Puthucheary et al., 2010).
4.2 Discussion of methods
The discrepancies noted between our results and these of some similar studies (Tables 4, 5) could be explained by at least seven points related to differences in:
1) Study designs: prospective observational cohort (Gloeckl et al., 2021) vs. case control (Liu K. et al., 2020) studies;
2) CRRP locations: outpatient (Betschart et al., 2021) vs. inpatient (Puchner et al., 2021) rehabilitation;
3) Some inclusion criteria such as inclusion of both males and females (Bouteleux et al., 2021; Daynes et al., 2021), which could have influenced the findings since COVID19 clinical data are sex-dependent (Marik et al., 2021); or inclusion of COVID19 patients having different ages (e.g., elderly (≥65 years) (Liu K. et al., 2020) vs. middle-aged (48 ± 15 years) (Bouteleux et al., 2021)) which could have influenced the findings (Liu Y. et al., 2020);
4) COVID19 patients’ profiles (e.g., no (Bouteleux et al., 2021), vs. several (Betschart et al., 2021; Gloeckl et al., 2021) comorbidities) and/or in the disease severity stages (e.g., mild/moderate vs. severe/critical) (Gloeckl et al., 2021);
5) CRRP’ components (e.g., exercise-training and education (Hermann et al., 2020; Betschart et al., 2021) vs. exercise-training alone (Bouteleux et al., 2021));
6) Durations and/or frequencies of CRRP (e.g., two sessions/week and 16 sessions (Betschart et al., 2021) vs. five sessions/week and three sessions (Gloeckl et al., 2021)); and
7) Time periods’ between the diagnosis of COVID19 and the start of CRRP [e.g., early rehabilitation for acute COVID19 (Puchner et al., 2021) vs. late rehabilitation for long-COVID19 (Daynes et al., 2021)].
4.3 Strengths and limitations
This study has three strong points. First, our study was conducted in an outpatient unit in a low-income country (e.g., Tunisia) and the different components were performed (i.e., exercise-training, education, and nutritional counseling). Second, our sample size was calculated according to a predictive equation (Serhier et al., 2020). Determination of the finest size is a central topic since it helps in avoiding an inadequate power to distinguish statistical effects (Mascha and Vetter, 2018), and it guarantees a representative sample to differentiate statistical significance (Serhier et al., 2020). Huge sample size is costly and exposes more participants to measures (Mascha and Vetter, 2018), but using insufficient participants may lead to lower “precision” in results. Third, both statistically and clinically significant approaches were applied. Nowadays, the statistically significant approach, with a “p-value” < 0.05 being considered significant, is disapproved (Yaddanapudi, 2016). The MCID of 30 m for the 6MWD (Singh et al., 2014) and one point for dyspnea (mMRC) were introduced (Crisafulli and Clini, 2010). For instance, in a German study (Gloeckl et al., 2021) (Table 4), it was demonstrated that post-CRRP dyspnea median (interquartile) value is significantly lower than the one measured pre-CRRP [2 (2-2) vs. 2 (1–2), p < 0.003]; but the zero mean difference between the two periods does not exceed the MCID of one point (Crisafulli and Clini, 2010).
The present study has two limitations. First, the lack of a control group is a major limitation. Indeed, the inclusion of a control group was reported only in few studies (Liu K. et al., 2020; Spielmanns et al., 2021) (Table 5). Several studies (Hermann et al., 2020; Betschart et al., 2021; Bouteleux et al., 2021; Daynes et al., 2021; Gloeckl et al., 2021; Piquet et al., 2021; Puchner et al., 2021) have included only one group (Table 4) and it was difficult to include a control group due to ethical considerations during the COVID19 pandemic. The lack of a control group did not allow us to “affirm” that our results are only attributable to CRRP. Indeed, one study reported that lung function data of most COVID19 patients improve spontaneously over 3-month period (Wu et al., 2021). Second, it would have been more interesting to explore the respiratory function using additional tests, such as plethysmography (Puchner et al., 2021), DLCO (Liu K. et al., 2020; Gloeckl et al., 2021; Puchner et al., 2021), and maximal inspiratory pressure (Puchner et al., 2021), and exercise data using a cardiopulmonary exercise test in order to determine the first ventilatory threshold. In COVID19 patients, the most frequent lung function impairment is altered DLCO (39%) (Torres-Castro et al., 2021). Due to the unavailability of equipment in our public health hospital, these examinations were not performed.
5 Conclusion
A 4-week CRRP in post-COVID19 patients improved dyspnea (mMRC), FEV1, 6MWD, 6-min walk work, resting heart-rate and DBP. CRRP has imposed itself as a standard of care for the treatment of post-COVID19 patients.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Farhat HACHED Hospital medical and research ethics committee (Approval number FH 2502/2021). The patients/participants provided their written informed consent to participate in this study.
Author contributions
ET conceived the study, participated in its design, the statistical analysis, performed the questionnaires and the exercise training and helped to draft the manuscript. WB conceived the study, participated in its design, the statistical analysis, performed the questionnaires and helped to draft the manuscript. CR helped to draft the manuscript. SA helped to draft the manuscript. InG performed the spirometry, HGS and 6MWT tests, and helped to draft the manuscript. AK helped to draft the manuscript. AS performed the spirometry, HGS and 6MWT tests, and helped to draft the manuscript. SS performed the spirometry, HGS and 6MWT tests, and helped to draft the manuscript. KM performed the spirometry, HGS and 6MWT tests, and helped to draft the manuscript. SJ helped to draft the manuscript. ImG helped to draft the manuscript. AH helped to draft the manuscript. WO helped to draft the manuscript. AA helped to draft the manuscript and coordinated the study. HBS conceived the study, participated in its design, performed the spirometry, HGS and 6MWT tests, performed the statistical analysis, helped to draft the manuscript and coordinated the study. All authors read and approved the final version of the manuscript.
Acknowledgments
Authors wish to thank Pr. Samir Boukattay for his invaluable contribution in the improvement of the quality of the writing in the present paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer IL declared to the handling editor a shared affiliation with all authors besides AA at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akwa L. G., Moses M. O., Emikpe A. O., Baffour-Awuah B., Asamoah B., Addai-Mensah O., et al. (2017). Lipid profile, cardiorespiratory function and quality of life of postmenopausal women improves with aerobic exercise. J. Hum. Sport Exerc. 12 (3), 698–709. doi:10.14198/jhse.2017.123.14
Aladin A. I., Whelton S. P., Al-Mallah M. H., Blaha M. J., Keteyian S. J., Juraschek S. P., et al. (2014). Relation of resting heart rate to risk for all-cause mortality by gender after considering exercise capacity (the Henry Ford exercise testing project). Am. J. Cardiol. 114 (11), 1701–1706. doi:10.1016/j.amjcard.2014.08.042
Ali M., Bonna A. S., Sarkar A. S., Islam A. (2022). Is coronavirus infection associated with musculoskeletal health complaints? Results from a comprehensive case-control study. J. Prim. Care Community Health 13, 21501319221114259. doi:10.1177/21501319221114259
Ammar A., Boukhris O., Halfpaap N., Labott B. K., Langhans C., Herold F., et al. (2021). Four weeks of detraining induced by COVID-19 reverse cardiac improvements from eight weeks of fitness-dance training in older adults with mild cognitive impairment. Int. J. Environ. Res. Public Health 18 (11), 5930. doi:10.3390/ijerph18115930
Aune D., Sen A., o'Hartaigh B., Janszky I., Romundstad P. R., Tonstad S., et al. (2017). Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality - a systematic review and dose-response meta-analysis of prospective studies. Nutr. Metab. Cardiovasc. Dis. 27 (6), 504–517. doi:10.1016/j.numecd.2017.04.004
Barker-Davies R. M., O'Sullivan O., Senaratne K. P. P., Baker P., Cranley M., Dharm-Datta S., et al. (2020). The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 54 (16), 949–959. doi:10.1136/bjsports-2020-102596
Ben Saad H., Babba M., Boukamcha R., Ghannouchi I., Latiri I., Mezghenni S., et al. (2014). Investigation of exclusive narghile smokers: Deficiency and incapacity measured by spirometry and 6-minute walk test. Respir. Care 59 (11), 1696–1709. doi:10.4187/respcare.03058
Ben Saad H., Ben Abdelkrim S. (2005). Daily exercise training. A practical session. Available from this URL: https://www.respir-sud.com/english/rehabilitation/12/105/2/D%c3%a9monstration.html; Last visit September 8 2022 [Online].
Ben Saad H., Ben Hassen I., Ghannouchi I., Latiri I., Rouatbi S., Escourrou P., et al. (2015). 6-Min walk-test data in severe obstructive-sleep-apnea-hypopnea-syndrome (OSAHS) under continuous-positive-airway-pressure (CPAP) treatment. Respir. Med. 109 (5), 642–655. doi:10.1016/j.rmed.2015.03.001
Ben Saad H., El Attar M. N., Hadj Mabrouk K., Ben Abdelaziz A., Abdelghani A., Bousarssar M., et al. (2013). The recent multi-ethnic global lung initiative 2012 (GLI2012) reference values don't reflect contemporary adult's North African spirometry. Respir. Med. 107 (12), 2000–2008. doi:10.1016/j.rmed.2013.10.015
Ben Saad H., Hamadou R., Ben Cheikh I., Chouchene A., Rejeb N., Zbidi A., et al. (2008). Réadaptation respiratoire des malades atteints d’une bronchopneumopathie chronique obstructive : Données préliminaires de l’expérience tunisienne. J. de Readaptation Medicale Pratique Form. en Med. Physique de Readaptation 28 (4), 138–147. doi:10.1016/j.jmr.2008.09.001
Ben Saad H. (2020). Interpretation of respiratory functional explorations of deficiency and incapacity in adult. Tunis. Med. 98 (11), 797–815.
Ben Saad H., Prefaut C., Tabka Z., Mtir A. H., Chemit M., Hassaoune R., et al. (2009). 6-minute walk distance in healthy North africans older than 40 years: Influence of parity. Respir. Med. 103 (1), 74–84. doi:10.1016/j.rmed.2008.07.023
Bessis S. (2020). The COVID-19 pandemic. Med. Mal. Infect. 50 (8), 8S20–8S24. doi:10.1016/S0399-077X(20)30779-4
Betschart M., Rezek S., Unger I., Beyer S., Gisi D., Shannon H., et al. (2021). Feasibility of an outpatient training program after COVID-19. Int. J. Environ. Res. Public Health 18 (8), 3978. doi:10.3390/ijerph18083978
Bouteleux B., Henrot P., Ernst R., Grassion L., Raherison-Semjen C., Beaufils F., et al. (2021). Respiratory rehabilitation for covid-19 related persistent dyspnoea: A one-year experience. Respir. Med. 189, 106648. doi:10.1016/j.rmed.2021.106648
Cadenas-Sanchez C., Sanchez-Delgado G., Martinez-Tellez B., Mora-Gonzalez J., Lof M., Espana-Romero V., et al. (2016). Reliability and validity of different models of TKK hand dynamometers. Am. J. Occup. Ther. 70 (4), 7004300010. doi:10.5014/ajot.2016.019117
Carda S., Invernizzi M., Bavikatte G., Bensmail D., Bianchi F., Deltombe T., et al. (2020). COVID-19 pandemic. What should physical and rehabilitation medicine specialists do? A clinician's perspective. Eur. J. Phys. Rehabil. Med. 56 (4), 515–524. doi:10.23736/S1973-9087.20.06317-0
Carter R., Holiday D. B., Nwasuruba C., Stocks J., Grothues C., Tiep B. (2003). 6-minute walk work for assessment of functional capacity in patients with COPD. Chest 123 (5), 1408–1415. doi:10.1378/chest.123.5.1408
Cheval B., Sieber S., Maltagliati S., Millet G. P., Formanek T., Chalabaev A., et al. (2021). Muscle strength is associated with COVID-19 hospitalization in adults 50 years of age or older. J. Cachexia Sarcopenia Muscle 12 (5), 1136–1143. doi:10.1002/jcsm.12738
Chuang M. L., Lin I. F., Wasserman K. (2001). The body weight-walking distance product as related to lung function, anaerobic threshold and peak VO2 in COPD patients. Respir. Med. 95 (7), 618–626. doi:10.1053/rmed.2001.1115
Crisafulli E., Clini E. M. (2010). Measures of dyspnea in pulmonary rehabilitation. Multidiscip. Respir. Med. 5 (3), 202–210. doi:10.1186/2049-6958-5-3-202
Cruz-Jentoft A. J., Bahat G., Bauer J., Boirie Y., Bruyere O., Cederholm T., et al. (2019). Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48 (1), 16–31. doi:10.1093/ageing/afy169
Daynes E., Gerlis C., Chaplin E., Gardiner N., Singh S. J. (2021). Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition - a cohort study. Chron. Respir. Dis. 18, 14799731211015691. doi:10.1177/14799731211015691
Delecluse C., Colman V., Roelants M., Verschueren S., Derave W., Ceux T., et al. (2004). Exercise programs for older men: Mode and intensity to induce the highest possible health-related benefits. Prev. Med. 39 (4), 823–833. doi:10.1016/j.ypmed.2004.03.023
Demeco A., Marotta N., Barletta M., Pino I., Marinaro C., Petraroli A., et al. (2020). Rehabilitation of patients post-COVID-19 infection: A literature review. J. Int. Med. Res. 48 (8), 300060520948382. doi:10.1177/0300060520948382
Dixit S., Borghi-Silva A., Bairapareddy K. C. (2021). Revisiting pulmonary rehabilitation during COVID-19 pandemic: A narrative review. Rev. Cardiovasc. Med. 22 (2), 315–327. doi:10.31083/j.rcm2202039
Fabre C., Chehere B., Bart F., Mucci P., Wallaert B., Grosbois J. M. (2017). Relationships between heart rate target determined in different exercise testing in COPD patients to prescribed with individualized exercise training. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 1483–1489. doi:10.2147/COPD.S129889
Ferris B. G. (1978). Epidemiology standardization project (American thoracic society). Am. Rev. Respir. Dis. 118 (6), 1–120.
Fletcher C. M., Elmes P. C., Fairbairn A. S., Wood C. H. (1959). The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br. Med. J. 2 (5147), 257–266. doi:10.1136/bmj.2.5147.257
Gamelin F. X., Berthoin S., Sayah H., Libersa C., Bosquet L. (2007). Effect of training and detraining on heart rate variability in healthy young men. Int. J. Sports Med. 28 (7), 564–570. doi:10.1055/s-2007-964861
Ghram A., Ayadi H., Knechtle B., Ben Saad H. (2022). What should a family physician know about nutrition and physical exercise rehabilitation' advices to communicate to 'long-term COVID-19' patients? Postgrad. Med. 134 (2), 143–147. doi:10.1080/00325481.2022.2035589
Gloeckl R., Leitl D., Jarosch I., Schneeberger T., Nell C., Stenzel N., et al. (2021). Benefits of pulmonary rehabilitation in COVID-19: A prospective observational cohort study. ERJ Open Res. 7 (2), 00108-2021. doi:10.1183/23120541.00108-2021
Greenland P., Daviglus M. L., Dyer A. R., Liu K., Huang C. F., Goldberger J. J., et al. (1999). Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: The chicago heart association detection project in industry. Am. J. Epidemiol. 149 (9), 853–862. doi:10.1093/oxfordjournals.aje.a009901
Haidar S. G., Kumar D., Bassi R. S., Deshmukh S. C. (2004). Average versus maximum grip strength: Which is more consistent? J. Hand Surg. Br. 29 (1), 82–84. doi:10.1016/j.jhsb.2003.09.012
Hermann M., Pekacka-Egli A. M., Witassek F., Baumgaertner R., Schoendorf S., Spielmanns M. (2020). Feasibility and efficacy of cardiopulmonary rehabilitation after COVID-19. Am. J. Phys. Med. Rehabil. 99 (10), 865–869. doi:10.1097/PHM.0000000000001549
Holland A. E., Spruit M. A., Troosters T., Puhan M. A., Pepin V., Saey D., et al. (2014). An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 44 (6), 1428–1446. doi:10.1183/09031936.00150314
Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. (2021). 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 397 (10270), 220–232. doi:10.1016/S0140-6736(20)32656-8
Jamil S., Mark N., Carlos G., Cruz C. S. D., Gross J. E., Pasnick S. (2020). Diagnosis and management of COVID-19 disease. Am. J. Respir. Crit. Care Med. 201 (10), P19–P20. doi:10.1164/rccm.2020C1
Jenkins A. R., Gowler H., Curtis F., Holden N. S., Bridle C., Jones A. W. (2018). Efficacy of supervised maintenance exercise following pulmonary rehabilitation on health care use: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 257–273. doi:10.2147/COPD.S150650
Lee H. M., Le H., Lee B. T., Lopez V. A., Wong N. D. (2010). Forced vital capacity paired with Framingham Risk Score for prediction of all-cause mortality. Eur. Respir. J. 36 (5), 1002–1006. doi:10.1183/09031936.00042410
Liu K., Zhang W., Yang Y., Zhang J., Li Y., Chen Y. (2020). Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 39, 101166. doi:10.1016/j.ctcp.2020.101166
Liu Y., Mao B., Liang S., Yang J. W., Lu H. W., Chai Y. H., et al. (2020). Association between age and clinical characteristics and outcomes of COVID-19. Eur. Respir. J. 55 (5), 2001112. doi:10.1183/13993003.01112-2020
Long B., Brady W. J., Koyfman A., Gottlieb M. (2020). Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 38 (7), 1504–1507. doi:10.1016/j.ajem.2020.04.048
Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P. A., Cuapio A., et al. (2021). More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 11 (1), 16144. doi:10.1038/s41598-021-95565-8
Mahler D. A., Wells C. K. (1988). Evaluation of clinical methods for rating dyspnea. Chest 93 (3), 580–586. doi:10.1378/chest.93.3.580
Marik P. E., DePerrior S. E., Ahmad Q., Dodani S. (2021). Gender-based disparities in COVID-19 patient outcomes. J. Investig. Med. 69 (4), 814–818. doi:10.1136/jim-2020-001641
Mascha E. J., Vetter T. R. (2018). Significance, errors, power, and sample size: The blocking and tackling of statistics. Anesth. Analg. 126 (2), 691–698. doi:10.1213/ANE.0000000000002741
Miller M. R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., et al. (2005). Standardisation of spirometry. Eur. Respir. J. 26 (2), 319–338. doi:10.1183/09031936.05.00034805
Murk W., Gierada M., Fralick M., Weckstein A., Klesh R., Rassen J. A. (2021). Analyse d’ensemble des complications de la COVID-19 : Étude d’auto-appariement pré- et post-exposition. Can. Med. Assoc. J. 193 (11), E389–E398. doi:10.1503/cmaj.201686-f
Nelson M. E., Rejeski W. J., Blair S. N., Duncan P. W., Judge J. O., King A. C., et al. (2007). Physical activity and public health in older adults: Recommendation from the American college of sports medicine and the American heart association. Circulation 116 (9), 1094–1105. doi:10.1161/CIRCULATIONAHA.107.185650
Piquet V., Luczak C., Seiler F., Monaury J., Martini A., Ward A. B., et al. (2021). Do patients with COVID-19 benefit from rehabilitation? Functional outcomes of the first 100 patients in a COVID-19 rehabilitation unit. Arch. Phys. Med. Rehabil. 102 (6), 1067–1074. doi:10.1016/j.apmr.2021.01.069
Puchner B., Sahanic S., Kirchmair R., Pizzini A., Sonnweber B., Woll E., et al. (2021). Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: An observational cohort study. Eur. J. Phys. Rehabil. Med. 57 (2), 189–198. doi:10.23736/S1973-9087.21.06549-7
Puthucheary Z., Harridge S., Hart N. (2010). Skeletal muscle dysfunction in critical care: Wasting, weakness, and rehabilitation strategies. Crit. Care Med. 38 (10), S676–S682. doi:10.1097/CCM.0b013e3181f2458d
Revel M. P., Parkar A. P., Prosch H., Silva M., Sverzellati N., Gleeson F., et al. (2020). COVID-19 patients and the radiology department - advice from the European society of radiology (ESR) and the European society of thoracic imaging (ESTI). Eur. Radiol. 30 (9), 4903–4909. doi:10.1007/s00330-020-06865-y
Rezende Barbosa M., Oliveira V. C., Silva A., Perez-Riera A. R., Vanderlei L. C. (2018). Effectiveness of functional training on cardiorespiratory parameters: A systematic review and meta-analysis of randomized controlled trials. Clin. Physiol. Funct. Imaging 38 (4), 539–546. doi:10.1111/cpf.12445
Schmidt J. F., Hansen P. R., Andersen T. R., Andersen L. J., Hornstrup T., Krustrup P., et al. (2014). Cardiovascular adaptations to 4 and 12 months of football or strength training in 65- to 75-year-old untrained men. Scand. J. Med. Sci. Sports 24 (1), 86–97. doi:10.1111/sms.12217
Sergysels R., Hayot M. (1997). Evaluation of exercise-induced dyspnea. Rev. Pneumol. Clin. 53 (5), 278–282.
Serhier Z., Bendahhou K., Ben Abdelaziz A., Bennani M. O. (2020). Methodological sheet n degrees 1: How to calculate the size of a sample for an observational study? Tunis. Med. 98 (1), 1–7.
Shi L., Wang Y., Wang Y., Duan G., Yang H. (2020). Dyspnea rather than fever is a risk factor for predicting mortality in patients with COVID-19. J. Infect. 81 (4), 647–679. doi:10.1016/j.jinf.2020.05.013
Simpson R., Robinson L. (2020). Rehabilitation after critical illness in people with COVID-19 infection. Am. J. Phys. Med. Rehabil. 99 (6), 470–474. doi:10.1097/PHM.0000000000001443
Singh R. B., Weydahl A., Otsuka K., Watanabe Y., Yano S., Mori H., et al. (2000). Can nutrition influence circadian rhythm and heart rate variability? Biomed. Pharmacother. 55 (1), s115–s124. doi:10.1016/s0753-3322(01)90016-2
Singh S. J., Puhan M. A., Andrianopoulos V., Hernandes N. A., Mitchell K. E., Hill C. J., et al. (2014). An official systematic review of the European respiratory society/American thoracic society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 44 (6), 1447–1478. doi:10.1183/09031936.00150414
Skjorten I., Ankerstjerne O. A. W., Trebinjac D., Bronstad E., Rasch-Halvorsen O., Einvik G., et al. (2021). Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur. Respir. J. 58 (2), 2100996. doi:10.1183/13993003.00996-2021
Soler X., Diaz-Piedra C., Ries A. L. (2013). Pulmonary rehabilitation improves sleep quality in chronic lung disease. COPD 10 (2), 156–163. doi:10.3109/15412555.2012.729622
Spielmanns M., Pekacka-Egli A. M., Schoendorf S., Windisch W., Hermann M. (2021). Effects of a comprehensive pulmonary rehabilitation in severe post-COVID-19 patients. Int. J. Environ. Res. Public Health 18 (5), 2695. doi:10.3390/ijerph18052695
Spruit M. A., Holland A. E., Singh S. J., Tonia T., Wilson K. C., Troosters T. (2020). COVID-19: Interim guidance on rehabilitation in the hospital and post-hospital phase from a European respiratory society and American thoracic society-coordinated international task force. Eur. Respir. J. 56 (6), 2002197. doi:10.1183/13993003.02197-2020
Tanaka H., Monahan K. D., Seals D. R. (2001). Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 37 (1), 153–156. doi:10.1016/s0735-1097(00)01054-8
Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., Solis-Navarro L., Burgos F., Puppo H., et al. (2021). Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 27 (4), 328–337. doi:10.1016/j.pulmoe.2020.10.013
Tsai A. G., Wadden T. A. (2013). In the clinic: Obesity. Ann. Intern. Med. 159 (5), ITC3-1–ITC3-15. doi:10.7326/0003-4819-159-5-201309030-01003
Welch C., Greig C., Masud T., Wilson D., Jackson T. A. (2020). COVID-19 and acute sarcopenia. Aging Dis. 11 (6), 1345–1351. doi:10.14336/AD.2020.1014
WHO (2021). Living guidance for clinical management of COVID-19. Available from this URl: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2; Last visit September 8 2022 [Online].
Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., et al. (2018). 2018 ESC/ESH guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: The task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J. Hypertens. 36 (10), 1953–2041. Erratum in: J Hypertens. 2019 Jan;37(1):226. doi:10.1097/HJH.0000000000001940
Wong A. W., Lopez-Romero S., Figueroa-Hurtado E., Vazquez-Lopez S., Milne K. M., Ryerson C. J., et al. (2021). Predictors of reduced 6-minute walk distance after COVID-19: A cohort study in Mexico. Pulmonology 27 (6), 563–565. doi:10.1016/j.pulmoe.2021.03.004
Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., et al. (2021). 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet. Respir. Med. 9 (7), 747–754. doi:10.1016/S2213-2600(21)00174-0
Xie Y., Xu E., Bowe B., Al-Aly Z. (2022). Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28 (3), 583–590. doi:10.1038/s41591-022-01689-3
Yaddanapudi L. N. (2016). The American Statistical Association statement on P-values explained. J. Anaesthesiol. Clin. Pharmacol. 32 (4), 421–423. doi:10.4103/0970-9185.194772
Yuksel M., Yildiz A., Demir M., Bilik M. Z., Ozaydogdu N., Aktan A., et al. (2014). Effect of sleep quality on hemodynamic response to exercise and heart rate recovery in apparently healthy individuals. Clin. Invest. Med. 37 (6), E352–E362. doi:10.25011/cim.v37i6.22240
Zhao H. M., Xie Y. X., Wang C.Chinese Association of Rehabilitation, M.Respiratory Rehabilitation Committee of Chinese Association of Rehabilitation, M.Cardiopulmonary Rehabilitation Group of Chinese Society of Physical, M. (2020). Recommendations for respiratory rehabilitation in adults with coronavirus disease 2019. Chin. Med. J. 133 (13), 1595–1602. doi:10.1097/CM9.0000000000000848
Keywords: handicap, health status, lung function test, SARS-Cov-2, walking, 6MWT
Citation: Toulgui E, Benzarti W, Rahmani C, Aissa S, Ghannouchi I, Knaz A, Sayhi A, Sellami S, Mahmoudi K, Jemni S, Gargouri I, Hayouni A, Ouanes W, Ammar A and Ben saad H (2022) Impact of cardiorespiratory rehabilitation program on submaximal exercise capacity of Tunisian male patients with post-COVID19: A pilot study. Front. Physiol. 13:1029766. doi: 10.3389/fphys.2022.1029766
Received: 27 August 2022; Accepted: 12 September 2022;
Published: 28 September 2022.
Edited by:
Mohamed Souhaiel Chelly, Higher Institute of Sport and Physical Education of Ksar Saïd, University of Manouba, TunisiaReviewed by:
Amine Ghram, University of Tehran, IranImed Latiri, Medical faculty Ibn Jazzar, University of Sousse, Tunisia
Copyright © 2022 Toulgui, Benzarti, Rahmani, Aissa, Ghannouchi, Knaz, Sayhi, Sellami, Mahmoudi, Jemni, Gargouri, Hayouni, Ouanes, Ammar and Ben saad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Achraf Ammar, acammar@uni-mainz.de
†These authors have contributed equally to this work and share first authorship
‡These authors share last authorship
 Emna Toulgui
Emna Toulgui Wafa Benzarti2†
Wafa Benzarti2† Amani Sayhi
Amani Sayhi Sana Sellami
Sana Sellami Achraf Ammar
Achraf Ammar Helmi Ben saad
Helmi Ben saad